Finely modified crystallites of unsubstituted zinc phthalocyanine for film deposition purposes
N. Y.
Borovkov
 *a,
I. V.
Kholodkov
ab,
N. V.
Kholodkova
b and
A. M.
Kolker
*a,
I. V.
Kholodkov
ab,
N. V.
Kholodkova
b and
A. M.
Kolker
 a
a
aG.A. Krestov Institute of Solution Chemistry of the Russian Academy of Sciences, Ivanovo, 153045, Russian Federation. E-mail: phthalocyanine2020@gmail.com
bIvanovo State University of Chemistry and Technology, Ivanovo, 153000, Russian Federation
First published on 5th November 2020
Abstract
The present work deals with unsubstituted zinc phthalocyanine (ZnPc), seeking to develop an advanced algorithm for solution-processed ZnPc films. Specifically, it reports on a detailed AFM study of ZnPc crystallites deposited from solution in the binary cyclopentanone–3-dimethylamino-1-propanol system. The crystallization behavior of ZnPc has been radically improved by means of simple aromatic substances added to the dye solution before processing. Varying the nature and quantity of an aromatic additive allows one to change the size, the habit, and even the dimensionality of ZnPc crystallites. Properly modified crystallites are elastic and capable of spontaneous spreading over a solid support. Besides, two alternative kinds of interparticle interactions, namely close agglomeration and seamless fusion, have been explicitly differentiated by AFM phase imaging. The modifying effect of aromatic substances on ZnPc is briefly discussed in terms of supramolecular intercalation of small organic molecules into growing dye crystallites.
Introduction
Metal phthalocyanines are intensely colored and highly stable dyes whose molecular assemblies are stabilized by the strong π–π stacking interaction.1 Of permanent interest are unsubstituted phthalocyanines (further MPcs) applicable in organic solar cells and field-effect transistors.2 Superior charge transport properties are exhibited by the three-dimensional (3D) β-phase of MPcs. Physically, this phase is represented by tiny rod-like crystallites inclined to neither self-assembling nor fusion.3–5 Therefore, much effort is demanded to deposit uniform MPc films, minimizing conductivity losses due to interparticle spacing. Typical MPcs are poorly soluble but easy to sublime; so, they are processed into thin films by vapor phase deposition.6 Nowadays, this method is fully developed7 and further technical progress demands more inventive approaches to crystalline MPc films.A known alternative to the customary method is solution processing. Common organic liquids are poor solvents for MPcs and need to be strengthened with a polar component, such as diethanolamine or trifluoroacetic acid.8 However, no reported solution-deposited MPc films exhibit distinct morphological features of vapor-deposited ones.9 Moreover, even individual MPc crystallites are not obtained by solution processing until now.
The film growth can follow three pathways leading to flat, island-like, or combined morphological patterns, but only the third one, otherwise known as the Stranski–Krastanov growth mode, allows finding a right balance between crystallinity and homogeneity of solution-processed films.10 This point was best elucidated by the researchers11 who deposited a continuous film of symmetrically substituted phthalocyanines by using the pen-writing technique. This film was constructed from dye crystallites and disordered interstitial matter, indicating that even under optimal experimental conditions the thin-film crystallization proceeded in a poorly controlled way. Therefore, to develop a good solution processing algorithm for MPc films, one needs skills in controlling the phase behavior of these dyes and, to be more particular, in generating stable low-dimensional MPc phases concurrently with 3D crystallites.
Very recently, we implement the thin-film crystallization of ZnPc from a solution in a binary system composed of cyclopentanone (CyP) and 3-dimethylamino-1-propanol (DMAP).12 The said aminoalcohol is fairly volatile and acts as a multifunctional additive to ZnPc solutions, allowing one concurrently to solubilize the dye, to improve the habit of crystallites, and to fully suppress the coffee-ring effect detrimental for uniform film deposition.13 Thus, the most serious barriers to progress towards a novel algorithm for crystalline ZnPc films were overcome. The present work aims to demonstrate how compact 3D crystallites of ZnPc are transformed into biphasic ones potentially applicable as building blocks for continuous crystalline films.
