Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Capture and displacement-based release of the bicarbonate anion by calix[4]pyrroles with small rigid straps†
Nam Jung
Heo
a,
Ju Ho
Yang
a,
Vincent M.
Lynch
b,
Byoung Joon
Ko
*c,
Jonathan L.
Sessler
 *b and
Sung Kuk
Kim
*b and
Sung Kuk
Kim
 *a
*a
aDepartment of Chemistry, Research Institute of Natural Science, Gyeongsang National University, Jinju, 660-701, Korea. E-mail: sungkukkim@gnu.ac.kr
bDepartment of Chemistry, The University of Texas at Austin, 105 E. 24th Street-Stop A5300, Austin, Texas 78712-1224, USA. E-mail: sessler@cm.utexas.edu
cNew Drug Development Center, Osong Medical Innovation Foundation, Chungbuk, Korea 28160. E-mail: kobjoon@kbiohealth.kr
First published on 24th July 2020
Abstract
Two-phenoxy walled calix[4]pyrroles 1 and 2 strapped with small rigid linkers containing pyridine and benzene, respectively, have been synthesized. 1H NMR spectroscopic analyses carried out in CDCl3 revealed that both of receptors 1 and 2 recognize only F− and HCO3− among various test anions with high preference for HCO3− (as the tetraethylammonium, TEA+ salt) relative to F− (as the TBA+ salt). The bound HCO3− anion was completely released out of the receptors upon the addition of F− (as the tetrabutylammonium, TBA+ salt) as a result of significantly enhanced affinities and selectivities of the receptors for F− once converted to the TEAHCO3 complexes. Consequently, relatively stable TEAF complexes of receptors 1 and 2 were formed via anion metathesis occurring within the receptor cavities. By contrast, the direct addition of TEAF to receptors 1 and 2 produces different complexation products initially, although eventually the same TEAF complexes are produced as via sequential TEAHCO3 and TBAF addition. These findings are rationalized in terms of the formation of different ion pair complexes involving interactions both inside and outside of the core receptor framework.
Introduction
An increasing appreciation of the role played by anions in a variety of biological and environmental systems, as well as a number of industrial processes, has made a variety of anions specific targets for selective recognition.1–3 Among such anions, small, basic anions such as the bicarbonate anion (HCO3−) and the fluoride anion (F−) have recently drawn particular attention. For example, the bicarbonate anion plays a critical role in physiological pH buffering and signal transduction in the human central nerve system as well as in triggering certain intracellular events.4,5 In addition, the bicarbonate anion is an essential element in the natural carbon cycle in which the bicarbonate anion largely generated from gaseous CO2 from the atmosphere acts as a key component in regulating and maintaining the pH of aquatic environments; it is thus necessary for a wide range of living organisms to survive.6 On the other hand, the fluoride anion plays a role in public health and is key to a number of chemical processes.7–9 In spite of their importance, only a handful of anion receptors capable of recognizing these basic anions with near-complete selectivity have been reported.10–13 Moreover, to our knowledge no receptors are known whose selectivity and inherent affinity for these two specific anions, e.g., the bicarbonate and fluoride anion, can be reversed by ion pairing effects. Here we report the synthesis of bicarbonate-selective receptors and the reversal of their inherent selectivity through ion pairing. As detailed below, this allows the bicarbonate anion to be bound and then released by treating with an appropriately chosen fluoride anion salt.The design of a receptor with high selectivity for relatively basic anions, such as the bicarbonate anion or the fluoride anion, is a recognized challenge in the anion binding community.10–12 Good size matching between the receptor and the anion, as well as appropriate spatial preorganization and orientation of the anion binding motifs within the receptor are time-honoured approaches to achieving selectivity.1–3,10–13 Preorganized geometries and structural rigidity can also contribute to the preparation of receptors targeting specific anions.1–3,10–13 Finally, the presence of one or more auxiliary hydrogen bond donors or acceptors within the receptor framework can impart selectivity for oxoanions, such as bicarbonate, by supporting additional interactions with the anions.13 In aggregate, these design considerations lead us to suggest that calix[4]pyrrole, a tetrapyrrolic macrocyclic compound featuring sp3-hybridized meso-carbons, would be an attractive framework for preparing anion receptors selective for the bicarbonate anion or the fluoride anion. Calix[4]pyrroles are relatively easy to functionalize and modify synthetically.14 For instance, both the meso-carbon atoms and the β-pyrrolic carbon atoms of the calix[4]pyrrole skeleton can be substituted with various functional groups.14 A particularly appealing set of calix[4]pyrroles are the so-called strapped calix[4]pyrroles, wherein the two diagonally opposing meso carbons are linked by a tether that can be used to introduce functional groups or modulate the size and rigidity of the binding cavity.14c,g For example, strapped calix[4]pyrroles bearing ancillary hydrogen bond donors on the strap show enhanced affinity for specific anions while those strapped with a small or rigid bridging element are selective for relatively small anions.13–15 Taking this into consideration, we designed and synthesized the HCO3−-selective anion receptors 1 and 2. These systems consist of a calix[4]pyrrole with small rigid straps that connect two diametrical phenol walls via pyridine and benzene linkers, respectively. As detailed below, receptors 1 and 2 in their ion free forms are able to recognize both the bicarbonate anion (as the TEA+ salt) and the fluoride anion (as the TBA+ salt) among various test anions in CDCl3 with higher affinity being seen for the former anion. Counterintuitively, as compared with the ion-free forms of receptors 1 and 2, their HCO3− complexes were found to bind the fluoride anion (as the corresponding TBA+ salt) with remarkably enhanced affinity. As a result, the bound bicarbonate anion is released from receptors 1 and 2 upon the addition of the fluoride anion (as the TBA+ salt) via anion metathesis. In contrast, the direct addition of TEAF to receptors 1 and 2 produces different complexation products initially, although eventually the same TEAF complexes are produced as generated via sequential TEAHCO3 and TBAF addition. These findings are explained in terms of ion pairing effects, which are operative both inside and outside of the receptor framework.
