Open Access Article
Open Access ArticlePreparation of Al/Fe-PILC clay catalysts from concentrated precursors: enhanced hydrolysis of pillaring metals and intercalation†
Carlos Andrés Vallejo a,
Luis Alejandro Galeano
a,
Luis Alejandro Galeano *a,
Raquel Trujillanob,
Miguel Ángel Vicente
*a,
Raquel Trujillanob,
Miguel Ángel Vicente b and
Antonio Gilc
b and
Antonio Gilc
aGrupo de Investigación en Materiales Funcionales y Catálisis GIMFC, Departamento de Química, Universidad de Nariño, Calle 18, Cra. 50 Campus Torobajo, 520002 Pasto, Colombia. E-mail: alejandrogaleano@udenar.edu.co; carlosvallejo@udenar.edu.co
bGIR-QUESCAT, Departamento de Química Inorgánica, Facultad de Ciencias Químicas, Universidad de Salamanca, Plaza de la Merced, s/n, 37008 Salamanca, Spain. E-mail: rakel@usal.es; mavicente@usal.es
cINAMAT-Departamento de Química Aplicada, Edificio de los Acebos, Universidad Pública de Navarra, Campus Arrosadía, 31006 Pamplona, Spain. E-mail: andoni@unavarra.es
First published on 6th November 2020
Abstract
The modification of bentonite with Al–Fe species from different concentrated precursors at both stages: (i) the preparation of the (Al/Fe)-mixed pillaring solution and (ii) intercalation itself, was studied at lab scale. The final solids were characterized by X-ray fluorescence (XRF), X-ray diffraction (XRD), Cationic Exchange Capacity (CEC), textural analyses by nitrogen adsorption–desorption at 77 K, and hydrogen-temperature programmed reduction (H2-TPR). Finally, the modified clays were assessed as active materials in the Catalytic Wet Peroxide Oxidation (CWPO) of phenol under very mild conditions through 1.0 h of reaction: T = 25.0 °C ± 0.1 °C, pH = 3.7, ambient pressure (76 kPa), and 0.5 g catalyst per dm3. Metal hydrolysis by the dissolution of elemental aluminium (final Total Metal Concentration TMC = 4.62 mol dm−3) achieved the best results, decreasing the volume of solution per mass unit of clay required to successfully expand the layered starting mineral by a factor of close to 75, in comparison with the widespread conventional preparation using highly diluted Al-based pillaring solutions. Even in the absence of any solvent for the clay dispersion, the intercalating/pillaring method was shown to be favourable, as a novel strategy promoting the process intensification and subsequent preparation of Al/Fe- and other Al-based pillared clays at larger scales. The best catalyst prepared from concentrated precursors exhibited 79.1% phenol conversion, 19.3% TOC mineralization, and pretty low iron leaching (0.037 mg Fe dm−3; ∼0.12% w/w) in such a short catalytic assessment; all these results were quite comparable or even exceeded those exhibited by the catalyst prepared from dilute precursors.
Introduction
Clay minerals are considered materials with high technological potential in heterogeneous catalysis and adsorption, since they constitute natural, low cost hydrous layered aluminosilicates.1 The structure of some clay minerals can be easily modified by different procedures, as through cationic exchange of their original cations by bigger ones (e.g. polymeric metal-containing inorganic cations such as the Keggin adduct [Al13O4(OH)24(H2O)12]7+) increasing their basal space, creating micro-porosity, and conferring superior mechanical stability as well as greater thermal stability to the final materials.1,2 Broadly speaking, the Al-based clay catalysts are prepared using a combination of a layered clay from the group of the smectites, together with a pillaring, hydrolysed solution of either aluminium, several mixed systems of Al with Fe, Cu, Co, etc.,3 or other metals.The conventional method of preparation of pillared clays (PILCs) consists of mixing a dilute clay aqueous dispersion (∼2.0% in weight) with the intercalating solution.4,5 The most common process for the preparation of this kind of clay catalysts involves at least three successive core steps: (1) preparation of the metal hydrolysed, intercalating solution; (2) intercalation of the clay with the intercalating solution (usually a dilute solution of the single or mixed metal precursor is slowly added over a dilute dispersion of the clay mineral in water); and (3) annealing to anchor the pillars onto the layered structure at 350–500 °C.3,4,6 The hydrolysis by slow addition of sodium hydroxide has been the most widely spread method,7 but the final concentration of metals (Al or mixed Al/M, M = transition metal) is restricted to very low Total Metal Concentrations (TMCs ∼0.1 mol dm−3 or less). Thus, the scaled-up preparation of pillared clays by such a conventional procedure would demand both large amounts of water and long times of reaction, which are clear drawbacks towards scaling-up.8,9 To overcome these, use of highly-concentrated (i) hydrolysed metal solutions and/or (ii) clay dispersions, becomes mandatory in the preparation of Al-based pillared clays, whereas both precipitation of the metal hydroxides and long times of hydrolysis should remain strongly prevented.2,4,6
The intensified preparation of Al-based PILCs (pillared interlayered clays) at laboratory scale has been studied by several authors within the past decade.10,11 Our preceding work,4 achieved increasing TMC of the pillaring precursor until 0.628 mol dm−3 and the content of the dispersed clay mineral up to 25.0% w/v in the preparation of Al/Fe-PILCs by controlling both the nature of the exchangeable cations in the mineral and using solvents with low dielectric constant to disperse the clay. Likewise, the hydrolysis of aluminium solutions by the microwave-assisted decomposition of urea has been claimed to achieve the preparation of Al-based PILCs with intercalating solutions of Al13-polycations up to TMC = 0.5 mol dm−3; several advantages of the microwave-assisted method over the conventional one of Al hydrolysis could be highlighted: (i) shorter reaction times, (ii) more efficient heating in the form of microwave radiation, and (iii) single-pot synthesis of interest from an industrial point of view.12 Other preparations of metal concentrated solutions6,13,14 have allowed to prepare PILC-catalysts of mixed systems as Al–Fe, Al–Ce or Al–Fe–Ce improving formation of the Al13-polymeric adduct. Moreover, long back Akitt et al.15 studied an alternative procedure of hydrolysis of aluminium solutions by addition of a fraction of the metal in its elemental form (Al0) to accomplish a more controlled process, apparently less susceptible against precipitation of the metal hydroxide even in highly concentrated solutions, which as long as we know has not been extensively explored in the preparation of mixed-metal (Al/M-) concentrated pillaring precursors. The same authors also studied electrolysis of AlCl3 solutions to yield pure Al13 with an OH−/Al ratio of 2.4.16
On the other hand, in order to either decrease the time or the operating volume in the stage of mineral's intercalation or still become simpler the process, throughout the past two decades several researchers have also studied different methods using concentrated reagents or dialysis bags.10 Other techniques such as microwave2,8,17,18 or ultrasound irradiation have been also used for this purpose;2,5,19 these methods allowed to decrease between 70–90% the synthesis and intercalation time, but they imply the consumption of an extra amount of energy. Other works dealing over the intercalation of Al or Al/Fe solutions20,21 reported the use of either an antiperspirant intercalating solution or the straightforward addition of raw clays (not previously swollen) over up to 40% clay's suspensions attaining Al-pillared materials with a significant increase in basal spacing (>17.0 Å), specific surface area (SBET > 182 m2 g−1), micropore surface area (Sμp > 77.5 m2 g−1), and improved thermal stability (up to 800 °C).
