Open Access Article
Open Access ArticleA clustering-triggered emission strategy for tunable multicolor persistent phosphorescence†
Qing
Zhou
 ,
Tianjia
Yang
,
Tianjia
Yang
 ,
Zihao
Zhong
,
Fahmeeda
Kausar
,
Ziyi
Wang
,
Yongming
Zhang
* and
Wang Zhang
Yuan
,
Zihao
Zhong
,
Fahmeeda
Kausar
,
Ziyi
Wang
,
Yongming
Zhang
* and
Wang Zhang
Yuan
 *
*
School of Chemistry and Chemical Engineering, Shanghai Key Lab of Electrical Insulation and Thermal Aging, Shanghai Electrochemical Energy Devices Research Center, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: ymzhang@sjtu.edu.cn; wzhyuan@sjtu.edu.cn
First published on 11th February 2020
Abstract
A clustering-triggered emission (CTE) strategy, namely the formation of heterogeneous clustered chromophores and conformation rigidification, for achieving tunable multicolor phosphorescence in single-component compounds is proposed. Non-conventional luminophores comprising just oxygen functionalities and free of π-bonding, i.e., D-(+)-xylose (D-Xyl), pentaerythritol (PER), D-fructose (D-Fru) and D-galactose (D-Gal), were adopted as a simple model system with an explicit structure and molecular packing to address the hypothesis. Their concentrated solutions and crystals at 77 K or under ambient conditions demonstrate remarkable multicolor phosphorescence afterglows in response to varying excitation wavelengths, because of the formation of diverse oxygen clusters with sufficiently rigid conformations. The intra- and inter-molecular O⋯O interactions were definitely illustrated by both single crystal structure analysis and theoretical calculations. These findings shed new light on the origin and simple achievement of tunable multicolor phosphorescence in single-component pure organics, and in turn, have strong implications for the emission mechanism of non-conventional luminophores.
Introduction
Currently, the development of smart luminescent materials with tunable multicolor emission upon external stimuli, such as temperature, light, electric/magnetic field, excitation intensity and pressure, is receiving increasing attention because of their unique photophysical properties and potential applications in information encryption, visual detection of UV lights, anticounterfeiting, sensing and bioassays.1–8 Thus far, despite the fact that multicolor luminescence has been successfully achieved through the modulation of crystallinity, molecular conformation/packing and composition of the compounds, or the combination of different emitters,5–13 it remains challenging to realize the tunability of persistent phosphorescence in pure organic single-component systems,14–20 particularly in single crystals.14 While there is growing interest in the achievement of pure organic persistent room temperature phosphorescence (p-RTP),21–27 little attention has been given to its tunability,14–20 presumably because of the high susceptibility of triplets28–38 and the difficulty in the construction of diverse triplet emissive populations.Very recently, tunable multicolor persistent phosphorescence from pure organics has been observed in certain nonaromatic luminophores,18–20 aromatic crystals14 and ionized or doped polymers,15–17 at cryogenic temperatures (i.e., 77 K) and/or even under ambient conditions. For example, Huang and co-workers demonstrated tunable p-RTP in 2,4,6-trimethoxy-1,3,5-triazine (TMOT) crystals,14 and Zhao et al. reported a tunable afterglow from blue to red by tuning the aggregation state of pyrene derivatives in poly(vinyl alcohol) (PVA) films.17 Meanwhile, tunable cryogenic phosphorescence was observed from concentrated solutions of sodium alginate (SA),18 poly(acrylic acid) (PAA),19 poly(acrylamide) (PAM)19 and natural proteins,20 the emission colors of which generally changed from blue to green with the variation in the excitation