Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Prescribed drugs containing nitrogen heterocycles: an overview
Majid M. Heravi * and
Vahideh Zadsirjan
* and
Vahideh Zadsirjan
Department of Chemistry, School of Science, Alzahra University, PO Box 1993891176, Vanak, Tehran, Iran. E-mail: mmh1331@yahoo.com; mmheravi@alzahra.ac.ir; Fax: +98 21 88041344; Tel: +98 21 88044051
First published on 15th December 2020
Abstract
Heteroatoms as well as heterocyclic scaffolds are frequently present as the common cores in a plethora of active pharmaceuticals natural products. Statistically, more than 85% of all biologically active compounds are heterocycles or comprise a heterocycle and most frequently, nitrogen heterocycles as a backbone in their complex structures. These facts disclose and emphasize the vital role of heterocycles in modern drug design and drug discovery. In this review, we try to present a comprehensive overview of top prescribed drugs containing nitrogen heterocycles, describing their pharmacological properties, medical applications and their selected synthetic pathways. It is worth mentioning that the reported examples are actually limited to current top selling drugs, being or containing N-heterocycles and their synthetic information has been extracted from both scientific journals and the wider patent literature.
1. Introduction
Medicinal and pharmaceutical chemistry are disciplines at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various other biological specialties, leading to the design, chemical synthesis and development of bio-active molecules, for being approved as prescribed and market purchasable pharmaceutical agents. Heterocyclic compounds, as the most important organic compounds, are frequently present in molecules of interest in medicinal chemistry.1 Among them, nitrogen containing heterocycles are of great importance to life science, since they are abundant in nature, existing as subunits in several natural products, for example vitamins, hormones and antibiotics. Some representative alkaloids and other nitrogen containing natural products, showing diverse biological activities, and several of them are even prescribed drugs such as serotonin,2 thiamine, which is also called vitamin B1,3 atropine,4 notorious morphine,5 codeine, (greater benefit may be gained when it is combined with acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen),6 papaverine,7 coniine,8 caffeine9 and nicotine.10Furthermore, N-based heterocycles are indispensable diet components such as thiamin (vitamin B1), riboflavin (vitamin B2), pyridoxol (vitamin B6), nicotinamide (vitamin B3).11,12 Nitrogen-containing heterocyclic compounds are not only present as the backbone in several biologically active natural products used as traditional medications or approved prescribed drugs, but some of their synthetic derivatives in different sizes, nowadays are prescribed and market purchasable drugs. The most famous are, diazepam, isoniazid, chlorpromazine, metronidazole, barbituric acid, captopril, chloroquinine, azidothymidine and anti-pyrine. Furthermore, most of the vitamins, nucleic acid, enzymes, co-enzymes, hormones, and alkaloids contain N-based heterocycles as scaffolds.13
Due to exhibiting diverse biological activities, nitrogen heterocyclic compounds have always been attractive targets to synthetic organic chemists. Since, several of them are prevalent in natural products, especially alkaloids, they have received much attention of synthetic community, especially those who are engaged with the total synthesis of natural products.14 As a result, the vast number of nitrogen heterocyclic compounds have been under continuous investigations from different points of view thus, found applications in pharmaceutical research and drug discovery.15,16 Recently, N-based heterocycles have attracted much interest of medicinal chemists and biologists due to broad range of biological activities and plentiful applications in the extensive fields of pharmacy.17
FDA databases has revealed that about 60% of unique small-molecule drugs, comprise N-based heterocycles, showing the structural significance of N-based heterocycles in drug design and drug discovery.18 The prevalence of N-heterocyles in biologically active compounds can be attributed to their stability and operational efficiency in human body and the fact of that the nitrogen atoms are readily bonded with DNA through hydrogen bonding. As a matter of fact, anti-cancer activities of N-based heterocycle agents are largely due to their tendency of interaction with DNA via hydrogen bonding.19
In 2014 Njardarson et al. published the first comprehensive analysis of the nitrogen based heterocycles.16 This analysis showed that indeed about 60% of small-molecule drugs contain a N-based heterocycle as common architectural cores. In 2011, Baumann et al. presented an overview of the key pathways to the synthesis of the best-selling five-membered ring heterocyclic medications regardless of their kinds of heterocycles.20 In the following, in 2013 the same authors presented an overview on the synthetic pathways to the best selling drugs comprising six-membered heterocyclic systems.21 In 2018, Ramazani and co-workers15 presented the recent advances in nitrogen-based heterocyles as useful cancer chemotherapy agents. Cancer is one of the foremost roots of death, globally. It is the result of mutation of the cells which regulate the genes and protein. Although, surgery and radiotherapy are the current therapy several drugs are also used as anticancer agents in spite of their undesired side effects. Some analogues of new isosteviol-fused pyrazoline, ursolic acid linked triazole or D-ribose linked exhibit anticancer activity at the nanomolar range.22 Furthermore showing segment resemblance with histidine imidazole molecule N-based-heterocycles can be linked with protein molecules more easily than some other heterocyclic scaffolds, thus, these types of N-heterocyclics are the most promising drugs for being designed and screened as anti-cancer drugs.23 We are interested in heterocyclic chemistry,24 especially those containing nitrogen atom.25–33
We are interested in heterocyclic chemistry,24 especially those containing nitrogen atom.25–33 In recent years, our group has also focused on the applications of name reactions in the total synthesis of natural products containing nitrogen heterocycles, showing diverse biological activities.34–42 Armed with these experiences, in this review we try to highlight the medical usages and selected synthetic pathways of approved and market purchasable prescribed medications, containing nitrogen based-heterocycles. Having collected and categorized of about 640 medications, comprising a based-nitrogen heterocycle, we had to be selective and sumarritive, limiting ourselves to most common of such pharmaceuticals, classifying them in accordance of their size of N-based heterocycles, in, four, five, six, and seven-membered rings. Moreover, the fused, bridged bicyclic nitrogen heterocycles have been also covered.
2. Synthesis of nitrogen prescribed drugs having
2.1 Four-membered heterocycles
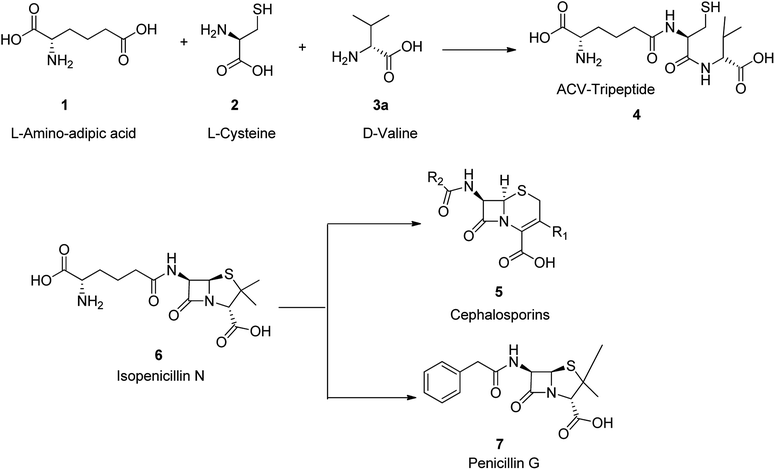
In general antimicrobial drugs are recognized as bacteriostatic (i.e., tetracyclines, sulfonamides) and as antibacterial (i.e., penicillin). Beta-lactam antibiotics are categorized to four groups. They are penicllins, cephalosporins, monobactams, and carbapenems. They all comprise a four-membered beta-lactam ring that is essential for displaying their antibactericidal activities. In 1929, penicillin was explored by Sir Alexander Fleming, who observed that one of his experimental cultures of staphylococcus was polluted with fungus that caused the bacteria to lyse.43,44 Since fungus belonged to the family penicillium, he called this bactericidal substance penicillin. A decade later, a research group at Oxford University could isolated a crude substance built of a few low-molecular substances that were named penicillins (F, G, K, O, V, X). Among the various penicillins (F, G, K, O, V, X), penicillin G (benzylpenicillin), was found the most effective. Since then, penicillin G, is used as an antibiotic to treat a number of bacterial infections.45There are three main and remarkable stages to the biosynthesis of penicillin G 7 (benzylpenicillin). Initially, three amino acids-L-α-aminoadipic acids, L-cysteine, L-valine are condensed to a tripeptide.46–48 Before the generation of this tripeptide, the amino acid L-valine is subjected to epimerization to turn out to be D-valine 3.49,50 This tripeptide is called δ-(L-α-aminoadipyl)-L-cysteine-D-valine (ACV) 4. The above epimerization and condensation reaction both are catalyzed by the enzyme δ-(L-α-aminoadipyl)-L-cysteine-D-valine synthetase (ACVS), a nonribosomal peptide synthetase or NRPS. In the second step of biosynthetic process of penicillin G 7, the catalyzed-isopenicillin N synthase (IPNS) oxidative transformation of linear ACV into the bicyclic intermediate isopenicillin N is taken place.46–49 Ultimately, by isopenicillin N 6, N-acyltransferase, is trans amidated in a way that the α-aminoadipyl side-chain of isopenicillin N 6 is eliminated and replaced by a phenylacetyl side-chain. This process is encoded by the gene penDE and considered as exceptional progression in providing penicillins G 7 (Scheme 1).46
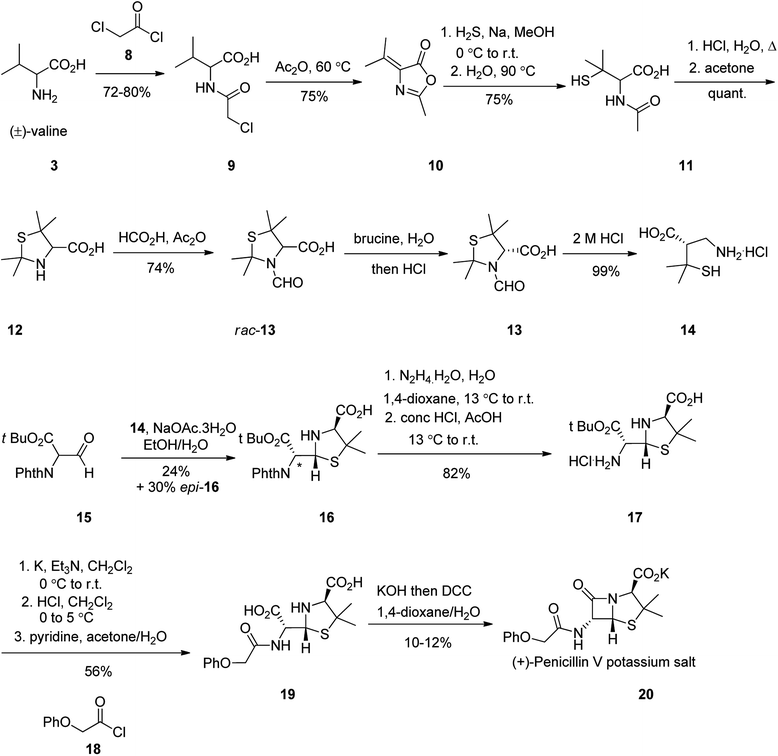
The total synthesis of penicillin V 20 was first achieved in 1948. It started with racemic valine 3, which was effectively converted into N-acetylpenicillamine 11. Formamide rac-13 upon resolution using brucine followed by hydrolysis, gave (−)-penicillamine hydrochloride 14. The latter was condensed with aldehyde 15 to give thiazolidine 16. The side-product epi-16 could be transformed into 16 using pyridine-induced epimerization. Elimination of protecting groups and assemblage of the phenoxyacetyl side chain provided penicilloic acid 19. Successive creation of the central amide bond was accomplished using DCC in basic conditions to afford penicillin V 20 as its potassium salt (Scheme 2).51
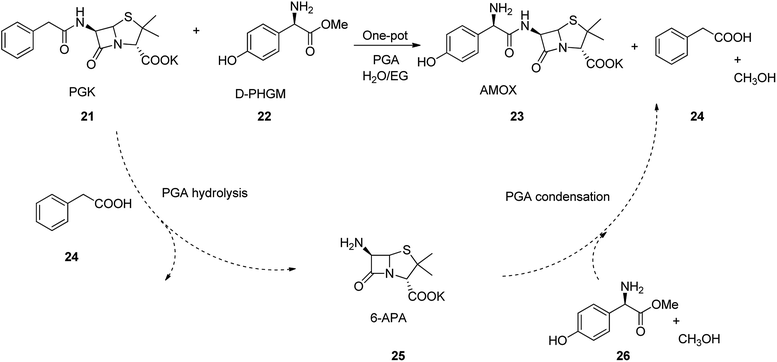
Amoxicillin is an antibiotic employed for the treatment of several bacterial infections, involving, strep throat, pneumonia skin infections middle ear infection, and urinary tract infections etc.45,52 Amoxicillin is one of the major β-lactam and best-selling antibiotics. It was discovered in 1958 and came into medical use in 1972 with much advantage over its precedents, for example it show higher spectrum of potency, high solubility, and high rate of absorption.53,54 Amoxicillin can also be prepared by enzymatic one-pot approach which has significant imminent application in its large scale production. The process began with 6-aminopenicillanic acid (6-APA) 25, which initially activated by a substrate, such as p-hydroxyphenylglycine methyl ester (HPGM) or p-hydroxyphenylglycine amide. It is well-recognized that PGA not only convert such substrates into an antibiotic, but also hydrolyzes penicillin G potassium salt (PGK) 21 into 6-APA 25. As a matter of fact, most of the β-lactam nuclei, e.g., 6-APA 25 and 7-ADCA employed in the enzymatic semi-synthetic process of β-lactam antibiotics are provided from the hydrolysis of PGK or cephalosporin C mediated by PGA. Thus, combination of the hydrolysis of PGK into 6-APA with the enzymatic catalysis is resulted in coupling reaction of 6-APA with p-hydroxyphenylglycine methyl ester (D-HPGM) to afford amoxicillin as the desired product. This one-pot approach avoids the number of steps in the production of β-lactam antibiotic, which not only skipping the isolation of 6-APA 25, but also effectively decrease the industrial cost production (Scheme 3).
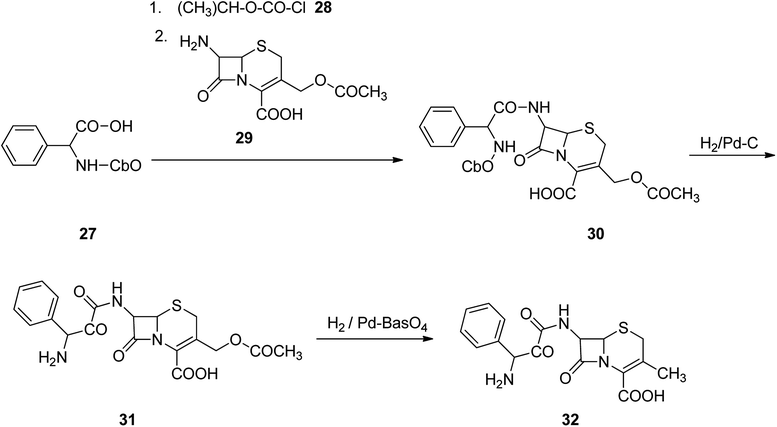
Cefalexin, is an antibiotic used for the treatment of several bacterial infections.55 Cefalexin used for treatment of definite bacterial infections, involving those grown of the middle ear, bone and joint, skin, and urinary tract, pneumonia, strep throat and to prevent bacterial endocarditis. Cefalexin was discovered in 1967.56–58 Initially, it was promoted in 1969 and 1970 under the brand names Keflex and Ceporex.59 Cefalexin under generic versions and under other trade names are, inexpensively market purchasable.60 Cephalexin is a first-generation cephalosporin antibiotic that was selected as the model medicine nominee to attain dose with better stability, palatability and attractive pediatric sophistication, economic and easy to take.61–68 Cephalexin, [6R-[6α,7β(R)]]-3-methyl-8-oxo-7-[(aminophenylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylic acid 32, indeed is an analog of ampicillin, due to the acyl segment present in the structure of 7-aminocephalosporanic acid, is just the same phenylglycine segment present in ampicillin.69 Cephalexin 32 is provided from cephalophenylglycine 31 that is in turn prepared by treating 7-aminocephalosporanic acid with a mixed anhydride which itself synthesized upon treatment of N-carbobenzoxyphenylglycine and isobutyl chloroformate in the presence of Et3N. Removal of the N-carbobenzoxy protective moiety from the obtained product 30 via hydrogentation in the presence of Pd on carbon catalyst provided a cephalophenyl-glycine 31 as an internal salt. Hydrogenation of the latter in the presence of Pd on barium sulfate leads to the deacetoxylation at the third position of 7-aminocephalosporanic acid, producing the desired prescribed antibiotic, cephalexin 32 (Scheme 4).70–72
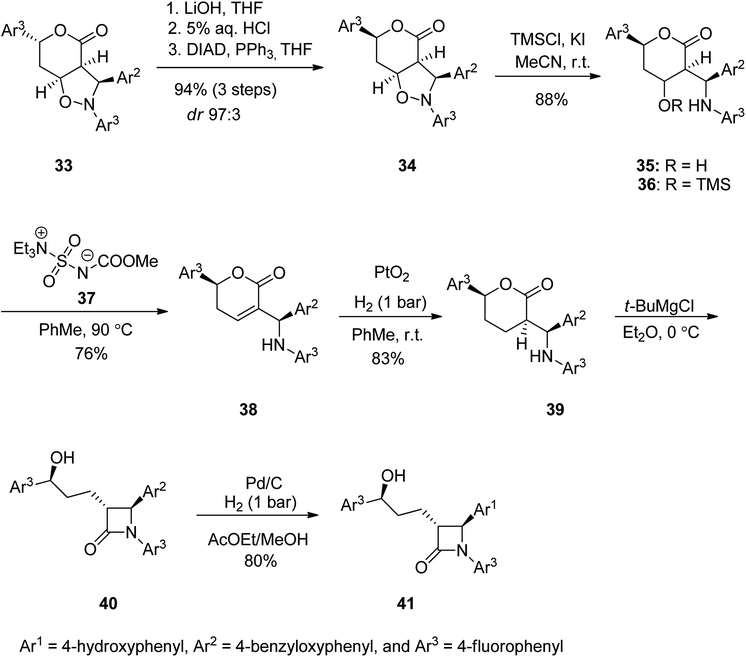
Ezetimibe is a medicine employed for the treatment of high blood cholesterol and some other lipid abnormalities. Ezetimibe was approved for medical use in the United States in 2002.73,74 Ezetimibe 41 is a strong β-lactamic cholesterol absorption inhibitor that decreases plasma (LDL-C).75–78 From the structural point of view, 41 has three para-substituted phenyl rings, a stereogenic benzylic hydroxyl, and two additional chiral centers at the 2-azetidinone skeleton.76–84 Ezetimibe 41 was synthesized starting with isoxazolidine 33 which was subjected to a ring opening of the lactone moiety upon treatment with LiOH with subsequent neutralization to a free carboxylic moiety followed by treatment of the resultant with Ph3P and DIAD at 0 °C to give compound 34 in high yield (80%) and high de purity (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 dr 9
9 dr 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In addition, the lessening of the reaction temperature to −10 °C resulted in further improvement in the chemical yield (94%) and de (24
1). In addition, the lessening of the reaction temperature to −10 °C resulted in further improvement in the chemical yield (94%) and de (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 dr 97
9 dr 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Next, the pure 34 (purified by chromatography) was subjected to N–O bond cleavage in 34 using TMSCl and potassium iodide in wet acetonitrile to give minolactone 35 in 88% yield that was enough pure for being used for the next step. Upon treatment of 35 with Burgess reagent 37 in toluene at 90 °C the desired unsaturated lactone 38 was obtained in satisfactory yield. The double bound of the latter was hydrogenated over PtO2, to proceed entirely anti to the aryl substituent of the lactone to produce compound 39 (83%, 98% ee) bearing three chiral centers with identical absolute configurations to those present in ezetimibe 41. The latter was reacted with t-BuMgBr in dry ether at 0 °C to give lactone 40 which using H2, Pd/C in AcOEt in MeOH to afford the desired target ezetimibe 41 in respectable yield (Scheme 5).85
3). Next, the pure 34 (purified by chromatography) was subjected to N–O bond cleavage in 34 using TMSCl and potassium iodide in wet acetonitrile to give minolactone 35 in 88% yield that was enough pure for being used for the next step. Upon treatment of 35 with Burgess reagent 37 in toluene at 90 °C the desired unsaturated lactone 38 was obtained in satisfactory yield. The double bound of the latter was hydrogenated over PtO2, to proceed entirely anti to the aryl substituent of the lactone to produce compound 39 (83%, 98% ee) bearing three chiral centers with identical absolute configurations to those present in ezetimibe 41. The latter was reacted with t-BuMgBr in dry ether at 0 °C to give lactone 40 which using H2, Pd/C in AcOEt in MeOH to afford the desired target ezetimibe 41 in respectable yield (Scheme 5).85
2.2. Five-membered heterocycles
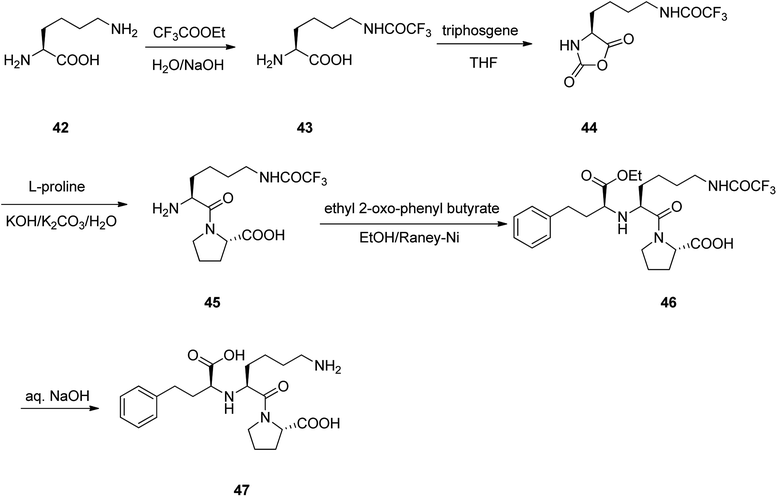
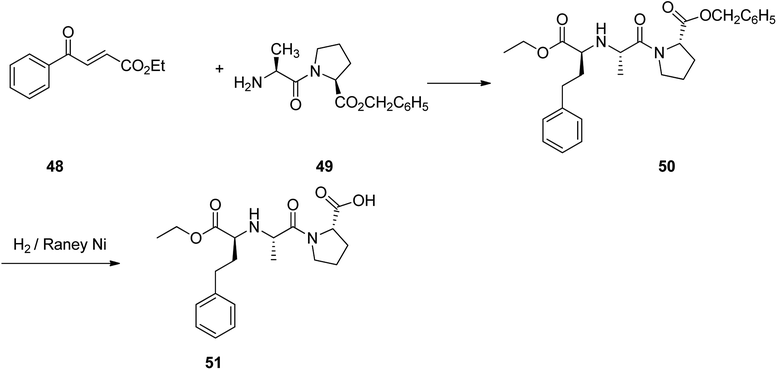
Lisinopril 47, is a drug of the angiotensin-converting enzyme (ACE) inhibitor family which is used, primarily in the treatment of hypertension, heart failure, and frequently utilized after heart attack.86 Lisinopril 47, chemically is named as N2-[(1S)-1-carboxy-3-phenylpropyl]-L-lysyl-L-proline, but sold under the brand name of PRINVIL® provided by Merck. Lisinopril 47 was patented in 1978, and approved for medical use in the United States in 1987.87–89 The synthetic pathway for lisinopril is depicted in Scheme 6. Its multistep synthesis, started with L-lysine 42 which upon treatment with ethyltrifluoro acetate afforded N6-trifluoroacetyl-L-lysine 43. The latter was reacted with triphosgene to provide N6-trifluoroacetyl-N2-carboxy-L-lysine anhydride 44 that was condensed with L-proline to give N6-trifluoroacetyl-L-lysyl-L-proline 45. The latter was condensed with ethyl 2-oxo-4-phenyl butyrate with subsequent hydrogenation using the RANEY® as catalyst to obtain N2-(1-(S)-ethoxycarbonyl-3-phenylpropyl)-L-N6-(trifluoroacetyl)-L-lysyl-L-proline 46. Lastly, upon the hydrolysis of the latter with sodium hydroxide, lisinopril 47 was obtained in pure form.86Enalapril 51, commercialized under the brand name Vasotec among others, is a drug, employed for the treatment of high blood pressure, kidney disease caused by diabetes and heart failure in which is frequently used with a diuretic, such as furosemide.90 Enalapril was patented in 1978, and approved as prescribed drug, coming to market in 1984. Enalapril, (S)-1-[N-[1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl]-L-proline 51, is prepared by treating the benzyl ester of L-alanyl-L-proline 49 with the ethyl ester of 3-benzoylacrylic acid 48 that affords the product 50, in which via hydrogenation in the presence of a RANEY® as catalyst eliminates the protective benzyl moiety, affording the desired prescribed drug enalapril 51.91 Some other alternative approaches to obtain enalapril have been also advocated (Scheme 7).92–96
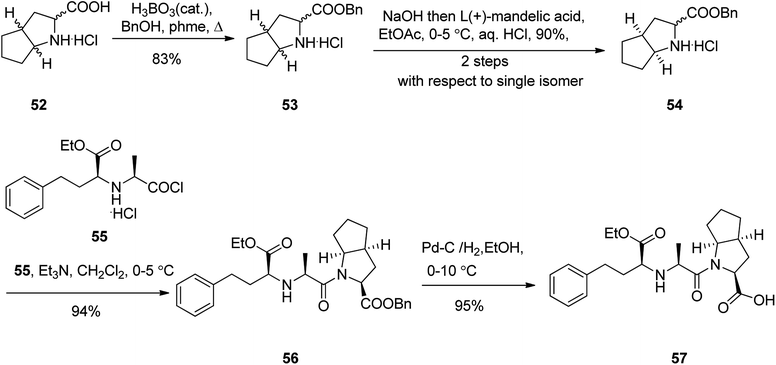
