Open Access Article
Open Access ArticleA simplified approach for the metal-free polymerization of propylene oxide†
Charlotte Vogler and
Stefan Naumann *
*
University of Stuttgart, Institute of Polymer Chemistry, 70569 Stuttgart, Germany. E-mail: stefan.naumann@ipoc.uni-stuttgart.de
First published on 9th December 2020
Abstract
Triethyl borane (Et3B), in combination with phosphazene-type superbases, has recently emerged as a powerful co-catalyst for the anionic polymerization of epoxides. Here, it is demonstrated that the monomer-activating property of Et3B can also compensate for the application of much gentler organobases. This not only results in simpler setups, but also significantly reduces nucleophilicity/basicity-derived side reactions. Notably, this principle applies to such a degree that simple 4-dimethylaminopyridine (DMAP) or 1,4-diazabicyclo[2.2.2]octane (DABCO) can serve to polymerize propylene oxide (PO). With suitable initiators, this results for example in very well-defined block copolyethers (ÐM ≤ 1.03) without requiring work-up to remove side products such as PPO homopolymer. Performance correlates nicely with the corresponding organobase proton affinities (PAs), and a limiting PA of 220–230 kcal mol−1 was identified for successful PO polymerization.
Introduction
The preparation of aliphatic polyethers,1,2 key materials in fields as diverse as rheology control, lubrication, cosmetics, drug delivery or polyol components,3–7 is under constant pressure to comply with economic and ecological requirements. These challenges are best met by polymerization processes which are as practicable and as efficient as possible, while simultaneously avoiding problematic aspects such as the necessity to include work-up or purification steps (removal of metal residues, side products). An interesting contribution in this regard is provided by organocatalytic epoxide polymerization,8–11 since metal-free synthesis is potentially cheap and recommendable if the resulting polymer is designed for sensitive employment (i.e., health applications, electronics). Unfortunately, however, many of the best available and robust organocatalysts, nitrogen bases such as 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 1,4-diazabicyclo[2.2.2]octane (DABCO) or 4-dimethylaminopyridine (DMAP), are unable to polymerize epoxides.One strategy to overcome this inability is to design more reactive organocatalysts with increased basicity; examples include phosphazene superbases, N-heterocyclic carbenes (NHCs) and N-heterocyclic olefins (NHOs) which can polymerize (substituted) epoxides.11–13 While very successful, this strategy also comes with its own downsides directly linked to the higher reactivity, namely increased transfer-to-monomer (phosphazenes),12 non-quantitative conversion (deactivation of NHCs by impurities/side reactions)13 or the occurrence of competing zwitterionic species (NHOs).11
As an alternative, it is also possible to employ organobases together with a cooperatively acting Lewis acid (LA, dual catalysis).14 The LA activates the epoxide for ring-opening and interacts with the propagating chain end (reducing its basicity), thus potentially increasing polymerization rates and selectivity at the same time.15 Depending on mechanistic and kinetic specifics, such polymerizations can be understood as Lewis pair polymerizations.16,17 A recent example for epoxide activation was found with Mg(HMDS)2, which in combination with NHOs enabled the preparation of high-molar mass poly(propylene oxide (PPO)).15
A very notable advance was revealed independently by Zhao and Zhang in 2018, when it was discovered that triethyl borane (Et3B) significantly facilitates the consumption of various epoxides when employed in combination with phosphazene-type organobases.18,19 In elegant studies it was shown that high molar masses and excellently controlled mass distributions could be realized in short reaction times, also enabling interesting block copolymer architectures.20
In a reversal of the strategy discussed above, it was the motivation of this work to identify a simplified setup for epoxide polymerization. To this end, Et3B was chosen as LA and investigated in combination with organobases of systematically decreasing reactivity. This approach was designed to map out how far the reactivity/basicity of the co-catalyzing organobase could be lowered (using proton affinity, PA, as reference) before compensation via monomer-activation by Et3B would not suffice anymore to allow for polymerization. As will be shown in the following, the limits of this strategy are to be found between DABCO, which can still be successfully employed, and pyridine, where no polymerization is observed. The overall result is a user-friendly and essentially side-reaction free method, which is also less economically punishing than application of phosphazenes, the so-far preferred cocatalyst for Et3B (for tBu–P4 a recent calculation suggests a cost of as high as 95.000 € per mol).21
Results and discussion
Initial experiments were conducted with PO as representative epoxide monomer, because it is technically relevant and more challenging to polymerize than EO, mainly on account of the occurring side reactions.22 As initiator, α,ω-dihydroxylated poly(ethylene glycol) (PEG) was selected; the resulting polymer is an amphiphilic triblock copolyether and as such of interest (“Poloxamer/Pluronics”).1 Importantly, employment of macroinitiators also allows for using GPC analysis as a tool for identifying and, within limits, also quantifying undesired side products.The BAB-type polymers were prepared (Scheme 1) by combining PEG 8k (Mn = 8.000 g mol−1) or PEG 20k (20.000 g mol−1) with Et3B, monomer and organocatalyst under inert gas conditions (N2). No further solvent was added. The reactions were typically conducted using low catalyst loadings (0.067 mol.-%) and a ratio of [–OH termini]/[PO] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 at reaction temperatures between T = 50–80 °C. Since PO acts both as substrate and solvent, increasing conversion resulted in a steadily rising viscosity. Consequently, reactions were usually not conducted beyond 40–50% conversion, where monomer consumption levelled off to be impractically slow. After removing residual PO in vacuo (potentially allowing for recycling the monomer), the polymer was directly analysed by 1H NMR (Fig. S4†) and GPC (Fig. S5†). This characterization was repeated after a single precipitation from pentane for comparison and to identify side product.
