Open Access Article
Open Access ArticleA designed second-sphere hydrogen-bond interaction that critically influences the O–O bond activation for heterolytic cleavage in ferric iron–porphyrin complexes†
Sarmistha
Bhunia
 a,
Atanu
Rana
a,
Atanu
Rana
 a,
Somdatta Ghosh
Dey
a,
Somdatta Ghosh
Dey
 *a,
Anabella
Ivancich
*a,
Anabella
Ivancich
 *b and
Abhishek
Dey
*b and
Abhishek
Dey
 *a
*a
aDepartment of Inorganic Chemistry, Indian Association for the Cultivation of Science, Kolkata, 700032, India. E-mail: icad@iacs.res.in
bCNRS, Aix-Marseille Univ, Laboratoire de Bioénergétique et Ingénierie des Protéines (UMR 7281), IMM FR3479, Marseille, France. E-mail: aivancich@imm.cnrs.fr
First published on 27th January 2020
Abstract
Heme hydroperoxidases catalyze the oxidation of substrates by H2O2. The catalytic cycle involves the formation of a highly oxidizing species known as Compound I, resulting from the two-electron oxidation of the ferric heme in the active site of the resting enzyme. This high-valent intermediate is formed upon facile heterolysis of the O–O bond in the initial FeIII–OOH complex. Heterolysis is assisted by the histidine and arginine residues present in the heme distal cavity. This chemistry has not been successfully modeled in synthetic systems up to now. In this work, we have used a series of iron(III) porphyrin complexes (FeIIIL2(Br), FeIIIL3(Br) and FeIIIMPh(Br)) with covalently attached pendent basic groups (pyridine and primary amine) mimicking the histidine and arginine residues in the distal-pocket of natural heme enzymes. The presence of pendent basic groups, capable of 2nd sphere hydrogen bonding interactions, leads to almost 1000-fold enhancement in the rate of Compound I formation from peracids relative to analogous complexes without these residues. The short-lived Compound I intermediate formed at cryogenic temperatures could be detected using UV-vis electronic absorption spectroscopy and also trapped to be unequivocally identified by 9 GHz EPR spectroscopy at 4 K. The broad (2000 G) and axial EPR spectrum of an exchange-coupled oxoferryl-porphyrin radical species, [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] with geff⊥ = 3.80 and geff‖ = 1.99, was observed upon a reaction of the FeIIIL3(Br) porphyrin complex with m-CPBA. The characterization of the reactivity of the FeIII porphyrin complexes with a substrate in the presence of an oxidant like m-CPBA by UV-vis electronic absorption spectroscopy showed that they are capable of oxidizing two equivalents of inorganic and organic substrate(s) like ferrocene, 2,4,6-tritertiary butyl phenol and o-phenylenediamine. These oxidations are catalytic with a turnover number (TON) as high as 350. Density Functional Theory (DFT) calculations show that the mechanism of O–O bond activation by 2nd sphere hydrogen bonding interaction from these pendent basic groups, which are protonated by a peracid, involves polarization of the O–O σ-bond, leading to lowering of the O–O σ*-orbital allowing enhanced back bonding from the iron center. These results demonstrate how inclusion of 2nd sphere hydrogen bonding interaction can play a critical role in O–O bond heterolysis.
O Por˙+] with geff⊥ = 3.80 and geff‖ = 1.99, was observed upon a reaction of the FeIIIL3(Br) porphyrin complex with m-CPBA. The characterization of the reactivity of the FeIII porphyrin complexes with a substrate in the presence of an oxidant like m-CPBA by UV-vis electronic absorption spectroscopy showed that they are capable of oxidizing two equivalents of inorganic and organic substrate(s) like ferrocene, 2,4,6-tritertiary butyl phenol and o-phenylenediamine. These oxidations are catalytic with a turnover number (TON) as high as 350. Density Functional Theory (DFT) calculations show that the mechanism of O–O bond activation by 2nd sphere hydrogen bonding interaction from these pendent basic groups, which are protonated by a peracid, involves polarization of the O–O σ-bond, leading to lowering of the O–O σ*-orbital allowing enhanced back bonding from the iron center. These results demonstrate how inclusion of 2nd sphere hydrogen bonding interaction can play a critical role in O–O bond heterolysis.
Introduction
Factors that can affect heterolytic O–O bond cleavage of metal peroxide species are of great contemporary interest.1,2 Heterolysis of metal peroxides generates high-valent metal oxo species which are very reactive and have been demonstrated to be able to catalyze oxidation of inert organic Compounds, a process that is highly relevant to the chemistry community.3–7 The inspiration for such chemistry is derived from naturally occurring heme metalloenzymes (monofunctional peroxidases,8 catalases,9 bifunctional catalase-peroxidases (KatGs),10 cyt P450 monooxygenases11 and P450 peroxygenases12) where detailed biochemical experiments heralded the presence of 1st and 2nd coordination sphere residues in the heme active site that are essential for the generation of high-valent catalytic intermediates. The resting state of peroxidases, peroxygenases and catalases is the ferric oxidation state of the heme iron and a catalytically-competent high-valent intermediate [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+], Compound I, is formed by the reaction with hydrogen peroxide or alternative two-electron oxidants. Upon reaction with substrates, the one-electron reduction of Compound I results in the formation of the FeIV
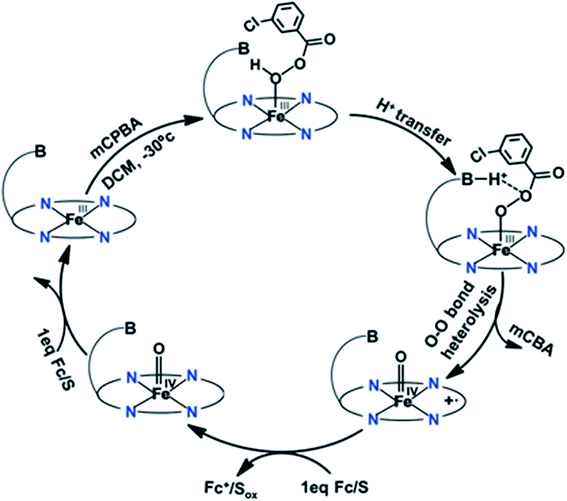
O Por˙+], Compound I, is formed by the reaction with hydrogen peroxide or alternative two-electron oxidants. Upon reaction with substrates, the one-electron reduction of Compound I results in the formation of the FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species known as Compound II, which undergoes further one electron reduction and regains back the resting ferric state.13 The Compound I intermediate is the strongest oxidant in the catalytic cycle of peroxidases which are involved in several activities like biosynthesis of hormones (cycloperoxidases), defense against pathogens (myeloperoxidase and horseradish peroxidase) and regulating cellular oxidative stress (CcP and GSH peroxidase).14,15 The oxidizing ability of high valent intermediates makes them valuable to the chemical industry as they can catalyze difficult chemical oxidations relevant to synthesis of fine chemicals, waste water decontamination of toxic phenol and polymerization.16,17 The ferric hydroperoxide (FeIII–OOH) intermediate, known as Compound 0,18,19 is the very short-lived species preceding the formation of Compound I in all hydroperoxidases20,21 A histidine residue is involved in the protonation of Compound 0 in the active site of peroxidases.22–24 Histidine acts as a base initially deprotonating the proximal oxygen atom of hydroperoxide bound to the heme iron, and then transfers the proton to a distal oxygen atom as an acid catalyst to facilitate the heterolytic O–O bond cleavage and water release, assisted by an arginine residue, leaving the oxygen atom bound to the iron (Fig. 1).20,24–27
O species known as Compound II, which undergoes further one electron reduction and regains back the resting ferric state.13 The Compound I intermediate is the strongest oxidant in the catalytic cycle of peroxidases which are involved in several activities like biosynthesis of hormones (cycloperoxidases), defense against pathogens (myeloperoxidase and horseradish peroxidase) and regulating cellular oxidative stress (CcP and GSH peroxidase).14,15 The oxidizing ability of high valent intermediates makes them valuable to the chemical industry as they can catalyze difficult chemical oxidations relevant to synthesis of fine chemicals, waste water decontamination of toxic phenol and polymerization.16,17 The ferric hydroperoxide (FeIII–OOH) intermediate, known as Compound 0,18,19 is the very short-lived species preceding the formation of Compound I in all hydroperoxidases20,21 A histidine residue is involved in the protonation of Compound 0 in the active site of peroxidases.22–24 Histidine acts as a base initially deprotonating the proximal oxygen atom of hydroperoxide bound to the heme iron, and then transfers the proton to a distal oxygen atom as an acid catalyst to facilitate the heterolytic O–O bond cleavage and water release, assisted by an arginine residue, leaving the oxygen atom bound to the iron (Fig. 1).20,24–27