Ramipril 57, is accessible in pharmacies under the brand name, Altace® as capsules.97 Ramipril 57 with a chemical name of 2-aza-bicyclo-[3.3.0]-octane-3-carboxylic acid is placed in angiotensin converting enzyme (ACE) inhibitors type drug,98 that are utilized as hypertensive, treatment of congestive heart failure. Just a few references can be found in literature describing the synthesis of ramipril 57 in detail.99 A highly operative, cost-effective, and more importantly enantioselective synthesis of ramipril was achieved and reported using an environmentally benign process. It started with esterification of racemic 2-aza-bicyclo-[3.3.0]-octane-3-carboxylic acid hydrochloride 52 with benzyl alcohol in refluxing toluene in the presence of boric acid as a catalyst, followed by a fully-bodied resolution using cheap and recyclable L-(+)-mandelic acid as vital steps to give the ester, the (S,S,S)-2-aza-bicyclo-[3.3.0]-octane-3-carboxylic acid benzyl ester 54 in 83%. The latter was then coupled with benzyl N-(2S-carbethoxy-3-phenyl propyl)-S-alanine acid chloride 55 in the presence of Et3N in CH2Cl2 to provide ramipril benzyl ester 56 in 94% yield. Lastly, ramipril benzyl ester 56 was hydrogenated over Pd/C in EtOH to afford, the desired target, optically pure ramipril 57 in 95% chemical yields (Scheme 8).100
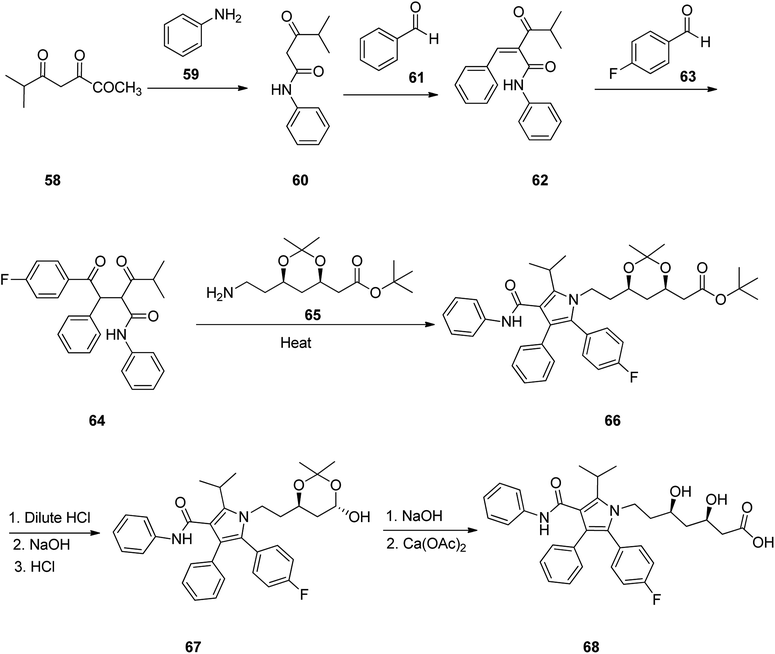
Atorvastatin 68 is placed among other suggested prescribed oral statin drugs, is sold under the brand name Lipitor. It is known to inhibit cardiovascular sickness by decreasing levels of low density lipoprotein (LDL) cholesterol level in blood. Atorvastatin was patented in 1986, and gained approval for being prescribed in US in 1996101 and currently is accessible as a generic drug.102 In 1989, Butler and co-workers, achieved and reported a successful synthetic approach for the atorvastatin 68 for the first time, comprising of six steps.103 This multistep approach started with 4-methyl-3-oxopentanoic acid methyl ester 58 which upon heating with aniline and ethylene diamine in toluene as solvent afforded 4-methyl-3-oxo-N-phenylpentanamide 60. The latter is reacted with benzaldehyde in hexane in the presence of catalytic amount of β-alanine and glacial acetic acid via Knoevenagel condensation provided 4-methyl-3-oxo-N-phenyl-2-(phenylmethylene)pentanamide 62. The latter was reacted with 4-fluorobenzaldehyde in the presence of catalytic amount of 3-ethyl-5-(2-hydroxyethyl)-4-methylthiazolium bromide and Et3N in ethanol at 80 °C to give diketone 64. The latter was then reacted with (4R-cis)-1,1-dimethyl-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane-4-acetate 65 in the presence of pivalic acid as catalyst in toluene-heptane as co-solvent system, to give poly-substituted Paal–Knorr pyrrole 66. Upon deprotection of 66 using dilute HCl and subsequent treatment of deprotected diol intermediate, with sodium hydroxide for the removal of tert-butyl ester group followed by acidification using HCl under mild heating lactone 67 was obtained. Lastly, treatment of the latter with sodium hydroxide, initially resulted in the cleavage of the lactone ring, followed by additional treatment of the corresponding sodium salt intermediate with 0.5 equivalent of calcium acetate to provide the desired target, atorvastatin calcium salt 68 (Scheme 9).104
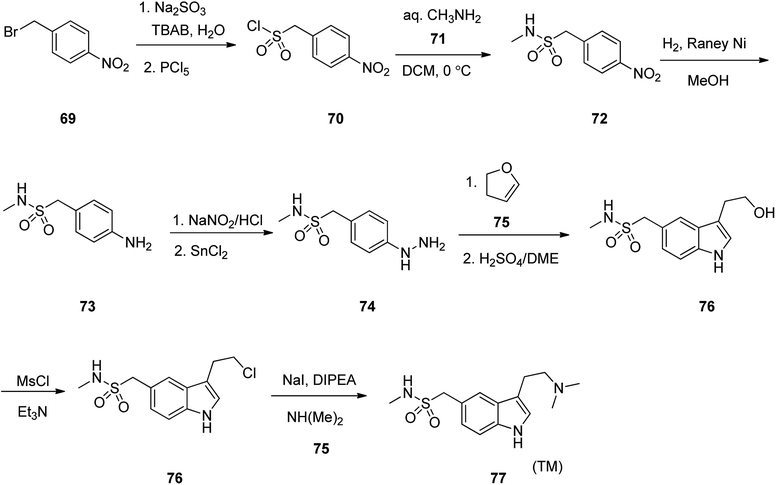
Sumatriptan, sold under the brand name Imitrex among others. Sumatriptan was patented in 1982 and approved for medical use in 1991.105 Sumatriptan (TM) 79 is a highly efficient and selective serotonin (5-HT1d) receptor agonist that is used on the treatment of migraine attacks.106 Literature survey revealed a plethora of information regarding the synthesis of sumatriptan, mostly patented107–112 In an attempt, starting with 1-(bromomethyl)-4-nitrobenzene 69, it was treated with Na2SO3 in TBAB with subsequent reaction of the resultant with PCl5 (4-nitrophenyl)methanesulphonyl chloride was obtained 70. The latter was then reacted with methyl amine 71 in dichloromethane to afford the corresponding sulfonamide 72. The latter upon catalytic hydrogenation over RANEY® transformed –NO2 group to –NH2 group gave compound 73. The latter was then treated with NaNO2/HCl, followed by reduction with SnCl2 gave the corresponding aryl hydrazine 74 as a key intermediate. The aryl hydrazine 74 was next, reacted with dihydrofuran 75 with subsequent treatment with anhy·H2SO4/DEM gave the corresponding indolyl alcohol 76. The latter was then treated with MsCl/Et3N to afford the corresponding chloride 77 which was subjected to amination with dimethyl amine 78 to give sumatriptan 79 (Scheme 10).113
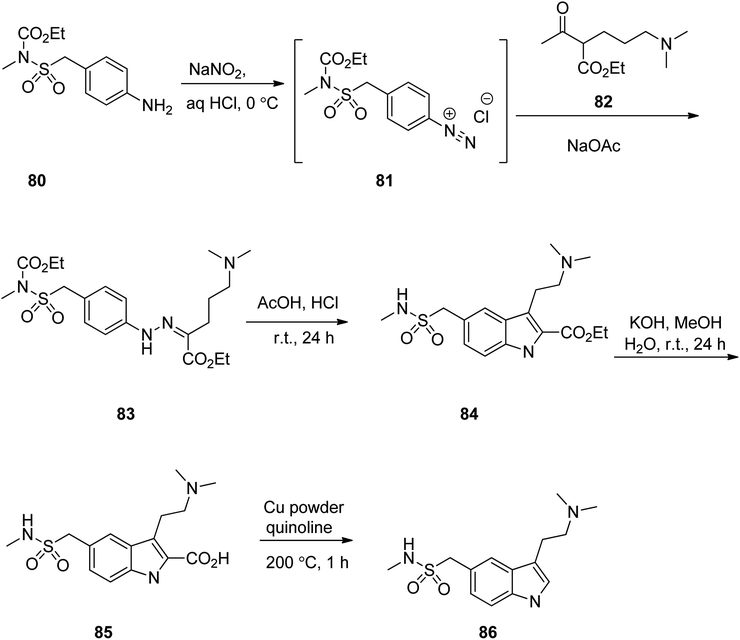
Sumatriptan (TM) could also be synthesized, starting with compound 80 which first was converted to 83 via reaction with NaNO2/HCl at 0 °C to give the corresponding diazonium salt 81, followed by direct reaction of the resultant with a β-ketoester under Japp–Klingemann reaction conditions in one-pot manner. Hydrazine 83 was subjected to intramolecular Fischer indole synthesis upon treatment with AcOH/HCl at room temperature to provide the expected corresponding indole derivative 84. The ester 84 upon treatment with KOH/MeOH at ambient temperature was hydrolyzed to the corresponding acid 85. The latter was then decarboxylated in the presence of Cu powder in quinolone at 200 °C to provide the desired sumatriptan in 80% yield (Scheme 11).114
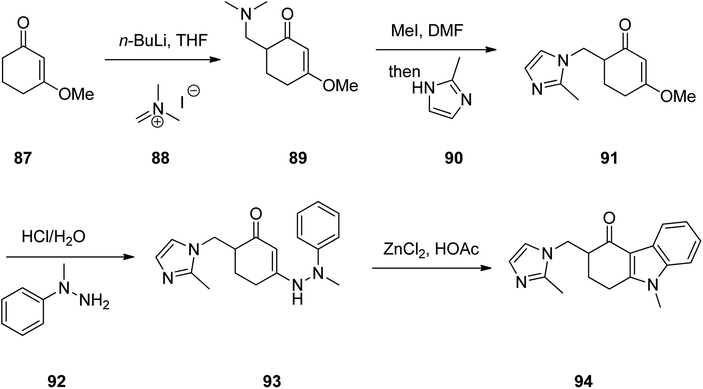
Ondansetron, 1,2,3,9-tetrahydro-9-methyl-3-(2-methyl-1H-imidazol-1-ylmethyl)-4H-carbazol-4-one hydrochloride dihydrate 94, commercialized under the brand name Zofran. Ondansetron was patented in 1984 and approved for medical use in 1990.115 It is a medication used to prevent nausea and vomiting caused by cancer chemotherapy, radiation therapy, or surgery.116,117 The pharmacologic and therapeutic applications of ondansetron have extensively been reviwed.118–124 It has been synthesized in five steps starting from 3-methoxycyclohex-2-en-1-one 87 which initially treated with CH2N+(CH3)2I− 88 in the presence of n-BuLi in THF to provide 6-((dimethylamino)methyl)-3-methoxycyclohex-2-enone 89 in 54% yield. Then, the latter was reacted with MeI in DMF and then reacted with 2-methyimidazole 90 to give 3-methoxy-6-((2-methyl-1H-imidazol-1-yl)methyl)cyclohex-2-enone 91 in one-pot fashion in 83% yield. The latter was first treated with HCl/H2O and then reacted with phenyhydrazine 92 to provide 6-((2-methyl-1H-imidazol-1-yl)methyl)-3-(2-methyl-2-phenylhydrazinyl)cyclohex-2-enone 93. The latter was finally subjected to Fischer indole synthesis at the presence of ZnCl2/HCl in which phenyl-methyl hydrazine and a cyclic 1,3-dione derivative are intramolecularly were cyclized to provide the fully substituted tricyclic core of the desired ondansetron 94 (Scheme 12).125
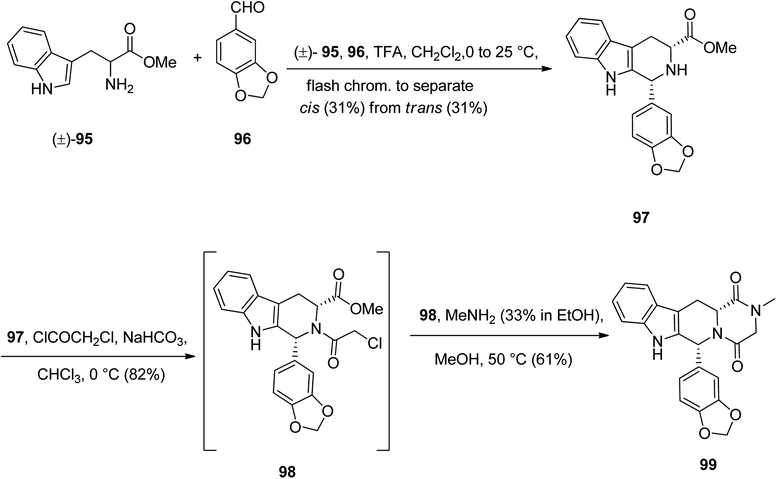
Tadalafil 99 under the brand name of Cialis acts is also used in erectile dysfunction (ED). The core structure in talalafil is a tetrahydro-β-carboline. Its synthesis is relatively straightforward and is relied on the work of Anand126 and co-workers via the straightforward multistep synthesis using four key framework, namely, D-tryptophan methyl ester 95, market accessible piperonal 96, chloroacetyl chloride, and methylamine.127
The pathway to provide tadalafil for clinical use is depicted in Scheme 13.128,129 Initially, commercially available tryptophan methyl ester 95 as racemic mixture was reacted with 96 under intermolecular Pictet–Spengler type reaction conditions at room temperature to give 97 as a mixture of the trans-isomer (31%) and the desired lower melting cis-isomer (31%), which was separated by flash chromatography. Noticeably, the later patents claim that the yield could be improved to 42% of the cis-isomer, with 28% of the trans-isomer if the Pictet–Spengler type reaction is performed at 4 °C instead of room temperature.130 The required cis-isomer 97 upon acetylation with chloroacetyl chloride afforded 98 which treated with methylamine, to give tadalafil 99 as clinically, pure form.131
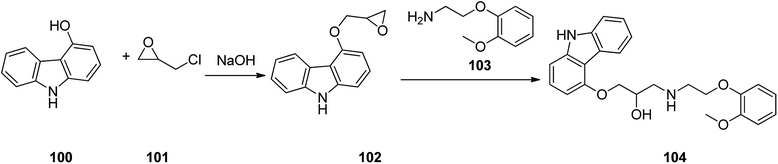
Carvedilol 104, sold in market under brand name of Coreg is actually a third-generation β1 and β2 blocker that also possesses α1-adrenergic-blocking potencies. Carvedilol 104 is actually produced via a two-step synthesis through reaction of 4-hydroxycarbazole 100 with epichlorohydrin in the presence of NaOH to provide 4-(2,3-epoxypropoxy)carbazole 101 which after isolation, treated with 2-(2-methoxyphe-noxy)ethanamine 103 to afford the desired target, carvedilol 104 (ref. 132) (Scheme 14). Alternative approaches for the formation of carvedilol have also been reported.133–138
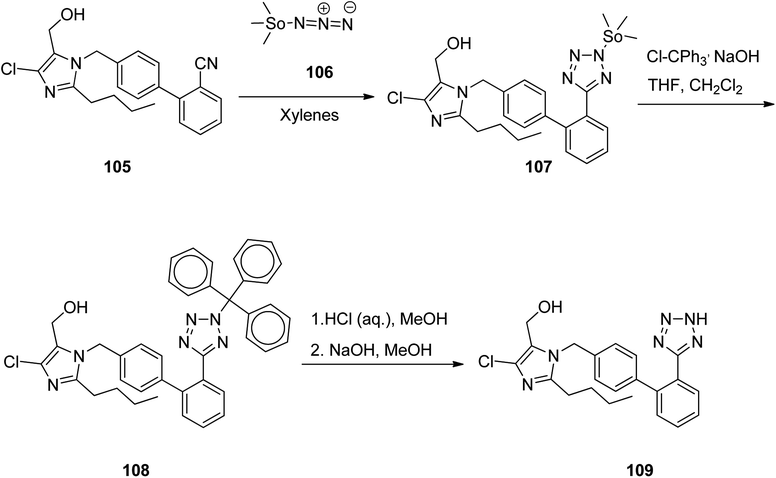
Losartan 109, marketed under the trade name Cozaar among other names, patented in 1986, and approved by FDA for being prescribed in 1995.139 It is a drug, chiefly prescribed for the treatment of high blood pressure. As illustrated in Scheme 15,140 the tetrazole ring of losartan 109 is constructed by treating of 1-[(2′-cyanobiphenyl-4-yl)methyl]-2-butyl-4-chloro-5-hydroxymethylimidazole 105 with trimethyltin azide 106. The aforementioned reaction affords a trimethylstannyl-substituted tetrazole compound 107, straightly. The trimethylstannyl motif is eliminated from the intermediate 107 by treating with trityl chloride. This reaction leads to the introduction of the trityl group to the tetrazole ring. In the final step, the trityl group is lost under acidic conditions to afford losartan 109. In this way, losartan 109 was obtained in 88.5% yield and 98.8% ee.141
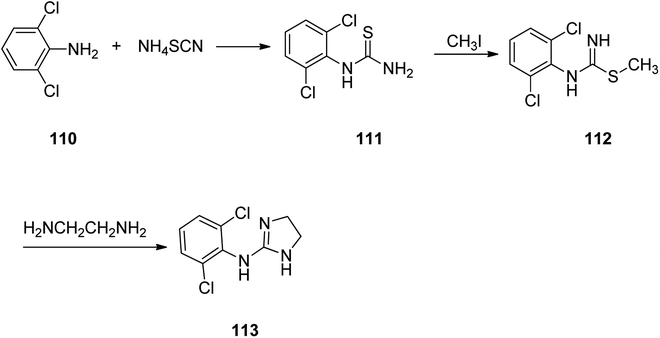
Clonidine, 2-(2,6-dichlorophenylamino)imidazoline 113, is also on market under the brand name Catapres. It is commonly prescribed for the treatment of high blood pressure, attention deficit hyperactivity disorder. Clonidine was patented in 1961 approved and came to market in 1966.142–144 It is an anti-hypertensive medicine with a clear-cut central site of act. The pharmacology of clonidine 113 has extensively been covered by Kobinger145 and Walland.146 Worthy to mention that introduction of the two chlorine atoms in ortho positions onto the 2-(arylimino)imidazolidine was found being vital for the biological activity of clonidine (Scheme 16).
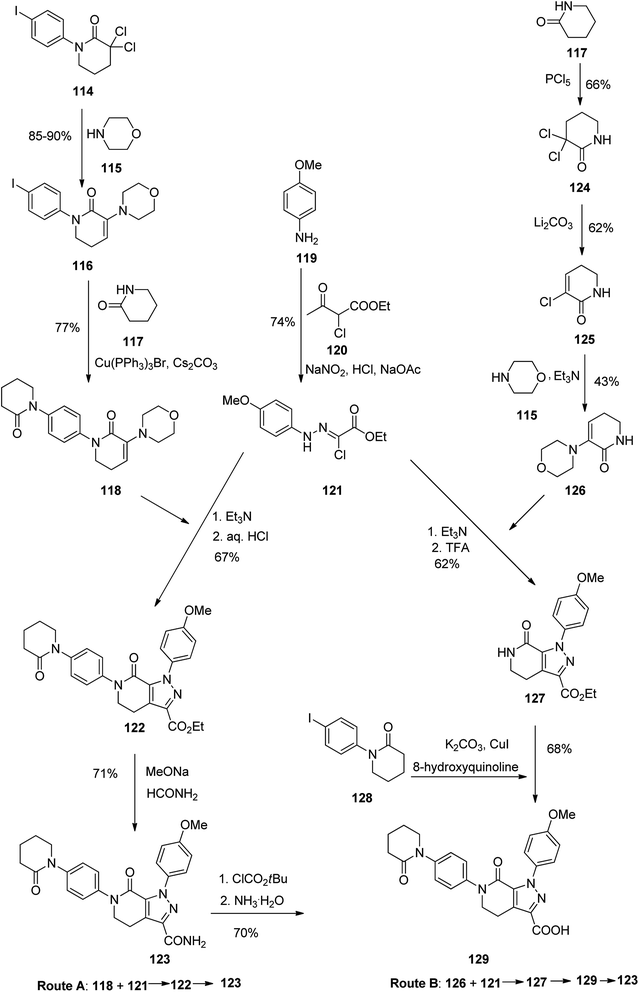
Apixaban, sold under the brand name eliquis was pataned in 2012 but approved in December 2019. Apixaban 123 is a powerful, selective, and orally bioavailable inhibitor of blood coagulation factor Xa (fXa).147,148 In addition, it has also exhibited promising in treatment of severe coronary syndrome (ACS),149 cerebrova scular ischemia, and cancer.150 Various pathways for the formation of apixaban 123 have been demonstrated, chiefly based on the application of expensive organic iodide.151–153 In the early age of drug discovery, 2003, Zhou and co-workers151 achieved and reported two approaches for the synthesis of 123 (Scheme 17, pathways A and B). In pathway A, initially, hydrazine 121, as a vital intermediate, was provided in two steps through the diazotization of 4-methoxyaniline 119 with subsequent Japp–Klingemann reaction with ethyl 2-chloroacetoacetate. Next, hydrazine 121 was exposed to an addition–elimination sequence with N-phenylvalerolactam 118 to afford pyrazolecarboxylate 122. Lastly, aminolysis of the latter with 10 equiv. of formamide and sodium methoxide (MeONa) resulted in the desired target 123. Particularly, N-phenylvalerolactam 118 was produced via an Ullmann reaction that treated with iodide 116 in 77% yield but organic cuprous compound Cu(PPh3)3Br as catalyst was needed. In pathway B, pyrazolecarboxylic acid 129 upon treatment with isobutyl chloroformate gave a mixed anhydride, which with subsequent aminolysis using ammonia, affording the desired target 123. In an alternative method, enamine 126 was reacted with key intermediate 121 via a sequential addition–elimination to afford pyrazololactam 127 which was subsequently undergone an Ullmann coupling reaction with organic iodide 128 using CuI to form 129 in 68% yield (Scheme 17).154
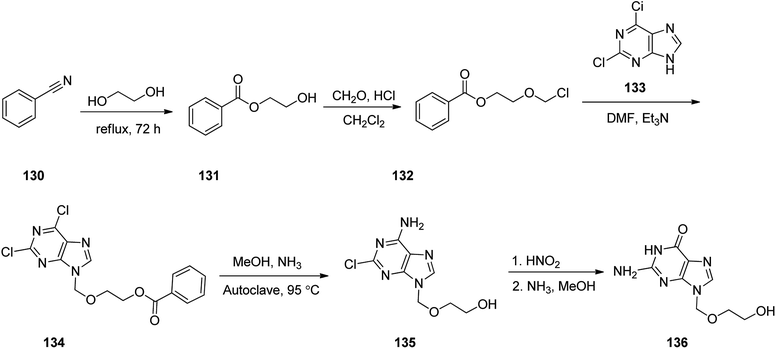
Aciclovir 136, was patented in 1974, and approved by FDA for medical use in 1981.155 It is an antiviral medicine,156 functioning by decreasing the production of the virus's DNA. It is prescribed for the treatment of herpes simplex virus infections, chickenpox, and shingles.157 Acyclovir 136 with chemical name of {9-[(2-hydroxyethoxy)methyl]guanine} (ACV), sold in market under the brand name of Zovirax.158 It functions selectively on herpes cells by specific inhibitory effects on repetition of herpes virus. In fact, acyclovir 136, the first effective antiviral agent and as mentioned above is a nucleoside analog.159 The synthetic pathway for acyclovir 136 was first disclosed in 1978.158 and then described in detail later.160 The synthetic route began with benzonitrile 130 which reacted with refluxing ethylene glycol to provide ethylene glycol monobenzoate 131. A cold mixture of the latter and paraformaldehyde in dry CH2Cl2 was saturated with hydrochloric acid, providing 1-benzoyloxy-2-chloromethoxyethane 132. Addition of the latter to a solution containing, 2,6-chloropurine 133 and Et3N in DMF, gave 2,6-chloro-9-(2-benzoyloxye-thoxymethyl)purine 134 in pure form. A solution of 134 in ammonia/methanol solution was heated in autoclave at 95 °C to give 2-chloro-9-(hydroxyethoxymethyl)adenine 135. As a matter of fact, the latter is the result of the known variances in the chemical reactivity in the 2- and 6-positions in the pyrimidine ring, leading to selective substitution of the 6-chloro group along with simultaneous deprotection of the side chain. The latter upon treatment with nitrous acid, followed by reaction of deaminated intermediate with methanolic ammonia to substitute the 2-chloro group, provided the desired target, acyclovir 136 a moderate yield (Scheme 18).161
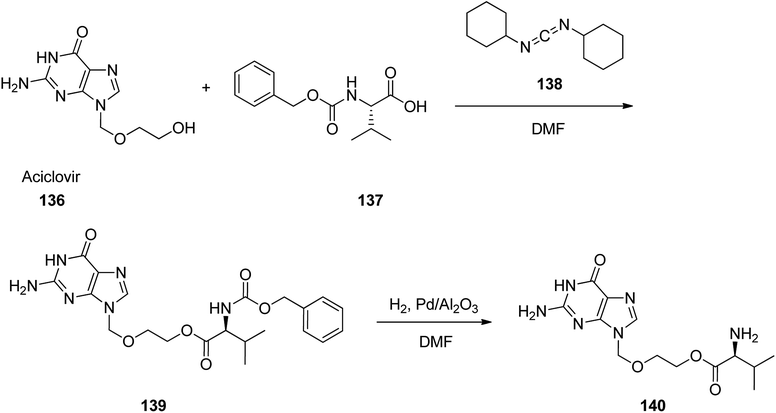
Valacyclovir, L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester, monohydrochloride 140 which is also available in market under the brand name vitrax. Valaciclovir was patented in 1987, approved by FDA, came into medical use in 1995.162 Valacyclovir 140 is a prodrug derived by esterifying acyclovir with L-valine. It is rapidly absorbed and well tolerated.163–168 A facile and multipurpose synthesis of valacyclovir 140,169,170 acyclovir 136 was condensed with N-carbobenzyloxy-L-valine 137 in the presence of dicyclohexylcarbodiimide in dimethylformamide to N-carbobenzyloxy-protected valacyclovir 139 that was subjected to palladium catalyzed deprotection (palladium/aluminium oxide in dimethylformamide) to provide valacyclovir 140. Later, an effective and scalable process was established, as illustrated in Scheme 19.171 The same method was performed with another masking group on an amino acid scaffold. Thus, valacyclovir was synthesized via reaction of N-(Boc)-L-valine with acyclovir employing 1-(3-dimethyl-aminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) as coupling agent and hydrochloric acid in the deprotection step.172
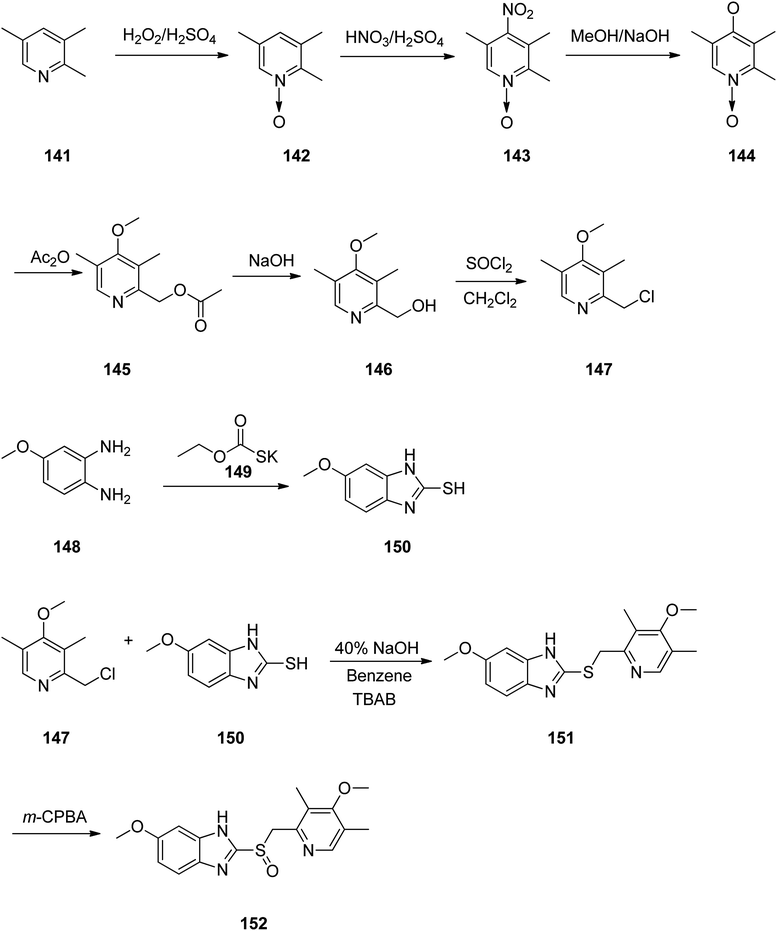
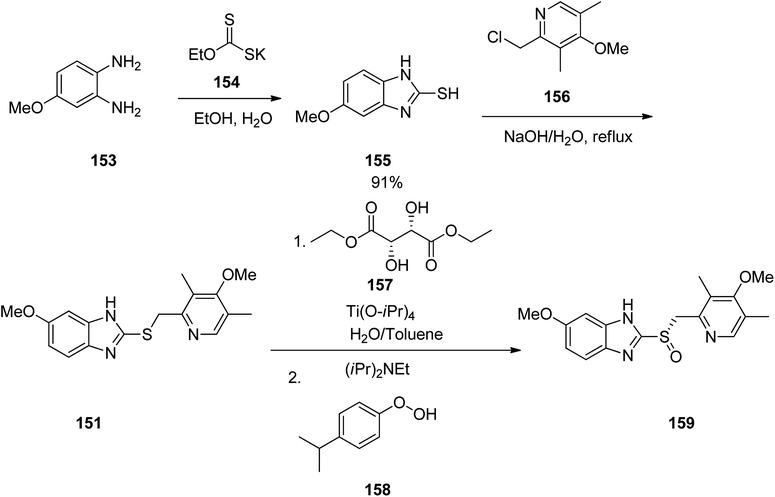
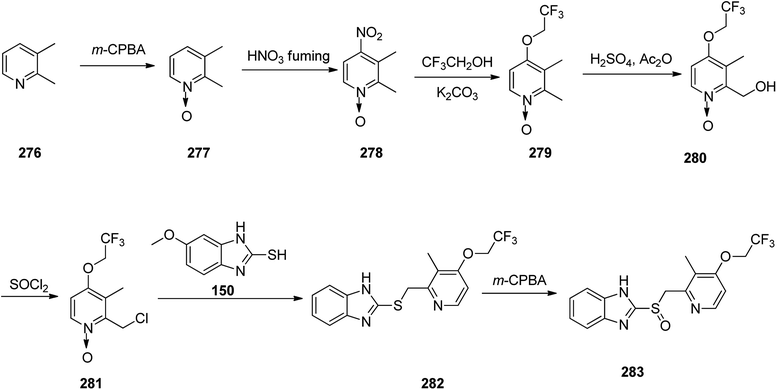
Omeprazole as a racemic mixture was the first recognized proton pump inhibitor (1979) having been marketed under the brand name Prilosec in 1988. As a proton pump inhibitor, omeprazole, like the others (such as lansoprazole, pantoprazol and rabeprazole) share the core structure of pyridinylmethylsulfinyl benzimidazoles.173 Different approaches have been reported for the synthesis of omeprazole 152.174–182 However, these approaches of synthesizing omeprazole are very close to each other and mainly relied on the first patent where its synthesis was revealed.183 The multi-step synthesis of omeparazole was started with 2,3,5-collidine 141 and was oxidized by H2O2 in HAOc to provide the N-oxide 142. The latter was nitrated in a mixture of nitric acid and sulfuric acids to provide the corresponding 4-nitro derivative 143. Then, the nitro group in 143 was substituted by OMe group in methanol/sodium hydroxide to give 144.184 The latter was heated in acetic anhydride that concurrently reduced the ring followed by acetylation (Boekelheide rearrangement) to provide the hydroxymethyl-pyridine acetyl derivative 145. In the following, the corresponding alcohol 146 was generated upon treatment with sodium hydroxide, with subsequent conversion to chloride-2-chloromethyl-4-methoxy-2,3,5-trimethylpyridine 147 employing SOCl2. On the other hand, condensation of 4-methoxy-o-phenylendiamine 148 with potassium ethylxantogenate 149 in the conventional fashion provided 2-mercapto-5-methoxy benzimidazole 150. Reaction of the latter with 2-chloromethylpyridine derivative 147 in the presence of sodium hydroxide in H2O/EtOH under reflux, or being performed under phase transfer catalysis conditions (benzene 40% sodium hydroxide, tetrabutyl ammonium bromide) provided thioether-5-methoxy-2-[((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)thio]-1H-benzimidzole 151 (pyrmetazole) that upon oxidation by 3-chloroperbenzoic acid in CH2Cl2 or H2O2 gave the corresponding sulfoxide, omeprazole 152 (Scheme 20). Several improved routes for the formation of omeprazole have been reported.185–193
Esomeprazole 159 is existed in the market under the brand names nexium among others, is a medicine which decreases the amount of stomach acid. It was patented in 1993, approved for medical use by FAD in 2000 and is available as a generic pharmaceutical.194,195 Structurally, esomeprazole is the (S)-(−)-isomer of omeprazole. It functions through blocking H+/K+-ATPase in the parietal cells of the stomach.196 Various methods have been reported for the synthesis of esomeprazole.197–200 The asymmetric synthesis of esomeprazole was achieved. The conjoint piece in their synthetic approach is the double condensation of a 1,2-diaminobenzene 153 with potassium ethylxanthate 154.201 An archetypal synthesis of the methoxybenzimidazole moiety existed in esomeprazole (omeprazole) is depicted in Scheme 21. For the synthesis of esomeprazole the succeeding steps comprise an S-alkylation as well as an asymmetric oxidation of the recently generated thioether.202,203 Practically, pyrmeprazole 151 was suspended in toluene and this solution was added to a water solution of (S,S)-diethyl tartrate and Ti(iso-proxide)4. To this solution, N,N-diisopropylethylamine and cumene hydroperoxide were added. In this way esomeprazole was obtained in 92%, chemical yield and the enantiomeric excess (ee) of crude sulphoxide was about 94%. The obtained esomeprazole was converted to its sodium salt as a solid with an enantiomeric excess of 99.5%, using conc. NaOH solution and CH3CN.
