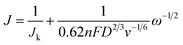Ultrathin bismuth nanosheets as an efficient polysulfide catalyst for high performance lithium–sulfur batteries†
Hongfei
Xu
,
Shubin
Yang
 and
Bin
Li
and
Bin
Li
 *
*
School of Materials Science & Engineering Beijing, Beihang University, 100191, China. E-mail: li_bin@buaa.edu.cn
First published on 14th November 2019
Abstract
Although many materials have sprung up as catalysts to improve polysulfide conversion in lithium–sulfur batteries, the catalytic mechanism is vague and a universal and efficient catalyst is still absent. Herein, we developed ultrathin polycrystalline bismuth (2D-Bi) nanosheets (thickness of ∼4 nm) as an electrocatalyst for polysulfide conversion. Such a 2D polycrystalline structure not only absorbs and immobilizes soluble polysulfides but also accelerates multistep polysulfide redox reactions, greatly inhibiting the polysulfide shuttling during charge–discharge cycles. Furthermore, we gained an in-depth understanding of the distinctive electrocatalysis mechanism of 2D-Bi nanosheets by steady-state and dynamic electrochemical testing methods. The results showed that with the 2D-Bi electrocatalyst, the exchange current density (i0) and electron transfer number (n) (i0 > 3 mA cm−2, n = 6.6 at 2.1 V) were significantly enhanced. As a consequence, a 2D-Bi modified Li–S battery exhibited good electrochemical performance with high reversible capacity, stable cycle life and superb rate performance (retaining a capacity of 408 mA h g−1 after 500 cycles at 10C). Ultimately, a deep exploration and comprehension of the interior redox process as well as the conversion mechanism of sulfides provided a great avenue for accelerating the large-scale application of Li–S batteries in the near future.
1. Introduction
Bismuth is one of the most stable elements among heavy metals, and it also possesses other superiorities such as being non-toxic and inexpensive. Lately, bismuth has drawn attention because of some unique and marvelous advantages in fields such as the electrochemical nitrogen reduction reaction (ENRR) and CO2 reduction reaction (CO2RR).1–3 For example, Bi nanocrystals were synthesized by Yan and his co-workers to act as the catalyst to promote the ENRR.4 Benefiting from the interaction between the p orbitals of bismuth and nitrogen absorbers as well as the semiconducting nature of bismuth which could provide a lower free-energy change for forming the *NNH intermediate, the obtained faradaic efficiency and ammonia yield reached a surprising record-high value. In addition to the auxo-action on the ENRR, mesoporous Bi nanosheets were used by Lu and his co-workers for selective CO2 reduction. The (012), (101), and (111) planes of bismuth can significantly stabilize the OCHO* intermediate while hindering the formation of COOH* and H*, and therefore a high catalytic activity and great formate selectivity were achieved. Enlightened by the remarkable electrochemical catalysis capability of Bi, we set about exploring if this material could play a special role in polysulfide conversions.Lithium–sulfur batteries have made great advances due to the progress of materials science and technology, but the complex conversion mechanism of polysulfides still needs to be investigated in detail. Recently, studies have been focused more on the sulfide kinetics as well as the Li–S battery chemical conversion processes.5–7 It has been proved that the electrochemical performance of Li–S batteries can be enhanced notably by adding a small number of catalytic materials.8–10 These catalytic materials are mainly focused on noble metals and strong polar materials.11–13 Noble metals such as Pt were first used as catalysts by Arava and his co-workers to expedite the redox reactions of polysulfides.14 They proved that the graphene/sulfur cathode with a Pt catalyst exhibited a significantly increased exchange current density, which was confirmed via potentiostatic polarization experiments. The accelerated transformation of polysulfides effectively inhibited the shuttling of polysulfides, leading to high coulombic efficiency and long-term cycling stability. A strong polar material, 2D MoN–VN heterostructure, was employed by Shi-Zhang Qiao and coworkers as a high-efficiency polysulfide conversion electrocatalyst.15In situ synchrotron XRD measurements were conducted to reveal the electrochemical conversion process. They found improved sulfur utilization efficiency by using a MoN–VN/S electrode because the α-S8 peaks disappeared completely after discharging.16 Although these catalysts have been proposed to enhance the electrochemical performance of the sulfur cathode, further exploration of efficient and universal catalysts is necessary, and more insight into the catalyst mechanism for polysulfide conversion is crucial.
In this work, ultrathin 2D-Bi nanosheets were synthesized by reducing precursor Bi2O2CO3 (BiOC) and found to be an effective multi-functional catalyst for sulfide conversion. The obtained 2D-Bi nanosheets with a high surface area can not only act as a trap to absorb polysulfides, but also as a multi-step accelerant to promote conversions between Li2S and S8. Static cyclic voltammetry (CV) tests of a symmetric cell and asymmetric cell were used to investigate the overall redox reactions while dynamic linear sweep voltammetry (LSV) via the rotating disk electrode (RDE) method was used to probe the sulfides' maximum catalytic performance and reaction mechanism. These steady-state and dynamic electrochemical test results indicated that 2D-Bi nanosheets are able to boost three main conversions, including high-order polysulfides to lower-order polysulfides, Li2S to S8 and the nucleation of Li2S. Benefiting from the aforementioned unique properties, the Li–S full cells assembled with a 2D-Bi modified separator exhibited excellent electrochemical performance in terms of high rate capability and cycling stability (reversible capacity of 1090 mA h g−1 at 0.5C; capacity retention of 408 mA h g−1 after 500 cycles even at 10C).