Results and discussion
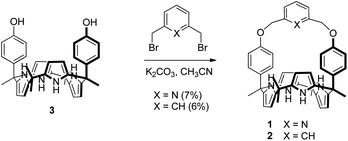
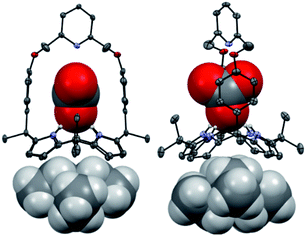
Receptors 1 and 2 were prepared by following the synthetic procedure depicted in Scheme 1. In contrast to the general strategy adopted for the synthesis of a strapped calix[4]pyrrole, receptors 1 and 2 were synthesized by installing the strap units linking the diagonal meso carbons in the final step. Briefly, cis-diphenolic calix[4]pyrrole 3 was synthesized following a literature procedure via the [2 + 2] condensation reaction of a meso-phenol substituted dipyrromethane with acetone in the presence of BF3·OEt2 as a Lewis acid. It was separated from the reaction mixture, which also contains the corresponding trans-isomer, by column chromatography.16 Subsequently, compound 3 was subjected to reaction with 2,6-bis(bromomethyl)pyridine and 1,3-bis(bromomethyl)benzene in the presence of K2CO3 in acetonitrile to give the strapped calix[4]pyrroles 1 and 2, respectively. Receptors 1 and 2 were fully characterized by standard spectroscopic means, such as 1H and 13C NMR spectroscopy and high resolution mass spectrometry (HRMS). The structure of receptor 1 was further confirmed by a single crystal X-ray diffraction analysis (Fig. 1). Single crystals of receptor 1 suitable for X-ray diffraction analysis were grown by subjecting a chloroform/methanol mixture containing receptor 1 to slow evaporation. The resulting crystal structure revealed that in the solid state the calix[4]pyrrole subunit of receptor 1 exists in the 1,3-alternate conformation with no solvent molecules found to interact with the receptor (Fig. 1).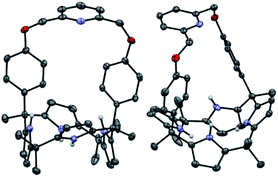 | ||
| Fig. 1 Two different views of the single X-ray crystal structure of receptor 1. Thermal ellipsoids are scaled to the 50% probability level. Most hydrogen atoms are omitted for clarity. | ||
The ability of receptors 1 and 2 to bind certain anions was assessed initially by means of 1H NMR spectroscopic studies carried out in CDCl3. When the receptors were treated with various anions (>10 equiv.) including F−, Cl−, Br−, I−, HCO3−, H2PO4−, HSO4−, and SO42−, only the F− and HCO3− anions caused the proton signals of receptors 1 and 2 to undergo chemical shift changes consistent with anion binding to the respective calix[4]pyrrole subunits (Fig. S1 and S2†). We thus infer that both receptors (1 and 2) bind F− and HCO3− selectively over other anions (Fig. S1 and S2†). This presumed selectivity is attributed to the small cavity size and the rigidity of the straps within receptors 1 and 2. It is important to note that in these studies all anions other than bicarbonate were used in the form of their respective tetrabutylammonium (TBA+) salts. The TBA+ salt of bicarbonate is not commercially available and our attempts to prepare and purify it proved unsuccessful, perhaps as a consequence of facile Hofmann elimination. Thus, the tetraethylammonium (TEA+) salt of HCO3− was used in the present study.