In this regard, the Al/Fe-PILCs offer great potential catalysing varied reactions of industrial interest due to the enhanced availability and good dispersion the iron achieves within the microporous channels of the clay material, whereas stabilized in the form of mixed oxides with Al. Several studies have shown that one of such potential applications for the Al/Fe pillared clays is the activation of the Catalytic Wet Peroxide Oxidation (CWPO) of dissolved pollutants in water,3,22–24 useful depleting low concentrated, hazardous compounds including emerging contaminants (ECs), phenolic compounds, natural organic matter and a wide variety of bio-refractory azo-dyes.3 The immobilized Fe cations supported in clays in the presence of hydrogen peroxide produce highly reactive oxidant hydroxyl radicals (HO˙) through the Fenton-like set of reactions.22,23,25,26 It has been well portrayed the high performance exhibited by this kind of catalysts in the CWPO reaction primarily depending on the following physicochemical properties: (i) high specific surface area and porosity, mainly represented in micropores; (ii) basal spacing in the range 17.0–20.0 Å; (iii) thermal but mainly chemical stability against leaching of the active transition metal in the strongly oxidizing environment of reaction.11,27
Accordingly, this research was first devoted to compare two methodologies of preparation of highly concentrated hydrolysed Al/Fe-intercalating solutions against the widely reported, conventional method of preparation in dilute medium by slow addition of sodium hydroxide, namely: (i) self-hydrolysis by controlled addition of elemental aluminium and (ii) microwave-assisted decomposition of urea, indirectly hydrolysing the mixed-metal solution. Afterwards, the best performing concentrated pillaring solution was then used to study three different methods for the clay intercalation at 50 g scale preparation varying the dispersion of the starting mineral as follows: (i) previously dispersed in ethanol, (ii) previously dispersed in water or (iii) with no previous dispersion at all. Likewise, the effect of the previous particle-size refining of the natural bentonite on the physicochemical and catalytic properties of the final Al/Fe-PILC was also determined. Every material was characterized by standard physicochemical techniques and comparatively assessed in a quick catalytic test of the CWPO degradation of phenol (PhO). These findings were essential to the later successful scaled-up preparation of the Al/Fe-PILC at a 10 kg pilot scale from concentrated precursors, we recently reported elsewhere.28
Experimental section
Materials
The starting material for the preparation of the clay catalysts was a Colombian bentonite, already carefully characterized,3 denoted as C2-N in its raw form. This material was refined by sedimentation of aqueous dispersions using the Stokes's law, allowing to enrich the fraction of particle diameters lower than 2.0 μm (C2-R). The mixed metal oligomer pillaring precursor was prepared employing AlCl3·6H2O (97%, Merck®) and FeCl3·6H2O (97%, Panreac®). The basic hydrolysing agents were either NaOH (≥99.0%, Merck®), elemental aluminium (powder pure, Panreac®) or urea (≥99.5%, Merck®). The clay dispersions were made in either twice-distilled water or ethanol (95.1–96.9% v/v ACS reagent Ph Eur, Merck®). Ammonium acetate (97.0%, Carlo Erba®) was used to determine Cationic Exchange Capacity (CEC) of the solids. Catalytic experiments were made using phenol (99.0–100%, Sigma-Aldrich®) and hydrogen peroxide (50% w/w, Chemí®).Preparation of the Al/Fe-mixed hydrolysed solution
Three Al–Fe mixed intercalating solutions, a dilute (reference solution) and two concentrated, were all prepared with identical targeted Atomic Metal Ratio of Fe(AMRFe = 5.0%) against the total concentration of metals.
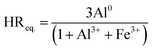 | (1) |
Preparation of the Al/Fe-clay catalysts
First, the best mixed-metal intercalating solution exhibiting the higher final concentration and stability (no dispersed particles) was assessed as a function of the expansion achieved between the clay layers; this study was made through preparations of 5.0 g of the final clay catalyst using the procedure later called (a). All solids were prepared with the Al/Fe-hydrolysed precursors in proper amount to obtain a final loading of 20 meq (Al3+ + Fe3+) per g clay. The intercalating procedures (a) and (b) were adapted from our previous work,4 whereas the method (c) was adopted from Kooli et al.:20For determining the best intercalating method and the effect of the previous particle-size refining (C2-R) of the starting mineral C2-N, the best concentrated pillaring solution was used in preparations at 50.0 g scale for each intercalating method. The materials are hereafter called by the starting clay mineral C2-N or C2-R, followed by the intercalating method used (EtOH, H2O or NS-not dispersion). The full physicochemical characterization and the catalytic behaviour of every solid in the degradation of phenol (PhO) were determined.
Analytical methods
The elemental analyses of the solid materials were carried out by X-ray Fluorescence (XRF) in a Bruker S8 Tiger 4 kW Wavelength Dispersive X-ray Spectrometer, with a Rh anode as an X-ray source, scintillation detector (heavy elements, from the Ti to the U), and flow (light elements, from the Na to the Sc); calibration curves were created using the Quant-Express method (Fundamental Parameters). Approximately 1.0 g of sample was used, sieved through 400 mesh, and calcined at a heating rate of 3.0 °C min−1 up to 950 °C to determine the loss on ignition.X-ray diffraction (XRD) patterns of the starting materials and the catalysts were recorded over both oriented specimens and powdered samples using a Bruker D8 Advance diffractometer, working at 40 kV and 30 mA with a scanning speed of 2.29° 2θ min−1, employing Cu Kα filtered radiation (λ = 1.5418 Å). Powders were analysed in the range of 2.0 to 70.0° 2θ, and oriented specimens in the range of 2.0 to 30.0° 2θ.
