Constructing 2D MOFs from 2D LDHs: a highly efficient and durable electrocatalyst for water oxidation†
Mengke
Cai
a,
Qinglin
Liu
a,
Ziqian
Xue
a,
Yinle
Li
a,
Yanan
Fan
a,
Aiping
Huang
b,
Man-Rong
Li
 a,
Mark
Croft
c,
Trevor A.
Tyson
d,
Zhuofeng
Ke
a,
Mark
Croft
c,
Trevor A.
Tyson
d,
Zhuofeng
Ke
 e and
Guangqin
Li
e and
Guangqin
Li
 *a
*a
aMOE Laboratory of Bioinorganic and Synthetic Chemistry, Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, People's Republic of China. E-mail: liguangqin@mail.sysu.edu.cn
bKey Laboratory of Polymer Composites and Functional Materials of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, People's Republic of China
cDepartment of Physics and Astronomy, Rutgers, The State University of New Jersey, 136 Frelinghuysen Road, Piscataway, New Jersey 08854, USA
dDepartment of Physics, New Jersey Institute of Technology, Newark, New Jersey 07102, USA
eKey Laboratory for Polymeric Composite and Functional Materials of Ministry of Education, School of Materials Science and Engineering, Sun Yat-Sen University, Guangzhou 510275, People's Republic of China
First published on 22nd November 2019
Abstract
Two-dimensional (2D) materials have been widely applied in electrochemical conversion technologies, especially toward the water oxidation reaction (WOR) in metal–air batteries and water splitting. Here, we demonstrate a facile ligand-assisted synthetic method, promoting the transformation of 2D layered double hydroxides (LDHs) into 2D metal–organic frameworks (MOFs). The CoFe-LDH precursor acts as an adjustable metal release source, controlling heterogeneous nucleation for 2D MOFs. Compared with most cobalt-based electrocatalysts, the optimized CoFe 2D MOFs exhibit a superior WOR performance on glassy-carbon electrodes (overpotential of 274 mV at 10 mA cm−2 and a Tafel slope of 46.7 mV dec−1) and long-term stability, due to the unique 2D characteristics and coupling effect between Co and Fe ions. More importantly, this work highlights the ability to transform 2D LDHs into 2D MOFs and reveals the intrinsic factors for excellent performance in the WOR.
Introduction
Water splitting by electrolysis (2H2O → O2 + 2H2) provides a potential and encouraging path for the generation of efficient, clean and renewable H2 fuel to support human civilization.1–3 However, the efficiency of water electrolysis is limited by large overpotentials, especially for the water oxidation reaction (WOR), and the use of expensive noble electrocatalysts hinders the long-term development for water electrolysis application.4–9 In recent years, further research has been conducted to explore cost-effective and efficient non-noble electrocatalysts for the WOR.10–12 Notably, two-dimensional (2D) materials have attracted very significant attention in the field of heterogeneous electrocatalysis due to their unique physical, chemical, and electronic properties. As a result, graphene,13–15 graphitic C3N4,16,17 transition metal dichalcogenides (TMDs),18–20 layered double hydroxides (LDHs),21–23 2D metal–organic frameworks (MOFs)24–28 and 2D covalent-organic frameworks (COFs)29,30 are considered as promising water splitting catalysts. Additionally, how to rationally design and synthesize 2D materials is also of great interest and challenge. The synthesis of 2D materials usually depends on chemical vapor deposition and physical exfoliation, involving both top–down and bottom–up approaches.31–33 Recently, 2D materials such as TMDs with a unique microstructure were obtained by means of precursor transformation, exhibiting superior catalytic properties compared with direct synthesis.21,26,34 Therefore, synthesizing 2D materials through precursor transformation is promising to significantly broaden the design and synthesis of electrocatalysts for energy storage and conversion.MOFs are constructed by coordination bonds between metal nodes/clusters and organic ligands with periodic reticular chemistry.35 Due to precise periodicity, nano-dimensional MOFs possess definite accessible active sites and well-defined structures and have exhibited promising performance towards water oxidation.24–26 However, the poor conductivity, low mass permeability and stability have limited their development in electrocatalysis. Thus, to design highly efficient electrocatalysts, construction of 2D MOFs with nanosheets could be an appropriate strategy to satisfy the following: nanolayers to strengthen electron transfer and mass transport;24,25,36,37 exposed active sites with large surface area, and coordinatively unsaturated metal sites.24,26,38–43
