Hydroxide promotes ion pairing in the NaNO2–NaOH–H2O system†
Trent R.
Graham
 *a,
Mateusz
Dembowski
*a,
Mateusz
Dembowski
 a,
Hsiu-Wen
Wang
a,
Hsiu-Wen
Wang
 b,
Sebastian T.
Mergelsberg
b,
Sebastian T.
Mergelsberg
 a,
Emily T.
Nienhuis
a,
Emily T.
Nienhuis
 a,
Jacob G.
Reynolds
c,
Calvin H.
Delegard
a,
Jacob G.
Reynolds
c,
Calvin H.
Delegard
 d,
Yihui
Wei
e,
Michelle
Snyder
a,
Ian I.
Leavy
a,
Steven R.
Baum
a,
Matthew S.
Fountain
a,
Sue B.
Clark
ae,
Kevin M.
Rosso
a and
Carolyn I.
Pearce
d,
Yihui
Wei
e,
Michelle
Snyder
a,
Ian I.
Leavy
a,
Steven R.
Baum
a,
Matthew S.
Fountain
a,
Sue B.
Clark
ae,
Kevin M.
Rosso
a and
Carolyn I.
Pearce
 *af
*af
aPacific Northwest National Laboratory, Richland, Washington 99352, USA. E-mail: trenton.graham@pnnl.gov; carolyn.pearce@pnnl.gov
bOak Ridge National Laboratory, Oak Ridge, Tennessee 37831, USA
cWashington River Protection Solutions, LLC, Richland, Washington 99352, USA
dTradeWind Services, LLC, Richland, Washington 99352, USA
eDepartment of Chemistry, Washington State University, Pullman, Washington 99164, USA
fDepartment of Crop & Soil Sciences, Washington State University, Pullman, WA 99164, USA
First published on 11th December 2020
Abstract
Nitrite (NO2−) is a prevalent nitrogen oxyanion in environmental and industrial processes, but its behavior in solution, including ion pair formation, is complex. This solution phase complexity impacts industries such as nuclear waste treatment, where NO2− significantly affects the solubility of other constituents present in sodium hydroxide (NaOH)-rich nuclear waste. This work provides molecular scale information into sodium nitrite (NaNO2) and NaOH ion-pairing processes to provide a physical basis for later development of thermodynamic models. Solubility isotherms of NaNO2 in aqueous mixtures with NaOH and total alkalinity were also measured. Spectroscopic characterization of these solutions utilized high-field nuclear magnetic resonance spectroscopy (NMR) and Raman spectroscopy, with additional solution structure detailed by X-ray total scattering pairwise distribution function analysis (X-ray PDF). Despite the NO2− deformation Raman band's insensitivity to added NaOH in saturated NaNO2 solutions, 23Na and 15N NMR studies indicated the Na+ and NO2− chemical environments change likely due to ion pairing. The ion pairing correlates with a decrease in diffusion coefficient of solution species as measured by pulsed field gradient 23Na and 1H NMR. Two-dimensional correlation analyses of the 2800–4000 cm−1 Raman region and X-ray PDF indicated that saturated NaNO2 and NaOH mixtures disrupt the hydrogen network of water into a new structure where the length of the O⋯O correlations is contracted relative to the typical H2O structure. Beyond describing the solubility of NaNO2 in a multicomponent electrolyte mixture, these results also indicate that nitrite exhibits greater ion pairing in mixtures of concentrated NaNO2 and NaOH than in comparable solutions with only NaNO2.
1. Introduction
In solutions of extreme alkalinity, a deficiency of water necessitates a contraction of both cation–cation and anion–anion distances, leading to local ion configurations of greater complexity than cation–anion ion pairs and solvent-separated ion pairs due to geometric packing constraints.1,2 These hydroxide (OH−)-bearing solutions are complex relative to other monovalent anions, due to proton transfer mechanisms and other transport pathways, facilitated by the reactive OH− ion.3–5 The convolution of contracted ion–ion distances, reactive OH− species, and low water activity, make it challenging to unravel the molecular scale interactions that influence the solubility of salts (e.g., sodium nitrite, NaNO2) at extreme alkalinity.Without knowledge of these molecular scale interactions, it is not possible to predict ion pairing processes and rheological properties of the solution, which are important in many industries.6–8 For example, in concentrated alkaline aqueous solutions, the sorption of nitric oxide can be leveraged to treat flue gas and simultaneously generate marketable salts.6 Sodium nitrite is also abundant in alkaline radioactive waste, currently stored in huge underground tanks at the U.S. Department of Energy legacy sites, such as the Hanford site, Washington State (USA). Within these radioactive wastes, NO2− is present in a concentrated mixture of electrolytes, with concentrations of sodium in excess of 10 moles per liter (M).7 Nitrite is also present at other radioactive waste sites, such as the Savannah River Site, in South Carolina (USA), where the influence of the NO2− ion has been investigated in sodium hydroxide (NaOH) recoveries using electrochemical separations.8 For both of these examples, disentangling the influence of specific ions, such as OH− and NO2−, on the solubility of ions and the rheology of concentrated electrolytes, is an ongoing effort.
In sodium hydroxide solutions, NO2− and nitrate (NO3−) were recently found to increase the solubility of gibbsite (Al(OH)3), which is an aluminum hydroxide mineral found in nuclear waste.9,10 However, addition of NO2− to alkaline sodium hydroxide solutions was also reported to decrease the solubility of amorphous zeolites in solutions also containing sodium aluminate and sodium nitrate.11 These contradictory results highlight the need to understand ion-specific effects and their influence on solution properties in mixtures of electrolytes.
Both measurements and theoretical calculations have provided insight into the solvation structure of separate systems of either sodium nitrite or sodium hydroxide and water. NO2− is classified as a chaotrope, a structure breaker, for its ability to dissolve and form hydrated ions that disrupt water structure to a greater extent relative to other monovalent ions.12 The NO2− ion has an N–O bond distance of 1.23 Å with an ∠ONO angle of 116°.13 Recent molecular dynamics simulations under infinitely dilute conditions showed that NO2− has 5 water molecules in the first solvation shell, and is asymmetrically solvated with water preferentially coordinated to the nitrite oxygens.14,15 However, at greater concentrations of NaNO2, there is insufficient water to form a complete solvation shell. For example, at NaNO2 concentrations of 5 moles per kilogram solvent (molal, m), the NO2− ion was associated with 3.7 water molecules, as measured using neutron diffraction.16 This is comparable but not identical to structures generated by classical molecular dynamics, which predicted NO2 to be solvated by 7–8 water, defined with a cutoff radial distance of 4 Å, even at concentrations of approximately 5 m NaNO2.17 In simple NaNO2 solutions without NaOH, the weak ion pairing between Na+ and NO2− is such that the solubility of NaNO2 can be modeled with parameters which indicate only weak ion pairing.18 In contrast, OH− is classified as a kosmotropic ion relative to NO2−,19 and the ion templates the structure of water and forms ion pairs with Na+ in aqueous solution.20 The ions in the NaOH solutions access a variety of local ion configurations to form corner-, edge-, and face-sharing polyhedral networks.21
Here, the physical rational behind the inclusion or omission of Na–NO2 ion-pair formation terms in solubility models describing the NaNO2–NaOH mixtures is explored via spectroscopy and diffraction. Solubility isotherms are presented for NaNO2 in mixtures containing NaOH and H2O. The concentration of NO2− in solution was measured using ion chromatography (IC) and total alkalinity (OH−) was measured by titration to a phenolphthalein endpoint. The NO2− and sodium solvation environment and the translational dynamics of H-bearing species and sodium ions were quantified using multinuclear NMR at 17.6 T (23Na, 15N, and 1H), Raman spectroscopy, and solution-state X-ray total scattering pairwise distribution function analysis (X-ray PDF). The results indicate that there is greater ion pairing in mixtures of concentrated NaNO2 and NaOH than in comparable solutions with only NaNO2 by the reduction in 1H and 23Na diffusivity, and the contraction of long-range correlations in X-ray total scattering. The new solubility data and molecular scale insight into the chemical environment of NO2− in concentrated alkaline electrolytes provides a foundational basis from which systems of greater complexity can be studied in order to better understand solubility, ion pairing, and rheology in alkaline solutions pertinent to flue gas treatment and nuclear waste processing.
