Open Access Article
Open Access ArticleHighly reactive photothermal initiating system based on sulfonium salts for the photoinduced thermal frontal cationic polymerization of epoxides: a way to create carbon-fiber reinforced polymers
Benoit Gacheta,
Maxime Lecompèrea,
Céline Croutxé-Barghorn a,
Dominique Burra,
Gildas L'Hostisb and
Xavier Allonas
a,
Dominique Burra,
Gildas L'Hostisb and
Xavier Allonas *a
*a
aLaboratoire de Photochimie et d'Ingénierie Macromoléculaires, Université de Haute Alsace, 3b rue Alfred Werner, 68093 Mulhouse, France. E-mail: xavier.allonas@uha.fr
bLaboratoire de Physique et Mécanique Textiles (LPMT), Université de Haute Alsace, 11 rue Alfred Werner, 68093 Mulhouse, France
First published on 18th November 2020
Abstract
A new combination of sulfonium salts has been investigated to cure opaque and thick carbon composite materials through photoinduced thermal frontal polymerization reaction. The photopolymerization occurs at the surface of the cycloaliphatic epoxide through the excitation of a triarylsulfonium salt and releases enough heat to decompose an alkyl-based sulfonium salt acting as a latent thermal initiator. Thus, a thermal front propagates into the medium leading to the polymerization of the whole sample. Thermal properties and optimal parameters are investigated to obtain frontal polymerization in the depth of the material. Front velocities were as high as 12.9 cm min−1 and were found to increase with an increasing concentration of thermal sulfonium salt. The effect of an addition of carbon filler is investigated with a concentration of up to 50 wt%, which allows the formation of a composite material with a high content of carbon without the need for thermal post curing.
Introduction
Fiber-reinforced polymers (composites) based on epoxy resins are commonly polymerized using a thermal process. A hardener, such as an amine, can react and open the epoxide ring to create a three-dimensional polymer network. This reaction can require a long processing time and is generally achieved at elevated temperature.1 On the other hand, epoxide monomers can be rapidly photopolymerized by using an onium salt as a photoinitiator, such as iodonium or sulfonium salt.2 However, in that case, due to the limited penetration of light in the resin, the depth curing of a composite is hardly achieved, especially when using opaque fibers like carbon or aramid ones.Nonetheless, in the past decade, a new approach was developed to overcome this drawback through dual-cure polymerization reactions. It has been shown that different frontal polymerization reactions can be used to cure the whole thickness of a composite, a process that takes advantage of a surface irradiation of the sample for initiating the reaction over the whole thickness.3 These reactions have been developed for both unsaturated polyester4–6 and epoxy7–14 resins. Recently, it has been found that 2,4,6-triphenylpyrylium salt can act as both photo- and thermal- initiator, leading to unprecedented efficiency in the photoinduced thermal frontal polymerization. Indeed, this system has the ability to initiate the cationic photopolymerization under visible light (395 nm) and also at room temperature through an activated reaction. However, the pyrylium salt is chemically unstable, leading to relatively poor pot-life.
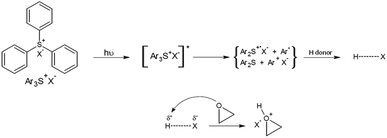
Sulfonium salts are well known as cationic photoinitiators for the polymerization of epoxy resin, especially since J. Crivello discovered and studied the triarylsulfonium salts in the 70 s.15,16 Under absorption of light, these compounds release Brönsted acids. The strength of these acids depends of its associated counter-ion (SbF6− > AsF6− > PF6− ≫ BF4−). Triarylsulfonium structures do not show any particular thermal activity, which makes them very stable. The initiation mechanism under light is shown in Scheme 1.
In the 80 s, it has been shown that some sulfonium salts can act as efficient thermal initiators for cationic polymerization of an epoxy resin.17,18 These interesting features were obtained by introducing an aliphatic group in the structure instead of a phenyl one, resulting in a lower thermal stability. However, these modifications also induced a lack of photochemical reactivity. It has been shown that several sulfonium salts can be used as thermal cationic initiators, their efficiencies and their thermal range of reactivity being tuned by modification of the alkyl group.19
In this paper, a novel dual-cure approach was developed using a blend of two sulfonium salts: a photoactive one was used to initiate the polymerization and a thermally active one used to cure the composite in depth. It is shown that the combination of these two initiators can yield a photoinduced thermal frontal polymerization of the epoxide resin. In addition, the thermal front was found to be sustained in the presence of various amounts of carbon fibers. This allows the manufacturing of carbon-reinforced composites with great ease and in a fast time scale.