Here, we report CoFe bimetal 2D MOFs as electrocatalysts for the WOR, for the first time, prepared from the transformation of the precursor CoFe-LDHs as the template. So far, there are extensive reports on 2D-LDHs containing Ni, Co, Fe and Mn exhibiting excellent catalytic properties in the WOR, especially for NiFe-LDHs known as the most promising catalyst.10,12,13,20,22,23 For Ni-based materials, the addition of Fe dramatically enhances WOR activity. But for Co-based materials, it is not well-documented and CoFe-based materials usually show more modest WOR activity compared with NiFe-based materials.49–53 So it is a significant challenge to construct novel CoFe-based materials with better activity than CoFe-LDHs and explore the relevant mechanism. More importantly, LDHs have adjustable and reasonable layer spacing, allowing ligands to attack inner Co and Fe from the interlayer, which makes them a good candidate to form 2D MOFs. Thus CoFe 2D-LDHs are chosen as precursors because of their bimetallic Co/Fe composition and 2D layered characteristics. Interestingly, with the transformation from 2D LDHs to 2D MOFs, the obtained electrocatalyst loaded on glassy-carbon (GC) electrodes demonstrate a low overpotential of 274 mV at 10 mA cm−2 under alkaline conditions. During the long-term stability test, the 2D MOFs loaded on nickel foam (NF) maintain highly stable activity for 70 h at a constant overpotential of 271 mV.
Results and discussion
The transformation was conducted via a ligand-assisted procedure by mixing the precursor CoFe-layered double hydroxide (CoFe-LDH) and terephthalic acid in DMF at various temperatures and times (Fig. 1), and the correspondingly transformed specimens were named LM-T-t (T: temperature/°C, t: time/hour). To clearly understand the process of transformation of CoFe-LDH into bimetallic 2D MOFs, powder X-ray diffraction (PXRD) tests were carried out on all specimens. In Fig. S1a,† the (003) and (006) diffractions can be observed at 2θ = 11.51° and 23.18° for the prepared CoFe-LDH, indexed as a bimetal ferrocobalt hydroxide with CO32− (carbonate) as the interlayer counterion. Interestingly, the (003) and (006) diffractions shifted to a lower angle, indicating interlayer ion-exchange for the CoFe-LDH. The basal interlayer spacing was calculated to be 0.77 nm for the pristine CoFe-LDH, shifting to 0.84 nm for both LM-100-12 and LM-130-12 based on the Bragg equation (2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = nλ). The PXRD patterns showed that the characteristic diffraction peaks of the (003) and (006) planes of CoFe-LDH disappeared, while the (200), (201) and (−201) facets of the 2D Co-MOFs emerged and became stronger with the increase of reaction temperature and time (Fig. S1a and b†). Finally, with excessive transformation time, another MOF phase diffraction peak arose in PXRD patterns, which was usually obtained by the coordination of Fe3+ with terephthalic acid and named MIL-88B(Fe) (Fig. S1b†) (No. 1415803, space group P63/mmc, Cambridge Crystallographic Date Centre).44–46
θ = nλ). The PXRD patterns showed that the characteristic diffraction peaks of the (003) and (006) planes of CoFe-LDH disappeared, while the (200), (201) and (−201) facets of the 2D Co-MOFs emerged and became stronger with the increase of reaction temperature and time (Fig. S1a and b†). Finally, with excessive transformation time, another MOF phase diffraction peak arose in PXRD patterns, which was usually obtained by the coordination of Fe3+ with terephthalic acid and named MIL-88B(Fe) (Fig. S1b†) (No. 1415803, space group P63/mmc, Cambridge Crystallographic Date Centre).44–46
Furthermore, N2-adsorption/desorption isotherms are shown in Fig. S2a and b.† The specimens LM-160-12, LM-160-24 and LM-160-36 all exhibited reversible microporous regions and adsorption hysteresis loops, categorized as microporous type-IV isotherms unlike those of the precursor CoFe-LDH, LM-100-12 and LM-130-12. Moreover, the hysteresis loop area of LM-160-12 was larger than that of the others, indicating the presence of multilevel pores and a unique hierarchical structure. Additionally, the Brunauer–Emmet–Teller (BET) surface area of LM-160-12 was calculated to be 105.1 m2 g−1 in Fig. S3,† higher than that of CoFe-LDH (39.2 m2 g−1), LM-100-12 (39.8 m2 g−1) and LM-130-12 (43.2 m2 g−1) and lower than that of LM-160-24 (174.5 m2 g−1) and LM-160-36 (247.8 m2 g−1), which was well consistent with XRD results. The total pore volume of specimens is shown in Fig. S3;† both LM-160-12 (0.151 cm3 g−1) and LM-160-24 (0.154 cm3 g−1) possessed nearly twice the pore volume of the precursor CoFe-LDH (0.085 cm3 g−1). The pore sizes of the specimens (Fig. S4 and S5†) were calculated using nonlocal density functional theory (NLDFT), showing that the mesoporous distribution of LM-160-12 was the largest compared with other specimens. The coexistence of the unique microporous and mesoporous structure in LM-160-12 may make it suitable for electrocatalysis.