2. Experimental methods
2.1 Solution preparation and solubility experiments
Solution preparation and solubility experiments with NaNO2–NaOH solutions were conducted in an Ar-atmosphere (Oxarc) glove box to minimize carbon dioxide uptake in the alkaline solutions. Sodium nitrite (NaNO2, Fischer, >97%) was dried in an oven at 80 °C to constant mass, with a mass loss of about 0.6 wt%. A 1, 3 and 6 m NaOH stock solution was prepared by dissolving sodium hydroxide (Sigma Aldrich, >98%) in N2-sparged water (18 MΩ cm) in a 250 mL fluorinated ethylene propylene Erlenmeyer bottle.To measure solubility from undersaturation, NaNO2 was added to the solutions in quantities expected to exceed the solubility of NaNO2 by a factor of 1.3. Details regarding the solubility prediction are available in the ESI† and summarized in Table S1. NaNO2 was weighed out into 15 ml Teflon tubes for experiments at ambient temperature (28 °C) and into 50 mL Teflon tubes for 40, 60 and 80 °C experiments. Then, 6 mL of the respective NaOH stock solution for experiments at 28 °C and 13 mL of the respective NaOH stock solution for experiments at 40, 60 and 80 °C was added. Systems were typically prepared in triplicate. Given that the conditions in Table 1 vary with temperature, concentrations are reported in units of molality (moles kg−1 solvent) and not molarity (moles L−1). For solubility experiments performed at 28 °C shown in Table 1, the PTFE lined reaction vessels were placed on a rocking platform to provide continuous agitation. For solubility experiments performed at 40, 60 and 80 °C also shown in Table 1, the reaction vessels were placed in an aluminum enclosure on a heating stir plate and mixed at 50–150 rpm via magnetic PTFE lined stir bar. The temperature stability during the experiment was ±1 °C, as measured by National Institute of Standards and Technology (NIST) traceable, calibrated thermometers (Fisher Digital Lollipop Thermometer and Fisher big-digit see-thru Thermometer). The thermometers were calibrated in compliance with ISO/IEC 17025:2005, ANSl/NCSL Z540-1-1994 Part 1, and ISO 9001.
| Solution + Solid composition | Solution composition | |||||
|---|---|---|---|---|---|---|
| NaOH (g) | NaNO2 (g) | H2O (g) | Temp (°C) | NaOH (molal, moles kg−1) | NaNO2 (molal, moles kg−1) | Density (g cm−1) |
| a Solution conditions were approached from both over and undersaturation, with results shown in Table S2 in the ESI. b Single measurement only. c Additional experiments characterized solids at approximately these conditions with Raman spectroscopy, X-ray diffraction, and scanning electron microscopy, with results showing that NaNO2 is the solid phase in Fig. S5–S7 in the ESI. | ||||||
| 0.0 | 107.6 | 100.0 | 27.6c | 0 | 13.1(4) | 1.378(4) |
| 4.0 | 98.7 | 100.0 | 27.6 | 0.9(1) | 11.9(1) | 1.379(8) |
| 12.0 | 84.3 | 100.0 | 27.6 | 2.8(1) | 10.1(1) | 1.372(7) |
| 24.0 | 61.0 | 100.0 | 27.6c | 5.4(2) | 7.1(1) | 1.391(4) |
| 4.0 | 116.6 | 100.0 | 41.1a | 0.9(1) | 13.5(1) | 1.375(1) |
| 12.0 | 98.7 | 100.0 | 41.1a | 2.9(1) | 11.5(1) | 1.357(13) |
| 24.0 | 78.0 | 100.0 | 41.1ab | 5.6 | 8.8 | 1.340 |
| 4.0 | 143.5 | 100.0 | 58.2 | 0.9(1) | 15.5(4) | 1.394(0) |
| 12.0 | 125.6 | 100.0 | 58.2 | 2.8(1) | 13.2(1) | 1.389(2) |
| 24.0 | 98.7 | 100.0 | 56.6 | 5.7(1) | 10.0(4) | 1.382(1) |
| 0 | 176.5 | 100.0 | 79.2c | 0 | 18.9(1) | 1.413(4) |
| 4.0 | 161.5 | 100.0 | 79.0 | 1.0(2) | 18.9(3) | 1.366(10) |
| 12.0 | 143.5 | 100.0 | 78.4 | 3.3(1) | 17.1(1) | 1.348(4) |
| 24.0 | 125.6 | 100.0 | 79.6c | 6.3(1) | 14.4(4) | 1.350(9) |
Solutions were sampled after 1 week for systems at 28, 40, and 60 °C and after 2 days for systems at 80 °C. Good agreement between measured and literature22 NaNO2 solubility in the absence of NaOH for control experiments at 28 and 80 °C, and the near equivalent solution compositions acquired from oversaturation and undersaturation approaches provides compelling evidence that solutions were essentially at equilibrium when compositions were determined. To measure solubility from oversaturation, the NaNO2 and NaOH solutions prepared at 80 °C were cooled to 40 °C which resulted in near equivalent NaNO2 concentrations. See Table S2 in the ESI for additional results.†
2.2 Ion chromatography
To determine the concentration of NaNO2, pipette tips were preheated to the solution temperature, and aliquots of approximately 0.2 mL were collected from the reaction vessels at temperature, and rapidly filtered through pre-heated 0.2 μm polyvinylidene difluoride filters. To confirm that filtering the solutions did not influence the results, NaNO2 concentrations were determined in: (i) solutions that had been filtered; and (ii) the supernatant from solutions following sedimentation, with the agreement in measured solution concentrations shown in Table S3 and Fig. S4 in the ESI.†For IC measurements of NO2− concentrations, samples were diluted with water. Two instruments were used for ion chromatography (IC) analysis: (i) a Dionex Reagent Free Ion Chromatography System 5000 (RFICS-5000) with an AS-AP auto-sampler, and (ii) a Dionex Reagent Free Ion Chromatography System 2000 (RFICS-2000) with an AS-1 auto-sampler. Calibration curves (multi-component anion solutions, SPEX CertiPrep) were collected periodically and solution blanks were included to validate lack of sample carryover. IC was also performed to validate the lack of inorganic contaminants. Specifically, the solution of 13 m NaNO2 and 5 m NaOH contained trace concentrations of chloride (<250 ppm), bromide (<500 ppm), fluoride (<100 ppm), phosphate (<750 ppm), and sulfate (<750 ppm), which are not considered significant. There are similarly insignificant carbonate impurities, based on Raman spectra of concentrated NaNO2 and NaOH solutions (Fig. S8, ESI†).
2.3 Total alkalinity
Total alkalinity [OH−] was measured using a titrator (Metrohm 888 Titrando) with Tiamo software (v 2.4), by titration with 0.02 N sulfuric acid (H2SO4) to a phenolphthalein endpoint (pH of 8.3).2.4 NMR spectroscopy
Single pulse direct excitation 23Na and 15N NMR and pulsed field gradient 23Na and 1H NMR were conducted on an Agilent spectrometer at a field strength of 17.6 T, using a broadband probe, operating at 25 °C. The temperature was calibrated with the 1H chemical shifts of ethylene glycol.23 The corresponding Larmor frequencies for 23Na, 15N, and 1H are 198.424, 76.039, and 750.130 MHz, respectively. Solutions were loaded into 5 mm PTFE sample liners, coaxially inserted into 5 mm NMR tubes in an Ar-filled glovebox.The single pulse direct excitation 23Na NMR spectra were acquired with a π/2 liquid-state pulse, an acquisition time of 600 ms, a delay between transients of 2 s, and 16 transients. Chemical shifts are internally referenced to a physically separated solution of 0.76 M NaCl in H2O, the resonance of which was assigned to 0 ppm. The 23Na NMR spectra were processed in Mestrenova (version 14.01-23559, released 2019-06-07, Mestrelab Research S.L.), where the free induction decay was zero-filled once, and the spectra are shown with 2 Hz of exponential line broadening. The single pulse direct excitation 15N NMR spectra were collected with a π/6 excitation pulse, an acquisition time (at) of 1.678 s, a delay between transients (d1) of 5 s, and 64 transients. Given the spin–lattice recovery coefficient (T1) of 15N is such that the expression {at + d1 < 5 T1} is true, only the chemical shifts of the 15N resonances are discussed here; the 15N intensities were not analyzed quantitatively because of saturation effects. NO2− concentrations were determined by IC, as described above. The 15N NMR spectra were externally referenced to acetonitrile, which was assigned a chemical shift of 130.2 ppm (relative to 0 ppm defined by 1 M solution of H15NO3 in D2O).24 The 15N NMR spectra were processed in Mestrenova, where the free induction decay was truncated to 0.6 s and the spectra are shown with 2 Hz of exponential line broadening. Single pulse direct excitation spectra were fit using purely Lorentzian line shapes.
Pulsed field gradient 23Na and 1H NMR spectra were also collected to investigate the translational motion of Na+ and H2O/OH− at millisecond timescales. For 23Na, diffusion coefficients were acquired with the vendor-supplied bipolar gradient pulse pair stimulated echo with convection compensation (Dbppste_cc). For 1H, diffusion coefficients were acquired with the vendor-supplied DOSY gradient compensated stimulated echo with spin lock and convection compensation sequence (DgcsteSL_cc). The π/2 pulse widths were calibrated for each sample for both nuclei. Acquisition with either pulse sequence used 16 spatially-dependent gradient pulse strength increments, where the spatially-dependent gradient pulse duration and amplitude were optimized for each sample. 23Na pulsed field gradient (PFG)-NMR experiments were conducted with an acquisition time of 800 ms and a delay between experiments of 2 s, and 1H PFG-NMR experiments were conducted with an acquisition time of 2.7 s and a delay between experiments ranging between 7 and 12 s, depending on T1. 23Na and 1H PFG-NMR spectra used diffusion delays of 40.5 and 30 ms, respectively, and the diffusion coefficients were calculated using single-component, spline-modeling in vNMRJ (v. 3.2). The gradient calibration value (gcal) used to determine absolute diffusion coefficients was calibrated using the self-diffusion coefficient of H2O at 25 °C.25
2.5 Raman spectroscopy
Raman spectra were collected on solutions with a Horiba LabRam HR spectrometer on a Nikon Ti-E inverted microscope with a 40× objective using a continuous 632.81 nm laser. Spectra were acquired between 100–4000 cm−1. Select regions were deconvoluted using Lorentzian line shape parameters following background correction with spline functions. Additional processing utilized 2DShige (v1.3, © Shigeaki Morita, Kwansei-Gakuin University, 2004–2005) to extract autocorrelation spectra from the generalized two-dimensional correlation spectra produced from (i) a concentration array of 1, 3, 6 m NaOH in H2O and (ii) a concentration array of saturated NaNO2 in NaOH solution (entries described in row 2–4, as listed in Table 1) using the NaOH concentration as the perturbation variable.2.6 X-ray total scattering
X-ray total scattering was measured to determine the atomic pair distribution function (PDF). Solutions were loaded into 2 mm ID polyimide capillaries at the Advanced Photon Source (APS), beamline 11-ID-B.26 Immediately after, scattered radiation from the incident beam (∼58.6 keV, λ = 0.2114 Å; beam size of 350 × 350 μm2) was measured in transmission mode using an amorphous-Si detector system manufactured by Perkin Elmer™ (2048 × 2048 pixels, 200 × 200 μm2 pixel size). The program GSAS-II was used to sum 2D scans and perform integration to 1D profiles using a polarization correction.27 The detector non-orthogonality and the sample-to-detector distance were calibrated using a CeO2 standard (NIST diffraction standard set 674a) diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 with glassy carbon. PDFgetX3 was used to process the 1D profiles obtained from GSAS-II and to subtract the background contributions associated with the polyimide capillary and air scattering.28 For PDFgetX3, the compositions were assumed to be that of the as-prepared solutions. The Qmax for the Fourier transformation was set to 24.26 Å−1. The resultant pairwise distribution function, G(r), was output from 0.0 to 16.0 Å with a step size of 0.1 Å. A default value of 0.9 was used for Rpoly, and a background factor of 1.0 was used to subtract the air-filled polyimide capillary. To better understand the X-ray weighted contributions of each i–j type pair (i, j = H, N, Na, and O atoms), the weighting factor (wij) was calculated as:
25 with glassy carbon. PDFgetX3 was used to process the 1D profiles obtained from GSAS-II and to subtract the background contributions associated with the polyimide capillary and air scattering.28 For PDFgetX3, the compositions were assumed to be that of the as-prepared solutions. The Qmax for the Fourier transformation was set to 24.26 Å−1. The resultant pairwise distribution function, G(r), was output from 0.0 to 16.0 Å with a step size of 0.1 Å. A default value of 0.9 was used for Rpoly, and a background factor of 1.0 was used to subtract the air-filled polyimide capillary. To better understand the X-ray weighted contributions of each i–j type pair (i, j = H, N, Na, and O atoms), the weighting factor (wij) was calculated as: 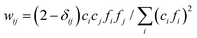 , where δij is the Kronecker delta, fi is the X-ray atomic scattering factor, and ci is the atomic fraction. The atomic fraction is defined by ci = Ni/N, where Ni and N are the number of atoms of type i and the total number of atoms in the solution, respectively.
, where δij is the Kronecker delta, fi is the X-ray atomic scattering factor, and ci is the atomic fraction. The atomic fraction is defined by ci = Ni/N, where Ni and N are the number of atoms of type i and the total number of atoms in the solution, respectively.
3 Results and discussion
3.1 Solubility measurements for NaNO2 and NaOH mixtures
There are few studies of the solubility of NaNO2 in the NaNO2–Na2O–H2O system in the literature. One such study details the solubility of NaNO2 in NaOH solutions at 20 and 25 °C, but no data above room temperature was collected,29 and other studies of NaNO2 solubility in NaOH solutions at elevated temperatures were in multicomponent systems containing significant amounts of sodium nitrate (NaNO3), tetrasodium ethylenediaminetetraacetate, trisodium citrate, sodium phosphate, sodium carbonate, and sodium sulfate.30 Therefore in this work, the solubility of NaNO2 was determined to close the identified knowledge gap associated with the NaNO2–Na2O–H2O system at temperatures greater than 25 °C. An experiment matrix was prepared with three variables: temperature and the concentrations of NaOH and NaNO2 (Table 1). The equilibrium concentration of NaNO2 in solutions containing a range of NaOH concentrations at 27, 40, 60 and 80 °C is shown in Fig. 1 and Table 1.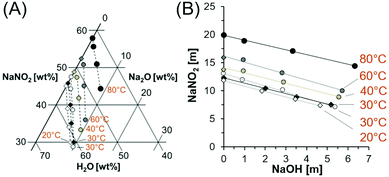 | ||
| Fig. 1 Compositions of saturated solutions in the NaNO2–Na2O–H2O system. (A) The datapoints from this study in the ternary diagram are marked with circles, with colors corresponding to the temperature of the solution. Datapoints marked with diamonds are comparable literature values in mixed NaNO2–Na2O–H2O systems at 20 and 30 °C and interpolated solubilities for the NaNO2–H2O system at 40 and 60 °C.22,29 The dotted lines are drawn only to guide the eyes. (B) The solubility data is also depicted on cartesian coordinates with units of molality. Temperatures are denoted with one significant figure, see Tables 1 and 2 and literature references29 for more precise temperature values. | ||
As shown in Fig. 1, the solubility of NaNO2 increases as a function of temperature and is inversely correlated with the NaOH concentration. The data compare well with prior literature trends of NaNO2 solubility in NaOH solutions at 20 and 25 °C,29 which are also shown in Fig. 1. Reported concentrations correspond to concentrations near equilibrium, as confirmed by the agreement of the solubility of NaNO2 in water at 27 °C and 80 °C with literature values.22Fig. 1B shows the linear dependence of NaNO2 solubility to the concentration of added NaOH. The regressed parameters (Table 2) indicate that the slope has a relatively small temperature dependence. The near equivalent slopes in Fig. 1B, which relate the decrease in solubility of NaNO2 as a function of NaOH, indicate that the addition of NaOH to a saturated solution of NaNO2 will maintain the total sodium concentration, while decreasing the amount of NO2−, in solution. This is consistent with the common ion effect, defined here by unity between moles of added Na-bearing salt and precipitation of an equivalent amount of a second Na-bearing salt on a mole basis.12
| Temperature [°C] | Slope [unitless] | y-Intercept [m] | R 2 [unitless] |
|---|---|---|---|
| 20 | −0.958 | 11.802 | 0.999 |
| 25 | −0.930 | 12.263 | 0.999 |
| 28 | −1.093 | 13.045 | 0.997 |
| 41 | −0.924 | 14.102 | 0.987 |
| 58 | −1.068 | 16.180 | 0.991 |
| 79 | −0.856 | 19.848 | 0.999 |
Solubility models are available in the literature for NaNO2–Na2O–H2O systems near ambient temperatures,18,31 and a Pitzer model of this system including these datasets is currently under development. A prior Pitzer model found that no ion pair association was required for sodium hydroxide up to 11 molal NaOH concentration but an ion pair association term was included in solutions of sodium nitrite.18 Recent work by another group also employed sodium nitrite ion-pair terms in a thermodynamic model of the NaNO2–H2O system in order to fit the water activity of aqueous NaNO2 solutions.32 This group inferred from their results that the ion pairs were poorly hydrated,32 which was proposed previously for large monovalent ions in aqueous solution.33
Analysis of empirical thermodynamic models is challenging because the stability of ion pairs in thermodynamic models does not necessarily correspond to the ion pair stability evaluated by spectroscopy.34 Often, the stability of ion pairs determined by spectroscopy is dependent on the spectroscopic method.34 This is likely because the different spectroscopic methods probe molecular interactions at different time scales. Therefore, in this work, the molecular nature of Na+, NO2−, OH−, and H2O and the chemical environment of the individual ions were investigated across a wide range of timescales and length scales using multinuclear NMR spectroscopy, Raman spectroscopy, and X-ray total scattering.
3.2 NMR spectroscopy
Spectroscopy and diffraction were performed on solutions with a total concentration of ions greater than 24 molal, which corresponds to approximately 2.3 moles of water per mole of ion assuming complete ion dissociation. This is far less than the predicted hydration number of nitrite at dilute conditions, which is around 4–8 water molecules per nitrite ion.14–17 These solution compositions are listed in Table 3 where the sodium nitrite concentration is determined at 27.6 °C and the solutions are composed of saturated sodium nitrite in 1, 3 and 5 m sodium hydroxide. The purpose of the spectroscopy and solution characterization is to provide a physical rational for the inclusion or omission of Na–NO2 ion-pair formation terms in solubility models describing the NaNO2–NaOH mixtures.| NaNO2 [m] | NaOH [m] | 15N maxima [ppm] | 15N fwhm [Hz] | 23Na maxima [ppm] | 23Na fwhm [Hz] |
|---|---|---|---|---|---|
| 5 | 0 | 497.9 | 5 | −0.5 | 16 |
| 13 | 0 | 498.8 | 5 | −1.1 | 23 |
| 12 | 1 | 498.8 | 5 | −0.8 | 23 |
| 10 | 3 | 499.0 | 5 | −0.3 | 28 |
| 7 | 5 | 499.3 | 5 | 0.6 | 38 |
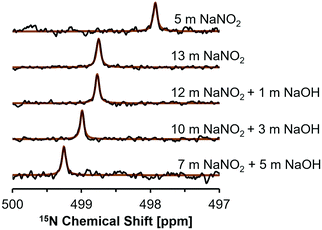
NMR studies were conducted on this subset of solutions to investigate for spectroscopic signatures of ion pairing by changes in the chemical environment of NO2− and Na+ ions. The 15N NMR nucleus is spin 1/2, and typically yields narrow resonances, but direct interrogation of this nucleus is usually challenging due to poor sensitivity resulting from its low relative abundance of 0.03%.35 One strategy to overcome this is to use 15N enrichment, which has been used to investigate nitrite in acidic or neutral conditions,36–38 however studies at high alkalinities are rare. High concentrations of nitrite facilitate natural abundance 15N studies, and recent studies utilized natural abundance samples to investigate photolysis products in NaOH and NaNO2 solutions.39 Here, 15N NMR spectra were acquired to investigate the effects of NaOH concentration on the shielding of 15NO2−. 15N NMR spectra acquired at a field strength of 17.6 T are shown in Fig. 2.
Single pulse, direct excitation 15N NMR spectra show that the NMR resonance of NO2− appears at higher frequencies as the NaNO2 concentration is increased from an undersaturated solution (5 m NaNO2) to a 13 m NaNO2 solution (Fig. 2). In saturated sodium nitrite solutions containing 1, 3, and 5 m NaOH there is a progressive increase in chemical shift from 498.8 ppm to 499.3 ppm, despite the decrease in NO2− concentration from 12 m to 7 m (Table 3). In general, the sensitivity of the 15N chemical shift in these samples can be attributed to: (i) changes in the bulk dielectric constant; (ii) hydrogen bonding, which is particularly important for these N-species that bear lone pair electrons; and (iii) ion-pairing processes, which also perturb the lone pair electrons of NO2− in the chemical environment surrounding the ion.35 When comparing the 15N concentration-dependent trend of the NaNO2 solutions with no added NaOH with studies in literature on the related nitrogen oxyanion, nitrate (NO3−), it is apparent that the increase in 15N chemical shift occurs with increasing salt concentration in both salt systems.40 However, when comparing trends it is important to note that the NaNO3 solutions reported in literature were of lower concentration (<1 m NaNO3).40
Contributions to the chemical shifts arising from intermolecular changes in NO2− was also considered, but changes in N–O bond distance or ONO angle is unlikely for the following reasons. While describing crystalline NaNO2, theoretical analysis of 15N NMR spectra for NaNO2 demonstrated that the chemical shift is extremely sensitive to the N–O bond distance, and also exhibits sensitivity to the ONO angle.13 Specifically, a decrease of N–O bond distance by 2 picometers (from 1.24 to 1.22 Å) with a conserved ONO angle of 115° corresponded to an increase in the 15N chemical shift by ca. 40 ppm. In comparison, widening the ONO angle by 2° (115 to 117°) with a conserved N–O distance of 1.22 Å decreased the 15N chemical shift by ca. 20 ppm.13 Given the 15N chemical shift of the solutions is observed to only vary by a few ppm in the saturated solutions, the N–O bond distance and ONO angle are likely conserved in the solutions described here. Given this, the higher frequencies of 15N resonances in mixtures of NaNO2 and NaOH is interpreted as an increase in ion–ion interactions in mixtures of NaOH and NaNO2 beyond those in single-component NaNO2 solutions at equivalent sodium concentrations.
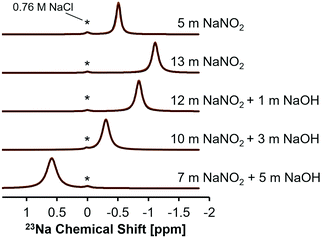
This interpretation of the 15N NMR data was further rationalized by single pulse direct excitation 23Na NMR studies (Fig. 3). 23Na is a 100% abundant, quadrupolar nuclei (spin 3/2) and, in electrolyte solutions, the chemical shift is sensitive both to ionic strength and anion composition.40 In the NaNO2–Na2O–H2O system studied here, the 23Na chemical shift progressively resonates at higher frequencies in NaNO2 solutions as the NaOH concentration increases, whereas the 23Na chemical shift moves to lower frequencies as the concentration of NaNO2 increases in solutions without NaOH.
Increasing the concentration of NaNO2 from 5 to 13 m corresponds with a decrease in the frequency of the 23Na resonance from −0.5 to −1.1 ppm. Given that concentrated, water-in-salt electrolytes have too few water molecules to satisfy ion hydration numbers found under dilute conditions, these solutions can be considered as intermediates between dilute electrolytes and molten salts. Thus, analysis of the chemical shift trend can benefit from comparison to the isotropic 23Na chemical shift of crystalline NaNO2 and NaOH. The isotropic 23Na chemical shift in crystalline NaNO2 is significantly lower, corresponding with −13 ppm at room temperature and −14 ppm at 517 K in molten form, relative to 1 M NaCl in H2O.41,42 Conversely, anhydrous sodium hydroxide has an isotropic 23Na chemical shift of ca. 26 ppm relative to 1 M NaCl in H2O.43 Increasing solution NaNO2 concentrations from 5 to 13 m corresponds with the 23Na chemical shift of those solutions approaching the negative isotropic 23Na chemical shift of crystalline NaNO2 (Fig. 3 and Table 3). Similarly, solutions with greater NaOH concentration approach the positive isotropic 23Na chemical shift of crystalline NaOH. These results indicate that both the 15N and 23Na chemical environment is changed by introducing NaOH to saturated NaNO2 solutions and therefore the introduction of hydroxide ions likely facilitates NO2 interactions with other solution constituents, such as Na+.
Additional pulsed-field gradient (PFG)-NMR experiments were used to investigate the translational motion of H-bearing species (H2O and OH−) and Na+, respectively. While translational motion within electrolyte solutions is often studied by conductivity measurements combined with viscosity measurements using falling ball, capillary, or rotational viscometry or with dielectric relaxation to measure rotational motion,44,45 an alternative description of translational motion across millisecond timescales can be obtained by determining diffusivity coefficients with PFG-NMR spectroscopy.
In the case of PFG-NMR, the displacement of H-bearing species (OH− and H2O) and Na+ ions are probed over millisecond timescales by the application of spatially-dependent, pulsed magnetic field gradients.46 The 1H PFG-NMR coefficients (Fig. 4), confirm that all solutions are more viscous than pure water, in which the various forms of translational mobility of protons in water lead to a diffusivity of ca. 23 EE-10 m2 s−1 at 25 °C.25 The mobility of the protonated species in NaNO2 and NaOH solutions is progressively reduced by addition of NaOH but, the diffusion coefficient of protonated species remains higher than that of Na+ across the range of concentrations. The diffusion coefficient of the Na+ ions in a mixture of NaNO2 and NaOH can be compared to literature 23Na PFG-NMR values of Na+ ions in NaOH solution (Fig. 4).47
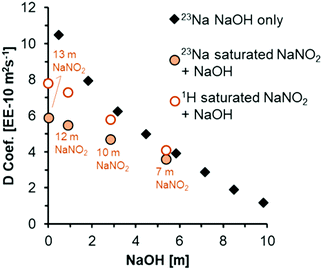 | ||
| Fig. 4 Diffusion coefficients acquired at 25 °C from analysis of 1H and 23Na PFG-NMR spectra of the solutions acquired at 17.6 T. To compare to literature values of 23Na diffusion in concentrated NaOH solutions (dataset labeled 23Na NaOH only),47 the x-axis is plotted as a function of NaOH concentrations. The concentration of NaNO2 is annotated for 1H and 23Na diffusion coefficients acquired from PFG-NMR for solutions composed of a mixture of NaNO2 and NaOH. | ||
There are only slight differences between the 23Na diffusion coefficient of 3.9 EE-10 m2 s−1 in 5 m NaOH, and 3.6 EE-10 m2 s−1 in the mixture of 5 m NaOH with 7 m NaNO2. Based on the 23Na diffusion coefficient of ca. 9.8 EE-10 m2 s−1 in the simple solution of 5 m NaNO2 (Table S3, ESI†), the diffusion coefficient of Na+ ions in NaNO2 solutions is larger, almost double that of NaOH solutions at equivalent molalities. Since the diffusion coefficients in the mixture of 5 m NaOH and 7 m NaNO2 are approximately equivalent to the diffusion coefficient in the absence of added NaNO2, it is suggested that NaOH concentration influences solution rheology more strongly than NaNO2 concentration, which is interpreted as being attributable to more extensive ion pairing in mixtures containing OH− ions. Although ion specific effects on rheological properties such as viscosity and diffusivity remain an active area of research, a similar enhanced contribution of OH− ions to the rheological properties relative to other ions in multicomponent electrolytes was also shown by the deviation of measured viscosities relative to those predicted by Young's rule for mixtures of electrolytes, and this is in agreement with the observations presented here.48 Given that the chemical environment of NO2− and Na+ as measured by 15N and 23Na NMR were found to be sensitive to NaOH addition to saturated NaNO2 solutions, Raman spectra were acquired to interrogate the optical properties of the H2O molecules, and also the NO2− and OH− ions.
3.3 Raman spectroscopy
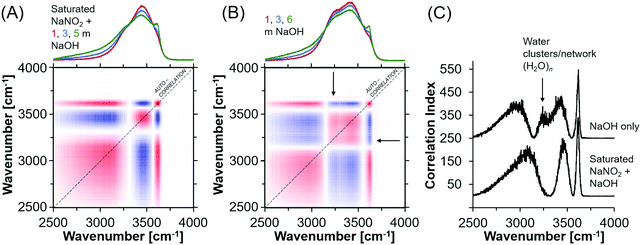
In general, oxyanion mixtures yield well resolved Raman bands sensitive to both the oxyanion composition49,50 and the chemical environment, as characterized by ion-pairing and solvation.51,52 Despite this, the Raman spectra exhibited very little change in the vibrational modes of the NO2− ion, which indicates that ion pairing between Na+ and NO2− is not strong enough to deform the geometry of the NO2− ion even in the presence of hydroxide ions. Fig. S8 (ESI†) shows the Raman spectra of the NaNO2 solutions, where the deformation band can be fit by a single Lorentzian function. Also shown in Fig. S8 (ESI†) are the other vibrational modes of NO2−, and the H2O bending and stretching modes. The insensitivity of the nitrite vibrational bands does not indicate that there are no NO2− ions in the first coordination shell of Na+. This is because splitting of the deformation band does not occur even in solid NaNO2, where there is direct contact between the Na+ and NO2− ions.31 Therefore the deformation bands of nitrite within ion-aggregates, contact and solvent separated ion-pairs, in addition to as solvated ions are likely poorly resolved from each other. For example, a series of Lorentzian bands assigned to solvated ions, solvent-separated ion-pairs, and ion aggregates, or sodium nitrite solutions was used to deconvolute the single quasi-Lorentzian symmetric stretching vibration in concentrated sodium nitrate solutions.53Given the insensitivity of the NO2− vibrational modes to ion pairing, further analysis focused on the OH− bending region of the Raman spectra. In particular, the H2O stretching mode is sensitive to the addition of NaOH and the relatively sharp band appearing ca. 3600 cm−1 and broad band near 3000 cm−1 are attributed to the solvated hydroxides [(OH−)·nH2O].20 Importantly, the OH stretching region is composed of many overlapping bands, leading to non-unique fitting results with Lorentzian deconvolutions. Therefore, synchronous autocorrelation Raman spectra of the OH− stretching region were acquired using the NaOH concentration as the perturbation variable. Analysis of synchronous autocorrelation Raman spectra is particularly useful for improving resolution of overlapping bands in crowded spectra,54 and the analysis was supplemented by comparison to additional solutions prepared with comparable NaOH concentrations without NaNO2. These solutions are 1, 3, and 6 m NaOH in H2O.
The synchronous correlation Raman spectra and corresponding autocorrelation function (Fig. 5) indicate that while the hydroxide peak near 3600 cm−1 does not vary between the two sets of solutions, there are discernable differences between the correlation index around 3250 cm−1 (filled arrow), which is a feature of the OH stretching region assigned to a network of water.20,55,56 This difference is apparent but less obvious in the traditional Raman spectra. Specifically, the water network vibrational modes are absent in concentrated NaNO2 and NaOH solutions. There are also shifts in the band energies at 3500 and 3000 cm−1, both of which are shifted to higher wavenumbers, and those bands are assigned to H2O with an incomplete tetrahedral network, such as small clusters of H2O, and to solvated hydroxide ions, respectively.57 Given that these differences indicate a perturbed solution structure at the molecular level in concentrated NaNO2 and NaOH solutions, X-ray total scattering measurements were performed to further describe the chemical environment of the ions and solvating water.
3.4 X-ray total scattering measurements
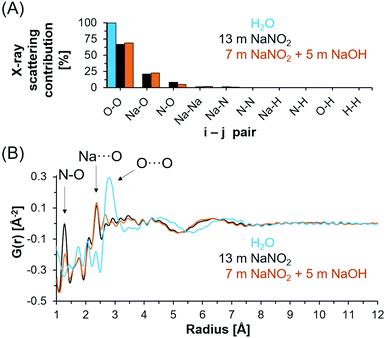
X-ray total scattering and PDF analysis was performed to complement the spectroscopic techniques employed to investigate the chemical environment of NO2−, Na+ and H-bearing species in the NaNO2–Na2O–H2O system. X-ray total scattering analysis is inherently insensitive to hydrogen and instead measures N, Na and O correlations. Based on the electron density of each element and the stoichiometry of the solution, the X-ray scattering contributions of each i–j pair can be calculated (Fig. 6A). The relative contribution of i–j pairs indicates that intra and intermolecular O–O bonds dominate the scattering signal for both deionized water and saturated solutions of NaNO2, both with and without NaOH.In Fig. 6B, the X-ray PDFs of deionized water, 5 m NaOH with 7 m NaNO2 and 13 m NaNO2 are shown. In deionized water, the tetrahedral structure of the water network is apparent from the broad O⋯O distances at 4.3 Å along with nearest neighbor O⋯O atom pairs near 2.8 Å.21 A reduction in both O⋯O correlations is observed when comparing deionized water with saturated NaNO2 and saturated NaNO2 with 5 m NaOH. This reduction in O⋯O correlation length is generally consistent with theoretical analysis of neutron diffraction experimental data of concentrated NaOH solutions.58
In addition, the X-ray PDF shows well resolved intramolecular (covalent) N–O bonds in NO2− at 1.2 Å with additional features attributed to intramolecular ONO correlations observed at ∼2.1 Å. The lack of change in the N–O correlation distance between samples is consistent with the few ppm shifts in 15N NMR spectra and insensitivity of the vibrational modes of the nitrite ion. Features of the Na+ solvation shell occur at approximately 2.3 Å, and include both Na⋯OH2 and Na⋯OH in hydroxide bearing solutions.59,60 The radial distance of the sodium solvation shell is consistent with prior ab initio molecular dynamics simulations of concentrated NaOH, in which an ensemble of ion cluster species was related to their individual X-ray PDF features at similar distances of 2.2–2.3 Å.21 Further comparison of scattering from solutions of 13 m NaNO2 and solutions of 7 m NaNO2 with 5 m NaOH indicates significantly more order in the solution containing OH− ions at distances greater than 3 Å. Specifically, the O⋯O peak observed at 2.8 Å in bulk water is significantly reduced in intensity and the maxima of the first long range oscillation O⋯O is reduced from 4.3 Å to 3.5 Å. This is interpreted as arising from the disruption of water's hydrogen bond network and the consequent collapse of the second neighboring hydration shell due to the ion solvation and association interactions. The correlations at distances greater than 3.5 Å in the concentrated electrolytes is then attributed to predominantly O⋯O correlations in hydrated ion networks, and these correlations occur at shorter distances in solutions containing NaOH and NaNO2−. The long-range order of the saturated solutions is distinct from simulated X-ray PDFs of crystalline NaNO2, NaOH·H2O, and NaOH polymorphs as shown in Fig. S10–S11 in the ESI.†
4. Conclusions
This study aimed to improve prediction of ion pairing processes, solution rheology properties, and ultimately the solubilities of NaNO2 in highly alkaline NaOH-containing aqueous solutions. Despite the ability for solubility to be modeled with only formalism of very weak Na+–nitrite ion pairs, the spectroscopy in this work indicates that the addition of NaOH perturbs the chemical environment of water, sodium, and nitrite compared to pure NaNO2 solutions of comparable amounts of sodium. The change in the chemical environment around water is shown directly by relatively large changes in the OH stretching region. In addition to the changes in the vibrational properties of H2O, 23Na and 15N NMR shows that shielding of 23Na+ and of 15NO2− are sensitive to NaOH concentration, with the chemical shift of both species increasing to higher frequencies as a function of NaOH concentration. NaOH concentration also significantly influences the rheological properties of the solution, progressively reducing the diffusion of Na- and H-bearing species. Analysis of X-ray PDF data indicated a reduction in length of O⋯O correlations in saturated nitrite solutions relative to deionized water. The reduction in O⋯O correlation length was greater for lower concentrations (7 m) of NaNO2 in the presence of NaOH, than for higher concentrations (13 m) of NaNO2 in H2O.While further work in related electrolytes is underway to better link the absence of fundamental vibrations of the water networks in the OH− stretching region to changes in long range O⋯O correlations obtained via analysis of X-ray PDF, the presence of this more ordered structure in concentrated electrolytes with NaOH correlates with less diffusion of H-bearing species and Na+ on millisecond timescales, and with the disappearance of the vibrational mode corresponding to H2O clusters in autocorrelation Raman spectra. The greater change in the chemical environment of H2O in mixed NaOH–NaNO2 systems relative to solutions with only NaNO2 is shown in X-ray PDF from the greater compaction of the long range O⋯O correlations, although this observation is convoluted with the other long range O⋯O correlations beyond that of H2O, such as those including contributions from oxygen correlations between NO2− and OH−. The results indicate that there is greater ion pairing in mixtures of concentrated NaNO2 and NaOH than in comparable solutions with only NaNO2. These studies, pairing solubility and spectroscopy to elucidate molecular-scale speciation of significant ions in multicomponent electrolyte mixtures of relevance to flue gas treatment and radioactive waste processing, generate new knowledge of solution processes that can reduce the technical risks associated with unit operations.
Conflicts of interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. Trent R. Graham, Mateusz Dembowski, Hsiu-Wen Wang, Sebastian T. Mergelsberg, Emily T. Nienhuis, Jacob G. Reynolds, Calvin H. Delegard, Yihui Wei, Michelle Snyder, Ian I. Leavy, Steven R. Baum, Matthew S. Fountain, Sue B. Clark, Kevin M. Rosso, and Carolyn I. Pearce.Acknowledgements
NMR spectroscopy, Raman spectroscopy, scanning electron microscopy, powder X-ray diffraction, and high-energy X-ray PDF were supported by IDREAM (Interfacial Dynamics in Radioactive Environments and Materials), an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES). The solubility experiments were conducted in support of the River Protection Project (RPP) Integrated Flowsheet group to identify and close flowsheet model and operations gaps and realize opportunities to reduce the waste treatment mission cost, schedule, and technical risk, funded by Washington River Protection Solutions. NMR, SEM, pXRD, and Raman spectroscopy were performed using facilities at the Environmental Molecular Science Laboratory (EMSL, grid.436923.9), a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research at Pacific Northwest National Laboratory (PNNL). PNNL is a multi-program national laboratory operated for DOE by Battelle Memorial Institute operating under Contract No. DE AC05-76RL0-1830. This project used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357, where data acquisition was performed at beam line 11-ID-B. Construction of the ternary diagram utilized Triplot (v 1.4).61References
- S. M. Chalanchi, I. Alkorta, J. Elguero and D. Quiñonero, Hydrogen Bond versus Halogen Bond in Cation–Cation Complexes: Effect of the Solvent, ChemPhysChem, 2017, 18, 3462–3468, DOI:10.1002/cphc.201700819.
- I. Mata, E. Molins, I. Alkorta and E. Espinosa, The paradox of hydrogen-bonded anion–anion aggregates in oxoanions: A fundamental electrostatic problem explained in terms of electrophilic-nucleophilic interactions, J. Phys. Chem. A, 2015, 199, 183–194, DOI:10.1021/jp510198g.
- D. Marx, A. Chandra and M. E. Tuckerman, Aqueous basic solutions: Hydroxide solvation, structural diffusion, and comparison to the hydrated proton, Chem. Rev., 2010, 110, 2174–2216, DOI:10.1021/cr900233f.
- M. E. Tuckerman, D. Marx and M. Parrinello, The nature and transport mechanism of hydrated hydroxide ions in aqueous solution, Nature, 2002, 417, 925–929, DOI:10.1038/nature00797.
- M. Hellström, M. Ceriotti and J. Behler, Nuclear Quantum Effects in Sodium Hydroxide Solutions from Neural Network Molecular Dynamics Simulations, J. Phys. Chem. B, 2018, 122, 10158–10171, DOI:10.1021/acs.jpcb.8b06433.
- N. J. Suchak, K. R. Jethani and J. B. Joshi, Absorption of Nitrogen Oxides in Alkaline Solutions: Selective Manufacture of Sodium Nitrite, Ind. Eng. Chem. Res., 1990, 29, 1492–1502, DOI:10.1021/ie00103a059.
- R. A. Peterson, E. C. Buck, J. Chun, R. C. Daniel, D. L. Herting, E. S. Ilton, G. J. Lumetta and S. B. Clark, Review of the Scientific Understanding of Radioactive Waste at the U.S. DOE Hanford Site, Environ. Sci. Technol., 2018, 52, 381–396, DOI:10.1021/acs.est.7b04077.
- D. T. Hobbs, Caustic recovery from alkaline nuclear waste by an electrochemical separation process, Sep. Purif. Technol., 1999, 15, 239–253, DOI:10.1016/S1383-5866(98)00105-1.
- P. Bénézeth, S. Hilic and D. A. Palmer, The Solubilities of Gibbsite and Bayerite Below 100 °C in Near Neutral to Basic Solutions, J. Solution Chem., 2016, 45, 1288–1302, DOI:10.1007/s10953-016-0470-1.
- M. Dembowski, M. M. Snyder, C. H. Delegard, J. G. Reynolds, T. R. Graham, H.-W. Wang, I. I. Leavy, S. R. Baum, O. Qafoku, M. S. Fountain, K. M. Rosso, S. B. Clark and C. I. Pearce, Ion–ion interactions enhance aluminum solubility in alkaline suspensions of nano-gibbsite (α-Al(OH)3) with sodium nitrite/nitrate, Phys. Chem. Chem. Phys., 2020, 8, 4368–4378, 10.1039/C9CP05856G.
- J. Addai-Mensah, J. Li, S. Rosencrance and W. Wilmarth, Solubility of amorphous sodium aluminosilicate and zeolite a crystals in caustic and nitrate/nitrite-rich caustic aluminate liquors, J. Chem. Eng. Data, 2004, 49, 1682–1687, DOI:10.1021/je049889f.
- J. G. Reynolds, Salt Solubilities in Aqueous Solutions of NaNO3, NaNO2, NaCl, and NaOH: A Hofmeister-like Series for Understanding Alkaline Nuclear Waste, ACS Omega, 2018, 3, 15149–15157, DOI:10.1021/acsomega.8b02052.
- P. J. Barrie, C. J. Groombridge, J. Mason and E. A. Moore, Nitrogen-15 nuclear magnetic shielding tensors in nitrite and nitrate ion. Experimental and theoretical determinations, Chem. Phys. Lett., 1994, 219, 491–496, DOI:10.1016/0009-2614(94)00111-1.
- S. Yadav and A. Chandra, Solvation Shell of the Nitrite Ion in Water: An Ab Initio Molecular Dynamics Study, J. Phys. Chem. B, 2020, 124, 7194–7204, DOI:10.1021/acs.jpcb.0c02221.
- S. Vchirawongkwin, C. Kritayakornupong, A. Tongraar and V. Vchirawongkwin, Hydration properties determining the reactivity of nitrite in aqueous solution, Dalton Trans., 2014, 32, 12164–12174, 10.1039/c4dt00273c.
- Y. Kameda, H. Arakawa, K. Hangai and O. Uemura, The Structure around the Nitrite Ion in Concentrated Aqueous Solutions, Bull. Chem. Soc. Jpn., 1992, 65, 2154–2156, DOI:10.1246/bcsj.65.2154.
- R. M. Cordeiro, M. Yusupov, J. Razzokov and A. Bogaerts, Parametrization and Molecular Dynamics Simulations of Nitrogen Oxyanions and Oxyacids for Applications in Atmospheric and Biomolecular Sciences, J. Phys. Chem. B, 2020, 124, 1082–1089, DOI:10.1021/acs.jpcb.9b08172.
- J. G. Reynolds, R. Carter and A. R. Felmy, A Pitzer interaction model for the NaNO3-NaNO2-NaOH-H2O system from 0 to 100 °C, Ind. Eng. Chem. Res., 2015, 54, 3062–3070, DOI:10.1021/acs.iecr.5b00016.
- A. M. Hyde, S. L. Zultanski, J. H. Waldman, Y. L. Zhong, M. Shevlin and F. Peng, General Principles and Strategies for Salting-Out Informed by the Hofmeister Series, Org. Process Res. Dev., 2017, 21, 1355–1370, DOI:10.1021/acs.oprd.7b00197.
- M. Moskovits and K. H. Michaelian, Alkali Hydroxide Ion Pairs. A Raman study, J. Am. Chem. Soc., 1980, 102, 2209–2215, DOI:10.1021/ja00527a010.
- D. Semrouni, H. W. Wang, S. B. Clark, C. I. Pearce, K. Page, G. Schenter, D. J. Wesolowski, A. G. Stack and A. E. Clark, Resolving local configurational contributions to X-ray and neutron radial distribution functions within solutions of concentrated electrolytes-a case study of concentrated NaOH, Phys. Chem. Chem. Phys., 2019, 13, 6828–6838, 10.1039/c8cp06802j.
- E. Washburn, International Critical Tables of Numerical Data, Physics, Chemistry and Technology, New York, New York, 1928 Search PubMed.
- D. S. Raiford, C. L. Fisk and E. D. Becker, Calibration of methanol and ethylene glycol nuclear magnetic resonance thermometers, Anal. Chem., 1979, 51, 2050–2051, DOI:10.1021/ac50048a040.
- I. Yavari and J. D. Roberts, Nitrogen-15 Nuclear Magnetic Resonance Spectroscopy. Carbodiimides, J. Org. Chem., 1978, 43, 4689–4693, DOI:10.1021/jo00419a001.
- P. T. Callaghan, M. A. Le Gros and D. N. Pinder, The measurement of diffusion using deuterium pulsed field gradient nuclear magnetic resonance, J. Chem. Phys., 1983, 79, 6372–6381, DOI:10.1063/1.445745.
- A. Hoeher, S. Mergelsberg, O. J. Borkiewicz, P. M. Dove and F. Marc Michel, A new method for in situ structural investigations of nano-sized amorphous and crystalline materials using mixed-flow reactors, Acta Crystallogr., Sect. A: Found. Adv., 2019, 75, 758–765, DOI:10.1107/S2053273319008623.
- B. H. Toby and R. B. Von Dreele, GSAS-II: The genesis of a modern open-source all purpose crystallography software package, J. Appl. Crystallogr., 2013, 46, 544–549, DOI:10.1107/S0021889813003531.
- P. Juhás, T. Davis, C. L. Farrow and S. J. L. Billinge, PDFgetX3: A rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions, J. Appl. Crystallogr., 2013, 46, 560–566, DOI:10.1107/S0021889813005190.
- V. F. Plekhotkin and L. P. Bobrovskaya, Russ. J. Inorg. Chem., 1970, 15, 842–844 Search PubMed.
- D. A. Reynolds and D. L. Herting, Solubilities of sodium nitrate, sodium nitrite, and sodium aluminate in simulated nuclear waste, Richland, WA, 1984 Search PubMed.
- R. K. Toghiani, V. A. Phillips, L. T. Smith and J. S. Lindner, Solubility in the Na + SO4 + NO3 and Na + SO4 + NO2 systems in water and in sodium hydroxide solutions, J. Chem. Eng. Data, 2008, 53, 798–804, DOI:10.1021/je700666t.
- A. A. Zavitsas, Ideal thermodynamic behaviors of aqueous electrolyte solutions at very high concentrations, Chem. Phys. Lett., 2020, 759, 137941, DOI:10.1016/j.cplett.2020.137941.
- R. M. Diamond, The aqueous solution behavior of large univalent ions. A new type of ion-pairing 1a, J. Phys. Chem., 1963, 67, 2513–2517, DOI:10.1021/j100806a002.
- G. Hefter, When spectroscopy fails: The measurement of ion pairing, Pure Appl. Chem., 2006, 78, 1571–1586, DOI:10.1351/pac200678081571.
- G. A. Webb and M. Witanowski, Nitrogen NMR and molecular interations, Proc. Indian Acad. Sci., 1985, 94, 241–290 CAS.
- G. K. Surya Prakash, L. Heiliger and G. A. Olah, 15N NMR Spectroscopic investigation of nitrous and nitric acids in sulfuric acid solutions of varying acidities, Inorg. Chem., 1990, 29, 4965–4968, DOI:10.1021/ic00349a027.
- R. L. Van Etten and J. M. Risley, 18O-Isotope-Induced Shifts in 15N Nuclear Magnetic Resonance Spectroscopy. 1. Isotope Shift in Nitrite and Its Application to the Study of Oxygen Exchange of Sodium [15N,18O2]Nitrite, J. Am. Chem. Soc., 1981, 103, 5633–5636, DOI:10.1021/ja00409a002.
- K. A. Thorn and M. A. Mikita, Nitrite Fixation by Humic Substances Nitrogen-15 Nuclear Magnetic Resonance Evidence for Potential Intermediates in Chemodenitrification, Soil Sci. Soc. Am. J., 2000, 64, 568–582, DOI:10.2136/sssaj2000.642568x.
- E. D. Walter, K. C. Schwarz, S. A. Kumar, Y. Chen, M. Sassi, Z. Wang and K. M. Rosso, Evolution of Radicals from the Photolysis of High Ionic Strength Alkaline Nitrite Solutions, J. Phys. Chem. A, 2020, 124, 3019–3025, DOI:10.1021/acs.jpca.9b11438.
- W. Makulski, Multinuclear Magnetic Resonance Study of Sodium Salts in Water Solutions, Magnetochemistry, 2019, 5, 1–12, DOI:10.3390/magnetochemistry5040068.
- S. V. Baryshnikov, E. V. Stukova, E. V. Charnaya, C. Tien, M. K. Lee, W. Böhlmann and D. Michel, Dielectric and NMR studies of nanoporous matrices loaded with sodium nitrite, Phys. Solid State, 2006, 48, 593–599, DOI:10.1134/S1063783406030292.
- A. G. Gorchakov, P. S. Sedykh, E. V. Charnaya, S. V. Baryshnikov, C. Tien and D. Michel, MAS NMR studies of nanoporous matrices filled with sodium nitrite, Phys. Solid State, 2009, 51, 2152–2156, DOI:10.1134/S1063783409100266.
- L. Gao, J. Chen, Y. Liu, Y. Yamauchi, Z. Huang and X. Kong, Revealing the chemistry of an anode-passivating electrolyte salt for high rate and stable sodium metal batteries, J. Mater. Chem. A, 2018, 6, 12012–12017, 10.1039/c8ta03436b.
- R. Buchner, P. Sipos, G. Hefter and P. M. May, Dielectric relaxation of concentrated alkaline aluminate solutions, J. Phys. Chem. A, 2002, 28, 6527–6532, DOI:10.1021/jp0143589.
- P. C. Ho, D. A. Palmer and R. H. Wood, Conductivity measurements of dilute aqueous LiOH, NaOH, and KOH solutions to high temperatures and pressures using a flow-through cell, J. Phys. Chem. B, 2000, 104, 12084–12089, DOI:10.1021/jp000845x.
- T. R. Graham, K. S. Han, M. Dembowski, A. J. Krzysko, X. Zhang, J. Hu, S. B. Clark, A. E. Clark, G. K. Schenter, C. I. Pearce and K. M. Rosso, 27Al Pulsed Field Gradient, Diffusion-NMR Spectroscopy of Solvation Dynamics and Ion Pairing in Alkaline Aluminate Solutions, J. Phys. Chem. B, 2018, 122, 10907–10912, DOI:10.1021/acs.jpcb.8b10145.
- J. Mitchell, Can sodium NMR provide more than a tracer for brine in petrophysics?, J. Pet. Sci. Eng., 2016, 146, 360–368, DOI:10.1016/j.petrol.2016.05.035.
- G. Hefter, P. M. May, P. Sipos and A. Stanley, Viscosities of concentrated electrolyte solutions, J. Mol. Liq., 2003, 103–104, 261–273, DOI:10.1016/S0167-7322(02)00145-9.
- F. Zapata, F. Ortega-Ojeda, C. García-Ruiz and M. González-Herráez, Selective monitoring of oxyanion mixtures by a flow system with Raman detection, Sensors, 2018, 18, 1–10, DOI:10.3390/s18072196.
- G. M. Maggioni, S. Kocevska, M. A. Grover and R. W. Rousseau, Analysis of Multicomponent Ionic Mixtures Using Blind Source Separation: A Processing Case Study, Ind. Eng. Chem. Res., 2019, 58, 22640–22651, DOI:10.1021/acs.iecr.9b03214.
- J. B. Gill, Solute-Solute Interactions in Liquid Ammonia Solutions: A Vibrational Spectroscopic View, Pure Appl. Chem., 1981, 53, 1365–1381, DOI:10.1351/pac198153071365.
- A. G. Miller and J. A. Macklin, Matrix Effects on the Raman Analytical Lines of Oxyanions, Anal. Chem., 1980, 52, 807–812, DOI:10.1021/ac50056a009.
- R. L. Frost and D. W. James, Ion–ion–solvent interactions in solution. Part 3.—Aqueous solutions of sodium nitrate, J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases, 1982, 78, 3223–3234, 10.1039/F19827803223.
- L. Ma, V. Sikirzhytski, Z. Hong, I. K. Lednev and S. A. Asher, Insight into Resolution Enhancement in Generalized Two-Dimensional Correlation Spectroscopy, Appl. Spectrosc., 2013, 67, 283–290, DOI:10.1366/11-06541.
- J. Stefanski, C. Schmidt and S. Jahn, Aqueous sodium hydroxide (NaOH) solutions at high pressure and temperature: insights from in situ Raman spectroscopy and ab initio molecular dynamics simulations, Phys. Chem. Chem. Phys., 2018, 20, 21629–21639, 10.1039/C8CP00376A.
- J.-B. Brubach, A. Mermet, A. Filabozzi, A. Gerschel and P. Roy, Signatures of the hydrogen bonding in the infrared bands of water, J. Chem. Phys., 2005, 122, 184509, DOI:10.1063/1.1894929.
- J. Stefanski, C. Schmidt and S. Jahn, Aqueous sodium hydroxide (NaOH) solutions at high pressure and temperature: insights from in situ Raman spectroscopy and ab initio molecular dynamics simulations, Phys. Chem. Chem. Phys., 2018, 20, 21629–21639, 10.1039/C8CP00376A.
- A. Botti, F. Bruni, S. Imberti, M. A. Ricci and A. K. Soper, Ions in water: The microscopic structure of concentrated NaOH solutions, J. Chem. Phys., 2004, 120, 10154–10162, DOI:10.1063/1.1705572.
- T. Megyes, S. Bálint, T. Grósz, T. Radnai, I. Bakó and P. Sipos, The structure of aqueous sodium hydroxide solutions: A combined solution X-ray diffraction and simulation study, J. Chem. Phys., 2008, 128, 1–12, DOI:10.1063/1.2821956.
- T. Radnai, P. M. May, G. T. Hefter and P. Sipos, Structure of Aqueous Sodium Aluminate Solutions: A Solution X-ray Diffraction Study, J. Phys. Chem. A, 1998, 102, 7841–7850, DOI:10.1021/jp981244x.
- D. J. Graham and N. G. Midgley, Graphical Representation of Particle Shape Using Triangular Diagrams: An Excel Spreadsheet Method, Earth Surf. Processes Landforms, 2000, 25, 1473–1477 CrossRef , DOI: 10.1002/1096-9837(200012)25:13<1473::AID-ESP158>3.0.CO;2-C.
Footnote |
| † Electronic supplementary information (ESI) available: Additional results from solubility studies approaching equilibrium concentrations from oversaturation and undersaturation, additional experimental results from studies comparing concentrations acquired with filtering versus sampling of supernatant, additional Raman results of nitrite vibrational bands including discussion of relevant literature, and tabulated 1H and 23Na diffusion coefficients. See DOI: 10.1039/d0cp04799f |
| This journal is © the Owner Societies 2021 |