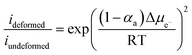Open Access Article
Open Access ArticleDesigning composite solid-state electrolytes for high performance lithium ion or lithium metal batteries
Tengfei
Zhang†
 *a,
Wenjie
He†
a,
Wei
Zhang
*a,
Wenjie
He†
a,
Wei
Zhang
 *b,
Tao
Wang
*b,
Tao
Wang
 a,
Peng
Li
a,
Peng
Li
 a,
ZhengMing
Sun
a,
ZhengMing
Sun
 b and
Xuebin
Yu
*c
b and
Xuebin
Yu
*c
aJiangsu Key Laboratory of Electrochemical Energy-Storage Technologies, College of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China. E-mail: zhangtengfei@nuaa.edu.cn
bJiangsu Key Laboratory of Advanced Metallic Materials, School of Materials Science and Engineering, Southeast University, Nanjing, 211189, China. E-mail: w69zhang@seu.edu.cn
cDepartment of Materials Science, Fudan University, Shanghai 200433, China. E-mail: yuxuebin@fudan.edu.cn
First published on 20th July 2020
Abstract
Solid-state electrolytes (SSEs) are capable of inhibiting the growth of lithium dendrites, demonstrating great potential in next-generation lithium-ion batteries (LIBs). However, poor room temperature ionic conductivity and the unstable interface between SSEs and the electrode block their large-scale applications in LIBs. Composite solid-state electrolytes (CSSEs) formed by mixing different ionic conductors lead to better performance than single SSEs, especially in terms of ionic conductivity and interfacial stability. Herein, we have systematically reviewed recent developments and investigations of CSSEs including inorganic composite and organic–inorganic composite materials, in order to provide a better understanding of designing CSSEs. The comparison of different types of CSSEs relative to their parental materials is deeply discussed in the context of ionic conductivity and interfacial design. Then, the proposed ion transfer pathways and models of lithium dendrite growth in composites are outlined to inspire future development of CSSEs.
Introduction
As efficient energy storage devices, batteries, including nickel-metal hydride (Ni-MH) batteries, lead-acid batteries and lithium-ion batteries (LIBs), can be effectively combined with renewable energy sources such as solar energy, wind energy and hydrogen energy, and such batteries are expected to be advanced energy storage systems and reduce fossil fuel dependence.1 In the past few decades, the energy density of LIBs has been higher than that of many other types of batteries; they have been widely used in the fields of personal electronics, hybrid and electrical vehicles, and grid energy conversion–storage systems.2,3 Although LIBs have appeared in daily life in many forms, currently available commercial LIBs still cannot meet the stringent or increasing demand for high-performance equipment in modern society.4–16 There is an urgent need to develop batteries with higher energy density and longer cycle life, along with an acceptable level of safety and an affordable price. Liquid electrolytes demonstrate high conductivity and excellent electrode surface wettability, but they often suffer from the problems of insufficient electrochemical and thermal stability, low ion selectivity and poor safety.17–25Concurrently, lithium metal, as a negative electrode, exhibits low redox potential (−3.045 V) and high theoretical specific capacity (3860 mA h g−1), while also being light-weight (M = 6.94 g mol−1, D = 0.534 g cm−3).26,27 However, as the charging and discharging process continues, the gradual growth of lithium dendrites can pierce the separator, leading to a short circuit in the battery.28 Furthermore, the electrolyte is a flammable substance. If leaked, it can cause serious safety accidents. In addition, an unstable solid electrolyte interphase (SEI) is formed between the liquid electrolyte and the electrode material.27,29–35 The appearance of an SEI will reduce battery capacity and shorten the cycle life of the battery. Therefore, LIBs using lithium metal as the negative electrode have poor cycling performance and serious safety problems. The emergence of SSEs is expected to enable lithium metal to be used as a negative material in all solid-state battery systems, resulting in a higher energy density than what is currently available. Replacing liquid electrolytes with SSEs can also effectively inhibit the generation of SEI films and improve the cycle performance of batteries. At the same time, the size of the battery is reduced, and its application scope is expanded. More importantly, compared to liquid electrolytes, SSEs (e.g., inorganic ceramic electrolytes) do not leak and are non-flammable, and thus, safety is greatly enhanced. The use of solid-state electrolytes instead of liquid electrolytes can not only overcome the liquid electrolyte durability problem, but also provide an important avenue for developing next-generation LIBs.
With the emergence of LIBs, research on SSEs continued. Manthiram et al. provided a detailed account of the development of SSEs in 2017.11,36 Solid electrolytes can be divided into two categories: solid polymer materials and inorganic materials. Typical conductive polymers are polyethylene oxide (PEO),37 polyacrylonitrile (PAN),38,39 polymethyl methacrylate (PMMA),40,41 and polyvinylidene fluoride (PVDF).42,43 In polymer electrolytes, the polymer long chains are partially shifted above the glass transition temperature, thereby creating a binding site for ion hopping, and the constant hopping of ions in a specific direction can achieve an ion transport effect. Typical inorganic lithium-ion conductive materials include lithium phosphorus oxynitride (LiPON),44 perovskite,45 sodium superionic conductor (NASICON),46–48 garnet,15,49–56 sulfide,10,17,57–64 halide,65–70 and hydride based materials.71–76 Among them, the high ionic conductivity of solid crystalline materials can be attributed to the large number of structural defects or special crystal structures. The former involves point defect-based ion diffusion mechanisms, including simple vacancy mechanisms and relatively complex diffusion mechanisms, while the latter involves usually two types of sublattices, which are composed of immobile and mobile ions. The ionic diffusion process in glass materials is similar to that of crystalline materials in that ions move between active sites.
High ionic conductivity, low ionic area specific resistance, high electronic area specific resistance, high ionic selectivity, a wide electrochemical stability window, good chemical compatibility, excellent thermal stability, excellent mechanical properties, simple fabrication processes, and environmental friendliness are the main properties of a good solid-state electrolyte.11 Much progress has been made in improving the above-mentioned properties. Perovskite-type oxide compounds, in the form of ABO3, show a lithium ionic conductivity as high as 10−3 S cm−1 in the bulk portion, which has been attributed to the presence of a significant amount of equivalent deficient sites for lithium ions to substitute and freely move in bulk crystals. Additionally, these compounds (e.g., Li3xLa2/3xTiO3) have a high grain boundary resistance that is related to the reduction of Ti4+ to Ti3+ upon contact with Li metal.77,78 Garnet-type materials, such as Li5La3Ta2O12 and Li5La3Nb2O12, have also been considered fast lithium-ion conductors since 2003.79 Since then, a new garnet-type ceramic of Li7La3Zr2O12 has been discovered with a high ionic conductivity (2.44 × 10−4 S cm−1).80 Although these ceramics are chemically stable with electrodes, their poor interfacial compatibility with lithium metal limits their applications in the field of solid-state batteries. Furthermore, the volume expansion and shrinkage of the electrode during the charging/discharging process will cause the electrolyte to crack and thereby lose its capacity. The mechanical flexibility of SSEs determines whether they easily crack.81 Therefore, electrolytes should have a moderate elastic modulus. In general, the mechanical flexibility of sulfide-type materials is better than that of oxide-type materials. Additionally, the replacement of oxygen ions with sulfur ions, which have a larger radius, can not only provide more migration space for lithium ions, but also reduce bonding strength. As a result, compared with oxides, sulfides exhibit higher lithium-ion conductivity with a value of approximately 10−2 to 10−4 S cm−1 at room temperature (RT).82 For example, Li10GeP2S12 exhibits an extremely high lithium ionic conductivity of 1.2 × 10−2 S cm−1 at RT owing to its three-dimensional framework structure.16 Notably, the low output and high cost of germanium limit the possibility of its large-scale production and application. Moreover, most sulfide solid electrolytes are not stable and can easily react with H2O, releasing a highly toxic gas: H2S.83 More recently, a complex hydride lithium superionic conductor, 0.7Li(CB9H10)–0.3Li(CB11H12), has been developed with excellent stability against lithium metal and a high conductivity of 6.7 × 10−3 S cm−1 at 298 K.84 However, the compatibility of the above material with cathode materials is poor due to the reducibility of complex hydrides. Unlike brittle crystalline solid inorganic electrolytes (SIEs), solid polymer electrolytes (SPEs) are highly flexible. Nonetheless, SPEs have not been applied in commercial batteries due to their low ionic conductivities (10−6 to 10−8 S cm−1) at RT and poor electrochemical stability (<4 V).85
Based on the excellent work of many researchers in analyzing various electrolytes, it is difficult for a single type of SSE to fully satisfy the challenging requirements mentioned above. This has led to a growing research interest in CSSEs, which aims to develop promising SSEs by combining the advantages and eliminating the drawbacks of both inorganic and organic solid electrolytes. A brief overview of CSSEs is first introduced (Fig. 1).11 Inspired by the radar plot from the work of Manthiram et al.11 (Fig. 1a), it is necessary to summarize the recent progress of composite solid-state electrolytes. Herein, this review systematically surveys recent CSSE progress, with special emphasis on the following aspects: polymer-based, oxide-based, hydride-based, sulfide-based, and halide-based SSEs. The conductivity, interphase behavior, electrochemical stability and properties of these electrolytes, along with their associated all-solid-state LIBs are also systematically summarized and characterized (Fig. 1b). We focus on the design of the electrolyte itself and discuss the challenges in developing these materials. Additionally, CSSEs are discussed with regard to their application in many Li battery systems, including Li-ion, Li-metal, and Li–sulfur batteries.
 | ||
| Fig. 1 (a) The superposed radar plot of different types of SSEs and requirements for SSEs. ASR, area-specific resistance. Reproduced from ref. 11 with permission from Springer Nature, copyright 2017. (b) Design of CSSEs from single type SSEs and corresponding physicochemical characteristics. | ||
Progress of CSSEs
Solid-state ionics is the science of ion transport in solids. The term “ionic” has been used for ionic conduction since the early days of electrochemical research, but mainly for liquid electrolytes. The field of solid-state ionics first started with the work of Faraday in heated solid electrolytes of Ag2S and PbF2 in 1833.86 In general, the mobility of ions in solids is very slow, and hardly contributes to electrical conductivity. However, high ionic mobility is observed in certain types of inorganic ceramics, organic polymers, and composite materials, and their ionic conductivities are comparable to that of a liquid. In the 1960s, a ceramic-based β-alumina (Na2O·11Al2O3) was found to possess a remarkable sodium-ion transport characteristic, and was successfully used in a high-temperature sodium–sulfur (Na–S) battery for grid energy conversion–storage systems.87 It is marked as a milestone and subsequently boosted the increase in practical applications of SSEs. For example, improved Na–S battery modules were developed and made commercially available by NGK Insulators Ltd. in 2000.88 Recently, increasing research has been made to realize the application of solid electrolytes. In particular, increasing attention has been turned from purely inorganic SSEs or purely organic SPEs to CSSEs. The research of these CSSEs has been focused on designing innovative superionic conductors, understanding the ion transport mechanism at the interface, and improving the electrochemical performance based on CSSEs. In the following sections, a comprehensive introduction to different CSSEs, including polymer-based, oxide-based, hydride-based, sulfide-based and halide-based CSSEs, will be presented and discussed.Polymer-based CSSEs
