Controlled growth of porous oxygen-deficient NiCo2O4 nanobelts as high-efficiency electrocatalysts for oxygen evolution reaction†
Shangzhi
Yao
ab,
Haoshan
Wei
ab,
Yong
Zhang
 *ab,
Xueru
Zhang
c,
Yan
Wang
*ab,
Xueru
Zhang
c,
Yan
Wang
 ab,
Jiaqin
Liu
ab,
Jiaqin
Liu
 d,
Hark Hoe
Tan
d,
Hark Hoe
Tan
 ef,
Ting
Xie
b and
Yucheng
Wu
ef,
Ting
Xie
b and
Yucheng
Wu
 *abc
*abc
aSchool of Materials Science and Engineering, Hefei University of Technology, Hefei 230009, Anhui, China. E-mail: zhangyong.mse@hfut.edu.cn; ycwu@hfut.edu.cn
bKey Laboratory of Advanced Functional Materials and Devices of Anhui Province, Hefei 230009, China
cInstrumental Analysis Center, Hefei University of Technology, Hefei 230009, China
dInstitute of Industry & Equipment Technology, Hefei University of Technology, Hefei 230009, Anhui, China
eChina International S&T Cooperation Base for Advanced Energy and Environmental Materials, Hefei 230009, Anhui, China
fDepartment of Electronic Materials Engineering, Research School of Physics and Engineering, The Australian National University, Canberra, ACT 2601, Australia
First published on 19th October 2020
Abstract
Owing to their distinctive chemical properties and cost-effectiveness, transition metal oxides (TMOs) promise intriguing potential in electrocatalysis applications. Herein, porous NiCo2O4 nanobelts with controlled oxygen deficiencies were synthesized based on a facile strategy of hydrothermal growth followed by annealing under an inert atmosphere. By finely adjusting annealing temperature and time, concentrations of oxygen deficiencies within the nanobelts could be modulated. The oxygen-deficient NiCo2O4 nanobelts exhibit superior oxygen evolution reaction (OER) performance at a relatively low overpotential which is superior to the values of prepared pristine NiCo2O4 electrocatalysts. In particular, they show excellent stability for 10 h at 10 mA cm−2. The enhanced OER activity and stability of the catalyst can be ascribed to the abundant oxygen deficiencies as well as porous architecture of the anisotropic nanobelts. This work paves a promising way in fabricating advanced electrocatalysts.
1. Introduction
With the increase of fossil energy consumption and concerns relating to climate change and environmental problems, the demand for green and renewable energy is gradually increasing.1–3 The oxygen evolution reaction (OER), as a vital half reaction of the water splitting reaction, is a promising way to solve the serious energy problem.4–10 However, compared to hydrogen evolution reaction (HER), OER demands a much higher overpotential to complete a sluggish four-electron oxidation process, which suffers from sluggish kinetics.11–14 Recently, a number of OER electrocatalysts have been applied, among which precious metal oxides are the most efficient like IrO2 and RuO2, but their high prices and exiguity hamper their extensive application commercially.15–19 Therefore, numerous studies have been devoted to developing highly active, durable, and cheap electrocatalysts like transition metal oxides.20–30Among all transition metal oxides, NiCo2O4 is one of the most promising alkaline medium materials as electrocatalysts, because of its fast charge transfer and plentiful catalytically active sites. However, its intrinsic conductivity is still lower than those of precious metal oxides, as well as its active sites.31–35 To overcome the limitation, Lee and co-workers synthesized a mesoporous NiCo2O4 nanoplatelet–graphene hybrid as a highly active bifunctional OER and ORR catalyst, owing to its improved electron transfer and more active sites facilitated by graphene as a support material.36 Lim et al. successfully prepared oxygen-deficient NiCo2O4 materials with oxygen vacancies which promoted the electrochemical activity for ORR.37 In light of the previous studies, introducing oxygen vacancies is a competitive strategy to improve both conductivity and the number of active sites.38–41 First, moderate oxygen vacancies can help to diffuse charge, thus getting a high conductivity and capacitance.42–44 Second, oxygen vacancies provide better adsorption of OH−.45,46 Finally, oxygen vacancies can serve as active sites for redox reactions, which play an important role in accelerating oxidation reaction kinetics.47–49
Presently, the common methods of creating oxygen vacancies can be classified into two ways: one is reducing metal oxides by strong reducing agents like NaBH4,50 and the other is annealing metal oxides in a reducing atmosphere like an NH3 or H2/Ar gas mixture.51,52 Compared with chemical reduction method, the annealing method shows more convenience, less pollution and better safety. But to date, less research has been concentrated on the impact of an inert atmosphere and corresponding annealing temperature on oxygen vacancy performance and resultant electrocatalytic properties.
In this paper, we prepared an oxygen-deficient NiCo2O4 nanobelt composite by a facile and safe hydrothermal method and subsequent Ar-annealing process. The impact of annealing temperature on oxygen vacancy concentration has further been investigated. The NiCo2O4 nanobelts rich in oxygen vacancies thermally annealed at 400 °C under an argon flow reach a current density of 10 mA cm−2 at 1.555 V versus the reversible hydrogen electrode (RHE) and show outstanding stability with no obvious current density loss at 10 mA cm−2 after 10 h in 1 M KOH. It is very interesting to propose that the introduction of oxygen vacancies and the resulting higher conductivity and more active sites have been proved to be essential factors for the improvement of electrocatalytic performance.