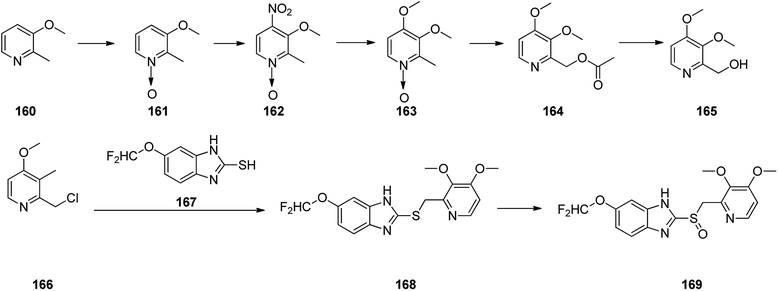
Pantoprazole 169 is the third proton pump inhibitor to be propelled for the treatment of peptic acid diseases. Research on pantoprazole commenced in 1985, and commercialized as an approved medication in Germany in 1994.204 Form that tine it has been listed as a generic medication and sold under the brand name Protonix among others. Pantoprazole 169 can be synthesized through the same approaches which employed for the above-mentioned synthesis for omeprazole and pantoprazole.205–207 Initially, sequential reaction is shown in Scheme 22, including the oxidation of 3-methoxy-2-methylpyridine 160 gave N-oxide 161, which upon selective nitration in the fourth position afforded 162. Then the nitro group in 162 was replaced by the methoxy group to give 163 which upon isomerization (Boekelheide rearrangement) furnished 164 which was hydrolyzed to give 2-(hydroxy)-3,4-dimethoxypyridine 165. The latter, was next converted to one of the vital starting materials, 2-(chloromethyl)-pyridine 166. Condensation of the latter with(difluoromethoxy)-2-mercapto-lH-benzimidazole 167 led to 2-((pyridin-2-ylmethyl)thio)-1H-benzo[d]imidazole derivative 168, which, after oxidation, provided the desired drug pantoprazole 169 (Scheme 22). The variation of suggested approach was also revealed in the chemical literature.208
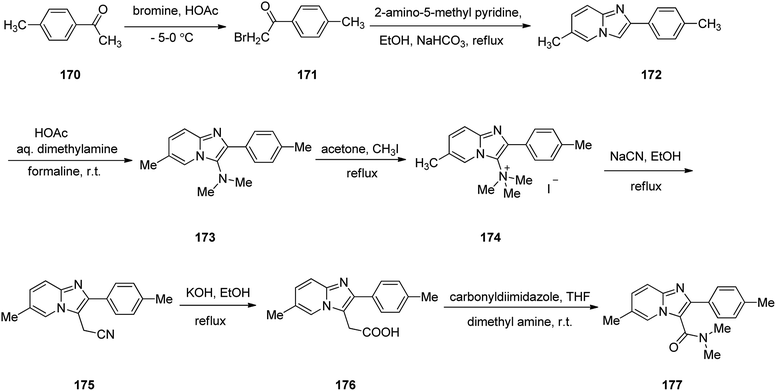
Zolpidem, sold under the brand name Ambien, among others, is a medication primarily used for the short term treatment of sleeping problems. Zolpidem was approved for being prescribed by FDA in 1992 but became accessible as a generic medication in 2007.209,210 It decreases the time to sleep onset by about 15 minutes and at larger doses helps people stay asleep longer.211 Common side effects include daytime sleepiness, headache, nausea, and diarrhea. Other side effects include memory problems, hallucinations, and abuse.211 Zolpidem 177 is a short stand-in, non-benzodiazepine imdazopyridine hypnotic medicine, sold as its tartrate salt.212 In addition, zolpidem 177 also have anxiolytic and anticonvulsant properties.213 The common synthesis of zolpidem 177 starts by the bromination of 4-methyl acetophenone 170 by bromine in acetic acid to afford its corresponding bromo derivative 171. The latter is condensed with 2-amino-5-methyl pyridine214 in the presence of NaHCO3 in refluxing EtOH to afford an imidazopyridine intermediate 172 that is subjected to Mannich reaction215 of dimethylamine and formaline in acetic acid at room temperature to afford the corresponding N,N-dimethyl aminoimidazopyridine 173. The latter was then transformed into its quaternary salt 174 upon treatment with CH3I in acetone under reflux. The latter was treated with sodium cyanide NaCN, in refluxing ethanol to afford cyano methylimidazopyridine derivative 175. Upon alkaline hydrolysis, the cyano compound 175, provided a pyridine acetic acid compound 176.216 Lastly, this essential intermediate 176 treated with carbonyl diimidazole followed by amidation using anhydrous dimethyl amine in THF provided the desired medicine zolpidem 177 (Scheme 23).217
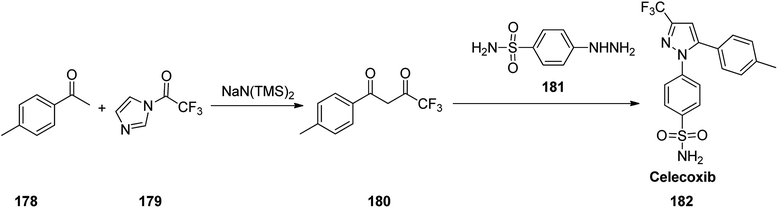
Celecoxib, came to market under the brand name celebrex among others. Celecoxib was patented in 1993 and approved for being used as medicine in 1999.218 Celecoxib 182, in fact is a 1,5-diarylpyrazole moiety integrates a sulfonamide or methylsulfonate pharmacophore at para position of N-aryl segment.219–221 It is a selective COX-II inhibitor established by Pfizer company and sold under the brand name of Celebrex®.222–224 Various approaches were reported for the formation of pyrazoles mostly based on 1,3-dipolar cycloadditions225–231 or condensation reactions232–236 as a key stage. It can be prepared by a reaction of 4-methyl-acetophenone 178 with N-(trifluoroacetyl)imidazole 179 in the presence of sodium bis(trimethylsilyl) amide to give the 4,4,4-trifluoro-1-(p-tolyl)butane-1,3-dione 180.222 Upon the reaction of the latter with 4-sulfamoylaphenylhydrazine 181 the desired compound, celecoxib 182 can be obtained (Scheme 24).237
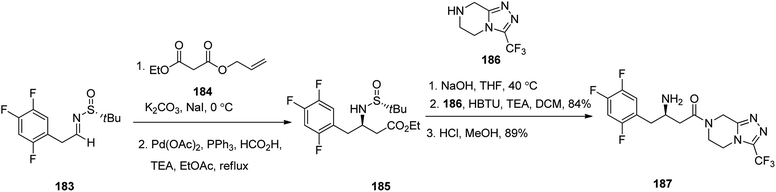
Sitagliptin 187, which is also in market under the trade name Januvia among other brand names, is a medicine used for the treatment of diabetes mellitus type 2. Sitagliptin 187, formerly named MK-0431, was developed by Merck Company and approved by FDA in 2006.238 Sitagliptin 187 was developed by Merck company and approved by FDA in 2006 then, delivered to market as phosphate salt under brand name of Januvia. Sitagliptin 187 is antihyperglycemic drug, is used in the treatment of type II diabetes.238 Notably, it is itemized as less favored than metformin or a sulfonylurea in the United Kingdom.239 Literature survey from 2005, revealed several synthetic formation for the preparation of sitagliptin 187.240,241 In 2017, Suh et al.242 achieved and reported a highly stereoselective approach for the satisfactory synthesis of sitagliptin. They commenced with tert-butyl sulfinyl aldimine 183 that was converted into β-amino-ester 185, as a sole stereoisomer via stereoselective enolate addition followed by palladium-catalyzed decarboxylation. Subsequently, the latter was subjected to saponification of the terminal ester and then peptide-like coupling with the piperazine scaffold 186 to afford the desired prescribed drug sitagliptin 187, with high chemical yield and excellent optical purity (Scheme 25).243
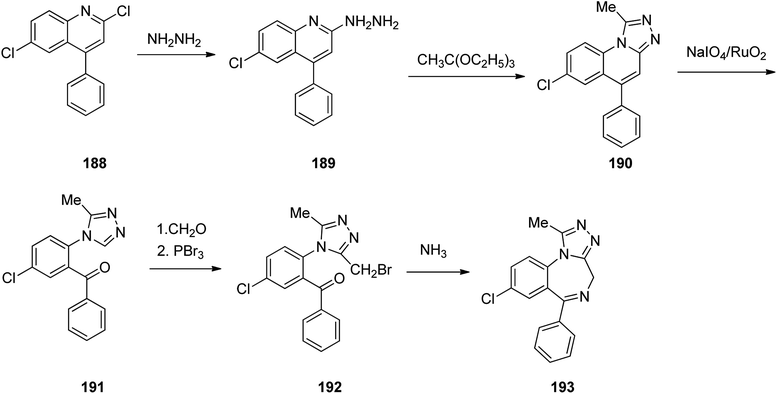
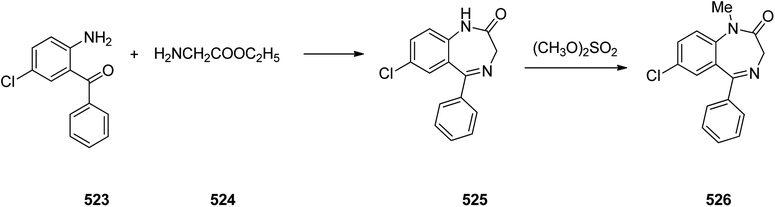
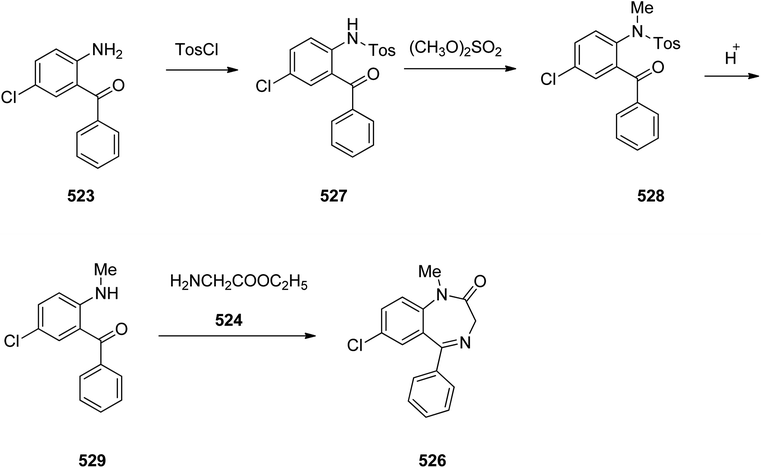
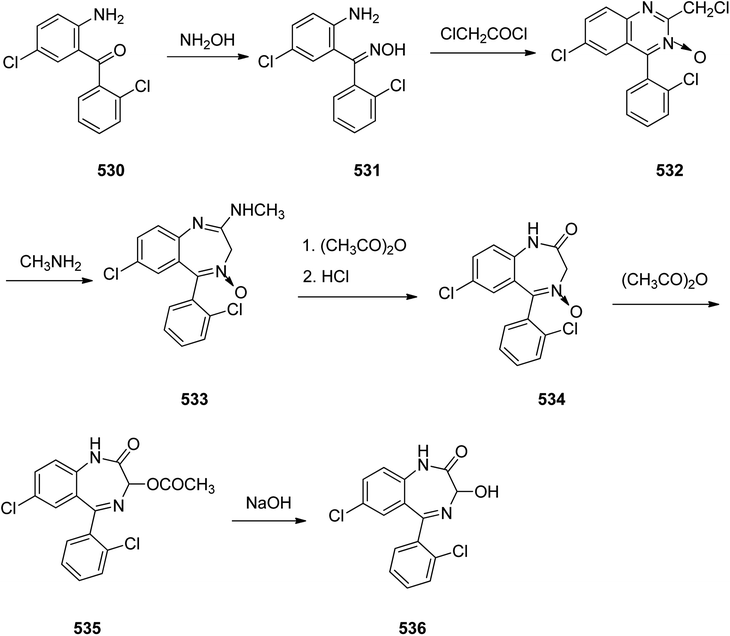
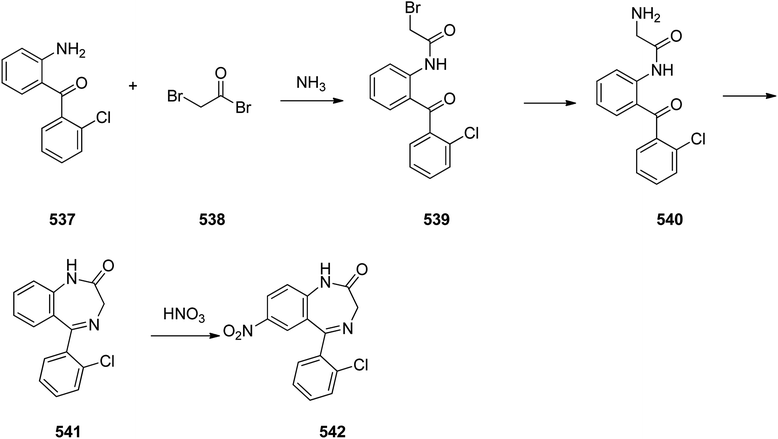
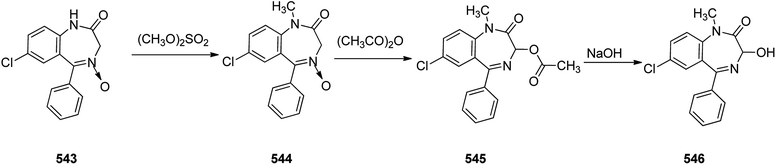
Benzodiazepines are a well-recognized class of compounds with a broad spectrum of central nervous system (CNS) related activities.244 They exhibited various kinds of biological potencies, for example antitumoral and anticonvulsive activities.245 One of the common intermediates for synthesis of benzodiazepines is aminobenzophenone.246 Alprazolam and diazepam are typical of this class of compounds. Benzodiazepines, such as alprazolam and diazepam with approved anxiolytic action and poorly expressed sedative-hypnotic potencies. Furthermore, alprazolam and diazepam are effective in treatment of panic disorders and agoraphobia.247 Clinical data proved that alprazolam is also helpful for treatment of depression. Alprazolam is short-lasting sedative taken orally in conditions of nervousness, panic disorders, anxiety which also treats depressive syndrome.248 In general, several compounds with promising sedative and toxic activities contain the 1,4-benzodiazepine framework.249 This class of active compound is obtainable through ring expansion of quinazolines, by ring contraction of benzoxadiazocines, and via synthesis starting from 2-aminobenzophenones.250 Alprazolam 193, sold under the brand name Xanax, among other trade names. Alprazolam was patented in 1971 and approved for being prescribed by FDA in 1981.251–253 It is most frequently taken orally for the short term controlling of anxiety disorders, specially panic disorder or general anxiety disorder (GAD).254 Alprazolam, is actually 8-chloro-1-methyl-6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepine 193.255 The similar strategy that is employed to prepare triazolam can be applied to synthesis alprazolam, with the exclusion which it starts from 2-amino-5-chlorobenzophenone as starting material.256–258 Worthy to notice a non-typical approach for the synthesis of alprazolam starting from 2,6-dichloro-4-phenylquinoline, has also been proposed. In this approach, 6-chloro-2-hydrazino-4-phenylquinoline 189 is treated with hydrazine and heating of this mixture with triethyl orthoacetate in xylene results in the corresponding triazole 190 via the heterocyclization. The latter is subjected to oxidative cleavage utilizing sodium periodate and ruthenium dioxide in an acetone/water as solvent to afford 2-[4-(3′-methyl-1,2,4-triazolo)]-5-chlorobenzophenone 191. Treatment of the latter with formaldehyde, followed by substitution of the resultant hydroxyl group by PBr3, affords 2-[4-(3′-methyl-5′-bromomethyl-1,2,4-triazolo)]-5-chlorobenzophenone 192. Replacement of the bromine atom in the latter with an amino group employing ammonia and the impulsive, intermolecular heterocyclization affords alprazolam 193 (Scheme 26).259–261
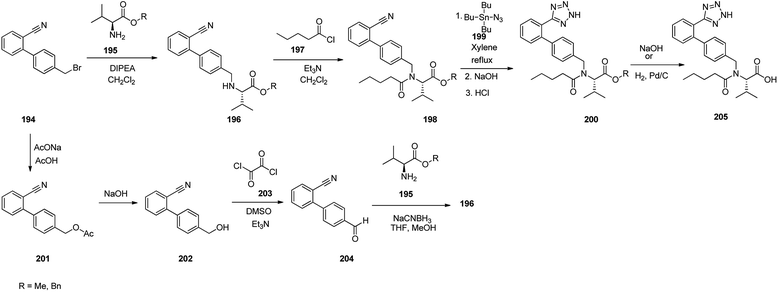
Valsartan 205 as the free acid, is a medication commercialized under the brand name ValsaDiovan®. Among other trade names, is prescribed, for the treatment of high blood pressure, heart failure, and diabetic kidney disease. Valsartan was patented in 1990, and came into medical use in 1996. Valsartan 205 is a non-peptide AT-II antagonist.262–264 Several approaches for the synthesis of valsartan 205 have been reported.265,266 An efficient synthetic pathway started with biphenylbromomethylnitrile 194 which upon alkylation using L-valine methyl or benzyl esters provided amines 196 which subsequently acylated with valeroyl chloride to provide N-valeryl derivatives 198. The latter was then refluxed with tributyltin azide in xylene affording tributyltin terazoles which subsequently was conventionally hydrolyzed to afford terazoles 200 in basic or acidic media. At the end, in the case of methyl ester, it was subjected to hydrolysis under basic conditions or in benzyl ester form, it was submitted to hydrogenation over the Pd catalyst, to provide the desired drug, valsartan 205. Alternatively, the same biphenylbromomethylnitrile 201, that was transformed into the corresponding acetate 201 which subsequently hydrolyzed to the corresponding benzyl alcohol 202. The latter under Swern oxidation condition (COCl2, DMSO, Et3N) was converted to aldehyde 204. Upon reductive amination of the latter using amino component, such as L-valine methyl ester and reductive agent such as sodium cyanoborohydride provided biphenyl nitrile 196. The latter by the same sequence of reactions (196 to 198 to 200) as mentioned above, provided the desired prescribed drug valsartan 205 (Scheme 27).267
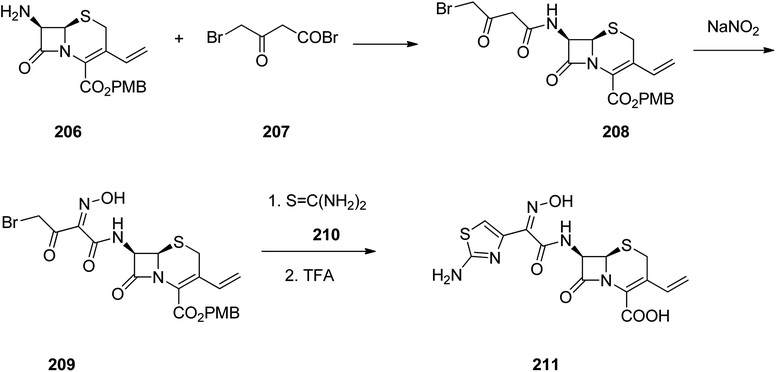
Cefdinir was patented in 1979 approved by FDA, for medical use in 1991, marketed under the brand name Omnicef, among others. It is a third-generation, semisynthetic cephalosporin antibiotic showing a broad spectrum of antibacterial activities.268–270 Cefdinir 211 is pinpointed by a vinyl group at C-3 position and a (Z)-2-(2-aminothiazol-4-yl)-2-(hydroxyimino)acetyl moiety at C-7 position, leading to a noticeable proliferation in its antimicrobial potency against Gram-positive and Gram-negative bacteria.271 It was synthesized through reaction of the primary amine 206 with 4-bromo-3-oxobutanoyl bromide 207 which resulted in the formation of the amide 208. Then, the active methylene group in the latter is nitrosated using sodium nitrite. The initial product impulsively is tautomerized to generate the oxime 209. The bromoketone assortment in this intermediate establishes a classical starting function for assembly of thiazole heterocycles. Reaction of oxime 209 with thiourea resulted in the formation of an aminothiazole moiety.272 Thus in this way the antibiotic cefdinir can be synthesized 211 (Scheme 28).269,272–274
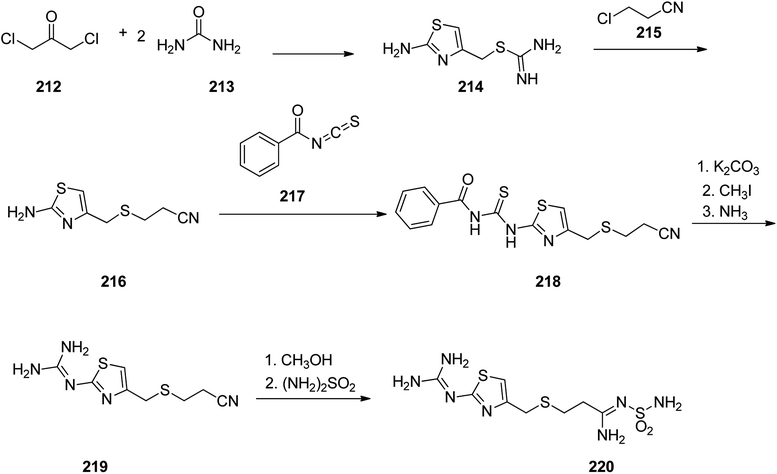
Famotidine 220, commercialized also under the trade name Pepcid among other brands, is a medicine that decreases stomach acid production. Famotidine 220 was patented in 1979 and passed clinical trials, approved by FDA in 1985.275,276 Fomatidine which is actually, 3-[[2-[(aminomethyl)amino]-4-thiazolyl]methyl]thio]-N-(aminosulfonyl)propanimidamide 220, can effectively be prepared in accordance with synthetic partway as illustrated in Scheme 29. 1,3-Dichloroacetone was reacted with two molecules of thiourea during which a thiazol ring is formed and the chlorine atom is substituted, providing an intermediate, 2-amino-5-chlormethylthiazol 214. The latter upon treatment with 2-chlorpropionitrile affords S-(2-aminothiazol-4-yl-methyl)-2-cyanoethane 216. The latter was then treated with benzoylizthiocyanate to afford benzoylthiourea derivative 218. This compound 218 was initially subjected into S-methylation using methyliodide and further submitted to cleavage by ammonia to afford 3-[[[2-(aminomethyl)amino]-4-thiazolyl]-methyl]thio]ethylcyanide 219. Sequential methanolysis of the nitrile group followed by reaction of the resulting iminoether with sulfonamide gives famotidine 220.277–282
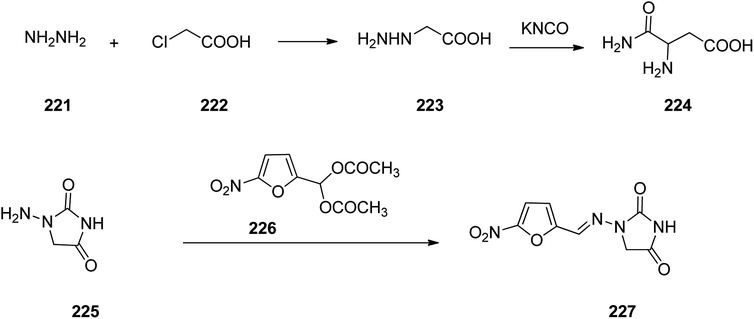
Nitrofurantoin 227, which was commercialized in 1953 under the trade names Macrobid®, Macrodantin®, and Furadantin®, is an antibiotic employed for the treatment of bladder and urinary tract infections. In spite of the development of a wide variety of new generation of antibiotics, nitrofurantoin vestiges a forefront for the treatment of easy urinary tract (pyelitis, pyelonephritis, cystitis, urethritis).283 Nitrofurantoin, 1-(5-nitrofurfurylidenamino)hydantoin 225, can deductively be prepared from hydrazinoacetic acid 223, that is provided upon treatment of chloroacetic acid with hydrazine. Treatment of the latter with potassium cyanate affords the semi-carbazidoacetic acid 224 that upon heating was cyclized into 1-aminoidantoin 225. When the latter was reacted with diacetylacetal of 5-nitrofurfurol the desired prescribed drug, nitrofurantoin 227 was obtained (Scheme 30).284–298
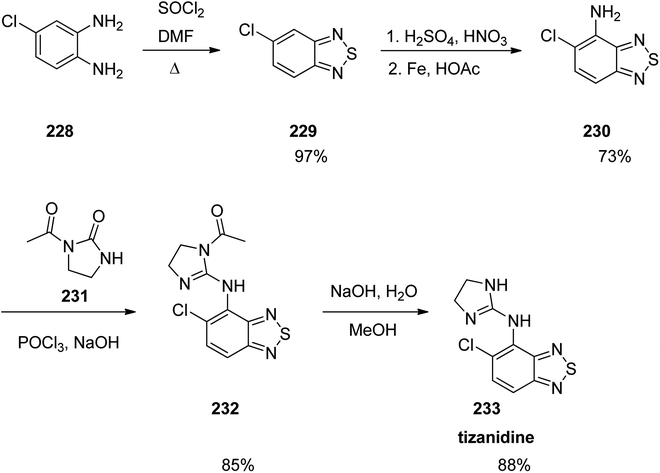
Tizanidine 233, came to market under the trade name Zanaflex among other brand names. It is a medicine which prescribed for the treatment of muscle spasticity because of spinal cord injury or multiple sclerosis.289 Tizanidine was approved for being prescribed by FD in 1996.289 It functions similar to baclofen or diazepam.290 Tizanidine is actually a substituted-1,3-benzothiadiazole 233. Treatment of an aromatic diamine 228 with SOCl2 in dimethylformamide gave the corresponding benzothiadiazole 229. The latter upon selective nitration followed by an Fe-mediated reduction gave the respective aniline 230 that is subjected to a nucleophilic substitution with 2-chloro-3,4-dihydroimidazole (produced in situ via the reaction of the urea 231 and POCl3). Elimination of the acetate group of the latter under basic conditions led to the desired medication tizanidine 233 (Scheme 31).291
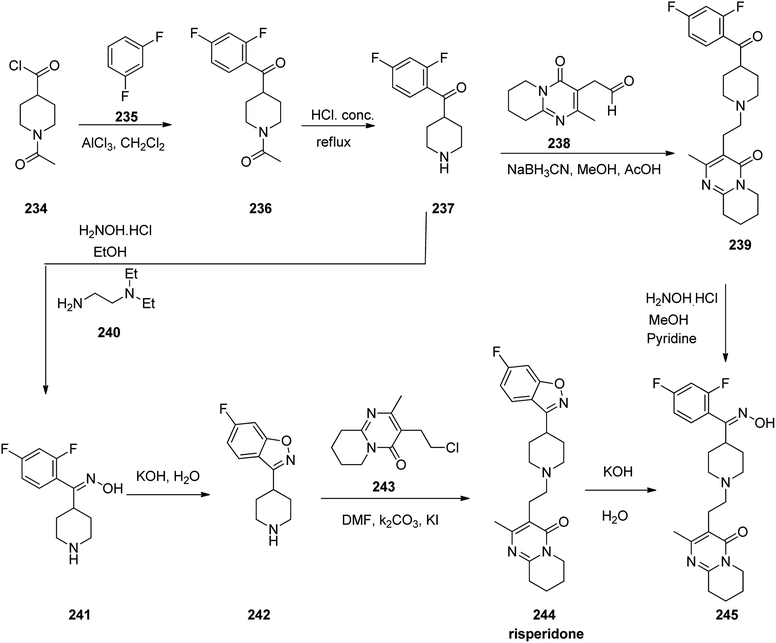
Risperidone 244, approved and came to market in 1993 under the trade name Risperdal among others.292 Risperidone is also active for Alzheimer's dementia, and substance abuse disorders.293–298 Risperidone 244 was prepared starting from 1-acetyl-4-piperidine-carbonyl chloride 234 that was employed to acylate 1,3-difluorobenzene 235 in CH2Cl2 in the presence of AlCl3 as Lewis acid. This reaction afforded 1-(4-(2,4-difluorobenzoyl)piperidin-1-yl)ethan-1-one 236. The protecting acetyl group of the latter was cleaved via hydrolysis in 6 N HCl under reflux condition which afforded (2,4-difluorophenyl)(piperidin-4-yl) methanone 237. The resultant product 237 was then transformed into respective oxime 241 upon treatment with NH2OH/HCl in EtOH using N,N-diethylenethanamine. The latter was then cyclized to 6-fluoro-3-(piperidin-4-yl)benzo[d]isoxazole 242 using 50% KOH solution of H2O under reflux condition. Finally, the latter was alkylated with 3-(2-chloroethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one 243 upon heating at 85–90 °C in DMF using Na2CO3 and KI to furnish the desired product, risperidone 244.299,300 Alternatively, compound 237 was transformed into 244 upon reductive alkylation of (2,4-difluorophenyl)(piperidin-4-yl)methanone 237 with aldehyde 238 using NaBH3CN which afforded compound 239, which was reasonably transformed into oxime 245 and further to the desired target compound, risperidone 244 (Scheme 32).301
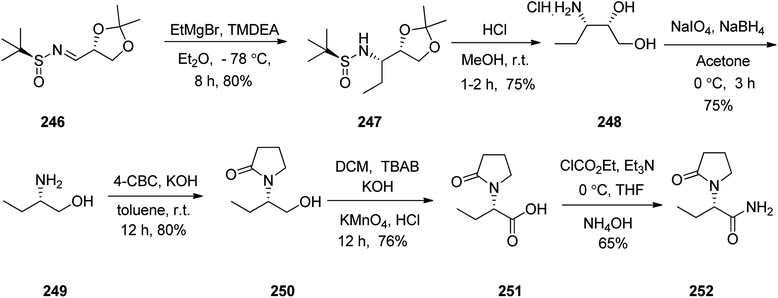
Levetiracetam 252, marketed under the trade name Keppra was approved as a medication for the treatment of epilepsy in 1999. An enantioselective synthesis of (−)-levetiracetam 252 was achieved and reported in six steps commencing from multipurpose novel optically active N-sulfinimine 246. In this strategy, the key step is asymmetric 1,2-addition of ethylmagnesium bromide (EtMgBr) to optically active N-sulfinimine prepared from (R)-glyceraldehyde acetonide and (S)-t-BSA, which afforded the respective sulfonamide 247 in high diastereoselectivity. Concurrent deprotection and deacetylation with subsequent cleavage using sodium periodate followed by reduction afforded ββ-amino alcohol 249. Subsequent reactions provided the targeted compound levetiracetam 252. The addition of the Grignard reagent to the imines 246 in tetrahydrofuran at −78 °C followed by deprotection of the t-butylsulfinyl group and 1,3-dimethylacetalin gave compound 247 which in acidic media (MeOH·HCl) was converted into the respective ββ-aminodiol 248. The latter, upon oxidation followed by reduction of amino diol using sodium periodate/sodium borohydride afforded the corresponding ββ-amino alcohol 249.302 The latter upon treatment with 4-chlorobutyryl chloride303 afforded 2-pyrrolidonealcohol 250. The latter upon oxidation with potassium permanganate gave 251, which in turn upon amidation provided the targeted (−)-levetiracetam 252. The spectral data of compound 252 was compared with those of previously reported in the literature and found being identical (Scheme 33).304
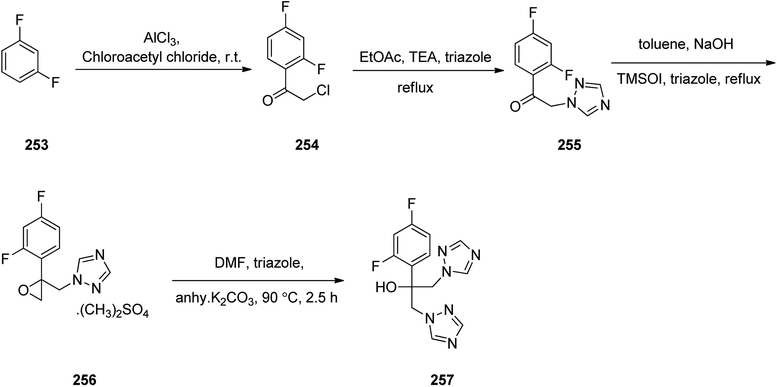
Fluconazole 257, is actually, α-(2,4-difluorophenyl)-α-(1H-1,2,4-triazol-1-yl-methyl)-1H-1,2,4-triazol-1-ethanol. Fluconazole was patented in 1981.305 It is an antifungal medicine prescribed for several fungal infections. Fluconazole was synthesized following a pathway as depicted in Scheme 34. 1,3-Difluorobenzene 253 reacted with chloroacetyl chloride in presence of aluminum chloride via Friedel–Crafts acylation reaction provided 2-chloro-1-(2,4-difluoro-phenyl)ethanone 254.306 Chloro compound 254 underwent nucleophilic substitution with 1,2,4-triazole in CH3COOEt in the presence of Et3N under reflux gave 1-(2,4-difluoro-phenyl)-2-[1,2,4]triazol-1-yl-ethanone 255. The latter upon treatment with trimethylsulfoxonium iodide in the presence of catalytic quantity of cetyltrimethylammonium bromide gave respective epoxy derivative 256 which upon the reaction with triazole under basic condition furnished fluconazole 257 (Scheme 34).307
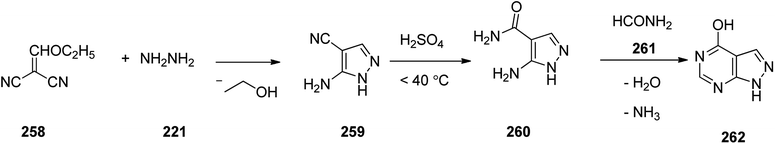
Allopurinol 262, is actually 1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one. It is marketed under the trend name Zyloprim and approved for being prescribed in the United States in 1966. It is a medication used to decrease high blood uric acid levels.308 Allopurinol 262, can be synthesized via a three steps pathway involving initial condensation of hydrazine 221 with ethoxymethylenemalononitrile 258 to afford 3-amino-4-cyanopyrazole 259, which, upon hydrolysis in acidic media (H2SO4) gave the corresponding amide 260. In last step, the latter was reacted with excess of formamide 261 under heating to furnish the desired medicationin, allopurinol 262 (Scheme 35).309
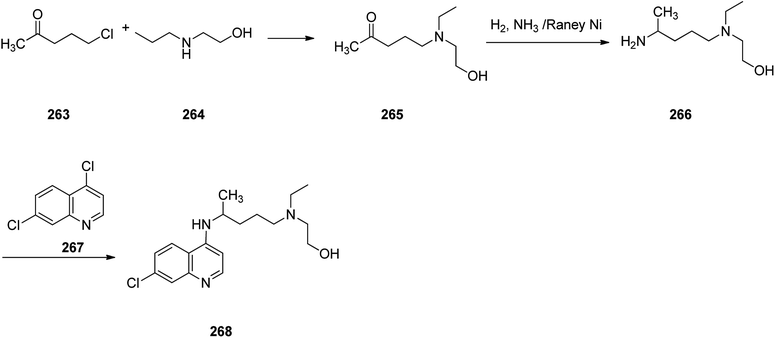
Hydroxychloroquine (HCQ) 268, which came to market under the trade name, Plaquenil, is a medication used for both prevention and treatment of certain kinds of malaria (chloroquine-sensitive malaria). Hydroxychloroquine was approved for being prescribed in the United States in 1955. Sometimes it is prescribed for the treatment of rheumatoid arthritis, lupus, and porphyria cutanea tarda.310 Interestingly, it is being used as an experimental medication for possible treatment for notorious coronavirus disease 2019 (COVID-19) which has very recently broken out and turned pandemic, in short period of time.311 Hydroxychloroquine 268, which is actually 7-chloro-4-[4-[ethyl(2-hydroxyethyl)amino]-1-methylbutylamino]quinoline 268, was synthesized in three steps starting from commercially available 1-chloro-4-pentanone as depicted in Scheme 36. Reaction of 1-chloro-4-pentanone with 2-ethylaminoethanol afforded the respective aminoketone 265 that was subjected to reductive amination to furnish 4-[ethyl(2-hydroxyethyl)amino]-1-methyl-butylamine 266. Reaction of the latter with 4,7-dichlroquinoline 267 gave the desired hydroxychloroquine 268.312,313
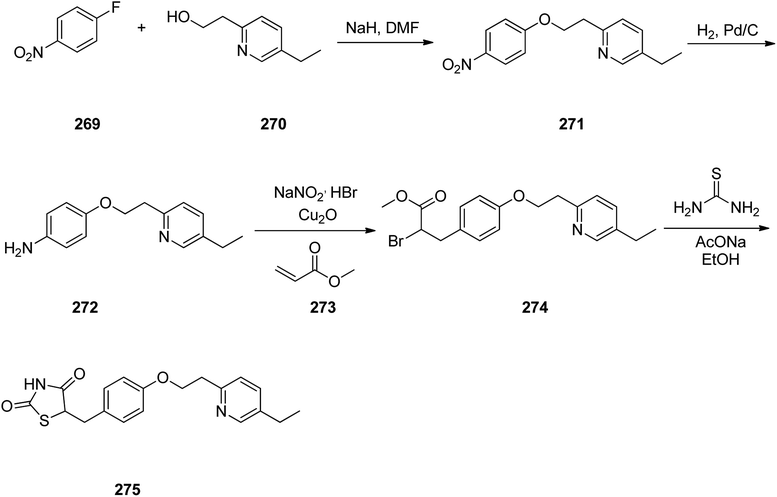
Pioglitazone 275, is a medication prescribed for the treatment of type 2 diabetes.314 It decreases insulin resistance in adipose tissue and liver.315 In addition, pioglitazone shows positive influences on lipid metabolism, regulate blood pressure, and endothelial function.316–322
Pioglitazone 275 was synthesized via a five step pathway, commencing from commercially available 1-fluoro-4-nitrobenzene 269. Condensation of the latter with 2-(5-ethyl-2-pyridyl)ethanol 270 provided pyridylethoxybenzene 271 that subsequently was hydrogenated using Pd on charcoal as catalyst to provide the anticipated aromatic amine 272. The latter upon diazotization in a mixture of acetone/methanol followed the workup with HBr, and coupling with methylacrylate in the presence of Cu2O (the Meerwein arylation) provided the methyl 2-bromo-propanoate derivative 274. The latter was subjected into cyclocondensation with thiourea, gave an imino compound as intermediate which upon hydrolysis provided the desired target pioglitazone 275 (Scheme 37).323,324
Lansoprazole 283, which reduces stomach acid, was patented in 1984 and approved for medical use in 1992.195 It is also known under the trade name Prevacid and prescribed for the treatment of peptic ulcer disease as well as Zollinger–Ellison syndrome.194 The most common method for the formation of lansoprazole reported by Nohara and Maki.325
This method was improved later and patented.326–328 The synthetic pathway for lansoprazole is depicted in the Scheme 38. In principle, it is the synthetic route of omeprazole, only divergent in details and characteristics, for instance, instead of 2,3,5-collidine as a starting material, 2,3-lutidine 276 was chosen, and the methoxy moiety in the fourth position of pyridine ring was changed by the 2,2,2-trifluoroethoxy moiety.
