Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A mechanistic investigation of low salinity water flooding coupled with ion tuning for enhanced oil recovery†
Rohit Kumar Saw and
Ajay Mandal *
*
Chemical Flooding Laboratory, Department of Petroleum Engineering, Indian Institute of Technology (Indian School of Mines), Dhanbad 826004, India. E-mail: ajay@iitism.ac.in
First published on 24th November 2020
Abstract
Oil recovery by low salinity water flooding (LSWF) from carbonate reservoirs has gained tremendous attention in recent years due to its cost-effectiveness and environment-friendly nature. The mechanisms of low salinity water flooding for enhanced oil recovery are very complex and depend on the mineralogy of the formation rock, properties of injection brine and reservoir fluids. The present work aimed at the optimization of salinity and concentration of potential determining ions (PDIs) in injection water for enhanced oil recovery from carbonate reservoirs. Initially, we conducted a series of experiments on the dilution effect of seawater (SW) with the help of rock/fluid and fluid/fluid interactions via interfacial tension (IFT), zeta potential and contact angle measurements. This offered an optimum salinity (20dSW) with an 11% increase in recovery of the original oil in place (OOIP) over the SW injection in secondary flooding mode. Then, the ion tuning was done on the optimum salinity (20dSW) by manipulating the PDIs (Ca2+, SO42− and Mg2+) while keeping ionic strength constant. The properties of ion tuned brine were optimized by zeta potential and contact angle measurements. The core flooding experiments performed with the injection of designed ion tuned water obtained by dilution and ion tuning of SW showed more than 20% OOIP as incremental recovery over the SW injection. Effluent analysis after the flooding confirms that the main mechanisms for enhanced oil recovery include calcite dissolution and wettability alteration due to interplay of PDIs.
1. Introduction
Out of all the proven oil reserves found in the world, carbonate reservoirs contain more than half of it.1 Carbonate reservoirs are mainly composed of calcite and dolomite with impurities such as quartz, anhydrite, aragonite and clay minerals.2 After primary recovery, most of the oil remains trapped in the reservoirs due to complex wettability of porous media and rock–fluid interactions3,4 which lead to enormous motivation to develop the techniques which are both cost effective and environment friendly for recovering as much oil as possible. Low salinity water flooding (LSWF) has proven its ability as one of the most promising approaches in this regard. Numerous experimental works have been reported in the literature using different types of measurements like IFT, zeta potential, contact angle, spontaneous imbibition, core flooding etc. using sea water, low salinity water and smart water as injection fluids particularly in carbonate reservoirs. Though significant contributions have been made by different researchers, the mechanisms of LSWF in carbonate reservoir are still not well understood due to the complex nature of the carbonate reservoirs and the conflicting reported results.5Past studies on LSWF in carbonate reservoir were mainly focused on two different approaches which are: (a) the successive dilution of formation brine/sea water and their injection into the reservoir in secondary and tertiary mode, thus altering the salinity and ionic strength of injection brine,6–9 and (b) the tuning the ionic composition of sea water keeping the ionic strength constant and by altering the concentration of SO42−, Ca2+, Mg2+ also termed as potential determining ions (PDIs) for carbonate reservoirs.10–14 Both the approaches have proved their ability to increase oil recovery.
Yousef et al.15 have demonstrated the positive effect of LSWF on limestone reservoir cores with the dilution of formation water and sea water as an injection fluid. They showed that injection of different dilutions of sea water sequentially led to an increase in incremental oil recovery of 7–8.5% from 2 times diluted, 9–10% from 10 times diluted and 1–1.6% from 20 times diluted sea water flooding. Zaeri et al.16 studied the effect of temperature, salinity, permeability and connate water saturation by spontaneous imbibition studies for LWSF. They also reported that the recovery at optimum salinity increased with an increase in temperature. In another study, Nasralla et al.17 showed the effect of salinity and mineralogy of reservoir carbonate cores on enhanced oil recovery by both imbibition test and core flooding. It was also stated that oil recovery by LSWF could not be increased upon further dilution after the optimum salinity.
The effect of PDIs of sea water and temperature in altering the wettability of rock towards more water wet was first investigated by Austad and their co-workers.12,18–21 It was observed that sea water spiked with different PDIs concentrations at constant ionic strength could enhance the oil recovery to a greater extent. Performance of ion tuned water flooding in carbonate rocks is generally dependent on reservoir temperature,22 crude oil composition, rock mineralogy6 and water chemistry of injection and formation water.23 According to some recent studies, the efficiency of LSWF in carbonate reservoirs is dependent on the following conditions: (i) combination of PDIs with a lower concentration of monovalent ions (ii) presence of anhydrite and (iii) temperature should be above 70 °C.24,25 However, the meeting of all the above criteria doesn't ensure the positive effect of LSWF due to the heterogeneity and complex nature of different reservoirs.
Changing the ionic composition has shown a significant impact on oil recovery. Shehata et al.26 evaluated the brine salinity and effect of ions both in the secondary and tertiary mode of oil recoveries in limestone rocks at 90 °C, and concluded that Ca2+, SO42− and Mg2+ played an important role in oil mobilization. In another study, it was shown that tuning the ionic composition of injection brine can reveal the answer to why the LSWF effect is not always observed.27 It is also reported that tuning the injection brine can lead to more favorable results. Purswani and Karpyn28 conducted the flooding experiment to understand the effects of PDIs (Ca2+, SO42− and Mg2+) on carbonate rocks with sea water. They showed that all chemically tuned brines showed a higher recovery than the plain sea water with maximum recovery obtained with Mg2+ spiked brine than the Ca2+ and SO42− spiked brine due to higher activity of Mg2+ ion at the higher temperature. They also proposed that chemical reaction includes salt precipitation, crude oil desorption/solubilization, and mineral dissolution. Authors19,29 have also shown that the tuning of the composition of sea water have an optimum condition for maximum oil recovery. They stated that increasing the sulfate concentration beyond 4 times can lead to a detrimental effect on oil recovery. Fathi et al.19 reported this optimum condition in NaCl free sea water whereas Awolayo et al.29 described this optimum condition in sea water.
From the prior literature survey, it is clear that both the methods of dilution and ion tuning of injection brine have got their individual benefits in extracting the original oil in place (OOIP). Combining these two potential methods can lead to a more efficient enhanced oil recovery method. In this paper, an attempt has been made to combine both the dilution and ionic tuning of injection water to enhance the recovery of oil in carbonate reservoirs. Sea water and diluted sea water are used as an injection fluid in most of the carbonate reservoirs in the offshore region. The effect of dilution of sea water was evaluated by measurement of rock–fluid and fluid–fluid properties by IFT, zeta potential and contact angle along with core flooding. The assessment of the salinity effect on interfacial and physicochemical properties was done to determine an optimal salinity. The properties were further improved by tuning the PDIs concentration 2 times, 3 times and 4 times of each of PDIs. This type of water is commonly known as ion tuned water. Core flooding experiments were performed to evaluate the efficiency of the designed ion tuned water for enhanced oil recovery. The synergistic effects of both the techniques i.e., salinity effect and ion tuning lead to the enhanced effect on oil recovery compared to that achieved individually.