Experimental
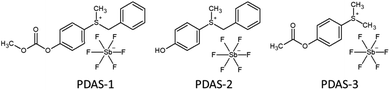
Triarylsulfonium hexafluoroantimonate salt (TAS, mixed in 50 wt% in propylene carbonate) was obtained from Merck. Phenyl-dialkyl sulfonium salts used as thermal initiators (Scheme 2) were obtained from Sanshin Chemical Industry and were dissolved in propylene carbonate at a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Carbon powder (glassy, spherical powder, 2–12 μm, 99.95% trace metals basis) and propylene carbonate (PC) were purchased from Merck. (3,4-Epoxycyclohexyl)methyl-3,4-epoxycyclohexyl carboxylate (DiEpox, Genomer 7210 from Rahn) was used as bifunctional epoxide. All these products were used without any purification.
3. Carbon powder (glassy, spherical powder, 2–12 μm, 99.95% trace metals basis) and propylene carbonate (PC) were purchased from Merck. (3,4-Epoxycyclohexyl)methyl-3,4-epoxycyclohexyl carboxylate (DiEpox, Genomer 7210 from Rahn) was used as bifunctional epoxide. All these products were used without any purification.
Photopolymerization analyses were achieved by using Real-Time FTIR setup based on a Vertex 70 from Bruker with a MIR source. MCT detector was used to record data every 120 ms. Samples with thicknesses of approximatively 12 μm were analysed at room temperature on KBr pellet. UV irradiation was carried out during 300 s at 230 mW cm−2 by a UFIBER LED from UWave company at 365 nm. Rate conversion was monitored by following the decrease of epoxy ring vibration band at 789 cm−1.
Differential Scanning Calorimetry (DSC) were carried out on a Q200 from TA instruments. Samples of approximatively 5 mg were sealed into hermetic pans. Analyses were performed under nitrogen flow at heat rate of 10 °C min−1.
Ultraviolet-visible spectroscopy analyses have been achieved on a Specord 210 from Analytik Jena.
Thermal analyses were recorded by IR thermal camera (FLIR Systems – model A325sc). UV irradiation was carried out on a 4 mm thick zone of 1 cm2, during 60 s at 250 mW cm−2 by a UFIBER LED at 365 nm. The formulation was placed horizontally in a polytetrafluoroethylene (PTFE) mould. The IR camera was mounted above the mould to follow the progress of the thermal front. A black wall was used to hide the light diffusion of the lamp. The post-experimental digital processing was carried out in order to determine the temperature rise along the thermal front.
Fiber composites were made by brush impregnation of 8 layers of 3 K plain weave carbon fabric, 204 gsm with dimensions of 15 cm by 2 cm. The resin content in the composite was 50 ± 2 wt%. Irradiation was carried out during 300 s at 250 mW cm−2 by a FireJet FJ200 LED (15 cm × 2 cm) at 365 nm from Phoseon. Temperature was recorded by type K thermocouples with multi-channel station DaqPRO 5300 between the ply 4 and 5.
Results and discussion
Photochemical behaviour
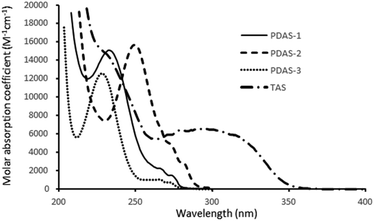
The UV-spectra of all sulfonium salts used in this paper were recorded in acetonitrile and their molar absorption coefficient are reported in Fig. 1.Although it has been shown that PDAS-1 can effectively lead to a photoinitiation reaction when exposed at short UV-light, none of the alkyl derivatives (PDAS-1, PDAS-2 or PDAS-3) absorb beyond 300 nm.20,21 As a consequence, a direct excitation of the aliphatic sulfonium salts is not possible under LED irradiation at 365 nm. By contrast, TAS absorbs the light until 365 nm with an absorption coefficient of 62 M−1 cm−1 at this wavelength. Fig. 2 shows the photopolymerization profiles obtained in the presence of alkyl-based sulfonium salt PDAS-1 or triarylsulfonium TAS.