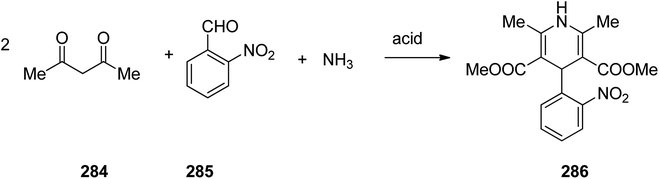
Nifedipine 286 is a famous medication used to regulate high blood pressure. It is also a calcium channel blocker of the dihydropyridine type. Nifedipine 286 was patented in 1967 and commercialized by Hofmann La Roch in 1981 under the trade name of Adalat among others. It is taken orally and comes in fast and slow release formulations.329 Nifedipine, actually is dimethyl ether 1,4-dihydro-2,6-dimethyl-4-(2′-nitrophenyl)-3,5-piridindicarboxylic acid 286, which is produced in large scale via Hantzsch 1,4-dihydropyridines (1,4-DHPs) synthesis. A multicomponent reaction, comprising two molecules of a β-dicarbonyl compound-methyl acetoacetate, 2-nitrobenzaldehyde and ammonia in acidic media and in one pot fashion produces the desired nifedipine 286. The sequence of the generation of intermediate has not been completely recognized (Scheme 39).330–333
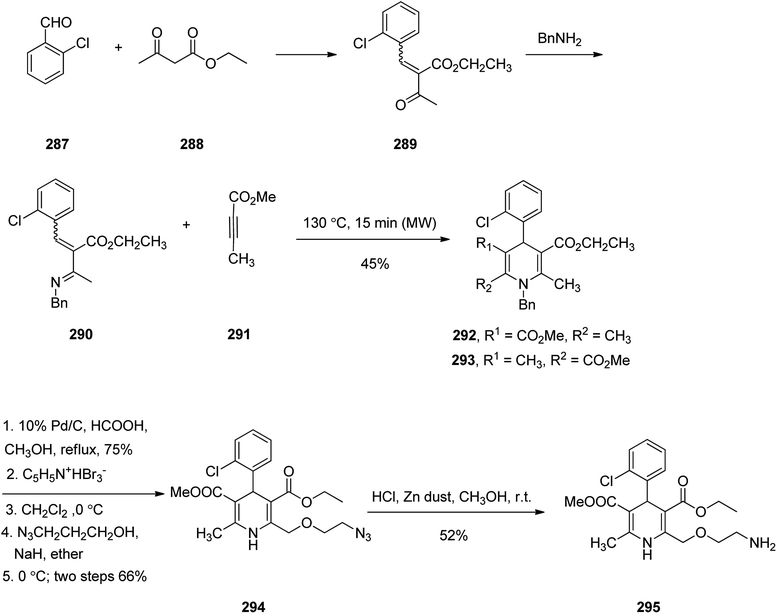
Amlodipine 295, is an approved drug used to treat high blood pressure and coronary artery disease. It is also presently sold under the brand name Norvasc.334 Amlodipine acts partially by swelling the size of arteries. It is in fact an efficient calcium channel blocker of the dihydropyridine category such as nefidipine which is a 1,4-dihydropyridine Ca2+ channel blockers and substantial antihypertensive drug.335,336 Amlodipine 295 was first patented in 1982 but approved as the prescribed drug in 1990.337 1,4-Dihydropyridines are frequently synthesized via an approach, explored by Hantzsch338 in 1882. This name reaction involves a simple procedure and straightforward isolation of product. Amlodipine 295 was synthesized as depicted in Scheme 40. Initially, the reaction of 2-chlorobenzaldehyde 287 with ethyl acetoacetate 288 under conventional heating gave the expected Knoevenagel product 289 as E,Z mixture in 70% yield. Then, compound 289 was reacted with benzylamine in presence of anhydrous MgSO4 under the microwave irradiation (MWI) at 70 °C to furnish imine compound 290 which was not isolated but subsequently the methyl butynoate 291 was added to the reaction mixture in the same vessel and exposed again to MWI to obtain Aza–Diels–Alder products 292 and 293 as a mixture in 45% combined yield. The reverse phase HPLC analysis of the reaction mixture confirmed that compound 292 was formed regioselectively over 293 with ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The structure of chief product 292 was elucidated by its spectroscopic data with those of authentic sample prepared via classical Hantzsch reaction followed by N-benzylation. Next, the side chain at C-2 in amlodipine 295 was successfully introduced via a procedure patented by Pfizer company.339 Product 292 obtained by Aza–Diels–Alder reaction upon treatment with formic acid followed by refluxing the reaction mixture in the presence of Pd/C resulted in the removal of benzyl group which was then upon bromination with pyridium tribromide gave the corresponding bromo compound. It was found that only C-2 position was selectively brominated over C-6 position.340 Reaction of the bromo compound with 2-azidoethanol in the presence of sodium hydride provided compound 294 in two steps good overall yield. At the end, reduction of azido group using zinc dust to amine was achieved to give the desired amlodipine 295 in satisfactory yield.341
3. The structure of chief product 292 was elucidated by its spectroscopic data with those of authentic sample prepared via classical Hantzsch reaction followed by N-benzylation. Next, the side chain at C-2 in amlodipine 295 was successfully introduced via a procedure patented by Pfizer company.339 Product 292 obtained by Aza–Diels–Alder reaction upon treatment with formic acid followed by refluxing the reaction mixture in the presence of Pd/C resulted in the removal of benzyl group which was then upon bromination with pyridium tribromide gave the corresponding bromo compound. It was found that only C-2 position was selectively brominated over C-6 position.340 Reaction of the bromo compound with 2-azidoethanol in the presence of sodium hydride provided compound 294 in two steps good overall yield. At the end, reduction of azido group using zinc dust to amine was achieved to give the desired amlodipine 295 in satisfactory yield.341
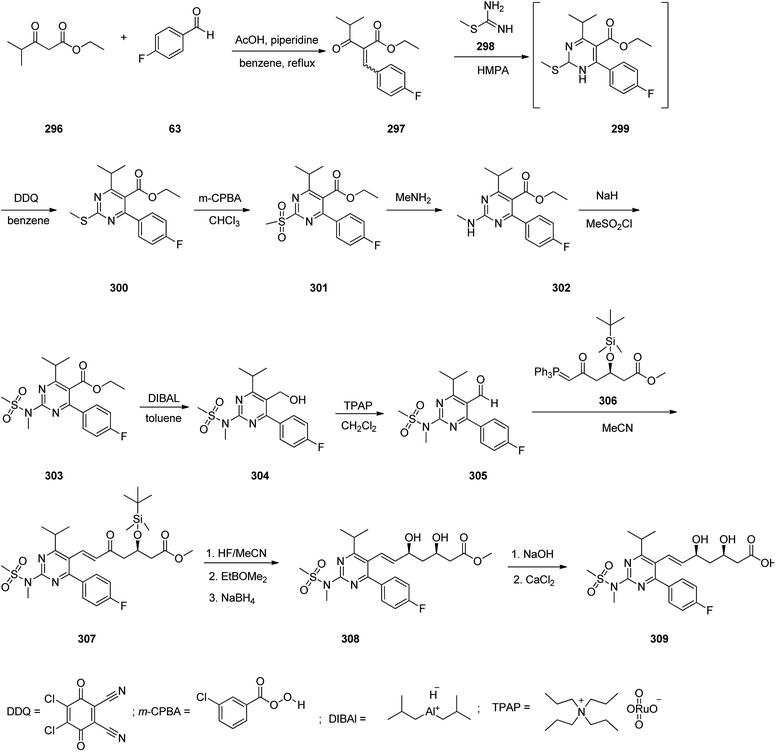
Rosuvastatin 309, is drug that inhibits the synthesis of cholesterol and promote the production of LDL-binding receptors in the liver. It was first patented in 1991 and marketed in 2003 under brand name of Crestor.342 A common synthesis of rosuvastatin commences from reaction of ethyl isobutyrylacetate 296 with p-fluorobenzaldehyde 63 that afforded the corresponding Knoevenagel condensation adduct benzylidene keto ester 297. The latter was upon cyclocondensation with S-methylisothiourea 298 in hexamethylphosphoramide (HMPA) at 100 °C provided the intermediate 299, which, without further purification, was subjected to dehydrogenation-aromatization with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) to provide the corresponding pyrimidine derivative 300. The sulfur scaffold in the resultant S-methyl pyrimidine 300 was oxidized employing m-chloroperbenzoic acid (m-CPBA) in CHCl3 to provide methylsulfonylpyrimidine 301. The latter was then converted into a methylamino derivative 302 through reaction with methylamine ethanol solution followed by treatment with methyl sulfonyl chloride in the presence of NaH to furnish the sulfonamide 303. Upon reduction of the ester group of the latter using diisobutylaluminium hydride (DIBAL) in toluene the corresponding primary alcohol 304 was obtained which upon oxidation using tetrapropylammonium perruthenate (TPAP) provided the respective aldehyde 305. Then, the latter was subjected to Wittig reaction with the optically active ylide-(3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidene hexanoate 306 in boiling acetonitrile to provide heptanoate 307 that was deprotected using hydrofluoric acid in acetonitrile, and the intermediate was reduced to using sodium borohydride in tetrahydrofuran to provide the ester 308 as syn-diol, regioselectively, typical for Wittig reaction. At last the latter treated with aqueous sodium hydroxide to provide the respective sodium salt that was converted into desired calcium salt, rosuvastatin 309 (Scheme 41).343,344
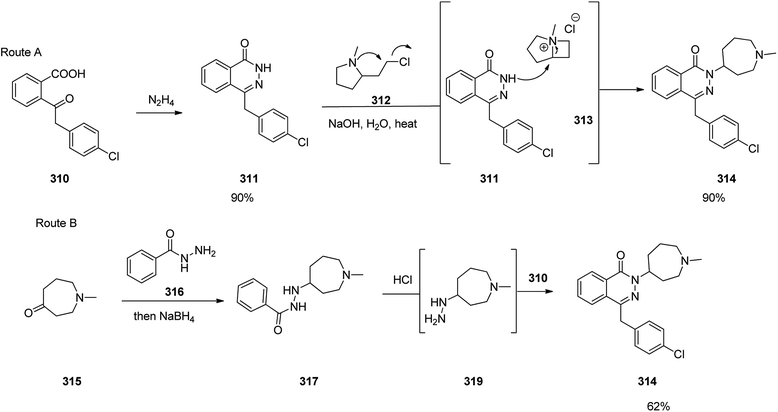
Azelastine is a medication which is primarily used as a nasal spray to treat allergic rhinitis (hay fever).345 Azelastine was first patented in 1971 and came into medical use under the trade name of Optivar in 1986.346 A brief synthesis of azelastine 314 was achieved and reported as shown in Scheme 42. It invoved the reaction of hydrazine with keto-acid 310 to give phthalazinone 311. The latter was reacted with 2-(2-chloroethyl)-N-methylpyrrolidine 312 in hot aqueous NaOH provided azelastine via a fascinating ring expansion. This ring expansion apparently proceeds via the intermediacy of [3.2.0]-framework 313 to afford the desired target azelastine 314 (Scheme 42, route A).347 On the other hand, the same keto-acid 310 can undergo condensation with substituted hydrazine 318 that was provided from an acid-mediated hydrolysis of acyl hydrazide 317 to give the desired azelastine 314 (Scheme 42, route B). Notably, by using a solid hydrazide 316 instead of volatile hydrazine this route is longer but it is a safer alternative at large scale production.
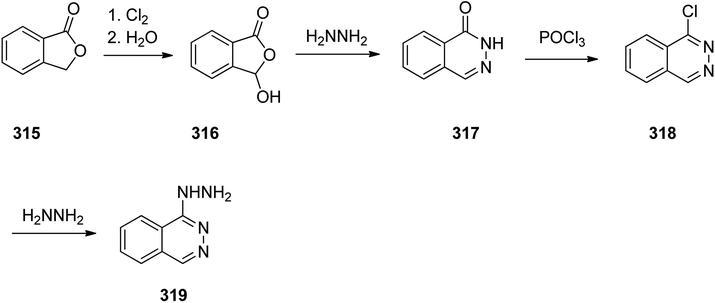
Hydralazine 319, is a medication used to treat high blood pressure and heart failure.348 Hydralazine 319, is also sold under the trade name Apresoline.349 Hydralazine, 1-hydrazinonaphthalazine 319, was synthesized via four steps reaction stating from the oxidative chlorination of phthalide 315 with concurrent hydrolysis of product that leads to hydroxyphthalide 316. The latter was reacted with hydrazine hydrate to give phthalazone 317, which upon treatment with phosphorous oxychloride to give 1-chloroph-thalazine 318. In the last steps substitution of the chlorine atom with hydrazine affords the desired hydralazine 319 (Scheme 43).350–352
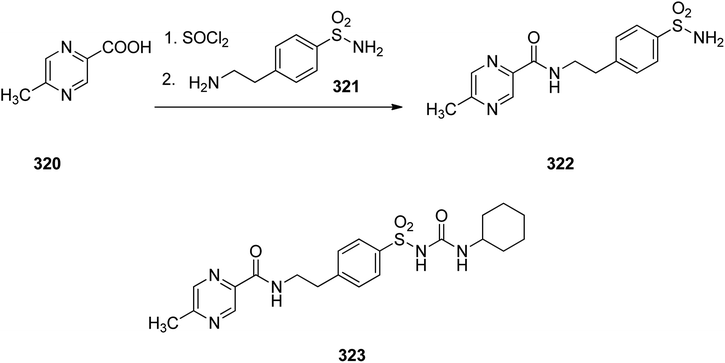
Glipizide 323, is an anti-diabetic medication of the sulfonylurea class prescribed for the treatment of type 2 diabetes and is used combined with a diabetic diet. It was approved for medical in 1984 and introduced to market under the brand name Glucotrol.353 Glipizide 323 is actually 1-cyclohexyl-3-[[p-[2-(5-methylpyrazincarboxamido)ethyl]phenyl]sulfonyl]urea 323. As depicted in Scheme 44, the synthesis of glipizide 323, is started with 6-methylpyrazincarboxylic acid 320 which is initially treated with SOCl2, leading to the respective chloride that is further reacted with 4-(2-aminoethyl)benzenesulfonamide 321, giving the expected amide 322. The resulting sulfonamide 322 upon reaction with cyclohexylisocyanate via conventional procedure resulted in formation of the desired glipizide 323.354–356
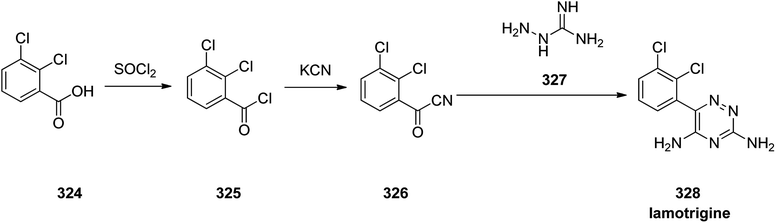
Lamotrigine 328, came to market in the Great Britain in 1991 as is an anticonvulsant medication prescibed for treatment and epilepsy and bipolar disorder. However, it was approved for being prescribed in the US in 1994.357 Nowaday, it is sole under trade name of Lamictal. There are two practical approaches for the formation of lamotrigine 328. The first approach,358,359 is relied on condensation of 2,3-dichlorobenzoyl cyanide 326 that is in turn provided by transformation of 2,3-dichlorobenzoic acid 324 to its acid chloride 325 by treatment with tionyl chloride. The latter was then reacted with copper cyanide to provide 2,3-dichlorobenzoyl cyanide 326. Condensation of the latter with aminoguanidine 327, proceeds smoothly to give the desired target lamotrigine 328 in about 16% yield (Scheme 45).360
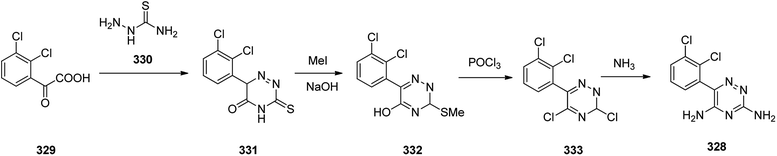
The alternative approach employed to provide lamotrigine 328 (ref. 361) involved, the reaction of 2-(2,3-dichlorophenyl)-2-oxoacetic acid 329 with thiosemicarbazide 330 to provide 3-thioxo-3,4-dihydro-1,2,4-triazin-5(6H)-one derivative 331 which subsequently methylated using MeI in aqueous NaOH provided the corresponding S-methylated product 332. The latter upon treatment with especially with phosphorous oxychloride, among other chlorinating agent, gave the S-methylated product 332 which was subjected to replacement of its thiomethyl and hydroxyl groups for chlorine, providing compound 333, which, upon treatment with NH3, gave the desired target lamotrigine 328 (Scheme 46). Some related methods for the formation of lamotrigines have been underlined in the two useful reviews.362,363
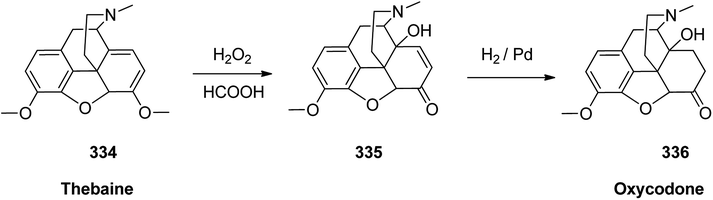
Oxycodone 336, sold under the brand name OxyContin among others, is an opioid medicine used for handle and alleviating of moderate to severe pain.364 Oxycodone was first semi-synthesized in Germany in 1916 from a natural product, thebaine, has been a common drug of abuse.365 Although the structure of oxycodone is similar to natourios morphine, it has shown better oral bioavailability, making it superior for pain alleviating in some clinical trials.366 Oxycodone 336 is prepared from thebaine (paramorphine) 334, that also known as codeine methyl enol ether which is an opiate alkaloid. Thebaine 334, is first converted into intermediate 14-hydroxycodeinone 335 upon oxidation using H2O2 in formic acid. Upon the selective hydrogenation of the double bond, in 335 the desired target oxycodone 336 was prepared (Scheme 47).367
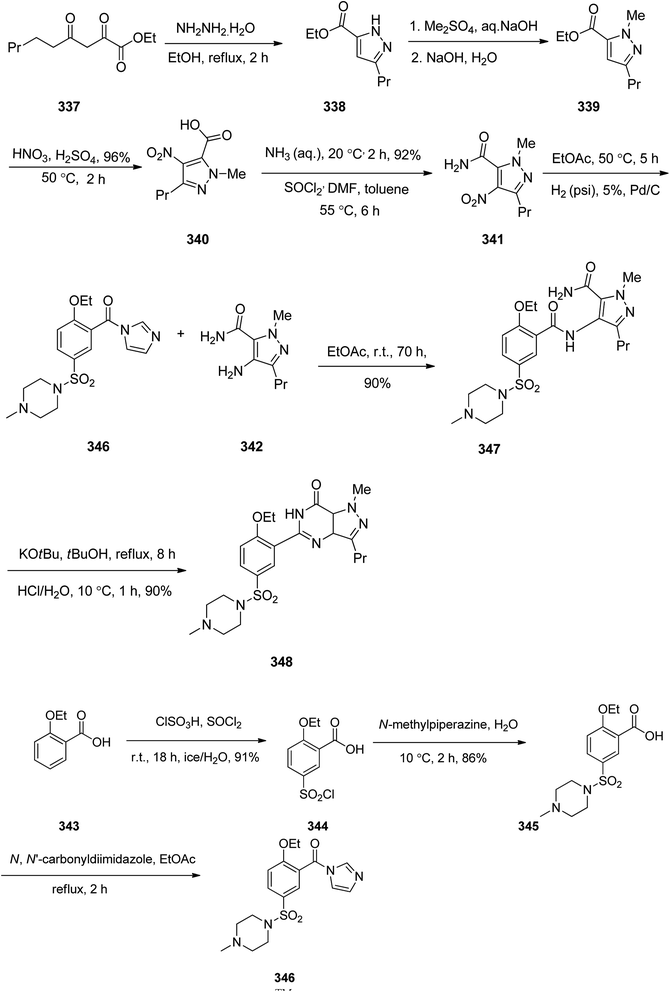
Sildenafil 348, has been used for the treatment of erectile dysfunction and pulmonary arterial hypertension. As a matter of fact, it was accidentally discovered by Pfizer in 1989 while the researchers were looking for a medication to treat heart-related chest pain.368 It was approved for being prescribed in the US and Europe in 1998.368 Sildenafil 348 is sold with the brand of Viagra by Pfizer. Sildenafil was initially examined against hypertension with little success but showed promising effects in the male sexual dysfunction.369 The synthetic pathway to sildenafil was achieved by the Pfizer research group.370 Sildenafil is prepared via the reaction of β-diketones with hydrazines. For the synthesis of sildenafil 346, diketone 337 was reacted with hydrazine hydrate in EtOH under reflux. The reaction proceeds through the formation of hydrazone A,371 which upon subsequent cyclization and dehydration provides the corresponding pyrazole 338. The latter upon treatment with Me2SO4, as source of a methyl nucleophile under basic condition (aq. NaOH) gave the corresponding pyrazole 339. The latter was dissolved in conc·H2SO4, next the fuming nitric acid was mixed with conc. H2SO4 and added to the pyrazole 339 which concurrently nitrated the pyrazole ring and hydrolysis of the ester moiety to carboxylic acid to give pyrazole 340. The latter was converted to 341 upon treatment with liq. NH3 in DMF. Then, the nitro group in 341 was reduced via hydrogenation in the presence of Pd as catalyst in ethyl acetate to afford the corresponding pyrazole 342. Sildenafil 348 was synthesized starting from 2-ethoxybenzoic acid 343 in molten form that was added gradually to a mixture of chlorosulfonic acid and thionyl chloride while the reaction temperature was kept below 25 °C. In this way a direct electrophilic aromatic substitution occurred in which the ethoxy group gave a common direction to the electrophile towards the expected ortho and para position. It was noticed that the addition of thionyl chloride for transformation of the intermediate sulfonic acid into the sulfonyl chloride is essential. In this stage, the reaction was quenched by addition of ice water in which 5-(chlorosulfonyl)-2-ethoxybenzoic acid 344 was precipitated out from reaction mixture. Next, the latter was reacted with N-methylpiperazine in water to give 2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl) benzoic acid 345. The carboxyl group of 345 was activated by a common and effective activating reagent, N,N′-carbonyldiimidazole (CDI) make it susceptible for nucleaphilic substitution.372 Thus the reaction of 345 with N,N′-carbonyldiimidazole (CDI) in refluxing acetic acid provided (2-ethoxy-5-(4-methylpiperazin-1-ylsulfonyl)phenyl)(1H-imidazol-1-yl)methanone 346. The latter was reacted with 342 in ethyl acetate at room temperature to give the desired amide 347 through the usual addition elimination mechanism. In the last step, the primary amide is deprotonated by potassium tert-butoxide making it more nucleophilic. This nitrogen as a nucleophile attack the other amide carbon closing the ring. Upon isomerization resulting in the formation of pyrimidone ring the synthesis of sildenafil 348 was completed. Worthy to mention, that the last step includes only water soluble solvents and reagents were used and the final product precipitates out of the aqueous solution upon reaching pH 7.5 to give sildenafil 348 with clinical quality from the filtration in which further purification is non-required (Scheme 48).373
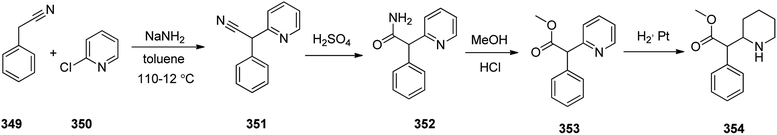
Methylphenidate 354, is a stimulating medicine used for treatment of attention deficit hyperactivity disorder (ADHD). It is sustaining attention, increases intellectual capacity, and enhance memory.374,375 Methylphenidate 354 initially synthesized and patented in 1944. It was first made in 1944 by CIBA and was approved for being prescribed in US in 1955.376 It is nowadays sold under the brand name Ritaline by Novartis Corporation.376 Methylphenidate 354 can be produced at large scale through reaction of phenylacetonitrile 349 with a 2-chloropyridin 350 at 110–112 °C in toluene in the presence of NaNH2 (sodium amide) that afforded 2-phenyl-2-(pyridine-2-yl)acetonitrile 351. The latter was then upon hydrolysis to the respective amide 352 that subsequently treated with hot hydrochloric acid in MeOH afforded methyl 2-phenyl-2-(pyridine-2-yl)acetate 353. The pyridine ring of the latter was hydrogenated to a piperidine ring in HAOc on the platinum or platinum oxide (PtO2) as catalyst afforded the desired target methylphenidate 354 (Scheme 49).377–379
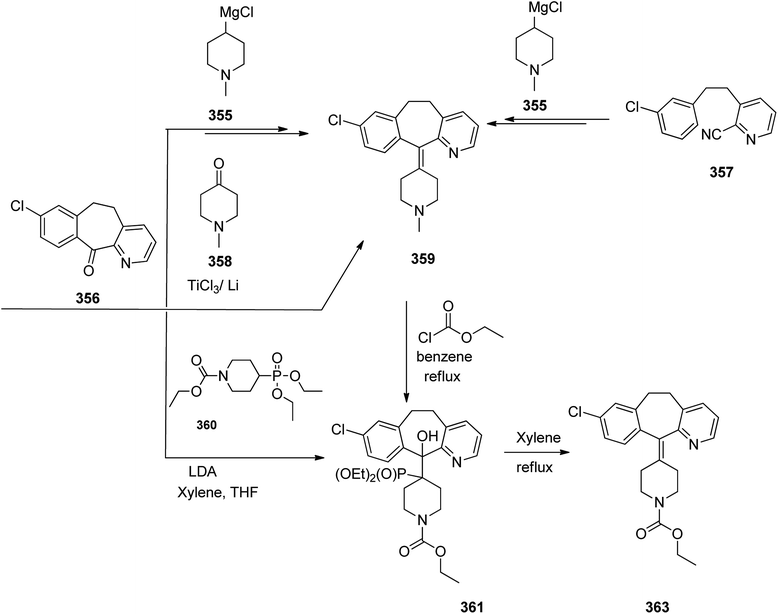
Loratadine 362, was found to treat several kinds of allergies including allergic rhinitis and hives. It was patented in 1980 and commercialized under trade name Claritin in 1988.380 It is also sold in combination with pseudoephedrine, a decongestant, known as loratadine/pseudoephedrine.381 As a matter of fact, loratadine, cetirizine and astemizole are second-generation antihistamines that have substituted first-generation antihistamines for example diphenhydramine and ketotifen.382–388 Loratadine 362 can be produced via various routes. It can be effectively synthesized, based on the formerly reported approaches,389 starting from the 8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-one ketone 356, which upon treatment with an appropriate Grignard reagent 355 afforded the respective tertiary carbinol that was subsequently dehydrated in acidic media giving the 8-chlorol-1-piperidiylidene derivative 359. The later then was treated with ethylchloroformate under refluxing benzene to afford compound 361 which was transformed to the desired target product, loratadine 362 by refluxing in xylene.390,391
Another approach was involved the construction of seven-membered ring scaffold through cyclizing intermediate ketone provided by the reaction of the same Grignard reagent 355 with tailor-made 3-(3-chlorophenethyl) picolinonitrile 357 in a different super acid systems media, preferably, a system comprising HF and BF3 resulted in the formation of compound 359 which similarly converted to compound 361 and then by refluxing in xylene was converted to the corresponding its ethyl carbamate which is actually the desired loratadine 362.392–396
A third strategy is relied on the use of low-valent titanium catalyzed reductive coupling between the two ketones which are available in hands. They were 8-chloro-5,6-dihydro-11H-benzo[5,6] cyclohepta[1,2-b]pyridin-11-one 356 and ethyl 4-oxopiperidine-1-carboxylate 358 which upon low-valent titanium assisted reductive coupling gave 8-chloroazatadine 359. The latter was converted to the desired loratadine 362, through above-mentioned two step reaction.397
Another approach employed the Wittig reaction in which initially ethyl 4-(diethox-ypho sphoryl)piperidine-1-carboxylate 360 was reacted with ketone 356 in presence of lithium diisopropyl amide in xylene-THF media to afford the β-hydroxyphosphonate 361, that was converted to loratadine 362 via thermal decomposition by being further refluxed in xylene 362 (Scheme 50).398
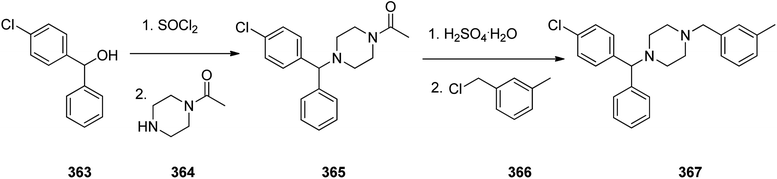
Meclizine 367, is also an antihistamine medication employed for treatment of motion disease and the sense like the world is rotating. Meclizine 367 was patented in 1951 and approved for being used as medication under the trade name Bonine in 1953.399 (4-Chlorphenyl)-phenylmethanol is halogenated with SOCl2 before adding acetylpiperazine. The acetyl group is cleaved with diluted sulfuric acid. An N-alkylation of the piperazine ring with 3-methylbenzylchloride completes the synthesis of meclizine 367 (Scheme 51).400
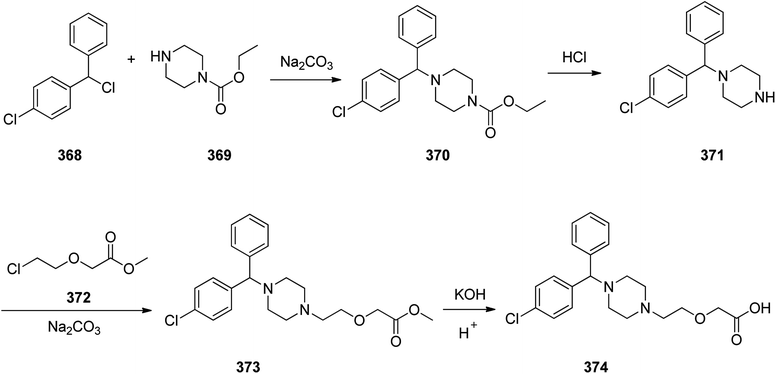
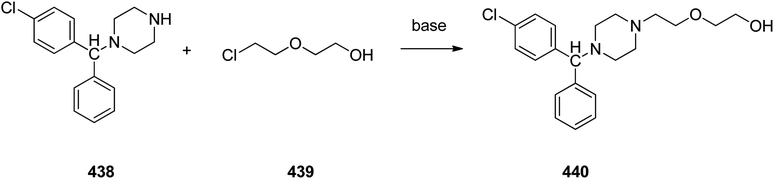
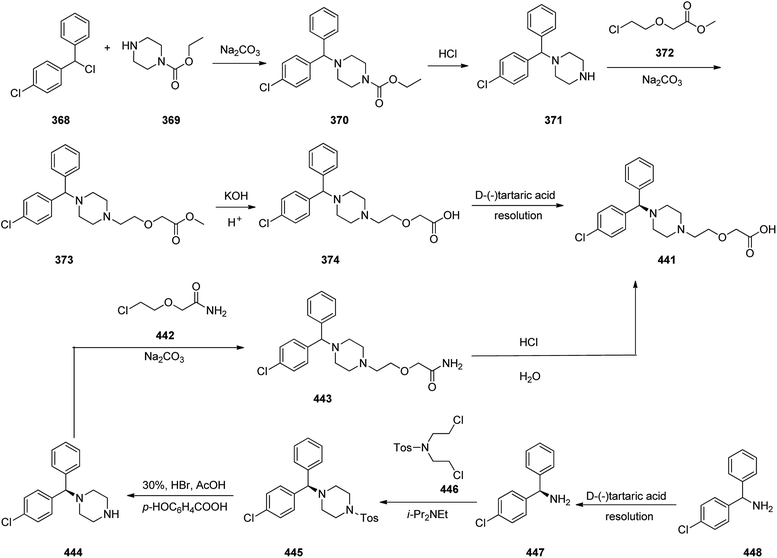
Cetirizine 374, is a second-generation antihistamine used for treatment for allergic rhinitis, dermatitis, and urticarial.401 It was patented in 1981 but marketed in 1987 (ref. 402) under the trade name of Zyrtec, (Zyrtec®1, Zirtec®). The pharmacological and medical properties and therapeutic efficiency of cetirizine have already been reviewed.403–407 Cetirizine 374 is prepared as a racemic mixture408,409 and its isomers can be separated.409 The first synthesis of cetirizine 374 commenced from 4-chlorobenzhydrylchloride 378, which was reacted with ethyl piperazine-1-carboxylate 369, in the presence sodium carbonate to provide compound 370. The latter was subjected to acidic hydrolysis (using HCl) to provide the benzhydrylpiperazine derivative 371. Next, the latter was treated with methyl 2-(2-chloroethoxy) acetate in the presence of Na2CO3 to provide the product 373. The resultant ester was readily subjected to basic hydrolysis to afford the corresponding carboxylic acid as racemate which in fact was the desired target, cetirizine 374 (Scheme 52).410
Levofloxacin 384, is an optically active antibiotic used to treat a number of bacterial infections involving acute bacterial sinusitis. Notably, it is approved for being used in the treatment of community-acquired pneumonia, H. pylori.411–420 Levofloxacin was first patented in 1985 and approved for being prescribed in 1996 under the brand name of Levaquin.421 Levofloxacin 384 is derived from the typical quinolones which have a more complex fused ring to the oxazinoquinoline core. (−)-Levofloxacin was found being twice as more active than ofloxacin.422 The synthetic pathway including resolution of racemic mixture to obtain (−)-levofloxacin 384 was designed and performed as depicted in Scheme 52. Based on this approach, the synthesis began from the reaction of 2,3-difluoro-6-nitrophenol 375 with chloroacetone 376 in the presence of K2CO3 and potassium iodide to provide 1-(2,3-difluoro-6-nitrophenoxy)propan-2-one 377. Notably, 2,3-difluoro-6-nitrophenol 375 was synthesized from 2,3,4-trifluoro-1-nitrobenzen by replacement of the ortho to the nitro group fluorine atom to the hydroxyl group through the reaction with potassium hydroxide in dimethyl sulfoxide. The resultant product 377 was hydrogenated using RANEY® in EtOH to afford a cyclic product, 7,8-difluoro-3-methyl-3,4-dihydro-2H-benzo[b][1,4]oxazine 378. The latter was reacted with diethyl ethoxymethylenemalonate 379 via the well-established Gould–Jacobs reaction at 130–140 °C to provide the expected benzoxazinyl methylenemalonate 380 that upon treatment with polyphosphoric ester at 140 to 145 °C provided 9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid ethyl ester 381. Upon hydrolysis of the latter in HAOc/conc·HCl benzoxazine-6-carboxylic acid was obtained 382. The resultant product 382 was reacted with N-methylpiperazine in DMSO resulted in displacement of a secondary amine smoothly, introduces an amino substituent at the C7 position selectively due to the activation by the C4 carbonyl group, giving ofloxacin 383 as racemate. Resolution of racemic mixture of 383 through optical, enzymatic, or crystallization approaches gave (−)-ofloxacin 384 (Scheme 53).423
Fentanyl 389 is an opioid used as a pain killer and sometimes combined with other medicine for anesthesia. Fentanyl was initially synthesized by Paul Janssen in 1960 and approved for clinical use in the US in 1968. Fentanyl is known more often by its brand name Sublimaze. Their modus operandi is supposed to include the binding to the transmembrane m-opioid receptors on cell surfaces leading to a flow of intracellular signals that finally leads to their biological effect.424,425 Several synthetic approaches have been developed for their construction since Janssen's first discovery.426–429 The approach is described herein were actually optimized to provide fentanyls in higher yields using a highly effective three-step synthetic method. The multistep synthesis of fentanyl 389, as illustrated in Scheme 54 was started with the alkylation reaction of commercially accessible 4-piperidone monohydrate hydrochloride 385 using 2-(bromoethyl)-benzene in the presence of cesium carbonate in acetonitrile at 80 °C to provide alkylated piperidone 387 in 88% yield. The latter upon reductive amination with aniline in the presence of sodium triacetoxyborohydride in HAOc provided the 4-piperidineamine 388 in excellent yield (91%) as appropriate precursor. Finally, the latter was acylated by propionyl chloride using Hunig's base to produce fentanyl 389 in 95% yield. Similarly, piperidineamine 388 was reacted with acetic anhydride using Hunig's base to produce acetylfentanyl 390 in 98% yield. Transformation of 389 and 390 were easily performed to give their corresponding hydrochloride and citrate salts in almost quantitative yields (Scheme 54).430
Donepezil 394, is a medicine prescribed usually to treat Alzheimer's disease. It was confirmed by FDA for being prescribed in 1996 to alleviate to what happens in Alzheimer's disease. Donepezil 394, shows a minor assistance in mental function and ability to function,431 but does exhibit any important change in the progression of the disease432,433 Donepezil is the most effective inhibitor of the AChE currently accessible on the market, also sold, under the brand name Aricept.434,435 Donepezil has been synthesized via two different pathways.436–438 The synthesis of donepezil was achieved and reported by Sugimoto and co-workers which is a convergent method leading to the production of donepezil hydrochloride 394. It includes aldol condensation of N-benzyl piperidine carboxaldehyde 392, with 5,6-dimethoxy indanone 391, under inert atmosphere and in the presence of n-butyl lithium and diisopropylamine in hexamethyl phosphoric amide (HMPA) at −78 °C, to produce olefinic compound 393. The double bond of 393 was catalytically reduced in the presence of 10% Pd/C in THF provided, donepezil as free base that was further transformed into its hydrochloride salt 394 upon treatment with HCl/MeOH and isopropyl ether 27% overall yield (Scheme 55, Method A).439
Alternatively, Elati research group accomplished and reported440–442 a route for the synthesis of donepezil hydrochloride 394. In this strategy, initially, 5,6-dimethoxy-1-indanone 391 was condensed with pyridine-4-aldehyde 395 to afford olefinic compound, 5,6-dimethoxy-2-(pyridine-4-yl)methyleneindan-1-one 396. The double bond of the latter was reduced in the presence of Pd carbon catalyst using HAOc in MeOH to produce compound 398 which was further alkylated with benzyl bromide 397 to give donepezil as free base 399. The last was then upon further treatment with HCl in mixture of MeOH, H2O and methyl tert-butyl ether produced donepezil hydrochloride 394 (Scheme 56, Method B).