Since 1973, significant attention has been paid to SPEs owing to their ease of synthesis, low shear modulus, low cost, compatibility with large-scale manufacturing processes and inherent mechanical toughness. However, SPEs exhibit a low oxidation voltage and poor thermal stability. Additionally, SPEs show particularly low ionic conductivity (10−6 to 10−8 S cm−1) at room temperature because the polymer chains are locked in a crystal lattice which hinders ion-pair dissociation. Improvements in ionic conductivity and interfacial resistance between the electrolyte and electrodes are still unable to satisfy the requirements for practical applications. To address these problems, various physical approaches and chemical strategies, such as polymer–inorganic material blending, architectural design of inorganic fillers, copolymerization, crosslinking and the introduction of ionic side groups, have been adopted.Researchers began to invest more effort to balance electrochemical stability and mechanical robustness in the development of composite polymer electrolytes (CPEs). The ionic conductivities of composite membranes were optimized as the content of Li7La3Zr2O12 changes. The composite membranes exhibited the highest ionic conductivity (4.42 × 10−4 S cm−1 at 55 °C) and maintained mechanical flexibility, consisting of an organic PEO matrix with the optimum composition (52.5% Li7La3Zr2O12).89 In contrast to conventional blending methods, Cui et al. reported a new approach for the preparation of ceramic–polymer electrolytes via in situ synthesis of ceramic filler particles in polymer electrolytes. The improved distribution of monodisperse ultrafine SiO2 particles in PEO helped increase the effective surface area and suppress the crystallization of PEO, thus facilitating polymer segmental motion for ionic conduction. All of these factors led to good ionic conductivity (1.2 × 10−3 S cm−1 at 60 °C, 4.4 × 10−5 S cm−1 at 30 °C) and greatly extended the electrochemical stability window up to 5.5 V.90 Chung et al. reported a novel composite electrolyte fabricated by a simple solution casting method. The composite electrolyte (Li6.4La3Zr1.4Ta0.6O12 (LLZTO) fillers in a PEO/LiClO4 matrix) exhibited low interfacial resistance and good Li-ion conductivity (4.8 × 10−4 S cm−1 at 60 °C).91
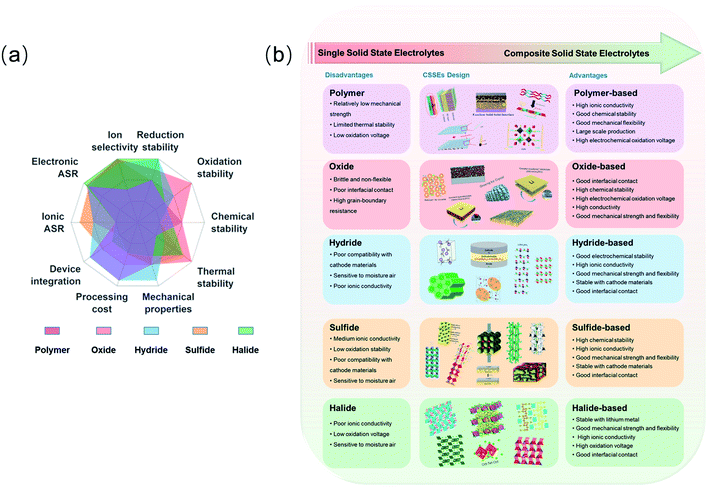
Li+-conducting oxides are considered better fillers than Li+-insulating oxides for improving Li+ conductivity and distribution in a composite electrolyte. To explore this possibility, a PEO/perovskite Li3/8Sr7/16Ta3/4Zr1/4O3 (LSTZ) electrolyte was prepared. An interphase layer was in situ formed during cycling with the electrolyte membrane, which indicated the strong interaction between F− and the surface Ta5+. This strong interaction improved Li-ion transport at the PEO/perovskite interface and suppressed lithium dendrite growth.92 More recently, Goodenough et al. used two Li+-insulating oxides, fluorite Gd0.1Ce0.9O1.95 (GDC) and perovskite La0.8Sr0.2Ga0.8Mg0.2O2.55 (LSGM), as ceramic fillers in the PEO matrix. Density functional theory (DFT) calculations and 7Li nuclear magnetic resonance (NMR) measurements confirmed the interaction between the oxide surface and the Li-salt anion in the polymer, which modified the activation energy for Li+ transport to obtain a high Li+ conductivity that was above 10−4 S cm−1 at 30 °C.93 Cui et al. reported the facile synthesis of Al3+/Nb5+ co-doped Li7La3Zr2O12 (LLZO) nanoparticles. The substitution of Li+ by Al3+ enhanced the stabilization of cubic LLZO at RT, and the substitution of Zr4+ by Nb5+ improved the ion conductivity. After optimization, the polymer electrolyte with 15 wt% LLZO showed an improved conductivity of 9.5 × 10−6 and 1.1 × 10−4 S cm−1 at 20 and 40 °C, respectively.94 There was a higher Li-ion conductivity in one/two-dimensional fillers than in zero-dimensional particles. One-dimensional Li0.33La0.557TiO3 (LLTO) nanofiber embedded in a PEO matrix, as shown in Fig. 2a–c, provided continuous ionic transport pathways and reduced interfacial resistance.95 Moreover, two-dimensional garnet nanosheets were first reported via co-precipitation with a graphene oxide (GO) template. The specially designed CPE containing garnet nanosheets could robustly isolate Li dendrites and exhibited a conductivity of 3.6 × 10−4 S cm−1 at RT.96
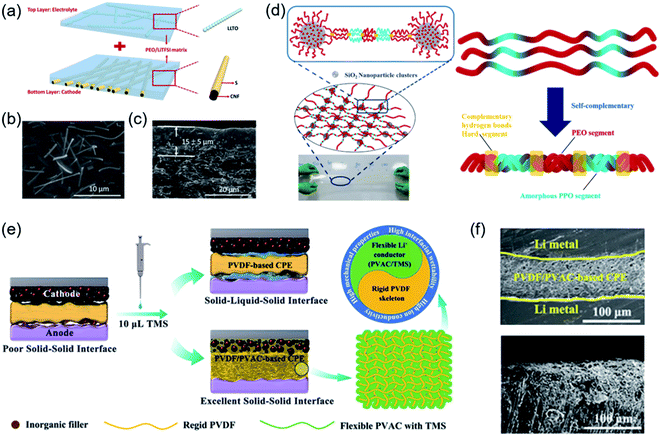 | ||
| Fig. 2 (a) Schematic illustration of the carbon nanofiber (CNF)/S-PEO/LLTO CSSE. (b) SEM images and (c) cross-sectional SEM image of the PEO/LLTO CSSE. Reproduced from ref. 95 with permission from Elsevier Inc., copyright 2019. (d) Graphical representation of the 3D framework in cross-linked nanocomposite polymer electrolytes (CNPEs). Reproduced from ref. 97 with permission from Elsevier Inc., copyright 2020. (e) Schematic illustration of the different interfacial characteristics between the PVDF-based CPE and PVDF/PVAC-based CPE. (f) Cross-sectional SEM images of the symmetric battery and Li metal after Li plating/stripping 200 h. Reproduced from ref. 101 with permission from Wiley-VCH, copyright 2020. | ||
Currently, the general strategies are adding integrated inorganic fillers to the SPE matrix or preparing polymer electrolytes with specific intermolecular interactions, which can not only improve the ionic conductivity but also sustain a high working voltage. Mai et al. provided a new approach to prepare cross-linked nanocomposite polymer electrolytes based on hydrophobic–hydrophilic–hydrophobic triblock copolymers (PPO–PEO–PPO) in Fig. 2d. In this enhanced framework, polymer-based composite electrolytes were generated from copolymers and surface-modified SiO2 nanoparticles, which led to the effective solvation of lithium salts and encapsulation of organic solvents. Thus, the electrolytes exhibited high ionic conductivity and their electrochemical stability window was extended to 6.5 V.97 The experiments, together with theoretical calculations, demonstrated that the gas releasing behaviour of PEO may be ascribed to the high oxidizing ability of delithiated LiCoO2. PEO was reported to decompose and release oxygen from LiCoO2 when more than 0.5Li+ was removed.98 The surface catalytic effect of delithiated LiCoO2 caused oxidation/dehydration of PEO-based SPBs and unexpected H2 generation at 4.2 V. To mitigate the surface catalytic effect, the surface of LiCoO2 was coated with a stable solid electrolyte Li1.4Al0.4Ti1.6(PO4)3 (LATP), thus avoiding direct contact with PEO and therefore extending the stable working voltage to over 4.5 V.99 Park et al. synthesized a self-standing and flexible CPE through the introduction of poly-(ethylene glycol)-dimethyl ether (PEGDME) to be plasticized in a PEO matrix with a uniformly distributed ceramic filler. After the addition of PEGDME, the stable working voltage of the PEO matrix was extended to 5.0 V.100 Besides PEO-based polymer electrolytes, a PVDF/polyvinyl acetate (PVAC)/LLZTO CPE was first fabricated to achieve high RT ionic conductivity and a high electrochemical stability window (4.5 V) (Fig. 2e). More importantly, the intermolecular interactions of tetramethylene sulfone (TMS) combined with PVAC or PVDF showed distinct differences. The Li/PVDF/PVAC-based CPE/Li symmetric battery shows a good inhibition of lithium dendrite growth in Fig. 2f. The PVAC/TMS layer formed on both the cathode and anode interfaces constructed an effective sulfurous Li+ transport pathway. Therefore, TMS with low flammability and excellent stability was able to selectively interact with only PVAC, which was helpful to enhance lithium-ion conductivity and electrode/electrolyte interfacial compatibility.101
Recently, sulfide electrolytes, such as Li2S–GeS2, Li2SeP2S5, Li2SeB2S3 and Li2SeSiS2, have attracted increasing attention due to their superior ion conductivity (∼10−2 S cm−1) and wide potential window (>10 V). Furthermore, sulfide electrolytes have cheaper precursors and simpler processes to provide the electrolyte with a large potential in all-solid-state lithium batteries (ASSLBs). However, little success has been achieved in adopting lithium metal anodes with sulfide-based electrolytes in ASSLBs. The main challenges are the interfacial instability and Li dendrite formation between Li metal and SSEs.
To solve these issues, Li10GeP2S12 (LGPS) was dispersed in a PEO-based polymer to fabricate SPE membranes. The inorganic LGPS in the organic PEO matrix impeded crystallization and weakened the interactions between the Li+ and PEO chains. The optimal SPE containing 1% LGPS electrolyte exhibited a maximum ionic conductivity of 1.21 × 10−3 S cm−1 at 80 °C and a broadened electrochemical window up to 5.7 V.102 Inspired by the similarity between the H bond and Li bond, hybrid solid electrolytes were prepared via an in situ coupling reaction. A commercialized silane coupling agent was used as a bridge builder to realize the chemical bonding interaction between LGPS, polyethylene glycol (PEG), and PEO (Fig. 3a and b). Hence, the optimal ceramic/polymer hybrid solid electrolyte (HSE) membrane provided an expressway for the transport of Li+, and the growth of lithium dendrites was suppressed.103 Li6PS5Cl is a promising solid electrolyte in ASSLBs. In the preparation process, S and Cl easily formed chemical bonds with the polymer groups and replaced other anion sites.104 A Li6PS5Cl/PEO composite electrolyte with enhanced mechanical properties and a stable interface was fabricated by a liquid-phase process. In particular, with an optimal value of 5 wt% PEO, the CSEs show an improved ionic conductivity and electrochemical window.105 Sun et al. reported a plastic crystal electrolyte (PCE) interlayer to address the interfacial challenge and lithium dendrite formation between sulfide electrolytes and Li metal. Using PCE as an interlayer, interfacial reactions could be avoided by preventing contact between the sulfide electrolytes and Li metal (Fig. 3c–e). In addition, submerging the cathode in a PCE matrix forms a continuous 3D-conduction pathway for Li+ on the cathode side.106 The synchrotron-based X-ray absorption spectra in Fig. 3e were used to analyze the interface between LGPS and Li metal, which suggests that using the PCE interlayer can prevent the reduction of LGPS by Li metal.
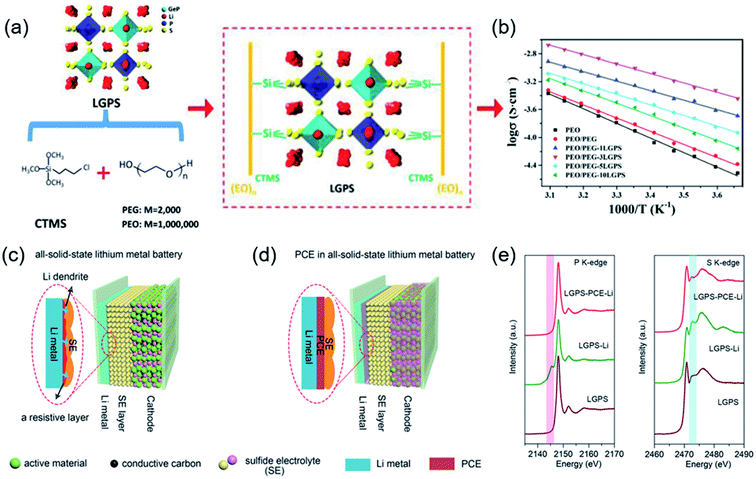 | ||
| Fig. 3 (a) Schematic illustration of the as-prepared HSE structure. Electrochemical characterization of the HSE membranes. (b) Arrhenius plot of different electrolytes from 0 to 50 °C. Reproduced from ref. 103 with permission from Wiley-VCH, copyright 2020. Schematic diagrams of (c) ASSLMBs and (d) ASSLMBs with the PCE interlayer. X-ray absorption (e) P K-edge and S K-edge spectra of LGPS before cycling, LGPS on the Li surface after cycling, and LGPS with the PCE interlayer after cycling, respectively. Reproduced from ref. 106 with permission from Wiley-VCH, copyright 2019. | ||
Oxide-based CSSEs
Oak Ridge Laboratory first synthesized LiPON in the 1990s.107 Since then, oxide electrolytes have attracted increasing attention. Compared to SPEs, oxide-based electrolytes are becoming a research hotspot owing to their chemical stability in air, good ion selectivity and wide electrochemical window.15 Unfortunately, an increasing number of studies have demonstrated that lithium dendrites occur in most oxide-based electrolytes owing to poor interfacial stability, large interfacial resistance and voids and cracks inside the electrolytes. To develop an interface with chemical and mechanical stability, researchers have adopted interfacial engineering by introducing artificial buffer layers to protect these SSEs against lithium dendrite growth.108,109 Nevertheless, simple physical contact could not solve the problems of large area specific resistance and dendrite formation. Due to a high shear modulus, the problem of interfacial contact was initially solved by designing a soft interface, such as polymer layers, amorphous oxides and metal coatings.110–112In practice, Ding et al. demonstrated that the coating layer of PEO (LiTFSI) could effectively prevent side reactions between the Li1.5Al0.5Ge1.5(PO4)3 (LAGP) and Li anode.113 It was exciting that the CSSE remained stable even at a high potential of 5.12 V. Considering the excellent performance of the polymer layer, Goodenough et al. developed a low-cost composite ceramic/polymer solid-state electrolyte (CPSE) containing up to 80 wt% garnet Li6.4La3Zr1.4Ta0.6O12 (LLZTO).56 Composites consisting of compositions ranging from “ceramic-in-polymer” to “polymer-in-ceramic” were found to be flexible and mechanically robust (Fig. 4a–d). The crystalline LLZTO particles not only increased the chain segment motion in a PEO matrix but also afforded an alternative Li+-conduction pathway. Compared with the “ceramic-in-polymer” electrolyte with high flexibility, the “polymer-in-ceramic” electrolyte was more suitable for large-scale application in electric vehicles owing to its high mechanical strength and safety. The PEO–LLZTO composite electrolyte showed the highest ionic electrolyte conductivity (above 10−4 S cm−1 at 55 °C) and suppressed Li dendrite growth. Coincidentally, to design an effective approach to achieve dendrite-free CSSEs, Sun et al. investigated the performance of composite electrolytes from “ceramic-in-polymer” to “polymer-in-ceramic” (Fig. 4e–g).114 The different sizes of garnet particles embedded in the electrolyte improved ionic conductivity and tensile strength. The satisfactory sandwich-type composite electrolyte with hierarchical garnet particles simultaneously achieved dendrite suppression and excellent interfacial contact with the Li metal in Fig. 4g. The “polymer-in-ceramic” interlayer with 80 vol% 5 μm LLZTO showed a high mechanical strength of 12.9 MPa and hindered Li dendrite propagation due to physical obstacles. The “ceramic-in-polymer” thin-film outer layers with 20 vol% 200 nm LLZTO particles created a smooth and flexible surface with a high t+ (Li+ transference number) of 0.47. Symmetric solid-state cells with Li maintained highly stable plating/stripping cycling for 400 h under 0.2 mA cm−2 at 30 °C. Full SSBs with a LiFePO4 cathode and Li metal anode delivered a RT specific capacity of 99.1 mA h g−1 with a good capacity retention of 82.4% after 200 cycles at 0.1C.
