Fabrication of a β-cyclodextrin-based self-assembly containing a redox-responsive ferrocene†
Bing
Jiang
a,
Huichuang
Guo
a,
Li
Zhao
 *a,
Baocai
Xu
a,
Ce
Wang
*a,
Baocai
Xu
a,
Ce
Wang
 a,
Changyao
Liu
a,
Changyao
Liu
 a and
Haiming
Fan
*b
a and
Haiming
Fan
*b
aSchool of Light Industry Science and Technology, Beijing Technology and Business University, Beijing 100048, P. R. China. E-mail: zhaol@btbu.edu.cn
bCollege of Petroleum Engineering, China University of Petroleum (East China), Qingdao 266555, P. R. China. E-mail: HaimingFan@gmail.com
First published on 14th November 2019
Abstract
The current research involves fabrication of a redox-responsive self-assembly system based on a ferrocene (Fc)-containing β-cyclodextrin (β-CD) derivative (βCD-EG-Fc). βCD-EG-Fc was synthesized, and its redox-sensitive self-assembly behavior was investigated using various techniques. On the basis of the intermolecular host–guest recognition between the β-CD group and the Fc moiety, βCD-EG-Fc primarily formed network-like structures and then vesicles following aging for a specified time. The formation of these structures was primarily driven by hydrogen bonding. Conversely, the oxidized molecules (βCD-EG-Fc+) self-assembled into cationic vesicles with the absence of host–guest complexation. Upon controlling the oxidation and reduction of Fc/Fc+, reversible aggregate transformation was achieved. The current study resulted in a deeper understanding of β-CD/Fc redox-responsive self-assemblies and contributed to the development of a single-component host–guest inclusion model.
Introduction
Recently, stimuli-responsive supramolecular self-assembly systems, constructed by host–guest approaches, have attracted significant attention due to their potential applications in many fields.1–4 Numerous external stimuli, including redox,5 light,6,7 pH,8,9 and temperature change10 have been employed to fabricate stimuli-responsive supramolecular self-assemblies. The regulation of external stimuli usually resulted in modification of steric structures of responsive groups. Furthermore, such alterations can influence their aggregation behavior.11–13 Of these stimuli, redox processes are employed to construct functional self-assemblies that respond to the reduction and oxidation of substances. This is a consequence of molecules with redox-active groups undergoing transitions between the reduced and oxidized states, resulting in a change in their molecular amphiphilicity.14 For example, ferrocene (Fc) can be converted into a positively charged hydrophilic group (Fc+) following oxidation; however, in the presence of a reductant, such as glutathione (GSH), this process can be reversed.15,16Host–guest interactions include non-covalent interactions between the host and guest groups with dynamic and reversible properties. Numerous stimuli-switchable self-assembly systems have been fabricated on the basis of host–guest recognition.17–20 Cyclodextrins (CDs) are one of the more frequently used hosts. A single CD molecule contains a hydrophobic inner cavity and a hydrophilic outer surface. Hydrophobic moieties can be incorporated into the cavities based on steric selective complexation, with their binding ability depending on size matching.1,21 Responsive guest groups undergo alterations before and after treatment with external stimuli, which influences their affinity towards CDs.22–24 Herein, adjustable host–guest binding ability endows CD-guest inclusions with the potential to construct stimuli-responsive self-assemblies.3,25,26 The frequently utilized β-cyclodextrin (β-CD) was selected because of its moderate cavity size, good modifiability, and low cost.27
In CD-based host–guest systems, Fc is a common guest unit for β-CD. Such systems can form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stable inclusions with a high association constant, whereas the oxidized state of Fc, i.e., Fc+, shows no binding affinity for β-CD due to its hydrophilic nature.28 Therefore, the redox stimulus can manipulate the formation and dissociation of β-CD/Fc inclusions, which provides the basis for the construction of redox-switchable self-assemblies. Consequently, these inclusions had been widely employed in the construction of redox-responsive host–guest supramolecular self-assembly systems.5,29,30