2. Experimental
2.1 Materials
Cobalt nitrate (Co(NO3)2·6H2O, 99.9%, Alfa Aesar), nickel nitrate (Ni(NO3)2·6H2O, 99.9%, Alfa Aesar), ethanedioic acid dihydrate (H2C2O4·2H2O, 99.5%, Alfa Aesar), ethylene glycol (C2H6O2, 99%, Alfa Aesar), potassium hydroxide (KOH, 99.9%, Alfa Aesar), ruthenium dioxide (RuO2, 99.9%, Sigma-Aldrich), Nafion (5% wt, Sigma-Aldrich). All chemicals were used directly without any further purification.2.2 Preparation of the NiCo2O4 nanobelts
The pristine NiCo2O4 nanobelts were synthesized by a hydrothermal treatment and subsequent annealing process. First, 1 mmol of Ni(NO3)2·6H2O and 2 mmol of Co(NO3)2·6H2O were dissolved in 15 mL deionized (DI) water and ultrasonicated for 5 min, named A. Simultaneously, 3 mmol of H2C2O4·2H2O was added into 45 mL C2H6O2 and ultrasonicated for 5 min, named B. Then, the aqueous solution of A was poured into the solution of B to mix into a homogeneous solution by vigorous magnetic stirring for 5 min. Then, the mixed solution was transferred into a Teflon-lined stainless-steel autoclave at 120 °C for 24 h. After the autoclave was cooled naturally to room temperature, the precipitates were separated by centrifugation, washed with DI water and ethanol six times, and dried in a vacuum oven at 60 °C for 8 h. Finally, the prepared pink powder sample was then calcined in air at 350 °C for 0.5 h. The synthesized black powder sample is denoted as P-NiCo2O4.2.3 Preparation of the oxygen-deficient NiCo2O4 nanobelts
To introduce oxygen vacancies into the NiCo2O4 nanobelts, the P-NiCo2O4 sample was annealed in a tube furnace under an argon flow for 2 h at different temperatures of 300 °C, 350 °C, 400 °C, 450 °C and 500 °C with a heating rate of 5 °C min−1. The Ar-treated NiCo2O4 powder samples are denoted as NiCo2O4-OV-X, where X represents the annealing temperature.2.4 Characterization
The surface morphologies of the powder samples were investigated by scanning electron microscopy (SEM, Hitachi SU8020) and transmission electron microscopy (TEM, JEM-2100F). The X-ray diffraction (XRD) patterns of all the samples were obtained at room temperature on an X'Pert Pro MPD (PANalytical B.V.) using a Cu target (λ = 0.154 nm) from 5 to 80 degrees. X-ray photoelectron spectroscopy (XPS) data were obtained with a Thermo ESCALAB250Xi using Al Kα radiation.2.5 Electrochemical measurements
5 mg of the obtained catalyst were dispersed in a mixture solution of 990 μL of ethanol and 10 μL of 5 wt% Nafion. The resulting mixture was dispersed by ultrasonication for 0.5 h to form a uniform suspension. 10 μL of catalyst ink was deposited onto the surface of a glassy carbon electrode (GCE, 3 mm in diameter), and the electrode was naturally dried at room temperature.The electrochemical tests were performed in a 1 M KOH aqueous electrolyte solution wherein the GC disk coated with the sample, Ag/AgCl electrode and graphite rod electrode were used as the working electrode (WE), reference electrode (RE), and counter electrode (CE), respectively. Each experiment was performed after the electrolyte solution was purged with O2 for 0.5 h to form an oxygen-saturated solution. Linear-sweep voltammetry (LSV) was performed with a scan rate of 5 mV s−1. The potentials herein were converted to RHE through the following equation:
| ERHE = EAg/AgO + 0.059 pH + 0.197 V | (1) |
| η = ERHE − 1.23 V | (2) |
Additionally, the Tafel slope was modelled by the Tafel equation:
η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a j + a | (3) |
3. Results and discussion
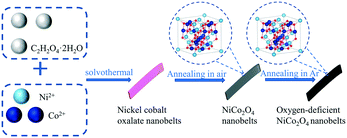
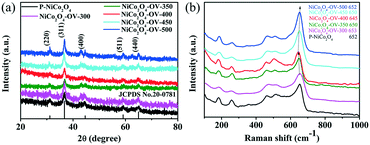