2. Experimental
2.1 Materials characterization
The crystal structures were identified via Powder X-ray diffraction (Rigaku D/max-2550 V) using Cu Kα (λ = 1.5406 Å) radiation. The morphologies and structures of the samples were characterized by field emission scanning electron microscopy (FESEM, ZEISS SUPRATM 55) at an accelerating voltage of 5–10 kV, and transmission electron microscopy (TEM, JEM 2010 (JEOL Ltd, Japan) operated at 120.0 kV). The sulfur content was determined by thermogravimetric analysis (TGA) by using a NETZSCH TG 209 F1 Libra.2.2 Synthesis of 2D-Bi
Raw materials including bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), hexadecyltrimethylammonium bromide (CTAB), urea (Acros, China), hydrazine hydrate (64%, Acros, China), and N-methyl-pyrrolidone (NMP, Sinopharm) were all purchased from Innochem without further purification.In a typical synthesis of BiOC nanosheets, 0.1 g Bi(NO3)3·5H2O was added into 40 mL NMP; then, 0.05 g CTAB and 1 g urea were also added into the above NMP solution. After 20 min of ultrasound and stir treatment to make all solutes dissolve, the uniform solution was transferred into a 100 mL Teflon-lined autoclave, and heated at 155 °C for 12 h. The solid product was collected by centrifugation and washed with ethanol and water three times. Then it was kept in a vacuum oven at 60 °C for 6 h after which white BiOC powder was obtained.
To obtain 2D-Bi nanosheets, a simple chemical one-step reduction method was used. First, the as-synthesized BiOC was added into 20 mL NMP and then 3 mL hydrazine hydrate was added. After that, the temperature was increased to 75 °C and the mixture was kept stirring for 30 min during which the colour gradually turned black. Finally, after centrifugation and being washed with NMP, ethanol, and water, the sample was dried in a vacuum oven at 60 °C.
2.3 LiPS adsorption study
Li2S6 was selected as the representative LiPS. Li2S6 solutions were prepared by reacting lithium sulfide (Li2S) and elemental sulfur in the desired mass ratio in DME/DOL (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent in an Ar-filled glovebox. The mass of 2D-Bi, Bi bulk, and KB (Ketjen Black) which were added into the above Li2S6 solution was 13 mg and the concentration of Li2S was 5 mmol L−1. We also tested the adsorption ability of 2D-Bi at a higher Li2S6 concentration of 15 mmol L−1. To simulate the real working environment in batteries, all the vials for the color test were left to stand and tested without vigorous stirring. The adsorption ability of 2D-Bi, Bi bulk, and KB was then qualitatively determined by using an ultraviolet-visible spectrometer (UV-3600, Japan).
1) solvent in an Ar-filled glovebox. The mass of 2D-Bi, Bi bulk, and KB (Ketjen Black) which were added into the above Li2S6 solution was 13 mg and the concentration of Li2S was 5 mmol L−1. We also tested the adsorption ability of 2D-Bi at a higher Li2S6 concentration of 15 mmol L−1. To simulate the real working environment in batteries, all the vials for the color test were left to stand and tested without vigorous stirring. The adsorption ability of 2D-Bi, Bi bulk, and KB was then qualitatively determined by using an ultraviolet-visible spectrometer (UV-3600, Japan).
2.4 Kinetics study
For the symmetric cell, Li2S6 was used to study the conversion process from Li2S6 to Li2S by a CV test. The coated materials on Al foil including 70 wt% 2D-Bi (or Bi bulk, KB, or BiOC), 20 wt% SP, and 10 wt% PVDF acted as the working electrode and counter electrode. The electrolyte was 20 μL as-prepared Li2S6 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) DOL/DME solution. CV was performed at a scan rate of 5 mV s−1 between −1 V and 1 V.
1 (v/v) DOL/DME solution. CV was performed at a scan rate of 5 mV s−1 between −1 V and 1 V.
For the asymmetric cell, Li2S was used to study the reverse conversion from Li2S to S8 by a CV test. The coated materials on Al foil for the working electrode were 70 wt% 2D-Bi (or Bi bulk, KB, or BiOC), 20 wt% SP, and 10 wt% PVDF. The counter electrode was Li2S, which was obtained by disassembling the anode of a full cell that had been discharged at 1.6 V for 8 h. The electrolyte for the working electrode was 20 μL Li2S6 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) DOL/DME solution and for the counter electrode it was 20 μL normal Li–S electrolyte (1 M lithium bis-(trifluoromethanesulfonyl)imide (LiTFSI) in a 1,3-dioxolane(DOL)–dimethoxyethane (DME) mixture (1
1 (v/v) DOL/DME solution and for the counter electrode it was 20 μL normal Li–S electrolyte (1 M lithium bis-(trifluoromethanesulfonyl)imide (LiTFSI) in a 1,3-dioxolane(DOL)–dimethoxyethane (DME) mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v)).
1, v/v)).
For the rotating disk electrode measurement, a commercial rotating disk electrode (RDE) (a PTFE embedded glassy carbon disc of 5.0 mm, with a geometric area of 0.196 cm2) was modified with catalyst inks (0.2 mg mL−1, the ink solution contains 800 mL deionized water, 200 mL isopropanol and 5 mL 5 wt% Nafion solution). A Li coil and a Pt coil were used as the reference electrode and counter electrode, respectively. These electrodes were immersed in a solution of 8 mL Li2S8 (4.29 × 10−6 mol cm−3 with 0.5 mol L−1 lithium bis(trifluoromethanesulfonyl)imide (LiTFSI)). For linear sweep voltammetry (LSV), the scan rate was 50 mV s−1 from 2.3 to 1.5 V. All the tests were conducted in an Ar-filled glovebox.
To determine the reaction kinetic current and electron transfer number, Levich–Koutecky plots of 1/Jkvs. ω−1/2 were first fitted with different potentials at each rotation rate. We can easily obtain the reaction kinetic current from the intercept. For the electron transfer number (n), the Levich–Koutecky equation was used:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), D is the diffusion coefficient of S82− (2.3 × 10−6 cm2 s−1), which was determined from former references, ν is the kinematic viscosity of the electrolyte (0.013 cm2 s−1), and C is the concentration of dissolved Li2S8 (4.29 × 10−6 mol cm−3).
485 C mol−1), D is the diffusion coefficient of S82− (2.3 × 10−6 cm2 s−1), which was determined from former references, ν is the kinematic viscosity of the electrolyte (0.013 cm2 s−1), and C is the concentration of dissolved Li2S8 (4.29 × 10−6 mol cm−3).