300 at reaction temperatures between T = 50–80 °C. Since PO acts both as substrate and solvent, increasing conversion resulted in a steadily rising viscosity. Consequently, reactions were usually not conducted beyond 40–50% conversion, where monomer consumption levelled off to be impractically slow. After removing residual PO in vacuo (potentially allowing for recycling the monomer), the polymer was directly analysed by 1H NMR (Fig. S4†) and GPC (Fig. S5†). This characterization was repeated after a single precipitation from pentane for comparison and to identify side product.
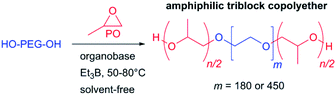
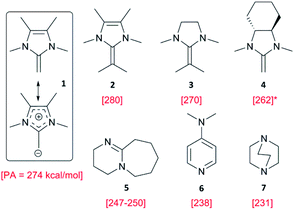
Starting with more reactive organobases, NHOs were screened (Fig. 1) in tandem with BEt3, which to the best of our knowledge constitutes the first such combination. These electron-rich, highly polar olefins can organopolymerize PO,11 but only the most reactive representatives of this class of compounds (2-alkylidene imidazolines such as 1 or 2) can do so without the help of monomer-activating Lewis acids.15 Compared to application alone, the dual catalytic approach delivers drastically accelerated monomer consumption, irrespective of the PEG macroinitiator (Fig. 2, S6–S8†). Molar mass distributions were found to be excellently controlled (ÐM ≤ 1.03, Fig. S5†). Thus, after only 2 h reaction time, 1-Et3B effects a conversion of 41% (Table 1, resulting in PPO122–PEO180–PPO122 according to 1H NMR), while 1 alone achieves lower conversion in more than the tenfold time (22 h, entries 1 and 3). Modifying reaction conditions to target even higher molar masses, a ratio of [–OH termini]/[PO] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 with 4 eq. of Et3B was employed, which delivers substantial PPO block lengths of n/2 = 129, 229 and 284 after 2 h, 3 h and 22 h, respectively (entries 6–8). Similar applies for 2, an NHO with increased basicity relative to 1 (entries 4, 9–10).23 It should be noted that regarding speed of conversion and degree of polymerization, these results are superior both relative to conventional anionic polymerization and to the application of even the most reactive organobases on their own.9,11,13
600 with 4 eq. of Et3B was employed, which delivers substantial PPO block lengths of n/2 = 129, 229 and 284 after 2 h, 3 h and 22 h, respectively (entries 6–8). Similar applies for 2, an NHO with increased basicity relative to 1 (entries 4, 9–10).23 It should be noted that regarding speed of conversion and degree of polymerization, these results are superior both relative to conventional anionic polymerization and to the application of even the most reactive organobases on their own.9,11,13
 | ||
| Fig. 1 Organobases, PA and mesomeric structures for NHO 1. * approximation.25 | ||
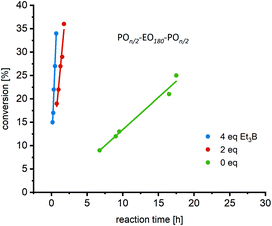 | ||
Fig. 2 Reaction time vs. conversion (1H NMR) for PO polymerization at 80 °C using NHO 1 and different equivalents of Et3B as cocatalyst: 1/PEO 8k [–OH]/PO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500. 1500. | ||
| # | Base | Initiator | Base/–OH/Et3B/PO molar ratio | T [°C] | t [h] | Conv.a [%] | POa n/2 | Mn (calc.)a [kg mol−1] | ĐMb |
|---|---|---|---|---|---|---|---|---|---|
| a Determined via 1H NMR analysis (CDCl3).b According to GPC (CHCl3).c n PO repeating units. | |||||||||
| 1 | 1 | PEO 8k | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 1500 |
50 | 22 | 21 | 63 | 15.2 | 1.04 |
| 2 | 2 | PEO 8k | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 1500 |
50 | 22 | 12 | 35 | 12.0 | 1.02 |
| 3 | 1 | PEO 8k | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 1500 |
50 | 2 | 41 | 122 | 22.1 | 1.03 |