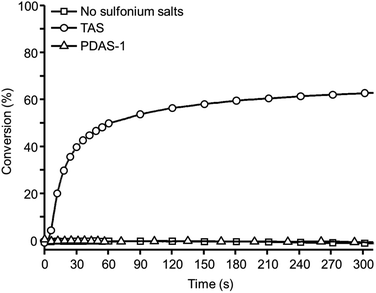 | ||
| Fig. 2 Kinetics of photopolymerization of DiEpox recorded by RT FTIR under LED irradiation at 365 nm and 230 mW cm−2. | ||
From Fig. 2, it is clear that the use of triarylsulfonium salt is necessary to photoinduced the polymerization process. Indeed, TAS leads to a fast photopolymerization of the epoxide with a relatively high final conversion of 63%. The rate of conversion of the reactive functions is 0.017 s−1. Due to the fast photopolymerization reaction in the presence of TAS, an increase in the temperature can be expected for thick sample. Therefore, pyrometric measurement was carried out in order to study the exothermicity generated during the photopolymerization. Several samples (4 mm thick cylinder of 1 cm diameter) of DiEpox with various amounts of TAS were prepared and then irradiated under a 365 nm LED at 250 mW cm−2 during 60 s. The IR camera was positioned to follow the temperature of the sample surface. The temperature does not change when DiEpox is irradiated in the absence of any initiator because of the lack of reaction, which is in agreement with RT-FTIR results. In the presence of TAS, the exotherm of the photopolymerization reaction is very high and an important temperature jump is detected.
Indeed, during the experiment, the surface temperature exceeds 250 °C with a concentration range of TAS between 1 and 3 wt% (Fig. 3). It is concluded that this photoinitiator is very effective to rapidly generate a large amount of heat which can be useful to initiated a thermal process in depth.
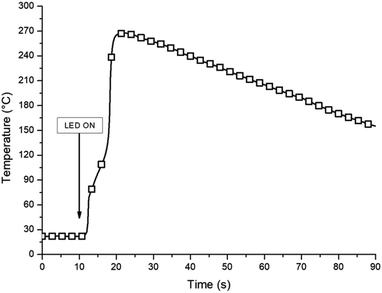 | ||
| Fig. 3 Temperature profile measured during the photopolymerization of DiEpox in the presence of 1 wt% of TAS. | ||
Thermal polymerization
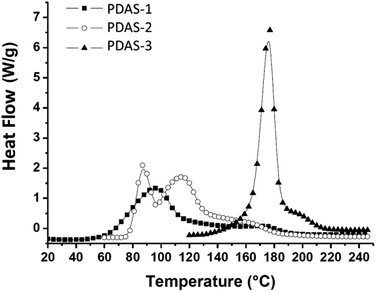
DSC experiments were performed on DiEpox in the presence of alkyl-based sulfonium salts to study the ability of these salts to thermally initiate the polymerization reaction. Fig. 4 shows that some exotherms can be detected when PDAS-1, PDAS-2 and PDAS-3 are used as thermal initiators. Fast polymerization reaction of epoxide with PDAS-2 was found, in agreement with results reported in the literature for glycidyl phenyl ether.22The corresponding enthalpies and onsets of polymerization peaks are collected in Table 1. The onset temperature Tonset is related to the temperature at which the sulfonium salt starts to decompose and is related to its stability. As can be seen, the reactivity was found to strongly depend of the sulfonium structure. The salts studied here presented a Tonset range between 62 °C and 164 °C. With regard to polymerization efficiency, PDAS-2 and PDAS-3 salts have a polymerization exotherm of about 650 J g−1, which is significantly higher than PDAS-1 (520 J g−1). The two salts PDAS-2 and PDAS-3 lead both to higher completion of the polymerization, but have a much higher decomposition temperature than PDAS-1.