For the Li2S nucleation measurement, 2D-Bi, Bi bulk, and KB were prepared as the Li2S deposition matrix and lithium foil served as the anode. Li2S8 solution (prepared in a similar way to Li2S6 solution) was used as the active material, and was dropped into the cathode and blank electrolytes, but no Li2S8 was added into the lithium anode. The assembled cells were discharged at 0.1 mA to 2.08 V and then kept at 2.07 V where Li2S nucleated and grew. The potentiostatic discharge ceased after about 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s and the nucleation/growth rate of Li2S was evaluated according to Faraday's law.
000 s and the nucleation/growth rate of Li2S was evaluated according to Faraday's law.
2.5 Preparation of modified separators
The slurry was prepared by mixing 25 mg 2D-Bi, 3 mg KB, and 3 mg PVDF in NMP solution. Then the slurry was coated on an 80 cm2 piece of Celgard 2400 PP separator. Afterward, the separator was placed in a 60 °C vacuum oven to make it dry before being punched into disks. The average catalyst loading was measured to be 0.3 mg cm−2.2.6 Li–S cell assembly and measurements
To prepare the cathode material, sulfur (Innochem, AR, ≥99.5%) was mixed with KB (Ketjen Black EC300J) at a mass ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and heated at 155 °C for 12 h. A slurry was prepared by mixing S-KB, SP, and PVDF at a mass ratio of 7
2 and heated at 155 °C for 12 h. A slurry was prepared by mixing S-KB, SP, and PVDF at a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in NMP and was cast on carbon cloth and dried at 55 °C for 20 hours under vacuum. These cathodes were tested in CR2032 coin cells with Li metal as a counter electrode. 1,3-Dioxolane and 1,2-dimethoxyethane (volume ratio 1
1 in NMP and was cast on carbon cloth and dried at 55 °C for 20 hours under vacuum. These cathodes were tested in CR2032 coin cells with Li metal as a counter electrode. 1,3-Dioxolane and 1,2-dimethoxyethane (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing 1 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), and 0.1 M LiNO3 were used as the electrolyte. The amount of electrolyte was controlled with an electrolyte/sulfur (E/S) ratio of 16 μL mg−1 for cells with a sulfur loading of 1.5 mg. The electrochemical properties were tested using a battery cycler (LAND-CT2001A). A galvanostatic charge–discharge test was performed in a voltage range from 1.6 to 2.8 V. CV tests were conducted on a CHI760E electrochemical workstation at a scan rate of 0.2 mV s−1.
1) containing 1 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), and 0.1 M LiNO3 were used as the electrolyte. The amount of electrolyte was controlled with an electrolyte/sulfur (E/S) ratio of 16 μL mg−1 for cells with a sulfur loading of 1.5 mg. The electrochemical properties were tested using a battery cycler (LAND-CT2001A). A galvanostatic charge–discharge test was performed in a voltage range from 1.6 to 2.8 V. CV tests were conducted on a CHI760E electrochemical workstation at a scan rate of 0.2 mV s−1.
2.7 Computational details
Density functional theory (DFT) calculations were carried out using the Vienna Ab initio Simulation Package (VASP). The general gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE) was employed for the exchange–correlation functional. The projector augmented wave (PAW) method was used to describe the electron–core interactions with a kinetic energy cutoff of 500 eV. Brillouin zones were sampled with a 2 × 2 × 1 Gamma special k-point grid for the 2 × 2 Bi(012) and 2 × 2 Bi(101) surface supercells. A vacuum layer of 20 Å in the vertical direction to the model slab was set to avoid lateral interactions between layers and their images. The top two layers were allowed to fully relax while the bottom two layers were fixed. The geometry optimization was stopped when the forces on all unconstrained atoms were less than 0.05 eV Å−1. The initial stable polysulfide molecule was obtained using the molecular mechanics (MM) method (Genmer) and GAUSSIAN 09 package.The binding energy Eb was defined as
| Eb = Etotal − Eslab − ELi2Sx |
3. Results and discussion
The ultrathin 2D-Bi nanosheets were synthesized via a one-step chemical reduction of the BiOC precursor (Bi2O2CO3) by using hydrazine hydrate in NMP solution (Fig. 1). After the time-controlled reduction process, orthorhombic BiOC gradually transformed into a trigonal Bi structure while its polycrystalline nature was retained. As can be seen from Fig. 2a, the scanning electron microscopy (SEM) measurement revealed that the reduced Bi shows a 2D sheet structure which is in contrast to commercial Bi bulk (ESI Fig. S1†). This special structure was derived from the BiOC precursor, which exhibits a hierarchical cluster structure and consists of many petal-like layers which could be attributed to the mild reduction conditions (ESI Fig. S2†). So after reduction in NMP solution, the 2D structure was well preserved and the height of each lamellar layer was approximately 4 nm according to atomic force microscopy (AFM) scanning results (Fig. 2c and the corresponding SEM images in Fig. S3†).17 The HRTEM image and the corresponding elemental distribution of BiOC are also displayed in Fig. S4.† One can find that the nanosheets of BiOC are very thin and flexible. With the extension of reduction time, BiOC was gradually reduced to Bi, and the sample's color turned from white to dark brown (Fig. S5†). Furthermore, transmission electron microscopy (TEM) image and the corresponding energy-dispersive spectroscopy mapping image (Fig. 2e) demonstrated the ultrathin structure of the as-prepared 2D-Bi. Profiting from this 2D structure, Bi nanosheets exhibit greatly increased surface area, outclassing commercial Bi bulk. In Fig. 2g and S6,† Brunauer–Emmett–Teller (BET) nitrogen adsorption isotherm experiments reveal a 2D-Bi surface area of 72.24 m2 g−1 while Bi bulk shows no clear N2 adsorption/desorption gradient.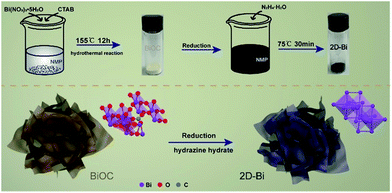 | ||
| Fig. 1 The synthesis route to the 2D-Bi nanosheets. The typical 2D structure of bismuth inherited from the BiOC precursor nanosheets due to the mild reduction conditions. | ||
The synthesized outspread 2D-Bi nanosheets are just like a huge seine and the crystal faces act as numerous meshes to trap the dissociative polysulfides. Selected area electron diffraction (SAED) confirms their polycrystalline properties and the diffraction rings are calibrated by using the formula Rd = Lλ, where L is the camera constant, d is the crystalline interplanar spacing, λ is the electron wavelength, and R is the diffraction ring distance. And the corresponding crystal faces are speculated and marked in the image.18,19 The HRTEM images in Fig. 2f and S7† also revealed that the 2D-Bi nanosheets exposed the (012), (101), and (104) planes and these planes play a major role in their strong affinity for polysulfides. In Fig. 2h, the X-ray diffraction (XRD) patterns confirm the complete transformation from BiOC to Bi. The peaks of the as-synthesized 2D-Bi and commercial Bi bulk share the same hexagonal characteristics as those of standard Bi patterns. According to the X-ray photoelectron spectroscopy (XPS) spectra in Fig. 2i, because of the adsorbed O2 on the surface, the Bi 4f spectra have a shift to the higher energy direction (Bi 4f5/2 (159.05 eV) and Bi 4f7/2 (164.37 eV)).20,21 The existential state of O2 could be further confirmed from O 1s spectra in Fig. S8† where the peaks at 531 eV could be ascribed to the adsorbed oxygen. Different from 2D-Bi, the Bi 4f spectrum of BiOC shows split peaks of B–O and B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.