2. Experimental setups and methodology
2.1 Crude oil
Crude oil contains thousands of different hydrocarbon molecules. Characterization of the individual molecule is not possible and hence group type characterization is generally employed for crude oil and Saturates–Aromatics–Resins–Asphaltene (SARA) analysis is one of them.30 Saturates and aromatics are classified as non-polar compounds whereas resin and asphaltene are classified as the polar compound. Resin and asphaltene are considered as surface active components which can act as a natural surfactants present in the crude oil/aqueous system, thus affecting the IFT.31 The acid number correlates the concentration of surface active components and is considered as an acidic crude oil if it is higher than 0.5 mg KOH per g.32Crude oil used in this study was dead crude oil and was procured from the Cambay basin of ONGC. Characterization of crude oil was done with the help of SARA and Fourier Transform Infrared spectroscopy (FTIR) analysis. The physical properties of crude oil are shown in Table S1 (ESI†). The acid number of crude oil was measured as per ASTM D664 and SARA analysis was done according to the procedure detailed in ASTM D2007. It can be said from experimental data that the crude oil is acidic in nature. The other polar components with phenolic, carboxyl, amine and amide functionalities are also present in crude oil as evidenced from FTIR analysis. A detailed description of functional groups present in crude oil is shown in Fig. S1 (ESI†). A closer examination shows that crude oil mainly consists of phenolic, carboxyl, amine and amide functionalities along with some other groups.
2.2 Characterization of core sample
Mineralogical composition of rocks plays a significant role in controlling the mechanisms of LSWF, hence its characterization is of utmost importance while planning for LSWF for any oil reservoirs.6,17 The reservoir cores were characterized with the help of X-ray diffraction (XRD) and is shown in Fig. S2 (ESI†). It can be seen that the rock sample is mainly composed of calcite. The rock sample was powdered and XRD analysis of the powdered sample was done by X-ray diffractometer (D8 Advance Bruker). The mineralogical composition of the carbonate rock was analyzed with the help of standard library data (ICDD).Semi-quantitative analysis was used to identify the mineralogy of carbonate rock by the Rietveld refinement method. It may be found that the rock is mainly composed of calcite mineral-79% and other minerals that are present in trace amount are quartz-4%, aragonite-12%, montmorillonite-3%, pyrite-2%. Carbonate reservoirs are generally found to be oil wet state. The initial oil-wet nature of the rock can be explained due to polar interactions, surface precipitation, acid/base interactions, and ion binding.33 Wettability alteration behavior of rocks is a complex phenomenon, which is dependent on interactions among oil/brine as well as rock/brine interfaces.27 Whilst changing the salinity of reservoir fluids, the extent and influence of the above-mentioned interactions weaken in order to cause gradual detachment of the crude oil and allow the injected brine phase to form a wetting film onto the rock surface.
2.3 Methodology and brine composition
All the brines were prepared with the help of deionized water by mixing the reagent grade salts (NaCl, MgCl2·6H2O, CaCl2·2H2O, NaHCO3, MgSO4, KCl and Na2SO4). Sea water was prepared by a standard procedure as mentioned in IS: 8770-1978 and further dilution of sea water were done with the help of deionized water. The formulation of sea water and its further dilution with other physical parameters are shown in Table S2 (ESI†). Similarly, to see the effect of ionic composition, ion tuned brine was also made and the formulation of ion tuned brine with different parameters are shown in Table S3 (ESI†). The brine solutions were filtered with a 0.45 μm filter paper to avoid any plugging.The stepwise dilution of sea water and the alteration of ionic composition along with the methodology for the present study is shown in Fig. 1. A prior compatibility study of various brines with FW was also conducted. The injection of incompatible brine may lead to scaling/precipitation which will in turn decrease the permeability of reservoirs leading to formation damage. To check for compatibility, FW was mixed with various concerned brines in equal ratio and was kept in a dark container for one week under the reservoir temperature of 90 °C. Mixed brines were visually analyzed and no precipitation was observed.
2.4 IFT
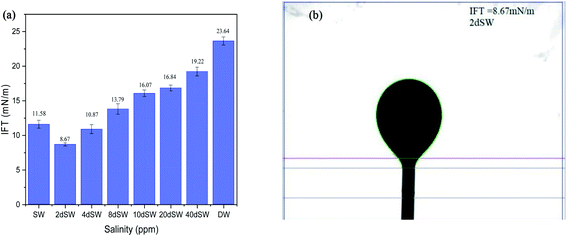
The measurement of interfacial tension between the aqueous solution (different salinity and ionic composition) and crude oil was done by the pendant drop method with the help of drop shape analyzer. A video was recorded until the oil drop detaches from the J needle tip and the last image before detachment was used for IFT determination. IFT apparatus was calibrated with the standard procedure at water/air interface and a standard solvent heptane and water interface. Only after calibration, the measurements were taken. Experiments were repeated thrice. All the IFT measurements were done at 90 °C and atmospheric pressure.2.5 Zeta potential measurement
Zeta potential was measured using Horiba scientific nano particle analyzer SZ-100. It measures the electrophoretic mobility of charged colloidal suspension. Samples of brine/rock were made according to the standard procedure. The solutions of brine/rock were prepared by adding 0.5 g of powdered rock into 50 ml of brine sample. Each solution was further sonicated for 3 min and left overnight to achieve equilibrium.34 After that, rock/brine solution was decanted and taken for measurement. For oil/brine zeta potential measurement, 0.5 ml of oil was mixed with respective 100 ml brine and sonicated for 3 minutes, left for some time and then taken for measurement.35 All the measurements were repeated 3 times for each sample. Also, the pH of each solution was measured as zeta potential is pH dependent. All the measurements of zeta potential and pH were made at 25 °C.2.6 Contact angle measurement
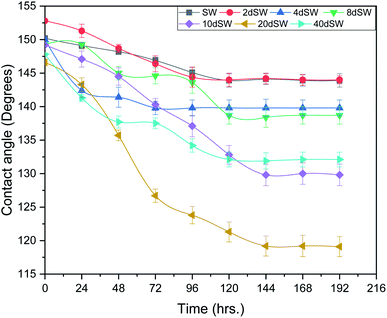
For contact angle measurement, small slices of core were cut from the carbonate cores. Slices were first polished with sand paper to make the surface smooth to minimize the hysteresis effect due to surface roughness on the contact angle. Then the slices of core were placed under vacuum for 24 h and then it was aged with formation brine for another 24 h, then the slices were put into the crude oil and aging was done for 4 weeks at reservoir temperature of 90 °C to restore the plate wettability.After the aging process has been completed, the slices were placed into the respective brine and where preheated. After the preheating, a crude oil drop was injected onto the rock slice. To see the behavior of oil drop with respect to time, the sealed vessel was put into oven which was maintained at 90 °C. Contact angle measurement has been carried out at reservoir temperature (90 °C) and at atmospheric pressure. The impact of pressure on wettability is weak to negligible whereas impact of temperature is predominant.36,37 This contact angle measurement procedure has also been used by various other researchers.29 Digital photographs were taken at a specific time interval and the contact angle was analyzed with the help of drop shape analyzer 25 (KRUSS) with in-built software DSA 4 in manual mode method. The first measurement was taken after 2 h so as to have a stable system and it was reported as 0 h reading. The contact angles were measured from the denser phase i.e. from brine phase which showed that a decrease in contact angle means that rock slice is going towards less oil wet condition. The rock is considered to be oil wet if the contact angle is in between 110° to 180°, neutral wet if the angle is in between 70° to 110° and water wet if contact angle is in between 0° to 70°.38 Contact angle measurements were repeated twice for each type of brine to eliminate the effect of local mineralogy and for its reproducibility concern.