wavelengths (λexs) from 312 to 365 nm at 77 K, because of the presence of multiple emissive species.18–20 Furthermore, bovine serum albumin (BSA)20 and ε-poly-L-lysine (ε-PLL)39 have even been shown to exhibit λex-dependent p-RTP. Despite these exciting advances, the understanding of the origination of such emissions, and moreover, the design rationale, remain elusive. For example, monomer and excimer phosphorescence were ascribed to the occurrence of tunable p-RTP in the TMOT system, where planar conformation was believed to play an important role in guaranteeing the formation of H-aggregates,14 whereas diverse clustered chromophores were thought to be the origin of the non-conventional luminophores.18–20,39
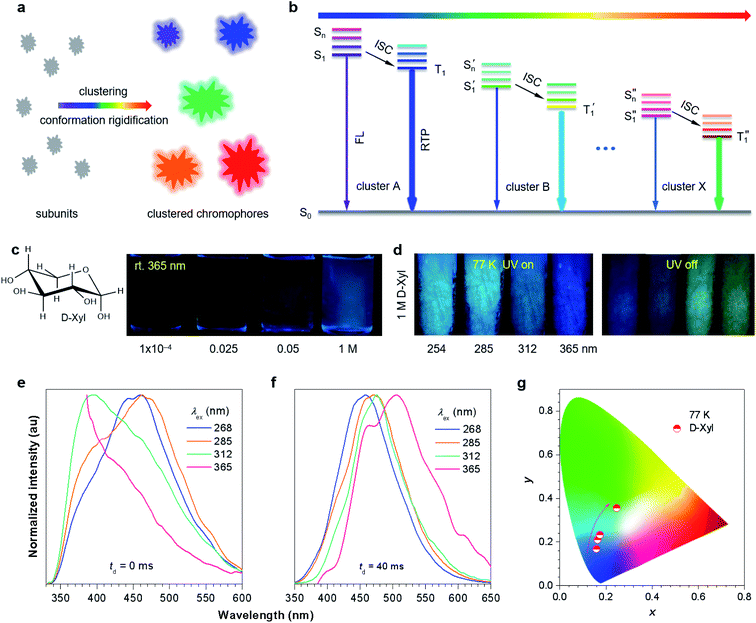
Taking together all the preceding reports, it is clear that the presence of multiple emissive centers alongside rigid conformations are necessary for tunable multicolor phosphorescence.14–20 These features, fortunately, are exactly the two key points required for achieving unusual luminescence from non-conventional luminogens devoid of classic conjugates.39–43 On account of the less effective delocalization of the subunits, individual non-conventional luminophores are virtually non-emissive.39,44–49 The clustering-triggered emission (CTE) mechanism was proposed to rationalize such photophysical properties.18–20,39,50 In this scenario, the clustering of non-conventional chromophores, with lone pairs (n) and/or π electrons to yield clustered chromophores with varying extended electron delocalization, are responsible for the λex-dependent emission (Fig. 1a).18–20,39,43
Inspired by the heterogeneous clustered chromophores of non-conventional luminophores, and considering the prompting effect of heteroatoms on the spin–orbit coupling (SOC), it is speculated that the CTE strategy is highly promising for tunable multicolor persistent phosphorescence (Fig. 1a and b). Although several atypical luminescent polymers have shown λex-dependent phosphorescence,15,18–20 their complicated chain entanglement makes it difficult to gain a clear picture of the electronic interactions among the subunits. Despite the fact that their molecular counterparts51,52 can provide more explicit conformations and molecular packing, there have been no reports concerning their tunable afterglows. To further the understanding of the origin of tunable persistent phosphorescence, it is urgently needed to establish a simple molecular model system. To simplify the interference of different subunits, we here chose luminophores such as those bearing just oxygen functionalities, namely pentaerythritol (PER), D-(+)-xylose (D-Xyl), D-fructose (D-Fru) and D-galactose (D-Gal) (Fig. S1, S2 and Table S1, ESI†). They are ready to form crystals comprising oxygen clusters, which would also allow access to structural diversity through electronic communications.53–55 Furthermore, multiple hydrogen bonds are beneficial for the formation of oxygen clusters with rigid conformations.51,52 Fortunately, even without any π segments, all the crystals demonstrated tunable multicolor p-RTP and more obvious tunability was achieved for them and their concentrated solutions at 77 K, thus confirming the speculation. Notably, although the triboluminescence of sugars has been reported, their photoluminescence (PL) behavior and mechanism have not yet been discussed in detail.56,57 The results given here also provide insights into the origin of the tunable phosphorescence in single-component systems and advance the understanding of the emission mechanism of non-conventional luminophores, including sugars.
Results and discussion
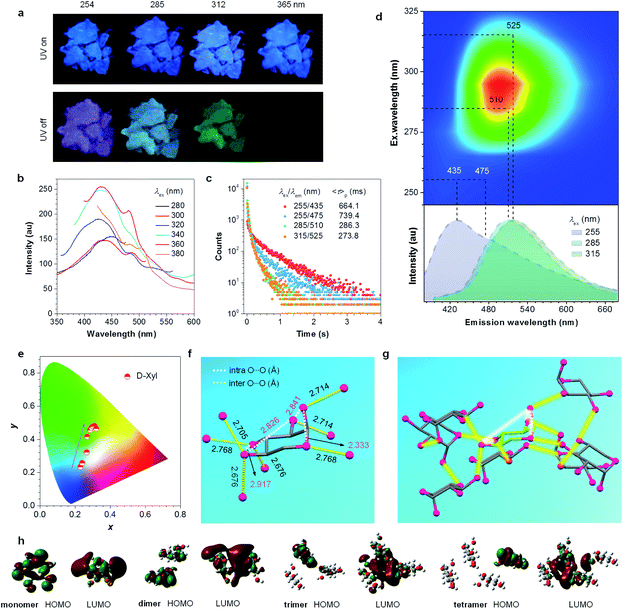