The morphology was characterized by scanning field-emission scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM). The SEM image (Fig. 2a) indicated that the CoFe-LDH nanosheets possessed a hexagonal shape with a lateral mean diameter of about 50–500 nm, consistent with reported work.21 After transformation via the ligand-assisted method at 160 °C for 12 hours (Fig. 2b), spindle-like thin nanosheets were obtained for LM-160-12. The TEM images (Fig. 2c and d) also revealed the large morphological changes and coincide well with Fig. 2a and b. Interestingly, upon tracking the morphology of the transformation (Fig. S6 and S7†), it suggested that the ligand-assisted transformation from 2D CoFe-LDHs to 2D MOFs resulted in obvious crystal dissociation, recrystallization, and irregular growth. Due to a lack of solution agitation and lack of surfactants in the solid–liquid reaction system, agglomeration and disordered growth happened in the long-term reaction, such as for LM-160-24 and LM-160-36. Encouragingly, high resolution transmission electron microscopy (HRTEM) and high angle annular dark field scanning transmission electron microscopy (HAADF-STEM) images clearly elucidated the spindle-like shape for LM-160-12 (Fig. 2e and f), and the nanosheets possessed a homogeneous distribution of Co, Fe and O elements, which was shown by energy-dispersive X-ray spectroscopy (EDXS) (Fig. 2g–i). Moreover, the AFM image of LM-160-12 in Fig. 2j apparently showed that the nanosheets consisted of ultrathin sheets, as shown in the red dashed circle, and the thickness of the ultrathin sheets was determined to be 4.84–7.39 nm (Fig. 2k). The thickness of a single coordination structural layer was calculated to be about 0.97 nm in reported CoNi 2D MOFs,24 suggesting that LM-160-12 ultrathin nanosheets included 5–8 coordination layers. The above results also revealed that LM-160-12 possessed characteristics of inherent 2D structural periodicity, which may provide exposed active surfaces for MOF-based electrocatalysts.47–49
To further identify the transformation process from LDHs to 2D MOFs, the active metal content and ratio of all specimens were measured by inductively coupled plasma-mass spectrometry (ICP-MS). Both Co and Fe content showed a downward trend with the increase of transformation temperature and time (Fig. 3a and b), because of Co and Fe ions coming only from the precursor CoFe-LDH in the transformation process. The molar ratio of Co/Fe, however, varied between 2.2 for LM-160-12 with the most 2D characteristics to 1.5 for LM-160-36 (Fig. 3c). This coincided well with the PXRD results that a high proportion of the iron content tends to form a microporous structure MIL-88B(Fe). The excessive doping of trivalent iron affected the coordination environment between bivalent cobalt and terephthalic acid to form 2D MOFs. Next, we carried out a dissociation experiment to monitor the cation release of the precursor CoFe-LDH, soaked in DMF at 160 °C measured by ICP-MS. As shown in Fig. 3d, the dissociation ratio of Co/Fe was between 3.5 for the initial 6 hours and decreased to 2.9 after 42 hours, which significantly exceeded the initial ratio of Co/Fe 2.0 for the precursor CoFe-LDH. In other words, the dissociation of cobalt was faster than that of iron at the beginning, which showed that the terephthalic ligand was more inclined to coordinate with cobalt in the early stage of heterogeneous nucleation. These suggested that precursor CoFe-LDH, an adjustable metal release source, played a key role in the ligand-mediated transformation process (Fig. 3e). As shown in Fig. 3e, f and S8,† the terephthalic ligand molecules separated the 2D bimetal layers along an axis, which were composed of pseudo octahedral [CoO6] and [FeO6]. Here, the unsaturated metal sites (green dashed circle) on the surface of LM-160-12 satisfied the desirable four-electron pathway for the WOR (Fig. 3f). However, there were four sets of non-equivalent octahedral [MO6], derived from different metal centers and ligands containing oxygen (Fig. 3g). So it is an enormous challenge to clarify the specific reaction pathways and active centers (Co or Fe).49–53
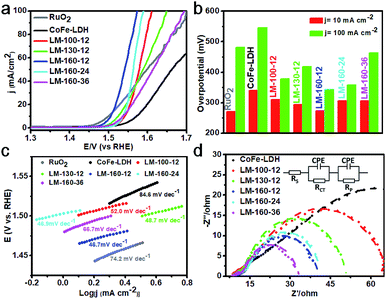
All electrochemical measurements for the WOR were performed with 1.0 M KOH solutions as the electrolyte. As shown in Fig. 4a, the linear sweep voltammetry (LSV) curves were recorded to compare each specimen's WOR catalytic activity. It should be noted that a rather small overpotential (274 mV) was required for LM-160-12 to deliver a current density of 10 mA cm−2 (Fig. 4b), which is comparable with that of commercial RuO2 (271 mV) and superior to most cobalt-based and pristine MOF electrocatalysts (Tables S1 and S2†). Interestingly, the specimen LM-160-24, possessing better crystallinity and specific surface area than LM-160-12, exhibited lower WOR catalytic activity. To understand how and why the weakly crystalline 2D MOF LM-160-12 possesses the best WOR electrocatalytic activity, correlative Tafel slopes were calculated and are shown in Fig. 4c. LM-160-12 showed the smallest Tafel slope (46.7 mV dec−1) compared with isostructural LM-160-24 (46.9 mV dec−1) and the other specimens, indicating better kinetic activity possibly due to fast mass transport and electron transfer. Electrochemical impedance spectroscopy (EIS) was also conducted to evaluate the electron transfer ability and understand the reaction kinetics (Fig. 4d). The equivalent circuit model (the inset of Fig. 4d) was set to fit the impedance responses, where Rs represents solution resistance, Rct represents electron transfer resistance, Rp represents surface porosity and CPE stands for constant phase element. The Rct and Rp values of LM-160-12 (47.8 and 3.4 ohm) were smaller than those of isostructural LM-160-24 (49.8 and 9.4 ohm), also suggesting faster electron transfer and ion diffusion in the WOR process (Table S3†).