| 4 | 2 | PEO 8k | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 1500 |
50 | 2 | 43 | 130 | 23.0 | 1.03 |
| 5 | 2 | BnOH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
50 | 18.5 | 49 | 49c | 3.0 | 1.03 |
| 6 | 1 | PEO 8k | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 3000 |
80 | 2 | 22 | 12 | 22.9 | 1.02 |
| 7 | 1 | PEO 8k | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 3000 |
80 | 3 | 38 | 229 | 34.5 | 1.03 |
| 8 | 1 | PEO 8k | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 3000 |
80 | 22 | 47 | 284 | 40.9 | 1.02 |
| 9 | 2 | PEO 8k | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 3000 |
80 | 2 | 29 | 174 | 28.1 | 1.04 |
| 10 | 2 | PEO 8k | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 3000 |
80 | 22 | 47 | 282 | 40.6 | 1.04 |
Perhaps even more importantly, however, the polymerizations also proceed in a more controlled manner. Although the polymers resulting from the action of NHO alone are well-defined (ÐM ≤ 1.04, main peak, entries 1–2), GPC analysis of the crude product reveals the presence of impurities (Fig. 3). By integration of the chromatogram it is found that these impurities typically make up 1–10%, depending on conditions (Table S1†). The identity of these impurities is well understood: for a nucleophilic NHO such as 1, the lower-molar mass side product is PPO homopolymer resulting from traces of zwitterionic polymerization,11,15 while strongly basic NHO 2 has been described to engender a high-molar mass impurity, potentially via chain condensation (see Fig. S9† for mechanisms).24 It should be noted that, while PPO homopolymer is easily separated from the triblock target structure, the same is not necessarily true for the latter type of impurities with its higher molar masses and similar polarity. Hence, it is especially noteworthy that in the presence of 2 eq. of Et3B these impurities are markedly reduced (Fig. 3, top, see also Fig. S10† for MALDI ToF MS analysis) or even virtually absent (Fig. 3, bottom). These results were obtained from crude samples, recommending this approach as a swift, work up-free access to amphiphilic triblock copolyethers.
 | ||
| Fig. 3 Direct comparison of non-purified POn/2–EO180–POn/2 derived from NHO alone (black) and NHO combined with 2.0 eq. of Et3B (orange). For reaction conditions, see Table 1, entries 1–4. | ||
In a next step of simplification, NHOs with decreased reactivity were employed (3–4). In the absence of LA, no conversion of PO is achieved with these organobases (Table S2†), as expected from previous work.11 In the presence of Et3B, in contrast, this changes profoundly (Table 2). Under standard conditions (80 °C, NHO/–OH/LA/PO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500), simple and mild 3 delivers 42% conversion in only 2 h reaction time; at this point, the polymerization solution is already too viscous to allow much further monomer consumption (48% conversion after 22 h, respectively, entries 1–2). The crude product is initially virtually side-product free; this only changes after prolonged reaction when conversion becomes impractically slow (see Table S1† for list of all polymerization results including quantification of side product). Removal of the methyl substituents on the exocyclic carbon (4), a modification that is well established to result in a further decreasing basicity,23 indeed engenders a muted reactivity. Under identical conditions, conversion drops to 6% and 26%, after 2 h and 22 h respectively. Nonetheless, in this case no meaningful amount of side product was detected via GPC; even after longer polymerization time (72 h) this was found to be <5%. The polyether obtained by the action of 4 was found to display atactic PPO blocks (Fig. S11†).