The Cationic Exchange Capacity (CEC) of the solids was determined by saturation with 45 mL of 2.0 mol dm−3 ammonium acetate solution per g of solid under reflux, followed by repeated washing with distilled water and centrifugation to eliminate the excess of adsorbed ammonium ions. The ammonium uptake was established by micro-Kjeldahl analysis. The results were interpreted in terms of the cationic compensation (CC) reached on the starting clay by the pillaring procedure for a given x sample (eqn 2):
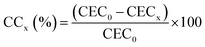 | (2) |
The textural analysis was carried out by determining the nitrogen adsorption–desorption isotherms at −196 °C obtained from a 100–200 mg sample in a 3-Flex Micromeritics Sorptometer, over a wide range of relative pressures; previously the samples were degassed at 300 °C for 12 h. The BET specific surface areas (SBET) were determined by the multipoint model, using the Keii–Rouquerol criteria to find the best linear BET fitting.29 The external surface area (Sext) and the surface corresponding to micropores (Sμp) were calculated using the t-plot (Harkins–Jura) method.
Hydrogen temperature-programmed reduction analyses (H2-TPR) were performed using a Chemisorb 2720 Micromeritics apparatus. Around 40–50 mg sample was heated from room temperature to 1000 °C at 10 °C min−1 under 50 mL min−1 of reactive gas (∼10.0% H2 in Ar). The consumption of hydrogen was measured with a thermal conductivity detector (TCD), where Ag2O (99.99%) was used as the external standard of calibration. The measured thermal events were interpreted in comparison with the sites usually exhibited by Al/Fe-PILCs, as proposed before:30 fraction of extra-structural FeOx aggregates, fraction of interlayered iron oxide aggregates (iron oxides “decorating” alumina pillars), Fe occupying structural sites of the clay, and finally the Fe presumably forming part of real mixed Al/Fe pillars.
Catalytic experiments
The starting as well as the modified materials were evaluated as active solids in the wet hydrogen peroxide-assisted catalytic oxidation of phenol (PhO). The experiments were carried out at 25.0 ± 0.1 °C and atmospheric pressure (76 kPa) in a 1500 cm3 semi-batch (Pyrex®) glass reactor equipped with a jacket for temperature control with thermostatic bath, and a peristaltic pump to feed the H2O2 solution under controlled flow with a mechanical stirrer 600 rpm (LB PRO® OS20-S). For each test, the reactor was loaded with 1.0 dm3 of PhO solution (26.1 mg dm−3 of total organic carbon) and 0.5 g of the solid catalyst under constant air bubbling (about 2.0 dm3 min−1). The pH of the solution was adjusted to 3.7 and constantly controlled in this value through the addition of drops of 0.1 mol dm−3 HCl or NaOH. Addition of 100 mL of the H2O2 solution (3.71 mmol dm−3, equivalent to the stoichiometric theoretical amount required for full mineralization of the PhO in the reactor by H2O2) started after 30 min of stirring and air bubbling (equilibrium period), under a flowrate of 1.67 cm3 min−1. The zero time of reaction was the starting of the addition of the hydrogen peroxide solution into the reactor; from that moment on, 25.0 mL-samples were taken each 15 minutes, during a total reaction time of 1.0 h. Every sample was micro-filtered (Millipore, Burlington, MA, USA, 0.45 μm filters) to remove the dispersed catalyst before the analyses. The measured catalytic parameters were the total organic carbon to calculate level of carbon mineralization (Shimadzu TOC-L CPH Analyser). The degradation of phenol was followed by the colorimetric analysis of 4-amino antipyrine (the standard method for quantification of phenols) from a spectral recording from 200 to 700 nm using the absorbance at 510 nm. At the end of every catalytic test, the remaining powdered catalyst was recovered by vacuum filtration and the chemical stability checked through the leached iron in the reaction effluent by atomic absorption spectroscopy (AAS).Results and discussion
Physicochemical features of the Al/Fe-mixed hydrolysed solutions
The physicochemical characteristics of the intercalating solutions Al/Fe-OH, Al/Fe-U/MW, and Al/FeAl0 are shown in Table 1. The hydroxide anions added in the first case or generated in situ in the second and third cases, successfully hydrolysed the metal chlorides leading to the formation of intercalating precursors with different distribution of highly condensed oligocations. The concentrated solutions prepared by the addition of powdered aluminium at different equivalent HReq. values (Al/Fe-Al01.50 and Al/Fe-Al02.58), as well as by microwave-assisted decomposition of urea (Al/Fe-U/MW) did not show the formation of precipitated solids despite the high concentrations of metal salts used in comparison with the dilute reference solution (Al/Fe-OH). This behaviour can be attributed to the rapid and uniform microwave heating in the case of Al/Fe-U/MW, and the controlled heating, addition of aluminium and gentle agitation in the case of Al/Fe-Al0; the second case was clearly quite less sensitive against high TMC values. In all the cases, the final pH increased, even when negative values were obtained in the highly acidic starting solution Al/Fe-U/MW.| Intercalating solution | [Al] (mol dm−3) | [Fe] (mol dm−3) | TMCf (mol dm−3) | HR | pHi | pHf | ρ (g dm−3) | EC(w) (mS cm−1) |
|---|---|---|---|---|---|---|---|---|
| a TMCf: final total metal concentration; HR: hydrolysis ratio (OH−/Al3+ + Fe3+), for Al/Fe-Al0 it was calculated from eqn (1) by Akitt et al.;15 ρ: density; EC: electric conductivity. w: experimental values for each highly concentrated Al/Fe intercalating solution determined by AAS. | ||||||||
| Al/Fe-OH | 0.054 | 0.002 | 0.06 | 2.00 | 1.97 | 3.86 | 0.999 | 22.4 |
| Al/Fe-U/MW | 2.20 | 0.12 | 2.32 | 1.50 | −0.52 | −0.33 | 1.410 | 47.8 |
| Al/Fe-Al01.50 | 4.39 | 0.23 | 4.62 | 1.50 | 0.43 | 0.57 | 1.280 | 85.6 |
| Al/Fe-Al02.58 | 3.98 | 0.27 | 4.25 | 2.58 | 1.22 | 3.32 | 1.330 | 58.6 |
The higher concentrations of metals also increased the final values of the electrical conductivity (EC) in comparison with the dilute precursor. However, for comparable TMC values, a lower EC in the solution may indicate a greater extent of oligomerization of the metals into Al13 and Al(13−x)Fex polycations; while the charge for each aluminium atom in a single, isolated Keggin-like polycation corresponds to +0.54 (7 positive charges distributed over 13 Al atoms), in the hypothetical free state of the Al3+ cation it is obviously (+3). Besides, the mobility of the polynuclear cations formed is of course much lower than that of the free cations in the (+3) oxidation state.30 In this way, the measured EC values decreased in the order Al/Fe-Al01.50 > Al/Fe-Al02.58 > Al/Fe-U/MW > Al/Fe-OH (Table 1); however, the specific conductivity per mol of dissolved metal was significantly lower for all concentrated precursors (13.8–20.6 mS cm−1 mol−1) in comparison with the dilute reference solution (373.3 mS cm−1 mol−1), where both Al/Fe-Al0 solutions displayed the lower couple of values, in turn decreasing inversely against HR. The method of conventional hydrolysis of course provides a smaller number of ions due to the lower concentration of metal precursors and the associative interactions of the ions constituting the mixed Keggin like Al/Fe poly-hydroxy-cation.30 Thus, it can be inferred that the methods of preparation of concentrated intercalating solutions here assessed allowed for most of the dissolved metals to get condensed in oligomer species, but particularly those prepared by the controlled dissolution of elemental aluminium, where the intercalating solution with higher hydrolysis ratio (2.58) exhibited a greater level of condensation of the oligomers.