| Compound | Tonset (°C) | ΔH (J g−1) |
|---|---|---|
| PDAS-1 | 62 | 520 |
| PDAS-2 | 78 | 650 |
| PDAS-3 | 164 | 650 |
Two different mechanisms were proposed to account for the cationic polymerization of spiroorthocarbonates initiated by aliphatic sulfonium salts, i.e. SN1 or SN2 reactions.18 Then, on the basis of substituent effects on benzyl sulfonium salts, it has been proposed that the thermal dissociation of the sulfonium salt leads to a neutral sulfide and an aliphatic cation, the latter being responsible for the epoxide polymerization through a SN1 reaction.23
As a consequence, the initiation reaction should depend on the stability of the intermediates. Therefore, the C–S bond dissociation energy (BDE) leading to the more stable cation was computed at the B3LYP/6-311++G** level on the basis of structures optimized at the B3LYP/6-31G* level. The BDE are collected in Table 2. From this table, it can be seen that the cleavage process strongly depends on the stability of the released cation. The formation of a methyl cation requires about 450 to 500 kJ mol−1. It is obvious that this could not be the cation released. By contrast, the formation of a benzyl cation in the case of PDAS-1 and PDAS-2 and a methoxycarbonyloxylphenyl cation in the case of PDAS-3 requires much less energy, between 144 and 363 kJ mol−1. Interestingly, the onset temperature for the polymerization of the resin is related to the lowest BDE values of the sulfonium salts. Indeed, it can be seen in Fig. 5 that the onset temperature linearly depends on the lowest bond dissociation energy of the sulfonium salts. This clearly underlines the fact that the cleavage reaction of the sulfonium salt is the limiting step in the thermal cationic polymerization of the cycloaliphatic epoxide.
| Compound | Cation formed | BDE (kJ mol−1) |
|---|---|---|
| PDAS-1 | methyl+ | 478 |
| benzyl+ | 144 | |
| PDAS-2 | methyl+ | 492 |
| benzyl+ | 161 | |
| PDAS-3 | methyl+ | 456 |
| methoxycarbonyloxylphenyl+ | 363 |
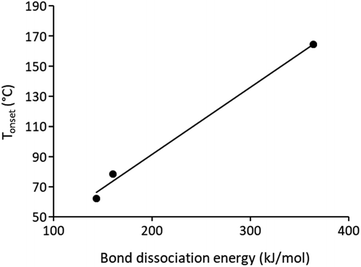 | ||
| Fig. 5 Plot of the onset temperature Tonset for the thermal curing of DiEpox with the bond dissociation energy of the sulfonium salt used as initiator. | ||
These results allow a rational design of the photothermal initiating system, by choosing the sulfonium salt exhibiting the best reactivity. It appears that PDAS-1 will be the most suitable for carrying out a frontal polymerization due to the lowest DBE and Tonset values. In addition, the whole thermogram obtained with PDAS-1 matches quite well with the maximum temperature reached by photopolymerization of this resin with TAS. A combination of these two sulfonium salts can be made to obtain a photoinduced thermal frontal polymerization of DiEpox.
Photoinduced thermal frontal polymerization
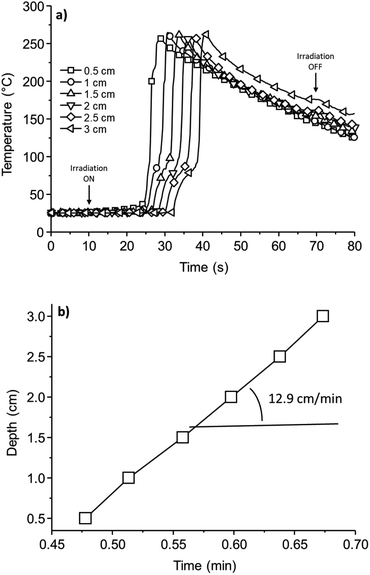
Having clarified the role of the photoinitiator and the reactivity of the thermal initiators, it is expected that a mixture of both initiating systems can be effective for photoinducing the thermal frontal polymerization of the cycloaliphatic epoxide. Therefore, formulations of DiEpox containing 1 wt% of triarylsulfonium salt and various amounts of thermal sulfonium salts were prepared. The samples were thus exposed to light during 60 s, and a IR thermal camera was used to follow the exotherm. A typical experiment is shown in Fig. 6. During irradiation, photopolymerization takes place at the surface and an increase in the temperature is observed as a consequence of the heat of reaction evolved in the medium. It could be seen that this temperature front is moving within the medium due to the decomposition of the thermal initiator and the resulting polymerization in successive layers. From this experiment, the front velocity can be obtained by plotting the time to reach the maximum temperature for each value of depth (Fig. 6b).When using PDAS-3, no photoinduced thermal frontal polymerization was observed. Although that the photopolymerization is achieved at the surface, the temperature jump is not high enough to decompose the thermal sulfonium salt. Therefore, even at PDAS-3 concentration up to 3 wt%, there is no sustainable thermal front which propagates into the medium. In the case of PDAS-2, an intermediate behaviour is observed. A low concentration of thermal initiator does not lead to an efficient thermal front. One has to increase the concentration of PDAS-2 up to 1 wt% in order to photoinduce a thermal front which could be sustained over the whole thickness of the sample.