Paroxetine 408, approved for being prescribed in 1992 by FDA, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It was commercialized under the trade names of Paxil and used for the treatment of social anxiety disorder, major depressive disorder, obsessive, panic disorder, and general anxiety disorder.443–450 Several routs for the synthesis of paroxetine were designed and suggested, designated in the recently published review.451 One of the rather practical approaches, being used in large-scale production of paroxetine 408 is started with 1-benzyl-4-piperidone 400 which upon the reaction with the Grignard reagent, 4-fluorophenyl magnesium bromide 401 afforded the corresponding tertiary alcohol 402. Upon the treatment of the latter with p-toluene sulfonic acid (PTSA), dehydration took place resulting in the formation of the corresponding tetrahydropyridine derivative 403. The latter was subjected to the Prins reaction conditions (using HCOH, HCl, H2SO4) to give the racemate of tetrahydropyridine-3-methanol that can be resolved using (2)-L-dibenzoyltartaric acid to afford 404. The latter was subjected to the stereoselective reduction over palladium/C catalyst, under acidic conditions in H2O resulted in formation of cis-(3R,4R) isomer of piperidine-3-methanol 405, due to retention of N-benzyl protective group. The obtained cis-alcohol 405 was reacted with methanesulfonyl chloride to give the corresponding cis-mesylate 406. The reaction of the latter with sodium sesamolate led to the formation of trans N-benzylparoxetine 407 that upon debenzylation upon hydrogenation over palladium/C catalyst afforded the desired target paroxetine 408 (Scheme 57).451
Clopidogrel 422, is also an antiplatelet medication prescribed to diminish the risk of heart disease and attacks in those at high risk. Clopidogrel was patented in 1982, and approved by FDA for being prescribed in 1997.452 Clopidogrel 422, came to market under the brand name of Plavix. Clopidogrel 422, was synthesized commencing with easily accessible 3-iodothiophene diacetate 409, which upon treatment with allyltrimethylsilane mediated by BF3·Et2O at −50 °C to give the expected 2-allyl-3-iodothiophene 411 in almost quantitative yield. The latter was then submitted to magnesium–halogen exchange to generate the corresponding heteroaryl magnesium followed by formylation to give aldehyde 412 in good yield. The latter was then underwent reductive amination using tert-butyl carbamate mediated by triethylsilane and trifluoroacetic acid to give Boc-protected amine 413 in good yield. Then, the latter was subjected to a dihydroxylation-oxidative cleavage of the terminal olefin in one pot fashion, affording the respective aldehyde which concurrently cyclized to give hemiaminal 414 in satisfactory yield. Ultimately, the last was subjected to reductive amination of hemiaminal 414 mediated triethylsilane and BF3·Et2O with subsequent in situ deprotection of the Boc-carbamate moiety gave the important intermediate 415 in good yield. The latest was then upon treatment with 2-bromo-2-(2-chlorophenyl)acetonitrile 416 mediated by NaHCO3 in MeOH gave the vital intermediate 2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridine-5(4H)-yl)acetonitrile 417. Delightfully, the direct alkaline hydrolysis of nitrile 417 to acid 419 was achieved virtually in quantitative yield when it was performed in the presence of phase transfer catalyst and in the mixed solvent and high concentration of inorganic strong base, while by monitoring the concentration of base (<20%), amide 418 can also be created selectively. Apparently, there is no method found in literature concerning the direct alkaline hydrolysis of nitrile 417 to acid 419. Next, metal salt of 419 was reacted with dimethyl sulfate, using TEBA (triethylbenzylammonium) in NaOH/MeOH afforded the desired expected compound 420, which in two steps gave the respective 421. By using 0.45–0.55 equiv. of L-CSA in toluene, a highly selective and efficient kinetic resolution took place giving optically pure clopidogrel with higher than 98.3% ee and 88% chemical yield. Clopidogrel 422 was obtained in even higher optical purity of above 99.5% ee, just by washing it with isopropanol at room temperature (Scheme 58).453
Ketoconazole 431, was patented in 1977 and approved in 1981 as an antifungal medicine, prescribed commonly for the treatment of several fungal infections. Ketoconazole 431, is mainly used to treat fungal skin infections such as versicolor, dandruff, tinea, seborrheic dermatitis, cutaneous candidiasis and pityriasis.454 Ketoconazole 431, is also sold under the trade name Nizoral. Ketoconazole 431, is in fact chemically named, cis-1-acetyl-4-[4-[2-(2,4-dichlorophenyl)-2-(1H-imidazole-1-ylmethyl)-1,3-dioxolan-4-ylmethyl]phenyl]piperazine 431. It can be synthesized from the reaction of 2,4-dichlorophenacyl bromide 423 with glycerol 424 affording cis-2-(2,4-dichlorophenyl)-2-bromoethyl-4-hydroxymethyl-1,3-dioxolane 425. The hydroxyl group of 425 can be benzoylated using benzoyl chloride followed by alkylating the resulting compound using imidazole to furnish, compound 428. Upon the alkaline hydrolysis of the latter which eliminates the benzoyl group and subsequent reaction of the resultant with methanesulfonyl chloride afforded 1-acetyl-4-(4-hydroxyphenyl)piperazine 429. Lastly, upon alkylating the latter with 430 gave ketoconazole 431 in satisfactory yield (Scheme 59).455–459
Mirtazapine 437, was approved by FDA for being prescribed in the United States in 1996 as an antidepressant. It primarily used to treat depression,460 depression combined by anxiety or for suffering sleeping.460,461 Mirtazapine 437 has a tetracyclic chemical structure distinct to other classes of antidepressants for example, those that are selective serotonin reuptake inhibitors, tricyclics or monoamine oxidase inhibitors.462,463 Mirtazapine 437, is in fact chemically known as 2-methyl-1,2,3,4,10,14b-hexahydrobenzo[c]pyrazino(1,2-a)pyrido[3,2-f]azepine. Various approaches have been achieved and reported in the literature for the production of mirtazapine.464–466 However, still some impurities are traced in the mirtazapine tablets, sold in the market,467 van der Burg and co-workers achieved and reported the synthesis of mirtazapine 437 started with 2-amino-3-cyanopyridine 432 which was reacted with N-methyl-1-phenyl-2,2′-iminodiethyl-chloride 433 to afford 1-(3-cyanopyridyl-2)-4-methyl-2-phenylpiperazine 434 (cyano-NMPP). The latter upon hydrolysis of its nitrile group under highly basic conditions (KOH/EtOH) at high temperatures (100 °C) for a long time (24 h) provided the 1-(3-carboxypyridyl-2)-4-methyl-2-phenylpiperazine 435. The vital intermediate-1-(3-hydroxy-methylpyridyl-2)-4-methyl-2-phenylpiperazine 436 was provided by reduction of 435 using lithium aluminium hydrate (LiAlH4) as an efficient reductive agent. Compound 436, upon treatment H2SO4 produced mirtazapine 437 (Scheme 60).468
Hydroxyzine 440 is selective antihistaminic medication, used in the treatment of itchiness, anxiety, and nausea. It is classified as a first generation antihistamine containing piperazine. Hydroxyzine 440 was first produced in 1956 and was approved for being prescribed in the United States. Nowadays, hydroxyzine 440 has been commercialized and sold under the trade name Atarax.469 Hydroxyzine 440 is chemically, 2-[2-[4-(p-chloro-α-phenylbenzyl)-1-piperazinyl]-ethoxy] ethanol which is produced by the alkylation reaction between 1-(4-chlorobenzohydril)piperazine 438 and 2-(2-hydroxyotoxy)ethylchloride 439 in the presence of an appropriate base (Scheme 61).470–475
Levocetirizine 441, approved by FDA in 2007, is an antihistamine prescribed for the treatment of allergic rhinitis.476 Levocetirizine 441 (ref. 477–480) signifies a third generation of antihistamines that was patented and approved after the second-generation such as cetirizine. The enantioriched levocetirizine 441 was obtained via a conventional resolution of cetirizine as racemate using D-(−)-tartaric acid. The synthesis of cetirizine as racemate408,409 commenced with 4-chlorobenzhydrylchloride 368, which, reacted with ethyl piperazine-1-carboxylate 369, to provide compound 370. The last was subjected to acid hydrolysis to provide the benzhydrylpiperazine derivative 371. The latter was then treated with methyl 2-(2-chloroethoxy) acetate in the presence of Na2CO3 to afford the product 373. The resultant ester was readily transformed into cetirizine in the form of free acid 374 and next the desired target product, levocetirizine 441, was isolated through the classic racemic resolution via crystallization of D-(−)-tartaric salt from the racemic mixture.409 Alternatively, each stereoisomer of cetirizine was asymmetrically synthesized. One alternate481 commenced with from each isomer of 4-chloro-benzhydrylamine 448, which separated with the utilization of (−)-, or (+)-tartaric acids (R)-(4-chlorophenyl)(phenyl)methanamine 447. The suitable enantiomer was then upon treatment with N,N-bis(2-chloroethyl)-4-methylbenzenesulfonamide 446 in diisopropylethylamine under reflux provided a tosyl derivative 445 that easily by crystallization from EtOH was purified. Reductive elimination of the N-tosyl group in 445 using 4-hydroxybenzoic acid (phenol component) in HBr/CH3COO gave product 443 in highly pure form. Then, upon alkylation of the latter with 2-(2-chloroethoxy)acetamide compound 443 was obtained that was hydrolyzed in acidic media (HCl) to provide the desired target levocetirizine 441 as a single stereoisomer (Scheme 62).
Trazodone 454, is a medication taken orally to treat main depressive and anxiety disorders, and also used as component with other drugs to treat alcohol dependence. Trazodone 454 was approved by FDA for being prescribed in US in 1981. Trazodone is also marketed as an antidepressant medication482 under several brand trade names. Trazodone 454 has been successfully synthesized from the reaction of 1,2,4-triazolo[4,3-a]pyridin-3(2H)-one 449 with 1-bromo-3-chloropropane 450a or 1,3-dibromopropane 450b to afford, 2-(3-halopropyl)[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one 451a/b which upon reaction with 1-(3-chlorophenyl) piperazine hydrochloride 452, in the presence of K2CO3 as a reaction medium, a PTC (phase transfer catalyst) field, under MWI gave the desired compound trazodone 454 (Scheme 63, Method I).
An alternative but similar process also gave rise to the production of trazodone 454. Reaction of 1,2,4-triazolo[4,3-a]pyridin-3(2H)-one 449 with chloropropyl-2-chloroarylpiperazine 453 gave trazodone 454, directly (Scheme 63, Method II). Moreover, these conditions can be also performed via “one-pot” fashion which has its own merit including, that isolation and purification of intermediates is non-required (Scheme 63, Method III).482
Doxazosin 460, first was patented in 1977 and approved for being prescribed to treat symptoms of an enlarged prostate and high blood pressure in 1988,483 and came to market a trade name of Cardura. Doxazosin 460 also showed a positive influence on coronary heart disease by reducing lipids.484,485 Doxazosin (doxazosin mesylate) 460 was synthesized in three steps starting from catechol 455 which was treated with 2,3-dibromopropionate in the presence of K2CO3 in acetone to afford ethyl 2,3-dihydro-1,4-benzodioxin-2-carboxylate 457. The latter was further refluxed with piperazine to give 1-(2,3-dihydro-1,4-benzodioxine-2-carbonyl) piperazine 458. The last was then condensed with 4-amino-2-chloro-6,7-dimethoxyquinazoline 459 to attain doxazosin mesylate 460. As depicted in Scheme 64, in the three steps synthesis of doxazosin mesylate 460 apparently five compounds are involved which are the stating materials and precursors, however, worthy to notice that a side product, a bis-amide (impurity-V), which is generated during the second step will be present as impurities in doxazosin mesylate 460.486
Aripiprazole, sold under the brand name Abilify among others, is an atypical antipsychotic. Aripiprazole 464 actually is an achiral quinolinone derivative, 7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one.487 These physicochemical assets fulfill the Lipinski's rule of five and give it with bioavailability, such as protein binding, and an acceptable metabolic profile.488 The first synthetic route and reporting its antipsychotic activity was revealed by Oshiro and co-workers in 1991,489 in 1998, Otsuka researchers designated a similar synthetic route for its free base, but under somewhat different conditions (Scheme 65).490,491 The two step synthesis started with the alkylation reaction of 7-hydroxy-3,4-dihydro-2(1H)-quinolinone 461 by reaction with 1,4-dibromobutane 450b using K2CO3 in DMF at 60 °C to afford 7-(4-bromobutoxy)-3,4-dihydro-2(1H)-quinolinone 462. The latter then treated with sodium iodide in MeCN under reflux and 1-(2,3-dichlorophenyl)piperazine,492 and Et3N were added to the reaction mixture, and refluxed in the same vessel to give the free base of aripiprazole as a white powder. This powdery substance can be dissolved in EtOH, treated with different acids to give the various corresponding salt. Other compounds, for example OPC-4392, aripiprazole's precursor, were synthesized following similar procedures by taking (4-bromobutoxy)-2(1H)-quinolinone (or a structural analog) and reacted with different phenylpiperizines.489,490 It should be mentioned that this protocol has since been optimized.492 Aripiprazole can also be produced by different simpler approaches.493–496
Finasteride 474, was patented in 1984 and approved in 1992 for being used as medicine to treat an enlarged prostate or hair loss in men.497–499 Finasteride came to market under the brand name Proscar. The synthesis of finasteride was patented and then published along with the chemistry of 4-azasteroids.500,501 Finasteride 474 was synthesized via multi steps pathway as depicted in Scheme 66. The synthesis commenced with 3-oxo-4-androstene-17β-carboxylic acid 465 which upon oxidative cleavage in a mixture of t-butyl alcohol and aqueous sodium carbonate with sodium periodate and potassium permanganate provided the corresponding diacid 466. The latter was subjected to ring closure with t-butyl amine in cold ethylene glycol and liquid NH3 and the solution was then gradually heated to 180 °C, in the same vessel generating the intermediate 3-oxo-4-aza-5-androstene-17β-carboxylic acid 467. The last was hydrogenated over Pt as catalyst to provide 4-azasteroid 468. The resultant acid was mixed with dicyclohexylcarbodiimide and N-hydroxybenzotriazole in dichloromethane and t-butyl amine to provide saturated azasteroid 472 that upon oxidation with benzeneseleninic anhydride in chlorobenzene provided the desired target finasteride 474 (Scheme 66).502
Ciprofloxacin 480 is a multipurpose antibiotic used for the treatment of a wide range of bacterial infections. It is extensively used to treat austere infections of the urinary, respiratory, and gastrointestinal tracts.503 It was patented in 1980 and approved in 1987.504 Ciprofloxacin is chemically (1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid) 480, the interesting feature of ciprofloxacin is to contain a quinolone ring bearing fluorine atom at the C-6 position of it bicyclic rings.505 Ciprofloxacin 480 was originally synthesized by Klaus Grohe (worked for Bayer)506 thus is also sold under the brand name of ciprofloxacin Byer. Its synthesis started with the reaction of 2,4,5-trifluoro benzoyl chloride 475 and amino acrylate 476 in the presence of TEA in chloroform to afford the corresponding condensed product 477. The latter was then reacted with cyclopropylamine to afford compound 478 which is subsequently cyclized in the presence of K2CO3 in DMSO to afford the corresponding fluroquinolinone 479. At last the latter was reacted with piperazine in the presence of HCl to afford ciprofloxacin hydrocgloride 480 (Scheme 67).507
Promethazine 483 is categorized in the list of first-generation antihistamine. It is prescribed for the treatment of allergies, sleeplessness, and nausea. Promethazine was first synthesized during 1940s by researchers of Rhône-Poulenc laboratories508 and approved for being used as medication in 1951. Promethazine, which chemically is 10-(2-dimethylaminopropyl)phenothiazine 483, was synthesized by alkylating phenothiazine 481 using 1-dimethylamino-2-propylchloride 482 (Scheme 68).509,510
A chemical compound, so-called 8-[4-[4-(2-pyrimidyl)-1-piperazinyl]butyl]-8-azaspiro[4,5]decan-7,9-dione was first synthesized in 1968 and after standard clinical trials, approved being used as medication in 1986 to treat anxiety disorders, particularly generalized anxiety disorder. Buspirone 489, was came to market under the trade name Buspar. Buspirone 489 is an extremely specific drug that could possibly represent a new chemical class of anxiolytics-azaspirones but has not been found to be effective in treating psychosis.255 Buspirone, 8-[4-[4-(2-pyrimidyl)-1-piperazinyl]butyl]-8-azaspiro[4,5]decan-7,9-dione 489, was synthesized started with 1-(2-pyrimidyl)piperazine 484 which reacted with 4-chlorobutyronitrile 485, to afford 4-(2-pyrimidyl)-1-(3-cyanopropyl)piperazine 486. The latter was hydrogenated in the presence of RANEY® to give, 1-(2-pyrimidyl)-4-(4-aminobutyl)piperazine 487 which upon reaction with 8-oxaspiro[4,5]decan-7,9-dione 488 afforded the desired compound, buspirone 489 (Scheme 69).255
Methotrexate 502, known as amethopterin, is a medication used as a component of chemotherapy and immune system suppressant. Methotrexate was first synthesized in 1947, and initially was used to treat cancer.511 Methotrexate 502, chemically is, N-[p-[[(2,4-diamino-6-piperidinyl)methyl]methylamino]-benzoyl]-L-(±)-glutamic acid 502. It was produced by a pathway as depicted in Scheme 70. Its multistep synthesis started with the reaction of 4-nitrobenzoyl chloride 490 with L-glutamic acid 491, to afford N-(4-nitrobenzoyl)glutamic acid 492. The nitro group of compound 490 was hydrogenated to an amino group in the presence of RANEY® that afforded N-(4-aminobenzoyl)glutamic acid 493. The latter was subjected to reductive methylation using formaldehyde and hydrogen that gave N-(4-methylaminobenzoyl)glutamic acid 494.512–515 On the other hand, 2,4,6-triaminopyrimidine 497 was prepared readily by treating malonic acid dinitrile with guanidine. Compound 497 was nitrosylated by anhydrous nitrous acid to afford 2,4,6-triamino-5-nitrosopyrimidine 498, which was subsequently reduced using NaBH4 to afford 2,4,5,6-tetraaminopyrimidine 499. Upon treating of the latter with 1,2-dibromopropionic aldehyde, the product of attaching bromine to acrolein, 2-amino-4-hydroxy-6-bromomethyl-pteridine 501 was obtained. In final step, the nitrogen atom of N-(4-methylaminbenzoyl)glutamic acid 494 was alkylated with the already prepared, bromide 501 to afford the desired target methotrexate 502 (Scheme 70).516–523
A notorious morphine 522 as a legal medication is actually a strong pain killer.524 Morphine was initially isolated from the unripe seed pods of opium poppy, Papaver somniferum.525–527 Sertürner, who first isolated this compound, originally named it morphium.528,529 The isolation morphine as a naturally occurring compound is believed to be the first classical isolation of an active ingredient from a plant.530 A number of structurally related alkaloids, including codeine, thebaine, and codeinone, have also been isolated from the same plant.531 Morphine, chemically is 4,5-epoxy-17-methymorphin-7-ene-3,6-diol 522. One of the suggested, delicate, multi-phase approaches to the morphine synthesis is depicted in Scheme 71. The suggested pathway, started morphine 522 is synthesized from 2,6-dioxynaphthelene 503 that is reacted with benzoyl chloride to give monobenzoate 504, which upon further treatment with nitrous acid is transformed into 1-nitroso compound 505. Then, the latter was hydrogenated over a Pd catalyst and the resultant was subjected to further soft oxidation by iron trichloride to afford 6-benzoyloxy-1,2-naphthoquinone 506. The latter was reduced to 6-benzoyloxy-1,2,-naphthohydroquinone that is methylated using dimethylsulfate as methylating agent to afford 5,6-dimethoxy-2-benzoate 507. The latter next underwent alkaline hydrolysis to give 5,6-dimethoxy-2-naphthol 508. The last was subjected to the same consecutive steps of synthesis, involving nitrozation, reduction and oxidation (using the same reagents, as above), 5,6-dimethoxy-1,2-napthoquinone 510 obtained. The latter was then condensed with ethyl cyanoacetate through Knoevenagel reaction, using potassium ferrocyanide, for the oxidation of the condensation product, gave rise to the formation of product 512 which upon hydrolysis and further decarboxylated gave 5,6-dimethoxy-4-cyanomethyl-1,2-naphthoquinone 513. The latter was subjected to 4 + 2 cycloaddition reaction with 1,3-butadiene to afford a modest yield of 3,4-dimethoxy-9,10-dioxy-13-cyanomethyl-5,8,9,10,13,14-hexahy-drophenanthrene 514. The latter was then hydrogenated, in the presence of a copper chromite catalyst resulted in the formation of ketolactam 515. The last was reduced using lithium aluminum hydride, resulting in the reduction of the both carbonyl groups and amide, followed by methylation of the secondary nitrogen atom using a mixture of formaldehyde and formic acid gave racemic methyl ester β-Δ6-dihydrodesoxycodeine 516. Then, the resulting product was treated with L-(+)-dibenzoyltartaric acid afforded the (+)-methyl ester of β-Δ6-dihydrodesoxycodeine. This submitted to hydration using hot, dilute sulfuric acid, to afford the methyl ester of β-dihydrothebainol 517. The latter was treated vigorously with KOH in diethyleneglycol in which partial demethylation to β-dihydrothe-bainol took place followed by the oxidation of which in potassium tert-butylate/benzophenone system afforded β-dihydrothebainone 518. The latter was subjected to further bromination using 3 mol of bromine in HAOc gave (−)-1-bromocodeinone 520 that is isolated in the form of 2,4-dinitrophenylhyrazone. Apparently, in this step a double bond between both C7–C8 and an oxide bridge between C4–C5 concurrently is formed. In addition, an epimerization occurs at C14, i.e. the isomorphinan system isomerizes into a morphinane system. Subsequently (−)-1-bromocodeinone 520 was reduced by lithium aluminum hydride (LiAlH4) afforded codeine 521 that upon demethylation by pyridine hydrochloride produced the desired morphine 522 (Scheme 71).532,533
2.3. Seven-membered heterocycles
Diazepam 526, was synthesized and patented in 1959534,535 and marketed and well-known as Valium. It is classified in the benzodiazepine family that stereotypically provides a calming effect.534 It is frequently prescribed for the treatment of wide a range of conditions, involving anxiety, muscle spasms and trouble sleeping.534 Structurally, diazepam, is actually 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one 526. As a matter of fact, it is the most simple of all among the biologically potent derivatives of 1,4-benzodiazepin-2-ones. Diazepam can be synthesized stating from 2-amino-5-chlorobenzophenone via different approaches. Couple of ways involve of the direct cyclocondensation of 2-amino-5-chlorobenzophenone or 2-methylamino-5-chlorobenzophenone upon treatment with the ethyl ester of glycine hydrochloride to afford 7-chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2-one 525. The amide nitrogen atom of 525 is methylated using dimethylsulfate results in the formation of diazepam 526 (Scheme 72).In the second approach, at first and before the cyclocondensation reaction, the nitrogen atom is methylated. Thus at first 2-amino-5-chlorobenzo-phenone is tosylated using p-toluenesulfonylchloride and the resultant tosylate 527 is converted to its N-sodium salt that is then methylated by dimethylsulfate to provide 2-N-tosyl-N-methyl-5-chlorobenzophenone 528. The latter is then hydrolyzed in an acidic medium, affording 2-methylamino-5-chlorobenzophenone 529 that is subjected to cyclocondensation via reaction with ethyl ester of glycine hydrochloride to afford the desired diazepam 526 (Scheme 73).536–540
Lorazepam 536, is also a benzodiazepine medicine. It is prescribed to treat anxiety disorders, trouble sleeping, active seizures including status epilepticus, alcohol withdrawal syndrome, and chemotherapy-induced nausea and vomiting. Lorazepam was first patented in 1963 and approved for being used as medication in 1977,541 sold under the brand name Ativan among others. Lorazepam, chemically is 7-chloro-4-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benodiazepin-2-one 536. It is prepared in six steps started with 2-amino-2′,5-dichlorobenzophenone 530 which reacted with hydroxylamine to afford 531. The last was then reacted with chloracetyl chloride to afford 6-chloro-2-chlormethyl-4-(2′-chlorophenyl)quinazolin-3-oxide 532, upon heterocyclizationto. The latter upon reaction with methylamine, as in the case of chlordiazepoxide, resulted in rearrangement and a ring expansion, providing 7-chloro-2-methylamino-5-(2′-chlorphenyl)-3H-1,4-benzodiazepin-4-oxide 533. The last underwent acetylation at the secondary nitrogen atom, using Ac2O followed by hydrolysis in the presence of HCl gave 7-chloro-5-(2′-chlorophenyl)-1,2-dihydro-3H-1,4-benzodiazepin-2-on-4-oxide 534. Treatment of the latter with Ac2O resulted in a Polonovski type rearrangement, affording a 3-acetoxylated benzodiazepine, 7-chloro-1,3-dihydro-3-acetoxy-5-(2′-chlorphenyl)-2H-benzodiazepin-2-one 535, which upon hydrolysis produced the desired product lorazepam 536 (Scheme 74).542–546
Clonazepam 542 was synthesized and patented in 1960 but approved by FDA in 1975.547 It is also sold under the trade name, Klonopin among others. It is a medication to prevent and treat seizures, panic disorder, and the movement disorder known as akathisia and as recreational drug. Clonazepam,548 which chemically is 5-(2-chlorphenyl)-1,3-dihydro-7-nitro-2H-1,4-benzodi-azepine-2-one 542 was produced in five steps starting from 2-chloro-2′nitrobenzophenon that was hydrogenated in the presence of RANEY® to afford 2-chloro-2-aminobenzophenon 537. The amino group of the latter was amidated using 2-bromoacetyl bromide to afford the bromacetamide 539 which was next converted into aminoacetamide 540 through the reaction with ammonia. Heating of the latter in pyridine as a basic solvent with pyridine, resulted in intramolecular cyclization to provide 5-(2-chlorophenyl)-2,3-dihydro-1H-1,4-benzodiazepine-2-one 541. The nitration of the latter in mild reaction conditions (KNO3 in H2SO4) led to the production of clonazepam 542 (Scheme 75).549–554
Temazepam 546, is accessible as a generic medication555 and was patented in 1962 whereas approved for being prescribed in 1969.556 It came to market under the brand names Restoril among others. It is a medication used to treat trouble sleeping and is an intermediate acting benzodiazepine and hypnotic.557 Temazepam, chemically is 7-chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one 546. It is produced in three steps from one of the intermediates in oxazepam synthesis, 7-chloro-5-phenyl-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-on-4-oxide 543. The latter was methylated at the nitrogen of the amide group in the first position of the benzodiazepine ring by dimethylsulfate as methylating agent that afforded 1-methyl-7-chloro-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-on-4-oxide 544. The latter was then underwent acetylation using acetic anhydride, affording 1-methyl-3-acetoxy-7-chloro-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one 545. This transformation is believed to proceed via Polonovski reaction. The acetyl group in the resulted compound 545 was removed via alkaline hydrolysis (NaOH) resulted in the formation of the desired temazepam 546 (Scheme 76).558–563
Olanzapine 552, which came to market under the brand name Zyprexa, is a typical antipsychotic. It was commercialized under the brand name Seroquel among others. It is used for the treatment of schizophrenia and bipolar disorder. Olanzapine was patented in 1971 and approved by FDA in 1996 for being prescribed.564 It shows a relatively wide range receptor binding profiles. It exhibits moderate affinity to α1- und α2-adrenergic receptors and slight affinity to muscarinergic M1 receptors.565 The multistep synthesis of quetiapine 555 was started from the reaction of o-chloronitrobenzene 547 with thiophenol 548 in the presence of NaOH in EtOH to afford the corresponding o-nitrodiphenyl sulfide 549. The latter was reduced by hydrogenation catalyzed by RANEY® to give the corresponding amine 550. The latter was reacted with phosgene to provide isocyanate 551, which upon heating in the presence of AlCl3 in o-dichlorobenzene as solvent to give a key intermediate dibenzo[b,f][1,4]thiazepine-11(10H)-one 552.566 The latter upon heating in POCl3, and dimethylaniline afforded the intermediate iminochloride 553, which was reacted with 2-(2-(piperazin-1-yl)ethoxy)ethanol 554 to provide the final desired product, quetiapine 555 (Scheme 77).567,568
Benazepril 565, is accessible as a generic medication prescribed for the treatment, hypertension, chronic renal failures, and diabetic kidney disease. It was patented in 1981 and came into market in 1990, sold under the brand name Lotensin among others.569 In 2003 Chang and coworkers570 demonstrated an enantioselective synthesis of a benazepril intermediate through a bioreductive reaction using baker's yeast, but the enantioselectivity was not ideal for a medication (80% ee). Benazepril 565, was prepared via a multistep involving the treatment of 2,3,4,5-tetrahydro-1H-(1) benzazepin-2-one 556 with PCl5 in hot xylene afforded 3,3-dichloro-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one 557, which is initially treated with NaOAC and hydrogenated over Pd/C in CH3COOH providing 3-chloro-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one 558. The latter was reacted with NaN3 in DMSO gave 3-azido-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one 559, which is then condensed with benzyl bromoacetate 560 in the presence of NaH in DMF affording 3-azido-1-(benzyloxycarbonylmethyl)-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one 561. The latter was treated with RANEY® in EtOH/water to give 3-amino-1-(benzyloxycarbonylmethyl)-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one 562, which was debenzylated upon hydrogenation with H2 over Pd/C in EtOH to furnish 3-amino-1-(carboxymethyl)-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one 563. At last, compound 563 was condensed with ethyl 3-benzylpyruvate 564 in the presence of sodium cyanoborohydride in MeOH/AcOH to give the desired medication, benazepril 565 (Scheme 78).571
Olanzapine 571, which came to market under the brand name Zyprexa among other trade names, is a typical antipsychotic. It is used for the treatment of schizophrenia and bipolar disorder. Olanzapine was patented in 1971 and approved by FDA in 1996 for being prescribed.572 The synthetic pathway of olanzapine 571 is illustrated in Scheme 79. 2-Amino-5-methylthiophene-3-carbonitrile 567 was reacted with o-chloronitrobenzene 566 to provide 2-phenylamino-thiophene-3-carbonitrile derivative 568. The nitro group of the latter was reduced to the amino group using SnCl2 which is concurrently cyclized to give 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine 569. The latter was reacted with N-methylpiperazine 570 to afford the desired amidine-olanzapine 571.573,574
3. Conclusion
Due to their inherent resourcefulness and versatility as well as exceptional physicochemical potencies, heterocyclic systems have dignified themselves as factual cornerstones of medicinal chemistry. The main stream of heterocyclic systems and archetypally common heterocycle scaffolds are present in most natural products and medications which are currently prescribed thus, market purchasable. Among them, nitrogen heterocycles are imposing since by quick glance at FDA databases their structural significance is unveiled. In the FDA list of approved drugs approximately 60% are nitrogen-based heterocycles, thus these heterocyclic systems are important in the drug design, drug discovery and engineering of medications. In this review, we tried to underscore the top and best-selling prescribed drugs, containing N-heterocyclic systems. Thus, in this review, we classified the N-heterocyle medications in accordance with their sizes. In addition, we tried to give the readers some elementary information about their biological and clinical applications. Furthermore, the selected synthetic pathways towards the production of those drugs as published in both chemical literatures and patents, were underlined. We wish this review attracts the attention of organic synthetic chemists, as well as biologists, pharmacists and general practitioners and specialists.Conflicts of interest
There is no conflict of interest.Acknowledgements
The authors appreciate partial financial supports from Alzahra University. M. M. H. is also thankful to Iran National Science Foundation (INSF) for the individual given grant.References
- P. Arora, V. Arora, H. Lamba and D. Wadhwa, Int. J. Pharma Sci. Res, 2012, 3, 2947–2954 Search PubMed.