The oxygen-deficient NiCo2O4 nanobelts were fabricated by a three-step process as shown in Scheme 1. First, the nickel cobalt oxalate nanobelts were synthesized by a hydrothermal method; then, the obtained sample was annealed at 350 °C for 0.5 h in an air atmosphere in order to prepare the NiCo2O4 nanobelts; finally, the nanobelts were continuously annealed at 300, 350, 400, 450 and 500 °C for 2 h by switching the air to argon atmosphere to achieve oxygen-deficient NiCo2O4 nanobelts.The XRD patterns of the prepared NiCo2O4 nanobelts are shown in Fig. 1a. As can be seen, five major diffraction peaks located at 31.1°, 36.7°, 44.6°, 59.0°, and 64.9° can be indexed to the (220), (311), (400), (511), and (440) planes of NiCo2O4 (JCPDS card no. 20-0781), respectively. Compared to those of the pristine NiCo2O4, no obvious peak position changes were seen in the XRD patterns, which implied that no new phase exists. Interestingly, the peak intensity of the Ar-annealed NiCo2O4 is lower, demonstrating the weak crystallinity of the oxygen-deficient NiCo2O4 samples. Also, there is a negative shift of the peaks owing to the introduction of the crystal defects like oxygen vacancies.53 As shown in Fig. 1b, the peaks at 184, 455, 505, and 648 cm−1 match up with the F2g, Eg, LO, and A1g bands of NiCo2O4, respectively. Significantly, the Raman peaks of the oxygen-deficient NiCo2O4 samples shift towards the negative direction, which implies the successful introduction of oxygen vacancies.54
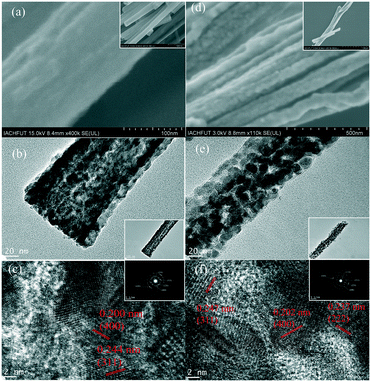
Fig. 2 shows the FESEM and TEM images of the P-NiCo2O4 and NiCo2O4-OV-400 samples. No obvious difference in the morphology between P-NiCo2O4 and NiCo2O4-OV-400 can be seen from the images. Both the NiCo2O4 samples are composed of well-defined nanobelts, indicating the favourable structural stability. After annealing at 400 °C, abundant NiCo2O4 nanoparticles grow in situ in the nanobelt, and even some particles grow to the edge of the nanobelt (Fig. 2e-inset), which formed mesoporous holes and therefore created a larger specific surface area. In addition, it can be seen from Fig. S1† that the annealing process makes the nanobelts curl and tend to form a tubular hollow structure, which is also consistent with the TEM results.55 The clear lattice spacings (Fig. 2f) of about 0.202 nm, 0.237 nm and 0.247 nm can be assigned to the (400), (222) and (311) planes of NiCo2O4-OV-400, respectively. The inset image shows the corresponding SAED pattern of NiCo2O4-OV-400, which presents diffraction rings corresponding to the (111), (220), (311), (400) and (440) crystal planes. Fig. S1b† clearly shows the energy dispersive spectroscopy maps of NiCo2O4-OV-400, implying an even distribution of Ni, Co, and O elements.
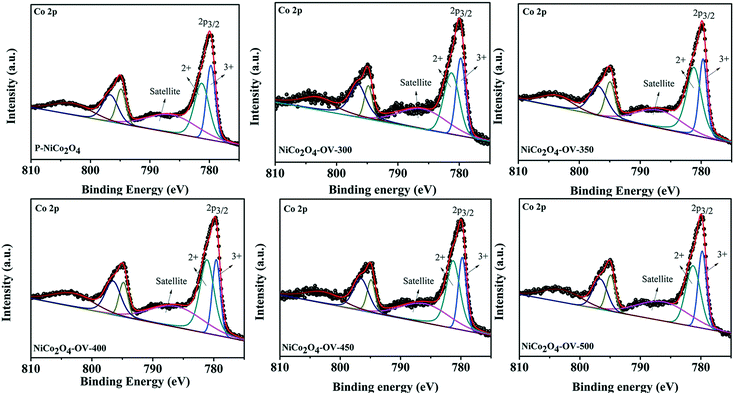
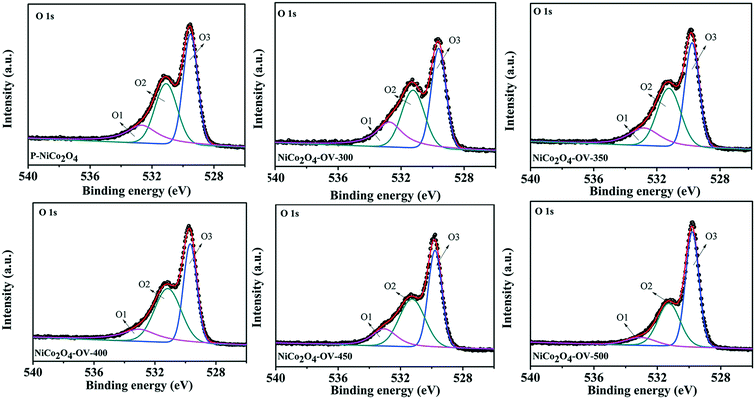
To verify the surface compositions and chemical states of P-NiCo2O4, NiCo2O4-OV-300, NiCo2O4-OV-350, NiCo2O4-OV-400, NiCo2O4-450 and NiCo2O4-OV-500, XPS test was also conducted. The survey spectrum of P-NiCo2O4 and NiCo2O4-OV-400 confirms the existence of Co, Ni, O, and C elements and no presence of any other impurity element (Fig. S2†). As shown in Fig. 3, the peaks at about 779.7, 781.2 and 787.5 eV can be indexed to Co3+, Co2+ and a satellite peak. As shown in Fig. 3, the Co2+/Co3+ ratios are 1.37 for P-NiCo2O4, 1.32 for NiCo2O4-OV-350, 1.78 for NiCo2O4-OV-350, 2.01 for NiCo2O4-OV-400, 1.88 for NiCo2O4-OV-450 and 1.57 for NiCo2O4-OV-500, respectively. Similar to Co 2p, as shown in Fig. S3,† Ni 2p3/2 can be carefully deconvoluted to Ni2+ at about 853.9 eV and Ni3+ at about 855.8 eV, as well as a satellite peak.56,57 The Ni2+/Ni3+ ratios are 0.27 for P-NiCo2O4, 0.259 for NiCo2O4-OV-300, 0.279 for NiCo2O4-OV-350, 0.343 for NiCo2O4-OV-400, 0.33 for NiCo2O4-OV-450 and 0.323 for NiCo2O4-OV-500, respectively. Obviously, the proportion of Co2+ and Ni2+ is increased mostly after annealing at 400 °C.