Furthermore, the electrochemical surface area (ECSA) was measured by evaluating the electrochemical double-layer capacitance (Cdl), which was proportional to the slope of current differences plotted against scanning rates. LM-160-12 possessed a Cdl value of 25.4 mF cm−2, nearly twice that of LM-160-24 (14.7 mF cm−2), significantly exceeding that of other specimens (Fig. S9–S11†). This ECSA result showed a volcanic type trend, differing from the observed ascending type for the BET surface area. It was clear that the ECSA is not exactly equal to the BET specific surface, so the unique 2D nanosheets' morphological characteristics had a vital impact on the electrocatalytic process. In addition, we compared the LSV curves normalized by the ECSA for all samples in Fig. S12,† where LM-160-12 was superior to others in intrinsic WOR activity and their WOR activity tendency was not entirely consistent with the LSV curves in Fig. 4a. Interestingly, the results indicate that the differences in the WOR performance not only originated from exposed active sites but also the intrinsic activity. To investigate the long-term electrocatalytic stability, we performed chronopotentiometric measurements to evaluate the 2D MOF LM-160-12 loaded on both glassy carbon electrode (GCE) and nickel foam (NF). As revealed in Fig. S13,† LM-160-12 loaded on the GCE showed 10 h stability with a slight decrease in activity, and it was supposed that bubble breakup resulted in catalyst shedding due to fast oxygen evolution. When replaced with applicable NF as the support, a highly stable current density of 10 mA cm−2 was obtained for at least 70 h. Importantly, the HRTEM, HAADF-STEM and EDXS mapping images (Fig. S14†) of LM-160-12 nanosheets loaded on NF after the long-term stability test were consistent with the observation before the reaction. The PXRD results (Fig. S15†) also illustrated that the diffraction peaks of 2D MOFs, (200), (201) and (−201) facets, were still retained modestly after the long-term reaction. Moreover, the valence electron structures before and after electrocatalysis were further confirmed by XPS. From the XPS results (Fig. S16†), the binding energy of Fe 2p3/2 shifted to low binding energy, while an opposite up-shift was observed for Co 2p3/2 at the same time. This suggested that long-term electrocatalysis promoted charge transfer from Fe to Co by the ligands containing oxygen, and deeper studies on electronic structures should be implemented.
Next, to further verify the bimetal interaction, XPS survey was carried out. As shown in Fig. S17,† four elements Co, Fe, O and C were detected on the surface for all specimens. And the molar ratio of Co/Fe measured by XPS was consistent with that detected by ICP-MS (Table S4†). After the transform from LDH to 2D MOFs, the O2− 1s (530.7 eV for CoFe-LDH) peak shifted to higher binding energies (531.2 eV for LM-160-12 and LM-160-24), respectively (Fig. S18†). Meanwhile, Fe3+ 2p3/2 shifted to low binding energy (Fig. 5a), and the same up-shift was obtained for Co2+ 2p3/2 (Fig. 5b). The above results indicated that some of the electrons were transferred from Co2+ to Fe3+ through the bridging oxygen, which could adjust the electron density of the metal sites. It is obvious that the peroxidation of Co2+ to a higher valence state of Co was induced by the π-donation between Fe3+ and bridging O2−, because enhanced π-donation triggered more partial charge transfer from Co2+ to O2−, which may contribute to the best activity of LM-160-12.