1500), simple and mild 3 delivers 42% conversion in only 2 h reaction time; at this point, the polymerization solution is already too viscous to allow much further monomer consumption (48% conversion after 22 h, respectively, entries 1–2). The crude product is initially virtually side-product free; this only changes after prolonged reaction when conversion becomes impractically slow (see Table S1† for list of all polymerization results including quantification of side product). Removal of the methyl substituents on the exocyclic carbon (4), a modification that is well established to result in a further decreasing basicity,23 indeed engenders a muted reactivity. Under identical conditions, conversion drops to 6% and 26%, after 2 h and 22 h respectively. Nonetheless, in this case no meaningful amount of side product was detected via GPC; even after longer polymerization time (72 h) this was found to be <5%. The polyether obtained by the action of 4 was found to display atactic PPO blocks (Fig. S11†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 (molar ratio)
1500 (molar ratio)
| # | Base | t [h] | Conv.a [%] | POa n/2 | Mn (calc.)a [kg mol−1] | ĐMb |
|---|---|---|---|---|---|---|
| a Determined via 1H NMR analysis (CDCl3).b Determined from GPC (CHCl3). | ||||||
| 1 | 3 | 2 | 42 | 127 | 22.7 | 1.03 |
| 2 | 3 | 22 | 48 | 145 | 24.7 | 1.03 |
| 3 | 4 | 2 | 6 | 18 | 10.0 | 1.02 |
| 4 | 4 | 22 | 26 | 79 | 17.1 | 1.03 |
| 5 | 5 | 2 | 30 | 90 | 18.4 | 1.02 |
| 6 | 5 | 22 | 45 | 135 | 23.6 | 1.02 |
| 7 | 6 | 22 | 22 | 65 | 15.5 | 1.02 |
| 8 | 7 | 22 | 5 | 16 | 9.8 | 1.03 |
Even the relatively gentle NHOs with saturated backbone (3–4) are known to be stronger organobases than typical nitrogen bases,23 so suitability of the latter for PO conversion is an attractive issue. As stated above, DBU, DABCO or DMAP cannot be used to prepare PPO if they are employed on their own, yet their successful application in combination with Et3B would be a major step towards a simple, fully commercially available polymerization setup for PO, with the additional benefit of low propensity for side reactions.
Polymerization experiments were first conducted with DBU (PA = 247–250 kcal mol−1, Fig. 1),26,27 a cyclic amidine which is routinely employed for organopolymerizations of other monomers.28–30 Despite the comparatively low basicity, conversion of PO was readily achieved with 30% and 45% conversion after 2 h and 22 h, respectively (Table 2, entries 5–6). Again, the complete absence of impurities was observed after the shorter reaction time, while analysis of the crude polymer after 22 h revealed the built-up of a low-molar mass impurity (Table S1†). Precipitation and consecutive GPC and NMR analysis found analytically pure polymer and identified the side product as PPO homopolymer.
Next, DMAP was investigated (PA = 238 kcal mol−1).31 This compound is known to be only able to convert monomers with high polymerizability (i.e., lactide),32 but is otherwise considered a relatively inferior organopolymerization catalyst. Surprisingly, in the presence of Et3B, PO is consumed to result in the corresponding amphiphilic polyether PPO65–PEO180–PPO65, equivalent to 22% conversion after 22 h, again underlining the striking impact of the cocatalyst (Table 2, entry 7). Importantly, repeat experiments substantiated the complete absence or only marginal appearance of side product even in the crude product.
Intriguingly, even the application of DABCO, which represents a further step down in basicity (PA = 231 kcal mol−1),33 results in successful polymerization. While conversion is expectedly slow (Table 2, entry 8), the reaction gently proceeds to finally arrive at >10% conversion after 48 h (Fig. 4, corresponding to PPO49–PEO180–PPO49) and it is obvious that longer polymerization times would concomitantly increase the achievable molar masses. Hence, especially for oligomeric or moderately high DPs of PPO the setup DABCO/Et3B might be of interest, the more so since this combination of (cheap) commercial cocatalysts delivers essentially side product-free polyethers as found by GPC analysis of the crude polymer.