1 stable inclusions with a high association constant, whereas the oxidized state of Fc, i.e., Fc+, shows no binding affinity for β-CD due to its hydrophilic nature.28 Therefore, the redox stimulus can manipulate the formation and dissociation of β-CD/Fc inclusions, which provides the basis for the construction of redox-switchable self-assemblies. Consequently, these inclusions had been widely employed in the construction of redox-responsive host–guest supramolecular self-assembly systems.5,29,30
Previous methods include a commonly adopted strategy, in which an Fc derivative was synthesized and subsequently mixed with β-CD or its derivatives in solution.5,29,31–35 For example, Yuan et al. reported several redox-switchable supramolecular self-assembly systems based on the host–guest recognition between β-CD derivatives and other molecules with an Fc terminal group.28,30 The construction of a redox-active self-assembly unimolecular platform based on the β-CD/Fc inclusion complexation has not been reported to a large extent. We have previously investigated the redox-responsive self-assembly behaviors of double-headed amphiphiles containing β-CD and Fc groups, linked via alkyl chains and based on intramolecular or intermolecular host–guest interactions.23,36 Zuo et al. reported a hydrophobic molecule containing a β-CD group and an Fc moiety, which was used in combination with a hydrophilic homopolymer to construct a redox-responsive supramolecular copolymer.5 Hao et al. synthesized two Fc-modified β-CD derivatives that self-assembled into fibrous structures upon intermolecular host–guest recognition.37
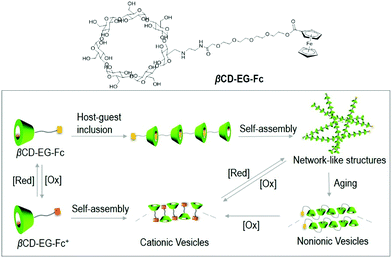
Further development of redox-switchable unimolecular self-assemblies based on β-CD/Fc host–guest complexes is vital for the fundamental study and potential applications. In this work, a β-CD derivative containing an Fc terminal moiety, βCD-EG-Fc was designed and synthesized (Scheme 1). The oligo(ethylene glycol, EG) chain was selected as the linker to enhance the solubility in aqueous solution. βCD-EG-Fc formed intermolecular host–guest inclusions, presenting network-like structures, which were transformed into non-ionic vesicles following aging for 1 week. The network-like structures and vesicles formed by the βCD-EG-Fc molecules were transformed into cationic vesicles after oxidation of Fc to Fc+. On the other hand, the reduction of Fc+ to Fc induced the reformation of the aforementioned network-like structures. The fabrication of this reversible redox-sensitive self-assembly through a host–guest approach may offer new prospects for the design of smart materials.
Experimental
Materials and methods
The detailed synthetic procedure for βCD-EG-Fc is presented in the ESI.† Ferrocenecarboxylic acid, ethyl diazoacetate, triethylamine, oxalyl chloride, 4-dimethylaminopyridine, triethylene glycol, boron trifluoride diethyl etherate, GSH, β-CD, Nile red, and FeCl3 were obtained from commercial sources.The solution was obtained by dissolving solid βCD-EG-Fc directly in ultrapure water/DMSO (9/1, v/v) (1 mM). A 100 mM solution of FeCl3 was prepared by dissolving the oxidant in acetonitrile. 0.1 mL of this solution was subsequently injected directly into 1 mL of the βCD-EG-Fc solution resulting in a solution of βCD-EG-Fc+. 10 mM GSH solution was prepared by dissolving GSH in water, and 50 μL of this solution was added to 1 mL of the βCD-EG-Fc+ solution to reduce the ferrocenium cation. Nile red-containing samples were prepared as follows: a 15 μL stock solution of Nile red in acetone (1 mg mL−1) was added to a test tube. Following volatilization of acetone, 1 mL of the desired solution was added. All solutions were allowed to rest at 25 °C overnight before any measurements were taken.