In order to obtain further insights into the anion binding features of receptors 1 and 2, detailed 1H NMR spectral titrations were performed with F− (as its TBA+ salt) and HCO3− (as its TEA+ salt) in CDCl3. For example, when receptor 1 was subjected to titration with F− and HCO3−, respectively, two sets of distinguishable signals were seen for the all observable protons in the 1H NMR spectra before saturation was reached (at ca. 2.62 and ca. 2.06 equiv. for F− and HCO3−, respectively; Fig. S3 and S4†). These peaks are attributable to the anion-free and the anion-bound forms of receptor 1, respectively. This leads to the conclusion that the corresponding association/dissociation equilibria are slow on the 1H NMR time scale, as typically seen for calix[4]pyrrole with high anion affinities. The presumed strong binding interactions between receptor 1 and F− and HCO3− were further supported by the observation of anion-induced chemical shift changes in the proton signals of receptor 1. For instance, upon the exposure of receptor 1 to F−, the pyrrolic NH proton signal in the 1H NMR spectrum initially appearing as a singlet at δ ≈ 6.83 ppm undergoes a remarkable downfield shift to δ ≈ 12.56 ppm (Δδ ≈ 5.73 ppm) and becomes split into a doublet (J = 44.6 Hz), as would be expected for a relatively symmetric system with 19F–1H coupling (Fig. S3†). In contrast, addition of F− causes the aromatic proton signals corresponding to the phenoxy walls (Hd) and β-pyrrolic protons (He) to move towards higher field (Δδ ≈ 0.13 ppm for Hd and Δδ ≈ 0.19 ppm for He, respectively) (Fig. S3†). Similar 1H NMR spectral changes were seen when receptor 1 was titrated with HCO3−, although a small quantity of HCO3− with respect to F− was needed to achieve saturation (Fig. S4†). On the basis of these 1H NMR spectral titrations, the binding constants (Ka) corresponding to the interaction of receptor 1 with F− and HCO3− were calculated to be approximately 240 and 1440 M−1, respectively (Table 1).17
| Guest | Host | |||
|---|---|---|---|---|
| 1 | 2 | 1·TEAHCO3 | 2·TEAHCO3 | |
| TBAF | 240 | 910 | 3330 | 5450 |
| TEAF | 380 | 980 | 340 | 430 |
| TEAHCO3 | 1440 | 2300 | — | — |
The relatively high selectivity of receptor 1 for HCO3− over F− is attributed, in part, to the fact that the counter cation, TEA+, used in the case of HCO3− possesses a relatively high charge density with respect to the TBA+ cation employed in the case of F−. For the bicarbonate complex, the TEA+ cation was believed to be co-bound with the HCO3− anion and to reside in the cone-shaped electron rich calix[4]pyrrole pocket leading to formation a receptor-separated ion pair complex.18 This presumption was supported by the finding that the proton signals of the TEA+ counter cation underwent an appreciable upfield shift when receptor 1 was subject to titration with TEAHCO3 (Fig. S4 and cf. Fig. S5†).
Further evidence for the proposed binding mode of receptor 1 with TEAHCO3 came from a single crystal X-ray diffraction analysis (Fig. 2). Single crystals suitable for such analyses were grown by subjecting a chloroform/acetonitrile solution containing receptor 1 and an excess of TEAHCO3 to slow evaporation. The resulting crystal structure revealed that one oxygen atom of the HCO3− anion forms hydrogen-bonds with all four NH protons of the calix[4]pyrrole unit at distances ranging from 2.74 and 2.95 Å (N⋯O interactions) (Fig. 2). As expected, the TEA+ cation is encapsulated by the electron rich calix[4]pyrrole cavity locked in the cone conformation presumably via electrostatic interactions (Fig. 2).
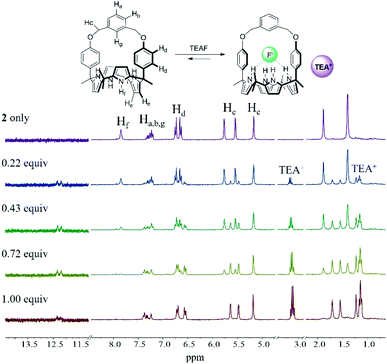
In analogy to what was seen with receptor 1, receptor 2 (capped with benzene in lieu of pyridine) is able to recognize F− and HCO3−via slow anion binding/release equilibria in CDCl3 (Fig. 3 and S7†). The binding constants corresponding to the interaction of receptor 2 with F− and HCO3− were estimated to be Ka = 910 M−1 and Ka = 2300 M−1, respectively (Table 1).17 These values were taken as an indication that receptor 2 is also selective for TEAHCO3 over TBAF.
The relatively high Ka values seen for HCO3− and F− in the case of receptor 2 as compared to receptor 1 are presumed to result from structural differences in the strap subunits. In the case of 1, electron repulsion between the bound anions and the nitrogen lone pair electrons of the pyridine strap would be expected. In contrast, in the case of receptor 2 and HCO3−, an additional hydrogen bonding interaction between the anion and the aryl C–H proton (Hg) on the strap of receptor 2 should contribute to an enhancement in the binding affinity.19
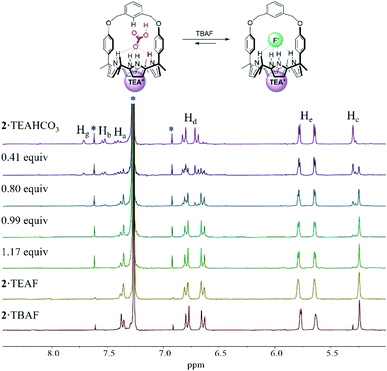
In order to evaluate further the selectivity of receptors 1 and 2 for TEAHCO3 relative to TBAF and vice versa, we also quantified their affinity for F− in the presence of an excess quantity of HCO3− (as its TEA+ salt) by 1H NMR spectroscopic titrations. For instance, when the TEAHCO3 complex of receptor 2 (2·TEAHCO3) was titrated with F− (as its TBA+ salt) in CDCl3, all proton signals corresponding to the complex of 2·TEAHCO3 gradually disappeared in the 1H NMR spectrum giving rise, at the same time, to a new set of proton resonances consistent with the F− complex of receptor 2 (Fig. 4 and S8†). These findings lead us to suggest that under these conditions the bound HCO3− anion is released from receptor 2 and replaced by the added F− anion via a slow exchange equilibrium to produce the corresponding 2·TEAF complex in quantitative yield. The Ka value corresponding to the binding of F− to the preformed TEAHCO3 complex of receptor 2 was enhanced by 6-fold and calculated to be ca. 5450 M−1 in CDCl3, which is remarkably high compared with what was seen when TBAF was added directly to 2 in its ion-free form (vide supra and Table 1).17 In a similar way, the HCO3− anion complexed with receptor 1 was also readily released from the receptor cavity upon the addition of the F− anion with attendant formation of the corresponding TEAF complex (1·TEAF). Again, this finding leads us to suggest that the preformed TEAHCO3 complex (1·TEAHCO3) is able to bind the F− anion with a 14-fold enhanced affinity relative to the corresponding ion free form (Fig. S9† and Table 1).
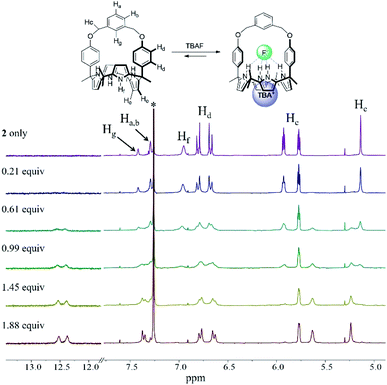 | ||
| Fig. 3 Partial 1H NMR spectra recorded during the titration of 2 (3 mM) with tetrabutylammonium fluoride (TBAF) in CDCl3. *Denotes the residual CHCl3 peak from the NMR solvent. | ||
The enhancement in the Ka value is rationalized in terms of F− binding to receptors 1 and 2 being enhanced by strong ion pairing with the pre-bound TEA+ cation (i.e., the counter cation added with the initial HCO3− anion). The tetraethylammonium cation with its relatively high charge density is expected to form stronger receptor-separated ion pair complexes than its TBA+ congener thus enhancing the apparent fluoride anion affinity (for a discussion of the ion pairing between F− and TEA+ and the receptors of this study, see Section S1 in the ESI†). The net result of this ion pairing is anion metathesis between the initially bound HCO3− anion and the added F− anion within the strapped cavities of these two receptors. Also contributing to an increase in the Ka value is the fact that pre-complexation of TEAHCO3 serves to lock the calix[4]pyrrole moiety into the cone conformation favourable for anion binding. (For a discussion of TEA+ cation interactions with the cavity formed when calix[4]pyrrole 2 is locked in its cone conformation, see Section S2 in the ESI†).
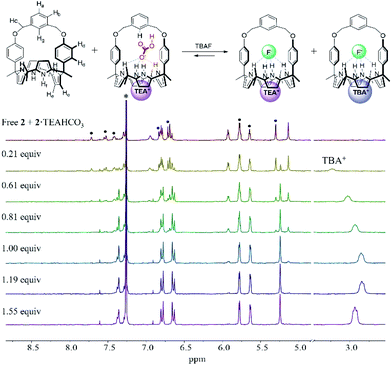
Further evidence that receptors 1 and 2 bind the fluoride anion via this proposed anion metathesis with remarkably improved efficiency came from 1H NMR titration experiments with the fluoride anion involving the use of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of the TEAHCO3 complexes and the anion free forms of receptors 1 and 2 (Fig. 5 and S10†). The mixtures were prepared by adding TEAHCO3 (0.6 equiv.) to CDCl3 solutions of receptors 1 and 2, respectively. In the resulting 1H NMR spectra, all observable proton signals appeared as two separate sets in nearly 1
1 mixtures of the TEAHCO3 complexes and the anion free forms of receptors 1 and 2 (Fig. 5 and S10†). The mixtures were prepared by adding TEAHCO3 (0.6 equiv.) to CDCl3 solutions of receptors 1 and 2, respectively. In the resulting 1H NMR spectra, all observable proton signals appeared as two separate sets in nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 integral ratio. These peaks were assigned to the anion free and TEAHCO3 complexed forms of receptors 1 and 2, respectively (Fig. 5 and S10†). When these mixtures were titrated with TBAF in CDCl3, a new set of proton signals corresponding to the fluoride complex of receptor 2 appeared, while those of its ion-free form and the TEAHCO3 complex gradually disappeared before saturation was observed upon the addition of ca. 1.2 equiv. of TBAF (Fig. 5). In this case, the TEAHCO3 complex of receptor 2 reached saturation more quickly than its ion free form (Fig. 3 and 5). Again, this finding supports our suggestions that (1) fluoride binding to receptor 2 in its cone conformation is favoured as the result of anion metathesis involving the preformed TEAHCO3 complexes and that (2) this complexation process is more favourable than the ostensibly similar, but chemically distinct, alternative of adding TBAF to the initial 1,3-alternate form of the ion-free receptor.
1 integral ratio. These peaks were assigned to the anion free and TEAHCO3 complexed forms of receptors 1 and 2, respectively (Fig. 5 and S10†). When these mixtures were titrated with TBAF in CDCl3, a new set of proton signals corresponding to the fluoride complex of receptor 2 appeared, while those of its ion-free form and the TEAHCO3 complex gradually disappeared before saturation was observed upon the addition of ca. 1.2 equiv. of TBAF (Fig. 5). In this case, the TEAHCO3 complex of receptor 2 reached saturation more quickly than its ion free form (Fig. 3 and 5). Again, this finding supports our suggestions that (1) fluoride binding to receptor 2 in its cone conformation is favoured as the result of anion metathesis involving the preformed TEAHCO3 complexes and that (2) this complexation process is more favourable than the ostensibly similar, but chemically distinct, alternative of adding TBAF to the initial 1,3-alternate form of the ion-free receptor.
TEA+ binding to the cone-shaped calix[4]pyrrole cavities of receptors 1 and 2via cation metathesis with TBA+ was also quantified by 1H NMR spectral titrations of the TBAF complexes of receptors 1 and 2 with TEAHCO3. For example, when the TBAF complexes of receptors 1 and 2 were titrated with TEAHCO3 in CDCl3, the signals corresponding to the N+CH2 protons of the TBA+ cation gradually shift to lower field before saturation is observed upon the addition of ≈1 equiv. of TEAHCO3 (Fig. S11 and S12†). By contrast, the CH3 protons of the TEA+, which resonate at ca. 1.35 ppm in the absence of a receptor (cf. Fig. S5†), are seen to resonate between 0.5 and 0.7 ppm as increasing aliquots of TEAHCO3 are added up to ≈1 equiv. Little or no changes were observed for any of the proton signals assigned to the receptors (Fig. S11 and S12†). Collectively, these findings are consistent with the TBA+ cation initially located within the calix[4]pyrrole cavity being replaced by TEA+ as it is added in the form of the counter cation to the TEAHCO3 salt used in these titrations while the fluoride anion remains fully bound (i.e., not displaced by the added HCO3− anion). The Ka values corresponding to this cation metathesis were calculated to be ca. 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 M−1 for receptor 1 and 15
960 M−1 for receptor 1 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 M−1 for receptor 2, respectively.20 By contrast, when the ion free form of receptor 2 was treated with BF4− (as the TEA+ salt), no evidence of either anion or TEA+ cation binding was observed (Fig. S13†). This is consistent with cation binding being dependent on anion binding and conversion of the initial 1,3-alternate conformation to the corresponding cone form.
150 M−1 for receptor 2, respectively.20 By contrast, when the ion free form of receptor 2 was treated with BF4− (as the TEA+ salt), no evidence of either anion or TEA+ cation binding was observed (Fig. S13†). This is consistent with cation binding being dependent on anion binding and conversion of the initial 1,3-alternate conformation to the corresponding cone form.
We also investigated the effect of the counter cation on the F− binding by receptors 1 and 2, This was done by replacing the TBA+ cation with the TEA+ cation. Two sets of separate signals corresponding to the ion-free forms and TEAF complexed forms, respectively, of receptors 1 and 2 were seen in the 1H NMR spectra upon titration with TEAF before saturation was reached upon of the addition of 2.18 equiv. and 1.72 equiv. of TEAF, respectively (Fig. S14 and S15†). Over the course of the titrations, the binding modes of the TEAF to ion pair receptors 1 and 2 were found to change from a receptor-bound contact ion pair to receptor-separated ion pair (for a discussion of the binding of the TEAF ion pair to receptors 1 and 2, see Section S3 in the ESI†). The binding constants (Ka) of receptors 1 and 2 for the F− anion as the TEA+ salt, estimated from the 1H NMR spectral titrations, were found to be 380 M−1 and 980 M−1, respectively. These values are somewhat higher than those for the corresponding TBA+ salt but are significantly reduced compared to those recorded when the preformed TEAHCO3 complexes of receptors 1 and 2 are titrated with TBAF salt (see above discussion and Table 1).18 This observation leads us to suggest that strong ion pairing occurs within the added TEAF salt and that this prevents in whole or in part the TEA+ cation from binding to the calix[4]pyrrole cavity. This reflects the greater energetics needed to separate the TEAF ion pair vs. forming a TEAF complex via addition of fluoride to a preformed TEA+ cation–receptor complex. As a result, TEAF initially binds to these receptors in the form of internal, as opposed to receptor-separated, ion pair complex. This sequence of binding events, which leads to the formation of the thermodynamically most stable TEAF complex of 2 in CDCl3, is summarised in Fig. 6.
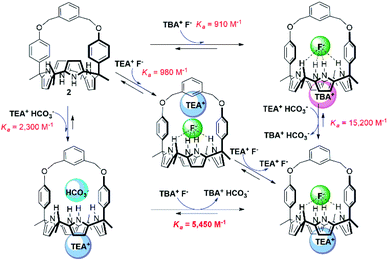 | ||
| Fig. 6 Hess diagram showing the binding modes and processes for the formation of the TEAF complex of receptor 2. | ||
In order to obtain greater insight into the release of bicarbonate from receptors 1 and 2 upon treatment with a fluoride anion source, we carried out 1H NMR spectral titrations with TEAF starting with the TEAHCO3 complexes of 1 and 2 (Fig. S17 and S18†). For these titrations, greater quantities of TEAF were necessary to achieve saturation than was required in the case of titration of the ion-free receptors with TEAF. This leads us to suggest that the binding affinities for F− (as its TEA+ salt) for the TEAHCO3 complexes of receptors 1 and 2 are decreased with respect to those of their ion-free forms (Ka = 380 M−1 for ion-free 1vs. Ka = 340 M−1 for 1·TEAHCO3 and Ka = 980 M−1 for ion-free 2vs. Ka = 430 M−1 for 2·TEAHCO3; cf.Table 1). These findings stand in striking contrast to what was seen when the TEAHCO3 complexes of receptors 1 and 2 was titrated with F− as the corresponding TBA+ salt (vide supra). Taken together, these findings provide support for the suggestions that (1) inherently strong ion pairing between TEA+ and F− outside the receptors impedes F− binding to the receptors while (2) their tendency to ion pair within the receptors favours fluoride binding. To the best of our knowledge, receptors 1 and 2 are the first examples of rationally designed receptors capable of binding ion pairs with different binding modes and affinities depending on the specific ion pair complex being formed.
In order to support the notion that tight ion pairing between TEA+ and F− could reduce the effective fluoride anion binding affinity in the case of receptors 1 and 2, we also performed 1H NMR spectral titrations involving the treatment of receptor 2 with TEAF in 10% aqueous DMSO-d6. Unfortunately, receptor 1 proved too insoluble in this medium to allow for its study. A mixture of 10% aqueous DMSO-d6 has a higher dielectric constant (εr) than CDCl3 (56.2 vs. 4.7)21 and was expected to favor dissociation of TEAF into its constituent ions. On the other hand, solvation of the individual ions was expected to increase. The balance between these competing effects was not known a priori. Flood and coworkers reported that the anion affinity of a given receptor is likely to be diminished in proportion to the solvent dielectric constant because of the solvation of the anion.21 However, when receptor 2 was subjected to titration with TEAF in 10% aqueous DMSO-d6, chemical shift changes consistent with F− anion binding were seen in the corresponding 1H NMR spectra with saturation being reached upon the addition of only ≈1 equiv. of TEAF (Fig. 7). The binding constant for this equilibrium was calculated to be Ka = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 M−1,17 a value that is significantly higher than what is seen in CDCl3 (Ka = 380 M−1). On this basis we conclude that the extent to which the TEAF fluoride anion source separates into its constituent ions, TEA+ and F−, plays a crucial role in regulating the effective anion affinities of the present set of receptors. In 10% aqueous DMSO-d6 no change in the chemical shift of the TEA+ resonances was seen as additional quantities of TEAF were added. This observation led us to suggest that in this solvent system the TEA+ cation remains solvated rather than being co-bound to the cone-shaped calix[4]pyrrole cavity (Fig. 7). We thus conclude that the effects of ion pairing both within and outside of receptors 1 and 2 play a critical role in regulating the observed overall anion binding affinities.
160 M−1,17 a value that is significantly higher than what is seen in CDCl3 (Ka = 380 M−1). On this basis we conclude that the extent to which the TEAF fluoride anion source separates into its constituent ions, TEA+ and F−, plays a crucial role in regulating the effective anion affinities of the present set of receptors. In 10% aqueous DMSO-d6 no change in the chemical shift of the TEA+ resonances was seen as additional quantities of TEAF were added. This observation led us to suggest that in this solvent system the TEA+ cation remains solvated rather than being co-bound to the cone-shaped calix[4]pyrrole cavity (Fig. 7). We thus conclude that the effects of ion pairing both within and outside of receptors 1 and 2 play a critical role in regulating the observed overall anion binding affinities.
Conclusions
In conclusion, the strapped calix[4]pyrroles 1 and 2 wherein two diametrical meso-phenol walls are linked via a rigid benzene and pyridine spacer, respectively, were found to bind the HCO3− anion (as the TEA+ salt) and the F− anion (as the TBA+ salt) with high selectivity over various other test anions in CDCl3. A higher affinity for the bicarbonate anion is seen over the fluoride anion under these conditions. In contrast, upon the addition of TBAF to the preformed TEAHCO3 complexes of receptors 1 and 2, the bound bicarbonate anion was released and replaced by the F− anion. This anion metathesis gives rise to the corresponding TEAF complexes. As compared to what is seen when TBAF is added to receptors 1 and 2 in their ion free forms, the fluoride anion binding affinities are increased when starting with the preformed TEAHCO3 complex, a result ascribed to the formation of a receptor separated F−–TEA+ ion pair complex. When receptors 1 and 2 in their ion free forms are titrated with TEA+ and F− (in the form of TEAF) TEAF complexes are formed in which the binding mode of TEA+ varies depending on the relative quantity of TEAF present with the same complex formed via anion metathesis eventually being formed. However, the observed binding affinity was unexpectedly low, a result ascribed to the TEAF salt remaining ion paired in CDCl3. The use of a more polar medium was found to increase the Ka values dramatically. The present findings provide support for the notion that ion pairing effects, occurring both inside and outside of the receptors, can play an important role in regulating the binding interactions between synthetic receptors and their targeted ions. In the present instance, these effects can be exploited to create systems that act as bicarbonate anion receptors from which HCO3− can be released via fluoride anion-mediated anion metathesis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1A4A2002831 to S. K. K.). The work in Austin was supported by the Office of Basic Energy Sciences, U.S. Department of Energy (DOE) (Grant DEFG02-01ER15186 to J. L. S.) and the Robert A. Welch Foundation (F-0018 to J. L. S.).Notes and references
- (a) J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, ed. J. F. Stoddart, Royal Society of Chemistry, Cambridge, 2006 Search PubMed; (b) A. Bianchi, K. Bowman-James and E. García-España, Supramolecular Chemistry of Anions, Wiley-VCH, New York, 1997 Search PubMed; (c) Anion Coordination Chemistry, ed. K. Bowman-James, A. Bianchi and E. García-España, Wiley-VCH, Weinheim, 2011 Search PubMed.
- (a) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS; (b) R. Martínez-Máñez and F. Sancenán, Chem. Rev., 2003, 103, 4419–4476 CrossRef PubMed; (c) P. A. Gale, N. Busschaert, C. J. E. Haynes, L. E. Karagiannidis and I. L. Kirby, Chem. Soc. Rev., 2014, 43, 205–241 RSC; (d) M. Wenzel, J. R. Hiscock and P. A. Gale, Chem. Soc. Rev., 2012, 41, 480–520 RSC; (e) P. A. Gale, Chem. Soc. Rev., 2010, 39, 3746–3771 RSC; (f) C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520–563 RSC; (g) P. A. Gale and G. Caltagirone, Chem. Soc. Rev., 2015, 44, 4212–4227 RSC.
- (a) S. O. Kang, R. A. Begum and K. Bowman-James, Angew. Chem., Int. Ed., 2006, 45, 7882–7894 CrossRef CAS PubMed; (b) S. O. Kang, J. M. Llinares, V. W. Day and K. Bowman-James, Chem. Soc. Rev., 2010, 39, 3980–4003 RSC; (c) J. H. Oh, J. H. Kim, D. S. Kim, H. J. Han, V. M. Lynch, J. L. Sessler and S. K. Kim, Org. Lett., 2019, 21, 4336–4339 CrossRef CAS PubMed.
- (a) Y. Chen, M. J. Cann, T. N. Litvin, V. Iourgenko, M. L. Sinclair, L. R. Levin and J. Buck, Science, 2000, 289, 625–628 CrossRef CAS PubMed; (b) B. J. Krieg, S. M. Taghavi, G. L. Amidon and G. E. Amidon, J. Pharm. Sci., 2014, 103, 3473–3490 CrossRef CAS PubMed; (c) R. D. Vaughan-Jones and K. W. Spitzer, Biochem. Cell Biol., 2002, 80, 579–596 CrossRef CAS PubMed; (d) A. Roos and W. F. Boron, Physiol. Rev., 1981, 61, 296–434 CrossRef CAS PubMed; (e) J. H. Zippin, L. R. Levin and J. Buck, Trends Endocrinol. Metab., 2001, 12, 366–370 CrossRef CAS PubMed.
- (a) J. R. Casey, Biochem. Cell Biol., 2006, 84, 930–939 CrossRef CAS PubMed; (b) Y. Liu, D.-Ke. Wang and L.-M. Chen, Biol. Reprod., 2012, 86, 1–13 Search PubMed.
- (a) D. M. Sigman and E. A. Boyle, Nature, 2000, 407, 859–869 CrossRef CAS PubMed; (b) N. Zeng, Adv. Atmos. Sci., 2003, 20, 677–693 CrossRef; (c) C. Heinze, S. Meyer, N. Goris, L. Anderson, R. Steinfeldt, N. Chang, C. L. Quéré and D. C. E. Bakker, Earth System Dynamics, 2015, 6, 327–358 CrossRef.
- (a) L. W. Ripa, Journal of Public Health Dentistry, 1993, 53, 17–44 CrossRef CAS PubMed; (b) M. S. McDonagh, P. F. Whiting, P. M. Wilson, A. J. Sutton, I. Chestnutt, J. Cooper, K. Misso, M. Bradley, E. Treasure and J. Kleijnen, Br. Med. J., 2000, 321, 855–859 CrossRef CAS PubMed; (c) C. Carstairs and R. Elder, Can. Hist. Rev., 2008, 89, 345–371 CrossRef; (d) P. Connet, Fluoride, 2007, 40, 155–158 Search PubMed; (e) R. G. Foulkes, Fluoride, 2007, 40, 229–237 Search PubMed; (f) R. J. Carton, Fluoride, 2006, 39, 163–172 CAS.
- (a) L. K. Kirk, Biochemistry of the Halogens and Inorganic Halides, Plenom Press. New York, 1991 CrossRef; (b) M. Kleerekoper, Endocrinol. Metab. Clin. North Am., 1998, 27, 441–452 CrossRef CAS PubMed; (c) D. Briancon, Revue du Rhumatisme, 1997, 64, 78–81 CAS.
- (a) Y. Michigami, Y. Kuroda, K. Ueda and Y. Yamamoto, Anal. Chim. Acta, 1993, 274, 299–302 CrossRef CAS; (b) P. Grandjean and P. J. Landrigan, Lancet, 2006, 368, 2167–2178 CrossRef CAS PubMed; (c) M. C. Friesen, G. Benke, A. Del Monaco, M. Dennekamp, L. Fritschi, N. de Klerk, J. L. Hoving, E. MacFarlane and M. R. Sim, Cancer, Causes Control, Pap. Symp., 2009, 20, 905–916 CrossRef PubMed; (d) E. Gazzano, L. Bergandi, C. Riganti, E. Aldieri, S. Doublier, C. Costamagna, A. Bosia and D. Ghigo, Curr. Med. Chem., 2010, 17, 2431–2441 CrossRef CAS PubMed; (e) O. Barbier, L. Arreola-Mendoza and L. M. Del Razo, Chem.-Biol. Interact., 2010, 188, 319–333 CrossRef CAS PubMed; (f) B. Spittle, Fluoride, 2011, 44, 117–124 CAS; (g) R. Sandhu, H. Lal, Z. S. Kundu and S. Kharb, Biol. Trace Elem. Res., 2011, 144, 1–5 CrossRef CAS PubMed.
- J. T. Davis, P. A. Gale, O. A. Okunola, P. Prados, J. C. Iglesias-Sánchez, T. Tomás and R. Quesada, Nat. Chem., 2009, 1, 138–144 CrossRef CAS PubMed.
- I. Suzuki, M. Ui and A. Yamauchi, J. Am. Chem. Soc., 2006, 128, 4498–4499 CrossRef CAS PubMed.
- E. Mulugeta, Q. He, D. Sareen, S.-J. Hong, J. H. Oh, V. M. Lynch, J. L. Sessler, S. K. Kim and C.-H. Lee, Chem, 2017, 3, 1008–1020 CAS.
- N. J. Heo, J. H. Oh, J. T. Lee, Q. He, J. L. Sessler and S. K. Kim, Org. Chem. Front., 2020, 7, 548–556 RSC.
- (a) P. A. Gale, J. L. Sessler, V. Král and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140–5141 CrossRef CAS; (b) P. A. Gale, J. L. Sessler and V. Král, Chem. Commun., 1998, 1–8 RSC; (c) C.-H. Lee, H. Miyaji, D.-W. Yoon and J. L. Sessler, Chem. Commun., 2008, 24–34 RSC and references therein. (d) S. K. Kim and J. L. Sessler, Acc. Chem. Res., 2014, 47, 2525–2536 CrossRef CAS PubMed and references therein. (e) I. Saha, J. T. Lee and C.-H. Lee, Eur. J. Org. Chem., 2015, 18, 3859–3885 CrossRef; (f) S. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. V. Rossom, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Nat. Chem., 2014, 6, 885–892 CrossRef CAS PubMed; (g) S. Peng, Q. He, G. I. Vargas-Zúñiga, L. Qin, I. Hwang, S. K. Kim, N. J. Heo, C.-H. Lee, R. Dutta and J. L. Sessler, Chem. Soc. Rev., 2020, 49, 865–907 RSC.
- (a) S. K. Kim, V. M. Lynch and J. L. Sessler, Org. Lett., 2014, 16, 6128–6131 CrossRef CAS PubMed; (b) R. Samanta, B. S. Kumar and P. K. Panda, Org. Lett., 2015, 17, 4140–4143 CrossRef CAS PubMed.
- J. Yoo, I.-W. Park, T.-Y. Kim and C.-H. Lee, Bull. Korean Chem. Soc., 2010, 31, 630–634 CrossRef CAS.
- Approximate binding constant calculated per, K. A. Connors, Binding Constants: The Measurement of Molecular Complex Stability, Wiley-Interscience, New York, 1987.
- (a) S. K. Kim, D. E. Gross, D.-G. Cho, V. M. Lynch and J. L. Sessler, J. Org. Chem., 2011, 76, 1005–1012 CrossRef CAS PubMed; (b) J. L. Sessler, D. E. Gross, W.-S. Cho, V. M. Lynch, F. P. Schmidtchen, G. W. Bates, M. E. Light and P. A. Gale, J. Am. Chem. Soc., 2006, 128, 12281–12288 CrossRef CAS PubMed.
- This presumption was supported by the finding that the aryl proton signal (Hg) undergoes a noticeable downfield shift (up to Δδ ≈ 0.29 ppm) in the 1H NMR spectra recorded as receptor 2 is titrated with HCO3− (Fig. S7†). In contrast to what was seen upon titration with TEAHCO3, the addition of TBAF induces an upfield shift in the benzene proton signal (Hg) of 2. Presumably, this reflects the presence of increased electron density within the receptor cavity as the result of fluoride anion binding (Fig. 3). No evidence of a hydrogen bond interaction between the aryl CH proton of the strap and the bound F− was observed.22 This latter absence is ascribed to the F− anion being small and a lack of flexibility in the strap unit that might otherwise allow the strap in receptor 2 to interact with the bound fluoride anion.22.
- Association constants (Ka) were evaluated using BindFit v5.0 available from URL http://app.supramolecular.org/bindfit/.
- Y. Liu, A. Sengupta, K. Raghavachari and A. H. Flood, Chem, 2017, 3, 411–427 CAS.
- D.-W. Yoon, D. E. Gross, V. M. Lynch, J. L. Sessler, B. P. Hay and C.-H. Lee, Angew. Chem., Int. Ed., 2008, 47, 5038–5042 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2010538 and 2010539. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc03445b |
| This journal is © The Royal Society of Chemistry 2020 |