O.
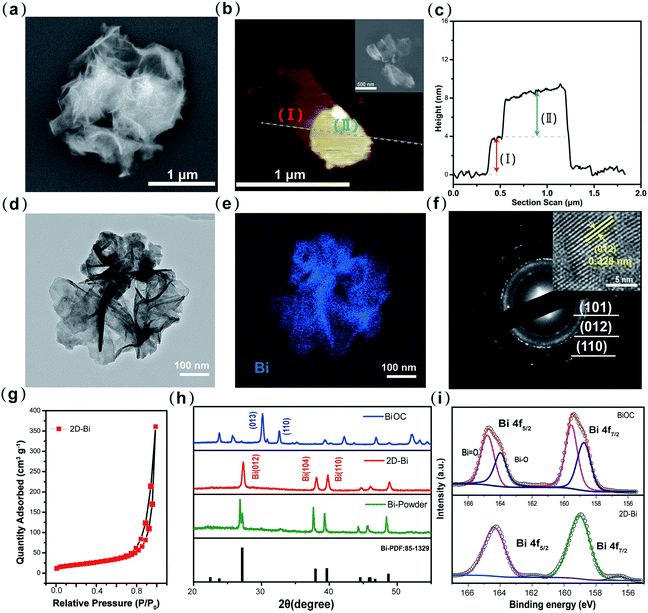
In order to confirm the strong interaction between 2D-Bi and polysulfides, a simple yet visual absorption test was performed where Li2S6 was selected as a representative polysulfide.22,23 As can be seen from Fig. 3a, the Li2S6 solution (5 μL 0.5 mol L−1 Li2S6 dissolved in 2.5 mL DOL/DME electrolyte) with 2D-Bi became lucid after adding 2D-Bi and leaving it to stand for 6 h. Other samples with different adsorbents (Bi bulk and Ketjen Black (KB)) only showed slight changes in color. Besides, when we increased the concentration of Li2S6 and kept the mass of 2D-Bi unchanged, the absorption phenomenon with time was still observable and one can clearly find the gradual sedimentation process of polysulfides (Fig. S9†). It could be attributed to the outstretched layer structure where plentiful exposed active sites ensure the strong chemical interaction between Bi and polysulfides.
The sharp contrast of adsorption capacity can be clearly revealed from the ultraviolet-visible (UV-Vis) spectra of the electrolyte supernatant liquid.24 The relative intensity of the absorption peaks demonstrates that 2D-Bi possesses the strongest affinity for polysulfides among these samples (2D-Bi, KB, and Bi bulk). Given the ultrathin nanostructure, 2D-Bi with a much higher surface area is able to catch more polysulfides. Then, to gain more accurate information on chemical interaction with polysulfides, X-ray photoelectron spectroscopy (XPS) was employed and the fitting results are shown in Fig. 3b.25 The main peaks of the pristine 2D-Bi spectrum located at 164.37 eV (Bi 4f5/2) and 159.05 eV (Bi 4f7/2) correspond to the binding of Bi/O2 in an air atmosphere. These peaks had a red-shift of 0.32 eV after absorbing polysulfides. This red-shift phenomenon could be explained by the absorption of polysulfides and the transfer of electrons from polysulfides to Bi. A similar phenomenon also occurred with Bi bulk, yet this shift is only half that of 2D-Bi (0.16 eV), which means a relatively weak binding.
To further confirm the affinity between Bi nanosheets and polysulfides, first-principles calculations were carried out to theoretically evaluate the adsorption energy.26 According to density functional theory (DFT) calculations, the binding energy (Eb) was defined as Eb = Etotal − Eslab − ELi2Sx, where ELi2Sx, Eslab, and Etotal are the energies of an isolated polysulfide molecule in the gas phase, the corresponding clean slab system (clean Bi(012) or Bi(101) surface) and the total energies of the adsorbed system, respectively. From Fig. 3c, the binding energies of polysulfides are 1.74, 1.22, 0.46, 0.38, and 0.34 eV (Li2S → Li2S8) over the Bi(012) face and 2.36, 1.34, 0.45, 0.32, and 0.39 eV (Li2S → Li2S8) over the Bi(101) face. The results suggest a relatively strong interaction between polysulfides and Bi, which is in good agreement with the visual adsorption results. Interestingly, the calculation results revealed that different lattice planes of Bi exhibited diverse adsorption capacities for Li2Sx. The (012) plane shows stronger absorbability for Li2S4 and Li2S6 while the (101) plane prefers to have a strong binding with Li2S and Li2S2 molecules. The advantage of this polycrystalline Bi contributes to a comprehensive restriction of polysulfide molecules. It can also be found that in ether-based electrolyte, there is a clear decrease in the affinity capability with the increase of polysulfide chain length.27 According to the above results and analysis, our synthesized 2D-Bi with high surface area exhibited superb affinity for polysulfides. And it is well known that efficient adsorption will facilitate the subsequent conversion from high-order polysulfides to Li2S.