For comparison, distilled glycerol (Gly) was first investigated as control. Liquid Gly is non-emissive at room temperature, whereas it is highly luminescent at 77 K with tunable afterglows from blue to light green by varying the λex (Fig. S3, ESI†). Encouraged by these results, it was speculated that heterogeneous oxygen clusters with sufficiently rigidified conformations in solids might provide tunable p-RTP. To verify it, D-Xyl, a saccharide with a much higher molecular weight and more hydroxyls than Gly (Fig. 1c) was investigated, and which would produce more effective hydrogen bonds to stiffen the molecular conformations. Aqueous D-Xyl solutions exhibited concentration enhanced emission and multiple emission maxima (Fig. 1c and S4, ESI†), which are explainable in terms of the CTE mechanism (Fig. S5, ESI†),43,50 considering the formation of different oxygen clusters and conformation rigidification.43,50,53–55 Furthermore, the presence of diverse oxygen clusters was also validated by looking at the various lifetimes at different maxima (Fig. S6 and Table S2, ESI†). Upon freezing to 77 K, with different λexs from 254 to 365 nm, the emission color of the concentrated D-Xyl solution (1 M) changed from cyan to blue (Fig. 1d), which originated from the ratiometric variation among the emission maxima/shoulders at 393, 443 and 462 nm (Fig. 1e). Moreover, after stopping the irradiations, bright afterglows with tunable colors from blue to greenish-yellow were observed (Fig. 1d). These afterglows corresponded to the cryogenic phosphorescence, whose spectra were red-shifted with increasing λex from 268 to 365 nm (Fig. 1f), further verifying the coexistence of diverse oxygen clusters. The Commission International de l'Eclairage (CIE) coordinates, which were derived from the PL spectra, were calculated and are shown in Fig. 1g, which again verifies the variation of the phosphorescence colors as observed.Considering the more rigidified conformations at crystals than in solutions, D-Xyl crystals were expected to possess tunable multicolor p-RTP at ambient conditions. Thus, the crystal emission was checked next. Comparing the PL efficiency (Φ) of 1 M solutions (0.6%, Table 1), with that of crystals, the crystals show a much higher efficiency (2.2%, Table 1), because of the further rigidification of the oxygen clusters. From being virtually non-luminescent in dilute solutions (i.e., 10−4 M, Φ = 0) to brightly emissive in crystals, D-Xyl exhibited apparent aggregation-induced emission (AIE) characteristics.58 Despite resembling blue PL under different λexs (Fig. 2a, upper set of images), the presence of multiple maxima with varying lifetimes of the prompt emissions (Fig. 2b and S7, ESI†) implied that there were concurrent diverse emissive species. Furthermore, as expected, distinct emission colors of blue, cyan, bluish-green and green were visualized after stopping the UV light of 254, 285, 312 and 365 nm (Fig. 2a and Video S1, ESI†), respectively. Such afterglows were ascribed to the p-RTP emissions, as evidenced by their long lifetimes (〈τ〉p) ranging from 274 to 739 ms (Fig. 2c and Table S3, ESI†). To gain a deeper insight into such an intriguing color-tunable p-RTP, the excitation–phosphorescence mapping of the crystals was performed. As λex changed from 245 to 325 nm, the p-RTP emission correspondingly exhibited an obvious bathochromic shift from blue to green alongside the peak/shoulder varying from 435/475 to 525 nm (Fig. 2d). Such tunable p-RTP colors in response to the variation in λex are also illustrated in the CIE coordinate diagram (Fig. 2e), from which the color evolution from blue to green is noticed. Specifically, the p-RTP spectra (td = 0.1 ms) with λexs of 255, 285 and 315 nm were peaking at 435/475, 510 and 525 nm (Fig. 2d), with CIE coordinates of (0.241, 0.257), (0.285, 0.456) and (0.311, 0.480) (Fig. 2e), respectively, which were highly consistent with the naked eye observation.