- P. M. T. Ferreira and L. S. Hernani, Tetrahedron Lett., 2002, 43, 4491–4493 Search PubMed.
- G. Daidone, B. Maggio and D. Schillari, Pharmazie, 1990, 45, 441–442 Search PubMed.
- A. M. Almerico, P. Diana, P. Barraja, G. Dattolo, F. Mingoia, A. G. Loi, F. Scintu, C. Milia, I. Puddu and P. La Colla, Il Farmaco, 1998, 53, 33–40 Search PubMed.
- E. J. Schaefer, J. R. McNamara, T. Tayler, J. A. Daly, L. Gleason, L. J. Seman, A. Ferrari and J. Rubenstein, Am. J. Cardiol., 2004, 93, 31–39 Search PubMed.
- E. Prommer, Role of codeine in palliative care, J. Opioid Manag., 2010, 7, 401–406 Search PubMed.
- J. K. Liu and W. T. Couldwell, Neurocrit. Care, 2005, 2, 124–132 Search PubMed.
- N. Neamati, A. Mazumder, S. Sunder, J. M. Owen, M. Tandon, J. W. Lown and Y. Pommier, Mol. Pharmacol., 1998, 54, 280–290 Search PubMed.
- D. Deidda, G. Lampis, R. Fioravanti, M. Biava, G. C. Porretta, S. Zanetti and R. Pompei, Antimicrob. Agents Chemother., 1998, 42, 3035–3037 Search PubMed.
- C. Kikuchi, H. Nagaso, T. Hiranuma and M. Koyama, J. Med. Chem., 1999, 42, 533–535 Search PubMed.
- N. K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C. H. Kim, A. K. Verma and E. H. Choi, Biomedical importance of indoles, Molecules, 2013, 18, 6620–6662 Search PubMed.
- M. S. Saini, A. Kumar, J. Dwivedi and R. Singh, Int. J. Pharma Sci. Res, 2013, 4, 66–77 Search PubMed.
- M. Al-Ghorbani, B. Begum, M. S. Zabiulla and S. A. Khanum, J. Chem. Pharm. Res., 2015, 7, 281–301 Search PubMed.
- (a) G. Keglevich, Green synthetic approaches for biologically relevant heterocycles, Elsevier, 2015, p. 559 Search PubMed; (b) D. Yang, B. An, W. Wei, L. Tian, B. Huang and H. Wang, ACS Comb. Sci., 2015, 17, 113–119 Search PubMed; (c) M. Srivastava, J. Singh, S. B. Singh, K. Tiwari, V. K. Pathak and J. Singh, Green Chem., 2012, 14, 901–905 Search PubMed; (d) M. K. Khan, D. Wang and M. G. Moloney, Synthesis, 2020, 52, 1602–1616 Search PubMed.
- Z. Hosseinzadeh, A. Ramazani and N. Razzaghi-Asl, Curr. Org. Chem., 2018, 22, 2256–2279 Search PubMed.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 Search PubMed.
- M. García-Valverde and T. Torroba, J. Invest. Dermatol. Symp. Proc., 2001, 6, 188–196 Search PubMed.
- (a) P. Martins, J. Jesus, S. Santos, L. R. Raposo, C. Roma-Rodrigues, P. V. Baptista and A. R. Fernandes, Molecules, 2015, 20, 16852–16891 Search PubMed; (b) O. O. Ajani, O. Y. Audu, D. V. Aderohunmu, F. E. Owolabi and O. A. Olomieja, Am. J. Drug Discovery Dev., 2017, 7, 1–24 Search PubMed.
- Y. Özkay, İ. Işıkdağ, Z. İncesu and G. Akalın, Eur. J. Med. Chem., 2010, 45, 3320–3328 Search PubMed.
- M. Baumann, I. R. Baxendale, S. V. Ley and N. Nikbin, Beilstein J. Org. Chem., 2011, 7, 442–495 Search PubMed.
- M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265–2319 Search PubMed.
- S.-L. Zhu, Y. Wu, C.-J. Liu, C.-Y. Wei, J.-C. Tao and H.-M. Liu, Eur. J. Med. Chem., 2013, 65, 70–82 Search PubMed.
- S. Baroniya, Z. Anwer, P. K. Sharma, R. Dudhe and N. Kumar, Pharm. Sin., 2010, 1, 172–182 Search PubMed.
- M. M. Heravi and V. Zadsirjan, Recent advances in the synthesis of benzo[b]furans, Adv. Heterocycl. Chem., 2015, 117, 261–376 Search PubMed.
- M. M. Heravi and B. Talaei, Diketene as privileged synthon in the syntheses of heterocycles Part 1: Four- and five-membered ring heterocycles, Adv. Heterocycl. Chem., 2017, 122, 43–114 Search PubMed.
- M. M. Heravi and B. Talaei, Ketenes as privileged synthons in the synthesis of heterocyclic compounds Part 3: Six-membered heterocyclesin, Adv. Heterocycl. Chem., 2016, 118, 195–291 Search PubMed.
- M. M. Heravi and V. Vavsari Fathi, Recent advances in application of amino acids: key building blocks in design and syntheses of heterocyclic compounds, Adv. Heterocycl. Chem., 2015, 114, 77–145 Search PubMed.
- M. M. Heravi and B. Talaei, Ketenes as privileged synthons in the syntheses of heterocyclic compounds Part 2: five-membered heterocycles, Adv. Heterocycl. Chem., 2015, 114, 147–225 Search PubMed.
- M. M. Heravi, S. Khaghaninejad and M. Mostofi, Pechmann reaction in the synthesis of coumarin derivatives, Adv. Heterocycl. Chem., 2014, 112, 1–50 Search PubMed.
- M. M. Heravi, S. Khaghaninejad and N. Nazari, Bischler-Napieralski reaction in the syntheses of isoquinolines, Adv. Heterocycl. Chem., 2014, 112, 183–234 Search PubMed.
- M. M. Heravi and T. Alishiri, Dimethyl acetylenedicarboxylate as a building block in heterocyclic synthesis, Adv. Heterocycl. Chem., 2014, 113, 1–66 Search PubMed.
- M. M. Heravi and B. Talaei, Ketenes as Privileged Synthons in the Syntheses of Heterocyclic Compounds. Part 1: Three-and Four-Membered Heterocycles, Adv. Heterocycl. Chem., 2014, 113, 143–244 Search PubMed.
- S. Khaghaninejad and M. M. Heravi, Paal-Knorr reaction in the synthesis of heterocyclic compounds, Adv. Heterocycl. Chem., 2014, 111, 95–146 Search PubMed.
- M. M. Heravi, V. Zadsirjan and M. Malmir, Molecules, 2018, 23, 943 Search PubMed.
- M. M. Heravi, T. B. Lashaki, B. Fattahi and V. Zadsirjan, RSC Adv., 2018, 8, 6634–6659 Search PubMed.
- M. M. Heravi, V. Zadsirjan and B. Farajpour, RSC Adv., 2016, 6, 30498–30551 Search PubMed.
- M. M. Heravi, M. Daraie and V. Zadsirjan, Mol. Diversity, 2015, 19, 577–623 Search PubMed.
- A. T. Kal-Koshvandi and M. M. Heravi, Chem. Rec., 2019, 19, 550–600 Search PubMed.
- M. M. Heravi and L. Mohammadkhani, J. Organomet. Chem., 2018, 869, 106–200 Search PubMed.
- A. T. Kal Koshvandi, M. M. Heravi and T. Momeni, Appl. Organomet. Chem., 2018, 32, e4210 Search PubMed.
- M. M. Heravi, S. Rohani, V. Zadsirjan and N. Zahedi, RSC Adv., 2017, 7, 52852–52887 Search PubMed.
- M. M. Heravi, B. T. Lashaki and N. Poorahmad, Tetrahedron: Asymmetry, 2015, 26, 405–495 Search PubMed.
- I. D. Vellar and T. B. Hugh, Howard Florey, Med. J. Aust., 2002, 177, 52–53 Search PubMed.
- R. Cruickshank, Sir Alexander Fleming, F.R.S., Nature, 1955, 175, 663 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 490 Search PubMed.
- C. W. James and C. Gurk-Turner, Cross-reactivity of beta-lactam antibiotics, Baylor University Medical Center Proceedings, 2001, 14, 106–107 Search PubMed.
- Q. Al-Abdallah, A. A. Brakhage, A. Gehrke, H. Plattner, P. Sprote and A. Tuncher, Adv. Biochem. Eng./Biotechnol., 2004, 88, 45–90 Search PubMed.
- A. A. Brakhage, Microbiol. Mol. Biol. Rev., 1998, 62, 547–585 Search PubMed.
- C. J. Schofield, J. E. Baldwin, M. F. Byford, I. Clifton, J. Hajdu, C. Hensgens and P. Roach, Curr. Opin. Struct. Biol., 1997, 7, 857–864 Search PubMed.
- J. F. Martín, S. Gutiérrez, F. J. Fernández, J. Velasco, F. Fierro, A. T. Marcos and K. Kosalkova, Expression of genes and processing of enzymes for the biosynthesis of penicillins and cephalosporins, Antonie van Leeuwenhoek, 1994, 65, 227–243 Search PubMed.
- J. C. Sheehan, Production of penicillins, US Pat., No. 3159617, 1 Dec. 1964.
- J. Roy, An introduction to pharmaceutical sciences production, chemistry, techniques and technology, Woodhead Pub, Cambridge, 2012, p. 239 Search PubMed.
- A. Bruggink, E. C. Roos and E. de Vroom, Org. Process Res. Dev., 1998, 2, 128–133 Search PubMed.
- M. A. Wegman, M. H. A. Jassen, F. van Rantwijk and R. A. Sheldon, Adv. Synth. Catal., 2001, 343, 559–576 Search PubMed.
- L. L. Brunton, 53, Penicillins, Cephalosporins, and other β-lactam antibiotics. goodman & gilman's pharmacological basis of therapeutics, McGraw-Hill, New York, 12th edn, 2011 Search PubMed.
- Neonatal formulary 5 drug use in pregnancy and the first year of life, ed. E. Hey, Blackwell, 5th edn, 2007, p. 67 Search PubMed.
- B. M. Robert and G. J. Billy, Penicillin conversion via sulfoxide, US Pat., 3275626, 1962.
- B. M. Robert and G. J. Billy, Certain 3-methyl-cephalosporin compounds, US Pat., 3507861, 2004.
- E. Ravina, The evolution of drug discovery: from traditional medicines to modern drugs, Wiley-VCH, Weinheim, 1. Aufl. edn, 2011, p. 267 Search PubMed.
- G. Hanlon and N. A. Hodges, Essential microbiology for pharmacy and pharmaceutical science, Wiley, Hoboken, 2012, p. 140 Search PubMed.
- J. L. Davis, J. H. Salmon and M. G. Papich, J. Vet. Pharmacol. Ther., 2005, 28, 425–431 Search PubMed.
- K. J. Tack, C. H. Keyserling, J. McCarty and J. A. Hedrick, Antimicrob. Agents Chemother., 1997, 41, 739–742 Search PubMed.
- M. R. Jacobs, R. N. Jones and P. A. Giordano, Diagn. Microbiol. Infect. Dis., 2007, 57, S55–S65 Search PubMed.
- F. A. Disney, H. Dillon, J. L. Blumer, B. A. Du dding, S. E. McLinn, D. B. Nelson and S. M. Selbst, Am. J. Dis. Child, 1992, vol. 146, pp. 1324–1327 Search PubMed.
- C. M. Perry and R. N. Brogden, Drugs, 1996, 52, 125–158 Search PubMed.
- F. K. Kees, U. Lukassek, K. G. Naber and H. Grobecker, Arzneim.-Forsch., 1991, 41, 843–846 Search PubMed.
- M. Ehrnebo, S. O. Nilsson and L. O. Boréus, J. Pharmacokinet. Biopharm., 1979, 7, 429–451 Search PubMed.
- K. H. Jones, Br. Med. J., 1977, 2, 232–233 Search PubMed.
- R. S. Vardanyan and V. J. Hruby, Chapter 32 Antibiotics, in Synthesis of Essential Drugs, 2006, p. 425 Search PubMed.
- C. W. Ryan, R. L. Simon and E. M. Van Heyningen, Chemistry of cephalosporin antibiotics. III. Deacetoxycephalosporins. Synthesis of cephalexin and some analogs, J. Med. Chem., 1969, 12, 310–313 Search PubMed.
- Eli Lilley & Co., Fr. Pat., 1524225, 1967.
- Eli Lilley & Co., Br. Pat., 1174335, 1967.
- Pharmaceutical Press, British National Formulary: BNF 76, Pharmaceutical Press, 76th edn, 2018, p. 196 Search PubMed.
- S. B. Rosenblum, Cholesterol absorption inhibitor ezetimibe (ZETIA â), in The Art of Drug Synthesis, John Wiley & Sons Inc Hoboken, New Jersey, 2007, pp. 183–196 Search PubMed.
- H. E. Bays, P. B. Moore, M. A. Drehobl, S. Rosenblatt, P. D. Toth, C. A. Dujovne, R. H. Knopp, L. J. Lipka, A. P. LeBeaut, B. Yang, L. E. Mellars, C. Cuffie-Jackson and E. P. Veltri, Clin. Ther., 2001, 23, 1209–1230 Search PubMed.
- R. H. Knopp, H. Gitter, T. Truitt, H. Bays, C. V. Manion, L. J. Lipka, A. P. LeBeaut, R. Suresh, B. Yang and E. P. Veltri, Eur. Heart J., 2003, 24, 729–741 Search PubMed.
- T. K. Thiruvengadam, A. R. Sudhakar and G. Wu, in Process Chemistry in the Pharmaceutical Industry, ed. K. G. Gadamasetti, Marcel Dekker, New York, 1999, pp. 221–242 Search PubMed.
- S. B. Rosenblum, T. Huynh, A. Afonso, H. R. Davis, N. Yumibe, J. W. Clader and D. A. Burnett, J. Med. Chem., 1998, 41, 973–980 Search PubMed.
- X. Fu, T. L. McAllister, T. K. Thiruvengadam, C.-H. Tann and D. Su, Tetrahedron Lett., 2003, 44, 801–804 Search PubMed.
- C. C. Sanchez, R. A. Dobarro, C. J. Bosch and D. D. Garcia, Patent WO2012004382A1, 2012.
- C. H. V. A. Sasikala, P. Reddy Padi, V. Sunkara, P. Ramayya, P. K. Dubey, V. Bhaskar Rao Uppala and C. Praveen, Org. Process Res. Dev., 2009, 13, 907–910 Search PubMed.
- M. Michalak, M. Stodulski, S. Stecko, A. Mames, I. Panfil, M. Soluch, B. Furman and M. Chmielewski, J. Org. Chem., 2011, 76, 6931–6936 Search PubMed.
- B. B. Shankar, M. P. Kirkup, S. W. McCombie, J. W. Clader and A. K. Ganguly, Tetrahedron Lett., 1996, 37, 4095–4098 Search PubMed.
- M. Sova, J. Mravljak, A. Kovač, S. Pečar, Z. Časar and S. Gobec, Synthesis, 2010, 2010, 3433–3438 Search PubMed.
- M. Sniezek, S. Stecko, I. Panfil, B. Furman and M. Chmielewski, J. Org. Chem., 2013, 78, 7048–7057 Search PubMed.
- R. A. V. Raghava, S. Garaga, C. Takshinamoorthy, A. Naidu and R. Dandala, Sci. Pharm., 2016, 84, 269–278 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based drug discovery, John Wiley & Sons, 2006, p. 467 Search PubMed.
- J. Fischer, T. Fodor and L. Dobay, Monatsh. Chem., 1988, 119, 645–647 Search PubMed.
- (a) M. T. Wu, A. W. Douglas, D. L. Ondeyka, L. G. Payne, T. J. Ikeler, H. Joshua and A. A. Patchett, J. Pharm. Sci., 1985, 74, 352–354 Search PubMed; (b) T. Aslan, Process for the production of lisinopril, US20070093664, 2002.
- R. Vardanyan and V. Hruby, Chapter 22 Antihypertensive drugs, Synthesis of essential drugs, Elsevier, 2006, pp. 295–310 Search PubMed.
- W. J. Greenlee, US Pat., 4442030, 1984.
- A. A. Patchett, E. Harris, E. W. Tristram, M. J. Wyvratt, M. T. Wu, D. Taub, E. R. Peterson, T. J. Ikeler, J. Ten Broeke, L. G. Payne and D. L. Ondeyka, Nature, 1980, 288, 280–283 Search PubMed.
- M. J. Wyvratt, E. W. Tristram, T. J. Ikeler, N. S. Lohr, H. Joshua, J. P. Springer, B. H. Arison and A. A. Patchett, J. Org. Chem., 1984, 49, 2816–2819 Search PubMed.
- E. E. Harris, A. A. Patchett, E. W. Tristram and M. J. Wyvratt, Aminoacid derivatives as antihypertensives, U.S. Pat., 4374829, 22 February 1983.
- E. E. Harris, A. A. Patchett, E. W. Tristram and M. J. Wyvratt, Amino acid derivatives as antihypertensives, US Pat., 4472380, 18 September 1984.
- E. E. Harris, A. A. Patchett, E. W. Tristram and M. J. Wyvratt. Eur. Pat. Appl., 12401, 1979.
- G. C. Malakondaiah, V. M. Gurav, L. A. Reddy, K. S. Babu, B. V. Bhaskar, P. P. Reddy, A. Bhattacharya and R. V. Anand, Synth. Commun., 2008, 38, 1737–1744 Search PubMed.
- (a) C. D. Bubbar, D. F. Blackburn, M. P. Wilson and T. W. Wilson, Ann. Pharmacother., 2007, 41, 129–132 Search PubMed; (b) K. L. McCall, D. Craddock and K. Edwards, Pharmacotherapy, 2006, 26, 1297–1306 Search PubMed.
- (a) K. Bankowski, T. Wachal, K. Sidoryk and W. A. Szelejewski, Process for the preparation of 2-[N-f(S)-1-ethoxycarbonyl-3-phenylpropylg-(S)-alanyl]-(1S,3S,5S)-2-azabicyclo-[3.3.0]-octane-3-carboxylic acid, PCT WO108366A1, 2005; (b) P. R. Babu, V. S. Hariharakrishnan, T. V. Srihari, K. H. Prasad, B. Ramesh and G. V. Mallaparaju, A process for industrially viable preparation of (S,S,S)-phenylmethyl-2-azabicyclo-[3.3.0]-octane-3-carboxylate tosylate, PCT WO049568A1, 2005; (c) G. P. Singh, H. M. Godbole, P. R. Mahajan and S. P. Nehate, Alpha-amino acid benzothiolylthioester as intermediates for manu-facturing of ACE inhibitors and process for preparation thereof, PCT WO010028A1, 2005; (d) M.-J. Tien and Y.-L. Liu, Method for producing angiotensin converting enzyme inhibitor, US Pat., 6541635B1, 2003; (e) Z.-X. Wang and C. McPhail, Process for the preparation of ramipril, US Pat., 6407262B1, 2002; (f) F. Hock, J. Scholtholt, H. Urbach, R. Henning, U. Lerch, W.-U. Nickel and W. Rüger, Aminoacid esters, pharmaceuticals containing them and the use thereof in learning disorders, US Pat., 5043346, 1991; (g) V. Teetz, R. Geiger, H. Urbach, R. Becker and B. Schölkens, Cis, endo-2-aza-bicyclo-[3.3.0]-octane-3-carboxylic acids, a process for their preparation, agents containing these compounds and their use, US Pat., 5061722, 1991; (h) E. H. Gold, B. R. Neustadt and E. M. Smith, Angiotensin-converting enzyme inhibitors, US Pat., 4587258, 1986; (i) B. R. Neustadt, E. H. Gold and E. M. Smith, Eur. Pat. Appl., 0050800A1, 1982.
- B. V. Bhaskar, M. S. Reddy, G. C. Malakondaiah, M. S. Reddy and K. Ravinder, Preparation of ramipril and stable pharmaceutical compositions, US Pat., Appl., 11/696372, 2007.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 473 Search PubMed.
- M. Müller, Angew. Chem., Int. Ed., 2005, 44, 362–365 Search PubMed.
- D. E. Butler, C. F. Deering, A. Millar, T. N. Nanninga and B. D. Roth, Improved process for trans-6-[2-(substituted-pyrrol-1-yl)alkyl]pyran-2-one inhibitors of cholesterol synthesis, WO Pat., 89/07598, 1989.
- H. W. Lee, Y. M. Kim, C. L. Yoo, S. K. Kang and S. K. Ahn, Biomol. Ther., 2008, 16, 28–33 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 531 Search PubMed.
- S. J. Hopkins, Drugs Today, 1992, 28, 155 Search PubMed.
- M. D. Dowle and I. H. Coates, Heterocyclic compounds, US Pat., 4816470 issued, March 28, 1989.
- A. W. Oxford, Indole derivative, US Pat., 5037845, issued, August 6, 1991.
- P. P. A. Humphrey, W. Feniuk, M. J. Perren, A. W. Oxford, R. T. Brittain, J. Prous and J. Castaner, Sumatriptan Succinate, Drugs Future, 1989, 14, 35–39 Search PubMed.
- R. C. Kevin, C. S. W. Jacqueline, L. Sandra and D. T. Richard, Org. Lett., 2004, 6, 79–82 Search PubMed.
- B. L. Pete, I. N. Bitter, K. L. N. Harsanyi and L. S. Toke, Heterocycles, 2000, 53, 665–673 Search PubMed.
- P. R. Brodfuehrer, B. C. Chen, T. R. Sattelberg, P. R. Smith, J. P. Reddy, D. R. Stark, S. L. Quinlan, J. G. Reid, J. K. Thottathil and S. Wang, J. Org. Chem., 1997, 62, 9192–9202 Search PubMed.
- C. Bhanja, S. Chakroborty and S. Jena, Asian J. Pharm. Anal. Med. Chem., 2014, 2, 168–175 Search PubMed.
- F. R. Japp and F. Klingemann, Chem. Ber., 1887, 20, 2942–2944 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 448 Search PubMed.
- D. Schnadower, Y. Finkelstein and S. B. Freedman, Curr. Opin. Gastroenterol., 2015, 31, 1–6 Search PubMed.
- S. B. Freedman, S. Ali, M. Oleszczuk, S. Gouin and L. Hartling, Evidence-based Child Health: A Cochrane Review Journal, 2013, 8, 1123–1137 Search PubMed.
- R. J. Milne and R. C. Heel, Drugs, 1991, 41, 574–595 Search PubMed.
- A. Markham and E. M. Sorkin, Drugs, 1993, 45, 931–952 Search PubMed.
- M. I. Wilde and A. Markham, Drugs, 1996, 52, 773–794 Search PubMed.
- D. R. Kohler and B. R. Goldspiel, Ondansetron: a serotonin receptor (5-HT3) antagonist for a nt ineopl astic chemotherapy-induced nausea and vomiting, DICP, 1991, 25, 367–380 Search PubMed.
- C. R. Roberson and C. H. McLeskey, Semin. Anesth., 1996, 15, 41–46 Search PubMed.
- C. R. Culy, N. Bhana and G. L. Plosker, Paediatric drugs, 2001, 3, 441–479 Search PubMed.
- Q. Xu, T. Liu, R. Tian, Q. Li and D. Ma, Front. Chem. China, 2009, 4, 63–68 Search PubMed.
- A. W. Oxford, C. D. Eldred, I. H. Coates, J. A. Bell, D. C. Humber and G. B. Ewan, Process for Preparing Tetrahydrocarbazolones, US Pat., 4739072, April 19, 1988.
- (a) A. K. Saxena, C. J. Padam, N. Anand and R. R. Dua, J. Med. Chem., 1973, 16, 560–564 Search PubMed; (b) A. Madrigal, M. Grande and C. Avendano, J. Org. Chem., 1998, 63, 2724–2727 Search PubMed.
- L. A. Sorbera, L. Martin and P. A. Leeson, J. Drugs Future, 2001, 26, 15–19 Search PubMed.
- A. C.-M. Daugan, Eur. Pat., EP0740668, 1998.
- A. Daugan, P. Grondin, C. Ruault, A.-C. Le Monnier de Gouville, H. Coste, J. Kirilovsky, F. Hyafil and R. Labaudiniere, J. Med. Chem., 2003, 46, 4525–4532 Search PubMed.
- M. W. Orme, J. C. Sawyer and L. M. Schultze, World Pat., WO02/036593, 2002.
- P. J. Dunn, Org. Process Res. Dev., 2005, 9, 88–97 Search PubMed.
- F. Wiedemann, W. Kampe, M. Thiel, G. Sponer and E. Roesch, K. Dietmann, Carbazolyl-4-oxypropanolamine derivatives, DE2815926, 1979.
- B. Anandkumar, R. B. Reddy, L. Gangaiah, G. Madhusudhan and K. Mukkanti, Pharmacol. Chem., 2011, 3, 620–626 Search PubMed.
- B. A. Kumar, K. V. Babu, R. K. Rao, K. Srinivas, G. Madhusudhan and K. Mukkanti, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2012, 51B, 1430–1435 Search PubMed.
- B. A. Kumar, Md. Ashrafuddin, V. Rajesh, S. Parveen and G. Madhusudhan, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2012, 51B, 780–784 Search PubMed.
- S. N. Rao, D. Sitaramaiah, K. Srimannarayana, C. N. Rao, P. S. Rao and K. S. Babu, Synth. Commun., 2011, 41, 85–93 Search PubMed.
- K. C. Naidu, V. K. Kumar, E. Naresh, C. Kameswararao, G. Madhusudhan and K. Mukkanti, Org. Chem.: Indian J., 2010, 6, 171–177 Search PubMed.
- B. A. Kumar, R. Vysabhattar, G. Ramadasu, K. Mukkanti and G. Madhusudhan, Org. Chem.: Indian J., 2010, 6, 70–73 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 470 Search PubMed.
- D. J. Carini, J. J. Duncia and P. C. Wong, Angiotensin II receptor blocking imidazoles, US Pat., 5138069, issued, August 11, 1992.
- A. R. A. Al-Majed, E. Assiri and N. Y. Khalil, Abdel-Aziz, H.A.;. Losartan: comprehensive profile, Profiles Drug Subst., Excipients, Relat. Methodol., 2015, 40, 159–194 Search PubMed.
- M. J. Neil, Curr. Clin. Pharmacol., 2011, 6, 280–287 Search PubMed.
- H. Stähle, Best Pract. Res., Clin. Anaesthesiol., 2000, 14, 237–246 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 550 Search PubMed.
- W. Kobinger, Rev. Physiol. Biochem. Pharmacol., 1978, 81, 39–100 Search PubMed.
- A. Walland, Clonidine, in Pharmacological and Biochemical Properties of Drug Substances, ed. M. E. Goldberg, American Pharmaceutical Association, Washington, DC, 1977, vol. 1, p. 67 Search PubMed.
- P. C. Wong and X. S. Jiang, Thromb. Haemost., 2010, 104, 302–310 Search PubMed.
- W. A. Schumacher, J. S. Bostwick, A. B. Stewart, T. E. Steinbacher, B. M. Xin and P. C. Wong, J. Cardiovasc. Pharmacol., 2010, 55, 609–616 Search PubMed.
- R. C. Becker, J. H. Alexander, L. K. Newby, H. Q. Yang, Y. C. Barrett, P. Mohan, J. Wang, R. A. Harrington and L. C. Wallentin, Thromb. Haemost., 2010, 104, 976–983 Search PubMed.
- A. G. G. Turpie, Arterioscler., Thromb., Vasc. Biol, 2007, 27, 1238–1247 Search PubMed.
- J. C. Zhou, L. M. Oh, P. Ma and H. Y. Li, Synthesis of 4,5-dihydro-pyrazolo[3,4-c]pyrid-2-ones, WO Pat., 2003/049681, June 19, 2003.
- D. J. P. Pinto, M. J. Orwat, S. Koch, K. A. Rossi, R. S. Alexander, A. Smallwood, P. C. Wong, A. R. Rendina, J. M. Luettgen, R. M. Knabb, K. He, B. M. Xin, R. R. Wexler and P. Y. S. Lam, J. Med. Chem., 2007, 50, 5339–5356 Search PubMed.
- T. G. Gant and M. Shahbaz, Pyrazole carboxamide inhibitors of factor Xa, WO Pat., 2010/030983, 18 March 2010.
- J. A. Jiang and Y. Ji, Synth. Commun., 2013, 43, 72–79 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 504 Search PubMed.