The complex reaction process that induced the sluggish electrochemical reactions and dissolution/shuttling of intermediate polysulfides is the culprit for poor rate capability and fast capacity decay of the sulfur cathode.28,29 Although various materials were developed to improve the sluggish electrochemical reactions, the efficiency is still below expectation and the mechanism is also enigmatic. To gain an in-depth understanding of the electrochemical reaction process of the sulfur cathode, three powerful methods of static cyclic voltammetry (CV), dynamic linear sweep voltammetry (LSV) and chronoamperometry were employed and the results analyzed.
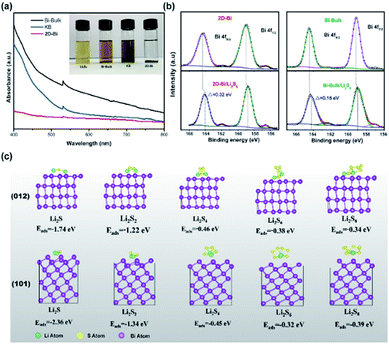
In order to figure out in which step or steps the 2D-Bi plays a role, we assembled a symmetric cell (2D-Bi as the working electrode and counter electrode) to study the transformation from Li2S6 to Li2S and an asymmetric cell (2D-Bi as a working electrode and 2D-Bi/Li2S as a counter electrode) to understand the reaction from Li2S to polysulfides. The control samples of KB, Bi bulk, and BiOC were also tested under the same conditions. As seen in Fig. 4a, for 2D-Bi, three typical reduction peaks can be identified at −0.03 (peak a), −0.21 (peak b), and −0.58 V (peak c), which are related to the step-wise conversion of S8 to S62−, S62− to S42−, and S42− to Li2S, respectively (Fig. 4c). As for Bi bulk, neither the number of peaks nor the intensity of the current is comparable with those of 2D-Bi, which could be attributed to the lack of sufficient surface area for attracting polysulfides. Fig. 4b shows the CV of the asymmetric cell. For 2D-Bi, because our original active material is Li2S for the counter electrode, the two reduction peaks located at −0.18 (peak d) and −0.6 V (peak e) represent the conversion of Li2S to S62− and S62− to S8, respectively. Bi bulk shows similar peaks while the polarization and current intensity are inferior to those of the 2D-Bi electrode. BiOC also shows weak facilitation to both conversion of Li2S6 and Li2S due to its inherent poor conductivity (Fig. S10 and S11†). According to the above analysis, one can find that 2D-Bi promotes both oxidation of Li2S to polysulfides and reduction of Li2S6 to the final Li2S as illustrated in Fig. 4c. In other words, the multi-step catalyst not only absorbs dissociative polysulfides to make the most of the active materials but also benefits the reverse reaction which guarantees good capacity retention.
The Rotating Disk Electrode (RDE) is widely used in electrocatalytic studies and here we recorded linear sweep voltammetry (LSV) curves at different rotation speeds in Li2S8 solution at a scan rate of 50 mV s−1 to probe the interior Sx2− reduction reaction.30,31Fig. 4d shows that the current density in both KB and 2D-Bi electrodes increases linearly with the increase of rotation rate. Interestingly, the current density of the 2D-Bi electrode exhibits a continuous increase from 2.0 to 1.5 V while the current density of the KB electrode reaches its limited diffusion current density at 1.9 V. It is worth noting that the stage from 2.0 to 1.6 V involves the final conversion (Li2S2 to Li2S) which contributes much capacity but is often impeded due to the sluggish reaction kinetics. This is well illustrated in Fig. S12† in which one can find that the last discharge stage of the Li–S cell with 2D-Bi contributes a capacity of 172 mA h g−1 while the contrast sample only delivers a capacity of 70 mA h g−1. To further confirm this, Levich–Koutecky plots derived from the negative scan disc current values and various potentials were fitted (Fig. S13 and S14 and Tables S1 and S2†).32 From the fitting results, we can easily calculate the electron transfer number as well as the reaction kinetic current. In Fig. 4e, the highest electron transfer number on the 2D-Bi electrode is 6.6 at 2.1 V and the lowest value is 4.7 at 1.9 V. In comparison, the highest electron transfer number on the KB electrode is 4.3 which occurs at 2.0 V and the lowest number is 3 at 1.8 V. Besides, the reaction kinetic current was recorded and the higher current density on the 2D-Bi electrode also represents the excellent reactivity from polysulfides to the final Li2S. Above we have used potential measurements to analyze the reciprocal conversion process between Li2S6 and Li2S. With 2D-Bi's help, sulfur is able to transform into Li2S sufficiently to improve the utilization; in the following anti-process, Li2S was easily oxidized to polysulfides and sulfur which could guarantee a full and fast circulation. The high catalytic activity can thus be considered from two aspects. First, 2D-Bi with high surface area and delocalized p-electrons near the edge sites can provide sufficient active sites to absorb free polysulfides which avoids the undesired active material loss. Second, these fixed active materials undergo a fast charge transfer on polycrystalline Bi surfaces, which allows for a sustainable conversion.
To further probe the liquid–solid nucleation of Li2S which accounts for the severe polarization and inert reaction, chronoamperometry was employed under a constant voltage of 2.08 V.33,34 After discharging under this voltage for 3 × 104 s, the integral areas of current peaks were calculated, resulting in Li2S nucleation capacities of 82.6, 69.9, and 29.3 mA h g−1 on 2D-Bi, Bi bulk, and KB, respectively. Besides, the current peaks increase in the order KB < Bi bulk < 2D-Bi. Clearly, the 2D-Bi electrode exhibits the highest activity towards Li2S precipitation and the fastest conversion rate for the nucleation process. It has been reported that the uniform and fast precipitation of Li2S during the discharge process contributes both to the rate performance and cycle life.35 From the above analysis, 2D-Bi is proved to be a good catalytic material which can effectively adjust the solid–liquid conversion and promote the forward–reverse redox reactions in Li–S batteries.