| Samples | D-Xyl | PER | D-Fru | D-Gal |
|---|---|---|---|---|
| λ ex,o/λem,o/Φ [nm]/[nm]/[%] | λ ex,o/λem,o/Φ [nm]/[nm]/[%] | λ ex,o/λem,o/Φ [nm]/[nm]/[%] | λ ex,o/λem,o/Φ [nm]/[nm]/[%] | |
| a λ ex,o = optimal excitation wavelength; λem,o = optimal emission wavelength; Φ = quantum efficiency. b Concentration: 1 M (D-Xyl, D-Fru and D-Gal), 0.1 M (PER). c Concentration: 1 × 10−4 M. | ||||
| Solutionb | 284/354/0.6 | 270/452/0.5 | 268/432/0.3 | 340/429/0.8 |
| Solutionc | 284/321/0.0 | 270/302/0.0 | 268/301/0.0 | 340/387/0.0 |
| Crystal | 360/429/2.7 | 270/460/2.2 | 280/495/1.8 | 280/451/4.1 |
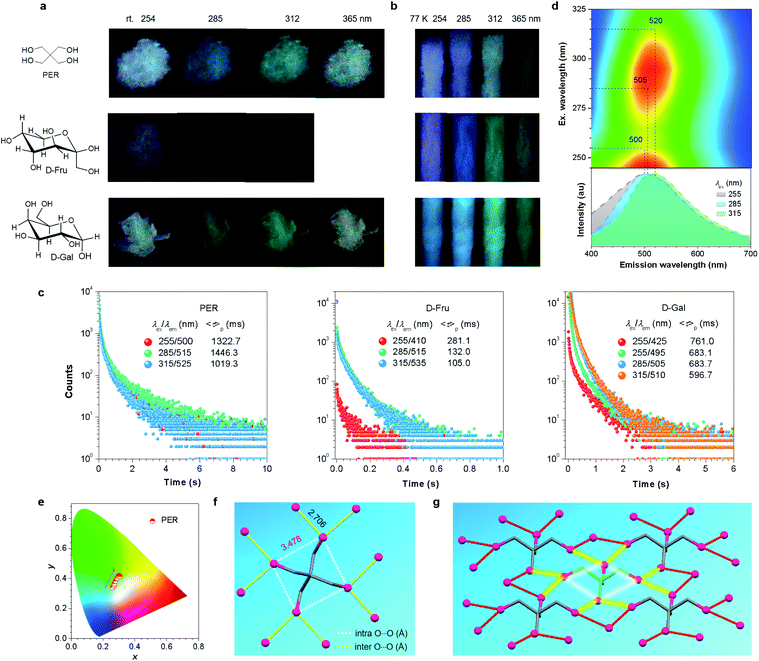
It was also noted that multiple maxima and/or broad full width at half maxima (FWHM) of greater than 116 nm (Fig. 2d) in the p-RTP spectra highly suggest the co-existence of varying emissive species even with a fixed λex. This was supported by the different 〈τ〉ps at 435 and 475 nm with the same λex of 255 nm (Fig. 2c), as well as the multi-exponential decay profiles for each monitored wavelength (Table 1). To further understand the tunable p-RTP, the single crystal structure of D-Xyl was determined. Notably, besides H–O⋯H–O (1.781, 1.859, 1.955 Å), O–H⋯C–H (2.717, 2.808, 2.863 Å) and H–O⋯H–C (2.559, 2.690 Å) hydrogen bonds, intermolecular short dihydrogen bonds of O–H⋯H–O (2.343 Å), C–H⋯H–O (2.395 Å) and C–H⋯H–C (2.342 Å) were also present (Fig. S8, ESI†), which remarkably restricted the molecular motions, thus providing rigid conformations in the crystals. Moreover, abundant intramolecular (2.333, 2.826, 2.841, 2.917 Å) and intermolecular (2.676, 2.705, 2.714, 2.768 Å) O⋯O electronic interactions were found (Fig. 2f), whose distances were much shorter than the sum of the vdW radii (3.04 Å), thus leading to effective 3D through-space conjugation of the lone pairs (Fig. 2g). Furthermore, the clustering of oxygens enriched the energy levels and narrowed the energy gaps of diverse clustered chromophores, which would promote the spin–orbit coupling (SOC) and consequent intersystem crossing (ISC) transitions. Therefore, derived from these oxygen clusters with varying energy gaps, strikingly excitation-dependent multicolor p-RTP were readily observable while waiting for sufficiently rigidified conformations through effective intra- and inter-molecular interactions (Fig. 1b). To further understand the mechanism, the HOMO and LUMO electron densities of monomer, dimer, trimer and tetramer of D-Xyl were calculated (Fig. S9, ESI†). Although the results remain preliminary, the electron density distribution of the monomer clearly indicated the intramolecular O⋯O electron delocalization (Fig. 2h). In addition, the LUMO levels of other aggregates clearly illustrated the extended delocalization among the neighboring molecules in excited states (Fig. 2h), which agreed well with the postulated hypothesis. Because of the preceding results, more luminogens with tunable p-RTP were confidently explored using the CTE strategy. To check the universality of it, the PL properties of PER, D-Fru and D-Gal were studied further (Fig. 3a, S10–S13, ESI† and Table 1). Resembling D-Xyl, they all exhibited AIE characteristics, as shown by D-Gal (Fig. S10 and S12, ESI†). In addition, the PL spectra of the crystals showed multiple maxima, which were associated with distinctive emission species (Fig. S13, ESI†). Although the observed p-RTP colors of their crystals did not vary considerably upon excitation with different λexs, from greenish-white to yellow/greenish-yellow (Fig. 3a and Video S2, ESI†), those of the cryogenic phosphorescence of their concentrated solutions demonstrated more apparent tunability (Fig. 3b and S11, ESI†), which presumably originated from the more widespread distribution of oxygen clusters in solutions and immensely rigidified conformations at 77 K. Time-resolved measurement revealed long 〈τ〉p values for the crystals, ranging from hundreds of microseconds to ∼1.45 s (Fig. 3c), which were among the longest values for both aromatic34,59–64 and non-conventional luminogens.15,18–20,51 Meanwhile, the multiple decay components of p-RTP indicated the simultaneous contribution from an ensemble of emissive species within a heterogeneous population (Tables S4–S6, ESI†), which was consistent with their unstructured spectra and broad FWHM of >131 nm (Fig. 3d and S14, S15, ESI†). Thus, tunable p-RTP can be readily realized from these crystals by changing the λex, which resulted in the formation of heterogeneous oxygen clusters.
Further excitation–phosphorescence mapping (Fig. 3d and S14, S15, ESI†) and the CIE coordinate diagrams (Fig. 3e and S14, S15, ESI†) clearly showed the peaks and color changes of p-RTP in response to the increasing λex, thus verifying their tunability. Taking PER as an example, as the λex changed from 245 to 325 nm, the p-RTP maximum was red-shifted from 500 to 520 nm, which were all located in the green region (Fig. 3d). Meanwhile, their CIE coordinate diagram shows nearly linear changes with increasing λex, accompanying the color variation from whitish-green to greenish-yellow (Fig. 3e). The p-RTP colors are mainly concentrated in the green region, and this may explain why the tunability of these crystals is less obvious when compared to that of D-Xyl.
Similar to D-Xyl, there were abundant intra- and inter-molecular O⋯O interactions (i.e., 3.478 and 2.706 Å in PER) in the crystals of these luminophores (Fig. 3f, g and S16, S17, ESI†), which constituted a 3D through-space electronic communication network among the oxygen atoms, thereby resulting in diverse clustered chromophores with varying electron delocalization. It was worth noting, that regardless of the fact that the distance among the intramolecular oxygens (3.478 Å) was greater than the sum of the van der Waals (vdW) radii (3.04 Å) in the PER crystals, the calculation results clearly indicate the through-space electron delocalization among the intramolecular oxygens, particularly in the LUMO levels (Fig. S18, ESI†), and this was presumably because of the shortened distance in the excited states.65 These interactions, together with O⋯H hydrogen bonds, H⋯H dihydrogen bonds and C⋯O short contacts (Fig. S19–S21, ESI†), collectively stiffen the oxygen clusters, thus enabling the noticeable p-RTP emissions. Taken together, because of the heterogeneous population of emissive clusters, tunable multicolor p-RTP can be achieved with adequatly rigidified conformations.
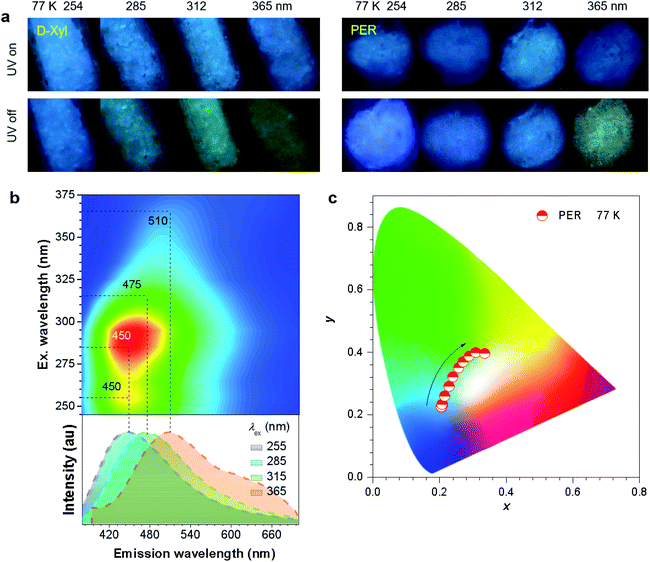
To further check the effect of cryogenic treatment on the tunability of phosphorescence, the PL emission of D-Xyl and PER crystals was investigated at 77 K. Both crystals exhibited intense blue PL upon irradiation with UV light from 254 to 365 nm (Fig. 4a). After the removal of the excitation, the crystals showed blue-shifted afterglows with much longer durations when compared to those under ambient conditions (Fig. 4, S22 and Video S3, S4, ESI†). The tunable range of cryogenic phosphorescence was notably, extended into both much bluer and much redder areas (Fig. 4c and S22c, ESI†), which was proved from the blue-shifted peaks at the same λex (Table 2), which should be ascribed to the emission enhancement for such emissive species in frozen states. Taking PER crystals as an example, the emission colors of blue, blue, cyan and yellowish-green were noticed after stopping the UV light at wavelengths of 254, 285, 312 and 365 nm (Fig. 4a and Video S4, ESI†), respectively, whose emission spectra showed improved fractions in the blue region when compared with those at room temperature (Fig. 4b). These results indicate enhanced color tunability for the persistent phosphorescence of such non-conventional luminophores at 77 K and corroborate the presence of diverse emissive oxygen clusters in the crystals.