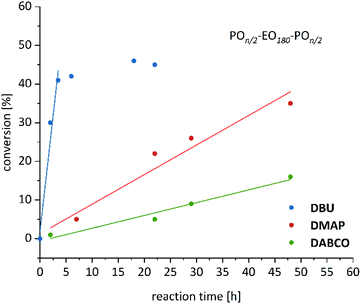 | ||
Fig. 4 Reaction time vs. conversion (1H NMR) for PO polymerization at 80 °C using organobase (5–7)/Et3B catalyst systems. Base/Et3B/PEO 8k [–OH]/PO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 (molar ratio). 1500 (molar ratio). | ||
Coherently, when pyridine was employed for a control reaction (PA = 219 kcal mol−1),34 no conversion at all was observed even after 48 h. Thus, it can be concluded that for PO conversion (no solvent, 80 °C) the minimum requirement of PA is located at 220–230 kcal mol−1. Since this range is well accessible for many nitrogen bases, as also shown above, and PA data are readily available in literature, this finding is expected to help evaluating (and possibly even predicting) the suitability of potential polymerization catalysts. In view of the obvious correlation of PA and performance, this also seems a straightforward way to adapt the desired rate of polymerization by choice of catalyst according to PA. To the best of our knowledge, only a single example for a nitrogen base/borane catalyst setup targeting polymerization of epoxides has been published to date.35
In a final set of experiments, it was probed whether this user-friendly setup was also applicable to epoxides other than PO. Screening allyl glycidyl ether (AGE), tert-butyl glycidyl ether (tBuGE) and 1-butylene oxide (BO), it is found that these higher epoxide homologues entail a notable drop in speed of conversion while at the same time require a higher minimum PA of the applied organobase (Table S3†). Thus, in contrast to what is observed for PO, DMAP was found not to be suitable under the applied conditions, while NHO 3 and also DBU could still engender conversion of all three monomers (7–15%). Amphiphilic polymers such as BO38–EO180–BO38 or tBuGE24–EO180–tBuGE24 were readily obtained, whereby notably polydispersity was again nicely controlled (ÐM = 1.03) and GPC analysis of the crude product did not display any observable impurities. For DBU, bulky tBuGE was slightly more suitable than the other epoxide congeners.
Conclusions
It was demonstrated that the beneficial impact of Et3B as co-catalyst for PO polymerization extends well beyond phosphazene-type superbases. Indeed, organocatalysts which are unable to polymerize this epoxide on their own are enabled by the presence of this Lewis acid to do so swiftly and in a well-controlled manner. By systematically decreasing the basicity (using proton affinity (PA) as a readily available guideline) of the organobase component in base/Et3B catalyst setups, a lower limit in the range of PA = 220–230 kcal mol−1 has been identified for monomer consumption to still occur. Importantly, this is a requirement which is readily met and surpassed by common nitrogen bases. Thus, operationally simple, fully commercially available catalyst combinations can be employed to generate PPO-based polymers in a user-friendly setup. Importantly, for the more reactive organobases, such as NHOs, the presence of Et3B not only accelerates PO enchainment but also reduces or even eliminates the occurrence of side reactions. More complex epoxides are not as easily converted and further research is necessary to improve monomer activation. The application of solvent might provide a future venue to increase viscosity-limited conversion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Dr Dongren Wang and B.Sc. Ralf Locke (University of Stuttgart) are gratefully acknowledged for support with MALDI ToF MS analysis and polymerizations. Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 358283783 – SFB 1333.References
- J. Herzberger, K. Niederer, H. Pohlit, J. Seiwert, M. Worm, F. R. Wurm and H. Frey, Chem. Rev., 2016, 116, 2170–2243 CrossRef CAS.
- A.-L. Brocas, C. Mantzaridis, D. Tunc and S. Carlotti, Prog. Polym. Sci., 2013, 38, 845–873 CrossRef CAS.
- M. Ionescu, Chemistry and Technology of Polyols for Polyurethanes, iSmithers Rapra Publishing, 2005 Search PubMed.
- P. Alexandridis, J. F. Holzwarth and T. A. Hatton, Macromolecules, 1994, 27, 2414–2425 CrossRef CAS.
- D. A. Chiapetta and A. Sosnik, Eur. J. Pharm. Biopharm., 2007, 66, 303–317 CrossRef.
- S. Gupta, R. Tyagi, V. S. Parmar, S. K. Sharma and R. Haag, Polymer, 2012, 53, 3053–3078 CrossRef CAS.
- R. Klein and F. R. Wurm, Macromol. Rapid Commun., 2015, 36, 1147–1165 CrossRef CAS.
- M. Fèvre, J. Pinaud, Y. Gnanou, J. Vignolle and D. Taton, Chem. Soc. Rev., 2013, 42, 2142 RSC.
- D. Taton, in Organic Catalysis for Polymerisation, ed. A. Dove, H. Sardon and S. Naumann, Royal Society of Chemistry, Cambridge, 2018, pp. 328–366 Search PubMed.
- S. Naumann and A. P. Dove, Polym. Chem., 2015, 6, 3185–3200 RSC.