2.7 Core flooding
The core flooding instrument consists of a Hassler type core holder, displacement pump (Teledyne Isco), calibrated collector, differential pressure gauge, slug container and an oven, schematic of which is shown in Fig. S3 (ESI†). The core holder was placed into the oven to stimulate the reservoir temperature and the displacement pump was used to inject the fluid with constant rate stored in a stainless-steel container. Injection fluid was heated with the help of a heating jacket before entering into the core holder. Overburden pressure of 2000 psi was applied with the help of a hydraulic pump by the injection of hydraulic oil between the rubber sleeve encasing the core and core holder's internal surface. A sample collector was used to collect the effluent samples. Carbonate cores were dried at 120 °C until the constant weight was achieved. Then the cores were saturated by formation water and left for 1 week so that ionic equilibrium could be achieved. Water permeability of cores were determined by injecting formation water at 90 °C with various injection rates of 0.5, 1.0, 1.5 and 2 ml min−1. The pressure drop across each core were monitored by digital pressure gauge until a steady state condition was reached and the permeabilities were calculated using Darcy's law. To establish the connate water saturation, dead crude oil was injected at a rate of 0.2 ml min−1 until only oil is produced at the outlet and no more water was produced. Then cores were kept in a sealed vessel filled with the crude oil in an oven for 4 weeks at a temperature of 100 °C. Table S4 (ESI†) shows the petrophysical properties of the core plugs used in this study. Core was remounted in the core holder for water flooding test and the fluid was injected in secondary mode at a flow rate of 0.2 ml min−1. All the experiments were conducted at a temperature of 90 °C and an overburden pressure of 2000 psi. Effluents were collected throughout the waterflooding experiment using the collector and the pressure drop was monitored with the help of differential pressure gauge installed across the core holder. Effluent collected was used to determine the oil recovery and ionic composition of produced brines.2.8 Effluent analysis by ion-tracking
The produced effluent by core flooding was collected to conduct effluent's ionic analysis of active ions (SO42−, Ca2+, Mg2+) with Metrohm ion chromatograph to gain a better insight into the mechanisms involved. The samples were first diluted with Millipore water and then filtered with a 0.02 μm syringe filter. Graphs were plotted as the ratio (C/C0) of produced concentration (C) of effluent PDIs and injected concentration (C0) of PDIs. Effluent analysis was carried out after some time the breakthrough has taken place.3. Results and discussions
This section is discussed in two parts as also shown in Fig. 1. In the first part, the dilution effect of sea water has been discussed with the help of measurement of IFT, contact angle and zeta potential. An optimum salinity was obtained by analysis of rock/fluid and fluid/fluid interactive properties. In the second part, the effect of PDIs on optimum dilution was evaluated by IFT, contact angle and zeta potential measurements. Finally, the core flooding experiments were performed to check the efficacy of the designed ion tuned water for enhanced oil recovery. The analysis of the ionic composition of PDIs of the effluent was done to unlock the mechanisms underneath the increased oil recovery.3.1 Effect of dilution of sea water (SW)
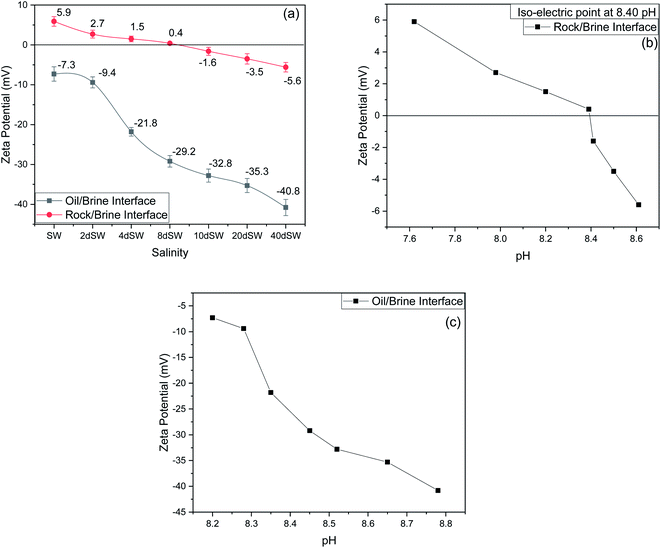
It can be seen from the results of zeta potential, as shown in Fig. 3a, that initially, the rock/brine interface was positively charged in sea water but as we dilute the sea water, the positive charge on the rock surface starts to shift to more negative, causing the double layer to expand. The value of zeta potential of carbonate rock dispersed in SW is positive,29 which is because of the abundant availability of calcite in carbonate rock and the value found is very close to the results reported by Alotaibi et al.44 Carbonate rock mainly constitutes of calcite, which is positively charged at neutral pH conditions.45–47 From literature, it is observed that the isoelectric point (IEP) of calcite surface is in between the pH value of 8.0 to 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46,47 and from our experimental result as shown in Fig. 3b, the isoelectric point is observed at pH of 8.40 which is in good agreement with literature. Charges present on calcite surfaces are found to be dependent on the isoelectric point. In this study, the charge at rock/brine interface are found to be positive at high salinity and pH lower than isoelectric point. Upon reducing the salinity and above the isoelectric point the zeta potential became negative. Hydrolysis reaction of carbonate in water generates the electric charge (mainly H+ and OH− ions). Therefore, surface charge is pH dependent and can be positive and negative. Following are the general reaction (eqn (1)–(5)) that takes place when calcium carbonate is dissolved in water
46,47 and from our experimental result as shown in Fig. 3b, the isoelectric point is observed at pH of 8.40 which is in good agreement with literature. Charges present on calcite surfaces are found to be dependent on the isoelectric point. In this study, the charge at rock/brine interface are found to be positive at high salinity and pH lower than isoelectric point. Upon reducing the salinity and above the isoelectric point the zeta potential became negative. Hydrolysis reaction of carbonate in water generates the electric charge (mainly H+ and OH− ions). Therefore, surface charge is pH dependent and can be positive and negative. Following are the general reaction (eqn (1)–(5)) that takes place when calcium carbonate is dissolved in water
| CaCO3(s) = CaCO3(aq.) | (1) |
| CaCO3(aq.) = Ca2+ + CO32− | (2) |
| CO32− + H2O = HCO3− + OH− | (3) |
| Ca2+ + HCO3− = CaHCO3+ | (4) |
| Ca2+ + OH− = CaOH+ | (5) |
At high pH concentration of negative species (HCO3− and CO32−) are high while at low pH positive species (Ca2+, CaHCO3+ and CaOH+) are higher leading to negative and positive charge respectively. At higher salinity, concentration of divalent ions is high due to which double layer is in compressed state. Upon dilution, less availability of divalent ions and high pH of calcite/water facilitates the negative species to realign themselves into the double layer and changes the surface charge. From the above discussion, we can say that below IEP, i.e., at lower pH value the surface charge will be positive and above IEP at higher pH values, surface charge will be negative. It is observed from the literature that change in polarity of zeta potential affects the wettability and subsequent oil recovery.48 It can be also be seen from Fig. 3a that the zeta potentials of oil/brine interface are negative in charge regardless of dilution. As dilution takes place, the negative charge at the oil/brine interface is found to be increasing in magnitude. The pH of low salinity brine/oil interface as shown in Fig. 3c ranges from 8.2–8.8, which is also in agreement with literature that brine/oil zeta potential is negative at pH greater than 3.49 It is also worth noting that as dilution takes place, the charges on oil/brine interface leads to higher stability. This can be an indicative measure for the emulsification at oil/brine interface and hence transportation of oil in the form of stable emulsion, leading to enhanced oil recovery. Negative charges on both the surfaces are very much desired as it also ensures the positive effect of LSWF. It is also worth noting that the water film will be more stable if there is a repulsion between the two interfaces. Here, from Fig. 3a, we can see that up to 8dSW the charge on the rock/brine interface is positive which will lead to attraction between the rock/brine and oil/brine interfaces leading to unstable water film, thus rendering the rock more oil wet. After the 8dSW, charges on the rock/brine interface became negative with dilution, which will lead to repulsion and a more stable water film for wettability alteration. From the results, it can be concluded that the dilution of sea water after a certain level has positive effect of LSWF, leading to a more stable water film. From the above experimental results, it is clear that potential candidates for wettability alteration are 10dSW, 20dSW and 40dSW.