Separators are in close contact with the cathode, and could be easily modified to improve the performance of Li–S batteries.36,37
In order to verify the actual effect of 2D-Bi nanosheets on Li–S batteries, CR2032 type coin cells were assembled where the separator (polypropylene, PP) was modified by being coated with a layer of 2D-Bi nanosheets (more details can be found in the Experimental part).38,39 Cyclic Voltammetry (CV) was first employed as an indicator of redox reactions in an electrochemical process.40 As shown in Fig. 5a, the CV curves of Li–S cells with a 2D-Bi coated separator (Bi-PP) and normal PP separator exhibit representative Li–S redox peaks: the peak at ∼2.3 V represents the step-wise conversion of high-order polysulfides, and the peak at ∼2.0 V corresponds to the formation of Li2S. Conversely, the partially overlapped peaks in the anodic scanning process are attributed to the sequential oxidation of Li2S and polysulfides. During these similar redox reactions, the cell with the 2D-Bi modified separator shows sharper peaks and a lower overpotential than the cell with the PP separator, and we believe that with all else held equal, it is the 2D-Bi that regulates the shuttling of polysulfides and further promotes the redox conversion process.
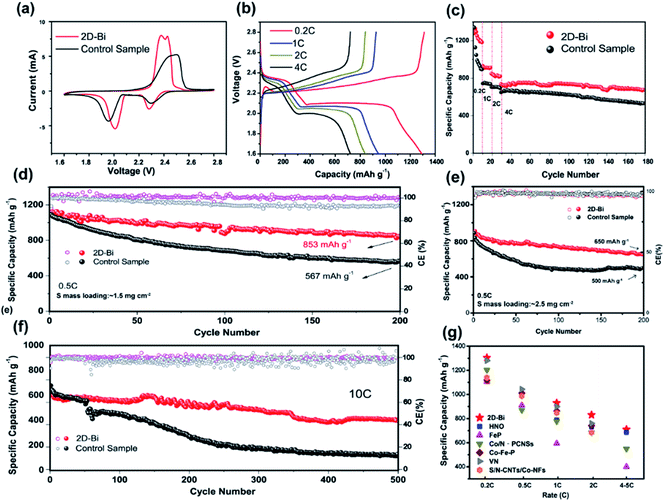
Then the rate capability and cycling performance were tested. As shown in Fig. 5b and c, the cell employing a 2D-Bi separator delivered superb rate capacities of 1305, 930, 830, and 710 mA h g−1 at 0.2, 1, 2, and 4C (1C = 1675 mA h g−1), which are much higher than those of the cell using a normal PP separator. Then we kept the rate at 0.5C and measured the cycling performance. Under conditions of 1.5 mg S mass loading and a high S content of 80 wt% (Fig. S15†), the cell with the 2D-Bi modified separator retained a capacity of 853 mA h g−1 (78% of the original capacity), while the capacity retention of the cell with the PP separator was only 567 mA h g−1. Furthermore, the 2D-Bi modified cell was still a strong performer (over 650 mA h g−1 after 200 cycles at 0.5C rate) when the S mass loading was increased to ∼2.5 mg cm−2 at 0.5C rate (Fig. 5e). For comparison, the precursor BiOC was also used to modify the separator. The electrochemical performance of cells with BiOC-modified separators was unsatisfactory, especially the high rate performance (∼200 mA h g−1 at 4C, Fig. S16 and S17†). It could easily lead one to the conclusion that materials with poor electrical conductivity such as BiOC impede electron transport and cause Li2S clogging, resulting in low sulfur utilization and large polarization, and this was proved from EIS spectra in Fig. S18.† The 2D-Bi modified Li–S cell showed much better performance than the control sample, although the degradation still existed. The reasons behind this can be described as follows. First, the cathode we used was a simple heated mixture of S and KB where the S content was as high as 80%. Some granuliform and unrestrained S caused deficient contact with the catalyst which could lead to performance degradation. Second, the mass loading of the catalyst on the separator was small compared with S loading plus the blade-coating method may cause nonuniform and poor dispersibility of 2D-Bi in NMP solution. These factors may cause incomplete catalysis on the cathode side.
In addition, we further studied the effect of 2D-Bi on the separator via observation of the separators and lithium disks after cycling (Fig. S19 and S20†). The separator where Bi and C elements distribute homogeneously was intact without any cracks and the Li2Sx deposition was uniform. From the SEM images of Li anodes after cycles, one can find the noteworthy discrepancy that the anode of the 2D-Bi modified cell showed a smooth morphology while that of the control cell was rugged. This irregularly deposited sediment may have contributed to the nucleation and growth of insoluble Li2S that formed from dissociative polysulfides. The phenomenon proves, from another angle, that 2D-Bi nanosheets can trap polysulfides and then promote their interconversion; thus, active materials can be utilized fully instead of them turning into dead Li2S on the anode.
For further investigation of the catalysis potential of the as-synthesized 2D-Bi nanosheets, cells were tested at a very high rate of 10C. As can be seen from Fig. 5f, the Li–S battery catalyzed by 2D-Bi nanosheets showed a very stable cycle life and its capacity remained at 408 mA h g−1 after 500 cycles. In comparison, the capacity of the cell with a normal PP separator experienced a cliff fall at around 50 cycles and then kept decreasing continuously until it reached a capacity of 180 mA h g−1 after 500 cycles. Combined with the above electrochemical tests, the positive impacts of 2D-Bi nanosheets on the Li–S battery cathode are well proven and here we attributed the superb performance to two main reasons. First, 2D-Bi nanosheets with high surface area could increase the trapping points for polysulfides, and these crystal surfaces' strong affinity for polysulfides ensures a fast redox reaction. Second, as confirmed from CV curves and RDE tests, 2D-Bi nanosheets can boost three main conversions: polysulfides to Li2S, Li2S to S8 and the deposition of Li2S. Last but not least, as shown in Fig. 5g, we compared the electrochemical performance in this study with that in previous excellent studies involving new catalysts in Li–S batteries. Other details and results are also summarized in Tables S3 and S4†.41–45 From the comparison, one can find that our synthesized main group element 2D-Bi surpasses many polar catalytic materials. Especially with the help of 2D-Bi catalysis, the rate performance is greatly enhanced (for instance, the capacities are 1305, 1090 and 710 mA h g−1 for 0.2C, 0.5C, and 4C, respectively, which rank at the top among these samples).