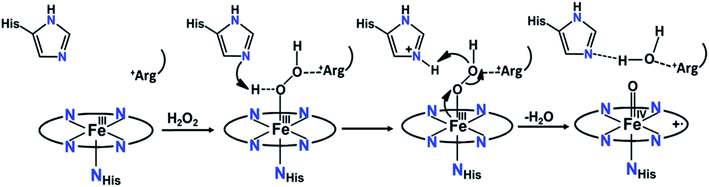 | ||
| Fig. 1 Mechanism for the formation of the Compound I intermediate in peroxidases, involving the His and Arg residues of the heme distal side.15,27,28 | ||
The arginine residue, being positively charged at neutral pHs, likely helps in polarization of the O–O bond and promotes heterolysis as its mutation leads to a two orders of magnitude reduction in the rates.28 This combined role of histidine and arginine residues in the heme distal side exerts a “Pull-effect” and results in facile heterolytic cleavage of the O–O bond.20,24,26,29 Such a “Pull effect” is also exhibited by other peroxidases with a similar distal acid-base residue.30–32 The arginine residue forms a hydrogen bond with the ferryl oxygen atom of Compound I and thus may stabilize the high valent reactive intermediates.28 The arginine residue enhances the binding of a substrate to the peroxidase active site as its mutation increases KM of the substrate.33–35 Peroxidases such as HRP show minimal heme degradation during catalysis, mostly occurring in the presence of very high molar excess of the oxidant.36,37
Over the last several decades, several synthetic systems were designed for the investigation of the “Push–Pull” effect and the formation of Compound I was examined using various oxidants.38–41 Watanabe and coworkers monitored the formation of Compound I in an organic medium with peracids in a series of synthetic iron-porphyrins by varying the substituents in their meso phenyl rings and also using substituted axial imidazole ligands.42 Groves and coworkers demonstrated that the nature of the bond cleavage process can be controlled by changing the polarity of solvents in some systems.43,44 Fujii and coworkers examined the spin coupling interaction between the ferryl iron and the porphyrin π-cation radical using EPR spectroscopy to probe the a1u/a2u character of the high-valent intermediates in several iron porphyrins with different substituents in meso phenyl rings and β-pyrroles.45 Nocera and co-workers introduced hanging carboxylic acid residues to impose 2nd sphere interactions with reactive intermediate mimicking the distal residues in the protein active site which promote heterolytic bond fission.46,47 Unfortunately, the reported rates for O–O bond heterolysis did not indicate a substantial role of the pendent carboxylic acids.46 In a very recent work, incorporation of a pendent amine group into the 2nd coordination sphere of a non heme iron complex has been reported to form selectively the FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate via heterolytic O–O bond cleavage with a lower activation energy barrier by the reaction of the non-heme Fe(II) complex with H2O2.5
O intermediate via heterolytic O–O bond cleavage with a lower activation energy barrier by the reaction of the non-heme Fe(II) complex with H2O2.5
To date, the role of the histidine residue as an acid-base catalyst in the distal site of peroxidases remains to be successfully modeled in any synthetic heme-based model system. Peroxidases generally oxidize organic phenols and aromatic amines using an outer sphere electron transfer and oxidation of substrates like epoxidation and sulfoxidation, traditionally performed by monooxygenases, is reported.48–53 So far, molecular mimics of the Compound I intermediate of peroxidases are reported to catalyze epoxidation and sulfoxidation with a low turnover number or yield.47,54,55 Yet, catalytic oxidation of phenols and amines, the typical substrates for peroxidases with a significant turnover number, has not been achieved.56–58 This is particularly important as the hydroperoxidase activity is a key technology for degradation of organic contaminants in water. Thus, facile generation of a synthetic reactive intermediate mimicking Compound I to be used for catalytic transformations and retarding its self-degradation is of interest to a broad community of chemists. Recently, mononuclear (ferric) iron porphyrins with pendent basic groups were reported (Fig. 2B and C) which could reduce oxygen to water with greater than 95% selectivity at pH 7 with rates >107 M−1 s−1.59 The origin of such high reactivity and much desired selectivity in simple mononuclear iron porphyrin was proposed to originate from specific proton transfer to the distal oxygen, akin to peroxidases, of a putative ferric hydroperoxide species from the pendent basic groups which remains protonated at pH 7. These proposals were supported by DFT calculations.59
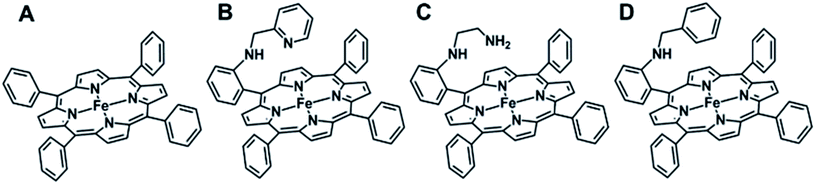 | ||
| Fig. 2 Molecular structures of iron–porphyrin complexes used in this study: (A) FeIIITPP, (B) FeIIIL2, (C) FeIIIL3 and (D) FeIIIMPh. | ||
In this work, we have used a series of ferric iron porphyrin complexes with pendent basic groups covalently attached to the amine group of the meso-phenyl ring (see Fig. 2) in the tetraphenylporphyrin architecture, in order to investigate the rates of O–O bond heterolysis and to characterize the ensuing intermediates formed. Compound I is detected upon oxidation of these ferric iron porphyrin complexes with a peracid (m-chloroperbenzoic acid), as clearly shown by the stopped-flow kinetics and EPR spectroscopic characterization. Pyridine and amine, designed as pendent groups emulating the distal His residue in natural peroxidases (see Fig. 1), translocate protons from the proximal to distal oxygen atom of bound m-chloroperbenzoic acid to facilitate the heterolytic cleavage pathway with much higher rates than the benzyl analogue (Fig. 2D, FeIIIMPh(Br)), a structurally analogous iron porphyrin without base, and also than any previously reported synthetic porphyrins thus with rates comparable to natural peroxidases. The results also suggest that the self-degradation of the FeIII porphyrin catalyst was prevented, most likely by the hydrogen bonding interaction of the pendent base with the FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O center of Compound I, thus allowing these synthetic hemes to efficiently catalyze the oxidation of organic and inorganic substrates using peracids. Density Functional Theory (DFT) calculations support the experimentally observed activation of a bound peroxide by pendent bases and help in developing a molecular orbital theory rationale of the elusive “pull effect”.
O center of Compound I, thus allowing these synthetic hemes to efficiently catalyze the oxidation of organic and inorganic substrates using peracids. Density Functional Theory (DFT) calculations support the experimentally observed activation of a bound peroxide by pendent bases and help in developing a molecular orbital theory rationale of the elusive “pull effect”.
Results and discussion
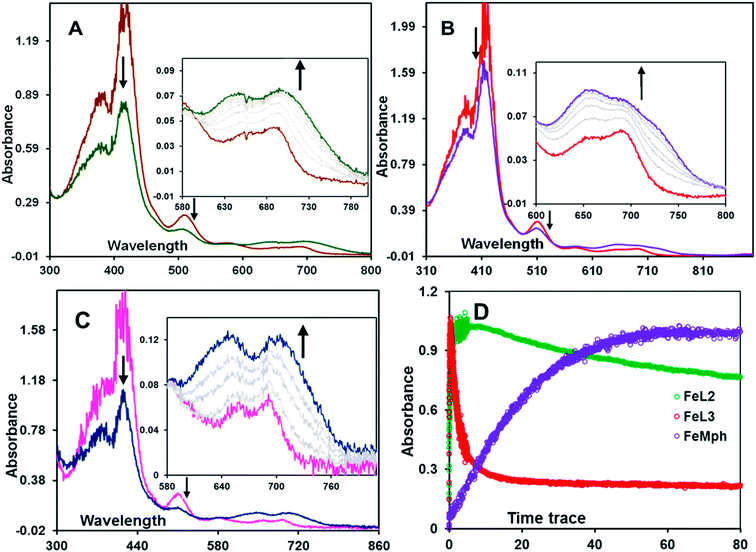
The formation of high valent iron-oxo intermediates was examined for four iron(III) porphyrin model complexes (Fig. 2) e.g., FeIIIL2(Br) and FeIIIL3(Br) each containing a distinct pendent (basic) residue pyridine and aliphatic amine, respectively, FeIIIMPh(Br) containing a mono benzyl group without additional basic residue and simple mononuclear iron porphyrin without any second sphere residue (FeIIITPP(Br)) (Fig. 2). m-CPBA was used as an oxidant. The pKa values of the pendent pyridine and amine groups in FeIIIL2(Br) (Fig. 2A) and FeIIIL3(Br) (Fig. 2B) complexes were determined to be 12.33 and 16.90, respectively, in CH3CN.60 The reactions were monitored at sub-zero temperatures using a stopped-flow UV-vis electronic absorption spectrometer.Upon reaction of FeIIIL2(Br) with m-CPBA in dichloromethane (DCM) at −30 °C, the electronic absorption spectrum of the ferric state showed a decrease in the intensity of the Soret (417 nm) and charge transfer bands at 510 nm, 594 nm and 695 nm. In addition, a new and rather broad band centered at ca. 705 nm was also observed (Fig. 3A). Similar changes were observed for the FeIIIL3(Br) complex under the same conditions where a broad band appeared at ca. 726 nm (Fig. 3B). In the case of the FeIIIMPh(Br) complex, where no basic residue is present, a new broad band at ca. 705 nm (Fig. 3C) was observed. The broad charge transfer band at ∼700 nm is consistent with the formation of the iron(IV)-oxo porphyrin cation radical intermediate, Compound I, previously reported in synthetic model complexes and in enzyme systems.43,46,61–65
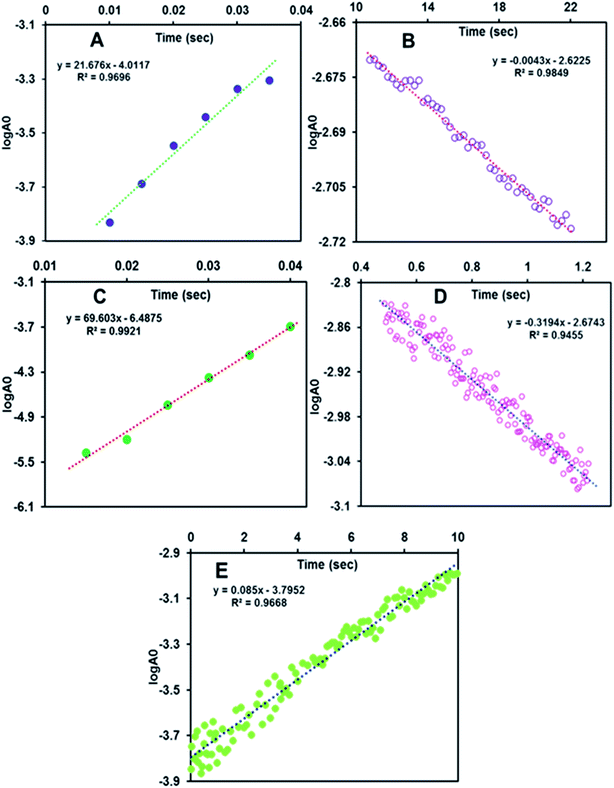