| λ ex [nm] | D-Xyl | PER | D-Fru | D-Gal |
|---|---|---|---|---|
| λ em [nm] | λ em [nm] | λ em [nm] | λ em [nm] | |
| 255 (rt) | 435/475 | 500 | 410 | 425/495 |
| 285 (rt) | 510 | 505 | 515 | 505 |
| 315 (rt) | 525 | 520 | 535 | 510 |
| 255 (77 K) | 425/470 | 450 | — | — |
| 285 (77 K) | 475 | 450 | — | — |
| 315 (77 K) | 480 | 475 | — | — |
Conclusions
In conclusion, a CTE strategy was proposed to achieve tunable multicolor persistent phosphorescence in non-conventional luminophores without any remarkable conjugates or even free of π segments, by virtue of the heterogeneous nature of the emissive clusters in these systems. The CTE strategy is proved effective, as demonstrated by a group of non-conventional luminophores comprising merely oxygen functionalities (–OH), i.e., D-Xyl, PER, D-Fru and D-Gal. The clustering of oxygens created enriched energy levels and narrowed the energy gaps, thus promoting SOC and allowing the consequent ISC transitions. By taking advantage of multistate O⋯O communicating networks and effective intra- and inter-molecular interactions, heterogeneous oxygen clusters were constructed, further achieving p-RTP color tunability assisted by a rigid local environment. The strategy and results presented should encourage the rational design of single-component molecules with tunable p-RTP emission colors in a variety of applications in chemistry. Furthermore, these findings, in turn, offer more fundamental implications for the underlying mechanism of non-conventional luminophores.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51822303) and the Medical-Engineering Cross Project of Shanghai Jiao Tong University (YG2016MS21).Notes and references
- J. Lee, P. W. Bisso, R. L. Srinivas, J. J. Kim, A. J. Swiston and P. S. Doyle, Nat. Mater., 2014, 13, 524–529 CrossRef CAS.
- H. Lee, J. Kim, H. Kim, J. Kim and S. Kwon, Nat. Mater., 2010, 9, 745–749 CrossRef CAS PubMed.
- R. Deng, F. Qin, R. Chen, W. Huang, M. Hong and X. Liu, Nat. Nanotechnol., 2015, 10, 237–242 CrossRef CAS PubMed.
- L. Pan, S. Sun, A. Zhang, K. Jiang, L. Zhang, C. Dong, Q. Huang, A. Wu and H. Lin, Adv. Mater., 2015, 27, 7782–7787 CrossRef CAS PubMed.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- O. Chen, D. E. Shelby, Y. Yang, J. Zhuang, T. Wang, C. Niu, N. Omenetto and Y. C. Cao, Angew. Chem., Int. Ed., 2010, 49, 10132–10135 CrossRef CAS PubMed.
- Y. Sagara, S. Yamane, M. Mitani, C. Weder and T. Kato, Adv. Mater., 2016, 28, 1073–1095 CrossRef CAS PubMed.
- Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878–3896 RSC.
- K. M. Lee, W. Y. Cheng, C. Y. Chen, J. J. Shyue, C. C. Nieh, C. F. Chou, J. R. Lee, Y. Y. Lee, C. Y. Cheng, S. Y. Chang, T. C. Yang, M. C. Cheng and B. Y. Lin, Nat. Commun., 2013, 4, 1544 CrossRef PubMed.
- M. J. Sun, Y. W. Zhong and J. Yao, Angew. Chem., Int. Ed., 2018, 57, 7820–7825 CrossRef CAS PubMed.
- M. Irie, T. Fulcaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- Z. Mao, Z. Yang, Y. Mu, Y. Zhang, Y. F. Wang, Z. Chi, C. C. Lo, S. Liu, A. Lien and J. Xu, Angew. Chem., Int. Ed., 2015, 54, 6270–6273 CrossRef CAS.
- T. H. Kim, K. S. Cho, E. K. Lee, S. J. Lee, J. Chae, J. W. Kim, D. H. Kim and J. Y. Kwon, Nat. Photonics, 2011, 5, 176–182 CrossRef CAS.
- L. Gu, H. Shi, L. Bian, M. Gu, K. Ling, X. Wang, H. Ma, S. Cai, W. Ning, L. Fu, H. Wang, S. Wang, Y. Gao, W. Yao, F. Huo, Y. Tao, Z. An, X. Liu and W. Huang, Nat. Photonics, 2019, 13, 406–411 CrossRef CAS.
- S. Cai, H. Ma, H. Shi, H. Wang, X. Wang, L. Xiao, W. Ye, K. Huang, X. Cao, N. Gan, C. Ma, M. Gu, L. Song, H. Xu, Y. Tao, C. Zhang, W. Yao, Z. An and W. Huang, Nat. Commun., 2019, 10, 4247 CrossRef PubMed.
- H. Wang, H. Shi, W. Ye, X. Yao, Q. Wang, C. Dong, W. Jia, H. Ma, S. Cai, K. Huang, L. Fu, Y. Zhang, J. Zhi, L. Gu, Y. Zhao, Z. An and W. Huang, Angew. Chem., Int. Ed., 2019, 58, 18776–18782 CrossRef CAS PubMed.
- Y. Su, Y. Zhang, Z. Wang, W. Gao, P. Jia, D. Zhang, C. Yang, Y. Li and Y. Zhao, Angew. Chem., Int. Ed., 2019, 58, 1–6 CrossRef.