However, the best results are obtained in the case of PDAS-1 and a frontal polymerization is observed at concentration as low as 0.3 wt%. Indeed, the photoinduced thermal front can be sustained at high temperature over the entire sample thickness. The front velocity is also much higher than in the case of PDAS-2, with values as high as 12.9 cm min−1.
Interestingly, increasing salt concentration increases the velocity of the front and the maximum temperature of the front is about 260–270 °C. The results obtained for all samples are collected in Table 3. They are in line with the reactivity of the thermal sulfonium salts as studied by DSC.
Filled systems and composites
The fast and efficient frontal polymerization obtained when using PDAS-1 opens the way to fiber-reinforced polymers. This topic is a particularly challenging and requires highly reactive photothermal systems. Indeed, when adding fillers at high content into the resin, the temperature jump decreases drastically. Therefore, in order to mimic this process, carbon filler was introduced into the resin. Different experiments were performed (Table 4) and finally optimal conditions were obtained by increasing the PDAS-1 concentration up to 1 wt% and 2 wt% for 25 wt% and 50 wt% of carbon filler, respectively, keeping constant the concentration of TAS (1 wt%). 50 wt% of carbon corresponds to the highest carbon content used in industry for manufacturing high performance carbon-fiber reinforced polymers. It should be noted that for 50 wt% of fillers and at least 2 wt% of PDAS-1 all the samples are actually cured over the whole thickness of 3 cm. To the best of our knowledge, there is no report of a sustainable polymerization front at such high carbon content.These results indicate that, even with fillers, a polymerization front can propagate in depth. The velocity of the fronts does not seem to be impacted by carbon content but rather by the concentration of PDAS. The temperature of the polymerization front tends to decrease compared to non-filled sample.
To confirm these results, different carbon-fiber reinforced polymer samples were prepared with 8 layers of plain weave carbon fabric. Due to the large irradiated surface of the composite with respect to the previous experiments made in PTFE mold, the optimal experimental conditions were found for 3.0 wt% of PDAS-1, irradiation during 300 s at 125 mW cm−2. Fig. 7 shows the temperature measured between plies 4 and 5, i.e. at the center of the sample, for these different runs.
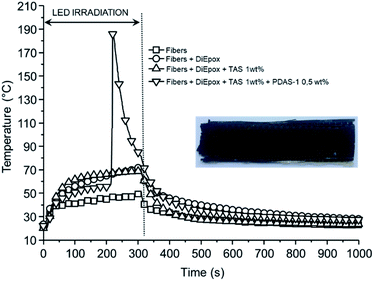 | ||
| Fig. 7 Temperature profile at the center of the carbon composite irradiated using a LED at 365 nm, 125 mW cm−2 during 300 s. Inset: final carbon composite obtained. | ||
For samples containing only carbon fiber, DiEpox and TAS, the maximum temperature reached at the center of the composite was about 70 °C as a consequence of the heat evolved by the lamp and that released by the photopolymerization at the surface.
By contrast, in the presence of PDAS-1, an important temperature jump occurs after 200 s of irradiation at the surface, showing the effect of the photoinduced thermal frontal polymerization. After curing, the sample is solid and a carbon composite is obtained (see inset Fig. 7).