Fig. 4 shows the XPS spectra of O 1s and there exist three major peaks of O1, O2, and O3 located at ∼532.9, 531.5, and 529.4 eV, respectively, coinciding with the hydroxyl species of water molecules adsorbed on the surface, defect sites and the bonding of oxygen atoms and metals.58 The O2 peak area ratio is 34.9 for P-NiCo2O4 and, similarly, increases with the annealing temperature and reaches a highest value of 38.9 for NiCo2O4-OV-400, then decreases at higher temperature. The largest area of the peak at 531.5 eV (O2) for the Ar-annealed NiCo2O4-OV-400 (Fig. 4) implies that the largest number of oxygen deficiencies existed in the as-prepared NiCo2O4 nanobelts. All the above results demonstrate that a mass of the Co2+ and a spot of Ni2+ ions is transformed by the reduction of Co3+ and Ni3+, respectively, owing to the formation of oxygen vacancies in the NiCo2O4 samples, which is consistent with the above XRD and Raman results. In addition, oxygen deficiencies coming from the annealing process under an inert atmosphere environment lead to the formation of a porous nanobelt structure.
As is well known, removing oxygen atoms in the crystal lattice sites (OC) to the gas state is a practical way to form oxygen vacancies (OVs).
| OC ↔ OV + 1/2O2(g) + e− | (4) |
From the formula,59 the reaction is favorable if the samples are annealed under an oxygen-deficient atmosphere, leading to the formation of oxygen vacancies. It is observed that the production of oxygen vacancies is essentially affected by the external partial pressure of oxygen. When the samples are annealed under low temperature conditions wherein the oxygen partial pressure is very low, the oxygen vacancy concentration increases as the temperature rises. Simultaneously, the proportion of M2+ (M = Ni/Co) increases as well. However, with the annealing temperature rising over 400 °C, the external O2 pressure is higher. Therefore, the reaction will be shifted to the left side, leading to a decrease of oxygen vacancy concentration.60 Meanwhile, the proportion of M2+ decreases. This explanation is supported and confirmed by the results shown in Fig. 5.
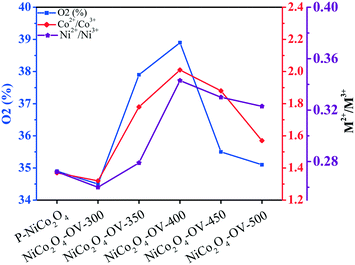 | ||
| Fig. 5 Area ratio of the O2 peak components and peak ratio of M2+/M3+ (M = Ni/Co) of the as-prepared samples. | ||
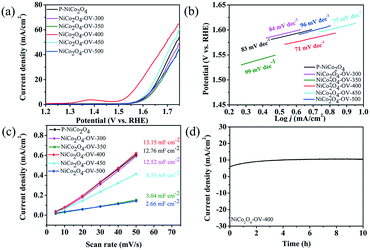
Electrocatalytic properties toward the OER of the samples were evaluated in 1 M KOH (Fig. 6a). Among these samples, NiCo2O4-OV-400 shows an outstanding activity at the lowest overpotential. The overpotential of NiCo2O4-OV-400 is about 325 mV (vs. RHE, reversible hydrogen electrode), which is much lower than that of P-NiCo2O4 (399 mV vs. RHE), NiCo2O4-OV-300 (404 mV vs. RHE), NiCo2O4-OV-350 (410 mV vs. RHE), NiCo2O4-OV-450 (387 mV vs. RHE), and NiCo2O4-OV-500 (400 mV vs. RHE), suggesting that the excellent electrocatalytic performance of NiCo2O4-OV-400 is apparently exhibited for the OER.
Further activity evaluation could be seen from the Tafel plots of Fig. 6b. The Tafel slope of the as-prepared NiCo2O4-OV-400 samples was calculated to be approximately 71 mV dec−1, which was the smallest of these obtained NiCo2O4 nanobelts, indicating that enhanced OER kinetics can be achieved by introducing oxygen vacancies. The CV method was tested to obtain the Cdl values, which can estimate the electrochemical surface area (Fig. 6c and S4†). The Cdl values are determined to be 12.79, 12.52, 3.04, 13.15, 8.55 and 2.66 mF cm−2 for P-NiCo2O4, NiCo2O4-OV-300, NiCo2O4-OV-350, NiCo2O4-OV-400, NiCo2O4-OV-450 and NiCo2O4-OV-500, respectively, suggesting that NiCo2O4-OV-400 can provide more active surface area to the electrolyte, which is consistent with the as-mentioned analysis of FESEM and XPS.
The long-term stability of the NiCo2O4-OV-400 nanobelt electrode was also tested as shown in Fig. 6d, in which no significant difference in the current density was observed after long-term testing, illustrating the high stability of the as-prepared catalyst.10 To determine the stability of NiCo2O4-OV-400, the morphology, structure and composition of the sample were characterized by SEM, TEM and XPS after the long-time stability test process. The sample undergoing the stability test is denoted as NCO-ST whose morphology and structure are shown in Fig. S5,† which shows that the nanobelt structure is well-maintained. Furthermore, the XPS spectra of O 1s of NCO-ST are provided in Fig. S6,† which exhibit similar peaks to NiCo2O4-OV-400 before the stability test. The strong O2 peak corresponding to oxygen deficiencies can still be observed in NCO-ST, confirming the stable oxygen-deficient structure of the nanobelts even after long-term reaction. A new peak appearing at 532 eV likely comes from the KOH electrolyte residue on the nanobelt surface which can be evidenced by the presence of K 2s in the XPS survey spectrum.61,62 The above results indicate the high activity and stability of the NiCo2O4-OV-400 catalyst.
For comparison, the OER performance of commercial RuO2 and the obtained NiCo2O4-OV-400 have been listed and discussed in Fig. S7,† which indicates that NiCo2O4-OV-400 has a very close overpotential and a much higher electrochemical stability compared to commercial RuO2. In addition, such OER catalytic activity of NiCo2O4-OV-400 is also superior to those of previously reported Ni or Co-based electrocatalysts. The detailed comparisons are shown in Table S1 (ESI†).
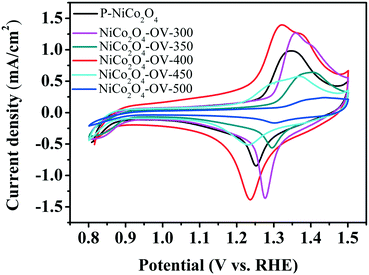
As observed in Fig. 7, there are two redox couples for the as-prepared NiCo2O4 nanobelts, respectively, which indicate that these electrocatalysts possess the same electrocatalysis mechanism. By comparing the CV curves of the different samples at the same scan rate of 10 mV s−1, the NiCo2O4-OV-400 nanobelt electrode has more remarkable peaks than the other as-prepared NiCo2O4 nanobelt electrodes, implying that in comparison to the other electrodes, the NiCo2O4-OV-400 electrode has more rapid redox reactions.63,64 Owing to the abundant surface oxygen vacancies of the material, which can promote the adsorption capacity of OH−, OER activity is promoted.
To get further insight into the OER mechanism, the CV (Fig. S8†) tests of NiCo2O4-OV-400 were carried out at a scan rate of 10 mV s−1 from 0.8 V to 1.5 V (vs. RHE). As shown in the magnified image in the inset, there were two oxidation peaks located at about 1.32 V and 1.38 V, which corresponded to the oxidation of Co2+ (Ni2+) to Co3+ (Ni3+) and further to Co4+ (Ni4+), respectively. Similarly, the corresponding reduction peaks located at 1.24 V and 1.36 V could be attributed to the reduction of Co4+ (Ni4+) to Co3+ (Ni3+) and further to Co2+ (Ni2+).63–66
According to all the above analysis, the OER mechanism of the NiCo2O4 electrode material can be briefly explained by the following four steps:
| M + OH− ↔ M − OH + e− | (5) |
| M − OH + OH− ↔ M − O + H2O + e− | (6) |
| M − O + OH− ↔ M − OOH + e− | (7) |
| M − OOH + OH− ↔ M + O2 + H2O + e− | (8) |
According to these results, the superior OER activity and excellent stability of the oxygen-deficient NiCo2O4 catalysts synthesized by hydrothermal treatment and subsequent annealing process can be explained and proved by the several following advantages. Firstly, the as-prepared spinel-type NiCo2O4 synthesized at different annealing temperatures in an inert atmosphere can maintain the original nanobelt structure with no presence of a new phase and morphology. It provides a stable structure and large active surface area. Secondly, the successful generation of appropriate oxygen vacancies in the catalysts can help to adjust the electronic states and further provide effective electrocatalytically active sites, stimulating the adsorption capacity of OH− and leading to a notably facilitated catalytic activity and electrochemical durability for the OER. And eventually, a series of NiCo2O4 samples with controllable oxygen vacancy concentration were prepared by adjusting the annealing temperature.