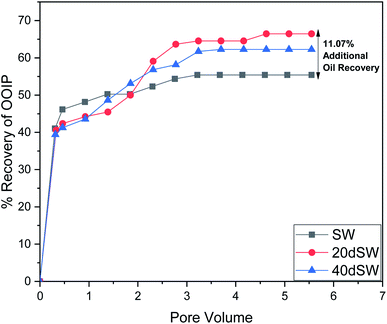
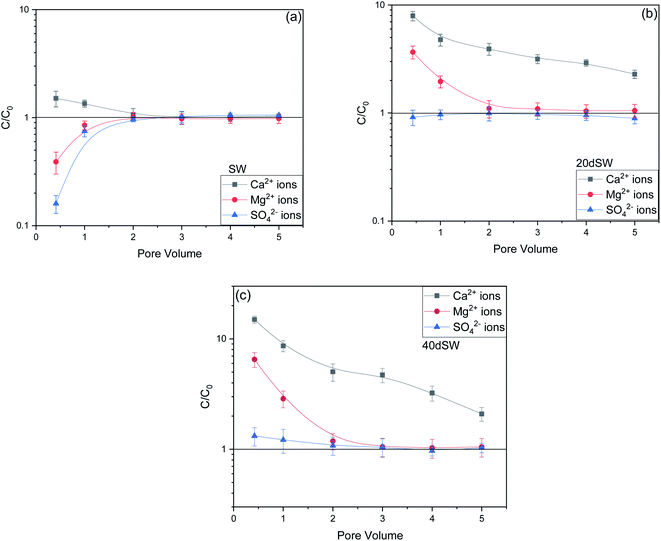
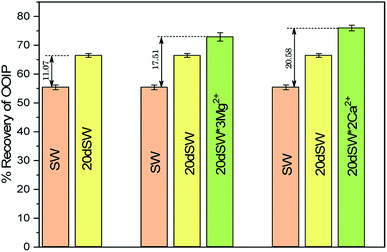
From the core flooding experiment, it can be clearly seen that the dilution of sea water has a positive impact on improved oil recovery up to a certain threshold salinity level. It can be seen from Fig. 5 that injection of SW in carbonate reservoirs in secondary mode provides a recovery of 55.38% of OOIP whereas the injection of 20dSW results a recovery of almost 66.45% of OOIP and 40dSW produces a recovery of 62.20% OOIP which was less than the recovery obtained at optimum salinity level, thus confirming our optimum salinity hypothesis in dilution of SW. Injection of optimum salinity SW produced 11.07% additional oil recovery compared to the flooding of SW alone. It is very interesting to note that 40dSW showed an increased oil recovery than the SW flooding which was due to low salinity effect. But the recovery is less than 20dSW, which may be due to the lesser concentration of potential determining ions. It is also worth noting that the LSWF effect started to act after 1.5–2.0 PV. This delay in the LSWF effect suggests that LSWF is not instantaneous in nature rather it is very much time dependent phenomenon. The effluent analysis as shown in Fig. 6, provides some important light on the dominant mechanisms for increased oil recovery due to the dilution effect. From Fig. 6a, it can be seen there is not much appreciable change in the ionic concentration of SW effluent which suggests that the marginal oil recovery by SW is because of applied viscous force. In the case of 20dSW and 40dSW, there is an appreciable change in the concentration of PDIs, which suggests that increased oil recovery is assisted by some other mechanism leading to wettability alteration. From Fig. 6b, it can be seen that the concentration of Ca2+ ions is higher than that of the injection brine. It shows that Ca2+ ions are being supplied to the brine, due to the calcite dissolution. As dilution takes place there is a reduction in the ions present in the brine. When the injection of diluted brine takes place, it disturbs the pre-existing equilibrium prevailing with the high salinity brine. Thus, ions from rock dissolve into the injected brine. During the process, the dissolution of rock is accompanied by the release of adsorbed polar components of crude oil, thus increasing the water wetness of rock and hence thereby increased oil recovery. A schematic explaining the above mechanism is shown in Fig. 7. Calcite dissolution takes place according to following eqn (6) and (7) and is also supported by many authors.4,8,51–53
| CaCO3 → Ca2+ + CO32− | (6) |
| CO32− + H2O → HCO3− + OH− | (7) |
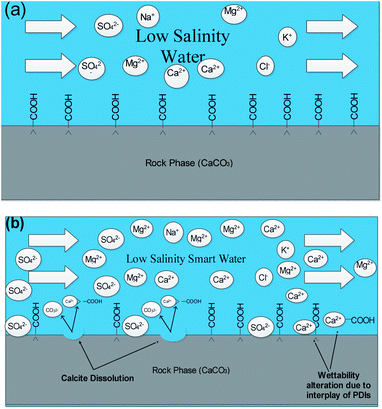 | ||
| Fig. 7 Schematic mechanisms prevailing at 20dSW oil recovery (a) injection of low salinity water (b) calcite dissolution and interplay of PDIs taking place. | ||
From Fig. 6b, it can also be seen that SO42− ions are getting adsorbed onto the rock surfaces which indicates that another mechanism that could be taking place simultaneously is wettability alteration due to interplay of PDIs. Due to this, SO42− ions gets adsorb onto the carbonate surfaces thus decreasing the positive surface charge density which in turn minimizes the electrostatic repulsive force between the positively charged carbonate surface and cations present in the brine. Due to this, co-adsorption of Ca2+ and other cations takes place to the surface. The Ca2+ ions thus react with the carboxylic acid groups that are bonded with the rock surface and thereby breaking the interactive interaction between oil and rock surface. This interaction of Ca2+ ions with carboxylic acid components lead to the formation of Ca2+–carboxylate complexes and their subsequent release from the rock surface alters the rock surface to a more water wet condition. For 40dSW, the same trend of effluent analysis has been observed indicating the calcite dissolution taking place except for the SO42− ions, which shows no variation indicating no interplay of PDIs. This could be due to the less availability of PDIs at 40dSW level and could be a reason for less recovery than 20dSW in which both the mechanism i.e., calcite dissolution and interplay of PDIs leading to wettability alteration is taking place. From the Fig. 6b and c, it has been observed that the initial concentration of Mg2+ ion at breakthrough is higher compared to the injection water (20dSW and 40dSW) which is because of production of FW with high Mg2+ ions at the initial stage of flooding. Increased recovery due to LSWF is also because of the fact that crude oil is bonded by the organometallic complex between the polar component of crude oil and ions present in the formation water. The low salinity brine breaks these complexes and thus releases the crude oil and ions into the low salinity water being injected. The breakdown of these complexes by low salinity waterflooding is also believed to be big reasons for improved oil recovery. The results obtained were found to be in line with the conducted zeta potential and contact angle experiments. Although zeta potential gave three candidates for optimum recovery, it was the contact angle study that clearly indicates an optimum salinity for improved oil recovery. The mechanism that clearly dominates the oil recovery due to dilution of SW is calcite dissolution along with interplay of PDIs which led to wettability alteration of rock surface. Here, zeta potential acted as a screening criterion for increased oil recovery by dilution of SW in carbonate reservoirs.