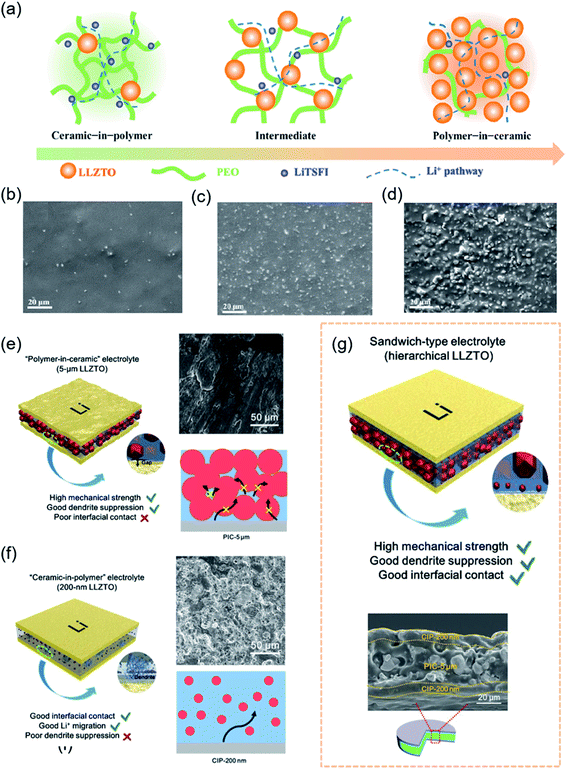 | ||
| Fig. 4 (a) Schematic illustration of PEO–LLZTO CSSE: “ceramic-in-polymer”; “intermediate”; “polymer-in-ceramic”, and corresponding (b–d) SEM images. Reproduced from ref. 56 with permission from Elsevier Inc., copyright 2018. The schematic illustrations of (e) PIC-5 μm, (f) CIP-200 nm, and (g) hierarchical sandwich-type CSSEs, and corresponding SEM images. Reproduced from ref. 114 with permission from Wiley-VCH, copyright 2019. | ||
The design of artificial structures can not only be beneficial for interfacial contact but also improve mechanical properties. Composite electrolytes with high loadings of ceramic fillers (>50 vol%) are necessary to achieve the required mechanical modulus.115 However, high loading can lead to a large interparticle contact resistance and insufficient particle–particle contact area, thus resulting in greatly decreased ionic conductivity. Therefore, designing a composite with an interconnected ceramic network takes advantage of the high ionic conductivity of the ceramic. Inspired by the structure of natural nacre, Yang et al. fabricated a “brick-and-mortar” arrangement of solid electrolytes with ceramic electrolyte microplatelets as the “brick” and polymer electrolytes as the “mortar” (Fig. 5a and b).116 Compared to pure ceramic electrolytes, the nacre-like ceramic/polymer composite electrolyte simultaneously exhibited high fracture strain and an ultimate flexural modulus to accommodate external deformation. The staggered microstructure provided a high fracture strain of 1.1% and a flexural modulus of 7.8 GPa. An ice template method was used to build a vertically aligned ceramic/polymer composite electrolyte (Fig. 5c and d), which was composed of LAGP, with a high ionic conductivity, and PEO.117 The vertical LAGP walls in the polymer provided fast ionic transport channels while retaining matrix flexibility. The ideal structure maximized the ionic conductivity of the composite electrolyte (1.67 × 10−4 S cm−1 at RT and 1.11 × 10−3 S cm−1 at 60 °C). In Chen's work, a composite solid electrolyte with a 3D-interconnected ceramic network was fabricated using a readily scalable processing method, as shown in Fig. 5e and f.118 The composite electrolyte had high ceramic loadings (77 wt% and 61 vol%) and demonstrated high ionic conductivity (3.5 × 10−5 S cm−1 at RT) as well as good mechanical strength (19.5 MPa).
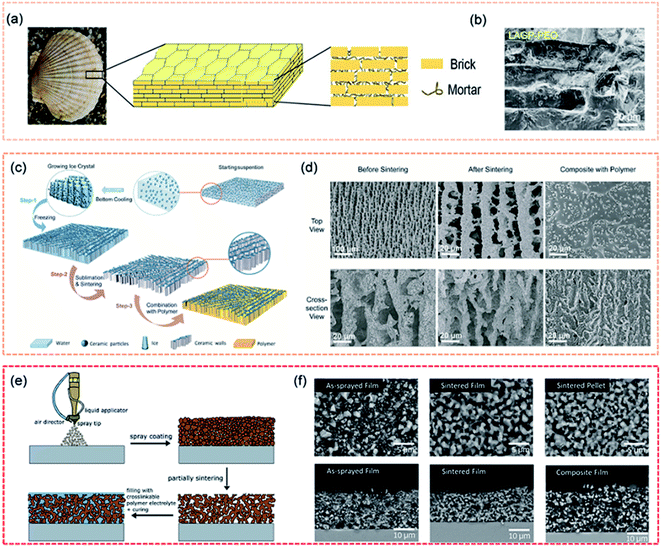 | ||
| Fig. 5 (a) Schematic of the staggered “brick-and-mortar” microstructure of the LAGP–PEO NCPE film, and the corresponding (b) cross-sectional SEM image. Reproduced from ref. 116 with permission from Wiley-VCH, copyright 2020. (c) The schematic of the preparation process of the ice-templated LAGP/PEO CSSE, and the corresponding (d) SEM images. Reproduced from ref. 117 with permission from Elsevier Inc., copyright 2019. (e) Schematic illustration of the fabrication procedure of the composite electrolyte film, and the corresponding (f) SEM images. Reproduced from ref. 118 with permission from Elsevier Inc., copyright 2020. | ||
In addition to excellent performance, the cost of processing should be considered in practical applications. Oxide electrolytes with superior ionic conductivity are synthesized at a high temperature, increasing the cost of the preparation process as well as the risk to the operation process.119 Therefore, developing a feasible method to address energy consumption and safety issues is reasonably significant for SSEs in practical applications. Liquid-phase sintering additives have received considerable attention because they have a low melting point and form a liquid phase. Li-based glass ceramics, such as Li2O, Li3BO3 (LBO), and LiSiO4, are utilized for densifying LLZO at low temperatures.120–123 In addition to borate electrolytes, halide electrolytes Li3OX (X = Cl and Br) also display good electrochemical stability with Li metal and a low melting temperature (Tm ≈ 282 °C).124,125 Taking advantage of the above merits, Li3OCl was introduced into the voids and boundaries of Ta-doped Li6.75La3Zr1.75Ta0.25O12 (LLZTO) particles at 350 °C (Fig. 6). In LLZTO–xLi3OCl CSSEs, amorphous Li3OCl, as a binder, filler and bridge, promoted the formation of an integrated continuous ion-conductive network among the LLZTO particles. Furthermore, Li3OCl, with excellent affinity, in situ reacted to form a stable and dense interfacial layer, which greatly decreased the interfacial resistance (from 1850 to 90 Ω cm2) and effectively suppressed lithium dendrite growth. The integrated CSSEs (LLZTO–2wt% Li3OCl) with compact and stable structures presented optimal ionic conductivity (2.27 × 10−4 S cm−1), and their electrochemical stability window was up to 10 V at RT.126 The above studies highlighted a novel strategy for developing integrated and compact CSSEs at ultralow temperature for high-performance ASSLBs.
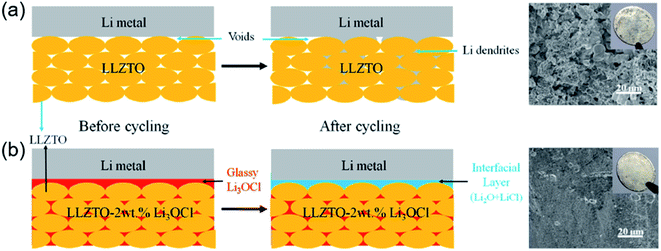 | ||
| Fig. 6 Schematics of Li deposition behavior using (a) LLZTO SSE and (b) LLZTO–2wt% Li3OCl CSE. Reproduced from ref. 126 with permission from Elsevier Inc., copyright 2018. | ||
Hydride-based CSSEs
In contrast to oxide and polymer SSEs, hydride SSEs belong to the complex hydride family and have been widely investigated as solid-state hydrogen-storage materials.127,128 To date, there has been little research on ionic conduction in complex hydrides owing to their poor ionic conductivity at moderate temperatures. In 2007, LiBH4 was reported as a fast-ion conductor by Orimo et al., and this is regarded as a turning point in the development of hydride SSEs.73 The ionic conductivity dramatically jumped by 3 orders of magnitude from 10−7 S cm−1 (303 K) to 10−3 S cm−1 (393 K), accompanied by a structural transition from orthorhombic to hexagonal (Fig. 7a).129 Furthermore, no polarization has been detected at the LiBH4–Li metal interface due to good reduction stability and low grain boundary resistance. The interfacial stability was even tested under a high current density of 2.8 mA cm−2.130 However, the fast 2D-Li+ conduction phenomenon could only occur in the high-temperature phase, which was a serious constraint for applying hydride SSEs in practical solid-state batteries. To improve the ionic conductivity of orthorhombic LiBH4 or prepare RT-stabilized hexagonal LiBH4, great efforts have been made, such as second-phase compositions, anion doping, and interfacial modifications. Therefore, a series of CSSEs have been synthesized based on hydrides.71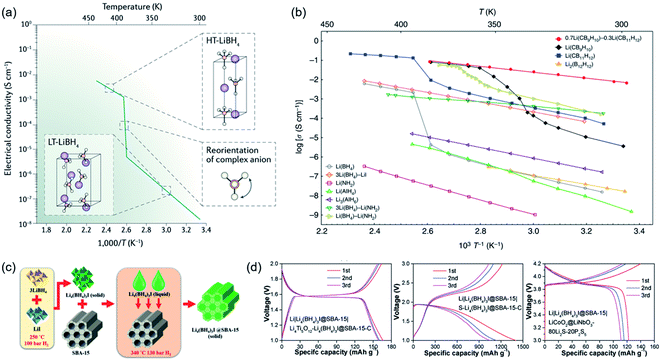 | ||
| Fig. 7 (a) Structural transition of LiBH4 from low temperature to high temperature phases triggered by the reorientation of complex anions. Reproduced from ref. 127 with permission from Springer Nature, copyright 2016. (b) Li+ ionic conductivity in complex hydrides. Reproduced from ref. 84 with permission from Springer Nature, copyright 2019. (c) Schematic diagram of the synthetic process of Li4(BH4)3I@SBA-15. (d) Full cell test based on the Li4(BH4)3I@SBA-15 composite electrolyte. Reproduced from ref. 135 with permission from Wiley-VCH, copyright 2019. | ||
A larger ionic radius (such as I−, 0.220 nm) can help increase the distance between alkali-metal ions and [BH4]− ions, which exhibit a low transition temperature.74 With efforts to obtain high conductivity at moderate temperature, the concept of partially replacing [BH4]− complex ions with I− ions was proposed and investigated. Among these conceptual materials, a LiBH4–LiI system was proven to be the most successful due to showing a high Li+ conductivity in the order of 10−5 S cm−1 for 3LiBH4–LiI at RT (Fig. 7b). No significant structural change was observed from differential scanning calorimetry (DSC) profiles, which revealed good structural stability and thermodynamic properties of the composite electrolyte.131 After a halide modification, the stability against Li was demonstrated by SEM and Li plating/stripping experiments. An all-solid-state Li–S battery with a 3LiBH4–LiI electrolyte exhibited high reversible capacity.132 Afterwards, researchers found that nanoconfined LiBH4 in the pores of ordered mesoporous silica scaffolds led to high Li+ conductivity due to the fast Li+ mobility at the interface between LiBH4 and SiO2.72,133,134 The above result also led to a lower phase transition temperature than for bulk LiBH4. More recently, inspired by the success of hydride–halide composites and nanoconfinement through mesoporous materials, Li4(BH4)3I was nanoconfined into SBA-15 via a two-step process by Zheng et al.(Fig. 7c and d).135 Li4(BH4)3I@SBA-15 exhibited a high conductivity of 2.5 × 10−4 S cm−1 at 35 °C with a Li-ion transference number of 0.97. Furthermore, the formation of a stable SEI between Li and the electrolyte could effectively suppress the growth of Li dendrites. More significantly, the good compatibility of Li4(BH4)3I@SBA-15 for ASSLBs was investigated with different cathodes, i.e., Li4Ti5O12, S and LiCoO2. On the other hand, the origin of dendrite growth in LiBH4 was clarified by Sun et al. in 2019. Li+ combines with electrons within the grain boundary/pore of the SSE, reducing to Li0 and eventually leading to a short circuit. Herein, the electronic conductivity of SSEs plays a significant role in dendrite formation. In this case, LiF was employed as an insulator for the LiBH4 SSE. The LiBH4–LiF CSSE exhibited amazing stability at 5.0 mA cm−2 for over 200 cycles, successfully inhibiting the growth of Li dendrites.130
A low activation energy is also needed to obtain a superionic conductor.136 For example, the Ea of 0.46 eV for Li4(BH4)3I@SBA-15 was lower than that of 0.56 eV for Li4(BH4)3I, which contributed to the high conductivity of the former. Thus, to weaken the electrostatic interaction between Li+ and [BH4]1−, sulfide materials were introduced into LiBH4 to form a hybrid system.137 A pseudo-binary system composed of a complex hydride and sulfide was reported by Orimo et al. in 2016.138 A new crystalline phase of 90LiBH4:10P2S5 with a possible orthorhombic structure was achieved with a high ionic conductivity of log(σ/S cm−1) = −3.0 at 300 K. A lower Ea for the new structure was calculated to be 0.38 eV compared with 0.53 eV for HT-LiBH4. A smooth charge transfer between the SSE and TiS2 electrode was certified to occur through a charge/discharge process. Unlike single complex hydrides, the new composite electrolyte exhibited no phase transition before 473 K and a wide electrochemical window of 0–5 V. Hydride-based CSSEs also experience stability issues as many candidates decompose on the cathode side.139 Many approaches have been developed to enhance the interfacial stability of CSSEs against cathodes, such as designing stable interfaces between the hydride SSEs and cathodes. To accommodate high-voltage cathodes, such as commercial LiCoO2, sulfides have recently been introduced to decrease the interfacial resistance between LiCoO2 and hydride-based SSEs by forming a stable Li-ion conductive cathode electrolyte interphase (CEI) layer.135,140 These hydride-based CSSEs can work well in all-solid-state LIBs with a LiCoO2 cathode, which exhibits typical high-voltage plateaus for charge/discharge.