Experimental
Materials
Highly disperse ZnPc was prepared from the sulfate salt under strictly non-aqueous conditions.12 The CyP solvent supplied by the Lvov Chemicals (Ukraine) was doubly distilled and a narrow fraction boiling at (130.5–131.0) °C was collected. Other organic solvents were obtained from Sigma–Aldrich and thoroughly distilled before using to dispose of high boiling admixtures.Procedures
All dye crystallites were obtained from the saturated (stock) solution (4.0 × 10−3 M ZnPc) in the binary CyP–DMAP system (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Small aliquots of liquid aromatic additives were injected into the stock solution to obtain a solution/additive ratio of 40
1, v/v). Small aliquots of liquid aromatic additives were injected into the stock solution to obtain a solution/additive ratio of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 20
1 or 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v. A ca. 4 μl droplet of the dye solutions was placed on a hydrophilic quartz glass from a Hamilton micro-syringe and volatile components were allowed to evaporate overnight at room temperature. Each solid deposit was annealed during 1 h at 70 ± 1 °C on a thermo-table of an Altami Polar 312 microscope (Altami, Russia).
1, v/v. A ca. 4 μl droplet of the dye solutions was placed on a hydrophilic quartz glass from a Hamilton micro-syringe and volatile components were allowed to evaporate overnight at room temperature. Each solid deposit was annealed during 1 h at 70 ± 1 °C on a thermo-table of an Altami Polar 312 microscope (Altami, Russia).
Instrumentation
The dye crystallites were imaged with a Solver P47 PRO atomic force microscope (NT-MDT, Zelenograd, Russia) operated in a semi-contact (tapping) mode under ambient conditions with a resolution of 256 pixels per scan line. Topographic and phase shift data were recorded simultaneously by using a single crystal silicon cantilever NSG010 (the force constant, 5.1 N m−1; the curvature radius, 6 nm; the free resonance frequency, 150 kHz). In all measurements, the driving frequency was 131.5 kHz and the oscillation amplitude of the cantilever was 250 ± 5 mV. To obtain the high phase imaging contrast, a ratio between the set point amplitude and free amplitude of oscillation (i.e., the rsp parameter14) was adjusted to 0.50. The AFM data were recorded by using the Nova 1.0.26 software (NT-MDT). The raw data were corrected by first-order flattening to eliminate distortions caused by casual movements of the scanner.Results and discussion
Imaging the ZnPc crystallites
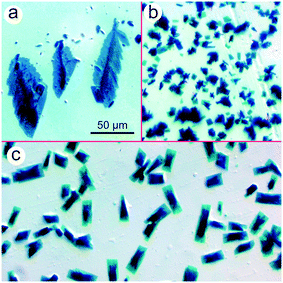
Amine-coordinated ZnPc molecules are known to precipitate from solutions in pairs,15 similar to metastable χ-ZnPc being intermediate between the low-dimensional α- and aforesaid β-phases.4,5 Such molecular pairs are packing loosely and, hence, there exists an opportunity to manipulate solid ZnPc–amine complexes by physicochemical means. For instance, they can be converted into β-ZnPc by gentle annealing.15 When DMAP is engaged as a dye partner, the ZnPc–amine complex is deposited from the solution in two forms: multiple prismatic crystallites with a low aspect ratio and occasional rod-like particles (Fig. 1). The particles are surrounded with light-blue smears, indicating that the dye matter tends to spread over the glass surface.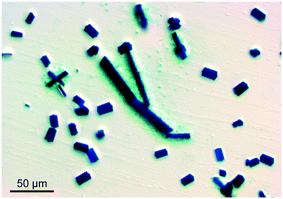 | ||
| Fig. 1 Prismatic and rod-like ZnPc crystallites deposited from the binary CyP–DMAP system followed by annealing at 70 °C. | ||
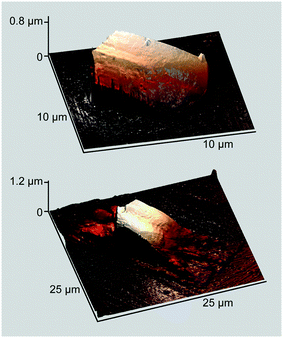
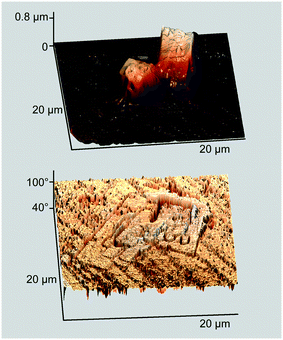
The AFM image allows estimating the height of these crystallites to be ca. 2.5 μm (Fig. 2, top). Of note are the structural differences between the two kinds of particles: the prismatic crystallites exhibit a terraced top surface and smooth side planes, while the rod-like ones are assembled from tightly fitted stalagmite-like units. Another remarkable feature is a ubiquitous pimpled ornament, indicating that the whole glass surface is covered with an ultrathin wetting layer formed by the ZnPc–DMAP complex.