- X. Dou, Q. Zhou, X. Chen, Y. Tan, X. He, P. Lu, K. Sui, B. Z. Tang, Y. Zhang and W. Z. Yuan, Biomacromolecules, 2018, 19, 2014–2022 CrossRef CAS PubMed.
- Q. Zhou, Z. Wang, X. Dou, Y. Wang, S. Liu, Y. Zhang and W. Z. Yuan, Mater. Chem. Front., 2019, 3, 257–264 RSC.
- Q. Wang, X. Dou, X. Chen, Z. Zhao, S. Wang, Y. Wang, K. Sui, Y. Tan, Y. Gong, Y. Zhang and W. Z. Yuan, Angew. Chem., Int. Ed., 2019, 58, 12667–12673 CrossRef CAS PubMed.
- W. Zhao, Z. He, J. W. Y. Lam, Q. Peng, H. Ma, Z. Shuai, G. Bai, J. Hao and B. Z. Tang, Chem, 2016, 1, 592–602 CAS.
- K. Narushima, Y. Kiyota, T. Mori, S. Hirata and M. Vacha, Adv. Mater., 2019, 31, 1807268 CrossRef PubMed.
- Z. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu and W. Huang, Nat. Mater., 2015, 14, 685–690 CrossRef CAS PubMed.
- R. Kabe and C. Adachi, Nature, 2017, 550, 384–387 CrossRef CAS PubMed.
- S. Hirata, K. Totani, J. Zhang, T. Yamashita, H. Kaji, S. R. Marder, T. Watanabe and C. Adachi, Adv. Funct. Mater., 2013, 23, 3386–3397 CrossRef CAS.
- Y. Su, S. Z. F. Phua, Y. Li, X. Zhou, D. Jana, G. Liu, W. Q. Lim, W. K. Ong, C. Yang and Y. Zhao, Sci. Adv., 2018, 4, eaas9732 CrossRef PubMed.
- S. Tao, S. Lu, Y. Geng, S. Zhu, S. A. T. Redfern, Y. Song, T. Feng, W. Xu and B. Yang, Angew. Chem., Int. Ed., 2018, 57, 2393–2398 CrossRef CAS PubMed.
- D. Li, F. Lu, J. Wang, W. Hu, X. M. Cao, X. Ma and H. Tian, J. Am. Chem. Soc., 2018, 140, 1916–1923 CrossRef CAS PubMed.
- Z. Yang, Z. Mao, X. Zhang, D. Ou, Y. Mu, Y. Zhang, C. Zhao, S. Liu, Z. Chi, J. Xu, Y. C. Wu, P. Y. Lu, A. Lien and M. R. Bryce, Angew. Chem., Int. Ed., 2016, 55, 2181–2185 CrossRef CAS PubMed.
- E. Lucenti, A. Forni, C. Botta, L. Carlucci, C. Giannini, D. Marinotto, A. Pavanello, A. Previtali, S. Righetto and E. Cariati, Angew. Chem., Int. Ed., 2017, 56, 16302–16307 CrossRef CAS PubMed.
- O. Bolton, K. Lee, H. J. Kim, K. Y. Lin and J. Kim, Nat. Chem., 2011, 3, 205–210 CrossRef CAS PubMed.
- G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747–751 CrossRef CAS PubMed.
- X. Chen, C. Xu, T. Wang, C. Zhou, J. Du, Z. Wang, H. Xu, T. Xie, G. Bi, J. Jiang, X. Zhang, J. N. Demas, C. O. Trindle, Y. Luo and G. Zhang, Angew. Chem., Int. Ed., 2016, 55, 9872–9876 CrossRef CAS PubMed.
- S. Xu, R. Chen, C. Zheng and W. Huang, Adv. Mater., 2016, 28, 9920–9940 CrossRef CAS PubMed.
- G. He, W. T. Delgado, D. J. Schatz, C. Merten, A. Mohammadpour, L. Mayr, M. J. Ferguson, R. McDonald, A. Brown, K. Shankar and E. Rivard, Angew. Chem., Int. Ed., 2014, 53, 4587–4591 CrossRef CAS PubMed.
- H. Wu, W. Chi, G. Baryshnikov, B. Wu, Y. Gong, D. Zheng, X. Li, Y. Zhao, X. Liu, H. Agren and L. Zhu, Angew. Chem., Int. Ed., 2019, 58, 4328–4333 CrossRef CAS PubMed.
- Z. He, H. Gao, S. Zhang, S. Zheng, Y. Wang, Z. Zhao, D. Ding, B. Yang, Y. Zhang and W. Z. Yuan, Adv. Mater., 2019, 31, 1807222 CrossRef PubMed.
- Z.-Y. Zhang, Y. Chen and Y. Liu, Angew. Chem., Int. Ed., 2019, 131, 6089–6093 CrossRef.
- X. Chen, W. Luo, H. Ma, Q. Peng, W. Z. Yuan and Y. Zhang, Sci. China: Chem., 2018, 61, 351–359 CrossRef CAS.