- E. de Clercq and H. J. Field, Br. J. Pharmacol., 2006, 147, 1–11 Search PubMed.
- P. I. Rafailidis, M. N. Mavros, A. Kapaskelis and M. E. Falagas, J. Clin. Virol., 2010, 49, 151–157 Search PubMed.
- H. J. Schaeffer, L. Beauchamp, P. Miranda de, G. B. Elion, D. J. Bauer and P. Collins, Nature, 1978, 272, 583–585 Search PubMed.
- T. Q. Hung, N. T. Thuong and T. Van Sung, Vietnam J. Chem., 2009, 47, 523 Search PubMed.
- H. J. Schaeffer, Compositions for treating viral infections and guanine acyclic nucleosides, US4199574, Apr 22, 1980.
- R. Vardanyan and V. Hruby, Antiviral Drugs in Synthesis of Best-Seller Drugs, 2016, ch. 34, p. 687 Search PubMed.
- S. S. Long, L. K. Pickering and C. G. Prober, Principles and practice of pediatric infectious disease, Elsevier Health Sciences, 2012, p. 1502 Search PubMed.
- E. P. Acosta and C. V. Fletcher, Valaciclovir, Ann. Pharmacother., 1997, 31, 185–191 Search PubMed.
- K. R. Beutner, Antiviral Res., 1995, 28, 281–290 Search PubMed.
- M. L. Smiley, A. Murray and P. De Miranda, Adv. Exp. Med. Biol. Antiviral Chemotherapy 4, 1996, 394, 33–39 Search PubMed.
- M. D. Antman and O. S. Gudmundsson, Valaciclovir: a prodrug of acyclovir, Biotechnol.: Pharm. Aspects, 2007, 5, 669–676 Search PubMed.
- S. K. Tyring, D. Baker and W. Snowden, J. Infect. Dis., 2002, 186, S40–S46 Search PubMed.
- C. M. Perry and D. Faulds, Drugs, 1996, 52, 754–772 Search PubMed.
- T. A. Krenitsky and L. M. Beauchamp, Acycloxic esters with valine and isoleucine as herpes virucides, EP308065, 1989.
- L. M. Beauchamp and T. A. Krenitsky, Drugs Future, 1993, 18, 619–628 Search PubMed.
- V. V. N. K. V. Prasada Raju, R. Vedantham, M. D. Khunt, V. T. Mathad, P. K. Dubey and A. K. Chakravarthy, Asian J. Chem., 2010, 22, 4092–4098 Search PubMed.
- M. Y. Etinger, L. M. Yudovich, M. Yuzefovich, G. A. Nisnevich, B. Z. Dolitzki, B. Pertsikov, B. Tishin and D. Blasberger, Synthesis and purification of valaciclovir, WO2003041647, 2003.
- A. A. Al-Badr, Omeprazole, in Profiles of drug substances, excipients and related methodology, 2010, vol. 35, pp. 151–262 Search PubMed.
- A. E. Brandstrom and B. R. Lamm, Processes for the preparation of omeprazole and intermediates therefore, US Pat., 4620008, 28 October 1986.
- C. Slemon and B. Macel, Preparation of omeprazole and lansoprazole, and intermediates useful therein, US Pat., 5470983, November 28, 1995.
- H. Cotton, T. Elebring, M. Larsson, L. Li, H. Sorensen and S. von Unge, Tetrahedron: Asymmetry, 2000, 11, 3819–3825 Search PubMed.
- A. Haessle, Jpn, Kokai Tokkyo Koho JP62–72666, 1983.
- J. E. Baldwin, R. M. Adlington and N. P. Crouch, Organic compound synthesis, US Pat., 6043371, 28 March 2000.
- S. P. Singh, S. M. J. Mukarram, D. G. Kulkami and M. Purohit, Synthetic Procedure for 5-Methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methylthio]-IH-benzimidazole Hydrochloride and its Conversion to Omeprazole, US Pat., 6245913B1, 12 June 2001.
- X. L. Liu, Synthesis of omeprazole, J. Shanxi Med. Univ., 2002, 33, 330–332 Search PubMed.
- G. W. Rao, W. X. Hu and Z. Y. Yang, Chin. J. Synth. Chem., 2002, 10, 297–301 Search PubMed.
- C. Vidaillac, J. Guillon, C. Arpin, I. Forfar-Bares, B. B. Ba, J. Grellet, S. Moreau, D. H. Caignard, C. Jarry and C. Quentin, Antimicrob. Agents Chemother., 2007, 51, 831–838 Search PubMed.
- U. K. Junggren and S. E. Sjostrand, Substituted pyridylsulfinylbenzimidazoles having gastric acid secretion properties, EP5129, 1979.
- A. E. Braendstroem and B. R. Lamm, 3,5-Dimethyl-4-methoxypyridine 1-oxides, EP103553, 1984.
- A. E. Braendstroem, Method for synthesis of omeprazole, WO9118895, 1991.
- V. Smahovsky, V. Oremus, K. Heleyova, P. Zlatoidsky, O. Gattnar, I. Varga, V. Stalmach and L. Jezek, Method of omeprazole preparation, WO9809962, 1998.
- M. Bekhazi and M. Zoghbi, Synthesis of omeprazole-type pyridine derivatives via 1,4-dihydropyridine intermediates, WO9729103, 1997.
- M. Zoghbi and L. Chen, Synthesis of pyridine derivatives useful as pharmaceutical intermediates under free radical conditions, WO9850361, 1998.
- A. Gustavsson and A. Kallstrom, Method for the synthesis of a benzimidazole compound, WO9722603, 1997.
- F. P. Clausen, K. K. Mccluskey, H. F. Preikschat and S. B. Pedersen, Process for the preparation of 2-[[(2-pyridinyl)methyl]sulfinyl]-1H-benzimidazoles, WO9840378, 1998.
- M. Kato, Y. Toyoshima and N. Iwano, Production of 2-(2-pyridylmethylsulfinyl)benzimidazole as ulcer inhibitors via S-oxidation using hydrogen peroxide and vanadium catalysts, EP302720, 1989.
- J. W. McManus, N. Anousis, B. N. Banks, H. Liu and L. Zhou, Preparation of omeprazole from pyrmetazole, US6191148, 2001.
- K. D. Prasad, Intermediates and an improved process for the preparation of omeprazole employing the said intermediates, US Pat., US6303787, 16 October 2001.
- Pharmaceutical Press, British national formulary: BNF 76, Pharmaceutical Press, 2018, p. 78 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 76th edn, 2006, p. 445 Search PubMed.
- R. Vardanyan and V. Hruby, Chapter 37-Proton Pump Inhibitors, Synthesis of best-seller drugs, 2016. pp. 765–781 Search PubMed.
- P. Erlandsson, R. Isaksson, P. Lorentzon and P. Lindberg, J. Chromatogr., 1990, 532, 305–319 Search PubMed.
- B. Kohl and J. Senn-Bilfinger, Enantiomerically pure (pyridylmethylsulfinyl)benzimidazoles useful as drugs, and their preparation from racemates, DE4035455, 1992.
- P. L. Lindberg and S. Von Unge, Process for the preparation of optically pure crystalline salts of omeprazole, WO9427988, 1994.
- H. Stepankova, J. Zezula, J. Hajicek and V. Kral, Process for preparation of esomeprazole via asymmetric oxidation using hydroperoxide and a chiral metal complex of lactic acid, WO2010091652, 2010.
- M. Uchida, S. Morita, M. Chihiro, T. Kanbe, K. Yamasaki, Y. Yabuuchi and K. Nakagawa, Chem. Pharm. Bull., 1989, 37, 1517–1523 Search PubMed.
- P. L. Lindberg and S. Von Unge, Compositions, US Pat., 5714504, Feb 3, 1998.
- H. Cotton, A. Kronström, A. Mattson and E. Möller, Novel form of S-omeprazole, WO Pat., 98/54171, Dec 3, 1998.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 130 Search PubMed.
- B. Kohl, E. Sturm, K. Klemm, R. Riedel, V. Figala, G. Rainer and H. Schaefer and J. Senn Bilfinger, [[(Dialkoxypyridyl)methyl]thio]benzimidazoles, EP166287, 1986.
- J. Senn-Bilfinger, H. Schaeffer, V. Figala, K. Kurt, K. Klemm, S. Hartmann and V. Figala, Fluoroalkoxy compounds, ZA8403288, 1984.
- B. Kohl, E. Sturm, J. Senn-Bilfinger, W. A. Simon, U. Krueger, H. Schaefer, G. Rainer, V. Figala and K. Klemm, (H+, K+)-ATPase inhibiting 2-[(2-pyridylmethyl)sulfinyl]benzimidazoles. 4, J. Med. Chem., 1992, 35, 1049–1057 Search PubMed.
- N. Palomo and E. Francisco, Pastor Del Castillo, A.; Molina, P.A. Process for preparing 2-(2-pyridylmethyl)-sulfinyl-1H-benzimidazoles and the intermediate compounds used therein, EP1992619, 2008.
- D. Riemann, C. Baglioni, C. Bassetti, B. Bjorvatn, L. Dolenc Groselj, J. G. Ellis, C. A. Espie, D. Garcia-Borreguero, M. Gjerstad, M. Gonçalves and E. Hertenstein, J. Sleep Res., 2017, 26, 675–700 Search PubMed.
- A. Qaseem, D. Kansagara, M. A. Forciea, M. Cooke and T. D. Denberg, Ann. Intern. Med., 2016, 165, 125–133 Search PubMed.
- E. Matheson and B. L. Hainer, Am. Fam. Physician, 2017, 96, 29–35 Search PubMed.
- B. Lemmer, Physiol. Behav., 2007, 90, 285–293 Search PubMed.
- K. Jean-Pierre and G. Pascal, Imidazo[1,2-a]pyridine derivatives and their application as pharmaceuticals, US4382938, 1983.
- L. Rafael, Process for preparing N,N,6-trimethyl-2-(4-methylphenyl)-imidazo-[1, 2-a]-pyridine-3-acetamide and salts thereof, US6407240, 18 June, 2002.
- A. Katsifis, F. Mattner, B. Dikic and V. Papazian, J. Label. Compd. Radiopharm., 1986, 23, 393 Search PubMed.
- L. Almirante and W. Murmann, New derivatives of imidazo[1,2-a]-pyridine and a process for the manufacture thereof, GB1076089, 1967.
- Y. Sumalatha, T. R. Reddy, P. P. Reddy and B. Satyanarayana, Arkivoc, 2009, 2, 315–320 Search PubMed.
- S. Dadiboyena and A. T. Hamme II, Curr. Org. Chem., 2012, 16, 1390–1407 Search PubMed.
- A. S. Kalgutkar, Expert Opin. Ther. Pat., 1999, 8, 831–849 Search PubMed.
- J. R. Vane, Y. S. Bakhle and R. M. Botting, Annu. Rev. Pharmacol. Toxicol., 1998, 38, 97–120 Search PubMed.
- A. G. Habeeb, P. N. P. Rao and E. E. Knaus, J. Med. Chem., 2001, 44, 3039–3042 Search PubMed.
- T. D. Penning, J. J. Talley, S. R. Bertenshaw, J. S. Carter, P. W. Collins, S. Docter, M. J. Graneto, L. F. Lee, J. W. Malecha, J. M. Miyashiro, R. S. Rogers, D. J. Rogier, S. S. Yu, G. D. Anderson, E. G. Burton, J. N. Cog-burn, S. A. Gregory, C. M. Koboldt, W. E. Perkins, K. Seibert, A. W. Veenhuizen, Y. Y. Zhang and P. C. Isakson, J. Med. Chem., 1997, 40, 1347–1365 Search PubMed.
- R. G. Kurumbail, A. M. Stevens, J. K. Gierse, J. J. McDonald, R. A. Stegeman, J. Y. Pak, D. Gildehaus, J. M. Miyashiro, T. D. Penning, K. Seibert, P. C. Isakson and W. C. Stallings, Nature, 1996, 384, 644–648 Search PubMed.
- M. C. Walker, R. G. Kurumbail, J. R. Kiefer, K. T. Moreland, C. M. Koboldt, P. C. Isakson, K. Seibert and J. K. Gierse, Biochem. J., 2001, 357, 709–718 Search PubMed.
- R. Huisgen, Angew. Chem., Int. Ed. Engl., 1963, 2, 565–598 Search PubMed.
- R. Huisgen, Angew. Chem., Int. Ed. Engl., 1963, 2, 633–645 Search PubMed.
- S. Dadiboyena and A. T. Hamme II, Tetrahedron Lett., 2011, 52, 2536–2539 Search PubMed.
- S. Dadiboyena, E. J. Valente and A. T. Hamme II, Tetrahedron Lett., 2010, 51, 1341–1343 Search PubMed.
- S. Dadiboyena, J. Xu and A. T. Hamme II, Tetrahedron Lett., 2007, 49, 1295–1298 Search PubMed.
- S. Dadiboyena and A. Nefzi, Tetrahedron Lett., 2012, 53, 2096–2099 Search PubMed.
- S. Dadiboyena, E. J. Valente and A. T. Hamme II, Tetrahedron Lett., 2009, 50, 291–294 Search PubMed.
- K. R. A. Abdellatif, M. A. Chowdhury, Y. Dong, Q.-H. Chen and E. E. Knaus, Bioorg. Med. Chem., 2008, 16, 3302–3305 Search PubMed.
- M. A. Chowdhury, Y. Dong, Q.-H. Chen, K. R. A. Abdellatif and E. E. Knaus, Bioorg. Med. Chem., 2008, 16, 1948–1956 Search PubMed.
- A. Zarghi, S. Kakhgi, A. Hadipoor, B. Daraee, O. G. Dadrass and M. Hedayati, Bioorg. Med. Chem. Lett., 2008, 18, 1336–1339 Search PubMed.
- A. Gadad, M. B. Palkar, K. Anand, M. N. Noolvi, T. S. Boreddy and J. Wag-wade, Bioorg. Med. Chem., 2008, 16, 276–283 Search PubMed.
- H. J. Seo, M. J. Kim, S. H. Lee, S.-H. Lee, M. E. Jung, M.-S. Kim, K. Ahn, J. Kim and J. Lee, Bioorg. Med. Chem., 2010, 18, 1149–1162 Search PubMed.
- R. Vardanyan and V. Hruby, Chapter 3, Analgesics, Synthesis of best-seller drugs, 2016, pp. 15–64 Search PubMed.
- M. Choy and S. Lam, Sitagliptin: a novel drug for the treatment of type 2 diabetes, Cardiol. Rev., 2007, 15, 264–271 Search PubMed.
- Pharmaceutical Press, British national formulary: BNF 76, Pharmaceutical Press, 76th edn, 2018, p. 681 Search PubMed.
- K. B. Hansen, J. Balsells, S. Dreher, Y. Hsiao, M. Kubryk, M. Palucki, N. Rivera, D. Steinhuebel, J. D. Armstrong, D. Askin and E. J. J. Grabowski, Org. Process Res. Dev., 2005, 9, 634–639 Search PubMed.
- P. R. Padi, P. B. Ireni, S. S. Padamata, K. Nerella, V. A. Ramasamy and R. R. Vangala, PCT INt Appl., WO2009/085990, 2009.
- S. K. Kang, G. H. Cho, H. J. Leem, B. K. Soh, J. Sim and Y.-G. Suh, Tetrahedron: Asymmetry, 2017, 28, 34–40 Search PubMed.
- G. Haufe and F. R. Leroux, Fluorine in life sciences: pharmaceutical, medicinal diagnostics, and agrochemicals, 2019, pp. 575–606 Search PubMed.
- C. E. Griffin, A. M. Kaye, F. Rivera Bueno and A. D. Kaye, Benzodiazepine pharmacology and central nervous system-mediated effects, Ochsner J., 2013, 13, 214–223 Search PubMed.
- S. H. Doss, V. B. Baghos, A. O. Abdelhamid and M. M. A. Halim, Molecules, 2000, 5, 816–825 Search PubMed.
- S. Cortez-Maya, E. Cortes Cortes, S. Hernández-Ortega, T. R. Apan and M. Martínez-García, Synth. Commun., 2012, 42, 46–54 Search PubMed.
- M. H. Bolli, J. Marfurt, C. Grisostomi, C. Boss, C. Binlcert, P. Hess, E. Treiber, A. Thorin, K. Morrison, S. Buchmann, D. Bur, H. Ramuz, M. Clozel, W. Fischli and T. Weller, J. Med. Chem., 2004, 47, 2776–2795 Search PubMed.
- F. J. B. Mendonça Júnior, L. Scotti, H. Ishiki, S. P. S. Botelho, M. S. Da Silva and M. T. Scotti, Med. Chem., 2015, 15, 630–647 Search PubMed.
- J. P. Liou, Y. L. Chang, F. M. Kuo, C. W. Chang, H. Y. Tseng, C. C. Wang, Y. N. Yang, J. Y. Chang, S. J. Lee and H. P. Hsieh, J. Med. Chem., 2004, 47, 4247–4257 Search PubMed.
- P. A. Albaugh, L. Marshall, J. Gregory, G. White, A. Hutchison, P. C. Ross, D. W. Gallagher, J. F. Tallman, M. Crago and J. V. Cassella, J. Med. Chem., 2002, 45, 5043–5051 Search PubMed.
- A. R. Massah, S. Gharaghani, H. A. Lordejani and N. Asakere, Med. Chem. Res., 2016, 25, 1538–1550 Search PubMed.
- K. Sealock, L. L. Lilley, S. R. Collins and J. S. Snyder, Pharmacology for Canadian Health Care Practice, Elsevier Health Sciences, 2016, p. 329 Search PubMed.
- T. J. Dodds, Prescribed benzodiazepines and suicide risk: A review of the literature, The Primary Care Companion for CNS Disorders, 2017, 19, 16r02037 Search PubMed.
- R. Goldberg, Drugs Across the Spectrum, Cengage Learning, 2009, p. 195 Search PubMed.
- K. Hemming, Advances in Heterocyclic Chemistry Volume 123, 2017, pp. 63–103 Search PubMed.
- H. B. Jackson, 6-Phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines, US Pat., 3987052, 19 October 1976.
- H. B. Jackson, Ger. Pat., 2012190, 1970.
- J. B. Hester Jr, D. J. Duchamp and C. G. Chidester, Tetrahedron Lett., 1971, 12, 1609–1612 Search PubMed.
- A. Walser and G. Zenchoff, J. Med. Chem., 1977, 20, 1694–1697 Search PubMed.
- J. Hester, Process for the production of triazolobenzodiazepines and intermediates, US Pat., 3709898, 1972.
- J. Hester, 7-Chloro-1-methyl-5-phenyl-s-triazolo (4,3-a) quinolines, US Pat., 3781289, 1973.
- J. V. Duncia, A. T. Chiu, D. J. Carini, G. B. Gregory, A. Johnson, W. A. LPrice, G. J. Wells, P. C. Wong, J. C. Calabrese and P. B. M. W. M. Timmermans, J. Med. Chem., 1990, 33, 1312–1329 Search PubMed.
- P. Bühlmayer, P. Furet, L. Criscione, M. de Gasparo, S. Whitebread, T. Schmidlin, R. Lattmann and J. Wood, Bioorg. Med. Chem. Lett., 1994, 4, 29–34 Search PubMed.
- M. J. Wyvratt and A. A. Patchett, Med. Res. Rev., 1985, 5, 483–531 Search PubMed.
- P. Buehlmayer, F. Ostermayer and T. Schmidlin, Preparation of [(tetrazolylbiphenyl)methyl] amines and analogs as angiotensin II antagonists, EP443983, 1991.
- P. Buhlmayer, P. Furet, L. Criscione, M. de Gasparo, S. Whitebread, T. Schmidlin, R. Lattmann and J. Wood, Bioorg. Med. Chem. Lett., 1994, 4, 29–34 Search PubMed.
- R. Vardanyan and V. Hruby, Chapter 22, Antihypertensive Drugs, Synthesis of Best-Seller Drugs, 2016, pp. 329–356 Search PubMed.
- K. V. Prasada Rao, R. Dandala, M. S. Sivakumaran, A. Rani and A. Naidu, Synth. Commun., 2007, 37, 2275–2283 Search PubMed.
- Y. Inamoto, T. Chiba, T. Kamimura and T. Takaya, J. Antibiot., 1988, 41, 828–830 Search PubMed.
- R. J. Ternansky, L. Christopher, F. Jordan, W. Bell, G. Theresea and S. Kasher, J. Antibiot., 1993, 46, 1897–1900 Search PubMed.
- F. Kees and H. Grobecker, Antibiot. Chemother., 1995, 47, 1–7 Search PubMed.
- M. González, Z. Rodríguez, B. Tolón, J. C. Rodríguez, H. Velez, B. Valdés, M. A. López and A. Fini, Il Farmaco, 2003, 58, 409–418 Search PubMed.
- T. Takaya, H. Takasugi, T. Masugi, H. Yamanaka and K. Kawabata, BE897864; eidem, US Pat., 4559334, 1984, 1985 both to Fujisawa Pharmaceuticals.
- H. Kamachi, Y. Narita, T. Okita, Y. Abe, S. Iimura, K. Tomatsu, T. Yamasaki, J. Okumura, T. Naito, T. Oki and H. Kawaguchi, J. Antibiot., 1988, 41, 1602–1616 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 444 Search PubMed.
- R. Vardanyan and V. Hruby, Chapter 16-Antihistamine Drugs, Synthesis of Essential Drugs, 2006, pp. 219–235 Search PubMed.
- Y. Hirata, I. Yanagisawa, Y. Ishi, S. Tsucamoto, N. Ito, Y. Isomura and M. Takeda, Guanidinothiazole compounds, process for preparation and gastric inhibiting compositions containing them, US Pat., 4283408, 11 August 1981.
- Y. Isomura, Y. Hirata, Y. Ishi, N. Ito, M. Takeda, I. Yasaginava and Sh Tsukamuto, Ger. Pat., 2951675, 1979.
- Y. Hirata, Y. Ishi, M. Takeda and I. Yasagisava, Ger. Pat., 3008056, 1980.
- Yamanouchi Pharma Co. Ltd., Br. Pat., 2052478, 1980.
- Yamanouchi Pharma Co. Ltd., Br. Pat., 2055800, 1979.
- I. Ishii, Y. Isomura, N. Ito, S. Tsukamoto, Y. Hirata, M. Takeda and I. S. Yanagisawa, Tsukamoto, Belg. Pat., 882071, 1980.
- R. Vardanyan and V. Hruby, Chapter 33 -Antimicrobial Drugs, Synthesis of Essential Drugs, 2006, pp. 499–523 Search PubMed.
- K. J. Hayes, Series of nitrofuran compounds comprising the azomethines of 5-nitro-2-acyl furans with 1-amino-hydantoins, U.S. Pat., 2610181, 1950.
- J. H. Koleman and W. Hayden, O'Keefe, Ch.J. Process of preparing 2-semicarbazidoacetic acid, US Pat., 2779786, January 29, 1957.
- J. G. Michels, Nitrating an n-(2-furyl) alkylidene hydrazine compound, US Pat., 2898335, August 4, 1959.
- G. Gever, O'Keefe, Ch.G. Process for the preparation of azomethines of 5-nitro-2-formyl furan with hydrazine compounds, US Pat., 2927110, March 1, 1960.
- A. Swirska, J. Lange and Z. Buczkowski, Przem. Chem., 1955, 11, 306–307 Search PubMed.
- Pharmaceutical Press, British national formulary: BNF 76, Pharmaceutical Press, 76th edn, 2018, p. 1094 Search PubMed.
- L. Kamen, H. R. Henney and J. D. Runyan, Curr. Med. Res. Opin., 2008, 24, 425–439 Search PubMed.
- J. Xu, Y. Shen, L. Xiang and Y. Deng, Zhongguo Yiyao Gongye Zazhi, 2005, 36, 593–595 Search PubMed.
- R. Vardanyan, Heterocyclic Drug Discovery, Chapter 7-Piperidine-based nonfused biheterocycles with C–N and C–C coupling, Piperidine-based drug discovery, 2017, pp. 241–267 Search PubMed.
- M. Corena-McLeod, Comparative pharmacology of risperidone and paliperidone, Drugs R&D, 2015, 15, 163–174 Search PubMed.
- D. Germann, N. Kurylo and H. F. Risperidone, Profiles Drug. Subst. Excip. Relat. Methodol, 2012, vol. 37, pp. 313–361 Search PubMed.
- A. A. Megens, F. H. Awouters, A. Schotte, T. F. Meert, C. Dugovic, C. J. Niemegeers and J. E. Leysen, Psychopharmacol, 1994, 114, 9–23 Search PubMed.
- L. J. Cohen, Risperidone, Pharmacotherapy, 1994, 14, 253–265 Search PubMed.
- H. Lane, C. Lee, Y. Liu and W. Chang, Pharmacogenom, 2005, 6, 139–149 Search PubMed.
- V. Madaan, D. P. Bestha, V. Kolli, S. Jauhari and R. C. Burket, Neuropsychiatr. Dis. Treat., 2011, 7, 611–620 Search PubMed.
- L. E. J. Kennis, Vandenberk J. 3-piperidinyl-substituted 1,2-benzisoxazoles and 1,2-benzisothiazoles, US4804663A, 1989.
- L. E. J. Kennis and J. Vandenberk, Preparation of 1,2-benzisoxazol-3-yl and 1,2-benzisothiazol-3-yl derivatives as antipsychotics, EP196132, 1986.
- D. Kim, M. Kang, J. S. Kim and J. Jeong, Arch. Pharmacal Res., 2005, 28, 1019–1022 Search PubMed.
- L. F. Jose, P. Albert and G. Antonio, WO2010/12789A1, 2010.
- A. K. Mandal, S. W. Mahajan, M. K. Sharma, A. Chetia and N. D. Chauhan, WO2006/095362A1, 2006.
- T. Imahori, K. Omoto, Y. Hirose and H. Takahata, Heterocycles, 2008, 76, 1627–1632 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 503 Search PubMed.
- G. D. Vaijanath, P. K. Pravin, D. G. Pradeep, M. S. Sanjukumar, P. Nalinakshya and K. Ashok, J. Pharm. Biomed. Anal., 2006, 42, 334–340 Search PubMed .
- M. Vasantha, S. D. Arikatla and M. Suryanarayana, Asian J. Biochem. Pharm. Res., 2011, 1, 201–206 Search PubMed.
- P. Pacher, A. Nivorozhkin and C. Szabó, Pharmacol. Rev., 2006, 58, 87–114 Search PubMed.
- R. K. Robins, Potential purine antagonists. I. Synthesis of some 4,6-substituted pyrazolo[3,4-d]pyrimidines1, J. Am. Chem. Soc., 1956, 78, 784–790 Search PubMed.
- R. Vardanyan and V. Hruby, Chapter 37 Drugs for treating protozoan infections, Synthesis of Essential Drugs, 2006, pp. 559–582 Search PubMed.
- A. R Surrey, U.S. Pat., 2.546.658, 1951.
- A. R. Surrey, 7-Chloro-4-[5-(n-ethyl-n-2-hydroxyethylamino)-2-pentyl] aminoquinoline, its acid addition salts, and method of preparation, US Pat., 2546658, Mar 27, 1951.
- A. R. Surrey and H. F. Hammer, J. Am. Chem. Soc., 1950, 72, 1814–1815 Search PubMed.
- Pharmaceutical Press, British national formulary: BNF 76, Pharmaceutical Press, 76th edn, 2018, p. 694 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 450 Search PubMed.
- P. S. Gillies and C. J. Dunn, Pioglitazone, Drugs, 2000, 60, 333–343 Search PubMed.
- J. Waugh, G. M. Keating, G. L. Plosker, S. Easthope and D. M. Robinson, Pioglitazone: a review of its use in type 2 diabetes mellitus, Drugs, 2006, 66, 85–109 Search PubMed.
- D. J. Betteridge, Diabetes, Obes. Metab., 2007, 9, 640–647 Search PubMed.
- U. Smith, Pioglitazone: mechanism of action, Int. J. Clin. Pract., Suppl., 2001, 121, 13–18 Search PubMed.
- M. S. Kostapanos, M. S. Elisaf and D. P. Mikhailidis, Curr. Pharm. Des., 2013, 19, 4913–4929 Search PubMed; J. Govindan and M. Evans, Diabetes Ther., 2012, 3, 1 Search PubMed.
- J. Dormandy, M. Bhattacharya and A.-R. van Troostenburg de Bruyn, Drug Saf., 2009, 32, 187–202 Search PubMed.
- R. E. Ryder, Br. J. Diabetes Vasc. Dis., 2011, 11, 113–120 Search PubMed.
- K. Meguro and T. Fujita, Thiazolidinedione derivatives and their use, EP193256, 1986.
- T. Sohda, Y. Momose, K. Meguro, Y. Kawamatsu, Y. Sugiyama and H. Ikeda, Arzneim. Forsch., 1990, 40, 37–42 Search PubMed.
- A. Nohara and Y. Maki, (Pyridylmethylthio)benzimidazoles and their sulfoxides, EP174726, 1986.
- A. Nohara and Y. Maki, Preparation of 2-[(2-pyridylmethyl)thio or -sulfinyl]benzimidazoles as antiulcer agents, US4689333, 1987.
- R. A. Bosch, B. P. Dalmases, O. F. Marquillas and J. M. Caldero Ges, Process for preparation of the antiulcer agent 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole [lansoprazole], ES2023609, 1992.
- V. A. Buxade, New process for the synthesis of a 2-(2-pyridylmethylsulfinyl)benzimidazole derivative [lansoprazole], and new intermediates prepared in the process, ES2060541, 1994.
- R. S. Vardanyan and V. J. Hruby, Chapter 19 Antianginal Drugs, Synthesis of Essential Drugs, 2006, pp. 257–267 Search PubMed.
- F. Bossert and W. Vater, Nifedipine ER 20, 4-aryl-1, 4-dihydropyridines, US Pat., 3485847, 23 Dec 23, 1969.
- F. Bossert and W. Vater, Pharmaceutical compositions and methods for producing coronary dilation with 4-aryl-1,4-dihydropyridine derivatives, US Pat., 3644627, 22 February, 1972.
- Farbenfbriken Bayer Actiengesellschaft, Br. Pat., 1173862, 1968.
- F. Bossert and W. Vater, S. Afr, Pat., 6801482, 1968.
- S. C. Kim, K. M. Choi and C. S. Cheong, Bull. Korean Chem. Soc., 2002, 23, 143–144 Search PubMed.
- D. J. Triggle, D. A. Langs and R. A. Janis, Med. Res. Rev., 1989, 9, 123–180 Search PubMed.
- S. Goldmann and J. Stoltefuss, Angew. Chem., Int. Ed. Engl., 1991, 30, 1559–1578 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 465 Search PubMed.
- A. Hantzsch, Justus Liebigs Ann. Chem., 1882, 215, 1–82 Search PubMed.
- S. F. Campbell, P. E. Cross and J. K. Stubbs, 2-(Secondary aminoalkoxymethyl) dihydropyridine derivatives as anti-ischaemic and antihypertensive agents, US. Pat., 4572909, 25 February, 1986.
- L. Sircar and K. R. Anderson, Tetrahedron Lett., 1988, 29, 6835–6838 Search PubMed.
- Y. A. Lee and S. C. Kim, J. Ind. Eng. Chem., 2011, 17, 401–403 Search PubMed.
- R. Vardanyan and V. Hruby, Chapter 20-Hypolipidemic and antihyperlipidemic drugs, Synthesis of Best-Seller Drugs, 2016, pp. 285–315 Search PubMed.
- M. Watanabe, H. Koike, T. Ishiba, T. Okada, S. Seo and K. Hirai, Bioorg. Med. Chem., 1997, 5, 437–444 Search PubMed.
- J. A. Pfefferkorn, Advances in the development of methods for the synthesis of second-generation HMG-CoA reductase inhibitors [fluvastatin sodium, (Lescol), rosuvastatin calcium (Crestor), pitavasstatin calcium (Livalo)], in Art of Drug Synthesis, ed. D. S. Johnson and J. J. Li, Wiley, 2007, pp. 169–182 Search PubMed.
- J. K. Aronson, Meyler's Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions, Elsevier, 2015, p. 782 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 548 Search PubMed.
- G. Scheffler, J. Engel, B. Kutscher, W. S. Sheldrick and P. Bell, Arch. Pharm., 1988, 321, 205–208 Search PubMed.
- C. G. Wermuth, The Practice of Medicinal Chemistry, Academic Press, 2011, p. 12 Search PubMed.
- R. S. Vardanyan and V. J. Hruby, Chapter 22-antihypertensive drugs, Synthesis of Essential Drugs, 2006, pp. 295–310 Search PubMed.
- M. Hartmann and J. Druey, Hydrazine derivatives of pyridazine compounds, US Pat., 2484029, 11 Oct 1949.
- M. Hartmann and J. Druey, Ger. Pat., 848818, 1945.
- J. Druey and B. H. Tingier, Helv. Chim. Acta, 1951, 34, 204 Search PubMed.
- R. S. Vardanyan and V. J. Hruby, Chapter 26 Insulin and synthetic hypoglycemic agents, Synthesis of Essential Drugs, 2006, pp. 343–348 Search PubMed.