Characterization
Transmission electron microscopy (TEM) measurements were carried out using a JEM-2100 transmission electron microscope, and the working voltage was 120 kV. The samples were stained using a 1.5% aqueous solution of uranyl acetate. Size distributions were measured via the dynamic light scattering (DLS) method with a 173° scattering angle using a Zetasizer Nano instrument (Malvern). The light source was a 22 mW He–Ne laser operating at 632.8 nm. A ZetaPALS Zeta Potential Analyzer was employed to measure the zeta potentials (Brookhaven). 1H nuclear magnetic resonance (1H NMR) experiments were carried out on a Bruker-600 (600 MHz) spectrometer. Ultraviolet–visible (UV-Vis) absorption spectra were recorded on a UV-3600 spectrophotometer (Shimadzu) at room temperature. A Hitachi F-4500 fluorescence spectrometer was used to measure the fluorescence emission of solutions. Fourier transform infrared (FT-IR) spectroscopy was conducted using a Nicolet iS 10 apparatus (ThermoFisher). The samples were freeze-dried prior to measurements.Results and discussion
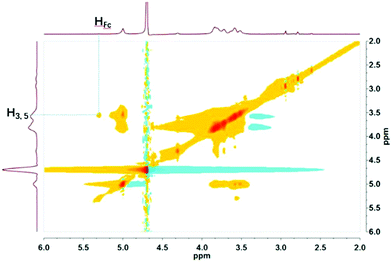
βCD-EG-Fc was first dissolved in water at a concentration of 1 mM, and the resulting solution showed apparent Tyndall scattering, indicating the formation of colloidal particles. However, brown precipitates were observed at the bottom of the container when the solution was left to stand for 3–4 days. Hence, DMSO was added to enhance the solubility of βCD-EG-Fc. The sample with a concentration of 1 mM in the H2O/DMSO (9/1, v/v) solvent mixture remained stable for over a month; therefore, this solution was used for further measurements.A βCD-EG-Fc molecule contains a host head (β-CD) and a guest tail (Fc) linked by an oligo(EG) spacer. On the basis of the host–guest recognition of β-CD and Fc, a host–guest inclusion complex is expected to form in the βCD-EG-Fc system. 2D 1H NMR is a powerful analytical technique, which can be used for detection of the host–guest interactions in supramolecular systems.38,39 As shown in Fig. 1, the Nuclear Overhauser Spectroscopy (NOESY) spectrum of the βCD-EG-Fc solution shows correlation between the Fc and β-CD cavity protons (H3,5), which indicates that the Fc moieties were embedded in the β-CD cavities.37 However, it is not possible to conclude whether the host–guest complexation occurs in an intermolecular or intramolecular manner based on the NOESY analysis alone.
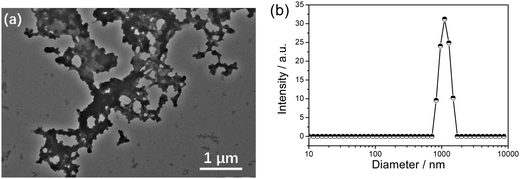
The freshly prepared βCD-EG-Fc solution showed apparent Tyndall scattering at 1 mM, indicating the presence of colloidal aggregates. TEM was employed to evaluate the morphology of the aggregates. As shown in Fig. 2a, network-like structures with a micron-scale size distribution were observed. The average hydrodynamic diameter obtained from the DLS result (Fig. 2b) is ∼1 μm. This self-assembly may be attributed both to the formation of intermolecular host–guest inclusions between the βCD-EG-Fc molecules and to the twisting into network-like structures.
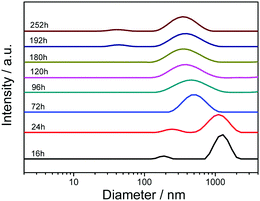
Interestingly, similar to our previous results, the aging effect was observed in the βCD-EG-Fc system.40 The transition process was traced both by TEM observation and DLS measurements. Clearly, based on the TEM images, the initial network-like structures were gradually transformed into spherical vesicles upon aging for 1 week (Fig. 3). In a βCD-EG-Fc solution aging for 16 h, network-like structures were widespread during the TEM observation, which quickly transformed into a profile of stacking vesicles at 24 h of aging. Fortunately, the vesicles in the separating state were captured after 72 h of aging. Subsequently, isolated vesicles were observed using TEM, accompanied by connected vesicles at 96 and 120 h of aging. When the aging time reached 180 h, scattered vesicles with a diameter ranging from ∼20 to 60 nm were noted. Increasing the aging time further did not bring any evident change, indicating that the aggregate transformation of the βCD-EG-Fc solution from network-like structures to vesicles occurred upon aging for approximately 1 week (∼180 h).