Additionally, a novel SSE from a solid solution of two closo-type complex hydrides, videlicet 0.7Li(CB9H10)–0.3Li(CB11H12), was reported with a high ionic conductivity of over 10−3 S cm−1 at 298 K (Fig. 8a–e).84 High stability against lithium metal was certified by lithium plating/stripping for 300 cycles with an extremely stable lithium-ion transfer capability at 0.2 mA cm−2. Based on a cathode loading of 2.5 mg cm−2, all-solid-state Li–S batteries (ASSLSBs) presented a good reversible capacity of 1239 mA h g−1 after 20 cycles at 1C (298 K); the notable energy densities were calculated to be over 2500 W h kg−1 at high current densities (1–3C). Moreover, based on the SEM observation it was found that dendrite growth was suppressed at the interface between 0.7Li(CB9H10)–0.3Li(CB11H12) and Li metal. Overall, these CSSE-based SSLSBs exhibited outstanding electrochemical performance at 298 K. Alternatively, approaches to improve the conductivity of LiBH4-based composites in regard to inducing defects and changing the atomic arrangement have been attempted. To decrease the volume density of Li ions in LiBH4, neutral molecules were brought into LiBH4. This new concept was first achieved by the utilization of ammonia absorption into LiBH4, leading to a structural transition and reducing the activation energy of Li-ion mobility (Fig. 8f).76 Li(NH3)nBH4 (0 < n ≤ 2) exhibited high ionic conductivity (2.21 × 10−3 S cm−1). A drastic increase in ionic conductivity occurred at approximately 37 °C, which is close to human body temperature, which shows its potential to be utilized in wearable devices. More recently, the Li+-conduction mechanism in LiBH4·1/2(NH3) was systematically investigated through crystal structure analysis and DFT calculations by Jensen et al.141 The molecular volume of NH3BH3 (v = 69.86 Å3 per unit cell) was much larger than that of NH3 (v = 37.79 Å3 per unit cell), which would intrinsically increase the cell volume of LiBH4 and lower the volume density of Li ions (Fig. 8g). Remarkably, a novel RT ultrafast CSSE (LiBH4·NH3BH3) was demonstrated with a conductivity up to 4.04 × 10−4 S cm−1 at 298 K.142
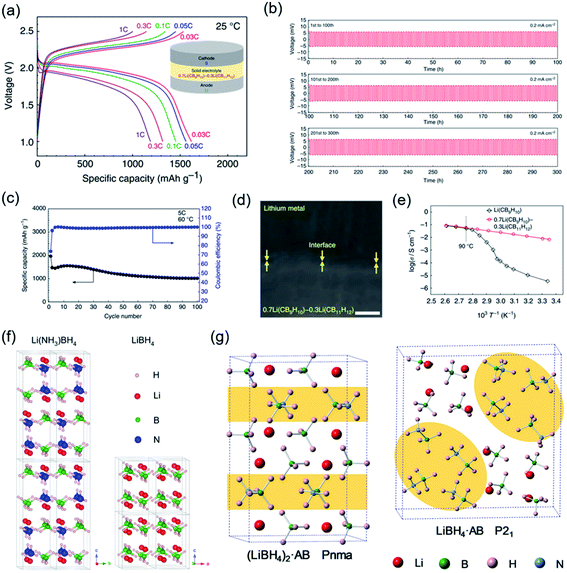 | ||
| Fig. 8 (a) Discharge–charge profiles at 298 K. (b) Stability of the composite electrolyte with lithium metal. (c) Cycling performance of the closo-type complex hydride-based Li–S battery. (d) FE-SEM image of the electrolyte/Li interface. (e) Ionic conductivity properties. Reproduced from ref. 84 with permission from Springer Nature, copyright 2019. (f and g) Crystal structures of Li(NH3)BH4, LiBH4, (LiBH4)2AB, and LiBH4AB. Reproduced from ref. 76 and 142 with permission from Elsevier Inc., copyright 2018 and permission from American Chemical Society, copyright 2019. | ||
In short, the formation of composites with other materials can effectively promote high ionic conductivity in LiBH4. Additionally, the original shortcomings of hydride-based SSEs, such as the ionic area specific resistance, thermal stability of composites, and oxidation stability with cathodes, have been overcome accordingly. Inspired by these studies, other nano/composites have been investigated, e.g., 2.0 × 10−5 S cm−1 for the LiBH4–C60 composite at 298 K.143 There are also several reports on ionic conductivities in hydride–nitride/imide systems,144 including but not limited to a LiBH4–Li3N system145 and a LiBH4–Li2NH system.146
Sulfide-based CSSEs
By replacing oxygen ions with sulfur ions, owing to their lower electronegativity and larger radius, sulfide SSEs present a weaker bonding strength between the sulfur and lithium ions and provide a wider migration tunnel for lithium ions. As a result, sulfide SSEs exhibit higher ionic conductivities, approximately 10−3 to 10−4 S cm−1 at RT, which are almost comparable to those of liquid electrolytes. In 1986, Li2S–SiS2 was first synthesized by twin roller quenching.147 Then, through further doping with a Li3PO4 electrolyte, the conductivity increased to 0.69 × 10−3 S cm−1.148 In 2011, a thio-lithium superionic conductor (thio-LISICON) Li10GeP2S12 was first reported to have a high ionic conductivity of 1.2 × 10−2 S cm−1 at RT (Fig. 9a), which greatly promoted the development of sulfide SSEs.16 To improve conductivity, the same group discovered a novel lithium superionic conductor, Li9.54Si1.74P1.44S11.7Cl0.3, based on a double substitution with aliovalent-ion doping (Fig. 9b).10 An exceptionally high conductivity of 2.5 × 10−2 S cm−1 was obtained. | ||
| Fig. 9 (a) Framework structure of Li10GeP2S12 and conduction pathways of lithium ions. Reproduced from ref. 13 with permission from Nature Publishing Group, copyright 2011. (b) Crystal structure of Li9.54Si1.74P1.44S11.7Cl0.3 and nuclear distributions of Li atoms. Reproduced from ref. 7 with permission from Nature Publishing Group, copyright 2016. (c) Schematic of the formation of the solid electrolyte in a polymer matrix membrane. Reproduced from ref. 151 with permission from Wiley-VCH, copyright 2015. (d) Schematic illustration of the preparation process of the LPS–LLZO CSSE. (e) The ionic conductivity properties of the LPS:LLZO mixture. (f) TEM image of the LLZO–LPS composite electrolyte (the core–shell structure) and the EELS map show a higher Li concentration across the LLZO–LPS interface (the bright part has a high concentration of Li). Reproduced from ref. 158 with permission from the Royal Society of Chemistry, copyright 2014. | ||
However, under current laboratory conditions, the cell-based energy density of ASSLBs is much lower than that of their competitors with liquid electrolytes. Because of the brittleness of electrolyte materials, the thickness of the electrolyte layer is often more than 1 mm to avoid the formation of cracks during high pressure stress.149 Synthesizing a sulfide/polymer composite electrolyte is an effective method to slim the electrolyte layer and maintain a high ionic conductivity. Kanno et al. prepared a thio-LISICON/silicone composite electrolyte sheet.150 Recently, an increasing number of compliant sulfide–polymer composite electrolytes have been successfully developed. Whiteley et al. produced a 64 μm-thick membrane with a sulfide loading of 77 wt% that exhibited a low shear modulus and high ionic conductivity of 10−4 S cm−1 at RT (Fig. 9c). An ultrathin solid-state membrane (100 μm) was fabricated based on 77.5Li2S–22.5P2S5 with a self-healing polymer matrix. It was applied as a separator (80 wt%) in ASSLBs with an FeS2-based cathode and achieved excellent rate capability and stable cycling for over 200 cycles.151 More recently, Nan et al. prepared a free-standing composite solid electrolyte membrane with 78Li2S–22P2S5 concentrations from 80 to 97 wt%. The sulfide/PEO and sulfide/PVDF composites showed ionic conductivities of 4–7 × 10−4 S cm−1 with a thickness of 120 μm.58 A moderate PEO content could help the composite electrolyte achieve good mechanical properties and a stable electrolyte/lithium interface.105,152–155 For example, with 5 wt% PEO, the proportional limit of Li6PS5Cl composite solid electrolytes was enhanced to a value of 60 MPa. The as-assembled cell exhibited a good capacity retention rate of 91% over 200 cycles at 0.05C and 303 K. Further characterization indicated that lithium dendrite growth could be effectively inhibited after the PEO modification.105 The formation of P–O–C bonds between the sulfide glass and oligomers was the key factor to ensure a high conductivity. It was also noted that the addition of small amounts of polymers improved ion conduction by lowering the glass transition temperature.156
In addition to combining with organic polymers, inorganic materials can act as fillers in sulfide-based electrolytes. Hood et al. reported the effect of oxide fillers in composites with β-Li3PS4 for the enhancement of the parent electrolyte. For example, 2 wt% Al2O3 increased the ionic conductivity of the parent electrolyte to 2.28 × 10−4 S cm−1 while maintaining electrochemical stability against metallic lithium up to 5 V.157 Similarly, the same group examined the composite electrolyte of a “hard” oxide (Li7La3Zr2O12, LLZO) and a “soft” sulfide (β-Li3PS4, LPS), which demonstrated an excellent conductivity of 5.36 × 10−4 S cm−1 at 298 K; the above conductivity was higher than that of its parent electrolytes (Fig. 9d and e). The improved conductivity was considered an effect of the space charge layer at the interface between the LLZO and LPS particles, which was believed to redistribute the ionic and electronic point defects (Fig. 9f).158 As both sulfide and hydride SSEs are very sensitive to humid air, the handling process is always conducted in a glove box in an inert gas atmosphere. Due to the similarity of the chemical stability of these two types of electrolytes, a composite of hydride (LiBH4) and sulfide (Li3PS4) showed the potential to improve the total lithium-ion conductivity. Tatsumisago et al. examined the effects of the addition of LiBH4 on the structures and properties of sulfide electrolytes. The conductivities of the composite SSEs increased with increasing LiBH4 content. The CSSEs had a wide electrochemical window up to 5 V vs. Li+/Li−. The glass with a composition of 2Li3PS4·LiBH4 showed the highest conductivity of 1.6 × 10−3 S cm−1 at RT.159
Halide-based CSSEs
To realize the application of ASSLBs at RT, current research efforts focus mostly on ionic conductivity and a wide electrochemical stability window. Compared to other SSEs, halide SSEs have had a relatively delayed development before 2018 because of their low ionic conductivity and low oxidation voltage.160,161 With the tireless efforts of countless researchers, a large breakthrough in halide SSEs occurred in 2018.68 Tetsuya Asano et al. successfully synthesized Li3YCl6 and Li3YBr6 with high ionic conductivities of 0.03–1.7 × 10−3 S cm−1 at RT.68 Afterwards, other halide SSEs with high ionic conductivity, such as Li3ErCl6,162,163 Li3InCl6 (ref. 70 and 164) and Li3−xM1−xZrxCl6 (M = Y, Er),165 were also successfully fabricated. In addition, recent experimental and theoretical results have further demonstrated that halide SSEs are quite promising owing to their wide electrochemical windows, good electrode stability, high humidity tolerance, and simple production processes. Halide SSEs and their application in ASSLBs are relentlessly advancing. These new developments make it necessary to revisit halide SSEs for potential applications in ASSLBs. Among halide SSEs, Li3MX6 (M = Sc, Y, Ho, Er, X = Cl, Br)-type SSEs have received wide attention. However, there is still a large gap between their experimental and theoretical results. To date, only a few halide SSEs, such as Li3YBr6 and Li3InCl6, have achieved a high ionic conductivity over 10−3 S cm−1 at RT. In addition, chloride-based SSEs showed an oxidation onset voltage of approximately 4 V, which cannot fully meet the electrolyte needs of high-voltage cathodes. Therefore, a tremendous amount of work is urgently required to improve ionic conductivity and optimize the electrochemical stability window in chloride-based SSEs.The Li3YBr6, Li3InCl6, and Li3InBr6 SSEs, which possess a cubic close-packed (ccp)-like anion arrangement, display relatively high ionic conductivities of 1–2 × 10−3 S cm.67,68,164,166 Furthermore, theoretical calculations demonstrated that halide SSEs with ccp anion sublattices could display high ionic conductivities.167 Inspired by the possibility of achieving fast Li+ migration in ccp halide SSEs, new routes have opened in the development of ASSLBs. Recently, LixScCl3+x SSEs (x = 2.5, 3, 3.5, and 4) were synthesized by a simple co-melting strategy from LiCl and ScCl3. The structural evolution and ionic diffusion mechanisms in LixScCl3+x were also systematically explored, and it was found that the vacancy concentration has the opposite trend with increasing x in LixScCl3+x (Fig. 10). The Li+ probability density and migration pathways were fitted based on AIMD simulations (Fig. 10a–c). The site occupations of metal/vacancies favoured Li+ migration within the local structure (Fig. 10e–g). As a result, the obtained Li3ScCl6 showed a high ionic conductivity of 3 × 10−3 S cm−1. Moreover, the all-solid-state LiCoO2/Li3ScCl6/In full cell exhibited a long cycle life and a wide electrochemical window of 0.9–4.3 V. Note that Li3ScCl6 was not stable towards Li in the initial cycles of plating/stripping.168 To enhance the ionic conductivity, the covalent substitution of metal ions was also an effective strategy to introduce vacancies in the mobile ion sublattice. Nazar et al. prepared a class of mixed-metal chloride solid-state electrolytes, Li3−xM1−xZrxCl6 (M = Y, Er) SSEs. These new halide SSEs exhibited high ionic conductivities (up to 1.4 mS cm−1 at RT) due to their unique new structures (Fig. 10h and i). In Fig. 10j, combining neutron and single-crystal X-ray diffraction methods, the evolution of new structures after a Zr substitution revealed trigonal to orthorhombic phase transition processes. Most importantly, chloride SSEs without any protective coating showed excellent oxidation stability. When using unprotected LiCoO2 as the cathode material, ASSLBs exhibited exceptional electrochemical oxidation stability up to 4.5 V at RT.165
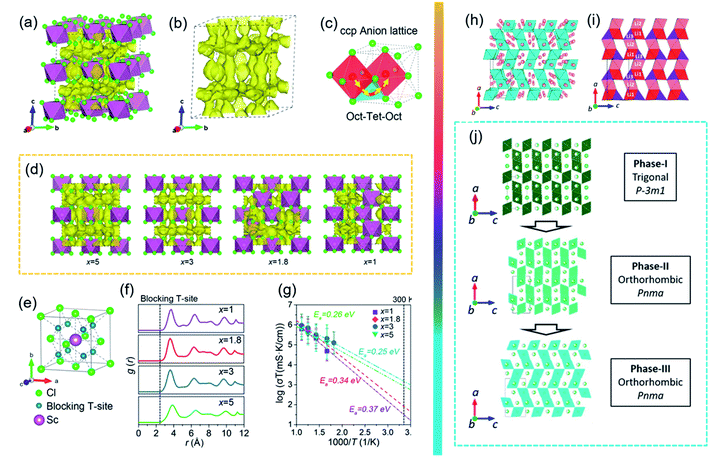 | ||
| Fig. 10 (a and b) The Li+ probability density based on AIMD simulations. (c) The Li+ migration pathways of the Li3ScCl6 structure. (d) The Li+ probability density marked by yellow isosurfaces of Li5ScCl8 (x = 5), Li3ScCl6 (x = 3), Li1.8ScCl4.8 (x = 1.8) and LiScCl4 (x = 1) structures along the a axis. (e) The blocking effect of Sc. (f) Radial distribution function (rdf) of Sc–Li ions in the LixScCl3+x (x = 1, 1.8, 3, and 5) SSEs. (g) Arrhenius plot of Li+ diffusivity in LixScCl3+x (x = 1, 1.8, 3, and 5) from AIMD simulations. Reproduced from ref. 167 with permission from the American Chemical Society, copyright 2020. (h) The crystal structure of Li2.5Er0.5Zr0.5Cl. (i) Li connectivity along the [100] direction. (j) Phase evolution of Li3M1−xZrxCl6 (M = Er, Y) upon Zr substitution. Reproduced from ref. 164 with permission from the American Chemical Society, copyright 2020. | ||
Remarkably, halide SSEs based on close-packed anion arrangements or covalent substitution of metal ions exhibited a high ionic conductivity. However, the chemical and electrochemical stability was difficult to satisfy with both high oxidation and low reduction voltages in full battery applications. One of the possible solutions is to combine halide SSEs and other SSEs to be compatible with cathode and anode materials, respectively. Unlike chloride SSEs, fluoride SSEs exhibit a wide electrochemical stability window (∼6 V). However, they have the lowest ionic conductivity among halide SSEs. To solve this problem, tuning the chemical composition of lithium difluoro(oxalato)borate (LiDFOB) and PEO can heighten the stability and improve the ionic conductivity in CSSEs,169,170 which undoubtedly proves the possibility of modifying CSSEs with other types of SSEs. Therefore, the lower performance compared to other SSEs can be enhanced by various physical approaches and chemical strategies. It is predicted that halide SSEs will become more deserving of in-depth attention, and their commercialization in ASSLBs will be realized in the near future.