3.2 Effect of potential determining ions (PDIs)
In this section, an effort has been made to see the effect of tuning the ionic composition in symbiotic association with the dilution effect of sea water. Basically, in this section we have tried to see the effect of PDIs (Ca2+, Mg2+, SO42−) by varying their concentration at the optimum value of dilution i.e., 20dSW that has been obtained from dilution approach earlier in this section. The effect was evaluated with the help of basic techniques such as IFT, contact angle and zeta potential measurements.From experimental data as shown in Fig. 9a for the rock/brine interface, it can be observed that increasing concentration of calcium and magnesium in the 20dSW, increase the magnitude of zeta potential at the rock/brine interface towards the more positive side which is due to the increased affinity of these ions toward the carbonate rock surface. Thereby making the surrounding surface more positively charged and hence shifting the charges present at rock/brine surface towards more positive side. Here p(PDI) represents the negative logarithm of PDI concentration in mol L−1 and decreasing p(PDI) values means there is an increase in the concentration of respective PDI. It can be seen that as pCa value decreases, the zeta potential becomes more positive which is consistent with increased adsorption of Ca2+ onto the calcite surfaces.54 A close to linear relationship is found between zeta potential and pCa, which is consistent with Nernstian behavior of calcite surface and suggests that the electrical double layer can be reasonably described by the Gouy–Chapman–Grahame model close to the isoelectric point. Similar results are also observed in the case of Mg2+ which also shows a close to linear relationship with respect to decreasing pMg concentration.12,55,56 It can be seen that both Ca2+ and Mg2+ ions behaves similarly with given carbonate rock surface.55 Apart from this, an opposite trend has been observed in the case of SO42− ions. The charge on the rock surface was increasing towards the more negative side with the increasing concentration of SO42− ions, because of increased affinity of SO42− ions towards the rock surface due to which rock surface becomes more negatively charged.
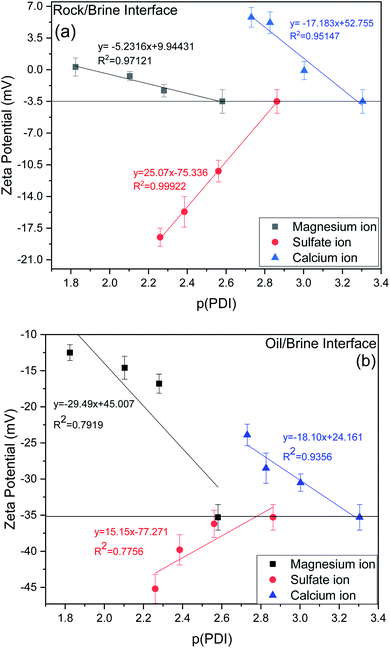 | ||
| Fig. 9 Variation of zeta potential (a) rock/brine interface (b) oil/brine interface of ion tuned brine at optimum dilution of 20dSW at 25 °C. | ||
For designing the optimum ionic concentration for the waterflooding process, it is very important to know the effect and charges of both rock/brine and oil/brine interfaces so that the positive effect of low salinity waterflooding can be harnessed.27 Although many studies have been conducted for quantifying the charges present at rock/brine interface with varying PDIs,11,12,28 the investigation on the variation of charges present at the oil/brine interface with varying PDIs needs to be explored in detail. For this purpose, the variation of zeta potential at oil/brine interface with varying PDIs are also studied and is shown in Fig. 9b. It can be seen that the addition of both the ions individually i.e. Ca2+ and Mg2+, decreases the negative charges at the oil/brine interface. This may be due to the interaction of carboxylic acid compounds present at the oil/brine interface with the gradually increasing concentration of Ca2+ and Mg2+ ions in the brine solution. The reduction in charge is found to be less upon the addition of Ca2+ ions than the addition of Mg2+ ions. This is due to the difference in charge density of the cations. Ca2+ ions are having less charge density and are weakly hydrated than the Mg2+ ions. Thus, it requires less entropy change resulting in favorable adsorption. This led to the formation of a stable Ca2+–carboxylate complexes at the interface leading to less reduction in charges and locking of the acidic component of oil at interface, leading to a stable emulsion system for increased oil recovery. Mg2+ ions possess high charge density and their strong hydration contributes to the repulsion from the oil/brine interface.57,58 Ca2+ and Mg2+ ions were found to follow the Hofmeister series for a stable emulsion formation where Ca2+ ions form a stable emulsion than Mg2+ ions also indicated by charges present at the interface as shown in Fig. 9b. An opposite trend is shown by the SO42− ions, it can be seen that upon increasing the SO42− ions concentration, zeta potential at oil/brine interface shifts to a more negative side. This is due to the adsorption of SO42− ions onto the oil/brine interface with the help of positively charged amide group present in the crude oil, as evident from FTIR of crude oil, shown in Fig. S1 (ESI†). Presence of carboxylic acid and SO42− ions lead to an increase in the magnitude of negative charge at the oil/brine interface upon the addition of SO42− ions. Zeta potential is dependent on pH, so it is imperative to discuss the effect of pH. Very few studies have shown the effect of pH due to variation of PDIs.54 The rock/brine solution pH of sulfate (8.44–8.50) and magnesium (8.66–8.70) did not vary much as the zeta potential of calcite is independent of pH if the concentration of calcium is kept constant.59,60 Also, the solution pH of Ca2+ ions varied insignificantly from 8.55 to 8.25. Since the pH was almost constant for each ion tuned water, so it can be inferred that the variation in zeta potential values are due to the ionic tuning of PDIs only. Thus, from the above results it can be said that, the tuning of PDIs in the solution has good potential to alter the charges of rock/brine and oil/brine interfaces leading to engineered interfaces for increased oil recovery.