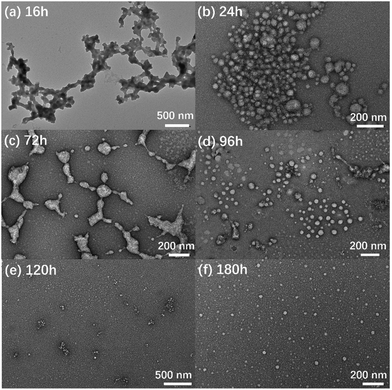 | ||
| Fig. 3 TEM images of βCD-EG-Fc aggregates upon aging: (a) 16 h; (b) 24 h; (c) 72 h; (d) 96 h; (e) 120 h; and (f) 180 h. | ||
The time-dependent DLS analysis revealed a change in the size of the βCD-EG-Fc aggregates and confirmed the aggregate transformation (Fig. 4). Initially, the DLS results showed the presence of aggregates in the fresh βCD-EG-Fc solution (observed within 24 h), and the average hydrodynamic diameter was determined to be >1 μm. After aging for 72 h, the average size of the βCD-EG-Fc aggregates gradually decreased to ∼500 nm. The size distribution then changed slowly, and the average diameter decreased to ∼275 nm after 180 h. At 192 and 252 h of aging, a peak at ∼40 nm appeared, which corresponded to the small vesicles that were at a disadvantage during the DLS measurements. The DLS peak observed at ∼275 nm for the βCD-EG-Fc solution aged for more than 180 h may be attributed to the residual connected aggregates. Hence, based on these results, the occurrence of the aggregate transformation from micron-sized aggregates to nanometer-scale ones was confirmed.
Similar transformation observed in our previous βCD-EG-Azo system was verified to be ascribed to the intermolecular host–guest inclusion complexation.40 Therefore, we can deduce that an intermolecular host–guest complexation occurred in the βCD-EG-Fc system in a “head-to-tail” manner and that the βCD-EG-Fc molecules formed linear intermolecular host–guest inclusions.41 The inclusions were found to be twisted structures and not linear ones, which could be attributed to the flexibility of the EG chain. Hence, the linear intermolecular host–guest inclusions were twisted and enclosed into loops and finally transformed into isolated vesicles following aging for 1 week.
According to the available literature reports, Fc is a redox-sensitive moiety. The oxidized state of Fc, i.e., Fc+, carries one positive charge, which leads to its hydrophilicity and dissociation from the cavity of β-CD. Therefore, the reversible redox transition of Fc/Fc+ can induce the reversible formation and dissociation of host–guest inclusion complexation between β-CD and Fc, which in turn endows the resulting self-assemblies with the feasibility of redox-responsive properties. The oxidation process was detected using UV-Vis absorption spectroscopy, and the results are shown in Fig. 5. Upon the addition of the oxidizing agent (FeCl3), the absorption intensity increased, whereas the overall spectrum became broad and featureless, indicating the oxidation of Fc to Fc+.15,42
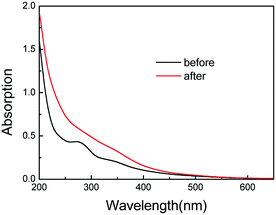 | ||
| Fig. 5 UV-Vis absorption spectra of the βCD-EG-Fc solution (0.1 mM) before and after the addition of the FeCl3 oxidizing agent. | ||
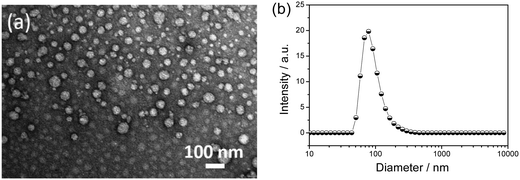
Moreover, both β-CD and Fc+ form the hydrophilic part of the βCD-EG-Fc+ molecule, whereas the EG chain can display hydrophobic or hydrophilic properties.43 Consequently, if the EG chain in the βCD-EG-Fc+ system was hydrophilic, no self-assembly aggregates would be formed in a solution of such a low concentration. A strong Tyndall effect was observed when the 1 mM βCD-EG-Fc+ solution was irradiated with a 632 nm laser, which indicated that the self-assembly occurred and colloidal-sized aggregates had formed. By considering the absence of host–guest interactions between β-CD and Fc+, it is assumed that the βCD-EG-Fc+ molecules acted as bola-type amphiphiles when the EG chain displayed hydrophobic character.43,44 As shown in Fig. 6, the aggregation behavior was investigated by TEM and DLS. Spherical vesicles with a diameter in the range of ∼20–100 nm were observed. The DLS results verified the presence of aggregates with an average hydrodynamic diameter of ∼80 nm, which is in good agreement with the TEM result.
Generally, asymmetrical bola amphiphiles containing two heads of different properties tend to arrange in an antiparallel packing manner.45–47 It was then necessary to examine how the βCD-EG-Fc+ molecules assembled in the vesicles. Since the two heads exhibit different electrical properties, the surface charge density can be detected through zeta potential measurements to evaluate the molecular arrangement. The ζ value was >+28 mV for 1 mM βCD-EG-Fc+ aqueous solution, indicating that the vesicle surfaces were covered by positively charged moieties. Therefore, the βCD-EG-Fc+ system possesses two possible packing modes: an antiparallel or a parallel mode, where all of the Fc+ cations are arranged towards water. The former is likely to be favored because of the electrostatic repulsion between the Fc+ moieties.
Owing to the reversible redox process between Fc and Fc+, the self-assembly of βCD-EG-Fc in an aqueous solution was expected to be sensitive to redox reagents and to show reversible redox-responsive character. Herein, the reversible redox self-assembly behavior was investigated by alternately exposing the solution to an oxidant or a reductant. Because of the distinguishing electrical properties of the oxidized and reduced state aggregates, zeta potential measurements could be used to easily track their transition. The ζ value would change during the oxidation and reduction processes (Fig. 7). The ζ value of the βCD-EG-Fc aggregates was 0.4 mV, which was somewhat low and comparable with water, indicating that the aggregates carry no charge. Addition of FeCl3 caused an increase of the ζ value to +28.9 mV, demonstrating the oxidation of Fc to Fc+ and the formation of cationic vesicles. Addition of a reductant (GSH) resulted in a sharp decrease to +4.7 mV, which proved the reduction of Fc+ to Fc and the transformation of the cationic vesicles back to neutral aggregates. This cycle could be repeated by the alternate addition of FeCl3 and GSH. The fluctuation in the ζ value for the βCD-EG-Fc aggregates around zero may be attributed to the disturbance in the countercharge binding.34 Undoubtedly, the reversible transformation between the neutral βCD-EG-Fc aggregates and the cationic βCD-EG-Fc+ vesicles was verified by the zeta potential measurements. Moreover, the aggregate morphology of the βCD-EG-Fc+ solution following the addition of GSH was analyzed by TEM (Fig. S1, ESI†), and the presence of network-like structures confirmed the reversible self-assembly.
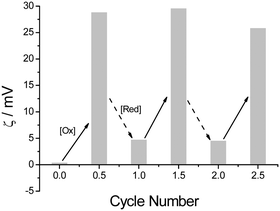 | ||
| Fig. 7 Variation of the ζ values of the βCD-EG-Fc solution with the alternate addition of FeCl3 and GSH. | ||
An investigation into the possible driving forces in the self-assembly process was also conducted. We speculate that two types of interactions, hydrogen bonding and hydrophobic effects, may be involved. Surface tension and fluorescence measurements were performed to explore the hydrophobic effects. The surface tensions were determined to be 68.4 mN m−1 for the βCD-EG-Fc solution and 66.2 mN m−1 for the βCD-EG-Fc+ solution, which are similar to those of water. Hydrophobic Nile red was selected to detect the hydrophobic domains, due to its sensitivity to micro-polarity. The fluorescence emission measurements (Fig. S2, ESI†) revealed that the local polarity inside the βCD-EG-Fc/βCD-EG-Fc+ aggregates was more hydrophilic than that in the 5 mM sodium dodecyl sulfate (SDS) and β-CD systems. Hence, considering the critical micelle concentration of SDS (8.2 mM) at 25 °C, a comparison of these spectra showed lack of typical hydrophobic domains in the βCD-EG-Fc/βCD-EG-Fc+ solutions.48 Thus, this indicated that the hydrophobic effects were weak and did not play a role of the main driving force during the self-assembly process of the βCD-EG-Fc/βCD-EG-Fc+ solution.
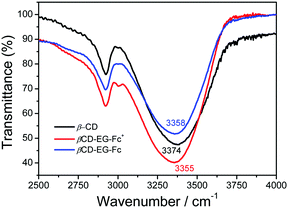
The hydroxyl groups on the outer surface of the β-CD and the EG chain of the βCD-EG-Fc molecule provide multiple hydrogen bonding sites; hence, hydrogen bonding may play an important role during the self-assembly of βCD-EG-Fc/βCD-EG-Fc+. To determine such bonding, FT-IR spectroscopy was employed, and the resulting FT-IR spectra are shown in Fig. 8. An absorption peak at 3374 cm−1 was seen for the pure β-CD sample, which shifted to 3358 cm−1 for the βCD-EG-Fc system and to 3355 cm−1 for βCD-EG-Fc+. Therefore, hydrogen bonding was confirmed for both the oxidized and reduced state systems, which offers a valuable contribution to the self-assembly process.48,49 Thus, we can speculate that an antiparallel packing manner was adopted in the βCD-EG-Fc+ system, which is in agreement with the zeta potential result showing its inability to form a hydrogen bond network between β-CDs.
A plausible schematic illustration of the redox-switchable self-assembly is proposed on the basis of the aforementioned results and their evaluation (Scheme 1). It is possible that the supramolecular host–guest inclusions were formed via the intermolecular host–guest recognition of the βCD-EG-Fc molecules. The inclusions intertwined into network-like structures, which distorted to form the membranes of vesicles based on the flexible EG chain. It can be deduced that the hydroxyl groups and oxygen atoms of the EG chain adjusted their spatial orientation to form a stable hydrogen-bonding network. An accompanying aggregate transformation from network-like structures to vesicles takes about 1 week. The oxidation of the Fc moiety generates a hydrophilic ferrocenium cation, which based on the FT-IR and zeta potential measurements may adopt an antiparallel packing manner and self-assembles into cationic vesicles in water. The reversible Fc/Fc+ redox triggers the formation and dissociation of host–guest complexes and further results in reversible redox-responsive self-assembly. This leads to the construction of a redox-responsive self-assembly based on a β-CD derivative with an Fc group linked by an EG chain.
Conclusions
In conclusion, a reversible redox-responsive self-assembly was fabricated by designing and synthesizing a β-CD derivative with an Fc terminal moiety, based on the distinct host–guest binding affinity of Fc/Fc+ for β-CD. Intermolecular host–guest inclusion complexation was observed, and the βCD-EG-Fc molecules formed network-like structures in a H2O/DMSO solvent mixture, which were transformed into vesicles upon aging. The oxidation of Fc resulted in the formation of cationic vesicles without host–guest complexation. The self-assembly was reversible as a consequence of the reversible transition between Fc and Fc+. Our research will contribute to a better understanding of the relationship between molecular structures and self-assembly behaviors and provide a platform for fabricating new stimuli-switchable materials.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (2017YFB0308701), the National Natural Science Foundation of China (21403010 and 21676003), and the Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (CIT&TCD201804026).Notes and references
- G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240–7303 CrossRef CAS PubMed.
- X. J. Loh, Mater. Horiz., 2014, 1, 185–195 RSC.
- H. Yamaguchi, Y. Kobayashi, R. Kobayashi, Y. Takashima, A. Hashidzume and A. Harada, Nat. Commun., 2012, 3, 603 CrossRef.
- X. Yao, J. Mu, L. Zeng, J. Lin, Z. Nie, X. Jiang and P. Huang, Mater. Horiz., 2019, 6, 846–870 RSC.
- C. Zuo, X. Dai, S. Zhao, X. Liu, S. Ding, L. Ma, M. Liu and H. Wei, ACS Macro Lett., 2016, 5, 873–878 CrossRef CAS.
- D. Chang, W. Yan, D. Han, Q. Wang and L. Zou, Dyes Pigm., 2018, 149, 188–192 CrossRef CAS.
- P. Y. Xing, H. Z. Chen, M. F. Ma, X. D. Xu, A. Y. Hao and Y. L. Zhao, Nanoscale, 2016, 8, 1892–1896 RSC.
- Z. Zhang, Q. Lv, X. Gao, L. Chen, Y. Cao, S. Yu, C. He and X. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 8404–8411 CrossRef CAS.
- M. Ni, N. Zhang, W. Xia, X. Wu, C. Yao, X. Liu, X. Y. Hu, C. Lin and L. Wang, J. Am. Chem. Soc., 2016, 138, 6643–6649 CrossRef CAS.
- K. Sun, C. Xiao, C. Liu, W. Fu, Z. Wang and Z. Li, Langmuir, 2014, 30, 11040–11045 CrossRef CAS.
- X. Li, J. Fei, Y. Xu, D. Li, T. Yuan, G. Li, C. Wang and J. Li, Angew. Chem., Int. Ed., 2018, 57, 1903–1907 CrossRef CAS.
- R. Sun, H. K. Bisoyi, M. Xie and Q. Li, Dyes Pigm., 2016, 132, 336–341 CrossRef CAS.
- J. Guo, H.-Y. Zhang, Y. Zhou and Y. Liu, Chem. Commun., 2017, 53, 6089–6092 RSC.
- Y. Gao, J. Lu, J. Wu, J. Hu and Y. Ju, RSC Adv., 2014, 4, 63539–63543 RSC.
- Y. Chang, K. Yang, P. Wei, S. Huang, Y. Pei, W. Zhao and Z. Pei, Angew. Chem., Int. Ed., 2014, 53, 13126–13130 CrossRef CAS.
- Y. Shi, H. Li, J. Cheng, T. Luan, D. Liu, Y. Cao, X. Zhang, H. Wei, Y. Liu and G. Zhao, Chem. Commun., 2017, 53, 12302–12305 RSC.
- W. Zhang, Y. Chen, J. Yu, X. Zhang and Y. Liu, Chem. Commun., 2016, 52, 14274–14277 RSC.
- H. Zou, Y. Lu, W. Yuan and S. Wang, Polym. Chem., 2017, 8, 661–665 RSC.
- B. V. K. J. Schmidt and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2017, 56, 8350–8369 CrossRef CAS.
- J. Wang, B. Li, X. Wang, F. Yang, H. Shen and D. Wu, Langmuir, 2016, 32, 13706–13715 CrossRef CAS.
- A. Harada, Y. Takashima and M. Nakahata, Acc. Chem. Res., 2014, 47, 2128–2140 CrossRef CAS.
- Y. Zheng, A. Hashidzume and A. Harada, Macromol. Rapid Commun., 2013, 34, 1062–1066 CrossRef CAS.
- H. Guo, B. Jiang, J. Zhou, L. Zhao, B. Xu and C. Liu, Colloids Surf., A, 2019, 579, 123683 CrossRef.
- Z. Du, K. Ke, X. Chang, R. Dong and B. Ren, Langmuir, 2018, 34, 5606–5614 CrossRef CAS.
- B. Liu, H. Zhou, S. Zhou and J. Yuan, Eur. Polym. J., 2015, 65, 63–81 CrossRef CAS.
- X. Zhang, X. Ma, K. Wang, S. Lin, S. Zhu, Y. Dai and F. Xia, Macromol. Rapid Commun., 2018, 39, e1800142 CrossRef.
- Z. Liu, J. Qiao, Y. Tian, M. Wu, Z. Niu and Y. Huang, Langmuir, 2014, 30, 8938–8944 CrossRef CAS.
- Q. Yan, J. Yuan, Z. Cai, Y. Xin, Y. Kang and Y. Yin, J. Am. Chem. Soc., 2010, 132, 9268–9270 CrossRef CAS.
- X. Chang, Z. Cheng, B. Ren, R. Dong, J. Peng, S. Fu and Z. Tong, Soft Matter, 2015, 11, 7494–7501 RSC.
- Q. Yan, A. Feng, H. Zhang, Y. Yin and J. Yuan, Polym. Chem., 2013, 4, 1216–1220 RSC.
- F. Szillat, B. V. K. J. Schmidt, A. Hubert, C. Barner-Kowollik and H. Ritter, Macromol. Rapid Commun., 2014, 35, 1293–1300 CrossRef CAS.
- Q. Li, X. Chen, X. Yue, D. Huang and X. Wang, Colloids Surf., A, 2012, 409, 98–104 CrossRef CAS.
- L. Liu, L. Rui, Y. Gao and W. Zhang, Polym. Chem., 2015, 6, 1817–1829 RSC.
- S. Hao, Q. Zhai, L. Zhao and B. Xu, Colloids Surf., A, 2016, 509, 116–122 CrossRef CAS.
- B. V. K. J. Schmidt, D. Kugele, J. von Irmer, J. Steinkoenig, H. Mutlu, C. Rüttiger, C. J. Hawker, M. Gallei and C. Barner-Kowollik, Macromolecules, 2017, 50, 2375–2386 CrossRef CAS.
- L. Zhao, S. Hao, Q. Zhai, H. Guo, B. Xu and H. Fan, Soft Matter, 2017, 13, 3099–3106 RSC.
- M. Ma, T. Luan, M. Yang, B. Liu, Y. Wang, W. An, B. Wang, R. Tang and A. Hao, Soft Matter, 2017, 13, 1534–1538 RSC.
- R. Dong, Y. Liu, Y. Zhou, D. Yan and X. Zhu, Polym. Chem., 2011, 2, 2771–2774 RSC.
- Z. Zhai, X. Yan, Z. Song, S. Shang and X. Rao, Soft Matter, 2018, 14, 499–507 RSC.
- H. Guo, J. Yang, J. Zhou, L. Zeng, L. Zhao and B. Xu, Dyes Pigm., 2018, 149, 626–632 CrossRef CAS.
- G. Yu, X. Zhao, J. Zhou, Z. Mao, X. Huang, Z. Wang, B. Hua, Y. Liu, F. Zhang, Z. He, O. Jacobson, C. Gao, W. Wang, C. Yu, X. Zhu, F. Huang and X. Chen, J. Am. Chem. Soc., 2018, 140, 8005–8019 CrossRef CAS.
- T. He, K. Li, N. Wang, Y.-X. Liao, X. Wang and X. Q. Yu, Soft Matter, 2014, 10, 3755–3761 RSC.
- J. Dey and S. Shrivastava, Soft Matter, 2012, 8, 1305–1308 RSC.
- R. Ghosh and J. Dey, Langmuir, 2014, 30, 13516–13524 CrossRef CAS.
- M. Berchel, C. Mériadec, L. Lemiègre, F. Artzner, J. Jeftić and T. Benvegnu, J. Phys. Chem. B, 2009, 113, 15433–15444 CrossRef CAS.
- Y. Wang, Z. Yu and B. Li, Colloid Polym. Sci., 2013, 292, 243–250 CrossRef.
- T. Shimizu, N. Kameta, W. Ding and M. Masuda, Langmuir, 2016, 32, 12242–12264 CrossRef CAS.
- L. Jiang, Y. Peng, Y. Yan, M. Deng, Y. Wang and J. Huang, Soft Matter, 2010, 6, 1731–1736 RSC.
- J. Wang, M. Yao, Q. Li, S. Yi and X. Chen, Soft Matter, 2016, 12, 9641–9648 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthetic procedure, TEM images of βCD-EG-Fc+ solution after reduction and the fluorescence spectra of NR-containing solutions are available. See DOI: 10.1039/c9sm02049g |
| This journal is © The Royal Society of Chemistry 2020 |