Mobile ion migration mechanism in CSSEs
A comprehensive summary of different CSSEs, including polymer-based, oxide-based, hydride-based, sulfide-based and halide-based CSSEs, is provided in Table 1. For practical application in ASSBs, in addition to the ionic conductivity, electrochemical stability window, chemical compatibility, and mechanical properties, other properties such as thermal stability, fabrication processes, cost, device integration and environmental friendliness are also important.11,171,172 In 2017, Manthiram et al. summarized the properties of the existing solid electrolyte materials and visualized those properties in radar plots.11 It is clear that single solid-state electrolytes have difficulties satisfying the increasing demands in our daily life. Even though significant progress has been achieved, huge challenges still remained for CSSEs to seek an equilibrium relationship, which minimizes the weaknesses of individual SSEs to enhance the overall performance. Therefore, it is important to summarize previous achievements in order to understand, design, and fabricate novel CSSEs. Fig. 11 briefly describes the migration mechanism of lithium ions in different kinds of composite solid-state electrolytes. For polymer-based electrolytes, the diffusion in the polymeric host is based on ether linkages. However, with the increase of inorganic fillers, the ion transport gradually transits to the newly formed interphase between the dispersed crystalline and polymer matrix (Fig. 11a).173 It is noticed that many solid-state electrolytes turn out to be superionic phases only at high temperature, such as hydride-based electrolytes (Fig. 2b).173 Typical approaches, including cationic/anionic doping and compositing, could provide an effective method to stabilize the metastable phase via interfacial interactions. Recent studies have suggested that the ion transport of sulfide- and halide-based SSEs strongly depends on the ion channels formed by the special crystal structure.174 For instance, the transformation of Li3PS4 from the glassy state to the crystalline state after heat treatment can effectively improve its ionic conductivity (Fig. 11c–f). In this case, understanding the basic conduction mechanism helps design better composite SSEs. In this section, we discuss how to combine hosts with dispersed phases to make an ideal solid-state electrolyte with high ionic conductivities and favourable electrochemical stability.| CSSEs | Conductivity (S cm−1) | Characteristics | Stability vs. Li/Li+ (V) | Cycle performance | Ref. | |
|---|---|---|---|---|---|---|
| Polymer-based | PEO/GDC/LSGM | 10−4, 30 °C | Reduced interfacial resistance | 4.8 | 100 cycles (Li‖CSSE‖NMC) | 93 |
| PEO/LLZO | 1.1 × 10−4, 40 °C | Reduced interfacial resistance | >4 | 200 cycles (Li‖CSSE‖S) | 94 | |
| PVDF–PVAC | 4.8 × 10−4, RT | Good flexibility | 4.8 | 200 cycles (Li‖CSSE‖LCO) | 101 | |
| PEO/PEG–LGPS | 9.83 × 10−4, RT | Good interfacial compatibility | 5.1 | Over 3200 h (Li‖CSSE‖Li) | 103 | |
| PCE/LGPS/PCE | 2.12 × 10−3, RT | Good thermal stability | 4.1 | 120 cycles (Li‖CSSE‖LFP) | 106 | |
| Oxide-based | PEO/LLZTO | 10−4, 55 °C | High mechanical strength | 5.0 | 100 cycles (Li‖CSSE‖LFP) | 56 |
| Sandwich-PEO/LLZTO | 9.1 × 10−5, 55 °C | Freestanding and flexible, 11.3 MPa | 5.03 | 200 cycles (Li‖CSSE‖LFP) | 114 | |
| LAGP–PEO | 1.3 × 10−3, 60 °C | High thermal stability | 4.5 | 300 cycles (Li‖CSSE‖LFP) | 116 | |
| Vertically aligned LAGP–PEO | 1.11 × 10−3, 60 °C | Good interfacial compatibility | 4.5 | 300 cycles (Li‖CSSE‖LFP) | 117 | |
| Hydride-based | Li4(BH4)3I@SBA-15 | 2.5 × 10−4, 35 °C | Good interfacial contact | 5.0 | 350 h (Li‖CSSE‖Li) | 135 |
| 90LiBH4:10P2S5 | 10−3, 27 °C | Good interfacial contact | 5.0 | 10 cycles (LiIn‖CPE‖TiS2) | 138 | |
| 0.7Li(CB9H10)–0.3Li(CB11H12) | 6.7 × 10−3, 25 °C | High stability and flexible | 5.0 | 300 h (Li‖CSSE‖Li) | 84 | |
| Li(NH3)nBH4 (0 < n ≤ 2) | 2.21 × 10−3, 40 °C | Good interfacial contact | >3.7 | 12 h (Li‖CSSE‖Li) | 76 | |
| Sulfide-based | Li9.54Si1.74P1.44S11.7Cl0.3 | 2.5 × 10−2 | Good interfacial contact | 2.6 | 1000 cycles (LTO‖CSSE‖LCO) | 10 |
| 78Li2S–22P2S5/polymer | 4–7 × 10−4, RT | Self-standing | 3.0 | 100 cycles (LiIn‖CPE‖S–CNT) | 58 | |
| LPS/LLZO | 5.36 × 10−4, 25 °C | Good interfacial stability and contact | 5.0 | 4500 min (Li‖CSSE‖Li) | 158 | |
| LPS–LiBH4 | 1.6 × 10−3, RT | Good interfacial contact | 5.0 | 5 cycles (Li‖CSSE‖TiS2) | 159 | |
| Halide-based | LixScCl3+x | 3 × 10−3, RT | Good interfacial stability | 4.3 | 160 cycles (Li‖CSSE‖LCO) | 168 |
| Li3−xM1−xZrxCl6 (M = Y, Er) | 1.4 × 10−3, RT | Good interfacial stability | 4.5 | 200 cycles (Li11Sn6‖CSSE‖LCO) | 165 | |
| Li3ErCl6 | 3.15 × 10−4, RT | Good oxidative stability | — | — | 163 | |
| PEO/LiDFOB–40wt% EMImTFSI | 1.85 × 10−4, 30 °C | Good interfacial contact | 4.0 | 50 cycles (Li‖CSSE‖LFP) | 170 |
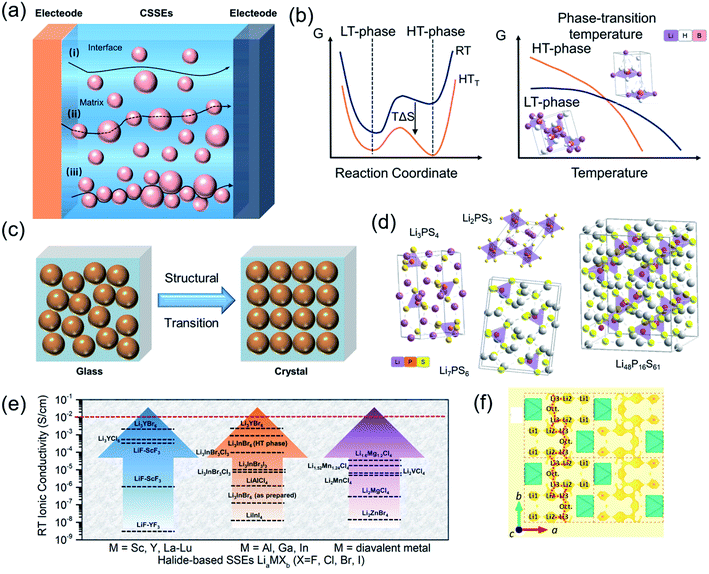 | ||
| Fig. 11 (a) Illustration of ion conduction paths in polymer-based CSSEs and inorganic material-based CSSEs. (b) Free energy diagram of crystalline SSEs with a phase transition. (c and d) Structural transition and common sulfide crystal structures. Reproduced from ref. 173 with permission from the Royal Society of Chemistry, copyright 2020. (e) Summary of the reported RT ionic conductivities of representative halide SSEs. Reproduced from ref. 174 with permission from the Royal Society of Chemistry, copyright 2020. (f) 1D migration pathway of the crystal structure of Li2.5Er0.5Zr0.5Cl in the ab plane. Reproduced from ref. 165 with permission from the American Chemical Society, copyright 2020. | ||
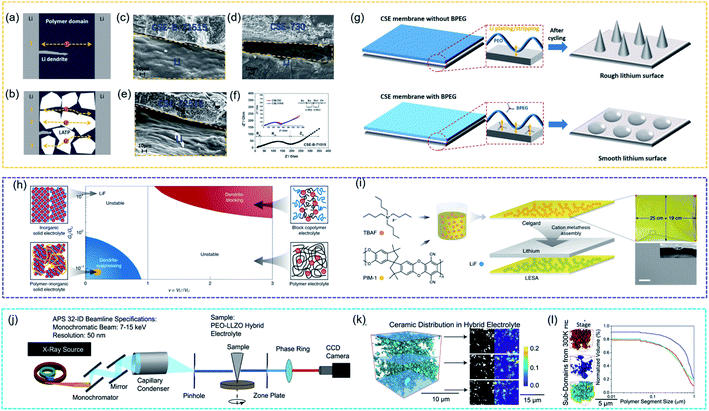
By combining isotope labelling and solid-state Li NMR to track the ion-diffusion pathway, Hu et al. first provided experimental evidence to show that Li ions favour the pathway through the LLZO ceramic phase, not the interface or the PEO polymer phase.175 After that, Pan et al. prepared a flexible composite solid electrolyte membrane consisting of inorganic solid particles Li1.3Al0.3Ti1.7(PO4)3 (LATP), PEO, and boronized polyethylene glycol (BPEG), which provided good mechanical strength and physically inhibited the free growth of lithium dendrites. The authors discussed the relationships between the stability against lithium dendrite formation and the combination of well-designed components. As proposed in Fig. 12a, the growth of lithium dendrites could easily penetrate the SPE membrane, while the close packing of the inorganic particles worked as a physical barrier to restrict the free growth of lithium dendrites in the CSE membrane. The post-cycling SEM images of the Li-electrolyte interfaces in Fig. 12b–d showed that CSE-B-71515 (LATP, PEO and BPEG in the weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) had the smoothest Li surface and Fig. 12e showed that CSE-B-71515 had the smallest impedance value. The main reason was the effect of BPEG on lithium dendrite formation in Fig. 12f. The addition of BPEG oligomers disorganized the crystallinity of the PEO domain and improved ionic conductivity. Additionally, the planar structure of the oligomers chemically enabled homogeneous lithium plating/stripping on the lithium metal and reduced the polarization effects.176
15) had the smoothest Li surface and Fig. 12e showed that CSE-B-71515 had the smallest impedance value. The main reason was the effect of BPEG on lithium dendrite formation in Fig. 12f. The addition of BPEG oligomers disorganized the crystallinity of the PEO domain and improved ionic conductivity. Additionally, the planar structure of the oligomers chemically enabled homogeneous lithium plating/stripping on the lithium metal and reduced the polarization effects.176
 | ||
| Fig. 12 Proposed lithium ion transfer pathways and lithium dendrite growth in SSEs (a) without inorganic particles and (b) with closely packed inorganic particles. (c–e) SEM images of the Li/CSE membrane interface in symmetrical cells after cycling, and corresponding to (f) electrochemical impedance spectra. (g) Schematic showing lithium plating/stripping processes with different CSE membranes. Reproduced from ref. 175 with permission from Wiley-VCH, copyright 2017. (h) Chemomechanical model underlying the successes and failures of SSEs during charging. (i) Schematic figure of prepared nano-LiF@PIM CSSEs. Scale bar, 1 μm. Reproduced from ref. 181 with permission from Nature Publishing Group, copyright 2020. (j) Schematic diagram of synchrotron nano-tomography characterization through the transmission X-ray microscopy technique. (k) Representative volume showing the ceramic and the polymer phase distribution within the CSSE. (l) Representative ceramic substructures and the polymer size distribution of the CSSE. Reproduced from ref. 186 with permission from Elsevier Inc., copyright 2020. | ||
However, the mechanism of dendrite formation and propagation remains unclear. Uneven Li+ transport across the electrode–electrolyte interface causes heterogeneous nucleation, which is tied to dendrite formation during electrodeposition. To solve the safety problem induced by lithium dendrites, high shear modulus (Gs) CSSEs have been prioritized. In this case, the mechanism associated with dendrite formation can be modelled using Butler–Volmer physics:177
CSSEs contain numerous solid/solid interfaces, including intrinsic and extrinsic interfaces, the former one is the contact region between the matrix phase and mixed media phase, and the later one is the contact surface between the electrode and electrolyte. These interfaces are responsible for ion-transportation, electrode/electrolyte compatibility, and stability in solid-state batteries. Understanding and evaluating interfacial properties have a significant effect on the rational design of ASSBs. Currently, there are few studies that report the effect of extrinsic interfaces on electrochemical performance. Extrinsic interfaces are responsible for high interfacial resistances, which can cause a catastrophic failure in capacity due to Li dendrites,182,183 dead Li,184 or interfacial delamination.185
Hatzell's group systematically evaluated the relationship between the electrolyte structure and extrinsic interfaces in solid-state batteries and investigated how extrinsic interfaces impacted electrochemical performance in ASSBs. By design, CSSEs with similar transport properties were manufactured and displayed varying interfacial properties. The mechanical, adhesion, and interfacial ion-transport properties in the extrinsic interfaces were estimated using an effective mean field theory model and atomic force microscopy techniques (AFM). They found that the difference in physical properties at the extrinsic interfaces depended on the distribution of inorganic particles in CSSEs. Hence, synchrotron X-ray nanotomography was performed to evaluate the heterogeneous distribution of inorganic materials. Then, combined with physics-based modelling, the 3D-structure of CSSEs was reconstructed to elucidate the origin of heterogeneous interfacial properties. These findings revealed that mechanical properties, rather than transport properties at the extrinsic interface, largely dictated the electrochemical performance. To achieve facile and long-life ASSBs, microstructural control over inorganic constituents may provide a pathway towards tailoring interfacial properties with spatial control, which can be achieved by controlling the CSSE nano- and microstructures (Fig. 12j and k).186 Despite the significant achievements in state-of-the-art CSSEs, the above detailed mechanistic study is insufficient to form a complete system to support the advanced performance of CSSEs and realize their application in ASSBs.