4. Conclusion
In summary, a main group metal element, Bi, is firstly introduced to act as an effective multi-step electrocatalyst for polysulfide conversions. The one-step chemically reduced Bi has an ultrathin 2D structure of around 4 nm, enabling a high surface area (72.24 m2 g−1) and many exposed active sites. Benefiting from this, this synthesized 2D-Bi is capable of absorbing and immobilizing soluble polysulfides. Theoretical calculations were also performed, and they offer the foundation for the strong adsorption between polycrystalline Bi(101) and (012) planes and Li2Sx (x = 1, 2, 4, 6, 8). Even more compelling is that 2D-Bi is conducive to three main conversions, including polysulfides to Li2S, Li2S to S8 and the deposition of Li2S. This was proved qualitatively and quantitatively by RDE tests combining the symmetric Li2S6 cell and asymmetric Li2S–Li2S6 cell. In addition, we compared the electrical performance of 2D-Bi modified cells and normal Li–S cells. Once again, with the catalysis capability of 2D-Bi, Li–S batteries show stable cycle life and superb rate performance (after 500 cycles at 10C current rate, the capacity retention of the battery with 2D-Bi is 2.5 times higher than that of a normal Li–S battery). Ultimately, we believe that a deep exploration and comprehension of the interior redox process as well as the conversion mechanism of sulfides will help in the exploration of non-noble metal catalysts and provide a great avenue for accelerating the large-scale application of Li–S batteries in the near future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (No. 2018YFB0104200).Notes and references
- Y. Wang, M.-M. Shi, D. Bao, F.-L. Meng, Q. Zhang, Y.-T. Zhou, K.-H. Liu, Y. Zhang, J.-Z. Wang, Z.-W. Chen, D.-P. Liu, Z. Jiang, M. Luo, L. Gu, Q.-H. Zhang, X.-Z. Cao, Y. Yao, M.-H. Shao, Y. Zhang, X.-B. Zhang, J. G. Chen, J.-M. Yan and Q. Jiang, Angew. Chem., Int. Ed., 2019, 58, 9464 CrossRef CAS PubMed.
- J. L. DiMeglio and J. Rosenthal, J. Am. Chem. Soc., 2013, 135, 8798 CrossRef CAS.
- S. Kim, W. J. Dong, S. Gim, W. Sohn, J. Y. Park, C. J. Yoo, H. W. Jang and J. L. Lee, Nano Energy, 2017, 39, 44 CrossRef CAS.
- Y.-C. Hao, Y. Guo, L.-W. Chen, M. Shu, X.-Y. Wang, T.-A. Bu, W.-Y. Gao, N. Zhang, X. Su, X. Feng, J.-W. Zhou, B. Wang, C.-W. Hu, A.-X. Yin, R. Si, Y.-W. Zhang and C.-H. Yan, Nat. Catal., 2019, 2, 448 CrossRef CAS.
- W.-G. Lim, S. Kim, C. Jo and J. Lee, Angew. Chem., Int. Ed., 2019, 58, 2 CrossRef.
- D. Liu, C. Zhang, G. Zhou, W. Lv, G. Ling, L. Zhi and Q.-H. Yang, Adv. Sci., 2018, 5, 1700270 CrossRef PubMed.
- J. He, G. Hartmann, M. Lee, G. S. Hwang, Y. Chen and A. J. E. Manthiram, Energy Environ. Sci., 2019, 12, 344 RSC.
- M. Zhao, H. J. Peng, Z. W. Zhang, B. Q. Li, X. Chen, J. Xie, X. Chen, J. Y. Wei, Q. Zhang and J.-Q. Huang, Angew. Chem., Int. Ed., 2019, 131, 3819 CrossRef.
- J. Xu, W. X. Zhang, H. B. Fan, F. L. Cheng, D. W. Su and G. X. Wang, Nano Energy, 2018, 51, 73 CrossRef CAS.
- B. Y. Hao, H. Li, W. Lv, Y. B. Zhang, S. Z. Niu, Q. Qi, S. J. Xiao, J. Li, F. Y. Kang and Q. H. Yang, Nano Energy, 2019, 60, 305 CrossRef CAS.
- B. Liu, S. Huang, D. Kong, J. Hu and H.-Y. Yang, J. Mater. Chem. A, 2019, 7, 7604 RSC.
- N. Wang, B. Chen, K. Q. Qin, E. Z. Liu, C. S. Shi, C. N. He and N. Q. Zhao, Nano Energy, 2019, 60, 332 CrossRef CAS.
- G. Babu, N. Masurkar, H. Al Salem and L. M. Arava, J. Am. Chem. Soc., 2017, 139, 171 CrossRef CAS.
- H. Al Salem, G. Babu, C. V. Rao and L. M. R. Arava, J. Am. Chem. Soc., 2015, 137, 11542 CrossRef CAS.
- C. Ye, Y. Jiao, H. Jin, A. D. Slattery, K. Davey, H. Wang and S.-Z. Qiao, Angew. Chem., Int. Ed., 2018, 57, 16703 CrossRef CAS.
- L. Li, L. Chen, S. Mukherjee, J. Gao, H. Sun, Z. Liu, X. Ma, T. Gupta, C. V. Singh and W. J. A. M. Ren, Adv. Mater., 2017, 29, 1602734 CrossRef.