- V. B. Ambrogi and W. Longemann, Ger. Pat., 2012138, 1969.
- V. Ambrogi and W. Longemann, Pyrazine derivatives and process for their preparation, US Pat., 3669966, 13 June, 1969.
- V. Ambrogi, K. Bloch, S. Daturi, P. Griggi, W. Longemann, M. A. Parneeti, T. Rabini and R. Tommasini, Arzneim.-Forsch., 1971, 21, 200–208 Search PubMed.
- S. D. Shorvon, E. Perucca and J. Engel, The Treatment of Epilepsy, John Wiley & Sons, Incorporated, 4th edn, 2015, p. 1321 Search PubMed.
- M. G. Baxter, A. R. Elphick, A. A. Miller and D. A. Sawyer, 1,2,4-Triazine derivatives, pharmaceutical compositions and intermediates utilized for their preparation, EP21121, 1981.
- M. G. Baxter, A. R. Elphick, A. A. Miller and D. A. Sawyer, Substituted aromatic compounds, CA1133938, 1982.
- R. Vardanyan and V. Hruby, Chapter 9-Antiepileptic Drugs, Synthesis of Best-Seller Drugs, 2016, pp. 155–177 Search PubMed.
- G. R. Lee, Preparation of lamotrigine, WO 9620935, 1996.
- Y. Qian, P.-C. Lv, L. Shi, R.-Q. Fang, Z.-C. Song and H.-L. Zhu, J. Chem. Sci., 2009, 121, 463–470 Search PubMed.
- J. Hlavac, R. Buchtik, J. Slouka, P. Hradil and I. Wiedermannova, Arkivoc, 2003, 1, 22–28 Search PubMed.
- J. V. Pergolizzi Jr, R. Taylor Jr, J. A. LeQuang and R. B. Raffa, J. Pain Res., 2018, 11, 301–311 Search PubMed.
- R. C. Dart, J. L. Iwanicki, N. Dasgupta, T. J. Cicero and S. H. Schnoll, J. Opioid Manag., 2017, 13, 365–378 Search PubMed.
- (a) T. Heiskanen and E. Kalso, Pain, 1997, 73, 37–45 Search PubMed; (b) S. Mercadante and E. Arcuri, Cancer Treat. Rev., 1998, 24, 425–432 Search PubMed; (c) C. P. Watson and N. Babul, Neurology, 1998, 50, 1837–1841 Search PubMed; (d) B. Silvestri, E. Bandieri, S. Del Prete, G. P. Ianniello, G. Micheletto, M. Dambrosio, G. Sabbatini, L. Endrizzi, A. Marra, E. Aitini, A. Calorio, F. Garetto, G. Nastasi, F. Piantedosi, V. Sidoti and P. Spanu, Clin. Drug Invest., 2008, 28, 399–407 Search PubMed.
- A. Kimishima, H. Umihara, A. Mizoguchi, S. Yokoshima and T. Fukuyama, Org. Lett., 2014, 16, 6244–6247 Search PubMed.
- I. Goldstein, A. L. Burnett, R. C. Rosen, P. W. Park and V. J. Stecher, Sex. Med. Rev., 2018, 7, 115–128 Search PubMed.
- R. Reddy, G. Srinivas, C. Takshinamoorthy, B. Gupta Peruri and A. Naidu, Sci. Pharm., 2016, 84, 447–455 Search PubMed.
- D. J. Dale, P. J. Dunn, C. Golightly, M. L. Hughes, P. C. Levett, A. K. Pearce, P. M. Searle, G. Ward and A. S. Wood, Org. Process Res. Dev., 2000, 4, 17–22 Search PubMed.
- N. K. Terrett, A. S. Bell, D. Brown and P. Ellis, Bioorg. Med. Chem. Lett., 1996, 6, 1819–1824 Search PubMed.
- J. Longépé, J. Prandi and J. M. Beau, Angew. Chem., Int. Ed. Engl., 1997, 36, 72–75 Search PubMed.
- M. A. Hussein, Y. O. Mosaad and N. A. E. K. Gobba, Med. Chem., 2018, 8, 136–146 Search PubMed.
- N. Gadoth, Curr. Drug Ther., 2013, 8, 171–180 Search PubMed.
- K. S. Renalson, A. B. Kalambe and D. Y. Panhekar, Int. J. Res. Dev. Pharm. Life Sci., 2014, 3, 10669 Search PubMed.
- K. W. Lange, S. Reichl, K. M. Lange, L. Tucha and O. Tucha, ADHD Attention Deficit and Hyperactivity Disorders, 2010, 2, 241–255 Search PubMed.
- M. Hartmann and L. Panizzon, Pyridine and piperidine derivatives, US2507631, 1950.
- C. F. M. Huntley, E. W. Kataisto, K. D. La Lumiere and H. A. Reisch, A process for the preparation of methylphenidate hydrochloride, WO2012080834, 2012.
- S. M. Stefanick, B. J. Smith, C. Barr and M. C. Dobish, A process for the preparation of methylphenidate, WO2015069505, 2015.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 549 Search PubMed.
- Y. Wang, J. Wang, Y. Lin, L. F. Si-Ma, D. H. Wang, L. G. Chen and D. K. Liu, Bioorg. Med. Chem. Lett., 2011, 21, 4454–4456 Search PubMed.
- J. Menardo, F. Horak, M. Danzig and W. Czarlewski, Clin. Ther., 1997, 19, 1278–1293 Search PubMed.
- A. Kantar, J. Rihoux and R. Fiorini, Eur. J. Pharm. Sci., 1996, 4, 101–107 Search PubMed.
- J. Tan, C. M. Reimert, B. Deleuran, C. Zachariae and C. J. Simonsen, J. Allergy Clin. Immunol., 1995, 95, 979–986 Search PubMed.
- W. W. Crawford, W. B. Klaustermeyer, P. H. Lee and I. M. Placik, Otolaryngol.--Head Neck Surg., 1998, 118, 668–673 Search PubMed.
- L. F. Delgado, A. Pferferman, D. Solé and C. K. Naspitz, Ann. Allergy, Asthma, Immunol., 1998, 80, 333–337 Search PubMed.
- A. Laak, M. Drooge, H. Timmerman and M. Gabrielle, Quant. Struct.-Act. Relat., 1992, 11, 348–363 Search PubMed.
- M. Drooge, G. Kelder and H. Timmerman, J. Comput.-Aided Mol. Des., 1991, 5, 357–370 Search PubMed.
- R. Vardanyan, Heterocyclic drug discovery Chapter 6- Piperidin-4-ylidene substituted tricyclic compounds, Piperidine-based drug discovery, 2017, pp. 223–239 Search PubMed.
- F. J. Villani, P. J. L. Daniels, C. A. Ellis, T. A. Mann, K. Wang and E. A. Wefer, J. Med. Chem., 1972, 15, 750–754 Search PubMed.
- F. J. Vilani, Antihistaminic 11-(4-piperidylidene)-5H-benzo-(5,6)-cyclohepta-[1,2-b]-pyridines, US4282233, 1981.
- F. J. Villani and J. K. Wong, Preparation of antihistaminic 8-(halo)-substituted 6,11-dihydro-11-(4-piperidylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridines and pharmaceutical compositions containing them, US4659716, 1987.
- D. P. Schumacher, B. L. Murphy and J. E. Clark, US. Process for preparing piperidylidene dihydrodibenzo(a,d)-cycloheptenes or aza-derivatives thereof, US4731447, 1988.
- P. S. Dorisu, J. B. Furanku, K. K. U. Jieshii, R. M. Buruusu and E. K. Jiyon, Piperidylidenedihydrodibenzo[a,d]cycloheptane analogs, JP61289087, 1986.
- D. P. Schumacher, B. L. Murphy, J. E. Clark, P. Tahbaz and T. A. Mann, J. Org. Chem., 1989, 54, 224–228 Search PubMed.
- J. J. Piwinski, J. K. Wong, M. J. Green, A. K. Ganguly, M. M. Billah, R. E. West Jr and W. Kreutner, J. Med. Chem., 1991, 34, 457–461 Search PubMed.
- A. Stampa, P. Camps, G. Rodriguez, J. Bosch and M. C. Onrubia, Process for the preparation of loratadine, US6084100, 2000.
- H. J. Doran, P. M. O'neill, M. Sharp and D. Corp, Process for preparing tricyclic compounds having antihistaminic activity, US Pat., 6271378, 2001.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 547 Search PubMed.
- J. H. Fuhrkop and G. Li, Organic Synthesis: Concepts and Methods, Wiley, 2003, p. 237 Search PubMed.
- Pharmaceutical Press, British national formulary: BNF 76, Pharmaceutical Press, 76th edn, 2018, p. 279 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 549 Search PubMed.
- M. P. Curran, L. J. Scott and C. M. Perry, Cetirizine, Drugs, 2004, 64, 523–561 Search PubMed.
- D. M. Campoli-Richards, M. M. Buckley and A. Fitton, Drugs, 1990, 40, 762–781 Search PubMed.
- C. M. Spencer, D. Faulds and D. H. Peters, Cetirizine: a reappraisal of its pharmacological properties and therapeutic use in selected allergic disorders, Drugs, 1993, 46, 1055–1080 Search PubMed.
- C. M. Spencer and S. Noble, Pediatric Drugs, 1999, 1, 51–73 Search PubMed.
- J. M. Portnoy and C. Dinakar, Expert Opin. Pharmacother., 2004, 5, 125–135 Search PubMed.
- E. Baltes, J. De Lannoy and L. Rodriguez, 2-[4-(Diphenylmethyl)-1-piperazinyl]acetic acids and their amides and pharmaceutical compositions, EP58146, 1982.
- E. Cossement, G. Motte, G. Bodson and J. Gobert, Process for preparation of cetirizine, its dihydrochloride, and optical isomers via hydrolysis and corresponding nitriles, GB2225321, 1990.
- R. Vardanyan and V. Hruby, Chapter 16-Antihistamine Drugs, Synthesis of Best-Seller Drugs, 2016, pp. 247–263 Search PubMed.
- R. Davis and H. M. Bryson, Drugs, 1994, 47, 677–700 Search PubMed.
- S. M. Wimer, L. Schoonover and M. W. Garrison, Clin. Ther., 1998, 20, 1049–1070 Search PubMed.
- D. S. North, D. N. Fish and J. J. Redington, Pharmacotherapy, 1998, 18, 915–935 Search PubMed.
- H. D. Langtry and H. M. Lamb, Drugs, 1998, 56, 487–515 Search PubMed.
- S. R. Norrby, Levofloxacin, Expert Opin. Pharmacother., 1999, 1, 109–119 Search PubMed.
- V. R. Anderson and C. M. Perry, Drugs, 2008, 68, 535–565 Search PubMed.
- S. J. Martin, J. M. Meyer, S. K. Chuck, R. Jung, C. R. Messick and S. L. Pendland, Ann. Pharmacother., 1998, 32, 320–336 Search PubMed.
- A. M. Noreddin and W. F. Elkhatib, Expert Rev. Anti-Infect. Ther., 2010, 8, 505–514 Search PubMed.
- K. F. Croom and K. L. Goa, Drugs, 2003, 63, 2769–2802 Search PubMed.
- M. Hurst, H. M. Lamb, L. J. Scott and D. P. Figgitt, Drugs, 2002, 62, 2127–2167 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 500 Search PubMed.
- R. Vardanyan and V. Hruby, Chapter 31-Antibacterial Drugs, Synthesis of Best-Seller Drugs, 2016, pp. 645–667 Search PubMed.
- I. Hayakawa, S. Atarashi, S. Yokohama, M. Imamura, K. Sakano and M. Furukawa, Antimicrob. Agents Chemother., 1986, 29, 163–164 Search PubMed.
- S. Sirohi, S. V. Dighe, E. A. Walker and B. C. Yoburn, Pharmacol., Biochem. Behav., 2008, 91, 115–120 Search PubMed.
- A. Duttaroy and B. C. Yoburn, Anesthesiology, 1995, 82, 1226–1236 Search PubMed.
- P. K. Gupta, G. Kumaran, P. Ambuja and R. C. Malhotra, J. Chem. Res., 2005, 7, 452–453 Search PubMed.
- A. J. A. Watson, A. C. Maxwell and J. M. J. Williams, J. Org. Chem., 2011, 76, 2328–2331 Search PubMed.
- M. Ghaffarzadeh, S. S. Joghan and F. Faraji, Tetrahedron Lett., 2012, 53, 203–206 Search PubMed.
- T. G. Gant and S. Sarshar, US Pat., 20100016365, 2010.
- C. A. Valdez, R. N. Leif and B. P. Mayer, PLoS One, 2014, 9, e108250 Search PubMed.
- J. S. Birks and R. J. Harvey, Donepezil for dementia due to Alzheimer's disease, Cochrane Database Syst. Rev., 2018, 6, CD001190 Search PubMed.
- Swedish Council on Health Technology Assessment, Dementia-Caring, Ethics, Ethnical and Economical Aspects: A Systematic Review, 2008, PMID 28876770 Search PubMed.
- Pharmaceutical Press, British national formulary: BNF 76, Pharmaceutical Press, 76th edn, 2018, p. 300 Search PubMed.
- H. Sugimoto, Y. Yamanish, Y. Iimura and Y. Kawakami, Curr. Med. Chem., 2000, 7, 303–339 Search PubMed.
- E. A. Van der Zee, B. Platt and G. Riedel, Behav. Brain Res., 2011, 221, 583–586 Search PubMed.
- H. Sugimoto, Y. Tsuchiya, K. Higurashi, N. Karibe, Y. Limural, A. Sasaki, Y. Yamanishi, H. Ogura, S. Araki, T. Kosasa, A. Kubota, M. Kosasa and K. Yamatsu, US Pat., US4895841, 1990.
- H. Sugimoto, Y. limura, Y. Yamanishi and K. Yamatsu, J. Med. Chem., 1995, 38, 4821–4829 Search PubMed.
- H. Sugimoto, Y. limura, Y. Yamanishi and K. Yamatsu, Bioorg. Med. Chem. Lett., 1992, 2, 871–876 Search PubMed.
- P. Costanzo, L. Cariati, D. Desiderio, R. Sgammato, A. Lamberti, R. Arcone, R. Salerno, M. Nardi, M. Masullo and M. Oliverio, ACS Med. Chem. Lett., 2016, 7, 470–475 Search PubMed.
- C. R. Elati, N. Kolla, S. Rao Chalamala, P. J. Vankawala, V. Sundaram, H. Vurimidi and V. T. Mathad, Synth. Commun., 2006, 36, 169–174 Search PubMed.
- K. K. V. S. R. Reddy, J. Moses Babu, P. Anil Kumar, E. R. R. Chandrasekhar, V. T. Mathad, S. Eswaraiah, M. S. Reddy and K. Vyas, J. Pharm. Biomed. Anal., 2004, 35, 1047–1058 Search PubMed.
- C. R. Elati, N. Kolla, S. R. Chalamala, P. J. Vankawala, V. Sundaram, H. Vurimidi and V. T. Mathad, US Pat., US2008/0114173A1, 2008.
- K. L. Dechant and S. P. Clissold, Drugs, 1991, 41, 225–253 Search PubMed.
- M. Bourin, P. Chue and Y. Guillon, Paroxetine: a review, CNS Drug Rev., 2001, 7, 25–47 Search PubMed.
- A. J. Wagstaff, S. M. Cheer, A. J. Matheson, D. Ormrod and K. L. Goa, Drugs, 2002, 62, 655–703 Search PubMed.
- W. E. Heydorn, Expert Opin. Invest. Drugs, 1999, 8, 417–441 Search PubMed.
- N. S. Gunasekara, S. Noble and P. Benfield, Drugs, 1998, 55, 85–120 Search PubMed.
- C. Pae and A. A. Patkar, Paroxetine: current status in psychiatry, Expert Rev. Neurother., 2007, 7, 107–120 Search PubMed.
- S. H. Snyderman, M. A. Rynn, K. Bellew and K. Rickels, Expert Opin. Pharmacother., 2004, 5, 1799–1806 Search PubMed.
- C. F. Caley and S. S. Weber, Ann. Pharmacother., 1993, 27, 1212–1222 Search PubMed.
- C. De Risi, G. Fanton, G. P. Pollini, C. Trapella, F. Valente and V. Zanirato, Tetrahedron: Asymmetry, 2008, 19, 131–155 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 453 Search PubMed.
- R. Vardanyan, Chapter 8, Piperidine-Based Fused Biheterocycles, Piperidine-based drug discovery, Elsevier, 2017, pp. 269–286 Search PubMed.
- Pharmaceutical Press, British national formulary: BNF 76, Pharmaceutical Press, 76th edn, 2018. p. 1198 Search PubMed.
- T. K. Agrawal and Y. Murti, Int. J. Pharma Sci. Res, 2011, 2, 3156–3160 Search PubMed.
- J. Heeres, L. J. J. Backx and J. H. Mostmans, Ger. Pat., 2804096, 1978.
- S. Gold de Sigman, Span. Pat., 512316, 1983.
- X. Cierra Dotti, Span. Pat., 539112, 1985.
- J. Heeres, L. J. J. Backx, J. H. Mostmans and J. Van Cutsem, J. Med. Chem., 1979, 22, 1003–1005 Search PubMed.
- S. A. Anttila and E. V. Leinonen, CNS Drug Rev., 2001, 7, 249–264 Search PubMed.
- D. J. Nutt, Hum. Psychopharmacol., 2002, 1, S37–S41 Search PubMed.
- T. De Boer, G. Maura, M. Raiteri, J. De Vos, J. Wieringa and R. P. Pinder, Neuropharmacology, 1988, 27, 399–408 Search PubMed.
- R. G. De Boer Th and H. H. G. Berendsen, Hum. Psychopharmacol., 1995, 10, 107S–118S Search PubMed.
- W. J. Van der Burg, Ger. Offen., 2614406, 1975Chem. Abstr., 1977, 86, 29883.
- A. Singer, A. Liberman and N. Finkelstein, PCT Int. Appl., W62782, 2000Chem. Abstr.. 2000, 133, 321900.
- K. Iishi and K. Yoshikawa, US Pat., 6437120, 2002Chem. Abstr.. 2001, 135, 33494.
- G. S. Wynia, G. Windhorst, P. C. Post and F. A. Maris, J. Chromatogr. A, 1997, 773, 339–350 Search PubMed.
- D. V. S. Rao, R. Dandala, C. Bharathi, V. K. Handa, M. Sivakumaran and A. Naidub, Arkivoc, 2006, 15, 127–132 Search PubMed.
- Pharmaceutical Press, British national formulary: BNF 74, British Medical Association, 76th edn, 2017, p. X Search PubMed.
- H. Morren, U.S. Pat., 2899436, 1959.
- H. Morren, Ger. Pat., 1049383, 1954.
- H. Morren, Ger. Pat., 1061786, 1954.
- H. Morren, Ger. Pat., 1068262, 1954.
- H. Morren, Ger. Pat., 1072624, 1954.
- H. Morren, Ger. Pat., 1075116, 1954.
- Pharmaceutical Press, British national formulary: BNF 76, Pharmaceutical Press, 76th edn, 2018, pp. 280–281 Search PubMed.
- L. Klimek, Drugs Today, 2009, 45, 213–225 Search PubMed.
- G. M. Walsh, Levocetirizine: an update, Curr. Med. Chem., 2006, 13, 2711–2715 Search PubMed.
- P. I. Hair and L. J. Scott, Drugs, 2006, 66, 973–996 Search PubMed.
- J. H. Day, A. K. Ellis and E. Rafeiro, Drugs Today, 2004, 40, 415–421 Search PubMed.
- C. J. Opalka, T. E. D'Ambra, J. J. Faccone, G. Bodson and E. Cossement, Synthesis, 1995, 7, 766–768 Search PubMed.
- J. Jaśkowska, P. Zaręba, P. Śliwa, E. Pindelska, G. Satała and Z. Majka, Molecules, 2019, 24, 1609 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 455 Search PubMed.
- A. Desiniotis and N. Kyprianou, Expert Opin. Ther. Targets, 2011, 15, 1405–1418 Search PubMed.
- J. L. Pool, A. A. Taylor and E. B. Nelson, Am. J. Med., 1989, 87, 57S–61S Search PubMed.
- R. Nageswara Rao, D. Nagaraju, A. K. Das and N. Jena, Separation, characterization, and quantitation of process-related substances of the anti-hypertensive drug doxazosin mesylate by reversed-phase LC with PDA and ESI-MS as detectors, J. Chromatogr. Sci., 2007, 45, 63–69 Search PubMed.
- A. B. Casey and C. E. Canal, ACS Chem. Neurosci., 2017, 8, 1135–1146 Search PubMed.
- E. Spina and J. De Leon, Basic Clin. Pharmacol. Toxicol., 2007, 100, 4–22 Search PubMed.
- Y. Oshiro, S. Sato and N. Kurahashi, Otsuka Pharmaceutical Co Ltd, assignee. Carbostyril derivatives, US Pat., US5006528, 1991 Apr 9.
- Y. Oshiro, S. Sato, N. Kurahashi, T. Tanaka, T. Kikuchi, K. Tottori, Y. Uwahodo and T. Nishi, J. Med. Chem., 1998, 41, 658–667 Search PubMed.
- C. B. Pollard and T. H. Wicker, J. Am. Chem. Soc., 1954, 76, 1853–1855 Search PubMed.
- A. Les, K. Badowska-Roslonek, M. Laszcz, A. Kamienska-Duda, P. Baran and L. Kaczmarek, Acta Pol. Pharm., 2010, 67, 151–157 Search PubMed.
- Y. Oshiro, S. Sato and N. Kurahashi, Preparation and formulation of 7-[(4-phenylpiperazino) butoxy]carbostyrils as dopaminergic neurotransmitter antagonists, EP367141, 1990.
- P. Kowalski and J. Jaskowska, Arch. Pharm., 2012, 345, 81–85 Search PubMed.
- F. Pettersson, H. Ponten, N. Waters, S. Waters and C. Sonesson, J. Med. Chem., 2010, 53, 2510–2520 Search PubMed.
- A. Les and W. Szelejewski, Przem. Chem., 2007, 86, 1174–1177 Search PubMed.
- T. L. Lemke and D. A. Williams, Foye's Principles of Medicinal Chemistry, Lippincott Williams & Wilkins, 6th edn, 2008, p. 1286 Search PubMed.
- U. Blume-Peytavi, D. A. Whiting and R. M. Trüeb, Hair Growth and Disorders, Springer Science & Business Media, 2008, p. 369 Search PubMed.
- E. L. Knezevich, L. K. Viereck and A. T. Drincic, Pharmacotherapy, 2012, 32, 54–66 Search PubMed.
- G. H. Rasmusson and G. F. Reynolds, 17β-Substituted 4-aza-5α-androstenones and their use as testosterone 5 α-reductase inhibitors, EP155096, 1985.
- G. H. Rasmusson, G. F. Reynolds, N. G. Steinberg, E. Walton, G. F. Patel, T. Liang, M. A. Cascieri, A. H. Cheung, J. R. Brooks and C. Berman, J. Med. Chem., 1986, 29, 2298–2315 Search PubMed.
- R. Vardanyan and V. Hruby, Chapter 27-Steroid Hormones, Synthesis of Best-Seller Drugs, 2016, pp. 459–493 Search PubMed.
- J. J. Heidelbaugh and H. Holmstrom, J. Fam. Pract., 2013, 62, 191–197 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 500 Search PubMed.
- R. Akhtar, M. Yousaf, S. A. R. Naqvi, M. Irfan, A. F. Zahoor, A. I. Hussain and S. A. S. Chatha, Synth. Commun., 2016, 46, 1849–1879 Search PubMed.
- K. Grohe and H. Heitger, Liebigs Ann. Chem., 1987, 29–37 Search PubMed.
- V. R. Arava and P. Umareddy, Der Pharma Chemica, 2018, 10, 174–178 Search PubMed.
- J. J. Li, Laughing Gas, Viagra, and Lipitor: The Human Stories behind the Drugs We Use, Oxford University Press, United Kingdom, 2006, p. 146 Search PubMed.
- P. Charpentier, Phenothiazines, US Pat., 2530451, 1950.
- S. B. Sidney and J. A. Nicholson, US. Pat., 2607773, 1952.
- W. Sneader, Drug Discovery: A History, John Wiley & Sons, 2005, p. 251 Search PubMed.
- S. S. Abolmaali, A. M. Tamaddon and R. Dinarvand, Cancer Chemother. Pharmacol., 2013, 71, 1115–1130 Search PubMed.
- Z. A. Khan, R. Tripathi and B. Mishra, Expert Opin. Drug Deliv., 2012, 9, 151–169 Search PubMed.
- A. K. Fotoohi and F. Albertioni, Leuk. Lymphoma, 2008, 49, 26–410 Search PubMed.
- B. Cai, A. Liao, K. K. Lee, J. S. Ban, H. S. Yang, Y. J. Im and C. Chun, Steroids, 2016, 116, 45–51 Search PubMed.
- D. R. Seeger, D. B. Cosulich, J. M. Smith and M. E. Hultquist, J. Am. Chem. Soc., 1949, 71, 1753–1758 Search PubMed.
- J. R. Piper and J. A. Montgomery, J. Heterocycl. Chem., 1974, 11, 279–280 Search PubMed.
- M. Chaykovsky, A. Rosowsky, N. Papathanasopoulos, K. K. Chen, E. J. Modest, R. L. Kisliuk and Y. Gaumont, J. Med. Chem., 1974, 17, 1212–1216 Search PubMed.
- J. A. Ellard, Ger. Pat., 2741270, 1977.
- J. Wiecko, Process for preparing methotrexate or an N-substituted derivative thereof and/or a di (lower) alkyl ester thereof and precursor therefor, US Pat., 4057548, 8 Nov 1977.
- J. Wiecko and J. Wiecko, 1978. Process for preparing pyrazine precursor of methotrexate or an N-substituted derivative thereof and/or a di (lower) alkyl ester thereof, US Pat., 4067867, 10 Jan 1978.
- J. A. Ellard, Synthesis of methotrexate, US Pat., 4080325, 21 March 1978.
- E. Catalucci, Ger. Pat., 2741383, 1977.
- G. B. Stefano, R. Ptáček, H. Kuželová and R. M. Kream, Endogenous morphine: up-to-date review 2011, Folia Biol., 2012, 58, 49–56 Search PubMed.
- M. Booth, Opium: a history, St. Martin’s Press, New York, 1998 Search PubMed.
- L. D. Kapoor, Opium poppy: botan y, chemistry, and pharmacology, The Haworth, New York, 1995 Search PubMed.
- D. T. Courtwright, Forces of habit drugs and the making of the modern world, Harvard University Press, Cambridge, Mass., 1st edn, 2009, pp. 36–37 Search PubMed.
- C. J. Mosher, Drugs and Drug Policy: The Control of Consciousness Alteration, SAGE Publications, 2013, p. 123 Search PubMed.
- G. L. Fisher, Encyclopedia of substance abuse prevention, treatment, & recovery, SAGE, Los Angeles, 2009, p. 564 Search PubMed.
- Molecular, clinical and environmental toxicology, ed. A. Luch, Springer, 2009, p. 20 Search PubMed.
- T. Jonsson, C. B. Christensen, H. Jordening and C. Frølund, Pharmacol. Toxicol., 1988, 62, 203–205 Search PubMed.
- M. Gates, J. Am. Chem. Soc., 1950, 72(228), 4839 Search PubMed , 1141.
- M. Gates and G. Tschudi, J. Am. Chem. Soc., 1952, 74, 1109 Search PubMed.
- N. E. Calcaterra and J. C. Barrow, ACS Chem. Neurosci., 2014, 5, 253–260 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 535 Search PubMed.
- L. H. Sternbach and E. Reeder, J. Org. Chem., 1961, 26, 4936–4941 Search PubMed.
- K. B. Nutley and L. H. Sternbach, Process for preparing, US Pat., 3109843, 1963.
- E. Reeder and L. H. Sternbach, 5-aryl-3h–1,4-benzodiazepin-2 (1h)-ones, US Pat., 3371085, 27 February, 1968.
- M. Gates, New synthesis of diazepam, J. Org. Chem., 1980, 45, 1675–1681 Search PubMed.
- M. Ishikura, M. Mori, T. Ikeda, M. Terashima and Y. Ban, J. Org. Chem., 1982, 47, 2456–2461 Search PubMed.
- S. C. Bell and L. L. C. Wyeth, 5-monocyclic aryl-1, 3-dihydro-2h-1, 4-benzodiazepin-2-ones, US Pat., 3296249, 3 Jan, 1967.
- S. C. Bell, Hydrolysis of l-acylated-j-acyloxy benzodiazepines, US Pat., 3176009, 1965.
- American Home Products Corp., Br. Pat., 1022642, 1962.
- S. C. Bell, Br. Pat., 1.057492, 1967.
- S. J. Childress and M. I. Gluckman, J. Pharm. Sci., 1964, 53, 577–590 Search PubMed.
- S. C. Bell, R. J. McCaully, C. Gochman, S. J. Childress and M. I. Gluckman, J. Med. Chem., 1968, 11, 457–461 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 535 Search PubMed.
- R. S. Vardanyan and V. J. Hruby, Chapter 9-Antiepileptic Drugs, Synthesis of Essential Drugs, 2006, pp. 125–133 Search PubMed.
- L. H. Sternbach, R. I. Fryer, O. Keller, W. Metlesics, G. Sach and N. Steiger, J. Med. Chem., 1963, 6, 261–265 Search PubMed.
- H. L. Newmark and J. Cariss, Oleaginous systems, US Pat., 3116203, 31 December 31, 1963.
- J. K. Clifton and H. L. Newmark, Parenteral-compoiaxions-containing, US Pat., 3123529, 1964.
- O. Kller, N. Steiger and N. H. Sternbach, Certificate of correction, US Pat., 3121114, 1964.
- N. Steiger and O. Keller, 2-Amino-2'-halo-5-nitro benzophenones, US Pat., 3203990, 31 August, 1965.
- J. A. Rachlin and A. Focella, Ammonolysis of 2-halo-2'-benzoyl-4'-nitroacetanilides, US Pat., 3335181, 8 August, 1967.
- Pharmaceutical Press, British national formulary: BNF 76, Pharmaceutical Press, 76th edn, 2018, p. 481 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 537 Search PubMed.
- S. R. Collins, RN-BC, Shelly Rainforth Collins, Pharmacology and the Nursing Process, Elsevier Health Sciences, 2015, p. 193 Search PubMed.
- R. S. Vardanyan and V. J. Hrub, Chapter 4-Soporific Agents (Hypnotics and Sedative Drugs), Synthesis of Essential Drugs, 2006, pp. 57–68 Search PubMed.
- American Home Products Corp., Br. Pat., 1022645, 1962.
- S. C. Bell and S. J. Childress, A Rearrangement of 5-Aryl-1, 3-dihydro-2H-1,4-benzodiazepine-2-one 4-Oxides1, J. Org. Chem., 1962, 27, 1691–1695 Search PubMed.
- S. C. Bell, US Pat., 3197467, 1965.
- E. Reeder and A. Stempel, Process for preparing 1, 3-dihydro-3-hydroxy-2h-1, 4-benzodiazepin-2-ones, US Pat., 3340253, 27 July, 1967.
- E. Reeder and A. Stempel, Aminobenzodiazepine compounds and methods, US. Pat., 3374225, 19 Marh 1968.
- M. Riedel, N. Müller, M. Strassnig, I. Spellmann, E. Severus and H. J. Möller, Neuropsychiatr. Dis. Treat., 2007, 3, 219–235 Search PubMed.
- M. Riedel, N. Müller, M. Strassnig, I. Spellmann, E. Severus and H. J. Möller, Neuropsychiatr. Dis. Treat., 2007, 3, 219–235 Search PubMed.
- J. Bruhwyler, E. Chleide and M. Mercier, Biobehav. Rev., 1990, 14, 357–363 Search PubMed.
- E. J. Warawa and B. M. Migler, EP240228, 1987.
- J. Schmutz, F. Kuenzle, F. Hunziker and A. Buerki, Helv. Chim. Acta, 1965, 48, 336–347 Search PubMed.
- J. Fischer and C. R. Ganellin, Analogue-based Drug Discovery, John Wiley & Sons, 2006, p. 468 Search PubMed.
- C. Y. Chang and T. K. Yang, Tetrahedron: Asymmetry, 2003, 14, 2239–2245 Search PubMed.
- B. Y. Hwang, M. Cha, H. Y. Park and B. G. Kim, Biotechnol. Bioprocess Eng., 2011, 16, 625–630 Search PubMed.
- C. A. Bunnell, B. A. Hendriksen, T. M. Hotten, S. D. Larsen and D. E. Tupper, Preparation of olanzapine solvates, EP733634, 1996.
- J. K. Chakrabarti, T. M. Hotten and D. E. Tupper, Preparation of 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno-[2,3-b][1,5]benzodiazepine, EP454436, 1991.
- C. M. Beasley Jr, J. K. Chakrabarti, T. M. Hotten and D. E. Tupper, 2-Methyl-4-(4-methyl-1-piperazinyl)-10H-thieno-[2,3-b][1,5]benzodiazepine for treatment of psychoactive substance disorders, US5817657, 1988.
| This journal is © The Royal Society of Chemistry 2020 |