Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective radical depolymerization of cellulose to glucose induced by high frequency ultrasound†
Somia
Haouache
ad,
Ayman
Karam
a,
Tony
Chave
 b,
Jonathan
Clarhaut
a,
Prince Nana
Amaniampong
b,
Jonathan
Clarhaut
a,
Prince Nana
Amaniampong
 a,
José M.
Garcia Fernandez
a,
José M.
Garcia Fernandez
 c,
Karine
De Oliveira Vigier
c,
Karine
De Oliveira Vigier
 a,
Isabelle
Capron
d and
François
Jérôme
a,
Isabelle
Capron
d and
François
Jérôme
 *a
*a
aInstitut de Chimie des Milieux et Matériaux de Poitiers, Université de Poitiers-CNRS, 1 Rue Marcel Doré, 86073 Poitiers, France. E-mail: francois.jerome@univ-poitiers.fr
bICSM, Univ Montpellier, CNRS, CEA, ENSCM, Bagnols-sur-Cèze, France
cInstitute for Chemical Research, CSIC and University of Sevilla, Americo Vespucio 49, Isla de la Cartuja, 41092 Sevilla, Spain
dINRA, Site de la Géraudière, 44316 Nantes, France
First published on 6th February 2020
Abstract
The depolymerization of cellulose to glucose is a challenging reaction and often constitutes a scientific obstacle in the synthesis of downstream bio-based products. Here, we show that cellulose can be selectively depolymerized to glucose by ultrasonic irradiation in water at a high frequency (525 kHz). The concept of this work is based on the generation of H˙ and ˙OH radicals, formed by homolytic dissociation of water inside the cavitation bubbles, which induce the cleavage of the glycosidic bonds. The transfer of radicals on the cellulose particle surfaces prevents the side degradation of released glucose into the bulk solution, allowing maintaining the selectivity to glucose close to 100%. This work is distinguished from previous technologies in that (i) no catalyst is needed, (ii) no external source of heating is required, and (iii) the complete depolymerization of cellulose is achieved in a selective fashion. The addition of specific radical scavengers coupled to different gaseous atmospheres and ˙OH radical dosimetry experiments suggested that H˙ radicals are more likely to be responsible for the depolymerisation of cellulose.
Introduction
The depolymerisation of cellulose1 to glucose has become an important reaction paving the way to various biobased chemicals such as ethanol, furandicarboxylic acid, caprolactam, sorbitol, levulinic acid, γ-valerolactone, among many others.2 The hydrolysis of cellulose to glucose is however difficult to achieve and often constitutes an obstacle in the synthesis of downstream products.3 Indeed, this reaction requires overcoming high energy barriers of about 30–40 kcal mol−1,3a,4 essentially because cellulose exhibits a highly cohesive hydrogen bond network,1,5 strong van der Waals interactions,6 and electronic effects.7 To date, the selective hydrolysis of cellulose to glucose is performed by enzymatic routes.8 Although this route is deployed on a large scale for the production of ethanol, the price of enzymes as well as low space time yield and costly downstream purification processes hamper the large development of this route in the chemical industry. Alternatively, acid catalysts can also depolymerize cellulose, but they require harsh conditions and thus unfortunately afford glucose in low yield due to the formation of tar-like materials.2,3The exploration of alternative technologies capable of selectively depolymerizing cellulose to glucose with a high efficiency is still an open scientific question.9 Supercritical water has emerged as a promising route for releasing glucose from cellulose10 and this process was even scaled-up by Renmatix.11 Solvent free technologies, aiming at producing concentrated feed of glucose, were also explored and one may cite the depolymerisation of cellulose by mechanocatalysis12 or by non-thermal atmospheric plasma (NTAP).13 With these solvent-free technologies, depolymerisation and repolymerization reactions occur simultaneously and processable water soluble oligosaccharides are obtained instead of monomeric glucose.