- S. Naumann, A. W. Thomas and A. P. Dove, Angew. Chem., Int. Ed., 2015, 54, 9550–9554 CrossRef CAS.
- O. Rexin and R. Mülhaupt, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 864–873 CrossRef CAS.
- J. Raynaud, W. N. Ottou, Y. Gnanou and D. Taton, Chem. Commun., 2010, 46, 3203–3205 RSC.
- E. Piedra-Arroni, A. Amgoune and D. Bourissou, Dalton Trans., 2013, 42, 9024–9029 RSC.
- P. Walther, A. Krauß and S. Naumann, Angew. Chem., Int. Ed., 2019, 58, 10737–10741 CrossRef CAS.
- M. Hong, J. Chen and E. Y.-X. Chen, Chem. Rev., 2018, 118, 10551–10616 CrossRef CAS.
- M. L. McGraw and E. Y.-X. Chen, Macromolecules, 2020, 53, 6102–6122 CrossRef CAS.
- Y. Chen, J. Shen, S. Liu, J. Zhao, Y. Wang and G. Zhang, Macromolecules, 2018, 51, 8286–8297 CrossRef CAS.
- C.-J. Zhang, H.-Y. Duan, L.-F. Hu, C.-H. Zhang and X.-H. Zhang, ChemSusChem, 2018, 11, 4209–4213 CrossRef CAS.
- S. Liu, T. Bai, K. Ni, Y. Chen, J. Zhao, J. Ling, X. Ye and G. Zhang, Angew. Chem., Int. Ed., 2019, 58, 15478–15487 CrossRef CAS.
- A. Benlahouès, B. Brissault, S. Boileau and J. Penelle, Macromol. Chem. Phys., 2018, 219, 1700463 CrossRef.
- G. Odian, Principles of Polymerization, Wiley-Interscience, S.l., 4th edn, 2004, pp. 548–553 Search PubMed.
- R. Schuldt, J. Kästner and S. Naumann, J. Org. Chem., 2019, 84, 2209–2218 CrossRef CAS.
- A. Balint, M. Papendick, M. Clauss, C. Müller, F. Giesselmann and S. Naumann, Chem. Commun., 2018, 54, 2220–2223 RSC.
- For organobase 4, this value is an approximation of a minimum PA based on ref. 23 on the assumption that the cycloaliphatic backbone substitution does not influence basicity significantly.
- M. Decouzon, J.-F. Gal, P.-C. Maria and E. D. Raczyńska, Rapid Commun. Mass Spectrom., 1993, 7, 599–602 CrossRef CAS.
- Z. Glasovac, V. Štrukil, M. Eckert-Maksić, D. Schröder, M. Kaczorowska and H. Schwarz, Int. J. Mass Spectrom., 2008, 270, 39–46 CrossRef CAS.
- S. Naumann, in Organic Catalysis for Polymerisation, ed. A. Dove, H. Sardon and S. Naumann, Royal Society of Chemistry, Cambridge, 2018, pp. 132–147 Search PubMed.
- F. Nederberg, B. G. G. Lohmeijer, F. Leibfarth, R. C. Pratt, J. Choi, A. P. Dove, R. M. Waymouth and J. L. Hedrick, Biomacromolecules, 2007, 8, 153–160 CrossRef CAS.
- H. A. Brown, A. G. de Crisci, J. L. Hedrick and R. M. Waymouth, ACS Macro Lett., 2012, 1, 1113–1115 CrossRef CAS.
- E. D. Raczyńska, J.-F. Gal and P.-C. Maria, Chem. Rev., 2016, 116, 13454–13511 CrossRef.
- F. Nederberg, E. F. Connor, M. Möller, T. Glauser and J. L. Hedrick, Angew. Chem., Int. Ed., 2001, 40, 2712–2715 CrossRef CAS.
- R. W. Alder, R. J. Arrowsmith, A. Casson, R. B. Sessions, E. Heilbronner, B. Kovač, H. Huber and M. Taagepera, J. Am. Chem. Soc., 1981, 103, 6137–6142 CrossRef CAS.
- R. V. Hodges, J. L. Beauchamp, A. J. Ashe and W.-T. Chan, Organometallics, 1985, 4, 457–461 CrossRef CAS.
- S. Zhu, Y. Zhao, M. Ni, J. Xu, X. Zhou, Y. Liao, Y. Wang and X. Xie, ACS Macro Lett., 2020, 9, 204–209 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08970b |
| This journal is © The Royal Society of Chemistry 2020 |