To probe the electronic and local structural motifs, X-ray absorption near-edge structure (XANES) was performed on the specimens. As shown in Fig. 5c and S19a,† the X-ray absorption curves of both LM-160-12 and LM-160-24 show Fe K-edge shifts quite close to, albeit somewhat lower, those of the reference La2FeVO6. This supports a formal chemical Fe valence state close to Fe3+ in both materials. At the Co K-edge, the XANES spectra of both specimens showed that the Co atom oxidation state is higher than +2 (Fig. 5d and S19b†). This is consistent with partial substitution of Fe in Co sites in MOFs, which led to charge transfer between both atoms, in accordance with a previous report on metal doping engineering in MOFs.54 The broad unresolved Co–K main edge peak near 7725 eV in LM-160-12, compared to the sharper features in the LM-160-24 spectrum (Fig. 5d) should also be noted. This broadening is consistent with a significant structural distortion of the nearest neighbours to Co sites in the former system compared to the latter.55 In the Fe and Co pre-edge region, two pre-edge peaks at around 7114.2 eV and 7709.8 eV were shown both in LM-160-12 and LM-160-24 (Fig. 5e and f). Both peaks were derived from the transition, from quadrupole allowed 1s to 3d and dipole allowed 1s to 3d-4p hybrid orbitals.56 Here non-centrosymmetric distortions enhance stronger transitions into the 3d–4p hybrid orbitals, and the enhanced pre-edge feature spectral weight for the LM-160-12 material supports a more strongly distorted and unsaturated local environment. In general, the combination of XANES, ECSA and morphological characterization clearly revealed that the existence of more coordination unsaturated metal sites and local structural distortion on LM-160-12 surfaces, compared with LM-160-24, resulted in excellent WOR activity. Hence, we propose that the excellent catalytic performance may mainly originate from the unique 2D characteristics rather than the coupling effect between Co and Fe, which not only provide more coordination unsaturated metal sites but also facilitate electron transport and transfer and ion diffusion.57–59
Conclusions
In summary, a novel ligand-assisted synthetic strategy is demonstrated to prepare bimetal 2D MOF nanosheets as highly active and durable electrocatalysts for the WOR, by the transformation from 2D LDHs. Furthermore, the precursor LDHs serve as an adjustable metal release source to control the heterogeneous nucleation process, forming layered bimetal 2D MOFs. The coupling effect between Co and Fe promotes the delocalization of Co 3d electron and enhances the WOR activity. More importantly, the layered bimetal 2D CoFe-MOFs possess more exposed active sites, rapid electron transfer and faster ion diffusion, which make major contributions to the superior activity in our work. We believe that the unique ligand-assisted synthetic strategy will extend opportunities towards constructing 2D MOF-based materials for widespread applications such as energy storage and conversion.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the 100 Talents Plan Foundation of Sun Yat-sen University, the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2017ZT07C069), Guangdong Natural Science Funds for Distinguished Young Scholar (No. 2015A030306027), and NSFC projects (201875287, 21821003, and 21890380). Part of this research used the QAS, 7-BM beamline at the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704.Notes and references
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- B. You and Y. Sun, Acc. Chem. Res., 2018, 51, 1571–1580 CrossRef CAS.