 | ||
| Fig. 2 Perspective AFM height and phase images of the sample in Fig. 1. | ||
More information is provided by the phase images that reflect variations in viscoelastic properties of a deposited matter.14 In the case under consideration, stiff and compliant regions of the displayed surface are represented by phase angles marked with light and dark colors, respectively (Fig. 2, bottom). The stiff region covers nearly the whole surface except for the crystallites' side planes that are outwardly smooth but actually disordered, as the researchers11 conclusively demonstrated. However, it is the compliant region that is meaningful in the context of this study. Judging from the topography image, two contiguous prismatic crystallites located in the lower corner seem seamlessly fused side-by-side, but this impression disappears when the phase image is allowed for. In fact, the two crystallites are separated by a boundary of the disordered matter, revealing the merely agglomerative nature of interparticle interaction. This observation fully complies with the afore-cited data11 and strongly suggests that no continuous MPc film can be constructed from 3D crystallites by wielding only mechanical tools.8
A merit of the binary CyP–DMAP system is the aliphatic nature of both components and, hence, an opportunity to control the thin-film crystallization of ZnPc by adding aromatics to the stock solution. Herein, three moderately volatile aromatic substances have been tested, namely benzyl alcohol (BzA), 2-chloroanisole (ClAn), and 3-chloropyridine (ClPy). These particular additives are chosen on account of their diverse affinities for ZnPc: BzA dissolves neither free ZnPc nor ZnPc–amine complexes, ClPy is a generally efficient solvent, while ClAn dissolves only complexes, thus being a latent solvent for ZnPc. When BzA is added to the stock solution, the initially uniform deposition pattern12 breaks up into two parts: a central spot that consists of non-modified prismatic crystallites and a narrow peripheral ring where large leaf-like pieces of the dye matter are located (Fig. 3a). In contrast, ClAn does not disrupt the deposition pattern and fully suppress the 3D crystallization, making the dye to precipitate in a form of minute trapezoidal plates (Fig. 3b). Most vividly, the ZnPc crystallites are affected by ClPy; specifically, they acquire a kind of the biphasic structure represented by a 3D core and low-dimensional periphery (Fig. 3c).
In more detail, the crystallites were visualized by AFM. When a very small aliquot of ClPy is added to the stock solution, brick-like particles with smooth surfaces and rounded ribs are obtained (Fig. 4, top). Further increasing the ClPy aliquot dramatically changes both the shape and morphology of the dye crystallites: a typical dye particle takes the shape of an asymmetric rectangular mountain with a smooth slope and a terraced foot (Fig. 4, bottom). Of note is a row of stalagmite-like protrusions observable on the brick-like particle but nearly absent on the heavier modified one.
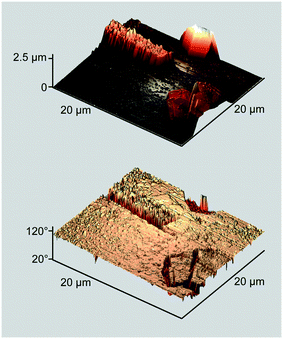
Another notable feature of the biphasic crystallites is the nature of interparticle interaction. Fig. 5 portrays a pair of obliquely oriented contiguous crystallites. The height image discloses a direct contact between the two 3D cores, while the phase image demonstrates a lack of structural boundaries between the cores. Again, nearly all the displayed surfaces are stiff, while strips of the compliant dye matter are markedly narrower than those in the non-modified sample (Fig. 2, bottom).