Summary and outlook
All solid-state batteries have experienced rapid progress and have drawn increasing attention owing to the introduction of high-energy density metal anodes. Developing advanced CSSEs is considered to be one of the most promising directions to achieve a breakthrough in solid-state battery technology. With more and more attempts, various hosts and dispersed phases are combined into potential CSSEs, which not only have high ionic conductivity, but also show good stability and properties. For instance, composites consisting of “oxides and polymers” are flexible and mechanically robust. A new strategy of forming a thin electrolyte is introduced by creating sulfide-in-polymer composites. Hydride-based CSSEs have good stability against metallic Li, which help overcome the side reaction problem of sulfides/oxides. Meanwhile, sulfide/oxide-based CSSEs also match high-voltage cathode materials. Although SSEs have been widely believed to be safer for ASSB application, such a point of view still lacks sufficient data support, in particular for batteries with Li as an anode electrode. Challenges including continuous interfacial side reactions, limited electrochemical window, poor air stability, large volume expansion/shrinkage, undesirable dendrite growth, inescapable heat generation and thermal runaway should be conquered for achieving better performance.187 For example, very recent progress suggests that the release of oxygen from SSEs at increased temperatures is found to be responsible for thermal runaway with Li. It is also demonstrated that oxide SSEs exhibit different thermal stability against highly reactive metallic Li.188 A further artificial layer (such as Al2O3, Li2CO3) can help stabilize the interface between Li and SSEs. For example, solid/solid interfacial architecturing through atomic layer deposition of Al2O3 on the SPE surface could suppress the “shuttle effect” of lithium polysulfide intermediates in the SPE and increase the interfacial compatibility between the metal lithium anode and the SPE.189 Considering the unique advantages of SSEs, some perspectives presented herein are based on CSSEs.Lithium dendrite growth is mostly discovered in single polymers or inorganic electrolytes based on recent research contributions. Among CSSEs, there are few reports on the growth of lithium dendrites, which is an open field for further research. Information on architecture and composite content, component contribution and ion-conduction tunnels, along with lithium deposition behaviour is required for the design of advanced CSSEs.
Currently, most syntheses of CSSEs are based on dissolving particles in a matrix, or compounding layer by layer. The interfacial contacts between two phases and the ion transport mechanism need further investigation. More importantly, advanced CSSEs need to be designed from nanoscale. Therefore, it also requires in situ/operando characterization techniques, such as time-of-flight secondary-ion mass spectrometry and in situ electrochemical spherical aberration electron microscopy, to provide an in-depth study on the interfacial dynamics and ion transport mechanism.
To further improve the capacity and energy density of CSSE based ASSBs, matching suitable and smart cathode materials according to the electrochemical window and stability is another key objective. This could be realized through increasing more sites for active substances by constructing efficient electronic and ionic conductive networks. The interfacial design between the cathode and anode would progress from micron to nanoscale, and the mechanism of ion transport would transit from interfacial contact into atomic contact, which may rely on advanced nanotechnology.
From traditional single SSEs to composite SSEs is a research trend that we believe. Many issues have been exposed along with thorough studies, but fortunately the pace has never stopped to face challenges. Fully exploiting their potential has high scientific and practical value for large-scale energy storage in ASSBs in the near future.
Abbreviations
| AFM | Atomic force microscopy |
| ASR | Area-specific resistance |
| ASSLBs | All-solid-state lithium batteries |
| BPEG | Boronized polyethylene glycol |
| CEI | Cathode electrolyte interphase |
| CPEs | Composite polymer electrolytes |
| CPSE | Ceramic/polymer solid-state electrolyte |
| CSSEs | Composite solid-state electrolytes |
| DFT | Density functional theory |
| DSC | Differential scanning calorimeter |
| GDC | Gd0.1Ce0.9O1.95 |
| GO | Graphene oxide |
| G s | Shear modulus |
| LAGP | Li1.5Al0.5Ge1.5(PO4)3 |
| LATP99: | Li1.4Al0.4Ti1.6(PO4)3 |
| LATP176: | Li1.3Al0.3Ti1.7(PO4)3 |
| LBO | Li3BO3 |
| LCO | LiCoO2 |
| LFP | LiFePO4 |
| LGPS | Li10GeP2S12 |
| LIBs | Lithium-ion batteries |
| LIPON | Lithium phosphorus oxynitride |
| LLTO | Li0.33La0.557TiO3 |
| LLZO | Li7La3Zr2O12 |
| LLZTO56,91,100 | Li6.4La3Zr1.4Ta0.6O12 |
| LLZTO126 | Li6.75La3Zr1.75Ta0.25O12 |
| LSGM | La0.8Sr0.2Ga0.8Mg0.2O2.55 |
| LSTZ | Li3/8Sr7/16Ta3/4Zr1/4O3 |
| NASICON | Sodium superionic conductor |
| Ni-MH | Nickel-metal hydride |
| NMR | Nuclear magnetic resonance |
| PAN | Polyacrylonitrile |
| PEG | Polyethylene glycol |
| PEGDME | Poly-(ethylene glycol)-dimethyl ether |
| PEO | Polyethylene oxide |
| PMMA | Polymethyl methacrylate |
| PPO–PEO–PPO | Hydrophobic–hydrophilic–hydrophobic triblock copolymers |
| PVAC | Polyvinyl acetate |
| PVDF | Polyvinylidene fluoride |
| RT | Room temperature |
| SEI | Solid electrolyte interphase |
| SIEs | Solid inorganic electrolytes |
| SPEs | Solid polymer electrolytes |
| SS | Stainless steel |
| SSE(s) | Solid-state electrolyte(s) |
| ASSLSBs | All-solid-state Li–S batteries |
| Thio-LISICON | Thio-lithium superionic conductor |
| TMS | Tetramethylene sulfone |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by “the Fundamental Research Funds for the Central Universities, No. NS2020039, Open Fund of Key Laboratory of Materials Preparation and Protection for Harsh Environment (Nanjing University of Aeronautics and Astronautics), and Ministry of Industry and Information Technology No. XCA20013-2. T. Z., W. Z. and X. Y. acknowledge support from the National Natural Science Foundation of China (51625102, 51802154 and 51903047). W. Z. acknowledges support from the Natural Science Foundation of Jiangsu Province (grant BK20180407). X. Y. acknowledges support from the Open Fund of Jiangsu Key Laboratory of Electrochemical Energy Storage Technologies No. EEST2019-2. W. H. acknowledges support from the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX20_0192).Notes and references
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- J.-M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- I. H. Son, J. Hwan Park, S. Kwon, S. Park, M. H. Rümmeli, A. Bachmatiuk, H. J. Song, J. Ku, J. W. Choi, J. Choi, S.-G. Doo and H. Chang, Nat. Commun., 2015, 6, 7393 CrossRef CAS PubMed.
- Q. Pang, X. Liang, C. Y. Kwok and L. F. Nazar, Nat. Energy, 2016, 1, 16132 CrossRef CAS.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- J. Wan, J. Xie, X. Kong, Z. Liu, K. Liu, F. Shi, A. Pei, H. Chen, W. Chen, J. Chen, X. Zhang, L. Zong, J. Wang, L. Q. Chen, J. Qin and Y. Cui, Nat. Nanotechnol., 2019, 14, 705–711 CrossRef CAS PubMed.
- Y. Wang, W. D. Richards, S. P. Ong, L. J. Miara, J. C. Kim, Y. Mo and G. Ceder, Nat. Mater., 2015, 14, 1026–1031 CrossRef CAS PubMed.
- A. Miura, N. C. Rosero-Navarro, A. Sakuda, K. Tadanaga, N. H. H. Phuc, A. Matsuda, N. Machida, A. Hayashi and M. Tatsumisago, Nat. Rev. Chem., 2019, 3, 189–198 CrossRef CAS.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, Nat. Energy, 2016, 1, 16030 CrossRef CAS.
- A. Manthiram, X. Yu and S. Wang, Nat. Rev. Mater., 2017, 2, 16103 CrossRef CAS.
- Y. Oumellal, A. Rougier, G. A. Nazri, J.-M. Tarascon and L. Aymard, Nat. Mater., 2008, 7, 916–921 CrossRef CAS PubMed.
- J. Janek and W. G. Zeier, Nat. Energy, 2016, 1, 1–4 Search PubMed.
- J. K. Nørskov, T. Bligaard, J. Rossmeisl and C. H. Christensen, Nat. Chem., 2009, 1, 37–46 CrossRef PubMed.
- X. Han, Y. Gong, K. Fu, X. He, G. T. Hitz, J. Dai, A. Pearse, B. Liu, H. Wang, G. Rubloff, Y. Mo, V. Thangadurai, E. D. Wachsman and L. Hu, Nat. Mater., 2017, 16, 572–579 CrossRef CAS PubMed.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS PubMed.
- Z. Lin, Z. Liu, N. J. Dudney and C. Liang, ACS Nano, 2013, 7, 2829–2833 CrossRef CAS PubMed.
- E. Rangasamy, Z. Liu, M. Gobet, K. Pilar, G. Sahu, W. Zhou, H. Wu, S. Greenbaum and C. Liang, J. Am. Chem. Soc., 2015, 137, 1384–1387 CrossRef CAS PubMed.
- W. S. Tang, M. Matsuo, H. Wu, V. Stavila, W. Zhou, A. A. Talin, A. V. Soloninin, R. V. Skoryunov, O. A. Babanova, A. V. Skripov, A. Unemoto, S.-I. Orimo and T. J. Udovic, Adv. Energy Mater., 2016, 6, 1502237 CrossRef.
- X. Wang, R. Xiao, H. Li and L. Chen, Phys. Rev. Lett., 2017, 118, 195901 CrossRef PubMed.
- S. J. Sedlmaier, S. Indris, C. Dietrich, M. Yavuz, C. Dräger, F. Von Seggern, H. Sommer and J. Janek, Chem. Mater., 2017, 29, 1830–1835 CrossRef CAS.
- H. Yamane, M. Shibata, Y. Shimane, T. Junke, Y. Seino, S. Adams, K. Minami, A. Hayashi and M. Tatsumisago, Solid State Ionics, 2007, 178, 1163–1167 CrossRef CAS.
- K. H. Park, Q. Bai, D. H. Kim, D. Y. Oh, Y. Zhu, Y. Mo and Y. S. Jung, Adv. Energy Mater., 2018, 8, 1800035 CrossRef.
- Y. Meesala, A. Jena, H. Chang and R. S. Liu, ACS Energy Lett., 2017, 2, 2734–2751 CrossRef CAS.
- Z. Zhang, Y. Shao, B. Lotsch, Y. S. Hu, H. Li, J. Janek, L. F. Nazar, C. W. Nan, J. Maier, M. Armand and L. Chen, Energy Environ. Sci., 2018, 11, 1945–1976 RSC.
- X. B. Cheng, R. Zhang, C. Z. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- F. Han, J. Yue, C. Chen, N. Zhao, X. Fan, Z. Ma, T. Gao, F. Wang, X. Guo and C. Wang, Joule, 2018, 2, 497–508 CrossRef CAS.
- H. Liu, X. B. Cheng, J. Q. Huang, H. Yuan, Y. Lu, C. Yan, G. L. Zhu, R. Xu, C. Z. Zhao, L. P. Hou, C. He, S. Kaskel and Q. Zhang, ACS Energy Lett., 2020, 5, 833–843 CrossRef CAS.
- Y. Zhu, X. He and Y. Mo, J. Mater. Chem. A, 2016, 4, 3253–3266 RSC.
- B. Zhang, Z. Lin, L. W. Wang and F. Pan, ACS Appl. Mater. Interfaces, 2020, 12, 6007–6014 CrossRef CAS PubMed.
- Y. Gao, Z. Yan, J. L. Gray, X. He, D. Wang, T. Chen, Q. Huang, Y. C. Li, H. Wang, S. H. Kim, T. E. Mallouk and D. Wang, Nat. Mater., 2019, 18, 384–389 CrossRef CAS PubMed.
- S. Wenzel, D. A. Weber, T. Leichtweiss, M. R. Busche, J. Sann and J. Janek, Solid State Ionics, 2016, 286, 24–33 CrossRef CAS.
- H. Zhong, L. Sang, F. Ding, J. Song and Y. Mai, Electrochim. Acta, 2018, 277, 268–275 CrossRef CAS.
- Y. Zhu, X. He and Y. Mo, ACS Appl. Mater. Interfaces, 2015, 7, 23685–23693 CrossRef CAS PubMed.
- Z. Hou, J. Zhang, W. Wang, Q. Chen, B. Li and C. Li, J. Energy Chem., 2020, 45, 7–17 CrossRef.
- A. Manthiram, ACS Cent. Sci., 2017, 3, 1063–1069 CrossRef CAS PubMed.
- D. E. Fenton, J. M. Parker and P. V. Wright, Polymer, 1973, 14, 589 CrossRef CAS.
- R. Xue, H. Huang, L. Chen and K. Wang, Solid State Ionics, 1993, 59, 1–4 CrossRef CAS.
- G. Dautzenberg, F. Croce, S. Passerini and B. Scrosati, Chem. Mater., 1994, 6, 538–542 CrossRef CAS.
- O. Bohnke, G. Frand, M. Rezrazi, C. Rousselot and C. Truche, Solid State Ionics, 1993, 66, 97–104 CrossRef CAS.
- O. Bohnke, G. Frand, M. Rezrazi, C. Rousselot and C. Truche, Solid State Ionics, 1993, 66, 105–112 CrossRef CAS.
- Y. Saito, H. Kataoka, E. Quartarone and P. Mustarelli, J. Phys. Chem. B, 2002, 106, 7200–7204 CrossRef CAS.
- V. Arcella, A. Sanguineti, E. Quartarone and P. Mustarelli, J. Power Sources, 1999, 81–82, 790–794 CrossRef CAS.
- X. Yu, J. Electrochem. Soc., 1997, 144, 524 CrossRef CAS.
- G. Y. Adachi, N. Imanaka and S. Tamura, Chem. Rev., 2002, 102, 2405–2429 CrossRef CAS PubMed.