- M. T. M. Koper, J. Electroanal. Chem., 2011, 660, 254–260 CrossRef CAS.
- J. Zhang, Q. Zhang and X. Feng, Adv. Mater., 2019, 31, 1808167 CrossRef.
- C. Tang, H.-F. Wang and Q. Zhang, Acc. Chem. Res., 2018, 51, 881–889 CrossRef CAS PubMed.
- F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu, L. Liardet and X. Hu, J. Am. Chem. Soc., 2018, 140, 7748–7759 CrossRef CAS PubMed.
- X. Li, J. Wei, Q. Li, S. Zheng, Y. Xu, P. Du, C. Chen, J. Zhao, H. Xue, Q. Xu and H. Pang, Adv. Funct. Mater., 2018, 28, 1800886 CrossRef.
- R. Zhu, J. Ding, Y. Xu, J. Yang, Q. Xu and H. Pang, Small, 2018, 14, 1803576 CrossRef PubMed.
- Q. Xiang, F. Li, W. Chen, Y. Ma, Y. Wu, X. Gu, Y. Qin, P. Tao, C. Song, W. Shang, H. Zhu, T. Deng and J. Wu, ACS Energy Lett., 2018, 3, 2357–2365 CrossRef CAS.
- H.-P. Guo, B.-Y. Ruan, W.-B. Luo, J. Deng, J.-Z. Wang, H.-K. Liu and S.-X. Dou, ACS Catal., 2018, 8, 9686–9696 CrossRef CAS.
- J. Chen, F. Zheng, S.-J. Zhang, A. Fisher, Y. Zhou, Z. Wang, Y. Li, B.-B. Xu, J.-T. Li and S.-G. Sun, ACS Catal., 2018, 8, 11342–11351 CrossRef CAS.
- D. Zhou, Z. Cai, X. Lei, W. Tian, Y. Bi, Y. Jia, N. Han, T. Gao, Q. Zhang, Y. Kuang, J. Pan, X. Sun and X. Duan, Adv. Energy Mater., 2018, 8, 1701905 CrossRef.
- X. Xu, H. Liang, F. Ming, Z. Qi, Y. Xie and Z. Wang, ACS Catal., 2017, 7, 6394–6399 CrossRef CAS.
- J.-S. Li, Y. Wang, C.-H. Liu, S.-L. Li, Y.-G. Wang, L.-Z. Dong, Z.-H. Dai, Y.-F. Li and Y.-Q. Lan, Nat. Commun., 2016, 7, 11204 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, Y. Zhu, Q. Cai, A. Vasileff, L. H. Li, Y. Han, Y. Chen and S.-Z. Qiao, J. Am. Chem. Soc., 2017, 139, 3336–3339 CrossRef CAS PubMed.
- J. Tian, Q. Liu, A. M. Asiri, K. A. Alamry and X. Sun, ChemSusChem, 2014, 7, 2125–2130 CrossRef CAS PubMed.