Here we report an alternative technology based on the use of high frequency ultrasound (HFUS). The ultrasonic irradiation of cellulose at a high frequency leads to a complete depolymerisation of cellulose to glucose, without any catalyst. In addition, the depolymerisation of cellulose induced by HFUS does not need any external source of heating or pressure, the energy being brought by the implosion of cavitation bubbles.14
When applied within a liquid, ultrasonic irradiation induce the nucleation, growth and collapse of gas and vapour filled bubbles. In contrast to the very popular low frequency ultrasound (<80 kHz) which mostly induces physical effects (shock waves, micro-jets, turbulences, etc.), irradiation of water at high frequencies (>150 kHz) mainly leads to the in situ formation of H˙ and ˙OH radicals resulting from the dissociation of water molecule.15 Once these cavitation bubbles implode, in situ formed radicals can recombine or react with solutes inducing chemical effects. Inspired by our previous works on NTAP,13 we conceived that these in situ produced radicals should theoretically induce the cleavage of the glycosidic bonds of cellulose.16 Being able to selectively depolymerize cellulose with H˙ and ˙OH radicals without side degradation of released glucose into the bulk solution is a challenging scientific task, which is addressed in this study.
When solid particles are present in an ultrasonic reactor, they act as nuclei for the formation and growth of cavitation bubbles. Close to a surface, the implosion of cavitation bubbles is very asymmetric and generates high-speed jets of liquid towards the surface, a good mean to concentrate radicals on a particle.17 Applied to cellulose, this physical principle should be an efficient mean to control the reaction selectivity by concentrating radicals on the cellulose particle surfaces, thus preventing side reactions of released glucose into the bulk solution.
Result and discussion
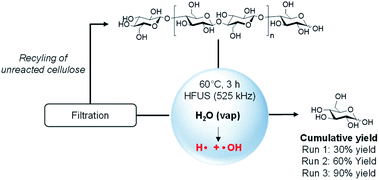
To demonstrate the potential of the above concept, we first subjected microcrystalline cellulose (MCC, Avicel PH 200) to an ultrasonic irradiation at 525 kHz (acoustic power density of 0.36 W mL−1) in water, at 60 °C, and under atmospheric pressure of air. Analysis of the products formed was performed by HPLC and the conversion of cellulose was monitored by size exclusion chromatography (SEC) and difference of weight. To our delight, after 3 h of irradiation, glucose was formed in 30% mass yield (Scheme 1). The reaction was fully selective to glucose, no other product was detected either by HPLC or mass spectrometry (Fig. S1†). 1H and 13C NMR confirmed the selectivity of the reaction and recorded NMR spectra of the crude product were rigorously similar to that of standard glucose (Fig. S2 and S3†). The absence of water-soluble low molecular weight oligosaccharides also suggests that the depolymerization occurs at the terminal position of the cellulosic chain. The yield of glucose also perfectly fits with the conversion of cellulose (30%), determined by measuring the difference of weight before and after HFUS treatment. Interestingly, the reaction also proceeded well at 40 °C and even 25 °C, without affecting the yield and the selectivity into glucose.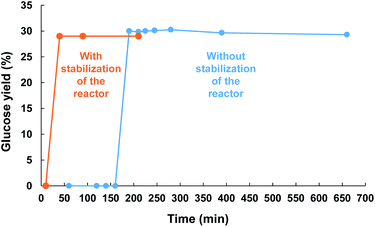 | ||
| Scheme 2 Kinetic profile of the reaction recorded at 60 °C under air (525 kHz, acoustic power: 0.36 W mL−1). Cellulose was introduced before (blue) or after (orange) warm-up of the reactor. | ||
To rationalize the high selectivity into glucose, the remaining MCC was removed by filtration after 3 h and the as-obtained aqueous solution of glucose was subjected again to ultrasonic irradiations. Pleasingly, in the absence of cellulose particles, ultrasonic irradiation of the homogeneous solution of glucose led to quick degradation, through uncontrolled oxidation reactions, with gluconic acid being formed as a primary product, as observed earlier.18 This counter experiment is in agreement with the advanced hypothesis that, in the presence of MCC, radicals preferentially react with the cellulose particles rather than with released glucose in the bulk solution, thus optimizing the selectivity to glucose. Analysis of the recovered cellulose by SEC confirmed its depolymerisation with a reduction in the weight-average molecular weight (Mw) and the number-average molecular weight (Mn) from 49 × 103 to 43 × 103 g mol−1 and 38 × 103 to 30 × 103 g mol−1, respectively, after 3 h of reaction (Fig. S4†). Furthermore, analysis of the linking pattern of the recovered cellulosic material showed the exclusive occurrence of β-(1 → 4)-linked glucose units, as expected for cellulose. These results rule out the possible occurrence of repolymerization reactions, which would have led to the formation of 1,6 glycosidic linkages, as previously observed by the mechanocatalytic12g or the NTAP technologies.13c FT-IR and XPS analyses confirmed that no oxidation or C–C bond cleavage of the recovered cellulose occurred in our conditions, at least it is below the detection limit of our apparatus (Fig. S5 and S6†). In addition, XRD analysis did not show any change in the crystallinity index before and after HFUS, which strongly suggest that the in situ produced H˙ and ˙OH radicals selectively cleave the glycosidic bonds of cellulose (Fig. S7†).