The formation of the 705 nm band of FeIIIL2(Br) maximizes within 5 s (Fig. 3D, green trace & S1A,† red trace). The species is quite stable at −30 °C and disappears in ca. 5 minutes (Fig. S4B,† blue trace). However, the formation of this band takes much longer time, i.e. around 1 min, in the case of FeIIIMPh(Br) (Fig. 3D & S1C,† violet trace). The absorbance at the 726 nm band for FeIIIL3(Br) maximizes within 500 ms (Fig. S1B,† green trace) and decays by 10 s (Fig. 3D, red trace & S4A,† green trace) at −30 °C. The rate constants for the formation of Compound I are 21.67 ± 1 s−1, 67.6 ± 2 s−1 and (8.5 ± 1) × 10−2 s−1 for FeIIIL2(Br), FeIIIL3(Br) and FeIIIMPh(Br), respectively (Fig. 4) at −30 °C (i.e. 243 K). A more than two orders of magnitude faster O–O bond heterolysis in FeIIIL2 and FeIIIL3 with respect to FeIIIMPh provides definitive proof of the advantage of the pendent nitrogenous bases during heterolytic cleavage. The first order rate constants for these two complexes are even much higher than those for previously reported model complexes (Table 1). The rate constants for the decay of these bands are (3.2 ± 0.5) × 10−1 s−1 and (5 ± 0.7) × 10−3 s−1 for FeIIIL2(Br) and FeIIIL3(Br), respectively, obtained from the first order exponential fits to the absorbance vs. time traces (Fig. 4). The Compound I intermediate for both of these complexes is converted to the corresponding Compound II (FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) species as indicated by the characteristic bands at 650 nm and 657 nm for FeIIIL2(Br) and FeIIIL3(Br), respectively, which is stable for several minutes at −30 °C (Fig. S3†). Note that the formation and decay of Compound I can be fit using a kinetic model for consecutive reactions (Fig. S4†) suggesting that the disproportionation of Compound II to form Compound I and ferric species is not observed here as has been reported for non-heme systems.66–68
O) species as indicated by the characteristic bands at 650 nm and 657 nm for FeIIIL2(Br) and FeIIIL3(Br), respectively, which is stable for several minutes at −30 °C (Fig. S3†). Note that the formation and decay of Compound I can be fit using a kinetic model for consecutive reactions (Fig. S4†) suggesting that the disproportionation of Compound II to form Compound I and ferric species is not observed here as has been reported for non-heme systems.66–68
| Catalyst | k heterolysis (s−1) | Temperature | ΔG≠ (kcal mol−1) | Ref. |
|---|---|---|---|---|
| a The molecular structures (6a, 6b, 6d and 6e) corresponding to ref. 41 are given in Fig. 6. Here, meta-chloroperbenzoic acid is used as an oxidant for all the catalysts except for ref. 53, 71 and 73 (where hydrogen peroxide is the oxidant). | ||||
| 6a | 16.6 × 10−3 | −80 °C | 12.78 | 41 |
| 6b | 4 × 10−3 | −80 °C | 13.33 | 41 |
| 6d | 3.94 × 10−3 | −40 °C | 16.19 | 41 |
| 6e | 0.5 × 10−4 | −80 °C | 14.14 | 41 |
| FeIIITMP | (6.5 ± 1) × 10−3 | −40 °C | 15.96 ± 0.1 | 45 |
| FeIII(HPX-CO2H) | (6 ± 3) × 10−3 | −40 °C | 15.99 ± 0.2 | 45 |
| FeIII(HPX-CO2Me) | (1 ± 0.2) × 10−2 | −40 °C | 15.76 ± 0.1 | 45 |
| FeIIIL2 | 21.67 ± 1 | −30 °C | 12.72 ± 0.02 | This work |
| FeIIIL3 | 69.6 ± 2 | −30 °C | 12.15 ± 0.01 | This work |
| FeIIIMPh | (8.5 ± 1) × 10−2 | −30 °C | 15.08 ± 0.05 | This work |
| HRP | 200–500 s−1 | 25 °C | 13–14 | 53 and 70 |
| H42A/H42V | (5–50) × 10−4 | 25 °C | 21 ± 1 | 71 and 73 |
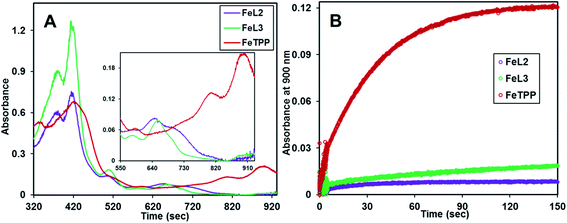
The reaction of m-CPBA with FeIIITPP(Br) under identical conditions did not show the formation of Compound I, rather it showed a very slow formation of isoporphyrin, a species isoelectronic with Compound I and an intermediate in heme degradation of the heme oxygenase cycle that has characteristic bands at 810 nm and 900 nm (Fig. 5A, red trace and Fig. S2†) suggesting the decay pathway of the porphyrin macrocycle of FeIIITPP(Br) consistent with previous literature (Scheme S1†).36,62,69 Alternatively, the FeIIIL2(Br) and FeIIIL3(Br) complexes form their high valent oxo species and decay to their resting ferric state in their reaction with mCPBA in contrast to FeIIITPP(Br) (Fig. 5A). Note that minor and sluggish formation of isoporphyrin is detected in FeIIIL2(Br), FeIIIL3(Br) and FeIIIMPh(Br) as indicated by the absorption spectra and time traces of 800 nm and 900 nm bands (Fig. 5A and B; S1A–S1C†) and is likely due to competitive binding of m-CPBA at the open site of the porphyrin, which logically follows a FeIIITPP-like reactivity (Fig. S2†). Overall, it is clear that the pendent basic groups avoid degradation of the heme upon its reaction with peracids.
The rates of Compound I formation from ferric porphyrins via heterolysis of the O–O bond of bound peracid (m-CPBA) are obtained at −30 °C. Since the kinetics for formation of the Compound I have been obtained at different temperatures than previously reported metalloporphyrin systems, a direct comparison of the rate constants is not possible (Fig. 6, Table 1). The activation energies (ΔG≠) for O–O bond heterolysis of FeIIIL2 and FeIIIL3 are obtained from the observed rates using the Eyring equation and compared to those calculated for different model iron porphyrins (Table 1) from their reported rates.42,46 The lowest energy barrier attained is 12.78 kcal mol−1 for complex 6a (Fig. 6) which has the most electron rich porphyrin ring among all of the synthetic models reported to date. The presence of a large number of electron donating methoxy substituents in 6a makes it facile to undergo faster heterolytic O–O bond cleavage than any other meso substituted porphyrins as reported by Watanabe et al.42 The ΔG# values of FeIIIL2(Br), FeIIIL3(Br) and FeIIIMPh(Br) for O–O bond heterolysis are calculated be 12.72 ± 0.02 kcal mol−1, 12.15 ± 0.01 kcal mol−1 and 15.08 ± 0.05 kcal mol−1, respectively. The activation energies of pendent base containing models match well with those of the earlier reported models (6a and 6b, Table 1). We have derived two other activation parameters i.e. ΔH# (2.34 ± 0.2 kcal mol−1) and ΔS# (−44.60 ± 1 cal mol−1 K−1) from the linear Arrhenius plot of the temperature dependent kinetic study for Compound I formation of FeIIIL2(Br). A remarkably low enthalpic barrier and large negative entropy of activation also support the facile O–O bond cleavage step. ΔG# (13.03 ± 0.3 kcal mol−1) is also determined from kinetics at different temperatures from the sum of ΔH# and ΔS# which is in good agreement with that obtained directly from the first order rate constants using the Eyring equation (details in the ESI†). It is important to note that ΔG# for O–O bond heterolysis is 3–4 kcal mol−1 lower in FeIIIL2 and FeIIIL3 relative to structurally analogous FeIIIMPh, FeIIITMP and iron-hangman porphyrins which have pendent carboxylic acid/ester groups (Table 1) directly establishing the efficacy of pendent basic groups in the enhancement of heterolytic O–O bond cleavage rates. Additionally, a three times faster rate and 0.5 kcal mol−1 lower ΔG# of FeIIIL3(Br) with an aliphatic amine (pka ∼ 16.9 in CH3CN) than those of FeIIIL2(Br) with a pyridine group (pka ∼ 12.33 in CH3CN) suggest that the O–O bond heterolysis is favored by pendent amine groups having higher basicity than the peracid. Therefore, these residues can easily act as efficient acid-base catalysts and are capable of deprotonating the distal oxygen atom of bound peracid and transferring the proton to a distal oxygen atom that is necessary for heterolysis. The rate of Compound I formation from m-CPBA in native HRP is 200–500 s−1 and two histidine mutants of HRP; H42A and H42V show 105 to 106 order lowering of the rate i.e. (5–50) × 10−4 s−1 at room temperature.53,70,71 The corresponding activation energy barrier for HRP (∼13–14 kcal mol−1) is much lower than the histidine mutants (21 ± 1 kcal mol−1) and comparable to that achieved in these synthetic models FeIIIL2(Br) and FeIIIL3(Br). Thus, the “pull effect” in peroxidases is successfully mimicked in synthetic models including pendent basic groups (analogous to the histidine residue) in the 2nd sphere of the iron porphyrin. Note that, mutation of the His42 residue in HRP leads to several orders of magnitude decrease in the rate of Compound I formation via O–O bond heterolysis,27,72,73 which is reasonably captured in two orders of magnitude reduction in the rate of Compound I formation in the FeMPh relative to FeL2 and FeL3.