From the experimental study as shown in Fig. 10, it is clear that there exists an optimum value of PDIs concentration obtained upon the tuning of optimum diluted seawater (20dSW). In case of Ca2+ ion tuning, optimum value is obtained at 20dSW × 2Ca2+ and for Mg2+ ion tuning, the optimum value is obtained at 20dSW × 3Mg2+. The effect of SO42− ion tuning on wettability alteration is complex as shown in Fig. 10c. Actually, the role of SO42− ion is different from that of Ca2+ and Mg2+ in ion tuned water flooding. The SO42− ions are preferentially adsorbed on the positively charge carbonate rock, which in turn displaces the polar oil components and makes the surface more water-wet. As the SO42− ions get adsorbed on the carbonate rock, its positive surface charge is decreased and consequently more Ca2+ gets attracted to the surface due to less electrostatic repulsion. Then Ca2+ removes the adsorbed polar oil component from the surface according to the following reaction as proposed by Rezaeidoust et al.:20
| RCOO−–Ca–CaCO3 (s) + Ca2+ + SO42− = RCOO–Ca+ + Ca–CaCO3 (s) + SO42− | (8) |
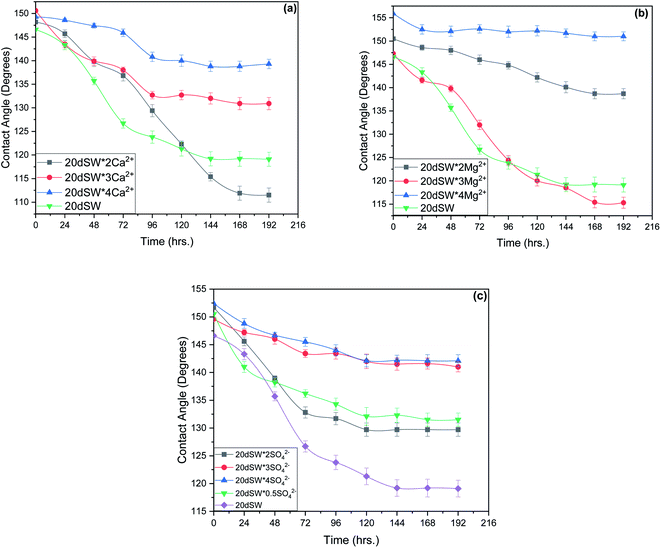 | ||
| Fig. 10 Time variant change in contact angle at 90 °C for (a) Ca2+ ion tuning (b) Mg2+ ion tuning and (c) SO42− ion tuning. | ||
Similarly, the oil detachment mechanism of Mg2+ in presence of SO42− is shown by eqn (9). In both the cases SO42− catalyzes the reactions.
| RCOO−–Ca–CaCO3 (s) + Mg2+ + SO42− = Mg–CaCO3 (s) + RCOO–Ca+ + SO42− | (9) |
An optimum concentration of SO42− ion is required to achieve the maximum benefits by ion tuned water flooding beyond this concentration, the effect of addition of SO42− ion is found to be detrimental.29 The present study indicates that the SO42− ion concentration corresponding to 20dSW is the optimum as it offers the maximum wettability alteration manifested through contact angle compared to other concentrations as shown in Fig. 10c.
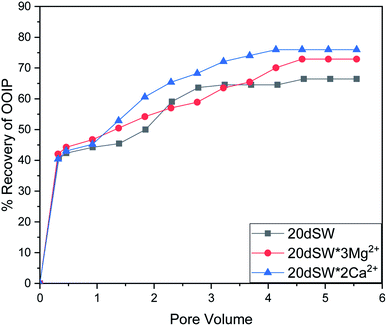 | ||
| Fig. 11 Oil recovery diagram for injection of 20dSW, 20dSW × 3Mg2+ ion and 20dSW × 2Ca2+ ion tuned brine in secondary mode at 90 °C (ion tuning effect coupled with dilution). | ||
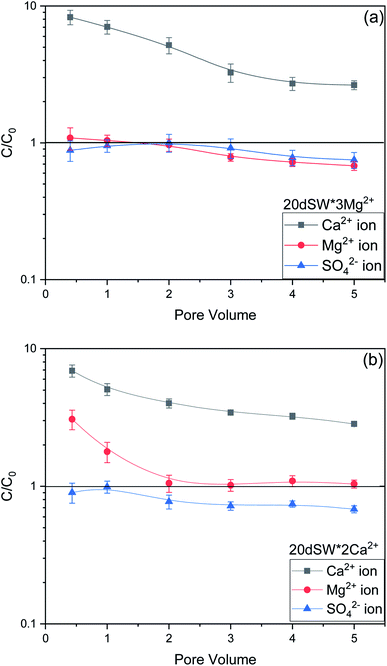
As discussed earlier, to have stable brine film between rock and oil, the charges both at rock/brine and oil/brine interfaces should be the same. From zeta potential result, it was found that as the concentration of Ca2+ was increased, zeta potential values shifted from negative to positive side as shown in Fig. 9a, whereas the zeta potential value on the oil/brine interface is negative as shown in Fig. 9b. To have positive effect of Ca2+ ion tuning, the zeta potential values should be on the negative side for the rock/brine interface. The negative charge on the rock/brine interface was found for 20dSW × 2Ca2+ brine only as shown in Fig. 9a. Contact angle experiments confirmed this result and thus offers an optimum salinity for Ca2+ ion tuning. The core flooding and effluent analysis of 20dSW × 2Ca2+ brine are shown in Fig. 11 and 12b respectively. The dominant mechanism proposed behind the increased oil recovery of 20dSW × 2Ca2+ is interplay of PDIs in combination with calcite dissolution, leading to wettability alteration of rock surfaces. The available SO42− ions get adsorbed onto the rock surfaces diminishing the positive charge present on carbonate rock to a larger extent, thus providing the chance for Ca2+ and Mg2+ ions to come and interact in the vicinity of attached crude oil components. Thus, the desorption of crude oil from the rock surface leads to increased oil recovery. A schematic of the above mechanism is also shown in Fig. 7. It has been observed that the initial concentration of Mg2+ ion at breakthrough is higher compared to the injection water (20dSW × 2Ca2+) which is because of production of FW with high Mg2+ ions at the initial stage of flooding and then slowly decreases due to subsequent injection of diluted ion tuned water and approaches to the original concentration of injection water. Among Ca2+, Mg2+ and SO42− ion tuning, maximum wettability alteration and subsequent oil recovery are found to be with 20dSW × 2Ca2+ brine which can be attributed to the higher activity of Ca2+ ions. At temperatures lower than 100 °C, Ca2+ ions have greater activity than the Mg2+ ions.5,12,21,64 Due to higher activity and increased concentration of Ca2+ ions, 20dSW × 2Ca2+ ion tuned water is able to detach more oil from the surface of carbonate reservoirs than Mg2+ ions brines. The Ca2+ and Mg2+ ions showed different optimum concentrations. The different response of Ca2+ and Mg2+ ions can be attributed to the activity of different ions which significantly varies with temperature. The addition of Ca2+ ions with increased activity, lead to a maximum recovery than the addition of Mg2+ ions. In case of Mg2+ ion addition, the Ca2+ ions were kept constant but at the same time the concentration of Na+ ions decreased significantly which ensured an unhindered activity of Ca2+ ions along with the Mg2+ ions. Since the activity of Mg2+ ions are less than Ca2+ ions at a temperature less than 100 °C this led to increased concentration of Mg2+ ions than the Ca2+ ions for an optimum value.