The kinetic profile of the reaction was next monitored at 60 °C and revealed an induction period of about 3 h. At the moment, we have no rational explanation for this induction period. It seems it corresponds to the time for the HFUS reactor to reach its optimal efficiency (see ESI† for additional information). For instance, when ultrasonic irradiation of neat water was performed for 3 h prior to addition of cellulose, the induction period was reduced to only 10 min. After this period, cellulose was quasi instantaneously depolymerized to glucose, indicating that the depolymerisation rate of cellulose was very high. Subjecting cellulose to HFUS for a reaction time higher than 3 h did not result in a further improvement of the glucose yield, meaning that the depolymerisation of cellulose has stopped.
To get more insight on this phenomenon, the amount of cellulose suspended in water was varied from 0.5 wt% to 5 wt%. Independently of the cellulose loading, the depolymerisation reaction always stopped when the concentration of glucose reached 16.7 mmol L−1 (i.e. 0.3 g L−1), suggesting that released glucose inhibits the depolymerisation of cellulose induced by HFUS. We speculated that, at a certain concentration of glucose, this latter can trap the radicals on the cellulose particle surface. More information on this aspect is provided hereinafter. Complete depolymerisation of cellulose by HFUS should be theoretically feasible by switching from batch to continuous or semi-continuous processes. In this context, the cellulose recovered by filtration after the first batch was re-suspended in pure water and subjected again to HFUS. Interestingly, the depolymerisation occurred but stopped again at a concentration of glucose of 16.7 mmol L−1, leading to a cumulative yield of glucose of 60% after two runs (2 × 0.3 g of glucose, Scheme 1). These experiments could be reproduced four consecutive times leading to a quantitative depolymerisation of cellulose to glucose (Scheme 1).
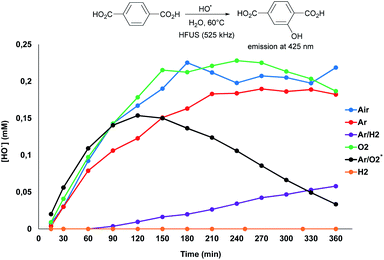
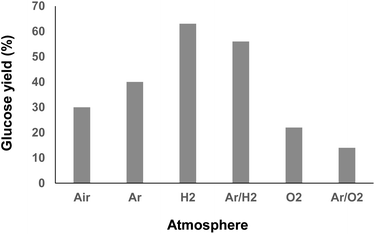
To explore the possibilities for further improving the glucose yield, we next investigated the reaction mechanism. It is widely accepted that irradiation of water at a high frequency leads to its homolytic dissociation to H˙ and ˙OH radicals.15 The H˙ radicals being difficult to observe due to fast recombination reactions, we first focused our investigations on the in situ produced ˙OH radicals. To this end, the formation of ˙OH radicals was monitored by fluorimetry using terephthalic acid (TPA) as an ˙OH radical scavenger (Scheme 3 and Fig. S8†).19 It is noteworthy that curves presented on Scheme 3 reach a maximum, and even decrease in some cases, which does not reflect the reality; it is actually due to the over-oxidation of the as-formed hydroxyterephthalic acid. Under air, the formation of ˙OH radicals was clearly evidenced by fluorimetry analysis. Ratio of specific heat, thermal conductivity and water solubility of gases impact the temperature of cavitation bubbles and thus the generation of radicals. In this context, the gaseous atmosphere was varied and changed from air to O2, Ar, H2 and mixtures of Ar/H2 and Ar/O2 (Scheme 3). In line with the state of the art on HFUS, the initial formation rate of ˙OH radicals was the highest in an Ar/O2 atmosphere.20 Conversely, addition of H2 to Ar dramatically inhibited the formation rate of ˙OH radicals, which was even inhibited when using neat H2 as a gaseous atmosphere, in line with previous report (Scheme 3).21 We noticed that the efficiency of HFUS-mediated cellulose depolymerisation was inversely proportional to the formation of ˙OH radicals, i.e. the higher the initial formation rate of ˙OH radicals, the lower the glucose yield (Scheme 4). For instance, cellulose was depolymerized to glucose with 63% yield under an H2 atmosphere (vs. 30% under air) while no ˙OH radical was formed in this case, suggesting that H˙ radicals are more likely to be involved in the reaction mechanism.