- L. Yuan, H. Yan, L. Bai, T. Bai, Y. Zhao, L. Wang and Y. Feng, Macromol. Rapid Commun., 2018, 39, 1800658 Search PubMed.
- C. Shang, N. Wei, H. Zhuo, Y. Shao, Q. Zhang, Z. Zhang and H. Wang, J. Mater. Chem. C, 2017, 5, 8082–8090 RSC.
- C. Hu, Y. Ru, Z. Guo, Z. Liu, J. Song, W. Song, X. Zhang and J. Qiao, J. Mater. Chem. C, 2019, 7, 387–393 RSC.
- Q. Zhou, B. Cao, C. Zhu, S. Xu, Y. Gong, W. Z. Yuan and Y. Zhang, Small, 2016, 12, 6586–6592 CrossRef CAS PubMed.
- W. I. Lee, Y. Bae and A. J. Bard, J. Am. Chem. Soc., 2004, 126, 8358–8359 CrossRef CAS PubMed.
- D. Wang and T. Imae, J. Am. Chem. Soc., 2004, 126, 13204–13205 CrossRef CAS PubMed.
- H. Lu, L. Feng, S. Li, J. Zhang, H. Lu and S. Feng, Macromolecules, 2015, 48, 476–482 CrossRef CAS.
- E. Zhao, J. W. Y. Lam, L. Meng, Y. Hong, H. Deng, G. Bai, X. Huang, J. Hao and B. Z. Tang, Macromolecules, 2015, 48, 64–71 CrossRef CAS.
- R. B. Restani, P. I. Morgado, M. P. Ribeiro, I. J. Correia, A. Aguiar-Ricardo and V. D. B. Bonifácio, Angew. Chem., Int. Ed., 2012, 51, 5162–5165 CrossRef CAS PubMed.
- V. D. B. Bonifácio, V. G. Correia, M. G. Pinho, J. C. Lima and A. Aguiar-Ricardo, Mater. Lett., 2012, 81, 205–208 CrossRef.
- Y. Gong, Y. Tan, J. Mei, Y. Zhang, W. Z. Yuan, Y. Zhang, J. Sun and B. Z. Tang, Sci. China: Chem., 2013, 56, 1178–1182 CrossRef CAS.
- M. Fang, J. Yang, X. Xiang, Y. Xie, Y. Dong, Q. Peng and Z. Li, Mater. Chem. Front., 2018, 2, 2124–2129 RSC.
- Y. Wang, X. Bin, X. Chen, S. Zheng, Y. Zhang and W. Z. Yuan, Macromol. Rapid Commun., 2018, 39, 1800528 CrossRef PubMed.
- W. Z. Yuan and Y. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 560–574 CrossRef.
- D. A. Tomalia, B. Klajnert-Maculewicz, K. A. M. Johnson, H. F. Brinkman, A. Janaszewska and D. M. Hedstrand, Prog. Polym. Sci., 2018, 90, 35–117 CrossRef.
- H. Zhang, Z. Zhao, P. R. McGonigal, R. Ye, S. Liu, J. W. Y. Lam, R. T. K. Kwok, W. Z. Yuan, J. Xie, A. L. Rogach and B. Z. Tang, Mater. Today, 2020, 32, 275–292 CrossRef.
- J. I. Zink, G. E. Hardy and J. E. Sutton, J. Phys. Chem. C, 1976, 80, 248–249 CrossRef CAS.
- F. G. Wick, J. Opt. Soc. Am., 1940, 30, 302–306 CrossRef CAS.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- M. Baroncini, G. Bergamini and P. Ceroni, Chem. Commun., 2017, 53, 2081–2093 RSC.
- S. Hirata, Adv. Opt. Mater., 2017, 5, 1700116 CrossRef.
- P. Long, Y. Feng, C. Cao, Y. Li, J. Han, S. Li, C. Peng, Z. Li and W. Feng, Adv. Funct. Mater., 2018, 28, 1800791 CrossRef.
- J. Wang, Z. Chai, J. Wang, C. Wang, M. Han, Q. Liao, A. Huang, P. Lin, C. Li, Q. Li and Z. Li, Angew. Chem., Int. Ed., 2019, 58, 17297–17302 CrossRef CAS PubMed.
- W. Zhao, T. S. Cheung, N. Jiang, W. Huang, J. W. Y. Lam, X. Zhang, Z. He and B. Z. Tang, Nat. Commun., 2019, 10, 1595 CrossRef PubMed.
- B. Zhou and D. Yan, Adv. Funct. Mater., 2019, 29, 1807599 CrossRef.
- R. D. Pensack, R. J. Ashmore, A. L. Paoletta and G. D. Scholes, J. Phys. Chem. C, 2018, 122, 21004–21017 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization data; photophysical data and chemical structure of the compounds. CCDC 1934968 (D-Xyl), 1934967 (PER), 1934969 (D-Fru) and 1934964 (D-Gal). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc06518k |
| This journal is © The Royal Society of Chemistry 2020 |