On the other hand, the Al/Fe-Al02.58 solution also displayed the higher final pH (Table 1; pHf = 3.32), which was comparatively closer to the reported optimal range of formation of Keggin-like polyoxocations in dilute systems.28,31 Meanwhile, an opposite behaviour was found for the density of the final intercalating solution in comparison with the pH, since higher TMC values of course significantly increased the density. Here it is worth noting that the Al/Fe-U/MW solution showed the highest density, even though close to half the TMC of both Al/Fe-Al0 solutions, revealing a significantly higher degree of oligomerization took place, not necessarily beneficial for the later interlayering of the clay mineral.
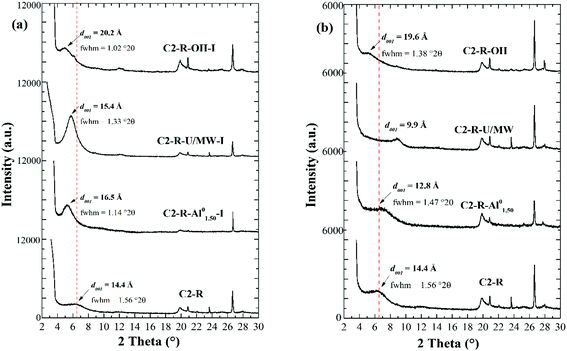
The XRD patterns of the oriented specimens of the intercalated materials (before the pillaring step) with the hydrolysed, concentrated solutions (Fig. 1a) keeping constant HR showed a better intercalation of the refined clay with the solution Al/Fe-Al0 (d001 = 16.7 Å) in comparison with the Al/Fe-U/MW solution (d001 = 15.4 Å); however, these values were both far lower than the one obtained with the dilute reference solution Al/Fe-OH (d001 = 20.2 Å). Nevertheless, it is remarkable that the low full width at high maximum value (fhwm = 1.14° 2θ) exhibited by the clay mineral modified with the Al/Fe-Al01.50 solution, even though it was prepared using a very high TMC, suggesting a high uniformity in the size of the intercalated oligocations formed by this hydrolysis procedure.28
On the other hand, the powder XRD patterns displayed by the same set of materials but after pillaring (Fig. 1b) revealed a huge decrease of the basal spacing with respect to their intercalated forms for both pillared materials prepared from concentrated precursors. The strong depletion in the basal spacing of C2-R-Al/Fe-Al01.50 upon pillaring (d001 = 13.3 Å) with respect to its intercalated form could be attributed to either low degree of oligomerization of the intercalating polycations or strongly hindered diffusion of them into the interlayer space of the clay, because of the highly concentrated clay dispersion used.28 Finally, the C2-R-Al/Fe-U/MW-P material showed a d001 signal typical of collapsed smectites (9.96 Å),28 corresponding to the thickness of the structural 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sheet; in this sense, it can be hypothesized that the MW treatment was enough to ensure the almost full decomposition of the added urea, but not to allow the condensation of the mixed-metal polycations in the proper extent to promote their Keggin-like form to be stabilized.
1 sheet; in this sense, it can be hypothesized that the MW treatment was enough to ensure the almost full decomposition of the added urea, but not to allow the condensation of the mixed-metal polycations in the proper extent to promote their Keggin-like form to be stabilized.
Accordingly, the best method for the preparation of concentrated, hydrolysed Al/Fe-intercalating solutions so far was the method (i) of hydrolysis in situ by dissolution of elemental aluminium (Al/Fe-Al0). The potential implementation of this procedure may allow to significantly intensify the process of obtaining this type of functional materials, since the prepared solution achieved to increase the concentration of metals by around 75 times as compared to the conventional, reference dilute method; for example, the interlayering of 1.0 g of clay requires 100 cm3 of dilute intercalating solution (Al/Fe-OH), whereas just 1.33 cm3 of the (Al/Fe-Al0) would be enough. Furthermore, according to SEM micrographs previously reported elsewhere,28 the Al/Fe-PILC obtained from a pillaring solution with TMCf 2.70 mol dm−3 prepared by this method of hydrolysis exhibited very similar morphology (4000×) in comparison with the one yielded from the mixed diluted intercalated solution Al/Fe-OH, both at lab scale.