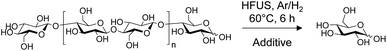
To independently assess the role of H˙ and ˙OH radicals in the reaction mechanism, control experiments were performed by adding two different radical scavengers during the ultrasonication of cellulose (Table 1). First, the ultrasonic irradiation of cellulose was performed under an Ar/H2 atmosphere in an aqueous solution of TPA (2.0 mM), with the aim of in situ trapping the ˙OH radicals. Consistent with our expectations, the glucose yield remained similar in the presence of 2.0 mM of TPA, supporting that ˙OH radicals have no major role in our case on the cellulose depolymerisation mechanism (Table 1, entry 2). This conclusion is also supported by FT-IR and XPS analyses which did not show oxidation, C–C bond cleavage or rearrangement of remaining cellulose. Next, the same reaction was performed by replacing TPA by carbon tetrachloride (CCl4). CCl4 is known to trap the H˙ radicals inside the cavitation bubbles.16 Despite the poor miscibility of CCl4 in water, addition of CCl4 completely inhibited the reaction, highlighting the important role of H˙ radicals in the depolymerisation of cellulose (Table 1, entry 3). This result is also consistent with our experiments under air, O2 and Ar/O2 for which the lowest yields in glucose were observed (Scheme 4). Indeed, H˙ radicals are known to be recombined with O2 to form ˙OH and ˙OOH radicals, as Niwano and Sivakumar previously observed by ESR spin trapping21 and dosimetry experiments,19 respectively. Hence, it is anticipated that the amount of free H˙ radicals is rather low under O2 and Ar/O2 atmosphere, explaining the low glucose yields obtained in these cases.
Altogether, these results show that the depolymerisation of cellulose observed under HFUS is enhanced under an H2 atmosphere, suggesting that H˙ radicals propelled onto the surface of cellulose particles can cleave the glycosidic bond of cellulose, presumably in a similar way as acid catalyst (protonation of the anomeric or the endocyclic oxygen of the terminal non-reducing glucopyranose unit).22 Depending on the nature of the gaseous atmosphere, the amount of H˙ radicals, and their recombination rate, can significantly differ. Although deeper investigations are needed at this stage to fully clarify the reaction mechanism, co-feeding the reactor with H2 seems to be an option to enhance the contact/reactivity of H˙ radicals with the cellulose surface and to ensure its extensive depolymerisation.
As above mentioned, at a certain concentration, glucose may interact with cellulose particle surfaces where it could locally trap radicals. Under oxygen free conditions, no change in pH (∼6.5) was observed, even at extended reaction times, ruling out a possible oxidation of glucose in this case. This was further supported by 13C NMR and mass spectrometry investigations, which did not show the formation of any C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. Furthermore, unlike TPA, when 16 mM of glucose was initially added to cellulose, the depolymerisation was completely inhibited, suggesting that glucose similarly behaves as CCl4 and scavenge H˙ radicals on the cellulose surface. As an evidence to it, we observed that, at extended ultrasonic irradiation time under H2 or Ar/H2 atmospheres, the glucose yield gradually decreased and fructose was concomitantly formed (Fig. S9†). This observation was supported by HPLC analysis and by 1H/13C NMR investigations, which clearly evidenced the selective formation of glucose and fructose in a 83/17 (glucose/fructose) ratio (Fig. S9–S11†). Although additional experiments are needed to fully rationalize the reaction mechanism, the absence of pH change and the partial isomerization of glucose to fructose under oxygen free atmosphere are strong arguments in favour of the involvement of H˙ radicals. This claim is also in agreement with our previous report which show, by density functional theory calculations, that H˙ radicals promote the ring opening of glucose,17a a known key step in its isomerization to fructose.
O group. Furthermore, unlike TPA, when 16 mM of glucose was initially added to cellulose, the depolymerisation was completely inhibited, suggesting that glucose similarly behaves as CCl4 and scavenge H˙ radicals on the cellulose surface. As an evidence to it, we observed that, at extended ultrasonic irradiation time under H2 or Ar/H2 atmospheres, the glucose yield gradually decreased and fructose was concomitantly formed (Fig. S9†). This observation was supported by HPLC analysis and by 1H/13C NMR investigations, which clearly evidenced the selective formation of glucose and fructose in a 83/17 (glucose/fructose) ratio (Fig. S9–S11†). Although additional experiments are needed to fully rationalize the reaction mechanism, the absence of pH change and the partial isomerization of glucose to fructose under oxygen free atmosphere are strong arguments in favour of the involvement of H˙ radicals. This claim is also in agreement with our previous report which show, by density functional theory calculations, that H˙ radicals promote the ring opening of glucose,17a a known key step in its isomerization to fructose.
Interestingly, previously reported technologies involving radical activation of cellulose such as photolysis, UV excitation, radiolysis by X-ray, γ-ray irradiation, electron beam, etc.,23 led also to glycosidic bond cleavage but with uncontrolled side dehydration, recombination or rearrangement of the glucosyl unit. Hence, in situ produced radicals alone cannot explain the very high selectivity into glucose observed using HFUS. One may suspect that, as in the case of supercritical water or the mechanocatalytic process, the implosion of cavitation bubbles on the surface of cellulose locally provides enough physical forces (pressure, shock waves, etc.) capable of inducing a conformational change of the glycosidic bond,7a which then become much more reactive.
Conclusions
We show here that HFUS is an alternative technology capable of selectively depolymerizing cellulose to glucose. The possible transfer of radicals produced inside the cavitation bubbles onto the cellulose particle surfaces was an efficient mean to prevent the concomitant side-degradation of glucose into the bulk solution. In contrast to previously reported technologies, this work is distinguished in that (i) no catalyst was needed, (ii) no external source of heating was required, and (iii) it proceeds in a very selective fashion. A deeper understanding of the reaction mechanism suggest that H˙ radicals are more likely to be responsible for the depolymerisation of cellulose. By recirculating unreacted cellulose into the HFUS reactor, it was possible to selectively and quantitatively depolymerize cellulose to glucose, which constitute one of the rare cases reported so far. A combined experimental-theoretical approach is now the topic of current investigations in our groups in order to get more insight on the reaction mechanism, including the observed induction period in Scheme 2.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors are grateful to the CNRS, the University of Poitiers and INRA for their financial supports. FJ and IC also strongly acknowledge the Agence Nationale de la Recherche for the funding of this work (project CELLOPLASM No. ANR-16-CE07-0003), including the PhD grant of SH. The GDR CNRS-INRA SYMBIOSE is also acknowledged for funding. JMGF acknowledges financial support from MCIU-AEI-FEDER (contract number RTI2018-097609-B-C21). Authors are also grateful to the region Nouvelle Aquitaine for the CPER and EU-FEDER support. We also gratefully acknowledge Karine Cahier, the BIBS (INRA, Nantes, France) and PLATINA (IC2MP, Poitiers, France) platforms for their technical assistance.Notes and references
- (a) D. Klemm, B. Heublein, h.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed; (b) D. Klemm, F. Kramer, S. Moritz, T. Lindström, M. Ankerfors, D. Gray and A. Dorris, Angew. Chem., Int. Ed., 2011, 50, 5438–5466 CrossRef CAS PubMed; (c) I. Siro and D. Plackett, Cellulose, 2010, 17(3), 459–494 CrossRef CAS; (d) R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40(7), 3941–3994 RSC.
- (a) M. Yabushita, H. Kobayashi and A. Fukuoka, Appl. Catal., B, 2014, 145, 1–9 CrossRef CAS; (b) P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC; (c) P. L. Dhepe and A. Fukuoka, ChemSusChem, 2008, 1(12), 969–975 CrossRef CAS PubMed; (d) J. S. Luterbacher, D. Martin Alonso and J. A. Dumesic, Green Chem., 2014, 16, 4816–4838 RSC; (e) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed; (f) M. J. Climent, A. Corma and S. Iborra Sara, Green Chem., 2011, 13(3), 520–540 RSC; (g) R. Palkovits, K. Tajvidi, J. Procelewska, R. Rinaldi and A. Ruppert, Green Chem., 2010, 12, 972–978 RSC; (h) S. Van De Vyver, J. Geboers, P. A. Jacobs and B. Sels, ChemCatChem, 2011, 3(1), 82–94 CrossRef CAS; (i) C. H. Zhou, X. Xia, C.-X. Lin, D.-S. Tong and J. Beltramini, Chem. Soc. Rev., 2011, 40(11), 5588–5617 RSC; (j) A. Arevalo-Gallegosa, Z. Ahmad, M. Asgher, R. Parra-Saldivar and H. M. N. Iqbal, Int. J. Biol. Macromol., 2017, 9, 308–318 CrossRef PubMed.
- (a) R. Rinaldi and F. Schüth, ChemSusChem, 2009, 2(12), 1096–1107 CrossRef CAS PubMed; (b) J. Wang, J. Xi and Y. Wang, Green Chem., 2015, 17(2), 737–751 RSC.
- (a) H. J. Heeres, B. Girisuta and L. P. B. M. Janssen, Ind. Eng. Chem. Res., 2007, 46, 1696–1708 CrossRef; (b) H. G. Higgins, V. Goldsmith and A. W. Mckenzie, J. Polym. Sci., 1958, 32, 247–252 CrossRef CAS; (c) Z. E. Dadach and S. Kaliaguine, Can. J. Chem., 1993, 71, 880–891 CrossRef CAS.
- Y. Nishiyama, P. Langan and H. Chanzy, J. Am. Chem. Soc., 2002, 124, 9074–9082 CrossRef CAS PubMed.
- (a) B. Lindman, G. Karlström and L. Stigsson, J. Mol. Liq., 2010, 156, 76–81 CrossRef CAS; (b) B. Medronho, A. Romano, M. G. Miguel, L. Stigsson and B. Lindman, Cellulose, 2012, 19, 581–587 CrossRef CAS; (c) O. Biermann, E. Hadicke, S. Koltzenburg and F. Muller-Plathe, Angew. Chem., Int. Ed., 2001, 40, 3822–3825 CrossRef CAS; (d) C. Yamane, T. Aoyagi, M. Ago, K. Sato, K. Okajima and T. Takahashi, Polym. J., 2006, 38, 819–826 CrossRef CAS.
- (a) C. Loerbroks, R. Rinaldi and W. Thiel, Chem.–Eur. J., 2013, 19, 16282–16294 CrossRef CAS PubMed; (b) G. A. Jeffrey, J. A. Pople, J. S. Binkley and S. Vishveshwara, J. Am. Chem. Soc., 1978, 100, 373–379 CrossRef CAS; (c) H. Thogersen, R. U. Lemieux, K. Bock and B. Meyer, Can. J. Chem., 1982, 60(1), 44–57 CrossRef; (d) M. K. Dowd, A. D. French and P. J. Reilly, Carbohydr. Res., 1992, 233, 15–34 CrossRef CAS PubMed; (e) C. J. Cramer, D. G. Truhlar and A. D. French, Carbohydr. Res., 1997, 298(1–2), 1–14 CrossRef CAS; (f) E. J. Cocinero, P. Çarçabal, T. D. Vaden, J. P. Simons and B. G. Davis, Nature, 2011, 469, 76–79 CrossRef CAS PubMed.
- As selected review articles: (a) N. Yan and S. Ding, Trend Chem, 2019, 1(5), 457–458 CrossRef; (b) L. N. Jayakody, J. J. Liu, E. J. Yun, T. L. Turner, E. J. Oh and Y. S. Jin, Biotechnol. Bioeng., 2018, 115(12), 2859–2868 CrossRef CAS PubMed; (c) L. O. Ingram and J. B. Doran, FEMS Microbiol. Rev., 1995, 16(2–3), 235–241 CrossRef CAS.
- F. Jérôme, G. Chatel and K. De Oliveira Vigier, Green Chem., 2016, 18, 3903–3913 RSC.
- (a) D. A. Cantero, M. D. Bermejo and M. J. Cocero, ChemSusChem, 2015, 8(6), 1026–1033 CrossRef CAS PubMed; (b) M. Sasaki, Z. Fang, Y. Fukushima, T. Adschiri and K. Arai, Ind. Eng. Chem. Res., 2000, 39(8), 2883–2890 CrossRef CAS; (c) S. Saka and T. Ueno, Cellulose, 1999, 6(3), 177–191 CrossRef CAS; (d) S. Kumar and R. B. Gupta, Ind. Eng. Chem. Res., 2008, 47(23), 9321–9329 CrossRef CAS; (e) J. Buffiere, P. Ahvenainen, M. Borrega, K. Svedstrom and H. Sixta, Green Chem., 2016, 18(24), 6516–6525 RSC; (f) C. M. Martinez, D. A. Cantero, M. D. Bermejo and M. J. Cocero, Cellulose, 2015, 22(4), 2231–2243 CrossRef CAS.
- http://renmatix.com/technology/plantrose-technology/how-it-works .
- (a) M. D. Kaufman Rechulski, M. Käldström, U. Richter, F. Schüth and R. Rinaldi, Ind. Eng. Chem. Res., 2015, 54, 4581–4592 CrossRef CAS; (b) Q. Zhang and F. Jérôme, ChemSusChem, 2013, 6(11), 2042–2044 CrossRef CAS PubMed; (c) S. M. Hick, C. Griebel, D. T. Restrepo, J. H. Truitt, E. J. Buker, C. Bylda and R. G. Blair, Green Chem., 2010, 12, 468–474 RSC; (d) J. Kano and F. Saito, Powder Technol., 1998, 98, 166–170 CrossRef CAS; (e) R. G. Blair, K. Chagoya, S. Biltek, S. Jackson, A. Sinclair, A. Taraboletti and D. Restrepo, Faraday Discuss., 2014, 170, 223–233 RSC; (f) N. Meine, R. Rinaldi and F. Schüth, ChemSusChem, 2012, 5, 1449–1454 CrossRef CAS PubMed; (g) A. Shrotri, L. K. Lambert, A. Tanksale and J. Beltramini, Green Chem., 2013, 15, 2761–2768 RSC; (h) M. Käldström, N. Meine, C. Farès, F. Schüth and R. Rinaldi, Green Chem., 2014, 16, 3528–3538 RSC; (i) F. Schüth, R. Rinaldi, N. Meine, M. Käldström, J. Hilgert and M. D. Kaufman Rechuslski, Catal. Today, 2014, 234, 24–30 CrossRef; (j) M. Käldström, N. Meine, C. Farès, R. Rinaldi and F. Schüth, Green Chem., 2014, 16, 2454–2462 RSC; (k) A. Karam, P. N. Amaniampong, J. M. García Fernández, C. Oldani, S. Marinkovic, B. Estrine, K. De Oliveira Vigier and F. Jérôme, Front. Chem., 2018, 6, 74 CrossRef PubMed.
- (a) M. Benoit, A. Rodrigues, Q. Zhang, E. Fourré, K. De Oliveira Vigier, J.-M. Tatibouët and F. Jérôme, Angew. Chem., Int. Ed., 2011, 50(38), 8964–8967 CrossRef CAS PubMed; (b) M. Benoit, A. Rodrigues, K. de Oliveira Vigier, E. Fourré, J. Barrault, J.-M. Tatibouët and F. Jérôme, Green Chem., 2012, 14(8), 2212–2215 RSC; (c) J. Delaux, C. Ortiz Mellet, C. Canaff, E. Fourré, C. Gaillard, A. Barakat, J. M. García Fernández, J.-M. Tatibouët and F. Jérôme, Chem.–Eur. J., 2016, 22(46), 16522–16530 CrossRef CAS PubMed.
- Y. T. Didenko and K. S. Suslick, Nature, 2002, 248, 394–397 CrossRef PubMed.
- (a) K. S. Suslick, Science, 1990, 247, 1439–1445 CrossRef CAS PubMed; (b) T. G. McKenzie, F. Karimi, M. Ashokkumar and G. G. Qiao, Chem.–Eur. J., 2019, 25, 5372–5388 CrossRef CAS PubMed; (c) L. H. Thompson and L. K. Doraiswamy, Ind. Eng. Chem. Res., 1999, 38, 1215–1249 CrossRef CAS; (d) K. S. Suslick, N. C. Eddingsaas, D. J. Flannigan, S. D. Hopkins and H. Xu, Acc. Chem. Res., 2018, 51, 2169–2178 CrossRef CAS PubMed.
- W. Li, X.-A. Xie, C.-Z. Tang, Y. Li, Y.-L. Wang, X. Wei and D. Fan, J. Fuel Chem. Technol., 2016, 44(4), 415–421 CrossRef CAS.
- (a) P. N. Amaniampong, Q. Thang Trinh, K. De Oliveira Vigier, D. Q. Dao, N. H. Tran, Y. Wang, M. P. Sherburne and F. Jérôme, J. Am. Chem. Soc., 2019, 141(37), 14772–14779 CrossRef CAS PubMed; (b) A. Philipp and W. Lauterborn, Acustica, 1997, 83, 223–227 Search PubMed; (c) D. G. Shchukin, E. Skorb, V. Belova and H. Möhwald, Adv. Mater., 2011, 23, 1922–1934 CrossRef CAS PubMed; (d) L. Zhang, V. Belova, H. Wang, W. Dong and H. Möhwald, Chem. Mater., 2014, 26, 2244–2248 CrossRef CAS; (e) L. Parizot, T. Chave, M.-E. Galvez, H. Dutilleul, P. Da Costa and S. I. Nikitenko, Appl. Catal., B, 2019, 241, 570–577 CrossRef CAS.
- P. N. Amaniampong, A. Karam, Q. Thang Trinh, K. Xu, H. Hirao, F. Jérôme and G. Chatel, Sci. Rep., 2017, 7, 40650 CrossRef PubMed.
- Y. Lida, K. Yasui, T. Tuziuti and M. Sivakumar, Microchem. J., 2005, 80(2), 159–164 CrossRef.
- (a) A. Weisser, H. W. Cooper and S. Snyder, J. Am. Chem. Soc., 1950, 72, 1769–1775 CrossRef; (b) R. Pflieger, T. Chave, G. Vite, L. Jouve and S. I. Nikitenko, Ultrason. Sonochem., 2015, 26, 169–175 CrossRef CAS PubMed; (c) E. J. Hart and A. Henglein, J. Phys. Chem., 1985, 89(20), 4342–4347 CrossRef CAS; (d) T. Chave, N. M. Navarro, P. Pochon, N. Perkas, A. Gedanken and S. I. Nikitenko, Catal. Today, 2015, 241, 55–62 CrossRef CAS.
- M. Kohno, T. Mokudai, T. Ozawa and Y. Niwano, J. Clin. Biochem. Nutr., 2011, 49(2), 96–101 CrossRef CAS PubMed.
- P. Bhaumik and P. L. Dhepe, Chapter 1: Conversion of Biomass into Sugars, in Biomass Sugars for Non-Fuel Applications, Green Chemistry Series, RSC, 2015, pp. 1–53 Search PubMed.
- (a) M. Wencka, K. Wichlacz, H. Kasprzyk, S. Lijewski and S. K. Hoffmann, Cellulose, 2007, 14, 183–194 CrossRef CAS; (b) H. Zegota and C. von Sonntag, Z. Naturforsch., B: J. Chem. Sci., 1977, 32(9), 1060–1067 Search PubMed; (c) J. C. Arthur, O. Hinojosa and V. W. Tripp, J. Appl. Polym. Sci., 1969, 13, 1497–1507 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc00020e |
| This journal is © The Royal Society of Chemistry 2020 |