4. Conclusions
In summary, oxygen-deficient NiCo2O4 materials have successfully been synthesized by a facile and simple hydrothermal method and subsequent annealing process in an argon atmosphere. The important role of oxygen vacancies was clearly demonstrated during the OER process. The oxygen-deficient NiCo2O4 catalysts annealed at 400 °C showed superior catalytic activities with lower overpotential (only 325 mV to reach 10 mA cm−2), and accelerated OER kinetics with strong long-term stability was observed compared to the other obtained NiCo2O4 nanobelt catalysts. It can be inferred that the introduction of oxygen vacancies in the synthesized NiCo2O4 electrocatalysts plays an essential part in promoting efficient electrocatalytically active sites and enhancing the electrochemical properties. In particular, a series of NiCo2O4 samples with controllable oxygen vacancy concentration have been prepared by changing the annealing temperature; meanwhile, the relationship between oxygen vacancy concentration, M2+/M3+ (M = Ni/Co) ratio and annealing temperature was determined, and a reasonable explanation was shown as well. Thus, this oxygen-deficient NiCo2O4 structure shows high-performance for the OER at a large current density, which provides a promising pathway toward developing advanced electrocatalysts for developing sustainable energy materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project is supported by the Key Project of Changfeng-HFUT Industrial Innovation Guidance Funds (JZ2020YDZJ0122), the National Natural Science Foundation of China (Grant No. 51772072, 51672065, and U1810204), and the Fundamental Research Funds for the Central Universities (PA2019GDQT002, PA2019GDZC0096). We also would like to thank the financial support from the 111 Project “New Materials and Technology for Clean Energy” (B18018).References
- C. Wei, R. R. Rao, J. Peng, B. Huang, I. E. L. Stephens, M. Risch, Z. J. Xu and Y. Shao-Horn, Adv. Mater., 2019, 31, 1806296 CrossRef.
- M. Tsutsumi, M. S. Islam, M. R. Karim, N. N. Rabin, R. Ohtani, M. Nakamura, L. F. Lindoy and S. Hayami, Bull. Chem. Soc. Jpn., 2017, 90, 950–954 CrossRef CAS.
- M. D. Hossain, Z. Liu, M. Zhuang, X. Yan, G.-L. Xu, C. A. Gadre, A. Tyagi, I. H. Abidi, C.-J. Sun, H. Wong, A. Guda, Y. Hao, X. Pan, K. Amine and Z. Luo, Adv. Energy Mater., 2019, 9, 1803689 CrossRef.
- N.-T. Suen, S.-F. Hung, Q. Quan, N. Zhang, Y.-J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- M. Tahir, L. Pan, F. Idrees, X. Zhang, L. Wang, J.-J. Zou and Z. L. Wang, Nano Energy, 2017, 37, 136–157 CrossRef CAS.
- X. Zhang, J. Luo, K. Wan, D. Plessers, B. Sels, J. Song, L. Chen, T. Zhang, P. Tang, J. R. Morante, J. Arbiol and J. Fransaer, J. Mater. Chem. A, 2019, 7, 1616–1628 RSC.
- E. Cossar, A. Barnett, F. Seland and E. Baranova, Catalysts, 2019, 9, 814 CrossRef CAS.
- Y. Guo, T. Park, J. W. Yi, J. Henzie, J. Kim, Z. Wang, B. Jiang, Y. Bando, Y. Sugahara, J. Tang and Y. Yamauchi, Adv. Mater., 2019, 31, 1807134 CrossRef.
- Y. Guo, J. Tang, H. Qian, Z. Wang and Y. Yamauchi, Chem. Mater., 2017, 29, 5566–5573 CrossRef CAS.
- Y. Guo, J. Tang, Z. Wang, Y.-M. Kang, Y. Bando and Y. Yamauchi, Nano Energy, 2018, 47, 494–502 CrossRef CAS.
- C. Li and J.-B. Baek, ACS Omega, 2020, 5, 31–40 CrossRef CAS.
- Z. Tan, H. Li, Q. Feng, L. Jiang, H. Pan, Z. Huang, Q. Zhou, H. Zhou, S. Ma and Y. Kuang, J. Mater. Chem. A, 2019, 7, 1607–1615 RSC.
- L. Hu, T. Xiong, R. Liu, Y. Hu, Y. Mao, M. S. Balogun and Y. Tong, Chem. – Eur. J., 2019, 25, 6575–6583 CrossRef CAS.
- J. Hu, S. Zheng, X. Zhao, X. Yao and Z. Chen, J. Mater. Chem. A, 2018, 6, 7827–7834 RSC.
- W.-H. Lai, L. Zhang, W. Hua, S. Indris, Z. Yan, Z. Hu, B. Zhang, Y. Liu, L. Wang, M. Liu, Y. Wang, J. Wang, Z. Hu, H.-K. Liu and S. Dou, Angew. Chem., Int. Ed., 2019, 58, 11868–11873 CrossRef CAS.
- T. Reier, M. Oezaslan and P. Strasser, ACS Catal., 2012, 2, 1765–1772 CrossRef CAS.
- X. Wang, L. Yu, B. Y. Guan, S. Song and X. W. Lou, Adv. Mater., 2018, 30, 1801211 CrossRef.
- H.-Y. Wang, S.-F. Hung, Y.-Y. Hsu, L. Zhang, J. Miao, T.-S. Chan, Q. Xiong and B. Liu, J. Phys. Chem. Lett., 2016, 7, 4847–4853 CrossRef CAS.
- Y. M. Hajar, L. Treps, C. Michel, E. A. Baranova and S. N. Steinmann, Catal. Sci. Technol., 2019, 9, 5915–5926 RSC.
- L. Han, S. Dong and E. Wang, Adv. Mater., 2016, 28, 9266–9291 CrossRef CAS.
- J. Wang, W. Cui, Q. Liu, Z. Xing, A. M. Asiri and X. Sun, Adv. Mater., 2016, 28, 215–230 CrossRef CAS.
- H. B. Tao, L. Fang, J. Chen, H. B. Yang, J. Gao, J. Miao, S. Chen and B. Liu, J. Am. Chem. Soc., 2016, 138, 9978–9985 CrossRef CAS.
- J. Hu, W. Chen, X. Zhao, X. Cao, J. Zhu and Z. Chen, Energy Technol., 2019, 7, 1900013 CrossRef.
- L. Li, H. Yang, J. Miao, L. Zhang, H.-Y. Wang, Z. Zeng, W. Huang, X. Dong and B. Liu, ACS Energy Lett., 2017, 2, 294–300 CrossRef CAS.
- Y.-T. Liao, V. C. Nguyen, N. Ishiguro, A. P. Young, C.-K. Tsung and K. C. W. Wu, Appl. Catal., B, 2020, 270, 118805 CrossRef CAS.
- H. Konnerth, B. M. Matsagar, S. S. Chen, M. H. G. Prechtl, F.-K. Shieh and K. C. W. Wu, Coord. Chem. Rev., 2020, 416, 213319 CrossRef CAS.
- Y.-T. Liao, B. M. Matsagar and K. C. W. Wu, ACS Sustainable Chem. Eng., 2018, 6, 13628–13643 CrossRef CAS.
- E. Doustkhah, J. Lin, S. Rostamnia, C. Len, R. Luque, X. Luo, Y. Bando, K. C. W. Wu, J. Kim, Y. Yamauchi and Y. Ide, Chem. – Eur. J., 2019, 25, 1614–1635 CrossRef CAS.
- C.-C. Chueh, C.-I. Chen, Y.-A. Su, H. Konnerth, Y.-J. Gu, C.-W. Kung and K. C. W. Wu, J. Mater. Chem. A, 2019, 7, 17079–17095 RSC.
- J. Wang, Y. Xu, B. Ding, Z. Chang, X. Zhang, Y. Yamauchi and K. C. W. Wu, Angew. Chem., Int. Ed., 2018, 57, 2894–2898 CrossRef CAS.
- C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen and X. W. Lou, Adv. Funct. Mater., 2012, 22, 4592–4597 CrossRef CAS.
- G. M. Tomboc, M. W. Abebe, A. F. Baye and H. Kim, J. Energy Chem., 2019, 29, 136–146 CrossRef.
- J. Wang, T. Qiu, X. Chen, Y. Lu and W. Yang, J. Power Sources, 2014, 268, 341–348 CrossRef CAS.
- Y. Huang, L. Sun, Z. Yu, R. Jiang, J. Huang, Y. Hou, F. Yang, B. Zhang, R. Zhang and Y. Zhang, Catal. Sci. Technol., 2020, 10, 2627–2643 RSC.
- W. Jin, J. Chen, H. Wu, N. Zang, Q. Li, W. Cai and Z. Wu, Catal. Sci. Technol., 2020, 10, 5559–5565 RSC.
- D. U. Lee, B. J. Kim and Z. Chen, J. Mater. Chem. A, 2013, 1, 4754–4762 RSC.
- D. Lim, H. Kong, C. Lim, N. Kim, S. E. Shim and S.-H. Baeck, Int. J. Hydrogen Energy, 2019, 44, 23775–23783 CrossRef CAS.
- D. Yan, Y. Li, J. Huo, R. Chen, L. Dai and S. Wang, Adv. Mater., 2017, 29, 1606459 CrossRef.
- Z. Xiao, Y. Wang, Y.-C. Huang, Z. Wei, C.-L. Dong, J. Ma, S. Shen, Y. Li and S. Wang, Energy Environ. Sci., 2017, 10, 2563–2569 RSC.
- C. Xie, D. Yan, W. Chen, Y. Zou, R. Chen, S. Zang, Y. Wang, X. Yao and S. Wang, Mater. Today, 2019, 31, 47–68 CrossRef CAS.
- Z. Liu, Z. Xiao, G. Luo, R. Chen, C.-L. Dong, X. Chen, J. Cen, H. Yang, Y. Wang, D. Su, Y. Li and S. Wang, Small, 2019, 15, 1904903 CrossRef CAS.
- J. H. Lin, Y. T. Yan, T. X. Xu, C. Q. Qu, J. Li, J. Cao, J. C. Feng and J. L. Qi, J. Colloid Interface Sci., 2020, 560, 34–39 CrossRef CAS.
- C. Jin, F. Lu, X. Cao, Z. Yang and R. Yang, J. Mater. Chem. A, 2013, 1, 12170–12177 RSC.
- D. Li, Y. Gong, M. Wang and C. Pan, Nano-Micro Lett., 2016, 9, 16 CrossRef.
- C. Guan, X. Liu, W. Ren, X. Li, C. Cheng and J. Wang, Adv. Energy Mater., 2017, 7, 1602391 CrossRef.
- X. Zhang, X. Li, R. Li, Y. Lu, S. Song and Y. Wang, Small, 2019, 15, 1903297 CrossRef CAS.
- Y. Li, G. Cheng, Z. Zhou, X. Liao, S. Han, F. Ye, M. Sun and L. Yu, ChemElectroChem, 2019, 6, 4429–4436 CrossRef CAS.
- W. Xu, F. Lyu, Y. Bai, A. Gao, J. Feng, Z. Cai and Y. Yin, Nano Energy, 2018, 43, 110–116 CrossRef CAS.
- M. Kuang, P. Han, Q. Wang, J. Li and G. Zheng, Adv. Funct. Mater., 2016, 26, 8555–8561 CrossRef CAS.
- Y. Wang, T. Zhou, K. Jiang, P. Da, Z. Peng, J. Tang, B. Kong, W.-B. Cai, Z. Yang and G. Zheng, Adv. Energy Mater., 2014, 4, 1400696 CrossRef.
- J. Kim, X. Yin, K.-C. Tsao, S. Fang and H. Yang, J. Am. Chem. Soc., 2014, 136, 14646–14649 CrossRef CAS.
- Y. Guo, Y. Tong, P. Chen, K. Xu, J. Zhao, Y. Lin, W. Chu, Z. Peng, C. Wu and Y. Xie, Adv. Mater., 2015, 27, 5989–5994 CrossRef CAS.
- W. Liu, J. Bao, L. Xu, M. Guan, Z. Wang, J. Qiu, Y. Huang, J. Xia, Y. Lei and H. Li, Appl. Surf. Sci., 2019, 478, 552–559 CrossRef CAS.
- B. Wei, J. Wu, G. Mei, Z. Qi, W. Hu and Z. Wang, Int. J. Hydrogen Energy, 2019, 44, 6612–6617 CrossRef CAS.
- K.-L. Yan, J.-Q. Chi, Z.-Z. Liu, B. Dong, S.-S. Lu, X. Shang, W.-K. Gao, Y.-M. Chai and C.-G. Liu, Inorg. Chem. Front., 2017, 4, 1783–1790 RSC.
- L. Du, X. Song, X. Liang, Y. Liu and M. Zhang, Appl. Surf. Sci., 2020, 526, 146706 CrossRef CAS.
- Y. Zhang, H. Wang, B. Liu, X. Zhao and Y. Wei, Appl. Surf. Sci., 2020, 504, 144598 CrossRef CAS.
- L. Sha, K. Ye, G. Wang, J. Shao, K. Zhu, K. Cheng, J. Yan, G. Wang and D. Cao, J. Power Sources, 2019, 412, 265–271 CrossRef CAS.
- Y. Liu, H. Zhang, H. Wan, W. Zhang, N. Jiang, G. Huang, Z. Wang, S. Luo and H. Sun, J. Alloys Compd., 2019, 787, 720–727 CrossRef CAS.
- H. Y. Xu, Y. H. Huang, S. Liu, K. W. Xu, F. Ma and P. K. Chu, RSC Adv., 2016, 6, 79383–79388 RSC.
- F. Buchner, M. Eckardt, T. Böhler, J. Kim, J. Gerlach, J. Schnaidt and R. J. Behm, ChemSusChem, 2020, 13, 3199–3211 CrossRef CAS.
- C. Guo, X. Liu, L. Gao, X. Ma, M. Zhao, J. Zhou, X. Kuang, W. Deng, X. Sun and Q. Wei, J. Mater. Chem. A, 2019, 7, 21704–21710 RSC.
- L. Qian, L. Gu, L. Yang, H. Yuan and D. Xiao, Nanoscale, 2013, 5, 7388–7396 RSC.
- J. He, Y. Sun, M. Wang, Z. Geng, X. Wu, L. Wang, H. Chen, K. Huang and S. Feng, J. Alloys Compd., 2018, 752, 389–394 CrossRef CAS.
- J. Béjar, L. Álvarez-Contreras, J. Ledesma-García, N. Arjona and L. G. Arriaga, J. Electroanal. Chem., 2019, 847, 113190 CrossRef.
- L. Yang, B. Zhang, B. Fang and L. Feng, Chem. Commun., 2018, 54, 13151–13154 RSC.
- S. Sun, X. Jin, B. Cong, X. Zhou, W. Hong and G. Chen, J. Catal., 2019, 379, 1–9 CrossRef CAS.
- H. Cheng, Y.-Z. Su, P.-Y. Kuang, G.-F. Chen and Z.-Q. Liu, J. Mater. Chem. A, 2015, 3, 19314–19321 RSC.
- S. N., S. L., P. S. Adarakatti, J. P. Hughes, S. J. Rowley-Neale, C. E. Banks and A. S., RSC Adv., 2019, 9, 24995–25002 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cy01669a |
| This journal is © The Royal Society of Chemistry 2021 |