Effect of the method of intercalation and the previous particle-size refining of the clay mineral
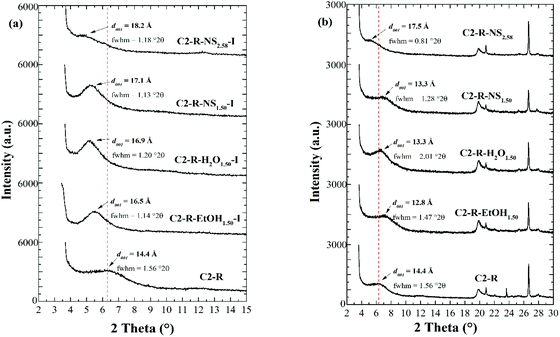
In the second part of this work, the effect of the previous particle-size refining of the starting material (C2-N vs. C2-R) and the type of dispersion used in the intercalation step (EtOH, H2O, or NS), in the preparation of the Al/Fe-PILC clay catalysts with the best concentrated intercalating solution (Al/Fe-Al0) was investigated. The solid from the raw clay mineral C2-N and the intercalating solution prepared via dispersion at 25% w/v in water (C2-N-H2O) was not manageable since the dispersed natural clay fully jellified after 30 minutes of agitation; this behaviour could be due to the higher dielectric constant of water as compard with ethanol, that has been reported to promote the destabilization of the clay dispersions.4 The SiO2/Al2O3 ratio provides information about the amount of quartz that may be present as non-swellable impurity in the starting clays.32 A high SiO2/Al2O3 ratio indicates a greater amount of quartz in the raw material. Thus, according to Table S1,† C2-N exhibited the highest SiO2/Al2O3 ratio (3.22), that then decreased after particle-size refinement showing significant removal of the quartz content in C2-R (2.76). As a result of the modification of the starting materials with the mixed Al/Fe oligomers, all products exhibited a decrease in their contents of exchangeable cations (Na+ and Ca2+) with respect to their starting materials (Table S1†), as evidence of the exchange and uptake of the intercalating metals in the interlayer space of the clay mineral.The oriented XRD patterns of the Al/Fe-intercalated forms of both starting materials C2-R and C2-N (Fig. 2a and S1a†) displayed a significant increase in the basal spacing, where the Al/Fe-Al01.50 concentrated solution provided proper interlayering oligomers regardless the intercalation method employed (EtOH, H2O, or NS). However, the materials prepared by intercalation in absence of any dispersion medium (NS) exhibited a slightly greater increase in the d001 spacing, reaching maximum values of 17.1 Å (C2-R-NS1.50-I) and 18.2 Å (C2-R-NS2.58-I). Moreover, when these materials were thermally treated (Al/Fe-pillared forms) their basal spacing (Fig. 2b and S1b†) decreased to such an extent that they even fall below the starting material C2-R (d001 = 14.4 Å), except for the material intercalated with the concentrated solution prepared at higher HR (C2-R-NS2.58); this behaviour suggested that the stability of the oligomers got significantly enhanced by increasing the level of hydrolysis, in other words, by promoting the formation of Keggin-like oligomer poly-oxocations, thanks to the final pH of the intercalating solution more closely approaching the optimal range typically achieved from dilute precursors.
Accordingly, the XRD analysis showed that the polynuclear cations formed in the Al/Fe-Al01.50 intercalating solution were so that condensed enough to formerly expand the basal spacing of the starting clay mineral, but not sufficiently stable to form Keggin-like Al/Fe-mixed oxide pillars upon the final heating at high temperature, which led to lower basal spacing values regardless the starting clay (Fig. 2b). Nevertheless, the d001 of these materials after pillaring was still greater than the thickness of the structural sheet (∼9.96 Å);28 thus, whereas the low HReq. of this intercalating solution allowed the formation of partially hydrolysed polycations, it did not allow their aggregation into stable Keggin-like three-dimensional structures. Thus, because of either the thermal treatment or the strongly acidic environment of the aluminosilicate interlayer space, the polycations got back disaggregated as in the case of the sample Al/Fe-Al1.50, which under heating just showed an incipient Al/Fe pillaring. This effect was clearly avoided when the hydrolysis ratio was increased as in the case of the intercalating solution Al/Fe-Al02.58 that clearly achieved the Al/Fe-pillaring.
On the other hand, the adsorption/desorption isotherms of the C2-N and C2-R starting materials belonged to type II according to the IUPAC classification,33 typical of mesoporous materials,20 whose measured surface area is mostly due to external area (Fig. 3 and S2†). Meanwhile, the isotherms of all Al/Fe-modified materials displayed intermediate behaviour between type I at low relative pressures, and type II at high p/po according to the BDDT classification,33 featuring mixed mesoporous and microporous solids.20 The specific surface area of the materials modified by the NS intercalation method was mainly represented by the generation of micropores with the largest physisorbed volumes on both starting materials. Besides, all solids obtained from the refined clay (C2-R) exhibited higher adsorbed volumes in comparison to those prepared from the natural mineral (C2-N), verifying the strong favourable effect of the previous particle-size refining on the textural properties of the final solids, including microporous surface and total volume of pores. Furthermore, the hysteresis loops were type H3 according to the IUPAC classification,33 typical of slit-shaped pores, indicating the stacking of parallel plates featuring this type of layered clay minerals was fairly preserved after the topotactic Al/Fe-modification.27,34
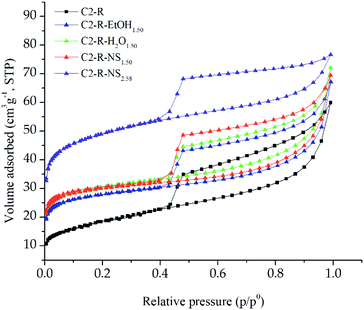 | ||
| Fig. 3 Nitrogen adsorption–desorption isotherms of the Al/Fe-PILC materials prepared as a function of the intercalating method from the refined starting clay. | ||
The BET specific surface area (SBET) of all modified materials (Table 2) significantly increased with respect to each starting material C2-N or C2-R, reaching maximum values of 181 m2 g−1 and 115 m2 g−1, both from the refined aluminosilicate and the intercalating solutions Al/Fe-Al02.58 and Al/Fe-Al01.50, respectively. It is worth noting that such increases were mostly due to the formation of new microporosity. The enhancement in the micropore surface area (Sμp) was greater for the materials obtained from the refined clay (C2-R), which showed that the previous particle-size refining strongly favours the improvement in the textural properties, probably because of the elimination of the non-expandable phases in the starting mineral, seriously improving the CEC and then the extent of the cationic exchange. Besides, the close correlation between the higher basal spacing achieved after the final heating step and the higher textural properties induced on the material is remarkable, both conditions accomplished in the solid C2-R-NS2.58.
| Material | Al2O3a (% w/w) | Fe2O3a (% w/w) | Feinc.a (% w/w Fe2O3) | CEC (meq/100 g) | CC (%) | SBET (m2 g−1) | Sμp (m2 g−1) | Sext (m2 g−1) |
|---|---|---|---|---|---|---|---|---|
| a Determined by XRF analyses. Feinc.: incorporated iron; CEC: cation exchange capacity; CC: compensated CEC after pillaring; SBET: specific superficial area; Sμp: micropore area; Sext: external surface area. | ||||||||
| C2-N | 16.52 | 6.86 | — | 108 | — | 36 | 10 | 26 |
| C2-N-EtOH1.50 | 25.90 | 8.42 | 1.56 | 66 | 64 | 71 | 48 | 23 |
| C2-N-NS1.50 | 26.39 | 8.02 | 1.16 | 71 | 34 | 85 | 73 | 12 |
| C2-R | 17.84 | 8.61 | — | 167 | — | 67 | 18 | 49 |
| C2-R-EtOH1.50 | 25.15 | 10.08 | 1.47 | 59 | 65 | 105 | 79 | 26 |
| C2-R-H2O1.50 | 25.96 | 9.86 | 1.25 | 62 | 63 | 115 | 86 | 29 |
| C2-R-NS1.50 | 26.34 | 10.22 | 1.61 | 75 | 55 | 115 | 93 | 22 |
| C2-R-NS2.58 | 26.41 | 10.18 | 1.57 | 115 | 31 | 181 | 117 | 64 |
Regarding the intercalation method, the BET specific surface area was greater for the materials prepared without any dispersion medium (NS). This result is very important in order to justify the strong intensification of the intercalation stage, since the direct addition of the aluminosilicate over the intercalating solution prevents the use of other solvents, minimize the time spent in the clay's pre-swelling stage as well as the working volume per unit mass of the solid prepared, without affecting the textural properties of the final material, instead, improving them.