- I. S. Amiinu, Z. Pu, X. Liu, K. A. Owusu, H. G. R. Monestel, F. O. Boakye, H. Zhang and S. Mu, Adv. Funct. Mater., 2017, 27, 1702300 CrossRef.
- J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. Dong, S. Liu, X. Zhuang and X. Feng, Angew. Chem., Int. Ed., 2016, 55, 6702–6707 CrossRef CAS.
- R. Liu, Y. Wang, D. Liu, Y. Zou and S. Wang, Adv. Mater., 2017, 29, 1701546 CrossRef.
- D. Wang, Q. Li, C. Han, Z. Xing and X. Yang, ACS Cent. Sci., 2018, 4, 112–119 CrossRef CAS PubMed.
- S. Yin, W. Tu, Y. Sheng, Y. Du, M. Kraft, A. Borgna and R. Xu, Adv. Mater., 2018, 30, 1705106 CrossRef PubMed.
- M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2013, 135, 8452–8455 CrossRef CAS.
- S. Zhao, Y. Wang, J. Dong, C.-T. He, H. Yin, P. An, K. Zhao, X. Zhang, C. Gao, L. Zhang, J. Lv, J. Wang, J. Zhang, A. M. Khattak, N. A. Khan, Z. Wei, J. Zhang, S. Liu, H. Zhao and Z. Tang, Nat. Energy, 2016, 1, 16184 CrossRef CAS.
- J. Huang, Y. Li, R. K. Huang, C. T. He, L. Gong, Q. Hu, L. Wang, Y. T. Xu, X. Y. Tian, S. Y. Liu, Z. M. Ye, F. Wang, D. D. Zhou, W. X. Zhang and J. P. Zhang, Angew. Chem., Int. Ed., 2018, 57, 4632–4636 CrossRef CAS.
- W. Cheng, X. Zhao, H. Su, F. Tang, W. Che, H. Zhang and Q. Liu, Nat. Energy, 2019, 4, 115–122 CrossRef CAS.
- S. Jin, ACS Energy Lett., 2019, 4, 1443–1445 CrossRef CAS.
- Y. Xu, B. Li, S. Zheng, P. Wu, J. Zhan, H. Xue, Q. Xu and H. Pang, J. Mater. Chem. A, 2018, 6, 22070–22076 RSC.
- D. Mullangi, V. Dhavale, S. Shalini, S. Nandi, S. Collins, T. Woo, S. Kurungot and R. Vaidhyanathan, Adv. Energy Mater., 2016, 6, 1600110 CrossRef.
- H. B. Aiyappa, J. Thote, D. B. Shinde, R. Banerjee and S. Kurungot, Chem. Mater., 2016, 28, 4375–4379 CrossRef CAS.
- D. Deng, K. S. Novoselov, Q. Fu, N. Zheng, Z. Tian and X. Bao, Nat. Nanotechnol., 2016, 11, 218–230 CrossRef CAS.
- A. Gupta, T. Sakthivel and S. Seal, Prog. Mater. Sci., 2015, 73, 44–126 CrossRef CAS.
- B. Mendoza-Sanchez and Y. Gogotsi, Adv. Mater., 2016, 28, 6104–6135 CrossRef CAS.
- D. McAteer, I. J. Godwin, Z. Ling, A. Harvey, L. He, C. S. Boland, V. Vega-Mayoral, B. Szydłowska, A. A. Rovetta, C. Backes, J. B. Boland, X. Chen, M. E. G. Lyons and J. N. Coleman, Adv. Energy Mater., 2018, 8, 1702965 CrossRef.
- J. S. Qin, D. Y. Du, W. Guan, X. J. Bo, Y. F. Li, L. P. Guo, Z. M. Su, Y. Y. Wang, Y. Q. Lan and H. C. Zhou, J. Am. Chem. Soc., 2015, 137, 7169–7177 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS.
- T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, I. X. F. X. Llabres and J. Gascon, Nat. Mater., 2015, 14, 48–55 CrossRef CAS.
- Z. Fang, B. Bueken, D. E. De Vos and R. A. Fischer, Angew. Chem., Int. Ed., 2015, 54, 7234–7254 CrossRef CAS.