The optimum salinity proposed for tailored ion tuned water was subjected to core flooding to validate the contact angle results for improved oil recovery. Core flooding with diluted SW and optimum diluted ion tuned water are shown in Fig. 5 and 11 respectively. SW and 20dSW showed a recovery factor of 55.38% and 66.45% OOIP, respectively. The brine 20dSW × 2Ca2+ showed the maximum recovery of 75.96% OOIP, 20dSW × 3Mg2+ showed maximum recovery of 72.89% OOIP. It can be seen that all the brines either obtained by dilution or dilution coupled with ion tuning showed improved oil recovery than the injection of SW. From the above discussions, it can be said that injection of different water recipe to the same reservoir can lead to different oil recovery. Thus, a comprehensive study is needed to optimize the oil recovery due to LSWF before injecting into the reservoir. A comparative graph of oil recovery by SW and all the optimized brines are shown in Fig. 13. From the figure, it is clear that both the approaches i.e., dilution and ion tuning have its own benefit on increased oil recovery and can be combined to harness the enhanced potential for increased oil recovery. Overall in this study, calcite dissolution and interplay between PDIs leading to wettability alteration of rock to less oil wet condition, played the dominant role both in dilution and ion tuning which gave rise to higher improved oil recovery.
4. Conclusions
In this study, the potential of oil recovery by low salinity waterflooding was evaluated by combining the dilution and ion tuning approaches in carbonate reservoir. Both methods showed positive effect on oil recovery. Effect of sea water dilution was investigated and the properties obtained were improved by ion tuning of Mg2+ and Ca2+ ions. Core flooding was done on all the optimum brines obtained from both the dilution and ion tuning. Effluent analysis was done to gain insight into the underlying mechanisms. The following conclusions can be drawn from the performed study:• Upon dilution of SW, charge present at carbonate surface at rock/brine interface shifted from positive to negative side.
• Contact angle studies upon dilution of SW showed that an optimum salinity at 20dSW exists.
• Core flooding conducted on 20dSW showed an increase of 11.07% of OOIP as compared to SW.
• Zeta potential studies conducted on ion tuned brines at optimum dilution showed that tuning of SO42−, Mg2+ and Ca2+ ions almost linearly affected the surface charges at rock/brine interface and can lead to an engineered rock/brine interface.
• Core flooding conducted with 20dSW × 3Mg2+ and 20dSW × 2Ca2+ ion tuned water showed an increase in oil recovery of 17.51% and 20.58% of OOIP respectively over SW.
• Calcite dissolution and interplay of PDIs were found to be the dominating mechanisms and lead to wettability alteration of carbonate rock, which in turn improved the oil recovery.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We gratefully acknowledge the financial assistance provided by Department of Science and Technology, Government of India, under FIST Project to the Department of Petroleum Engineering, Indian Institute of Technology (ISM), Dhanbad, India and Central Research Facility (CRF) for laboratory facilities.References
- H. D. Klemme and G. F. Ulmishek, Am. Assoc. Pet. Geol. Bull., 1991, 75, 1809–1851 CAS.
- R. J. Reeder, Carbonates: mineralogy and chemistry, Reviews in Mineralogy, Mineral. Soc. Amer., 1990, vol. 11 Search PubMed.
- T. Austad, S. Strand, M. V. Madland, T. Puntervold and R. I. Korsnes, International Petroleum Technology Conference, IPTC-11370-MS, Society of Petroleum Engineers, 2007 Search PubMed.
- S. Chandrasekhar, H. Sharma and K. K. Mohanty, SPE Annual Technical Conference and Exhibition, SPE-181700-MS, Society of Petroleum Engineers, 2016 Search PubMed.
- E. W. Al-Shalabi and K. Sepehrnoori, J. Pet. Sci. Eng., 2016, 139, 137–161 CrossRef CAS.
- H. Mahani, A. L. Keya, S. Berg, W. B. Bartels, R. Nasralla and W. R. Rossen, Energy Fuels, 2015, 29, 1352–1367 CrossRef CAS.
- S. Strand, S. C. Henningsen, T. Puntervold and T. Austad, Energy Fuels, 2017, 31, 4687–4693 CrossRef CAS.
- A. A. Yousef, S. Al-Saleh, A. U. Al-Kaabi and M. S. Al-Jawfi, Can. Unconv. Resour. Int. Pet. Conf., SPE-137634-MS, 2010 Search PubMed.
- A. Zahid, E. H. Stenby and A. A. Shapiro, SPE Europec/EAGE Annual Conference, SPE-154508-MS, 2012 Search PubMed.
- S. Strand, E. J. Høgnesen and T. Austad, Colloids Surf., A, 2006, 275, 1–10 CrossRef CAS.
- P. Zhang and T. Austad, Colloids Surf., A, 2006, 279, 179–187 CrossRef CAS.
- P. Zhang, M. T. Tweheyo and T. Austad, Colloids Surf., A, 2007, 301, 199–208 CrossRef CAS.
- O. Karoussi and A. A. Hamouda, Energy Fuels, 2007, 21, 2138–2146 CrossRef CAS.
- P. Purswani and Z. T. Karpyn, Fuel, 2019, 235, 406–415 CrossRef CAS.
- A. A. Yousef, S. H. Al-Saleh, A. Al-Kaabi and M. S. Al-Jawfi, SPE Reservoir Eval. Eng., 2011, 14, 578–593 CrossRef CAS.
- M. R. Zaeri, R. Hashemi, H. Shahverdi and M. Sadeghi, Pet. Sci., 2018, 15, 564–576 CrossRef CAS.
- R. A. Nasralla, H. Mahani, H. A. van der Linde, F. H. M. Marcelis, S. K. Masalmeh, E. Sergienko, N. J. Brussee, S. G. J. Pieterse and S. Basu, J. Pet. Sci. Eng., 2018, 164, 640–654 CrossRef CAS.
- T. Austad, S. Strand, E. J. Høgnesen and P. Zhang, SPE Int. Symp. Oilf. Chem., SPE-93000-MS, 2005 Search PubMed.
- S. J. Fathi, T. Austad and S. Strand, Energy Fuels, 2011, 25, 5173–5179 CrossRef CAS.
- A. Rezaeidoust, T. Puntervold, S. Strand and T. Austad, Energy Fuels, 2009, 23, 4479–4485 CrossRef CAS.
- P. Zhang, M. T. Tweheyo and T. Austad, Energy Fuels, 2006, 20, 2056–2062 CrossRef CAS.
- Y. Zhang and H. Sarma, Abu Dhabi Int. Pet. Exhib. Conf., SPE-161631-MS, 2012 Search PubMed.
- A. Hiorth and S. Evje, Netw. Heterog. Media, 2010, 5, 217–256 Search PubMed.
- T. Austad, S. F. Shariatpanahi, S. Strand, C. J. J. Black and K. J. Webb, Energy Fuels, 2012, 26, 569–575 CrossRef CAS.
- T. Austad, Enhanc. Oil Recover. F. Cases, Gulf Professional Publishing, Amsterdam, 2013, ch. 13, pp. 301–332 Search PubMed.
- A. M. Shehata, M. B. Alotaibi and H. A. Nasr-El-Din, SPE Reservoir Eval. Eng., 2014, 17, 304–313 CrossRef CAS.
- M. D. Jackson, D. Al-Mahrouqi and J. Vinogradov, Sci. Rep., 2016, 6, 1–13 CrossRef.
- P. Purswani and Z. T. Karpyn, Fuel, 2019, 235, 406–415 CrossRef CAS.
- A. Awolayo, H. Sarma and A. M. AlSumaiti, SPE EOR Conf. Oil Gas West Asia, SPE-169662-MS, 2014 Search PubMed.
- N. Aske, H. Kallevik and J. Sjo, Energy Fuels, 2001, 15, 1304–1312 CrossRef CAS.
- T. E. Havre, J. Sjöblom and J. E. Vindstad, J. Dispersion Sci. Technol., 2003, 24, 789–801 CrossRef CAS.
- B. S. Rana, D. W. Cho, K. Cho and J. N. Kim, Fuel, 2018, 231, 271–280 CrossRef CAS.
- J. S. Buckley, Y. Liu and S. Monsterleet, SPE J., 1998, 3, 54–61 CrossRef.
- H. Mahani, A. L. Keya, S. Berg and R. Nasralla, SPE J., 2017, 22, 053–068 CrossRef CAS.
- M. Takeya, M. Shimokawara, Y. Elakneswaran, T. Nawa and S. Takahashi, Fuel, 2019, 235, 822–831 CrossRef CAS.
- O. S. Hjelmeland and L. E. Larrondo, SPE Reservoir Eng., 1986, 1, 321–328 CrossRef CAS.
- Y. Zhang, J. Zeng, J. Qiao, X. Feng and Y. Dong, Energy Fuels, 2018, 32, 9010–9019 CrossRef CAS.
- W. G. Anderson, J. Pet. Technol., 1986, 38, 1125–1144 CrossRef CAS.
- J. S. Buckley, T. Fan, Crude Oil/Brine Interfacial Tensions, Society of Petrophysicists and Well-Log Analysts, 2007, vol. 48, pp. 175–185 Search PubMed.
- C. S. Vijapurapu and D. N. Rao, Int. Symp. Oilf. Chem., SPE-80273-MS, 2003 Search PubMed.
- A. Sari, Q. Xie, Y. Chen, A. Saeedi and E. Pooryousefy, Energy Fuels, 2017, 31, 8951–8958 CrossRef CAS.
- P. C. Myint and A. Firoozabadi, Curr. Opin. Colloid Interface Sci., 2015, 20, 105–114 CrossRef CAS.
- S. T. Dubey and P. H. Doe, SPE Reservoir Eng., 1993, 8, 195–200 CrossRef CAS.
- M. B. Alotaibi, H. A. Nasr-El-Din and J. J. Fletcher, SPE Reservoir Eval. Eng., 2011, 14, 594–603 CrossRef CAS.
- P. Somasundaran and G. Agar, J. Colloid Interface Sci., 1967, 24, 433–440 CrossRef CAS.
- W. G. Anderson, J. Pet. Technol., 1987, 39, 1605–1622 CrossRef CAS.
- O. S. Pokrovsky, J. Schott and F. Thomas, Geochim. Cosmochim. Acta, 1999, 63, 3133–3143 CrossRef CAS.
- D. A. Al Mahrouqi, J. Vinogradov and M. D. Jackson, SPE Latin American and Caribbean Petroleum Engineering Conference, SPE-177242-MS, 2015 Search PubMed.
- K. Takamura and R. S. Chow, Colloids Surf., 1985, 15, 35–48 CrossRef CAS.
- R. A. Nasralla, M. A. Bataweel, H. A. Nasr-el-din and A. Texas, SPE Offshore Eur. Oil Gas Conf. Exhib., SPE-146322-MS, 2011 Search PubMed.
- A. N. Awolayo, H. Sarma and L. X. Nghiem, Energy Fuels, 2018, 32, 8921–8943 CrossRef CAS.
- A. A. Hamouda and E. Maevskiy, Energy Fuels, 2014, 28, 6860–6868 CrossRef CAS.
- A. Hiorth, L. M. Cathles and M. V. Madland, Transp. Porous Media, 2010, 85, 1–21 CrossRef CAS.
- D. Al Mahrouqi, J. Vinogradov and M. D. Jackson, Adv. Colloid Interface Sci., 2017, 240, 60–76 CrossRef CAS.
- A. Alroudhan, J. Vinogradov and M. D. Jackson, Colloids Surf., A, 2016, 493, 83–98 CrossRef CAS.
- L. Chen, G. Zhang, L. Wang, W. Wu and J. Ge, Colloids Surf., A, 2014, 450, 1–8 CrossRef CAS.
- A. P. Dos Santos and Y. Levin, Langmuir, 2012, 28, 1304–1308 CrossRef CAS.
- S. Taylor and H. Chu, Colloids Interfaces, 2018, 2, 40 CrossRef CAS.
- D. S. Cicerone, A. E. Regazzoni and M. A. Blesa, J. Colloid Interface Sci., 1992, 154, 423–433 CrossRef CAS.
- I. Sondi, J. Bišćan, N. Vdović and S. D. Škapin, Colloids Surf., A, 2009, 342, 84–91 CrossRef CAS.
- R. L. Folk and L. S. Land, Am. Assoc. Pet. Geol. Bull., 1975, 59, 60–68 CAS.
- L.-C. Escorcia, E. Gomez-Rivas, L. Daniele and M. Corbella, Geofluids, 2013, 13, 221–231 CrossRef CAS.
- B. B. Hanshaw, W. Back and R. G. Deike, Econ. Geol., 1971, 66, 710–724 CrossRef CAS.
- A. Awolayo, H. Sarma and L. Nghiem, Energies, 2018, 11, 3020 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08301a |
| This journal is © The Royal Society of Chemistry 2020 |