Table 2 exhibits the contents of Al and Fe in the form of their oxides found on every starting and modified material, obtained by X-ray Fluorescence (XFR). Besides, the percentages of incorporated Fe are also shown; these analyses confirmed the proper incorporation of both metals in all the modified materials, with higher contents of both aluminium and iron incorporated in C2-R-NS1.50 (8.50% w/w Al2O3 and 1.61% w/w Fe2O3). Among the materials prepared from the refined mineral (C2-R), a significantly lower incorporation of iron was observed for the solid previously dispersed in water (C2-R-H2O1.50), probably related to the greater gelation of the clay dispersion because of the high dielectric constant of this medium.4 Meanwhile, among the materials obtained from the natural mineral (C2-N) a higher percentage of iron incorporation was attained when the previously intercalated material was dispersed in ethanol, which agrees with the greater CEC compensation, confirming that the incorporation of metals was carried out predominantly via cationic exchange.
The refined material C2-R favoured the intercalation in the absence of dispersion medium (NS). Although quite similar CEC compensations were observed for the materials obtained from the raw clay against the refined clay (Table 2), probably better physicochemical properties were obtained in the C2-R derived materials in part, because of the significantly higher CEC displayed by the starting refined mineral. The absence of dispersion medium (NS) mainly affected the efficiency of cationic compensation, probably because, as explained before, the higher the level of condensation of the metal polycations, the lower the mean cationic charge of every atom of metal intercalated. However, the straight addition of the clay over the intercalating solution resulted quite favourable for the intensification purpose.
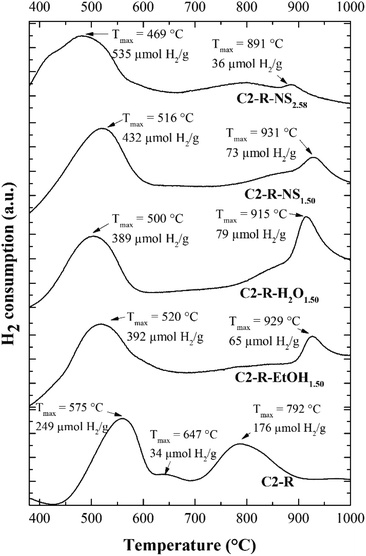
The H2-TPR plots of the starting materials (Fig. 4 and S3†) displayed two noticeable reduction effects that can be attributed to (i) the reduction of iron on the external surface (iron contaminating phases), which occurred at lower temperatures between 445 °C and 600 °C, and (ii) the reduction of structural iron within the sheets of the clay at temperatures between 690 °C and 875 °C. The small shoulder evidenced only in the starting materials at very similar temperatures (645 °C for C2-N and 647 °C for C2-R) was noticeable, probably corresponding to one of the following: (a) iron oxides of more difficult access since after the heating through the analysis some iron ions could migrate to less accessible positions for hydrogen,35 (b) the subsequent reduction of magnetite to wüstite (Fe3O4 → FeO),36 or more likely related to (c) the release of gases from the starting materials, not previously heated at 500 °C as the pillared ones. The lowest temperatures of reduction and lowest total consumption of hydrogen were found for the unrefined C2-N material, suggesting that the refining of the clay mineral did not retire a significant amount of contaminating iron oxide phases.
On the other hand, irrespective of the starting clay (natural or particle-size refined), all Al/Fe-modified materials displayed a first reduction effect in the range of 450–650 °C. In this set of materials, such effect can be ascribed to external iron oxides, together with iron within the more accessible tetrahedral sheets of the clay, whose reduction might occur at lower temperatures facilitated by the prior expansion of the clay layers upon pillaring.19,28 These materials also showed a second reduction signal at even higher temperatures between 890 °C and 960 °C, which was not observed in the starting materials; it has been attributed to less reducible, true mixed species of Al/Fe (pillars) incorporated into the interlayer space of the clays, as previously proposed,30 but the possible reduction of the less accessible octahedral iron sites in the structural sheets of the clay material allowed by the thermal delamination at that high temperature, should be also carefully considered to explain it.
Among the materials modified from C2-R, the highest reduction temperatures were achieved over 890 °C; the material C2-R-NS1.50 displayed a Tmax of 931 °C, which indicated either a stronger interaction of the added iron with the pillars or the layered aluminosilicate, or a higher thermal stability developed in this material. Moreover, the rest of materials showed values not far below from that.
Catalytic behaviour of the prepared materials in the CWPO degradation of phenol
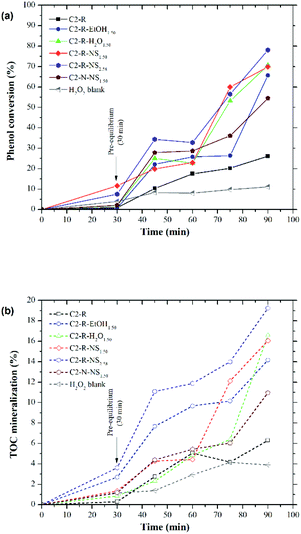
The evolution of the phenol conversion and TOC mineralization (%) throughout the CWPO catalytic reaction in the presence of every prepared Al/Fe-modified clay as a function of the dispersion medium employed in the intercalation stage is compared in Fig. 5 and S4.† The starting material C2-R showed very low levels of both phenol degradation (26.1%) and TOC mineralization (6.30%). Likewise, among the modified materials from C2-R, the conversion of phenol ranged between 66.0% and 80.0% and the TOC mineralization between 14.2% and 19.3% after 60 min of reaction. The H2O2 blank experiment (only peroxide without addition of catalyst) revealed the very important role played by the catalysts in the H2O2 activation, since the removal did not exceed 11.0% of phenol and 3.89% of TOC mineralization after 60 min of reaction in the presence of the stoichiometric amount of peroxide. It also allowed to infer both the significant role played by the incorporated iron in Al/Fe-pillared clays, and the minor one played by the structural iron in the starting mineral to explain the recorded phenol conversion and TOC mineralization.In comparison, the highest value among the modified materials from the natural clay was reached using C2-N-NP1.50 solid, exhibiting 54.5% of phenol conversion and 10.9% of TOC mineralization. The conversions displayed by the starting materials were clearly very low to explain the values obtained in the presence of the modified ones, ruling out the possible conversion by the structural Fe content in the sheets of the starting clays. The materials modified from the refined mineral (C2-R) exhibited enhanced conversion of phenol after 30 minutes of reaction compared to those prepared from the natural material (C2-N). The adsorption of phenol occurred a little bit faster in the first group and hence the degradation also, which is attributed to the presence of mostly expanded clay phases leading to enhanced textural properties (Table 2).
The conversion of phenol was greater for the intercalated solid prepared without any dispersion medium (NS), followed by that dispersed in H2O and finally the one dispersed in EtOH. The greatest conversions of phenol were achieved in the presence of materials derived from C2-R, in a good correlation with: (i) greater area of micropores (Sμp = 117 m2 g−1), wherein the most active catalytic sites could be predominantly located in this kind of structures, (ii) greater difference in basal spacing (d001 = 17.5 Å) after calcination with respect to its starting material, which allowed greater accessibility of organic substrates and phenol to the active sites, (iii) percentage of compensated CEC (CC = 31%) and incorporated iron (Feinc. = 1.57% w/w Fe2O3), revealing a strong correlation between the ability of the polycations to compensate the clay's layer charge and the catalytic performance of the clays upon Al/Fe pillaring in the CWPO reaction,28,30 and (iv) higher reduction temperature of the active metal (Tmax > 890 °C) probably confirming that C2-R-NS2.58 was the catalyst that also exhibited the highest thermal stability.
Even in the materials in which the expansion at the end of the pillaring procedure was not very efficient (C2-R-NS1.50 and C2-R-H2O1.50, both exhibiting d001 = 13.3 Å), the insertion of the oxides and oxy-hydroxides improved the catalytic behaviour, in close correlation with the increase in the micropore's surface (Sμp). The H2-TPR results allowed to suppose that H2O2 achieved to diffuse onto the iron species mainly immobilized within the microporous cavities (Fe decorating alumina pillars or making part of truly mixed pillars). The material C2-R-NS2.58 showed the highest phenol conversion (78.1%) and mineralization (19.3% of TOC mineralized to CO2 and H2O), correlating with the best physicochemical properties in comparison to the rest of the prepared materials. The measured catalytic performance for the prepared materials in these quick experiments was not intended to display very high levels of either phenol degradation or mineralization, but just to get a confident comparison between the clay catalysts prepared to be correlated with their recorded physicochemical properties.
Last but not least, the concentration of the active metal (Fe) in the aqueous effluents of reaction after the catalytic reactions in the presence of the prepared catalysts from the raw starting mineral were always below 0.028 mg Fe dm−3 (0.10% w/w respect to the iron content in C2-N-NS1.50), and less than 0.044 mg Fe dm−3 (0.12% w/w respect to the iron content in C2-R-NS2.58) for those from the refined clay. Such a low range of concentrations of iron leaching safely ruled out any significant contribution of homogeneous Fenton over the recorded catalytic response; furthermore, recent studies in a continuous flow CSTR reactor showed the iron leaching from an Al/Fe-PILC clay catalyst prepared from concentrated precursors here described in depth, stabilizing around 0.05 mg dm−3 over more than ten consecutive catalytic runs in the CWPO degradation of phenol,37 displaying interesting stability of the active transition metal in the CWPO degradation of contaminants in water.
Conclusions
The study of the two alternative methodologies for the preparation of highly concentrated, mixed Al/Fe intercalating solutions showed the self-hydrolysis by controlled addition of part of the aluminium content in form the powdered metal (Al/Fe-Al0) to be the most efficient one. This method allowed an indirect control of the hydrolysis ratio by means of the starting (Al3+/Al0) quotient, whereas yielding stable, particle-free hydrolysed solutions in concentrations around 75 times higher than the one widely reported for the preparation of Al-based pillared clays. The clay interlayered with the best performing intercalating solution ([Al + Fe]f = 4.25 mol dm−3, (Al3+/Al0) = 86/14, HR = 2.58, AMRFe = 5.0%) exhibited excellent intercalating power, enhanced thermal stability and physicochemical properties relatively close to those found in a reference solution (prepared in dilute conditions). It was also unequivocally determined that a previous particle-size refining of natural bentonites displayed a stressed favourable impact, especially on the final textural properties of the Al/Fe-PILC, enhanced expansion of the aluminosilicate's basal spacing (d001) and stronger anchoring of the pillars to the host layered structure improving its thermal stability.On the other hand, by comparison of three intercalation methods it was realized that in absence of any dispersion medium, that is, the straight addition of the powdered clay over the intercalating solution is a simpler procedure with great potential further intensifying the preparation of Al/Fe- and other Al-based-PILCs. Such a strategy led to solids with great physicochemical properties and catalytic performance in the CWPO degradation of phenol in a period of just 60 min (78.1% phenol conversion and 19.3% of TOC mineralization). Since most of the studies about the CWPO degradation of phenol in the presence of different types of solid catalysts have been performed at either times of reaction mostly exceeding 3.0 h, different catalyst's concentration, pH or peroxide dosing,26,38–40 a straight comparison with the results here reported might be confusing; however, other studies have already underlined the excellent performance of the Al/Fe-PILCs catalysing the degradation of phenol and other model molecules at very mild conditions of ambient temperature and pressure.2,37
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from CT&I Fund of the SGR – Colombia to CWPO for better drinking water quality project (BPIN 2014000100020), is kindly acknowledged. The Spanish authors are grateful for financial support from the Spanish Ministry of Economy, Industry and Competitiveness (AEI/MINECO), and the European Regional Development Fund (ERDF) through project MAT2016-78863-C2-R. AG also thanks Santander Bank for funding via the Research Intensification Program.Notes and references
- F. Bertella and S. B. C. Pergher, Materials, 2017, 10, 712 CrossRef.
- A. Olaya, G. Blanco, S. Bernal, S. Moreno and R. Molina, Appl. Catal., B, 2009, 93, 56–65 CrossRef CAS.
- H. J. Munoz, C. Blanco, A. Gil, M. A. Vicente and L. A. Galeano, Materials, 2017, 10, 1364 CrossRef.
- L. A. Galeano, P. F. Bravo, C. D. Luna, M. Á. Vicente and A. Gil, Appl. Catal., B, 2012, 111–112, 527–535 CrossRef CAS.
- A. Olaya, S. Moreno and R. Molina, Appl. Catal., A, 2009, 370, 7–15 CrossRef CAS.
- N. R. Sanabria, M. A. Centeno, R. Molina and S. Moreno, Appl. Catal., A, 2009, 356, 243–249 CrossRef CAS.
- J. Barrault, M. Abdellaoui, C. Bouchoule, A. Majesté, J. M. Tatibouët, A. Louloudi, N. Papayannakos and N. H. Gangas, Appl. Catal., B, 2000, 27, L225–L230 CrossRef CAS.
- A. Olaya, S. Moreno and R. Molina, Catal. Commun., 2009, 10, 697–701 CrossRef CAS.
- S. Moreno, E. Gutierrez, A. Alvarez, N. G. Papayannakos and G. Poncelet, Appl. Catal., A, 1997, 165, 103–114 CrossRef CAS.
- M. A. Vicente, A. Gil and F. Bergaya, in Developments in Clay Science, ed. F. Bergaya and G. Lagaly, Elsevier, Amsterdam, The Netherlands, 2nd edn, 2013, vol. 5, pp. 523–557 Search PubMed.
- C. Catrinescu, D. Arsene and C. Teodosiu, Appl. Catal., B, 2011, 101, 451–460 CrossRef CAS.
- M. V. Sivaiah, S. Petit, J. Brendlé and P. Patrier, Appl. Clay Sci., 2010, 48, 138–145 CrossRef CAS.
- Y. Guo, G. Li, C. Zhao, Q. Zhao, J. Wang and Z. Luan, Sep. Purif. Technol., 2009, 69, 221–223 CrossRef CAS.
- Z. Chen, Z. Luan, J. Fan, Z. Zhang, X. Peng and B. Fan, Colloids Surf., A, 2007, 292, 110–118 CrossRef CAS.
- J. W. Akitt and A. Farthing, J. Chem. Soc., Dalton Trans., 1981, 7, 1624–1628 RSC.
- J. W. Akitt and A. Farthing, J. Chem. Soc., Dalton Trans., 1981, 7, 1606–1608 RSC.
- B. Gonzalez, A. H. Perez, R. Trujillano, A. Gil and M. A. Vicente, Materials, 2017, 10, 886 CrossRef.
- Y. Fernández, J. A. Menéndez, A. Arenillas, E. Fuente, J. H. Peng, Z. B. Zhang, W. Li and Z. Y. Zhang, Solid State Ionics, 2009, 180, 1372–1378 CrossRef.
- N. R. Sanabria, R. Molina and S. Moreno, Catal. Lett., 2009, 130, 664–671 CrossRef CAS.
- F. Kooli, Microporous Mesoporous Mater., 2013, 167, 228–236 CrossRef CAS.
- D. E. W. Vaughan, Catal. Today, 1988, 2, 187–198 CrossRef CAS.
- A. M. García, R. A. Torres-Palma, L. A. Galeano, M. Á. Vicente and A. Gil, in Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment, ed. A. Gil, L. A. Galeano and M. A. Vicente, Hdb Env Chem, Springer International Publishing AG, 2017, vol. 67, pp. 99–132 Search PubMed.
- J. H. Ramírez and L. A. Galeano, in Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment, ed. A. Gil, L. A. Galeano and M. A. Vicente, Hdb Env Chem, Springer International Publishing AG, 2017, vol. 67, pp. 69–98 Search PubMed.
- L. A. Galeano, M. Á. Vicente and A. Gil, Catal. Rev., 2014, 56, 239–287 CrossRef CAS.
- Z. Liu, H. Ma, J. Liu, L. Xing, L. Cheng, J. Yang, B. Mao and Q. Zhang, Asia-Pac. J. Chem. Eng., 2018, 13, e2156 CrossRef.
- M. Munoz, P. Domínguez, Z. M. de Pedro, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2017, 203, 166–173 CrossRef CAS.
- P. Banković, A. Milutinović-Nikolić, Z. Mojović, N. Jović-Jovičić, M. Perović, V. Spasojević and D. Jovanović, Microporous Mesoporous Mater., 2013, 165, 247–256 CrossRef.
- H. J. Muñoz, C. Vallejo, C. Blanco, A. Gil, M. Á. Vicente, J. H. Ramírez and L. A. Galeano, Green Chem., 2018, 20, 5196–5208 RSC.
- J. Rouquerol, P. Llewellyn and K. Sing, in Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, ed. J. Rouquerol, P. Llewellyn and K. Sing, Elsevier, Amsterdam, The Netherlands, 2nd edn, 2013, pp. 467–527 Search PubMed.
- L. A. Galeano, A. Gil and M. A. Vicente, Appl. Catal., B, 2010, 100, 271–281 CrossRef CAS.
- M. N. Timofeeva, S. T. Khankhasaeva, Y. A. Chesalov, S. V. Tsybulya, V. N. Panchenko and E. T. Dashinamzhilova, Appl. Catal., B, 2009, 88, 127–134 CrossRef CAS.
- C. L. Chin, Z. A. Ahmad and S. S. Sow, Appl. Clay Sci., 2017, 143, 327–335 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CAS.
- J. Carriazo, Appl. Clay Sci., 2012, 67–68, 99–105 CrossRef CAS.
- W. Najjar, S. Perathoner, G. Centi and A. Ghorbel, Stud. Surf. Sci. Catal., 2007, 170, 1425–1431 CrossRef.
- J. Carriazo, R. Molina and S. Moreno, Rev. Colomb. Quim., 2007, 36, 213–225 CAS.
- J. H. Ramírez, L. A. Galeano, G. Pinchao, R. A. Bedoya and A. Hidalgo, J. Environ. Chem. Eng., 2018, 6, 2429–2441 CrossRef.
- A. Devard, P. Brussino, F. Albana-Marchesini and M. A. Ulla, J. Environ. Chem. Eng., 2019, 7, 103201 CrossRef CAS.
- Y. Li, X. Zhang, D. Zhang, Y. Li, X. Wang and S. Wang, RSC Adv., 2017, 7, 43681–43688 RSC.
- Y. Yang, Y. Yan, H. Zhang and X. Wu, Sep. Purif. Technol., 2020, 237, 116452 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08948f |
| This journal is © The Royal Society of Chemistry 2020 |