- H. Hu, B. Guan, B. Xia and X. W. Lou, J. Am. Chem. Soc., 2015, 137, 5590–5595 CrossRef CAS.
- S. Lin, C. S. Diercks, Y.-B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Science, 2015, 349, 1208 CrossRef CAS.
- S. Choi, W. Cha, H. Ji, D. Kim, H. J. Lee and M. Oh, Nanoscale, 2016, 8, 16743–16751 RSC.
- D. X. Xue, Y. Belmabkhout, O. Shekhah, H. Jiang, K. Adil, A. J. Cairns and M. Eddaoudi, J. Am. Chem. Soc., 2015, 137, 5034–5040 CrossRef CAS.
- K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurin and M. Eddaoudi, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC.
- P. He, X.-Y. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2017, 56, 3897–3900 CrossRef CAS.
- T. Tanasaro, K. Adpakpang, S. Ittisanronnachai, K. Faungnawakij, T. Butburee, S. Wannapaiboon, M. Ogawa and S. Bureekaew, Cryst. Growth Des., 2017, 18, 16–21 CrossRef.
- Z. Xue, Y. Li, Y. Zhang, W. Geng, B. Jia, J. Tang, S. Bao, H.-P. Wang, Y. Fan, Z.-w. Wei, Z. Zhang, Z. Ke, G. Li and C.-Y. Su, Adv. Energy Mater., 2018, 8, 1801564 CrossRef.
- C. G. Morales-Guio, L. Liardet and X. Hu, J. Am. Chem. Soc., 2016, 138, 8946–8957 CrossRef CAS PubMed.
- P. Zhou, Y. Wang, C. Xie, C. Chen, H. Liu, R. Chen, J. Huo and S. Wang, Chem. Commun., 2017, 53, 11778–11781 RSC.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS PubMed.
- M. Gorlin, P. Chernev, J. Ferreira de Araujo, T. Reier, S. Dresp, B. Paul, R. Krahnert, H. Dau and P. Strasser, J. Am. Chem. Soc., 2016, 138, 5603–5614 CrossRef PubMed.
- D. Wang, J. Zhou, Y. Hu, J. Yang, N. Han, Y. Li and T.-K. Sham, J. Phys. Chem. C, 2015, 119, 19573–19583 CrossRef CAS.
- J. Y. Chen, L. Dang, H. Liang, W. Bi, J. B. Gerken, S. Jin, E. E. Alp and S. S. Stahl, J. Am. Chem. Soc., 2015, 137, 15090–15093 CrossRef CAS PubMed.
- M. B. Stevens, C. D. M. Trang, L. J. Enman, J. Deng and S. W. Boettcher, J. Am. Chem. Soc., 2017, 139, 11361–11364 CrossRef CAS.
- Y. Mun, M. J. Kim, S.-A. Park, E. Lee, Y. Ye, S. Lee, Y.-T. Kim, S. Kim, O.-H. Kim, Y.-H. Cho, Y.-E. Sung and J. Lee, Appl. Catal., B, 2018, 222, 191–199 CrossRef CAS.
- Q. Qian, T. A. Tyson, C. C. Kao, M. Croft, S. W. Cheong, G. Popov and M. Greenblatt, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 024430 CrossRef.
- B. Wang, X. Wang, J. Zou, Y. Yan, S. Xie, G. Hu, Y. Li and A. Dong, Nano Lett., 2017, 17, 2003–2009 CrossRef CAS.
- Y. Xu, Q. Li, H. Xue and H. Pang, Coord. Chem. Rev., 2018, 376, 292–318 CrossRef CAS.
- Y. T. Xu, Z. M. Ye, J. W. Ye, L. M. Cao, R. K. Huang, J. X. Wu, D. D. Zhou, X. F. Zhang, C. T. He, J. P. Zhang and X. M. Chen, Angew. Chem., Int. Ed., 2019, 58, 139–143 CrossRef CAS PubMed.
- F.-L. Li, P. Wang, X. Huang, D. J. Young, H.-F. Wang, P. Braunstein and J.-P. Lang, Angew. Chem., Int. Ed., 2019, 58, 7051–7056 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta09397d |
| This journal is © The Royal Society of Chemistry 2020 |