- B. E. Francisco, C. R. Stoldt and J. C. M'Peko, Chem. Mater., 2014, 26, 4741–4749 CrossRef CAS.
- J. Płcharski and W. Weiczorek, Solid State Ionics, 1988, 28–30, 979–982 CrossRef.
- F. Zheng, M. Kotobuki, S. Song, M. O. Lai and L. Lu, J. Power Sources, 2018, 389, 198–213 CrossRef CAS.
- X. Huang, C. Liu, Y. Lu, T. Xiu, J. Jin, M. E. Badding and Z. Wen, J. Power Sources, 2018, 382, 190–197 CrossRef CAS.
- K. Fu, Y. Gong, B. Liu, Y. Zhu, S. Xu, Y. Yao, W. Luo, C. Wang, S. D. Lacey, J. Dai, Y. Chen, Y. Mo, E. Wachsman and L. Hu, Sci. Adv., 2017, 3, e1601659 CrossRef PubMed.
- G. T. Hitz, D. W. McOwen, L. Zhang, Z. Ma, Z. Fu, Y. Wen, Y. Gong, J. Dai, T. R. Hamann, L. Hu and E. D. Wachsman, Mater. Today, 2019, 22, 50–57 CrossRef CAS.
- N. Zhao, W. Khokhar, Z. Bi, C. Shi, X. Guo, L. Z. Fan and C. W. Nan, Joule, 2019, 3, 1190–1199 CrossRef CAS.
- B. Liu, Y. Gong, K. Fu, X. Han, Y. Yao, G. Pastel, C. Yang, H. Xie, E. D. Wachsman and L. Hu, ACS Appl. Mater. Interfaces, 2017, 9, 18809–18815 CrossRef CAS PubMed.
- R. Murugan, V. Thangadurai and W. Weppner, Angew. Chem., Int. Ed., 2007, 46, 7778–7781 CrossRef CAS PubMed.
- K. Fu, Y. Gong, T. Gregory Hitz, W. Dennis McOwen, Y. Li, S. Xu, Y. Wen, L. Zhang, C. Wang, G. Pastel, J. Dai, B. Liu, H. Xie, Y. Yao, E. D. Wachsman and L. Hu, Energy Environ. Sci., 2017, 10, 1568–1575 RSC.
- L. Chen, Y. Li, S. P. Li, L. Z. Fan, C. W. Nan and J. B. Goodenough, Nano Energy, 2018, 46, 176–184 CrossRef CAS.
- X. Li, X. Li, J. Liang, C. Wang, J. Luo, R. Li and X. Sun, Energy Environ. Sci., 2018, 11, 2828–2832 RSC.
- Y. Zhang, R. Chen, S. Wang, T. Liu, B. Xu, X. Zhang, X. Wang, Y. Shen, Y. H. Lin, M. Li, L. Z. Fan, L. Li and C. W. Nan, Energy Storage Mater., 2020, 25, 145–153 CrossRef.
- S. Chen, D. Xie, G. Liu, J. P. Mwizerwa, Q. Zhang, Y. Zhao, X. Xu and X. Yao, Energy Storage Mater., 2018, 14, 58–74 CrossRef.
- H. D. Lim, X. Yue, X. Xing, V. Petrova, M. Gonzalez, H. Liu and P. Liu, J. Mater. Chem. A, 2018, 6, 7370–7374 RSC.
- H. Muramatsu, A. Hayashi, T. Ohtomo, S. Hama and M. Tatsumisago, Solid State Ionics, 2011, 182, 116–119 CrossRef CAS.
- R. Xu, S. Zhang, X. Wang, Y. Xia, X. Xia, J. Wu, C. Gu and J. Tu, Chem.–Eur. J., 2018, 24, 6007–6018 CrossRef CAS PubMed.
- Q. Zhang, J. P. Mwizerwa, H. Wan, L. Cai, X. Xu and X. Yao, J. Mater. Chem. A, 2017, 5, 23919–23925 RSC.
- F. Han, J. Yue, X. Fan, T. Gao, C. Luo, Z. Ma, L. Suo and C. Wang, Nano Lett., 2016, 16, 4521–4527 CrossRef CAS PubMed.
- W. Weppner and R. A. Huggins, Phys. Lett. A, 1976, 58, 245–248 CrossRef.
- E. J. Plichta, J. Electrochem. Soc., 1992, 139, 1509 CrossRef CAS.
- Y. Tomita, A. Fuji-I, H. Ohki, K. Yamada and T. Okuda, Chem. Lett., 1998, 223–224 CrossRef CAS.
- T. Asano, A. Sakai, S. Ouchi, M. Sakaida, A. Miyazaki and S. Hasegawa, Adv. Mater., 2018, 30, 1803075 CrossRef PubMed.
- X. Li, J. Liang, N. Chen, J. Luo, K. R. Adair, C. Wang, M. N. Banis, T. Sham, L. Zhang, S. Zhao, S. Lu, H. Huang, R. Li and X. Sun, Angew. Chem., Int. Ed., 2019, 131, 16579–16584 CrossRef.
- X. Li, J. Liang, J. Luo, M. Norouzi Banis, C. Wang, W. Li, S. Deng, C. Yu, F. Zhao, Y. Hu, T. K. Sham, L. Zhang, S. Zhao, S. Lu, H. Huang, R. Li, K. R. Adair and X. Sun, Energy Environ. Sci., 2019, 12, 2665–2671 RSC.
- M. Matsuo and S. Orimo, Adv. Energy Mater., 2011, 1, 161–172 CrossRef CAS.
- D. Blanchard, A. Nale, D. Sveinbjörnsson, T. M. Eggenhuisen, M. H. W. Verkuijlen, Suwarno, T. Vegge, A. P. M. Kentgens and P. E. De Jongh, Adv. Funct. Mater., 2015, 25, 184–192 CrossRef CAS.
- M. Matsuo, Y. Nakamori, S. I. Orimo, H. Maekawa and H. Takamura, Appl. Phys. Lett., 2007, 91, 224103 CrossRef.
- H. Maekawa, M. Matsuo, H. Takamura, M. Ando, Y. Noda, T. Karahashi and S. Orimo, J. Am. Chem. Soc., 2009, 131, 894–895 CAS.
- P. López-Aranguren, N. Berti, A. H. Dao, J. Zhang, F. Cuevas, M. Latroche and C. Jordy, J. Power Sources, 2017, 357, 56–60 CrossRef.
- T. Zhang, Y. Wang, S. Isobe, N. Hashimoto, Y. Kojima, T. Song, H. Miyaoka, K. Shinzato, H. Miyaoka, T. Ichikawa, S. Shi and X. Zhang, Joule, 2018, 2, 1522–1533 CrossRef CAS.
- H. T. T. Le, R. S. Kalubarme, D. T. Ngo, H. S. Jadhav and C. J. Park, J. Mater. Chem. A, 2015, 3, 22421–22431 RSC.
- C. Ma, K. Chen, C. Liang, C. W. Nan, R. Ishikawa, K. More and M. Chi, Energy Environ. Sci., 2014, 7, 1638–1642 RSC.
- V. Thangadurai, H. Kaack and W. J. F. Weppner, J. Am. Ceram. Soc., 2003, 86, 437–440 CAS.
- R. Murugan, V. Thangadurai and W. Weppner, Angew. Chem., Int. Ed., 2007, 46, 7778–7781 CrossRef CAS PubMed.
- S. Ramakumar, C. Deviannapoorani, L. Dhivya, L. S. Shankar and R. Murugan, Prog. Mater. Sci., 2017, 88, 325–411 CrossRef CAS.
- M. Murayama, R. Kanno, M. Irie, S. Ito, T. Hata, N. Sonoyama and Y. Kawamoto, J. Solid State Chem., 2002, 168, 140–148 CrossRef CAS.
- J. C. Bachman, S. Muy, A. Grimaud, H. H. Chang, N. Pour, S. F. Lux, O. Paschos, F. Maglia, S. Lupart, P. Lamp, L. Giordano and Y. Shao-Horn, Chem. Rev., 2016, 116, 140–162 CrossRef CAS PubMed.
- S. Kim, H. Oguchi, N. Toyama, T. Sato, S. Takagi, T. Otomo, D. Arunkumar, N. Kuwata, J. Kawamura and S. Orimo, Nat. Commun., 2019, 10, 1081 CrossRef PubMed.
- Y. Aihara, S. Arai and K. Hayamizu, Electrochim. Acta, 2000, 45, 1321–1326 CrossRef CAS.
- M. Faraday, Philos. Trans. R. Soc. London, 1833, 123, 23–54 CrossRef.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- About NAS Batteries|Products|NGK INSULATORS, LTD., https://www.ngk-insulators.com/en/product/nas/about/index.html, accessed 5 April 2020.
- J. H. Choi, C. H. Lee, J. H. Yu, C. H. Doh and S. M. Lee, J. Power Sources, 2015, 274, 458–463 CrossRef CAS.
- D. Lin, W. Liu, Y. Liu, H. R. Lee, P. C. Hsu, K. Liu and Y. Cui, Nano Lett., 2016, 16, 459–465 CrossRef CAS PubMed.
- S. H. S. Cheng, K. Q. He, Y. Liu, J. W. Zha, M. Kamruzzaman, R. L. W. Ma, Z. M. Dang, R. K. Y. Li and C. Y. Chung, Electrochim. Acta, 2017, 253, 430–438 CrossRef CAS.
- H. Xu, P. H. Chien, J. Shi, Y. Li, N. Wu, Y. Liu, Y. Y. Hu and J. B. Goodenough, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 18815–18821 CrossRef CAS PubMed.
- A. N. Wu, P. H. Chien, Y. Qian, Y. Li, N. S. Grundish, B. Xu, H. Jin, Y. Y. Hu, G. Yu and J. B. Goodenough, Angew. Chem., Int. Ed., 2020, 59, 4131–4137 CrossRef PubMed.
- X. Tao, Y. Liu, W. Liu, G. Zhou, J. Zhao, D. Lin, C. Zu, O. Sheng, W. Zhang, H. W. Lee and Y. Cui, Nano Lett., 2017, 17, 2967–2972 CrossRef CAS PubMed.
- P. Zhu, C. Yan, J. Zhu, J. Zang, Y. Li, H. Jia, X. Dong, Z. Du, C. Zhang, N. Wu, M. Dirican and X. Zhang, Energy Storage Mater., 2019, 17, 220–225 CrossRef.
- S. Song, Y. Wu, W. Tang, F. Deng, J. Yao, Z. Liu, R. Hu, Alamusi, Z. Wen, L. Lu and N. Hu, ACS Sustainable Chem. Eng., 2019, 7, 7163–7170 CrossRef CAS.
- S. Tang, Q. Lan, L. Xu, J. Liang, P. Lou, C. Liu, L. Mai, Y. C. Cao and S. Cheng, Nano Energy, 2020, 71, 104600 CrossRef.
- J. N. Zhang, Q. Li, C. Ouyang, X. Yu, M. Ge, X. Huang, E. Hu, C. Ma, S. Li, R. Xiao, W. Yang, Y. Chu, Y. Liu, H. Yu, X. Q. Yang, X. Huang, L. Chen and H. Li, Nat. Energy, 2019, 4, 594–603 CrossRef CAS.
- K. Nie, X. Wang, J. Qiu, Y. Wang, Q. Yang, J. Xu, X. Yu, H. Li, X. Huang and L. Chen, ACS Energy Lett., 2020, 5, 826–832 CrossRef CAS.
- J. H. Cha, P. N. Didwal, J. M. Kim, D. R. Chang and C. J. Park, J. Membr. Sci., 2020, 595, 117538 CrossRef CAS.
- X. Yu, L. Wang, J. Ma, X. Sun, X. Zhou and G. Cui, Adv. Energy Mater., 2020, 1903939 CrossRef CAS.
- Y. Zhao, C. Wu, G. Peng, X. Chen, X. Yao, Y. Bai, F. Wu, S. Chen and X. Xu, J. Power Sources, 2016, 301, 47–53 CrossRef CAS.
- K. Pan, L. Zhang, W. Qian, X. Wu, K. Dong, H. Zhang and S. Zhang, Adv. Mater., 2020, 2000399 CrossRef CAS PubMed.
- J. Auvergniot, A. Cassel, D. Foix, V. Viallet, V. Seznec and R. Dedryvère, Solid State Ionics, 2017, 300, 78–85 CrossRef CAS.
- J. Zhang, C. Zheng, J. Lou, Y. Xia, C. Liang, H. Huang, Y. Gan, X. Tao and W. Zhang, J. Power Sources, 2019, 412, 78–85 CrossRef CAS.
- C. Wang, K. R. Adair, J. Liang, X. Li, Y. Sun, X. Li, J. Wang, Q. Sun, F. Zhao, X. Lin, R. Li, H. Huang, L. Zhang, R. Yang, S. Lu and X. Sun, Adv. Funct. Mater., 2019, 29, 1900392 CrossRef.
- N. J. Dudney, J. B. Bates, R. A. Zuhr, C. F. Luck and J. D. Robertson, Solid State Ionics, 1992, 53–56, 655–661 CrossRef CAS.
- H. Duan, Y. X. Yin, Y. Shi, P. F. Wang, X. D. Zhang, C. P. Yang, J. L. Shi, R. Wen, Y. G. Guo and L. J. Wan, J. Am. Chem. Soc., 2018, 140, 82–85 CrossRef CAS PubMed.
- M. Du, K. Liao, Q. Lu and Z. Shao, Energy Environ. Sci., 2019, 12, 1780–1804 RSC.
- A. Jena, Y. Meesala, S. F. Hu, H. Chang and R. S. Liu, ACS Energy Lett., 2018, 3, 2775–2795 CrossRef CAS.
- Y. Shao, H. Wang, Z. Gong, D. Wang, B. Zheng, J. Zhu, Y. Lu, Y. S. Hu, X. Guo, H. Li, X. Huang, Y. Yang, C. W. Nan and L. Chen, ACS Energy Lett., 2018, 3, 1212–1218 CrossRef CAS.
- W. Feng, X. Dong, X. Zhang, Z. Lai, P. Li, C. Wang, Y. Wang and Y. Xia, Angew. Chem., Int. Ed., 2020, 59, 5346–5349 CrossRef CAS PubMed.
- C. Wang, Y. Yang, X. Liu, H. Zhong, H. Xu, Z. Xu, H. Shao and F. Ding, ACS Appl. Mater. Interfaces, 2017, 9, 13694–13702 CrossRef CAS PubMed.
- H. Huo, Y. Chen, J. Luo, X. Yang, X. Guo and X. Sun, Adv. Energy Mater., 2019, 9, 1804004 CrossRef.
- S. Kalnaus, A. S. Sabau, W. E. Tenhaeff, N. J. Dudney and C. Daniel, J. Power Sources, 2012, 201, 280–287 CrossRef CAS.