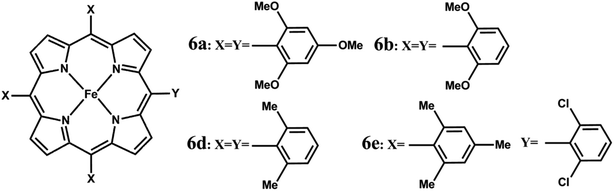 | ||
| Fig. 6 Structures of model iron-porphyrins referred to in Table 1. | ||
Identification of the high-valent intermediates by EPR spectroscopy
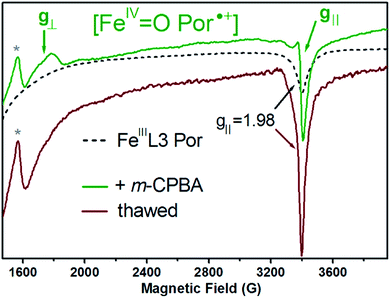
To further identify and characterize the catalytically-relevant high-valent intermediates (Compound I and Compound II) proposed from the spectral changes in the electronic absorption spectra (Fig. 3 and S3A†), we applied 9 GHz cw-EPR spectroscopy at liquid helium temperatures, and monitored the reaction of the FeIIIL3 porphyrin complex with m-CPBA (both in dichloromethane). The reaction of the FeIIIL3 porphyrin complex with 10-fold molar equivalents of m-CPBA was done by manual mixing and directly in a quartz EPR tube placed in a ca. −100 °C bath, in order to trap the short-lived intermediates. In particular, we aimed to characterize the short-lived intermediate assigned to Compound I, showing an electronic absorption CT band at 705 nm and appearing within 500 ms upon a reaction of the FeIIIL3 Por complex with m-CPBA at −30 °C (Fig. 3A and S3A†). The subsequent intermediate with an electronic absorption CT band at 657 nm, and that was assigned to Compound II, proved to be stable for several minutes at −30 °C (Fig. S3A†). It is of note that the concentrations of the FeIIIL3 porphyrin complex used for the EPR experiments differ substantially to those used in the electronic absorption experiments, thus the yields and life time of the intermediates are not expected to be the same (see below).The FeIIIL3 complex shows an axial EPR spectrum with observed geff values at g⊥ = 6.04 and g‖ = 1.98 (Fig. S5† and 7, black dotted traces), which is typical for heme iron in the ferric high-spin state. Upon reaction with m-CPBA a new and distinct EPR signal was observed: an axial and very broad (2000 G) EPR spectrum, extending between the two resonances with observed g-values of 3.80 and 1.99 (Fig. 7, green trace) that is consistent with the expected axial EPR spectrum of an exchange-coupled oxoferryl-porphyrin radical species, [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] with geff⊥ = 3.80 and geff‖ = 1.99, as previously reported for the porpholactone ferryl radical complexes74 and iron porphyrins that are highly nonplanar, but not ruffled.45,75 The fact that this new broad and axial EPR signal could only be detected at temperatures lower than 30 K (due to the line broadening effect76) well agrees with the expected behavior of a radical in magnetic interaction (exchange-coupling) with the ferryl moiety. Interestingly, the EPR spectrum is virtually identical, including the overall width (ca. 250 G) of the resonance at g = 3.80 (g⊥) and the narrow and asymmetric resonance at g = 1.99 (g‖), to that of the previously reported [FeIV
O Por˙+] with geff⊥ = 3.80 and geff‖ = 1.99, as previously reported for the porpholactone ferryl radical complexes74 and iron porphyrins that are highly nonplanar, but not ruffled.45,75 The fact that this new broad and axial EPR signal could only be detected at temperatures lower than 30 K (due to the line broadening effect76) well agrees with the expected behavior of a radical in magnetic interaction (exchange-coupling) with the ferryl moiety. Interestingly, the EPR spectrum is virtually identical, including the overall width (ca. 250 G) of the resonance at g = 3.80 (g⊥) and the narrow and asymmetric resonance at g = 1.99 (g‖), to that of the previously reported [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] intermediate of nitrophorin, a heme-containing enzyme showing substantial peroxidase-like activity.77 When thawing and incubating the EPR sample for 15 min at 20 °C, the resulting EPR spectrum (Fig. 7, dark red trace) showed the complete disappearance of the broad axial signal of the [FeIV
O Por˙+] intermediate of nitrophorin, a heme-containing enzyme showing substantial peroxidase-like activity.77 When thawing and incubating the EPR sample for 15 min at 20 °C, the resulting EPR spectrum (Fig. 7, dark red trace) showed the complete disappearance of the broad axial signal of the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] species, as expected if considering the short-lived character of this intermediate demonstrated by the stopped-flow electronic absorption experiments (Fig. S3A,† brown trace).
O Por˙+] species, as expected if considering the short-lived character of this intermediate demonstrated by the stopped-flow electronic absorption experiments (Fig. S3A,† brown trace).
Compound II, a reaction intermediate (absorption CT band at 657 nm, Fig. S3A†) formed subsequently to the Compound I in the stopped-flow absorption experiments is an integer spin species ([FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]), thus EPR-silent. The fact that the EPR signal of the trapped [FeIV
O]), thus EPR-silent. The fact that the EPR signal of the trapped [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] species does not fully account for the conversion of the ferric signal upon reaction with m-CPBA (as estimated by the decrease of the ferric EPR signal; see Fig. S5†) is fully consistent with the subsequent formation of the oxoferryl intermediate, an EPR-silent species. In addition, the higher stability of this intermediate, as shown by the stopped-flow experiments (Fig. S3A,† green trace), is consistent with the partial recovery of the ferric signal, reflected by the increase of its g‖ = 1.98 component (Fig. 8, dark red trace and Fig. S5,† dark green trace) when thawing the EPR sample for 15 min at 20 °C (see above). Further thawing and incubation of the sample (at 20 °C for 60 min) showed a small further increase in the intensity of the ferric signal (see Fig. S6†), thus confirming that the [FeIV
O Por˙+] species does not fully account for the conversion of the ferric signal upon reaction with m-CPBA (as estimated by the decrease of the ferric EPR signal; see Fig. S5†) is fully consistent with the subsequent formation of the oxoferryl intermediate, an EPR-silent species. In addition, the higher stability of this intermediate, as shown by the stopped-flow experiments (Fig. S3A,† green trace), is consistent with the partial recovery of the ferric signal, reflected by the increase of its g‖ = 1.98 component (Fig. 8, dark red trace and Fig. S5,† dark green trace) when thawing the EPR sample for 15 min at 20 °C (see above). Further thawing and incubation of the sample (at 20 °C for 60 min) showed a small further increase in the intensity of the ferric signal (see Fig. S6†), thus confirming that the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] species is rather stable at room temperature, as previously reported for natural peroxidases. For example, in the case of the bi-functional peroxidases KatGs, the [FeIV
O] species is rather stable at room temperature, as previously reported for natural peroxidases. For example, in the case of the bi-functional peroxidases KatGs, the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] species is very short-lived while the [FeIV
O Por˙+] species is very short-lived while the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] intermediate converts back to the ferric resting state only after 12 min at 20 °C, provided that all the oxidant has been used up.78 This implies that in the FeIIIL3 porphyrin complex the hydrogen bonding stabilizes the oxoferryl intermediate.
O] intermediate converts back to the ferric resting state only after 12 min at 20 °C, provided that all the oxidant has been used up.78 This implies that in the FeIIIL3 porphyrin complex the hydrogen bonding stabilizes the oxoferryl intermediate.
It is of note that Dawson and coworkers, using rapid-scan stopped flow absorption spectroscopy, reported that m-CPBA can play both the roles of an oxidant and a substrate in the formation of the catalytic intermediates of C. fumago chloroperoxidase.79 It was concluded that m-CPBA acts as an oxidant in the conversion of the ferric enzyme to the Compound I intermediate, and also as a one-electron donor (i.e. ‘substrate’) in the subsequent reductions of Compound I to Compound II, and of Compound II back to the ferric native enzyme.79 In the case of the FeIIIL3 porphyrin complex reacting with m-CPBA, further incubation at −100 °C for 3 s showed an increase of the ferric EPR signal and no detectable change of the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] EPR signal (Fig. S6†). This result can be rationalized as the reaction of the [FeIV
O Por˙+] EPR signal (Fig. S6†). This result can be rationalized as the reaction of the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] intermediate with the remaining m-CPBA, as in the case of chloroperoxidase. It is of note that intramolecular e− transfer reactions can readily occur at such low temperatures.78 Taken together, these EPR experiments confirm that the [FeIV
O] intermediate with the remaining m-CPBA, as in the case of chloroperoxidase. It is of note that intramolecular e− transfer reactions can readily occur at such low temperatures.78 Taken together, these EPR experiments confirm that the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] intermediate formed by the peroxidase-like reaction (Compound 0–Compound I) of the FeIIIL3 porphyrin complex with m-CPBA is a short-lived species even at subzero temperatures, and that the subsequent intermediate, the [FeIV
O Por˙+] intermediate formed by the peroxidase-like reaction (Compound 0–Compound I) of the FeIIIL3 porphyrin complex with m-CPBA is a short-lived species even at subzero temperatures, and that the subsequent intermediate, the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] species (Compound II), formed most possibly by the reaction of the [FeIV
O] species (Compound II), formed most possibly by the reaction of the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] intermediate with m-CPBA, is relatively stable at room temperature. In addition, the [FeIV
O Por˙+] intermediate with m-CPBA, is relatively stable at room temperature. In addition, the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] intermediate can react slowly with m-CPBA as a one-electron donor, resulting in a partial cycling back to the ferric resting state.
O] intermediate can react slowly with m-CPBA as a one-electron donor, resulting in a partial cycling back to the ferric resting state.
The Compound I formed in most synthetic (ferric) iron porphyrin complexes readily attacks the meso position of the porphyrin macrocycle leading to the formation of an isoporphyrin, an intermediate leading to the eventual degradation of the iron porphyrin complex (Scheme S1†). Accordingly, the UV-vis absorption spectrum of FeIIITPP(Br) upon reaction with m-CPBA showed the appearance of the broad absorption bands at 800–900 nm (Fig. 5, red trace & S2†) consistent with the isoporphyrin species. At variance, FeIIIL2(Br) and FeIIIL3(Br) complexes showed very minor formation of isoporphyrin (Fig. 5A and B, violet and green traces in Fig. 5B) and converted to Compound II then eventually cycled back to the ferric complex, as indicated by the UV-Vis spectra and consistent with the EPR spectroscopic characterization (see above). These results suggest that not only the basic groups in FeIIIL2 and FeIIIL3 accelerate the heterolysis of the peroxide inciting the formation of Compound I, but also decelerate the electrophilic attack of Compound I on the porphyrin ring. This attribute implies that FeIIIL2(Br) and FeIIIL3(Br) should be able to catalyze oxidation of substrates by m-CPBA.
Catalytic oxidation of substrates
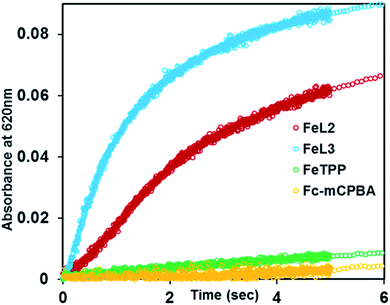
The reaction of FeIIIL2(Br) and FeIIIL3(Br) with m-CPBA in the presence of excess ferrocene (Fc) as an external sacrificial electron donor was monitored. The formation of a new band at 620 nm, characteristic of ferrocenium (Fc+), indicates the oxidation of ferrocene by the porphyrin complexes (Fig. 8 and S7†). In the presence of excess ferrocene and m-CPBA, both FeIIIL2 and FeIIIL3 can catalyze its oxidation with turnover numbers (TONs) of 67 and 96, respectively, and turnover frequencies (TOFs) of 2.68 s−1 and 6.40 s−1, respectively (Table 2). The catalytic turnover entails oxidation of two molar equivalents of ferrocene per Compound I species, i.e. Compound I + 2Fc → FeIIIL + 2Fc+ (Scheme 1). This result is expected, as Eo of Fc is lower than those of both Compound I and Compound II. Similar to the case of peroxidases, the organic substrate 2,4,6-tri tertiarybutyl phenol (TBPH) is oxidized to the corresponding phenoxy radical, as shown by the absorption spectra with bands at 380 nm, 400 nm and 625 nm (Fig. S7B†) and the EPR spectrum measured at 77 K shows a signal at g = 2 consistent with an organic radical (Fig. S8A†).80 Both FeIIIL2(Br) and FeIIIL3(Br) can catalyze the oxidation of TBPH with TONs of 25 and 350, respectively, with TOFs of 1.41 s−1 and 0.63 s−1, respectively (Table 2). Similarly, the two-electron oxidation of o-phenylene diamine (OPD) to 2,3-diaminophenazine with a characteristic absorption feature at 450 nm for these two iron porphyrins (Fig. S10†) yields TONs of 68 and 61, respectively, and the corresponding TOFs are 0.85 s−1 and 2 s−1, respectively. Note that these are the first examples of catalytic oxidation of phenols and amines by one electron (normal peroxidase activity) using synthetic heme complexes, and using peracid as an oxidant.| Substrate (2 mM) | FeIIIL2(Br) (4 μM) | FeIIIL3(Br) (4 μM) | ||
|---|---|---|---|---|
| TONa | TOFb (s−1) | TONa | TOFb (s−1) | |
| a The maximum number of product molecules obtained from per mole of the catalyst. b TON per unit time (sec). c Stopped flow kinetics at −30 °C. d UV-visible kinetic measurements at room temperature. The other conditions are described in the Experimental section. | ||||
| Ferrocene (Fc)c | 67 | 2.68 | 96 | 6.40 |
| 2,4,6-Tri tertiarybutyl phenol (TBPH)d | 25 | 1.41 | 350 | 0.63 |
| o-Phenylene diamine(OPD)d | 68 | 0.85 | 61 | 2 |
DFT calculations
Having shown that facile O–O heterolysis in FeIIIL2(Br) and FeIIIL3(Br) allows rapid formation of Compound I which at its turn can oxidize ferrocene and TBPH, we performed DFT calculations to gain theoretical insight into the effect of pendent basic groups covalently attached to iron-porphyrins in relation to such O–O bond cleavage. The structures of m-CPBA bound metal complexes are optimized in the PCM model using dichloromethane as a solvent. In general, all attempts of optimizing the peracid bound ferric state inevitably lead to spontaneous O–O heterolysis forming Compound I and m-chloroperbenzoic acid (Fig. 9) likely due to the acidic proton of mCPBA (pKa = 7.5 in water). Alternatively, the electronic structure contribution of the pendent basic groups to O–O bond heterolysis can be evaluated with H2O2 which has a higher pKa (11.75). Note that the use of hydrogen peroxide is more relevant to the chemistry of peroxidases. The structures with bound hydroperoxide resulting in low spin ferric porphyrins with protonated pendent basic groups are optimized (Fig. S11†). In the case of [FeIIIL2-OOH]H+, the –N1H group and protonated pyridine (N2H+) act as H-bond donors to the distal oxygen atom. The optimized geometry shows that the O–O bond length for [FeIIIL2-OOH]H+ is elongated to 1.88 Å in the protonated structure (Fig. S11†) which is much longer than those of FeIIITPP-OOH (the O–O bond length is 1.46 Å) and free H2O2.59The same trend also occurs for protonated FeIIIL3-OOH, where the protonated aliphatic amine group (–NH3+) elongates the O–O bond length to 1.86 Å (Fig. S11†). All these optimized geometries suggest that strong H-bond donation to the distal oxygen atom of the FeIII–OOH species facilitates activation of the O–O bonds. These appended groups in the studied porphyrin complexes appear to be oriented in such a way to allow selective delivery of protons to the distal oxygen atom with simultaneous weakening of the O–O bond, both factors beneficial for heterolytic O–O bond cleavage.59
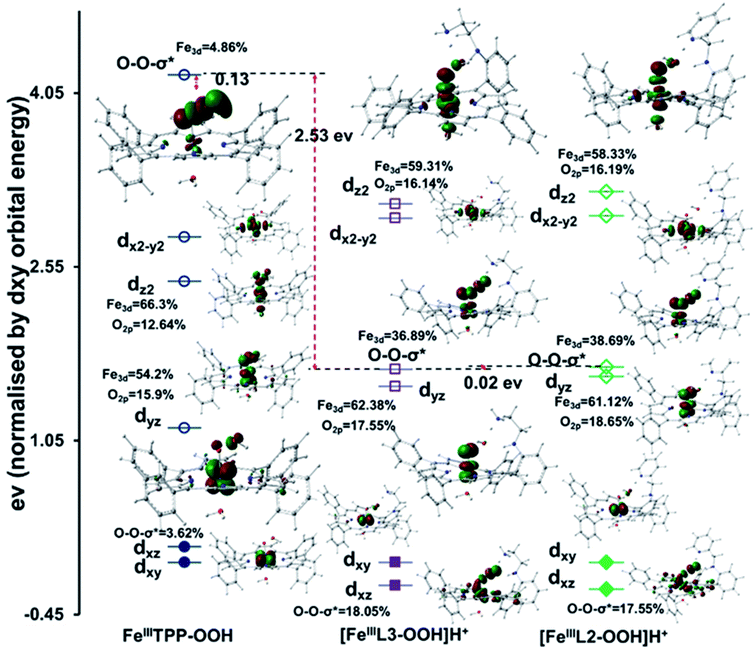
The substantial activation of the O–O bond of a FeIII–OOH species with H-bonding by a protonated base is the root of the “pull effect” proposed in peroxidase active sites where an analogous FeIII–OOH species is produced, Compound 0.24,81,82 The DFT calculated wave functions show that the Fe dπ character in the O–O σ* orbitals increases from 4.86% in the non H-bonded system to 36.89% and 38.69% in porphyrins having protonated OOH]H+, respectively (Fig. 10).
The enhanced back-bonding is facilitated by the lowering of the O–O σ* orbital energies due to hydrogen bonding to the pendent basic groups of [FeIIIL3-OOH]H+ and [FeIIIL2-OOH]H+ groups (Fig. 10) which polarize the electron density of the O–O bond towards the distal OH group reducing the overlap between the two O2p orbitals and lowering the energy of the O–O σ* orbital. This polarization is magnified when the amines are protonated. This is evident from the calculated Mulliken charge density values on distal oxygen atoms for [FeIIIL3-OOH]H+ (Op = −0.30 and Od = −0.60) and [FeIIIL2-OOH]H+ (Op = −0.29 and Od = −0.61) which are more negative than those of the FeIIITPP analogue (Op = −0.293 and Od = −0.383) without any hydrogen bonding residue (Fig. S12†). Thus the “pull effect” in this case may be interpreted as pulling the energy of the O–O σ* orbital down substantially by hydrogen bonding to a protonated basic residue to allow better back-bonding into the O–O σ* orbital from the filled Fe dπ orbital of the metal. The O2p coefficient increases from 3.62% in FeIIITPP-OOH to 18.05% in [FeIIIL3-OOH]H+ and 17.55% in [FeIIIL2-OOH]H+ in the dπ orbital signifying increased covalent donation from the proximal oxygen to the empty Fe dπ orbital i.e. strong π bonding (Fig. 10). A similar increase in σ covalency is observed in the dz2 orbital. These are consistent with the shortening of the Fe–O bond upon elongation of the O–O bond taking the reactant FeIII–OOH to the product [Fe(IV) ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+].
O Por˙+].
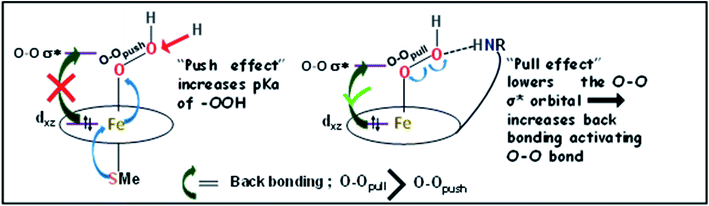
In nature, the alternative mechanism of activating a FeIII–OOH used in Cyt P450 and CPO is the “push effect” of an axial thiolate ligand. The anionic thiolate ligand enhances the electron density of its trans axial ligand increasing the basicity. This enhanced pKa (∼12) leads to a protonated Compound II in Cyt p450/CPO when Compound II is deprotonated in peroxidases/myoglobin with a pKa of around 3.83,84 DFT calculations on a hypothetical thiolate bound FeIIITPP-OOH species show a O–O bond length of 1.46 Å which is shorter than that of [FeIIIL2-OOH]H+ with a protonated pendent base (1.88 Å) and the Fe–O bond length of thiolate bound FeIIITPP-OOH is calculated to be 1.85 Å which is shorter than that of [FeIIIL2-OOH]H+ (Fig. S11†). The Mulliken charge on the hydroperoxide unit of the thiolate bound FeIIITPP-OOH species is calculated to be Op = −0.362 and Od = −0.422. The negative charge of the distal oxygen atom of the bound hydroperoxide is substantially more negative than a FeIIITPP-OOH without axial thiolate (Op = −0.293, Od = −0.383) (Fig. S12†). The MO diagram of the thiolate bound FeIIITPP-OOH shows much less electron donation by the bound –OOH to the Fe dσ and dπ orbitals consistent with greater anionic charge on the bound hydroperoxide relative to FeIIITPP-OOH. There is very little Fe3d character in O–O σ* suggesting the lack of back-bonding from the filled Fe3d consistent with the short O–O distance (Fig. S13†). Thus the “push effect” of the thiolate is limited to push electron density to the bound –OOH ligand increasing its basicity causing its protonation at physiological pH (Fig. 11). Once protonated, the resulting FeIII–OOH2+ species will have a very weak O–O bond which would cleave almost spontaneously. Alternatively, the “pull effect” of the pendent basic group results from polarization of the electron density of the peroxide resulting in lowering the O–O σ* energy (Fig. 11) which allows greater back-bonding into O–O σ* from the filled 3d orbitals of the iron due to better energy match, and thus weakening of the O–O σ bond activates the O–O bond for cleavage.
Conclusion
Inclusion of pendent basic groups in iron porphyrins, mimicking the 2nd sphere interactions present in the distal site of HRP, enables facile O–O bond heterolysis of peracids having barriers lower than naturally occurring enzymes. The Compound I generated by these iron porphyrin complexes with 2nd sphere coordination is capable of oxidizing two equivalents of substrates (both organic and inorganic) thus making them functional models of natural peroxidases. The introduced basic groups, mimicking the His–Arg base pair in the active site of horseradish peroxidase, not only enable facile heterolysis but also stabilize the Compound I and Compound II intermediates. More importantly, electrophilic attack on the porphyrin macrocycle is prevented and such intermediates effectively oxidize organic and inorganic substrates, using an outer-sphere electron transfer mechanism. Accordingly, these synthetic complexes act as efficient catalysts with reasonable TONs and TOFs. Investigations are underway to understand how the hydrogen bonding tunes the reactivity of the high valent oxoferryl species from electrophilic inner-sphere oxidation (monooxygenase-like) to outer-sphere electron transfer (peroxidase-like).Experimental procedures
Instrumentation
All UV-visible absorption spectra were recorded on an Agilent 8453 diode array spectrophotometer in a cuvette of 1 cm path length at room temperature unless otherwise mentioned. Rapid mixing stopped flow kinetics were performed on a SFM 4000 spectrophotometer (halogen-deuterium lamp as the light source) equipped with a low temperature chamber. The 4 K 9 GHz EPR spectra were recorded on a Bruker EleXsys E500 spectrometer equipped with a standard Bruker ER4102 X-band resonator and a liquid helium cryostat (Oxford Instruments, ESR 900). EPR quartz tubes of 4 mm external diameter were used. A JEOL FA200 spectrometer was used for the measurements at 77 K.Materials
All reagents were of the highest grade commercially available and were used without further purification. The iron-porphyrins (FeIIIL2(Br), FeIIIL3(Br) and FeIIITPP(Br)) studied here were synthesized according to previously reported procedures.59 3-Chloroperoxybenzoic acid (m-CPBA) was purchased from Aldrich (77%) and purified by washing with pH 7.40 phosphate buffer and recrystallized from pentane to remove 3-chlorobenzoic acid. Purity (>95%) was determined by 1H NMR. Solvents were purchased from RANKEM with HPLC grade for spectroscopic purpose. 2,4,6-Tritertiary butyl phenol (TBPH) and ferrocene (Fc) were also purchased from Sigma-Aldrich.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), MAPP (50 mg, 0.079 mmol) was added. After adding benzaldehyde (30 μl, 0.316 mmol) and trifluoroacetic acid (28 μl, 0.371 mmol) to the solution, the solution turned green. After 2 h, 5 equivalent of NaBH4 (16 mg, 0.417 mmol) was added to the reaction and was further stirred for 12 h. The colour again changed to reddish brown. The solvent was removed through a rotary evaporator and the compound was extracted with dichloromethane. The organic layer was dried with Na2SO4 and the solvent was evaporated to isolate the compound. Then the desired compound was eluted with a 50% DCM–hexane mixture through column chromatography using neutral alumina and purple coloured solid was isolated (Mph, Scheme S2†). Yield: (45 mg, 80%); 1H NMR (500 MHz,CDCl3, 25 °C): δ, ppm = 9.03 (m, 8H), 8.30 (d, 6H), 7.95 (d, 1H), 7.83 (m, 9H), 7.66 (t, 1H), 7.13 (m, 7H), 7.05 (m, 2H), 4.55 (d, 2H), 3.88(t, NH), −2.62 (s, 2H); UV-Vis (CH2Cl2): λmax = 418 nm, 516 nm, 551 nm, 591 nm, 651 nm; ESI-MS (positive ion mode in ACN): m/z (%) = 720.29 (100) [MPh]H+.
3), MAPP (50 mg, 0.079 mmol) was added. After adding benzaldehyde (30 μl, 0.316 mmol) and trifluoroacetic acid (28 μl, 0.371 mmol) to the solution, the solution turned green. After 2 h, 5 equivalent of NaBH4 (16 mg, 0.417 mmol) was added to the reaction and was further stirred for 12 h. The colour again changed to reddish brown. The solvent was removed through a rotary evaporator and the compound was extracted with dichloromethane. The organic layer was dried with Na2SO4 and the solvent was evaporated to isolate the compound. Then the desired compound was eluted with a 50% DCM–hexane mixture through column chromatography using neutral alumina and purple coloured solid was isolated (Mph, Scheme S2†). Yield: (45 mg, 80%); 1H NMR (500 MHz,CDCl3, 25 °C): δ, ppm = 9.03 (m, 8H), 8.30 (d, 6H), 7.95 (d, 1H), 7.83 (m, 9H), 7.66 (t, 1H), 7.13 (m, 7H), 7.05 (m, 2H), 4.55 (d, 2H), 3.88(t, NH), −2.62 (s, 2H); UV-Vis (CH2Cl2): λmax = 418 nm, 516 nm, 551 nm, 591 nm, 651 nm; ESI-MS (positive ion mode in ACN): m/z (%) = 720.29 (100) [MPh]H+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBPH = 1
TBPH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500. The kinetics were recorded at room temperature and using a time interval of 0.5 s. Oxidation of TBPH to a 2,4,6-tri tertiary butyl phenoxyl (TBP) radical was monitored using the time traces of the characteristic absorption bands at 380 nm, 400 nm and 635 nm. Similar conditions were applied for OPD oxidation to the corresponding 2,3-diaminophenazine formation with an absorption feature at 450 nm.
500. The kinetics were recorded at room temperature and using a time interval of 0.5 s. Oxidation of TBPH to a 2,4,6-tri tertiary butyl phenoxyl (TBP) radical was monitored using the time traces of the characteristic absorption bands at 380 nm, 400 nm and 635 nm. Similar conditions were applied for OPD oxidation to the corresponding 2,3-diaminophenazine formation with an absorption feature at 450 nm.
The reactions with ferrocene as a substrate were very rapid at room temperature and thus were performed in the stopped-flow set-up immersed in a cold bath (−30 °C). In a typical experiment, three syringes were filled with ferrocene (2 mM, 3 ml), m-CPBA (1 mM, 5 ml) and iron porphyrin (4 μM, 2 ml), respectively, and volumes of these were injected to achieve a final concentration ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 for iron porphyrins
500 for iron porphyrins![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m-CPBA
m-CPBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ferrocene of the mixture.
ferrocene of the mixture.
y = y0 + A0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−kt) exp(−kt) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A0 = kt + log(y − y0) A0 = kt + log(y − y0) |
The above first order monophasic equation was used to fit the kinetics data and thus obtained the first order rate constants of initial formation and decay from the slope of the linear fit in the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A0vs. time trace plot.
A0vs. time trace plot.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m-CPBA
m-CPBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 1
catalyst = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) from the ratio of the oxidized product concentration to the initial catalyst concentration. For this purpose, we have determined the product concentration (c) from measured absorbance (A) and known molar extinction coefficient (ε) using equation A = ε × c × l (where, the path length of cuvette l = 1 cm).
500) from the ratio of the oxidized product concentration to the initial catalyst concentration. For this purpose, we have determined the product concentration (c) from measured absorbance (A) and known molar extinction coefficient (ε) using equation A = ε × c × l (where, the path length of cuvette l = 1 cm).
For ferrocenium hexafluorophosphate, the molar extinction co-efficient (ε) value from the slope of absorbance vs. concentration dependence plot (Fig. S9†) was calculated to be 350.34 M−1 cm−1. The ε values 404 M−1 cm−1 and 11.1 mM−1 cm−1 for TBPH and OPD, respectively, were obtained from an earlier report.80,86 TON is the number of substrate molecules oxidized into the product by per mole of the catalyst. TOFs have been calculated by dividing the TON with the time required for maximum product conversion estimated at the highest absorbance (Table 2). The TOF thus obtained represents an average rate over the entire duration of the reaction and not an initial rate.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por˙+] to the [FeIV
O Por˙+] to the [FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] intermediate.
O] intermediate.
For the 77 K EPR measurement of the TBP radicals, samples were prepared in an EPR quartz tube using 80 μl of 2 mM TBPH, 10 μl of 10 mM m-CPBA and 10 μl of 44 μM FeL2/FeL3, so that the concentration ratio of the final resulting sample was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 for iron porphyrins: m-CPBA
500 for iron porphyrins: m-CPBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBPH. The reaction mixture was kept in a −80 °C bath for 1 min before flash freezing in liquid nitrogen.
TBPH. The reaction mixture was kept in a −80 °C bath for 1 min before flash freezing in liquid nitrogen.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
This work was funded by the Department of Science and Technology, India (EMR/2016/008063) and Council for Scientific and Industrial Research (CSIR) (to A. D.), the French National Center for Scientific Research (CNRS/UMR 7281) and the French EPR Federation/TGE RENARD (IR3443) (to A. I). S. B. and A. R. acknowledge IACS Integrated PhD Programme for fellowships.References
- S. Chatterjee, K. Sengupta, B. Mondal, S. Dey and A. Dey, Acc. Chem. Res., 2017, 50, 1744–1753 CrossRef CAS PubMed.
- S. M. Adam, I. Garcia-Bosch, A. W. Schaefer, S. K. Sharma, M. A. Siegler, E. I. Solomon and K. D. Karlin, J. Am. Chem. Soc., 2017, 139, 472–481 CrossRef CAS PubMed.
- M. Bhadra, J. Y. C. Lee, R. E. Cowley, S. Kim, M. A. Siegler, E. I. Solomon and K. D. Karlin, J. Am. Chem. Soc., 2018, 140, 9042–9045 CrossRef CAS PubMed.
- R. Cao, C. Saracini, J. W. Ginsbach, M. T. Kieber-Emmons, M. A. Siegler, E. I. Solomon, S. Fukuzumi and K. D. Karlin, J. Am. Chem. Soc., 2016, 138, 7055–7066 CrossRef CAS PubMed.
- K. Cheaib, M. Q. E. Mubarak, K. Sénéchal-David, C. Herrero, R. Guillot, M. Clémancey, J.-M. Latour, S. P. de Visser, J.-P. Mahy, F. Banse and F. Avenier, Angew. Chem., 2019, 131, 864–868 CrossRef.
- A. W. Schaefer, M. T. Kieber-Emmons, S. M. Adam, K. D. Karlin and E. I. Solomon, J. Am. Chem. Soc., 2017, 139, 7958–7973 CrossRef CAS PubMed.
- L. Nurdin, D. M. Spasyuk, L. Fairburn, W. E. Piers and L. Maron, J. Am. Chem. Soc., 2018, 140, 16094–16105 CrossRef CAS PubMed.
- T. L. Poulos, Chem. Rev., 2014, 114, 3919–3962 CrossRef CAS PubMed.
- A. Díaz, P. C. Loewen, I. Fita and X. Carpena, Arch. Biochem. Biophys., 2012, 525, 102–110 CrossRef PubMed.
- A. Ivancich and P. C. Loewen, Electron Transfer in Catalases and Catalase-Peroxidases, Encyclopedia of Biophysics, European Biophysical Societies' Association (EBSA) 2018, ed. G. Roberts and Watts A., Springer, Berlin, Heidelberg, 2018, DOI:10.1007/978-3-642-35943-9_51-1.
- E. J. Mueller, P. J. Loida and S. G. Sligar, in Cytochrome P450: Structure, Mechanism, and Biochemistry, ed. P. R. O. de Montellano, Springer US, Boston, MA, 1995, pp. 83–124, DOI:10.1007/978-1-4757-2391-5_3.
- A. W. Munro, K. J. McLean, J. L. Grant and T. M. Makris, Biochem. Soc. Trans., 2018, 46, 183–196 CrossRef CAS PubMed.
- F. R. S. D. Keilin and T. Mann, Proc. R. Soc. London, Ser. B, 1937, 122, 119 Search PubMed.
- F. L. Muller, M. S. Lustgarten, Y. Jang, A. Richardson and H. Van Remmen, Free Radicals Biol. Med., 2007, 43, 477–503 CrossRef CAS PubMed.
- M. Zamocky, C. Jakopitsch, P. G. Furtmüller, C. Dunand and C. Obinger, Proteins: Struct., Funct., Bioinf., 2008, 72, 589–605 CrossRef CAS PubMed.
- S. Ghasempur, S.-F. Torabi, S.-O. Ranaei-Siadat, M. Jalali-Heravi, N. Ghaemi and K. Khajeh, Environ. Sci. Technol., 2007, 41, 7073–7079 CrossRef CAS PubMed.
- O. Kirk, T. V. Borchert and C. C. Fuglsang, Curr. Opin. Biotechnol., 2002, 13, 345–351 CrossRef CAS PubMed.
- Y. Watanabe, H. Nakajima and T. Ueno, Acc. Chem. Res., 2007, 40, 554–562 CrossRef CAS PubMed.
- L. A. Blumenfeld, R. M. Davydov, N. S. Fel, S. N. Magonov and R. O. Vilu, FEBS Lett., 1974, 45, 256–258 CrossRef CAS PubMed.
- T. L. Poulos and J. Kraut, J. Biol. Chem., 1980, 255, 8199–8205 CAS.
- D. Dolphin, A. Forman, D. C. Borg, J. Fajer and R. H. Felton, Proc. Natl. Acad. Sci., 1971, 68, 614–618 CrossRef CAS PubMed.
- T. Matsui, S.-i. Ozaki, E. Liong, G. N. Phillips and Y. Watanabe, J. Biol. Chem., 1999, 274, 2838–2844 CrossRef CAS PubMed.
- A. N. P. Hiner, E. L. Raven, R. N. F. Thorneley, F. García-Cánovas and J. N. Rodríguez-López, J. Inorg. Biochem., 2002, 91, 27–34 CrossRef CAS PubMed.
- S.-i. Ozaki, M. P. Roach, T. Matsui and Y. Watanabe, Acc. Chem. Res., 2001, 34, 818–825 CrossRef CAS PubMed.
- E. Derat and S. Shaik, J. Phys. Chem. B, 2006, 110, 10526–10533 CrossRef CAS PubMed.
- J. N. Rodríguez-López, D. J. Lowe, J. Hernández-Ruiz, A. N. P. Hiner, F. García-Cánovas and R. N. F. Thorneley, J. Am. Chem. Soc., 2001, 123, 11838–11847 CrossRef PubMed.
- J. E. Erman, L. B. Vitello, M. A. Miller, A. Shaw, K. A. Brown and J. Kraut, Biochemistry, 1993, 32, 9798–9806 CrossRef CAS PubMed.
- G. I. Berglund, G. H. Carlsson, A. T. Smith, H. Szöke, A. Henriksen and J. Hajdu, Nature, 2002, 417, 463 CrossRef CAS PubMed.
- B. D. Howes, J. N. Rodriguez-Lopez, A. T. Smith and G. Smulevich, Biochemistry, 1997, 36, 1532–1543 CrossRef CAS PubMed.
- T. L. Poulos, S. T. Freer, R. A. Alden, S. L. Edwards, U. Skogland, K. Takio, B. Eriksson, N. Xuong, T. Yonetani and J. Kraut, J. Biol. Chem., 1980, 255, 575–580 CAS.
- I. Fita and M. G. Rossmann, J. Mol. Biol., 1985, 185, 21–37 CrossRef CAS PubMed.
- M. Sundaramoorthy, J. Terner and T. L. Poulos, Structure, 1995, 3, 1367–1378 CrossRef CAS PubMed.
- J. N. Rodríguez-López, M. A. Gilabert, J. Tudela, R. N. F. Thorneley and F. García-Cánovas, Biochemistry, 2000, 39, 13201–13209 CrossRef PubMed.
- S. Bhakta, A. Nayek, B. Roy and A. Dey, Inorg. Chem., 2019, 58, 2954–2964 CrossRef CAS PubMed.
- J. N. Rodriguez-Lopez, A. T. Smith and R. N. F. Thorneley, J. Biol. Chem., 1996, 271, 4023–4030 CrossRef CAS PubMed.
- R. Nakajima and I. Yamazaki, J. Biol. Chem., 1980, 255, 2067–2071 CAS.
- S. Marklund, Arch. Biochem. Biophys., 1973, 154, 614–622 CrossRef CAS PubMed.
- J. H. Dawson, Science, 1988, 240, 433 CrossRef CAS PubMed.
- Y. Watanabe and T. Ueno, Bull. Chem. Soc. Jpn., 2003, 76, 1309–1322 CrossRef CAS.
- S. R. Bell and J. T. Groves, J. Am. Chem. Soc., 2009, 131, 9640–9641 CrossRef CAS PubMed.
- N. Jin, D. E. Lahaye and J. T. Groves, Inorg. Chem., 2010, 49, 11516–11524 CrossRef CAS PubMed.
- K. Yamaguchi, Y. Watanabe and I. Morishima, J. Am. Chem. Soc., 1993, 115, 4058–4065 CrossRef CAS.
- J. T. Groves and Y. Watanabe, J. Am. Chem. Soc., 1988, 110, 8443–8452 CrossRef CAS.
- J. T. Groves and Y. Watanabe, J. Am. Chem. Soc., 1986, 108, 7834–7836 CrossRef CAS PubMed.
- H. Fujii, T. Yoshimura and H. Kamada, Inorg. Chem., 1996, 35, 2373–2377 CrossRef CAS PubMed.
- J. D. Soper, S. V. Kryatov, E. V. Rybak-Akimova and D. G. Nocera, J. Am. Chem. Soc., 2007, 129, 5069–5075 CrossRef CAS PubMed.
- C. J. Chang, L. L. Chng and D. G. Nocera, J. Am. Chem. Soc., 2003, 125, 1866–1876 CrossRef CAS PubMed.
- F. Hollmann, P.-C. Lin, B. Witholt and A. Schmid, J. Am. Chem. Soc., 2003, 125, 8209–8217 CrossRef CAS PubMed.
- S. Shaik, S. Cohen, Y. Wang, H. Chen, D. Kumar and W. Thiel, Chem. Rev., 2010, 110, 949–1017 CrossRef CAS PubMed.
- C. J. Thibodeaux, W.-c. Chang and H.-w. Liu, Chem. Rev., 2012, 112, 1681–1709 CrossRef CAS PubMed.
- H. B. Dunford and B. B. Hasinoff, Biochemistry, 1970, 9, 4930–4939 CrossRef CAS PubMed.
- E. G. Hrycay and S. M. Bandiera, in Monooxygenase, Peroxidase and Peroxygenase Properties and Mechanisms of Cytochrome P450, ed. E. G. Hrycay and S. M. Bandiera, Springer International Publishing, Cham, 2015, pp. 1–61, DOI:10.1007/978-3-319-16009-2_1.
- M. I. Savenkova, J. M. Kuo and P. R. Ortiz de Montellano, Biochemistry, 1998, 37, 10828–10836 CrossRef CAS PubMed.
- J. T. Groves and T. E. Nemo, J. Am. Chem. Soc., 1983, 105, 5786–5791 CrossRef CAS.
- B. Meunier, Chem. Rev., 1992, 92, 1411–1456 CrossRef CAS.
- X. Huang and J. T. Groves, Chem. Rev., 2018, 118, 2491–2553 CrossRef CAS PubMed.
- T. G. Traylor, W. A. Lee and D. V. Stynes, J. Am. Chem. Soc., 1984, 106, 755–764 CrossRef CAS.
- T. G. Traylor and R. Popovitz-Biro, J. Am. Chem. Soc., 1988, 110, 239–243 CrossRef CAS.
- S. Bhunia, A. Rana, P. Roy, D. J. Martin, M. L. Pegis, B. Roy and A. Dey, J. Am. Chem. Soc., 2018, 140, 9444–9457 CrossRef CAS PubMed.
- I. Kaljurand, T. Rodima, I. Leito, I. A. Koppel and R. Schwesinger, J. Org. Chem., 2000, 65, 6202–6208 CrossRef CAS PubMed.
- H. Fujii, J. Am. Chem. Soc., 1993, 115, 4641–4648 CrossRef CAS.
- I. Garcia-Bosch, S. K. Sharma and K. D. Karlin, J. Am. Chem. Soc., 2013, 135, 16248–16251 CrossRef CAS PubMed.
- S. Yokota and H. Fujii, J. Am. Chem. Soc., 2018, 140, 5127–5137 CrossRef CAS PubMed.
- W. D. Hewson and L. P. Hager, J. Biol. Chem., 1979, 254, 3182–3186 CAS.
- M. M. Palcic, R. Rutter, T. Araiso, L. P. Hager and H. B. Dunford, Biochem. Biophys. Res. Commun., 1980, 94, 1123–1127 CrossRef CAS PubMed.
- Z. Pan and M. Newcomb, Inorg. Chem. Commun., 2011, 14, 968–970 CrossRef CAS PubMed.
- Z. Pan and M. Newcomb, Inorg. Chem., 2007, 46, 6767–6774 CrossRef CAS PubMed.
- X. Lu, X.-X. Li, M. S. Seo, Y.-M. Lee, M. Clémancey, P. Maldivi, J.-M. Latour, R. Sarangi, S. Fukuzumi and W. Nam, J. Am. Chem. Soc., 2019, 141, 80–83 CrossRef CAS PubMed.
- Z. Cong, T. Kurahashi and H. Fujii, J. Am. Chem. Soc., 2012, 134, 4469–4472 CrossRef CAS PubMed.
- D. M. Davies, P. Jones and D. Mantle, Biochem. J., 1976, 157, 247–253 CrossRef CAS PubMed.
- S. L. Newmyer and P. R. O. de Montellano, J. Biol. Chem., 1995, 270, 19430–19438 CrossRef CAS PubMed.
- T. L. Poulos, Arch. Biochem. Biophys., 2010, 500, 3–12 CrossRef CAS PubMed.
- M. I. Savenkova, S. L. Newmyer and P. R. Ortiz de Montellano, J. Biol. Chem., 1996, 271, 24598–24603 CrossRef CAS PubMed.
- K. Jayaraj, A. Gold, R. N. Austin, L. M. Ball, J. Terner, D. Mandon, R. Weiss, J. Fischer, A. DeCian, E. Bill, M. Müther, V. Schünemann and A. X. Trautwein, Inorg. Chem., 1997, 36, 4555–4566 CrossRef CAS PubMed.
- K. Ayougou, D. Mandon, J. Fischer, R. Weiss, M. Müther, V. Schünemann, A. X. Trautwein, E. Bill, J. Terner, K. Jayaraj, A. Gold and R. N. Austin, Chem.–Eur. J., 1996, 2, 1159–1163 CrossRef CAS.
- J. T. Colvin, R. Rutter, H. J. Stapleton and L. P. Hager, Biophys. J., 1983, 41, 105–108 CrossRef CAS PubMed.
- R. Singh, R. E. Berry, F. Yang, H. Zhang, F. A. Walker and A. Ivancich, Biochemistry, 2010, 49, 8857–8872 CrossRef CAS PubMed.
- A. Ivancich, L. J. Donald, J. Villanueva, B. Wiseman, I. Fita and P. C. Loewen, Biochemistry, 2013, 52, 7271–7282 CrossRef CAS PubMed.
- D. P. Collins, I. S. Isaac, E. D. Coulter, P. W. Hager, D. P. Ballou and J. H. Dawson, J. Porphyrins Phthalocyanines, 2013, 17, 63–72 CrossRef CAS.
- E. R. Altwicker, Chem. Rev., 1967, 67, 475–531 CrossRef CAS.
- E. Derat, S. Shaik, C. Rovira, P. Vidossich and M. Alfonso-Prieto, J. Am. Chem. Soc., 2007, 129, 6346–6347 CrossRef CAS PubMed.
- H. K. Baek and H. E. Van Wart, Biochemistry, 1989, 28, 5714–5719 CrossRef CAS PubMed.
- K. L. Stone, R. K. Behan and M. T. Green, Proc. Natl. Acad. Sci., 2006, 103, 12307–12310 CrossRef CAS PubMed.
- T. H. Yosca, R. K. Behan, C. M. Krest, E. L. Onderko, M. C. Langston and M. T. Green, J. Am. Chem. Soc., 2014, 136, 9124–9131 CrossRef CAS PubMed.
- J. P. Collman, J. I. Brauman, K. M. Doxsee, T. R. Halbert, E. Bunnenberg, R. E. Linder, G. N. LaMar, J. Del Gaudio, G. Lang and K. Spartalian, J. Am. Chem. Soc., 1980, 102, 4182–4192 CrossRef CAS.
- O. V. Ignatenko, I. Gazaryan, E. A. Mareeva, T. A. Chubar, V. A. Fechina, P. Savitsky, A. M Rojkova and V. Tishkov, Catalytic Properties of Tryptophanless Recombinant Horseradish Peroxidase, 2000 Search PubMed.
- J. P. Perdew, Phys. Rev. B, 1986, 33, 8822–8824 CrossRef PubMed.
- S. Miertuš, E. Scrocco and J. Tomasi, Chem. Phys., 1981, 55, 117–129 CrossRef.
- L. Noodleman and W.-G. Han, JBIC, J. Biol. Inorg. Chem., 2006, 11, 674–694 CrossRef CAS PubMed.
- M. G. Ullmann, L. Noodleman and D. A. Case, JBIC, J. Biol. Inorg. Chem., 2002, 7, 632–639 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc04388h |
| This journal is © The Royal Society of Chemistry 2020 |