 | ||
| Fig. 5 Perspective AFM height and phase images of a “Siamese” ZnPc crystallite deposited as in Fig. 4, bottom. | ||
Thus, the use of ClPy provides vital benefits to the thin-film crystallization of ZnPc. First of all, it allows obtaining the low-dimensional phase fully integrated with 3D crystallites. The ClPy-modified crystallites are capable of both microscopic spreading over the glass surface and seamless fusion concurrently with the vertical growth. This additive is indifferent to the wetting layer and in no case impairs the uniformity of the solid dye deposits. The additives' affinity for the ZnPc molecule does not matter at all; so, the modifying effect of ClPy can be discussed in terms of supramolecular intercalation rather than Ostwald ripening.4,5
Mechanistic remarks
The growth of solution-processed films is determined by colloidal factors responsible for the uniform distribution of a film-forming matter over a solid support. To bring the film growth under control, a main channel of structure formation should be redirected from bulk solution to the solid–liquid interface.16 In the case under consideration, the π–π interaction between dye molecules prevails over the dye–surface attraction, resulting in the formation of compact 3D crystallites. The wetting layer favors attaching the crystallites to the glass surface but is unable to support the microscopic spreading of the dye matter. Therefore, to greatly improve processability of the stock solution, one had to think up how the π–π interaction could be purposely attenuated. The idea to address this challenge by using a combination of aliphatic and aromatic small molecules follows from the classic work.3When CyP evaporates from the stock solution, the DMAP molecules occupy well accessible voids in growing dye crystallites, also tending to penetrate into voids of higher energy. The immature crystal lattice succumbs to this impact and suffers a partial collapse, which entails the formation of pseudo-crystalline rod-like particles as well as stalagmite- and pimple-like defects on normal crystallites. If well polarizable aromatic molecules are present in the solution, they selectively intercalate into the latter voids to form a stable ternary host–guest system. As a result, there arise a novel kind of 3D crystallite, which is still compact but exhibits a number of useful physicochemical features, such as elasticity, fusibility, and spreadability.
Allowing for the data presented above, a current opinion about allegedly poor film-forming properties of ZnPc whose rigid molecule bears no plasticizing substituents should be reconsidered. The nature and quantity of the aromatic additives can be widely varied; so, there exist no obstacles to developing multi-component ZnPc solutions for crystalline film deposition.17
In conclusion, two points of future concern are to be noted. In general, to smoothly transform an array of dye crystallites into a uniform film, the crystallites are to be seamlessly fused irrespective of their in-plane orientation. Although the fusion of well modified ZnPc crystallites is unrestrained by the structural factor, it should be thermodynamically stimulated in order to suppress detrimental colloidal processes, such as dewetting and cracking.18 Besides, an extra problem of thin-film crystallization is revealed in the case of the ClAn modifier (Fig. 3b): despite the full transformation of 3D crystallites into low-dimensional lamellas, the latter exhibit no adhesion to glass and tend to agglomerate rather than to be laid flat as expected. Again, this feature is connected with a competition between small molecules. Because the modifier evaporates slower than DMAP, there arise microscopic pools of ClAn serving as agglomeration centers for the fully formed dye particles. Obviously, to dispose of this side effect, a non-volatile dye-compatible surfactant would be extremely useful.
Very recently, we proposed to improve the morphology of crystalline ZnPc films by adding tailor-made phthalocyanines capable of forming low-dimensional phases.19 Allowing for the mechanism of ZnPc crystallization from solution, amphiphilic phthalocyanines with hydroxyl-substituted aliphatic chains20 could be chosen for this purpose. To our mind, such highly specific surfactants might act in unison with DMAP, increasing adhesion of ZnPc crystallites to glass and promoting the fusion of crystallites due to intermolecular H bonding.
Conclusions
This contribution outlines an easy way to process unsubstituted ZnPc into thin films from solutions based on the binary ketone–aminoalcohol system. The thin-film crystallization of ZnPc is controlled by using simple aromatic substances added to the dye solution before processing. The properly modified dye crystallites are fusible, spreadable, and, hence, potentially applicable as building blocks for the crystalline films of ZnPc.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Russian Foundation for Basic Research (the grant 19-03-00090a). The authors thank “The Center for the Collective Use of Scientific Equipment of the Ivanovo State University of Chemistry and Technology” for assistance in the AFM measurements.References
- Z. Ni, R. Li and J. Jiang, New Progress in Monomeric Phthalocyanine Chemistry: Synthesis, Crystal Structures and Properties, in Structure and Bonding, ed. X.-T. Wu, Springer-Verlag, Berlin, Heidelberg, 2009, vol. 133, pp. 121–160, DOI:10.1007/430_2008_13.
- (a) T. Inabe and H. Tajima, Phthalocyanines – Versatile Components of Molecular Conductors, Chem. Rev., 2004, 104, 5503–5533, DOI:10.1021/cr030649x; (b) C.-F. Lin, M. Zhang, S.-W. Liu, T.-L. Chiu and J.-H. Lee, High Photoelectric Conversion Efficiency of Metal Phthalocyanine/Fullerene Heterojunction Photovoltaic Device, Int. J. Mol. Sci., 2011, 12, 476–505, DOI:10.3390/ijms12010476; (c) O. A. Melville, B. H. Lessard and T. P. Bender, Phthalocyanine-Based Organic Thin-Film Transistors: A Review of Recent Advances, ACS Appl. Mater. Interfaces, 2015, 7, 13105–13118, DOI:10.1021/acsami.5b01718.
- T. Kobayashi, N. Uyeda and E. Suito, The n-Donor Complex Formation and Polymorphic Transformation of Zinc Phthalocyanine in Organic Suspension Media, J. Phys. Chem., 1968, 72, 2446–2456, DOI:10.1021/j100853a029.
- J. R. Fryer, R. B. McKay, R. R. Mather and K. S. W. Sing, The Technological Importance of The Crystallographic and Surface Properties of Copper Phthalocyanine Pigments, J. Chem. Technol. Biotechnol., 1981, 31, 371–387, DOI:10.1002/jctb.503310152.
- F. Iwatsu, Crystal Behavior of Zinc Phthalocyanine Films in Alcohols, J. Cryst. Growth, 1985, 71, 629–638, DOI:10.1016/0022-0248(85)90371-9.
- M. Cook and I. Chambrier, Phthalocyanine Thin Films: Deposition and Structural Studies, in The Porphyrin Handbook, ed. K. Kadish, K. Smith and M. Guillard, Academic Press, New York, 2003, ch. 108, vol. 17, pp. 37–127, DOI:10.1016/B978-0-08-092391-8.50008-X.
- R. R. Lunt, B. E. Lassiter, J. B. Benziger and S. R. Forrest, Organic Vapor Phase Deposition for the Growth of Large Area Organic Electronic Devices, Appl. Phys. Lett., 2009, 95, 233305, DOI:10.1063/1.3271797.
- (a) T. Komino, M. Matsuda and H. Tajima, The Fabrication Method of Unsubstituted Planar Phthalocyanine Thin Films by a Spin-Coating Technique, Thin Solid Films, 2009, 518, 688–691, DOI:10.1016/j.tsf.2009.07.063; (b) J.-L. Su, M.-Z. Xue, N. Ma, Q.-R. Sheng, Q. Zhang and Y.-G. Liu, Dissolution of Copper Phthalocyanine and Fabrication of Its Nanostructure Film, Sci. China, Ser. B: Chem., 2009, 52, 911–915, DOI:10.1007/s11426-009-0021-3; (c) H. A. Afify, A.-S. Gadallah, M. M. El-Nahass and M. A. Khedr, Effect of Thermal Annealing on the Structural and Optical Properties of Spin Coated Copper Phthalocyanine Thin Films, J. Mol. Struct., 2015, 1098, 161–166, DOI:10.1016/j.molstruc.2015.06.016; (d) F. Ghani, H. Gojzewski and H. Riegler, Nucleation and Growth of Copper Phthalocyanine Aggregates Deposited From Solution on Planar Surfaces, Appl. Surf. Sci., 2015, 351, 969–976, DOI:10.1016/j.apsusc.2015.06.020.
- (a) S. Karan and B. Mallik, Templating Effects and Optical Characterization of Copper (II) Phthalocyanine Nanocrystallites Thin Film: Nanoparticles, Nanoflowers, Nanocabbages, and Nanoribbons, J. Phys. Chem. C, 2007, 111, 7352–7365, DOI:10.1021/jp070302o; (b) S. Karan and B. Mallik, Nanoflowers Grown from Phthalocyanine Seeds: Organic Nanorectifiers, J. Phys. Chem. C, 2008, 112, 2436–2447, DOI:10.1021/jp709780a; (c) A. Chowdhury, B. Biswas, M. Majumder, M. K. Sanyal and B. Mallik, Studies on Phase Transformation and Molecular Orientation in Nanostructured Zinc Phthalocyanine Thin Films Annealed at Different Temperatures, Thin Solid Films, 2012, 520, 6695–6704, DOI:10.1016/j.tsf.2012.07.013.
- H. Brune, Growth Modes, in Encyclopedia of Materials: Science and Technology, ed. K. H. J. Buschow, M. C. Flemings, E. J. Kramer, P. Veyssière, R. W. Cahn, B. Ilschner and S. Mahajan, Elsevier Ltd., 2nd edn, 2001, pp. 3683–3692, DOI:10.1016/B0-08-043152-6/00657-4.
- Z. Pan, N. Rawat, I. Cour, L. Manning, R. L. Headrick and M. Furis, Polarization-Resolved Spectroscopy Imaging of Grain Boundaries and Optical Excitations in Crystalline Organic Thin Films, Nat. Commun., 2015, 6, 8210, DOI:10.1038/ncomms9201.
- N. Y. Borovkov, E. G. Odintsova, V. E. Petrenko and A. M. Kolker, Amine-Assisted Solubilization of Unsubstituted Zinc Phthalocyanine for Film Deposition Purposes, RSC Adv., 2019, 9, 33969–33975, 10.1039/c9ra07453h.
- D. Mampallila and H. B. Eral, A Review on Suppression and Utilization of the Coffee-Ring Effect, Adv. Colloid Interface Sci., 2018, 252, 38–54, DOI:10.1016/j.cis.2017.12.008.
- L.-C. Xu and C. A. Siedlecki, Atomic Force Microscopy, Comprehensive Biomaterials, 2011, vol. 3, pp. 23–35, DOI:10.1016/B978-0-08-055294-1.00083-0.
- B. Przybył and J. Janczak, Structural Characterisation and DFT Calculations of Three New Complexes of Zinc Phthalocyanine with n-Alkylamines, Dyes Pigm., 2014, 100, 247–254, DOI:10.1016/j.dyepig.2013.09.020.
- A. P. Sommer and N. Rozlosnik, Formation of Crystalline Ring Patterns on Extremely Hydrophobic Super-Smooth Substrates: Extension of Ring Formation Paradigms, Cryst. Growth Des., 2005, 5, 551–557, DOI:10.1021/cg0496989.
- Y. Socol and I. S. Guzman, Fast Ring-Shape Self-Assembling in Water-Based Ink-Jetted Droplets, J. Phys. Chem. B, 2006, 110, 18347–18350, DOI:10.1021/jp0637577.
- (a) C. V. Thompson, Solid-State Dewetting of Thin Films, Annu. Rev. Mater. Res., 2012, 42, 399–434, DOI:10.1146/annurev-matsci-070511-155048; (b) A. F. Routh, Drying of Thin Colloidal Films, Rep. Prog. Phys., 2013, 76, 046603, DOI:10.1088/0034-4885/76/4/046603.
- N. Y. Borovkov and A. M. Kolker, Crystallization Behavior of Copper Tetra-tert-butylporphyrazine in Ultrathin Films, J. Phys. Chem. C, 2020, 124, 24171–24178, DOI:10.1021/acs.jpcc.0c06706.
- M. Vélez, S. Vieira, I. Chambrier and M. J. Cook, Molecular Order within Langmuir-Blodgett Films of Two Amphiphilic Octasubstituted Phthalocyanines Studied by Atomic Force Microscopy, Langmuir, 1998, 14, 4227–4231, DOI:10.1021/la980118h.
| This journal is © The Royal Society of Chemistry 2021 |