- A. Li, X. Liao, H. Zhang, L. Shi, P. Wang, Q. Cheng, J. Borovilas, Z. Li, W. Huang, Z. Fu, M. Dontigny, K. Zaghib, K. Myers, X. Chuan, X. Chen and Y. Yang, Adv. Mater., 2020, 32, 1905517 CrossRef CAS PubMed.
- X. Wang, H. Zhai, B. Qie, Q. Cheng, A. Li, J. Borovilas, B. Xu, C. Shi, T. Jin, X. Liao, Y. Li, X. He, S. Du, Y. Fu, M. Dontigny, K. Zaghib and Y. Yang, Nano Energy, 2019, 60, 205–212 CrossRef CAS.
- M. J. Palmer, S. Kalnaus, M. B. Dixit, A. S. Westover, K. B. Hatzell, N. J. Dudney and X. C. Chen, Energy Storage Mater., 2020, 26, 242–249 CrossRef.
- M. Huang, T. Liu, Y. Deng, H. Geng, Y. Shen, Y. Lin and C. W. Nan, Solid State Ionics, 2011, 204–205, 41–45 CrossRef CAS.
- K. Tadanaga, R. Takano, T. Ichinose, S. Mori, A. Hayashi and M. Tatsumisago, Electrochem. Commun., 2013, 33, 51–54 CrossRef CAS.
- R. Takano, K. Tadanaga, A. Hayashi and M. Tatsumisago, Solid State Ionics, 2014, 255, 104–107 CrossRef CAS.
- N. C. Rosero-Navarro, T. Yamashita, A. Miura, M. Higuchi and K. Tadanaga, Solid State Ionics, 2016, 285, 6–12 CrossRef CAS.
- R. H. Shin, S. I. Son, Y. S. Han, Y. Do Kim, H. T. Kim, S. S. Ryu and W. Pan, Solid State Ionics, 2017, 301, 10–14 CrossRef CAS.
- M. H. Braga, N. S. Grundish, A. J. Murchison and J. B. Goodenough, Energy Environ. Sci., 2017, 10, 331–336 RSC.
- X. Lü, J. W. Howard, A. Chen, J. Zhu, S. Li, G. Wu, P. Dowden, H. Xu, Y. Zhao and Q. Jia, Adv. Sci., 2016, 3, 1500359 CrossRef PubMed.
- Y. Tian, F. Ding, H. Zhong, C. Liu, Y. B. He, J. Liu, X. Liu and Q. Xu, Energy Storage Mater., 2018, 14, 49–57 CrossRef.
- R. Mohtadi and S. Orimo, Nat. Rev. Mater., 2016, 2, 16091 CrossRef.
- S. Orimo, Y. Nakamori, J. R. Eliseo, A. Zu and C. M. Jensen, Chem. Rev., 2007, 107, 4111–4132 CrossRef CAS PubMed.
- A. Unemoto, T. Ikeshoji, S. Yasaku, M. Matsuo, V. Stavila, T. J. Udovic and S.-I. Orimo, Chem. Mater., 2015, 27, 5407–5416 CrossRef CAS.
- F. Mo, J. Ruan, S. Sun, Z. Lian, S. Yang, X. Yue, Y. Song, Y. Zhou, F. Fang, G. Sun, S. Peng and D. Sun, Adv. Energy Mater., 2019, 9, 1902123 CrossRef CAS.
- A. Borgschulte, R. Gremaud, S. Kato, N. P. Stadie, A. Remhof, A. Züttel, M. Matsuo and S.-I. Orimo, Appl. Phys. Lett., 2010, 97, 031916 CrossRef.
- K. Kisu, S. Kim, H. Oguchi, N. Toyama and S. Orimo, J. Power Sources, 2019, 436, 226821 CrossRef CAS.
- P. Ngene, P. Adelhelm, A. M. Beale, K. P. De Jong and P. E. De Jongh, J. Phys. Chem. C, 2010, 114, 6163–6168 CrossRef CAS.
- S. Das, P. Ngene, P. Norby, T. Vegge, P. E. De Jongh and D. Blanchard, J. Electrochem. Soc., 2016, 163, 2029–2034 CrossRef.
- F. Lu, Y. Pang, M. Zhu, F. Han, J. Yang, F. Fang, D. Sun, S. Zheng and C. Wang, Adv. Funct. Mater., 2019, 1809219 CrossRef.
- X. He, Y. Zhu and Y. Mo, Nat. Commun., 2017, 8, 1–7 CrossRef PubMed.
- A. El kharbachi, Y. Hu, K. Yoshida, P. Vajeeston, S. Kim, M. H. Sørby, S. Orimo, H. Fjellvåg and B. C. Hauback, Electrochim. Acta, 2018, 278, 332–339 CrossRef CAS.
- A. Unemoto, H. Wu, T. J. Udovic, M. Matsuo, T. Ikeshoji and S. I. Orimo, Chem. Commun., 2016, 52, 564–566 RSC.
- W. D. Richards, L. J. Miara, Y. Wang, J. C. Kim and G. Ceder, Chem. Mater., 2016, 28, 266–273 CrossRef CAS.
- Y. Pang, X. Wang, X. Shi, F. Xu, L. Sun, J. Yang and S. Zheng, Adv. Energy Mater., 2020, 10, 1902795 CrossRef CAS.
- Y. Yan, J. B. Grinderslev, Y.-S. Lee, M. Jørgensen, Y. W. Cho, R. Černý and T. R. Jensen, Chem. Commun., 2020, 56, 3971–3974 RSC.
- H. Liu, Z. Ren, X. Zhang, J. Hu, M. Gao, H. Pan and Y. Liu, Chem. Mater., 2020, 32, 671–678 CrossRef CAS.
- J. A. Teprovich, H. R. Colón-Mercado, P. A. Ward, B. Peters, S. Giri, J. Zhou, S. Greenway, R. N. Compton, P. Jena and R. Zidan, J. Phys. Chem. C, 2014, 118, 21755–21761 CrossRef CAS.
- M. N. Guzik, R. Mohtadi and S. Sartori, J. Mater. Res., 2019, 34, 877–904 CrossRef CAS.
- L. Zhan, Y. Zhang, X. Zhuang, H. Fang, Y. Zhu, X. Guo, J. Chen, Z. Wang and L. Li, Solid State Ionics, 2017, 304, 150–155 CrossRef CAS.
- Y. Zhang, L. Zhan, X. Zhuang, Y. Zhu, N. Wan, X. Guo, J. Chen, Z. Wang and L. Li, J. Alloys Compd., 2017, 695, 2894–2901 CrossRef CAS.
- A. Pradel and M. Ribes, Solid State Ionics, 1986, 18–19, 351–355 CrossRef CAS.
- S. Kondo, K. Takada and Y. Yamamura, Solid State Ionics, 1992, 53–56, 1183–1186 CrossRef CAS.
- K. Takada, T. Inada, A. Kajiyama, H. Sasaki, S. Kondo, M. Watanabe, M. Murayama and R. Kanno, Solid State Ionics, 2003, 158, 269–274 CrossRef CAS.
- T. Inada, T. Kobayashi, N. Sonoyama, A. Yamada, S. Kondo, M. Nagao and R. Kanno, J. Power Sources, 2009, 194, 1085–1088 CrossRef CAS.
- J. M. Whiteley, P. Taynton, W. Zhang and S.-H. Lee, Adv. Mater., 2015, 27, 6922–6927 CrossRef CAS PubMed.
- B. Chen, Z. Huang, X. Chen, Y. Zhao, Q. Xu, P. Long, S. Chen and X. Xu, Electrochim. Acta, 2016, 210, 905–914 CrossRef CAS.
- S. Wang, X. Zhang, S. Liu, C. Xin, C. Xue, F. Richter, L. Li, L. Fan, Y. Lin, Y. Shen, J. Janek and C. W. Nan, J. Materiomics, 2020, 6, 70–76 CrossRef.
- J. Zheng, P. Wang, H. Liu and Y. Y. Hu, ACS Appl. Energy Mater., 2019, 2, 1452–1459 CrossRef CAS.
- L. Cong, Y. Li, W. Lu, J. Jie, Y. Liu, L. Sun and H. Xie, J. Power Sources, 2020, 446, 227365 CrossRef CAS.
- A. Hayashi, T. Harayama, F. Mizuno and M. Tatsumisago, J. Power Sources, 2006, 163, 289–293 CrossRef CAS.
- Z. D. Hood, H. Wang, Y. Li, A. S. Pandian, M. Parans Paranthaman and C. Liang, Solid State Ionics, 2015, 283, 75–80 CrossRef CAS.
- E. Rangasamy, G. Sahu, J. K. Keum, A. J. Rondinone, N. J. Dudney and C. Liang, J. Mater. Chem. A, 2014, 2, 4111–4116 RSC.
- A. Yamauchi, A. Sakuda, A. Hayashi and M. Tatsumisago, J. Power Sources, 2013, 244, 707–710 CrossRef CAS.
- B. Kubíková, J. Mlynáriková, O. Beneš, E. Mikšíková, J. Priščák, A. Tosolin and M. Boča, J. Mol. Liq., 2018, 268, 754–761 CrossRef.
- N. Garg, A. K. Mishra, H. K. Poswal, A. K. Tyagi and S. M. Sharma, J. Solid State Chem., 2015, 229, 164–172 CrossRef CAS.
- S. Muy, J. Voss, R. Schlem, R. Koerver, S. J. Sedlmaier, F. Maglia, P. Lamp, W. G. Zeier and Y. Shao-Horn, iScience, 2019, 16, 270–282 CrossRef CAS PubMed.
- R. Schlem, S. Muy, N. Prinz, A. Banik, Y. Shao-Horn, M. Zobel and W. G. Zeier, Adv. Energy Mater., 2020, 10, 1903719 CrossRef CAS.
- X. Li, J. Liang, N. Chen, J. Luo, K. R. Adair, C. Wang, M. N. Banis, T. Sham, L. Zhang, S. Zhao, S. Lu, H. Huang, R. Li and X. Sun, Angew. Chem., 2019, 131, 16579–16584 CrossRef.
- K. H. Park, K. Kaup, A. Assoud, Q. Zhang, X. Wu and L. F. Nazar, ACS Energy Lett., 2020, 5, 533–539 CrossRef CAS.
- K. Yamada, K. Kumano and T. Okuda, Solid State Ionics, 2006, 177, 1691–1695 CrossRef CAS.
- S. Wang, Q. Bai, A. M. Nolan, Y. Liu, S. Gong, Q. Sun and Y. Mo, Angew. Chem., Int. Ed., 2019, 58, 8039–8043 CrossRef CAS PubMed.
- J. Liang, X. Li, S. Wang, K. R. Adair, W. Li, Y. Zhao, C. Wang, Y. Hu, L. Zhang, S. Zhao, S. Lu, H. Huang, R. Li, Y. Mo and X. Sun, J. Am. Chem. Soc., 2020, 142, 7012–7022 CrossRef CAS PubMed.
- A. R. Polu, D. K. Kim and H. W. Rhee, Ionics, 2015, 21, 2771–2780 CrossRef CAS.
- A. R. Polu and H. W. Rhee, Int. J. Hydrogen Energy, 2017, 42, 7212–7219 CrossRef CAS.
- E. Quartarone and P. Mustarelli, Chem. Soc. Rev., 2011, 40, 2525–2540 RSC.
- Y.-S. Hu, Nat. Energy, 2016, 1, 1–2 Search PubMed.
- Z. Zou, Y. Li, Z. Lu, D. Wang, Y. Cui, B. Guo, Y. Li, X. Liang, J. Feng, H. Li, C. W. Nan, M. Armand, L. Chen, K. Xu and S. Shi, Chem. Rev., 2020, 120, 4169–4221 CrossRef CAS PubMed.
- X. Li, J. Liang, X. Yang, K. R. Adair, C. Wang, F. Zhao and X. Sun, Energy Environ. Sci., 2020, 13, 1429–1461 RSC.
- J. Zheng, M. Tang and Y.-Y. Hu, Angew. Chem., Int. Ed., 2016, 55, 12538–12542 CrossRef CAS PubMed.
- L. Yang, Z. Wang, Y. Feng, R. Tan, Y. Zuo, R. Gao, Y. Zhao, L. Han, Z. Wang and F. Pan, Adv. Energy Mater., 2017, 7, 1–9 Search PubMed.
- C. Monroe and J. Newman, J. Electrochem. Soc., 2004, 151, A880–A886 CrossRef CAS.
- Z. Ahmad and V. Viswanathan, Phys. Rev. Lett., 2017, 119, 056003 CrossRef PubMed.
- C. Monroe and J. Newman, J. Electrochem. Soc., 2003, 150, A1377–A1384 CrossRef CAS.
- P. Albertus, S. Babinec, S. Litzelman and A. Newman, Nat. Energy, 2018, 3, 16–21 CrossRef CAS.
- C. Fu, V. Venturi, J. Kim, Z. Ahmad, A. W. Ells, V. Viswanathan and B. A. Helms, Nat. Mater., 2020, 19, 758–766 CrossRef CAS.
- M. B. Dixit, M. Regala, F. Shen, X. Xiao and K. B. Hatzell, ACS Appl. Mater. Interfaces, 2019, 11, 2022–2030 CrossRef CAS PubMed.
- F. Shen, M. B. Dixit, X. Xiao and K. B. Hatzell, ACS Energy Lett., 2018, 3, 1056–1061 CrossRef CAS.
- M. J. Zachman, Z. Tu, S. Choudhury, L. A. Archer and L. F. Kourkoutis, Nature, 2018, 560, 345–349 CrossRef CAS PubMed.
- J. Zheng and Y. Y. Hu, ACS Appl. Mater. Interfaces, 2018, 10, 4113–4120 CrossRef CAS PubMed.
- M. B. Dixit, W. Zaman, N. Hortance, S. Vujic, B. Harkey, F. Shen, W. Y. Tsai, V. De Andrade, X. C. Chen, N. Balke and K. B. Hatzell, Joule, 2020, 4, 207–221 CrossRef CAS.
- R. Chen, Q. Li, X. Yu, L. Chen and H. Li, Chem. Rev., 2020, 120(14), 6820–6877 CrossRef CAS PubMed.
- R. Chen, A. M. Nolan, J. Lu, J. Wang, X. Yu, Y. Mo, L. Chen, X. Huang and H. Li, Joule, 2020, 4, 812–821 CrossRef CAS.
- Z. Fan, B. Ding, T. Zhang, Q. Lin, V. Malgras, J. Wang, H. Dou, X. Zhang and Y. Yamauchi, Small, 2019, 15, 1903952 CrossRef CAS PubMed.
Footnote |
| † T. Z. and W. H. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |