Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Enantioselective synthesis of isochromans and tetrahydroisoquinolines by C–H insertion of donor/donor carbenes
Leslie A.
Nickerson
,
Benjamin D.
Bergstrom
,
Mingchun
Gao
,
Yuan-Shin
Shiue
,
Croix J.
Laconsay
,
Matthew R.
Culberson
,
Walker A.
Knauss
,
James C.
Fettinger
,
Dean J.
Tantillo
and
Jared T.
Shaw
*
Chemistry Department, University of California, Davis, One Shields Ave, Davis, CA 95616, USA. E-mail: jtshaw@ucdavis.edu
First published on 6th May 2020
Abstract
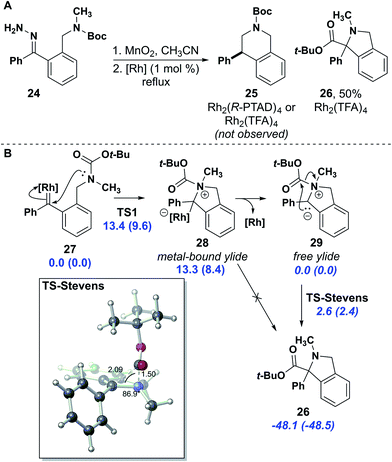
Correction for ‘Enantioselective synthesis of isochromans and tetrahydroisoquinolines by C–H insertion of donor/donor carbenes’ by Leslie A. Nickerson et al., Chem. Sci., 2020, 11, 494–498, DOI: 10.1039/C9SC05111B.
The electron pushing arrows in Fig. 6B were, unbeknownst to the authors, converted by ChemDraw (version 18.0) into double-headed resonance arrows during the final stages of galley proof review and went unnoticed until the article appeared in print. This event was traced to a version-specific bug in the software that has been resolved in a subsequent update. A corrected figure is provided here.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2020 |