- J. Zhou, L. Wang, M. Yang, J. Wu, F. Chen, W. Huang, N. Han, H. Ye, F. Zhao, Y. Li and Y. Li, Adv. Mater., 2017, 29, 1702061 CrossRef PubMed.
- H. Yang, N. Han, J. Deng, J. H. Wu, Y. Wang, Y. P. Hu, P. Ding, Y. F. Li, Y. G. Li and J. Lu, Adv. Energy Mater., 2018, 8, 1801536 CrossRef.
- J. Zhou, J. Chen, M. Chen, J. Wang, X. Liu, B. Wei, Z. Wang, J. Li, L. Gu and Q. J. A. M. Zhang, Adv. Mater., 2019, 31, 1807874 CrossRef PubMed.
- B. Afsin and M. J. S. l. Roberts, Spectrosc. Lett., 1994, 27, 139 CrossRef CAS.
- P. Madhusudan, J. R. Ran, J. Zhang, J. G. Yu and G. Liu, Appl. Catal., B, 2011, 110, 286 CrossRef CAS.
- Z. Hao, L. Yuan, C. Chen, J. Xiang, Y. Li, Z. Huang, P. Hu and Y.-H. Huang, J. Mater. Chem. A, 2016, 4, 17711 RSC.
- J. Xie, H. J. Peng, J. Q. Huang, W. T. Xu, X. Chen and Q. Zhang, Angew. Chem., Int. Ed., 2017, 56, 16223 CrossRef CAS PubMed.
- H. Lin, S. Zhang, T. Zhang, S. Cao, H. Ye, Q. Yao, G. W. Zheng and J. Y. Lee, ACS Nano, 2019, 13, 7073 CrossRef CAS PubMed.
- R. Fang, S. Zhao, Z. Sun, D.-W. Wang, R. Amal, S. Wang, H.-M. Cheng and F. J. E. S. M. Li, Energy Storage Materials, 2018, 10, 56 CrossRef.
- X. Tao, J. Wang, Z. Ying, Q. Cai, G. Zheng, Y. Gan, H. Huang, Y. Xia, C. Liang, W.-K. Zhang and Y. Cui, Nano Lett., 2014, 14, 5288 CrossRef CAS.
- N. N. Rajput, V. Murugesan, Y. Shin, K. S. Han, K. C. Lau, J. Z. Chen, J. Liu, L. A. Curtiss, K. T. Mueller and K. A. Persson, Chem. Mater., 2017, 29, 3375 CrossRef CAS.
- X. Yang, X. Gao, Q. Sun, S. P. Jand, Y. Yu, Y. Zhao, X. Li, K. Adair, L.-Y. Kuo, J. Rohrer, J. Liang, X. Lin, M. N. Banis, Y. Hu, H. Zhang, X. Li, R. Li, H. Zhang, P. Kaghazchi, T.-K. Sham and X. Sun, Adv. Mater., 2019, 31, 1901220 CrossRef PubMed.
- H. Xu, Y. Shi, S. Yang and B. Li, J. Power Sources, 2019, 430, 210 CrossRef CAS.
- Y.-C. Lu, Q. He and H. A. Gasteiger, J. Phys. Chem. C, 2014, 118, 5733 CrossRef CAS.
- J. He, Y. Chen and A. Manthiram, Adv. Energy Mater., 2019, 9, 1900584 CrossRef.
- J. Herranz, A. Garsuch and H. A. Gasteiger, J. Phys. Chem. C, 2012, 116, 19084 CrossRef CAS.
- Y. Song, W. Zhao, L. Kong, L. Zhang, X. Zhu, Y. Shao, F. Ding, Q. Zhang, J. Sun, Z. J. E. Liu and E. Science, Energy Environ. Sci., 2018, 11, 2620 RSC.
- G. Zhou, H. Tian, Y. Jin, X. Tao, B. Liu, R. Zhang, Z. W. Seh, D. Zhuo, Y. Liu, J. Sun, J. Zhao, C. Zu, D. S. Wu, Q. Zhang and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 840 CrossRef CAS.
- Y. Song, W. Zhao, N. Wei, L. Zhang, F. Ding, Z. Liu and J. Sun, Nano Energy, 2018, 53, 432 CrossRef CAS.
- N. Zhang, B. Li, S. Li and S. Yang, Adv. Energy Mater., 2018, 8, 1703124 CrossRef.
- S.-H. Chung and A. Manthiram, Adv. Mater., 2014, 26, 7352 CrossRef CAS.
- Y. Song, S. Zhao, Y. Chen, J. Cai, J. Li, Q. Yang, J. Sun and Z. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 5687 CrossRef CAS PubMed.
- S.-H. Chung and A. Manthiram, Adv. Mater., 2014, 26, 7352 CrossRef CAS PubMed.
- Y. C. Jeong, J. H. Kim, S. Nam, C. R. Park and S. J. Yang, Adv. Funct. Mater., 2018, 28, 1707411 CrossRef.
- Q. Zhang, Y. Wang, Z. W. Seh, Z. Fu, R. Zhang and Y. Cui, Nano Lett., 2015, 15, 3780 CrossRef CAS.
- J. Shen, X. Xu, J. Liu, Z. Liu, F. Li, R. Hu, J. Liu, X. Hou, Y. Feng, Y. Yu and M. Zhu, ACS Nano, 2019, 13, 8986 CrossRef CAS.
- J. S. Kim, T. H. Hwang, B. G. Kim, J. Min and J. W. Choi, Adv. Funct. Mater., 2014, 24, 5359 CrossRef CAS.
- Y. Chen, W. Zhang, D. Zhou, H. Tian, D. Su, C. Wang, D. Stockdale, F. Kang, B. Li and G. J. A. n. Wang, ACS Nano, 2019, 13, 4731 CrossRef CAS PubMed.
- L. Ma, H. Lin, W. Zhang, P. Zhao, G. Zhu, Y. Hu, R. Chen, Z. Tie, J. Liu and Z. Jin, Nano Lett., 2018, 18, 7949 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta11079h |
| This journal is © The Royal Society of Chemistry 2020 |