Conclusions
A combination of aliphatic and aromatic sulfonium salts was found very efficient to photopolymerize thick and opaque carbon fiber reinforced polymers. The heat released by photopolymerization of cycloapliphatic epoxide allows reaching a temperature above 260 °C which was sufficient to initiate decomposition of thermal latent initiator. Photoinduced thermal frontal polymerization thus takes place in the medium with velocities as high as 13 cm min−1. It is found that the material was cured over 3 cm within a couple of minutes even with carbon content of 50 wt%. This novel dual-cure initiating system opens the way to create photocomposites without thermal post-curing treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
XA thank Prof. M. Tsunooka for helpful discussion. The companies Mäder and Magyar are acknowledged for their support. This work is funded in the framework of the “Programme d'investissements d'avenir” (PIA) operated by ADEME.Notes and references
- F. González Garcia, M. E. Leyva, M. G. Oliveira, A. A. A. De Queiroz and A. Z. Simões, J. Appl. Polym. Sci., 2010, 117, 2213 CrossRef.
- X. Allonas, Photopolymerization, Cationic in Encyclopedia of Polymer Science and Technology, Wiley, 2019 Search PubMed.
- J. A. Pojman, Frontal Polymerization, in Polymer Science: A Comprehensive Reference, ed. K. Matyjaszewski and M. Möller, Elsevier BV, Amsterdam, 2012, vol. 4, p. 957 Search PubMed.
- M. Retailleau, A. Ibrahim and X. Allonas, Polym. Chem., 2014, 5, 6503 RSC.
- P. Carion, A. Ibrahim, X. Allonas, C. Croutxé-Barghorn and G. L'Hostis, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 898–906 CrossRef CAS.
- P. Garra, F. Morlet-Savary, C. Dietlin, J. P. Fouassier and J. Lalevee, Macromolecules, 2016, 49, 9371 CrossRef CAS.
- A. Mariani, S. Bidali, S. Fiori, M. Sangermano, G. Malucelli, R. Bongiovanni and A. Priola, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 2066 CrossRef CAS.
- D. Maréchal, A. Criqui, X. Allonas and M. Lecompère, WO/2015/136180 (A1), 2015.
- D. Bomze, P. Knaack and R. Liska, Polym. Chem., 2015, 6, 8161–8167 RSC.
- D. Maréchal, X. Allonas, M. Lecompère and A. Criqui, Macromol. Chem. Phys., 2016, 217, 1169–1173 CrossRef.
- D. Bomze, P. Knaack, T. Koch, H. Jin and R. Liska, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 3751–3759 CrossRef CAS.
- M. Lecompère, X. Allonas, D. Maréchal and A. Criqui, Polym. Chem., 2017, 8, 388–395 RSC.
- M. Lecompère, X. Allonas, D. Maréchal and A. Criqui, Macromol. Rapid Commun., 2017, 38, 1600660 CrossRef.
- N. Klikovits, R. Liska, A. D'Anna and M. Sangermano, Macromol. Chem. Phys., 2017, 218, 1700313 CrossRef.
- J. V. Crivello and J. H. W. Lam, J. Org. Chem., 1978, 43, 3055 CrossRef CAS.
- J. V. Crivello and J. H. W. Lam, J. Polym. Sci., Part A: Polym. Chem., 1979, 17, 977 CAS.
- S. P. Pappas and L. H. Hill, J. Coat. Technol., 1981, 53, 43 CAS.
- T. Endo and H. Arita, Makromol. Chem., Rapid Commun., 1985, 6, 137 CrossRef CAS.
- F. Hamazu, T. Koizumi, T. Endo and Y. Yamamoto, EP0393893 (A1), 1990.
- F. Hamazu, S. Akashi, T. Koizumi, T. Takata and T. Endo, Makromol. Chem., Rapid Commun., 1992, 13, 203 CrossRef CAS.
- F. Hamazu, S. Akashi, T. Koizumi, T. Takata and T. Endo, J. Photopolym. Sci. Technol., 1992, 5, 247 CrossRef CAS.
- F. Hamazu, S. Akashi, T. Koizumi, T. Takata and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 1991, 29, 1675 CrossRef CAS.
- K. Morio, H. Murase, H. Tsuchiya and T. Endo, J. Appl. Polym. Sci., 1986, 32, 5727 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |