Open Access Article
Open Access ArticleThe crucial roles of guest water in a biocompatible coordination network in the catalytic ring-opening polymerization of cyclic esters: a new mechanistic perspective†
Sheng-Chun
Chen
a,
Fei-Hang
Zhang
a,
Kun-Lin
Huang
b,
Feng
Tian
a,
Zhi-Hui
Zhang
 a,
Renxian
Zhou
a,
Renxian
Zhou
 c,
Xue-Jun
Feng
a,
Xiaoying
Zhou
c,
Xue-Jun
Feng
a,
Xiaoying
Zhou
 a,
Ming-Yang
He
a,
Jiande
Gu
*d,
Qun
Chen
*a and
Chuan-De
Wu
a,
Ming-Yang
He
a,
Jiande
Gu
*d,
Qun
Chen
*a and
Chuan-De
Wu
 *c
*c
aJiangsu Key Laboratory of Advanced Catalytic Materials and Technology, School of Petrochemical Engineering, Changzhou University, Changzhou 213164, P. R. China. E-mail: chenqunjpu@yahoo.com
bCollege of Chemistry, Chongqing Normal University, Chongqing 401331, P. R. China
cDepartment of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: cdwu@zju.edu.cn
dDrug Design & Discovery Center, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, CAS, Shanghai 201203, P. R. China. E-mail: jiandegu@simm.ac.cn
First published on 27th February 2020
Abstract
The ring-opening polymerization (ROP) of cyclic esters/carbonates is a crucial approach for the synthesis of biocompatible and biodegradable polyesters. Even though numerous efficient ROP catalysts have been well established, their toxicity heavily limits the biomedical applications of polyester products. To solve the toxicity issues relating to ROP catalysts, we report herein a biocompatible coordination network, CZU-1, consisting of Zn4(μ4-O)(COO)6 secondary building units (SBUs), biomedicine-relevant organic linkers and guest water, which demonstrates high potential for use in the catalytic ROP synthesis of biomedicine-applicable polyesters. Both experimental and computational results reveal that the guest water in CZU-1 plays crucial roles in the activation of the Zn4(μ4-O)(COO)6 SBUs by generating μ4-OH Brønsted acid centers and Zn–OH Lewis acid centers, having a synergistic effect on the catalytic ROP of cyclic esters. Different to the mechanism reported in the literature, we propose a new reaction pathway for the catalytic ROP reaction, which has been confirmed using density functional theory (DFT) calculations, in situ diffuse reflectance IR Fourier transform spectroscopy (DRIFTS), and matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS). Additionally, the hydroxyl end groups allow the polyester products to be easily post-modified with different functional moieties to tune their properties for practical applications. We particularly expect that the proposed catalytic ROP mechanism and the developed catalyst design principle will be generally applicable for the controlled synthesis of biomedicine-applicable polymeric materials.
Introduction
The ring-opening polymerization (ROP) of cyclic esters/carbonates is a crucial approach for synthesizing biodegradable polyesters.1 As they have been developed for more than 65 years, numerous efficient ROP catalysts have been reported in the literature,2 including tin(II)-bis(2-ethylhexanoate),3 aluminum alkoxides,4 Ln(OR)3 (Ln = La and Y; R = i-Pr and n-Bu),5 and the oxo-metal alkoxides M5(μ5-O)(OR)13 (M = Fe, Y, La, Sm and Yb; R = Et and Pr).6 However, the toxicity of these catalysts has heavily limited the biomedical applications of the produced polyesters. Therefore, developing efficient biocompatible ROP catalysts remains a crucial and essential issue for the manufacture of biomedicine-applicable polyesters.7One of the key elements involved in the development of efficient ROP catalysts is understanding the catalytic mechanism of ROP reactions both experimentally8 and theoretically.9 Dittrich and Schulz first proposed a three-step coordination–insertion mechanism catalyzed using metal-alkoxide complexes (Scheme 1).10 Density functional theory (DFT) calculations based on Al-alkoxide- and Sn-alkoxide-catalyzed ROP reactions showed that the three-step coordination–insertion mechanism is kinetically applicable.11 However, there are many experimental phenomena that could not be reasonably explained using the three-step ROP mechanism, such as the important roles played by protic agents (e.g., water and alcohols) in ROP reactions. Therefore, unveiling the ROP reaction mechanism is of significant importance for the development of efficient ROP catalysts.
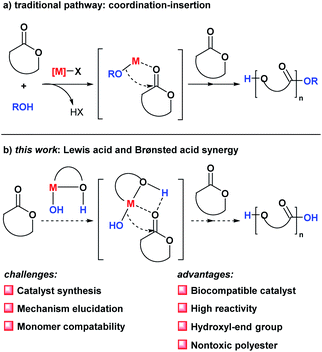 | ||
| Scheme 1 Coordination–insertion mechanisms for ROP reactions: (a) metal-alkoxide catalysts; and (b) bifunctional catalysts. | ||
Metal–organic frameworks (MOFs) are a class of emerging porous materials that are constructed from coordination bond connections between metal ions/clusters and organic linkers.12 MOFs built from oxo-metal clusters, e.g., M3O(COO)6 in MIL-101 and MIL-100 (M = Fe, Cr and Al);13 Zn4O(COO)6 in the IRMOF series14 and UMCM-1;15 Zr6O4(OH)4(COO)12 in the UiO series;16 and others,17 with coordinatively unsaturated metal sites have been used as highly efficient Lewis acid catalysts in numerous organic transformations. The catalytic efficiency can be highly improved if synergistic Brønsted acid/base sites are present in the organic linkers.18 Since discrete oxo-metal complexes are highly active in catalyzing ROP reactions, MOFs consisting of oxo-metal cluster secondary building units (SBUs) might also be highly active in ROP reactions. Therefore, the toxicity issues relating to polyesters could be solved by developing biocompatible MOFs as ROP catalysts, such as through choosing biocompatible metal ions (e.g., ZnII, FeIII, ZrIV, CaII and MgII) and organic linkers (e.g., naturally or medically relevant molecules) as building units. Herein, we report a biocompatible coordination network, {[Zn4(μ4-O)(tzmb)3]·0.5H2O}n (CZU-1), built from Zn4(μ4-O)(COO)6 SBUs and the aromatase inhibitor letrozole derivative 4,4′-(1H-1,2,4-triazol-1-yl)methylene-bis(benzoate) (tzmb), which demonstrates high catalytic efficiency in the solvent-free ROP of cyclic esters for the production of biocompatible polyesters. DFT calculation results indicate that the guest water molecules in CZU-1 play crucial roles in the activation of the Zn4(μ4-O)(COO)6 SBUs through generating active Brønsted acid μ4-OH and Lewis acid Zn(II)–OH sites (Scheme 2). We found that the ROP reaction pathway is different to the three-step coordination–insertion mechanism given in the literature, thus providing new insights for the development of efficient biocompatible ROP catalysts.
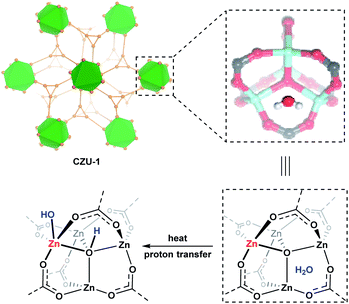 | ||
| Scheme 2 Hydroxyl-based Brønsted acid generation via temperature-promoted proton transfer from lattice water to the oxo-bridge of CZU-1. | ||
Results and discussion
Synthesis and characterization
CZU-1 was synthesized by heating a mixture of Zn(OAc)2·2H2O, H2tzmb and NaOH in water at 180 °C for 3 days (see the ESI† for details). The formula of CZU-1 was established on the basis of single-crystal X-ray diffraction and elemental analysis studies. CZU-1 crystallizes in the trigonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group, built from oxo-centered tetranuclear Zn4(μ4-O)(COO)6 SBUs connected by tzmb linkers (Fig. 1). The geometry of the tetranuclear Zn4(μ4-O)(COO)6 SBUs is similar to that observed in the IRMOF series,14 in which four ZnII atoms, coordinated to the carboxyl groups and triazole N atoms of tzmb, are connected by a μ4-O atom. The connections between the 9-connected Zn4(μ4-O)(COO)6 SBUs and 3-branched tzmb result in an interesting 3D network, which can be simplified as a 3,9-connected binodal net with the point symbol {42·6}3{46·621·89}, corresponding to xmz topology.19 It is worth noting that the lattice water molecules are involved in weak hydrogen-bonding with the carboxyl O atom (O⋯Owater = 3.465 Å), triazole N atom (N⋯Owater = 3.760 Å) and μ4-O atom (μ4-O⋯Owater = 3.603 Å), and should be easily activated during catalysis.
space group, built from oxo-centered tetranuclear Zn4(μ4-O)(COO)6 SBUs connected by tzmb linkers (Fig. 1). The geometry of the tetranuclear Zn4(μ4-O)(COO)6 SBUs is similar to that observed in the IRMOF series,14 in which four ZnII atoms, coordinated to the carboxyl groups and triazole N atoms of tzmb, are connected by a μ4-O atom. The connections between the 9-connected Zn4(μ4-O)(COO)6 SBUs and 3-branched tzmb result in an interesting 3D network, which can be simplified as a 3,9-connected binodal net with the point symbol {42·6}3{46·621·89}, corresponding to xmz topology.19 It is worth noting that the lattice water molecules are involved in weak hydrogen-bonding with the carboxyl O atom (O⋯Owater = 3.465 Å), triazole N atom (N⋯Owater = 3.760 Å) and μ4-O atom (μ4-O⋯Owater = 3.603 Å), and should be easily activated during catalysis.
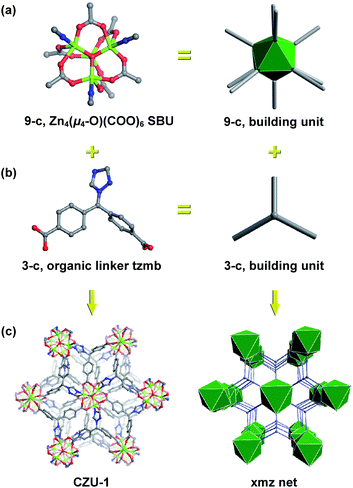 | ||
| Fig. 1 The combination of 9-connected tetranuclear Zn4(μ4-O)(COO)6 SBUs (a) and tripodal tzmb linkers (b) results in the 3D framework of CZU-1, displaying an augmented xmz net (c). | ||
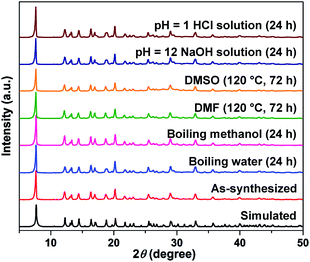
CZU-1 is thermally and chemically stable. Thermogravimetric analysis (TGA) showed that the decomposition temperature of CZU-1 is about 420 °C (Fig. S7†). Powder X-ray diffraction (PXRD) patterns showed that the robust structure of CZU-1 was retained after a crystalline sample was heated at 390 °C in air for 6 h (Fig. S8†). CZU-1 is also highly stable in various chemical environments (Fig. 2, S9 and S10†), such as being suspended in strongly acidic or basic aqueous solution (pH = 1 or 12) at room temperature, in hot water (100 °C), and in various organic solvents (DMF and DMSO) at 120 °C.
CZU-1 catalyzed solvent-free ROP reactions
The ROP reaction conditions were optimized via measuring the catalytic activity of CZU-1 during the bulk polymerization of L-lactide (L-LA) (Fig. S11 and S12†). When fixing the catalyst loading (0.025 mol% CZU-1), the optimized reaction temperature is 160 °C, resulting in the near-quantitative polymerization of L-LA monomers within 36 h, obtaining high molecular weight poly(L-LA) (PLLA) (up to 32 kDa) with a polydispersity index (PDI) of 1.52. Decreasing or increasing the reaction temperature leads to low molecular weight PLLA. Moreover, L-LA conversion markedly decreased when the reaction temperature was below 160 °C. At the optimized reaction temperature (160 °C), the best CZU-1 catalyst loading is 0.025 mol%. Even though the molecular weight of PLLA could be improved with lower catalyst loading, the reaction time was then markedly prolonged (60 h for 0.01 mol% CZU-1).Considering that the lattice water in CZU-1 could not be directly removed by heating under vacuum, we used hydrated zinc salt Zn(OAc)2·2H2O and anhydrous zinc salt Zn(OTf)2 as control catalysts to illustrate the roles played by water in the ROP reaction. The hydrated zinc salt Zn(OAc)2·2H2O is highly active for the bulk polymerization of L-LA (99% conversion) under identical conditions, while the anhydrous zinc salt Zn(OTf)2 is inactive for the ROP reaction, indicating that water plays important roles in the ROP reaction. Compared with Zn(OAc)2·2H2O, CZU-1 allows the ability to better control the molecular weight distribution (PDI = 1.52 for CZU-1 and 2.13 for Zn(OAc)2·2H2O) under identical conditions; this should be ascribed to a substantial decrease in the occurrence of side transesterification reactions, which could be controlled by the bulky tetranuclear active sites present in CZU-1.
To check the stability of CZU-1, we carried out leaching and recycling experiments. When the filtrate of the reaction mixture after catalysis was used instead of CZU-1 under identical conditions, the conversion of L-LA was negligible (Fig. S14†). Inductively coupled plasma-mass spectrometry (ICP-MS) analysis showed that no zinc species leached into the supernate. These results demonstrate the heterogeneous nature of the catalyst. CZU-1 can be simply recovered via centrifugation and reused over successive runs while retaining the majority of its high catalytic efficiency (Fig. S15†). PXRD patterns, X-ray photoelectron spectra (XPS) and FT-IR spectra of recovered CZU-1 samples are almost identical to those of as-synthesized ones, proving the structural integrity of CZU-1 during catalysis (Fig. S16–S18†). Because the pore size of CZU-1 is very small and the molecular size of PLLA is very large, the ROP reaction should be catalyzed by the active sites on the surface of CZU-1.
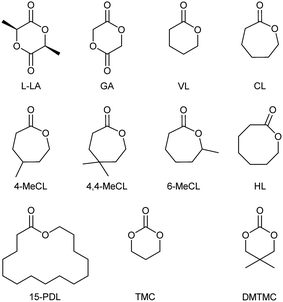
Under the optimized ROP reaction conditions (160 °C and 0.025 mol% catalyst loading), as shown in Table 1, the CZU-1 catalyst exhibits good substrate compatibility with a range of cyclic mono- and diesters, as well as carbonates; these afforded the corresponding polymers with high conversions (>99%). The ROP of GA generated the PGA product with Mn = 45.41 kDa and PDI = 1.39 (entry 2). Unsubstituted 6-, 7-, 8- and 15-membered-ring monoesters were transformed into the corresponding products with Mn values in the range of 16.72–27.83 kDa (entries 3–6). The bulk ROPs of 4-methyl, 4,4′-dimethyl- and 6-methyl-substituted CL analogues also proceeded smoothly with complete substrate conversions (entries 7–9). Trimethylene carbonate (TMC) and its 2,2′-dimethyl-substituted analogue 2,2′-dimethyltrimethylene carbonate (DMTMC) could also be easily polymerized by the CZU-1 catalyst under identical conditions (entries 10 and 11). Having a methyl substituent at the 4- or 6-position of CL barely affected the reaction rate, but it did reduce the molecular weight of the corresponding product. A similar substituent effect was also observed for the TMC-type substrates. These results indicate that steric hindrance from different substrates could affect the polymerization degree.
| Entry | Monomer | Polymer | Conv.b (%) | M n (kDa) | PDI |
|---|---|---|---|---|---|
a Reaction conditions: [monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CZU-1] = 4000 [CZU-1] = 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 160 °C; 36 h.
b Determined via1H NMR spectroscopy.
c
M
n and PDI were determined via GPC in THF in the presence of a polystyrene (PS) standard.
d 190 °C; 36 h.
e [Monomer] 1; 160 °C; 36 h.
b Determined via1H NMR spectroscopy.
c
M
n and PDI were determined via GPC in THF in the presence of a polystyrene (PS) standard.
d 190 °C; 36 h.
e [Monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CZU-1] = 200 [CZU-1] = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 5 days.
f GPC in hexafluoroisopropanol (HFIP) in the presence of a poly(methyl methacrylate) (PMMA) standard. 1; 5 days.
f GPC in hexafluoroisopropanol (HFIP) in the presence of a poly(methyl methacrylate) (PMMA) standard.
|
|||||
| 1 | L-LA | PLLA | >99 | 32.60 | 1.52 |
| 2d,f | GA | PGA | >99 | 45.41 | 1.39 |
| 3 | VL | PVL | >99 | 16.72 | 1.51 |
| 4 | CL | PCL | >99 | 22.25 | 1.36 |
| 5 | HL | PHL | >99 | 17.57 | 1.48 |
| 6e,f | PDL | PPDL | >99 | 27.83 | 1.03 |
| 7 | 4-MeCL | P(4-MeCL) | >99 | 17.23 | 1.43 |
| 8 | 4,4′-MeCL | P(4,4′-MeCL) | >99 | 13.80 | 1.59 |
| 9 | 6-MeCL | P(6-MeCL) | >99 | 11.36 | 1.60 |
| 10 | TMC | PTMC | >99 | 12.74 | 1.62 |
| 11 | DMTMC | PDMTMC | >99 | 8.45 | 1.59 |
|
|||||
In contrast to the well-studied solution ROP reactions involving cyclic esters catalyzed by zinc catalysts (Table S2 in the ESI†), there are few zinc-based catalysts for bulk ROP reactions reported in the literature; however, bulk polymerization is very important for practical applications, because it is organic solvent free and has minimized numbers of undesired side reactions, and large-scale production is easy.20 Additionally, even though there are a few reported zinc complexes that are active for the bulk polymerization of L-LA, their catalytic properties are far inferior to those of CZU-1.
DFT calculations based on the CZU-1 catalyzed solvent-free ROP reaction
According to the structure of Zn4(μ4-O)(COO)6 (Fig. S19†) in CZU-1, the direct coordination of the alkoxy oxygen of a cyclic ester (using glycolide as an example) monomer with a zinc atom is impossible because of steric hindrance. Therefore, activation of the Zn4(μ4-O)(COO)6 cores in CZU-1 should be the first step in the catalytic ROP reaction.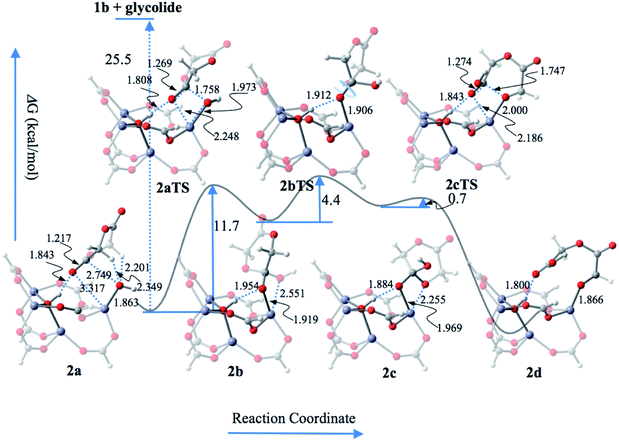 | ||
| Fig. 4 The reaction profile of the initiation of the ROP reaction catalyzed by Zn4(μ4-O)(COO)6 from CZU-1. Color scheme: O, red; H, light grey; C, gray; Zn, blue. | ||
The hydroxyl group at the activated Zn4(μ4-O)(COO)6 core can undergo the nucleophilic attack of the carbonyl group of glycolide, resulting in the formation of a new C–O bond between glycolide and the hydroxyl group. Meanwhile, the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O π-bond of the monomer is broken via the four-membered-ring transition state 2aTS. The corresponding activation energy barrier is 11.7 kcal mol−1, which is much lower than that for the activation of the Zn4(μ4-O)(COO)6 cluster unit. The shortened μ4-OH⋯O H-bond (1.808 Å in 2aTSvs. 1.843 Å in 2a) indicates the importance of μ4-OH in this elementary reaction step.
O π-bond of the monomer is broken via the four-membered-ring transition state 2aTS. The corresponding activation energy barrier is 11.7 kcal mol−1, which is much lower than that for the activation of the Zn4(μ4-O)(COO)6 cluster unit. The shortened μ4-OH⋯O H-bond (1.808 Å in 2aTSvs. 1.843 Å in 2a) indicates the importance of μ4-OH in this elementary reaction step.
This elementary step leads to the transformation of the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond into a C–O single bond in the intermediate product (2b) via a bifurcated bonding pattern. The carbonyl C adopts an sp3 bonding pattern in 2b, which is favorable for the formation of a ring-opening intermediate (2c) via rotating the C–O bond to relocate the acyl oxygen atom. The low activation energy barrier of the transition state (2bTS) (4.4 kcal mol−1) indicates that the corresponding C–O single bond is easily rotated. In the subsequent ring rupture step, the activation energy barrier (0.7 kcal mol−1) is much lower than that for the transformation from a carbonyl C
O double bond into a C–O single bond in the intermediate product (2b) via a bifurcated bonding pattern. The carbonyl C adopts an sp3 bonding pattern in 2b, which is favorable for the formation of a ring-opening intermediate (2c) via rotating the C–O bond to relocate the acyl oxygen atom. The low activation energy barrier of the transition state (2bTS) (4.4 kcal mol−1) indicates that the corresponding C–O single bond is easily rotated. In the subsequent ring rupture step, the activation energy barrier (0.7 kcal mol−1) is much lower than that for the transformation from a carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond to a C–O single bond (2aTS, 11.7 kcal mol−1). This process is accompanied by the formation of a new carbonyl C
O double bond to a C–O single bond (2aTS, 11.7 kcal mol−1). This process is accompanied by the formation of a new carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond in the transition state 2cTS and the ring ruptured product 2d. The initiation step is then completed through the formation of the ring-opened monomer–catalyst complex 2d.
O double bond in the transition state 2cTS and the ring ruptured product 2d. The initiation step is then completed through the formation of the ring-opened monomer–catalyst complex 2d.
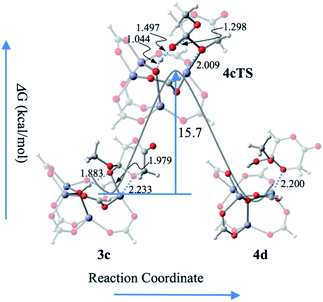 | ||
| Fig. 6 The reaction profile of the deprotonation of μ4-OH for the termination of the ROP reaction process. Color scheme: O, red; H, light grey; C, gray; Zn, blue. | ||
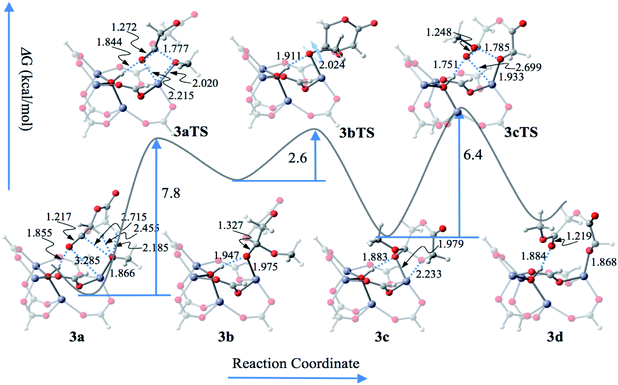
According to the coordination–insertion polymerization mechanism, the molecular-weight is controlled by the ratios kpropagation/kinitiation and kpropagation/ktermination.1 Based on the present mechanism, the molecular weight should be evaluated according to either the ratio exp(−G3aTS/RT)/exp(−G1aTS/RT) for the former or the ratio exp(−G4cTS/RT)/exp(−G3cTS/RT) for the latter.
It is worth noting that the carbonyl oxygen atom of glycolide undergoes strong hydrogen bonding with μ4-OH in all reaction steps, including the initiation and propagation processes, which is similar to the mechanisms proposed for guanidine triazabicyclodecene,21 thiomidate,2g and other proton shuttle catalysts.22 In other words, the Brønsted acid center μ4-OH in activated Zn4(μ4-O)(COO)6 plays key roles in the catalytic ROP reaction.
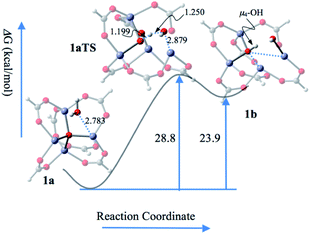
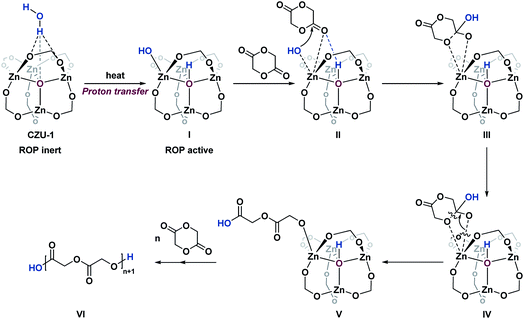
On the basis of the above computational studies, a novel reaction pathway for the CZU-1 catalyzed ROP process is proposed, which includes activation, initiation, propagation, and termination processes (Scheme 3). First, proton transfer from a guest water molecule to the oxo bridge of the Zn4(μ4-O)(COO)6-based cluster leads to a hydroxo-bridged intermediate I, creating a Brønsted acid μ4-OH moiety. Second, an unprecedented ROP initiation model II, based upon the synergy of the Lewis acid Zn(II)–OH site and Brønsted acid μ4-OH, was obtained. Then, the nucleophilic attack of the carbonyl group by the hydroxyl group of the activated Zn(II)–OH site generates the intermediate III, which then undergoes ring-opening to afford the intermediate V. Further chain propagation proceeds through the synergistic initiation cycle. Finally, the termination of the growing chain, caused by the deprotonation of μ4-OH, results in the formation of the polyester product VI with hydroxyl end groups. The rate controlling step of this reaction pathway is the activation step, with an activation energy of 28.8 kcal mol−1. This reaction is unlikely to take place at room temperature. When heated to 160 °C, the rate of this step is estimated to be 0.014 s−1, indicating that an observable reaction should thus be expected.
Experimental verification of the proposed mechanism for the ROP reaction
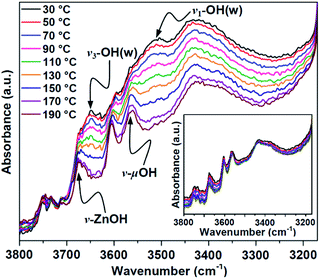
According to the mechanism proposed above, the activation process consists of proton transfer from water to μ4-O in Zn4(μ4-O)(COO)6, generating a Brønsted acid center, μ4-OH, to initiate the ROP reaction. To verify this prediction, temperature-dependent IR spectra of CZU-1 were collected via in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). The broad peaks centered at 3510 and 3645 cm−1 (the O–H stretching vibrations of lattice water) shift to 3560 and 3675 cm−1, respectively, when the temperature is raised to 130 °C (Fig. 7). The peak centered at 3560 cm−1 corresponds to the O–H stretching vibration of μ4-OH.23 It is worth noting that the temperature-dependent proton transfer process from guest water to the oxo-bridge of CZU-1 is irreversible (Fig. 7, inset), which is favorable for the ROP reaction.DFT calculations predicted that the polyester products feature hydroxyl end groups that are controlled by the initiating μ4-OH group. To determine the terminal end groups of the resulting polymer, we performed MALDI-TOF MS experiments on the PCL product. MALDI-TOF MS showed a single distribution of a series of peaks at 114n + 18 + 23 with a charge of +1, which can be assigned to n(CL) + H2O + Na+ (Fig. 8). This result confirmed the presence of hydroxyl end groups in the resultant polyester chains, which is consistent with the μ4-OH moieties acting as initiating groups, thus supporting the proposed mechanism obtained via DFT calculations.
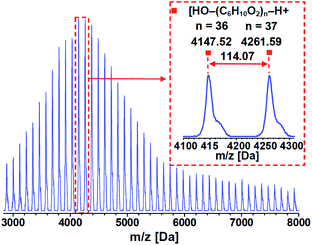 | ||
Fig. 8 MALDI-TOF MS from a PCL sample (88% conversion, Mn = 12.6 kDa and PDI = 1.06) prepared via the ROP of CL with [CZU-1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CL] = 1 [CL] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500. 500. | ||
Toxicity tests
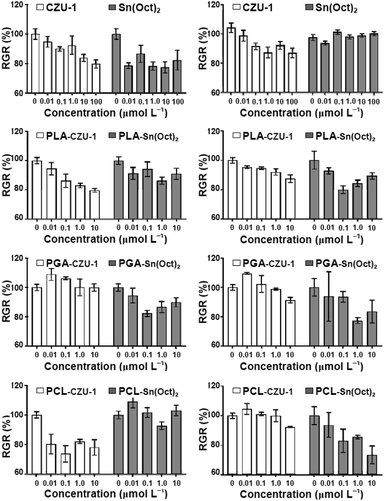
Fibroblasts (FBs) are a type of cell involved in the synthesis of extracellular matrices and collagen, the structural frameworks of human and animal tissues and connective tissues, which play a critical role in wound healing. Smooth muscle cells (SMCs), known as myocytes, grow around blood vessels, bronchia, the stomach and the bladder to control starch. Both types of cells are commonly involved in wound healing after clinical surgery and come into close contact with artificial biomaterials, such as the surgical sutures used in surgery. In this work, three pairs of different polyesters [PLLA-CZU-1/PLLA-Sn(Oct)2, PGA-CZU-1/PGA-Sn(Oct)2, and PCL-CZU-1/PCL-Sn(Oct)2], containing equimolar quantities of Zn or Sn, were prepared via the bulk ROP of cyclic esters (L-LA, GA, and CL) using CZU-1 and Sn(Oct)2 catalysts, respectively. We further conducted a series of FB- and SMC-based cytotoxicity studies on the catalysts and polyesters to investigate possible toxicity effects on these two types of cells, and compared PLLA, PGA and PCL with and without Sn.As shown in Fig. 9, the data from toxicity studies for these six polyester materials demonstrated that, up to 10 μmol L−1, PGA-CZU-1 did not affect the growth rates of both cell types, while PLLA-CZU-1, PCL-CZU-1 and CZU-1 showed dose-dependent effects on FBs. Toward SMCs, PLLA-CZU-1, PCL-Sn(Oct)2 and CZU-1 affected cell growth dose-dependently. Compared with Sn(Oct)2, CZU-1 generated better outcomes for FBs, and there was not much difference in the case of SMCs. Interestingly, the data from this study reveals that the RGR% values of some polyester materials are above those of untreated control cells (100%), indicating that these polyester materials did not affect cell growth, which is good for keeping tissue intact. Among these six materials, PCL-CZU-1 showed strong non-toxic potential, generating the possibility that it could be used in medical artificial biomaterials for ophthalmology, drug delivery systems, surgical mending, bone fixing and tissue repair.
Conclusions
In summary, we demonstrated for the first time the solvent-free ROP of cyclic esters catalyzed using a biocompatible coordination network, CZU-1, consisting of oxo/hydroxo-bridged Zn4(μ4-O)(COO)6 SBUs and guest water; this showed great potential for the ROP synthesis of biocompatible polyesters. DFT calculations revealed that the guest water in CZU-1 played crucial roles in the key steps of the ROP reactions by generating active Brønsted acid μ4-OH and Lewis acid Zn–OH sites via proton transfer from water to μ4-O in the Zn4(μ4-O)(COO)6 SBU, which is different to mechanisms reported in the literature for solvent-free ROP reactions. The mechanistic research results highlight the potential of combining Brønsted and Lewis acid sites in ROP catalysts to synergistically improve the catalytic efficiency. Temperature-dependent in situ DRIFTS experiments confirmed the presence of an irreversible intermolecular proton transformation process. DFT calculation and MALDI-TOF MS analysis results showed that the resulting polyester chains feature hydroxyl end groups, which can be readily post-modified for biomedical applications. We hope that this newly developed mechanism will stimulate extensive research into biocompatible ROP catalysts for the solvent-free ROP of cyclic esters, generating biocompatible polyesters for biomedical applications.Abbreviations
| ROP | Ring-opening polymerization |
| MOFs | Metal–organic frameworks |
| SBUs | Secondary building units |
| Tzmb | 4,4′-(1H-1,2,4-Triazol-1-yl)methylene-bis(benzoate) |
| DMF | N,N′-Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| THF | Tetrahydrofuran |
| HFIP | Hexafluoroisopropanol |
| L-LA | L-Lactide |
| GA | Glycolide |
| VL | δ-Valerolactone |
| CL | ε-Caprolactone |
| 4-MeCL | 4-Methyl-ε-caprolactone |
| 4,4-MeCL | 4,4-Dimethyl-ε-caprolactone |
| 6-MeCL | 6-Methyl-ε-caprolactone |
| HL | 7-Heptalactone |
| PDL | 15-Pentadecalactone |
| TMC | Trimethylene carbonate |
| DMTMC | 2,2-Dimethyltrimethylene carbonate |
| PLLA | Poly(L-lactide) |
| PGA | Polyglycolide |
| PVL | Poly(δ-valerolactone) |
| PCL | Poly(ε-caprolactone) |
| PHL | Poly(7-heptalactone) |
| PPDL | Poly(15-pentadecalactone) |
| P(4-MeCL) | Poly(4-methyl-ε-caprolactone) |
| P(4,4′-MeCL) | Poly(4,4′-dimethyl-ε-caprolactone) |
| P(6-MeCL) | Poly(6-methyl-ε-caprolactone) |
| PTMC | Poly(trimethylene carbonate) |
| PDMTMC | Poly(2,2-dimethyltrimethylene carbonate) |
| PMMA | Poly(methyl methacrylate) |
| PS | Polystyrene |
| GPC | Gel-permeation chromatography |
| M n | Number average molecular weight |
| M w | Weight average molecular weight |
| PDI | Polydispersity index |
| FBs | Fibroblasts |
| SMCs | Smooth muscle cells |
| FCS | Fetal calf serum |
| DRs | Dissolution rates |
| SEM | Standard error of mean |
| RGR | Relative growth rate |
| DFT | Density functional theory |
| DRIFTS | Diffuse reflectance IR Fourier transform spectroscopy |
| MALDI-TOF MS | Matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy |
| HRMS | High resolution mass spectroscopy |
| FT-IR | Fourier transform infrared |
| TGA | Thermogravimetric analysis |
| PXRD | Powder X-ray diffraction |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| XPS | X-ray photoelectron spectroscopy |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (21676030, 11775037 and 21872122), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Advanced Catalytic and Green Manufacturing Collaborative Innovation Center, Changzhou University and Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology (ZZZD201807 and BM2012110).Notes and references
- (a) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147–6176 CrossRef CAS PubMed; (b) M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC; (c) M. J. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486–494 RSC; (d) C. M. Thomas, Chem. Soc. Rev., 2010, 39, 165–173 RSC; (e) A. Tardy, J. Nicolas, D. Gigmes, C. Lefay and Y. Guillaneuf, Chem. Rev., 2017, 117, 1319–1406 CrossRef CAS PubMed; (f) G. Becker and F. R. Wurm, Chem. Soc. Rev., 2018, 47, 7739–7782 RSC.
- (a) A. K. Sutar, T. Maharana, S. Dutta, C.-T. Chen and C.-C. Lin, Chem. Soc. Rev., 2010, 39, 1724–1746 RSC; (b) S. Dagorne, M. Normand, E. Kirillov and J.-F. Carpentier, Coord. Chem. Rev., 2013, 257, 1869–1886 CrossRef CAS; (c) Y. Sarazin and J.-F. Carpentier, Chem. Rev., 2015, 115, 3564–3614 CrossRef CAS PubMed; (d) A. B. Kremer and P. Mehrkhodavandi, Coord. Chem. Rev., 2019, 380, 35–57 CrossRef CAS; (e) D. M. Lyubov, A. O. Tolpygin and A. A. Trifonov, Coord. Chem. Rev., 2019, 392, 83–145 CrossRef CAS; (f) S. Naumann, P. B. V. Scholten, J. A. Wilson and A. P. Dove, J. Am. Chem. Soc., 2015, 137, 14439–14445 CrossRef CAS PubMed; (g) X. Zhang, G. O. Jones, J. L. Hedrick and R. M. Waymouth, Nat. Chem., 2016, 8, 1047–1053 CrossRef CAS PubMed; (h) B. Lin and R. M. Waymouth, J. Am. Chem. Soc., 2017, 139, 1645–1652 CrossRef CAS; (i) T. Stoesser and C. K. Williams, Angew. Chem., Int. Ed., 2018, 57, 6337–6341 CrossRef CAS.
- H. R. Kricheldorf, I. Kreiser-Saunders and A. Stricker, Macromolecules, 2000, 33, 702–709 CrossRef CAS.
- T. M. Ovitt and G. W. Coates, J. Am. Chem. Soc., 1999, 121, 4072–4073 CrossRef CAS.
- (a) W. M. Stevels, M. J. K. Ankoné, P. J. Dijkstra and J. Feijen, Macromolecules, 1996, 29, 3332–3333 CrossRef CAS; (b) X. Wang, J. L. Brosmer, A. Thevenon and P. L. Diaconescu, Organometallics, 2015, 34, 4700–4706 CrossRef CAS.
- (a) V. Simic, N. Spassky and L. G. Hubert-Pfalzgraf, Macromolecules, 1997, 30, 7338–7340 CrossRef CAS; (b) E. Martin, P. Dubois and R. Jerome, Macromolecules, 2000, 33, 1530–1535 CrossRef CAS; (c) B. J. O'Keefe, S. M. Monnier, M. A. Hillmyer and W. B. Tolman, J. Am. Chem. Soc., 2001, 123, 339–340 CrossRef PubMed.
- (a) R. H. Platel, L. M. Hodgson and C. K. Williams, Polym. Rev., 2008, 48, 11–63 CrossRef CAS; (b) E. Piedra-Arroni, C. Ladavière, A. Amgoune and D. Bourissou, J. Am. Chem. Soc., 2013, 135, 13306–13309 CrossRef CAS PubMed; (c) D. S. Bolotin, V. Korzhikov-Vlakh, E. Sinitsyna, S. N. Yunusova, V. V. Suslonov, A. Shetnev, A. Osipyan, M. Krasavin and V. Y. Kukushkin, J. Catal., 2019, 372, 362–369 CrossRef CAS.
- (a) P. Dubois, N. Ropson, R. Jerome and P. Teyssie, Macromolecules, 1996, 29, 1965–1975 CrossRef CAS; (b) N. Nomura, R. Ishii, M. Akakura and K. Aoi, J. Am. Chem. Soc., 2002, 124, 5938–5939 CrossRef CAS PubMed; (c) L. R. Rieth, D. R. Moore, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2002, 124, 15239–15248 CrossRef CAS PubMed; (d) I. Yu, A. Acosta-Ramirez and P. Mehrkhodavandi, J. Am. Chem. Soc., 2012, 134, 12758–12773 CrossRef CAS PubMed; (e) R. Ligny, M. M. Hänninen, S. M. Guillaume and J.-F. Carpentier, Angew. Chem., Int. Ed., 2017, 56, 10388–10393 CrossRef CAS PubMed.
- (a) M. Ryner, K. Stridsberg, A.-C. Albertsson, H. von Schenck and M. Svensson, Macromolecules, 2001, 34, 3877–3881 CrossRef CAS; (b) E. L. Marshall, V. C. Gibson and H. S. Rzepa, J. Am. Chem. Soc., 2005, 127, 6048–6051 CrossRef CAS; (c) Y. Sarazin, B. Liu, T. Roisnel, L. Maron and J.-F. Carpentier, J. Am. Chem. Soc., 2011, 133, 9069–9087 CrossRef CAS; (d) C. Robert, T. E. Schmid, V. Richard, P. Haquette, S. K. Raman, M.-N. Rager, R. M. Gauvin, Y. Morin, X. Trivelli, V. Guérineau, I. del Rosal, L. Maron and C. M. Thomas, J. Am. Chem. Soc., 2017, 139, 6217–6225 CrossRef CAS PubMed.
- W. Dittrich and R. C. Schulz, Angew. Makromol. Chem., 1971, 15, 109–126 CrossRef CAS.
- (a) H. von Schenck, M. Ryner, A.-C. Albertsson and M. Svensson, Macromolecules, 2002, 35, 1556–1562 CrossRef CAS; (b) J. L. Eguiburu, M. J. Fernandez-Berridi, F. P. Cossío and J. S. Román, Macromolecules, 1999, 32, 8252–8258 CrossRef CAS.
- (a) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS; (b) J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. B. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC; (c) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC; (d) J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC; (e) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974 CrossRef CAS; (f) Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC; (g) A. Schoedel, M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2016, 116, 12466–12535 CrossRef CAS PubMed; (h) W. P. Lustig, S. Mukherjee, N. D. Rudd, A. V. Desai, J. Li and S. K. Ghosh, Chem. Soc. Rev., 2017, 46, 3242–3285 RSC.
- (a) C. Serre, F. Millange, S. Surblé and G. Férey, Angew. Chem., Int. Ed., 2004, 43, 6286–6289 CrossRef CAS PubMed; (b) P. Horcajada, C. Serre, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 5974–5978 CrossRef CAS PubMed; (c) L. Hamon, C. Serre, T. Devic, T. Loiseau, F. Millange, G. Férey and G. De Weireld, J. Am. Chem. Soc., 2009, 131, 8775–8777 CrossRef CAS PubMed.
- (a) H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS; (b) M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed; (c) A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998–17999 CrossRef CAS PubMed; (d) H. Deng, C. J. Doonan, H. Furukawa, R. B. Ferreira, J. Towne, C. B. Knobler, B. Wang and O. M. Yaghi, Science, 2010, 327, 846–850 CrossRef CAS PubMed.
- K. Koh, A. G. Wong-Foy and A. J. Matzger, Angew. Chem., Int. Ed., 2008, 47, 677–680 CrossRef CAS PubMed.
- (a) M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS; (b) H. Fei, J. Shin, Y. S. Meng, M. Adelhardt, J. Sutter, K. Meyer and S. M. Cohen, J. Am. Chem. Soc., 2014, 136, 4965–4973 CrossRef CAS PubMed; (c) B. An, J. Zhang, K. Cheng, P. Ji, C. Wang and W. Lin, J. Am. Chem. Soc., 2017, 139, 3834–3840 CrossRef CAS PubMed; (d) X.-P. Wu, L. Gagliardi and D. G. Truhlar, J. Am. Chem. Soc., 2018, 140, 7904–7912 CrossRef CAS PubMed.
- (a) Y. Liu, J. F. Eubank, A. J. Cairns, J. Eckert, V. C. Kravtsov, R. Luebke and M. Eddaoudi, Angew. Chem., Int. Ed., 2007, 46, 3278–3283 CrossRef CAS PubMed; (b) S.-T. Zheng, T. Wu, F. Zuo, C. Chou, P. Feng and X. Bu, J. Am. Chem. Soc., 2012, 134, 1934–1937 CrossRef CAS PubMed; (c) K. Wang, D. Feng, T.-F. Liu, J. Su, S. Yuan, Y.-P. Chen, M. Bosch, X. Zou and H.-C. Zhou, J. Am. Chem. Soc., 2014, 136, 13983–13986 CrossRef CAS PubMed; (d) T. Ikuno, J. Zheng, A. Vjunov, M. Sanchez-Sanchez, M. A. Ortuño, J. L. Fulton, D. M. Camaioni, Z. Li, D. Ray, B. L. Mehdi, N. D. Browning, O. K. Farha, J. T. Hupp, C. J. Cramer, L. Gagliardi and J. A. Lercher, J. Am. Chem. Soc., 2017, 139, 10294–10301 CrossRef CAS PubMed; (e) P. Ji, K. Manna, Z. Lin, X. Feng, A. Urban, Y. Song and W. Lin, J. Am. Chem. Soc., 2017, 139, 7004–7011 CrossRef CAS PubMed; (f) Y. Keum, S. Park, Y.-P. Chen and J. Park, Angew. Chem., Int. Ed., 2018, 57, 14852–14856 CrossRef CAS PubMed.
- (a) C.-Y. Sun, S.-X. Liu, D.-D. Liang, K.-Z. Shao, Y.-H. Ren and Z.-M. Su, J. Am. Chem. Soc., 2009, 131, 1883–1888 CrossRef CAS PubMed; (b) R. Srirambalaji, S. Hong, R. Natarajan, M. Yoon, R. Hota, Y. Kim, Y. H. Ko and K. Kim, Chem. Commun., 2012, 48, 11650–11652 RSC; (c) J. Jiang and O. M. Yaghi, Chem. Rev., 2015, 115, 6966–6997 CrossRef CAS PubMed; (d) B. Li, K. Leng, Y. Zhang, J. J. Dynes, J. Wang, Y. Hu, D. Ma, Z. Shi, L. Zhu, D. Zhang, Y. Sun, M. Chrzanowski and S. Ma, J. Am. Chem. Soc., 2015, 137, 4243–4248 CrossRef CAS PubMed; (e) W.-Y. Gao, H. Wu, K. Leng, Y. Sun and S. Ma, Angew. Chem., Int. Ed., 2016, 55, 5472–5476 CrossRef CAS PubMed; (f) Y.-B. Huang, J. Liang, X.-S. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126–157 RSC; (g) C. A. Trickett, T. M. Osborn Popp, J. Su, C. Yan, J. Weisberg, A. Huq, P. Urban, J. Jiang, M. J. Kalmutzki, Q. Liu, J. Baek, M. P. Head-Gordon, G. A. Somorjai, J. A. Reimer and O. M. Yaghi, Nat. Chem., 2019, 11, 170–176 CrossRef CAS PubMed; (h) W. Gong, X. Chen, H. Jiang, D. Chu, Y. Cui and Y. Liu, J. Am. Chem. Soc., 2019, 141, 7498–7508 CrossRef CAS PubMed; (i) P. Liu, E. Redekop, X. Gao, W.-C. Liu, U. Olsbye and G. A. Somorjai, J. Am. Chem. Soc., 2019, 141, 11557–11564 CrossRef CAS PubMed.
- J. Jia X. Lin, C. Wilson, A. J. Blake, N. R. Champness, P. Hubberstey, G. Walker, E. J. Cussen and M. Schröder, Chem. Commun., 2007, 8, 840–842 Search PubMed.
- (a) J. Börner, U. Flörke, K. Huber, A. Döring, D. Kuchling and S. Herres-Pawlis, Chem. – Eur. J., 2009, 15, 2362–2376 CrossRef PubMed; (b) Q. Shi, J. Yang and X. Lü, Inorg. Chem. Commun., 2015, 59, 61–62 CrossRef CAS.
- A. Chuma, H. W. Horn, W. C. Swope, R. C. Pratt, L. Zhang, B. G. Lohmeijer, C. G. Wade, R. M. Waymouth, J. L. Hedrick and J. E. Rice, J. Am. Chem. Soc., 2008, 130, 6749–6754 CrossRef CAS PubMed.
- N. Susperregui, D. Delcroix, B. Martin-Vaca, D. Bourissou and L. Maron, J. Org. Chem., 2010, 75, 6581–6587 CrossRef CAS PubMed.
- D. Armentano, G. D. Munno, T. F. Mastropietro, M. Julve and F. Lloret, J. Am. Chem. Soc., 2005, 127, 10778–10779 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details relating to synthesis and characterization; PXRD patterns; 1H/13C NMR spectra; TGA curves; IR, elemental analysis, HRMS, XPS, ICP-MS, in situ DRIFTS, GPC, and MALDI-TOF MS data; X-ray structure determination information; cytotoxicity assays; DFT calculations; and catalytic test results. CCDC 1842876. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc06024c |
| This journal is © The Royal Society of Chemistry 2020 |