Efficient photocatalytic chemoselective and stereoselective C–C bond formation over AuPd@N-rich carbon nitride†
Heyan
Jiang
 *,
Jie
Xu
,
Sishi
Zhang
,
Hongmei
Cheng
,
Cuicui
Zang
and
Fengxia
Bian
*
*,
Jie
Xu
,
Sishi
Zhang
,
Hongmei
Cheng
,
Cuicui
Zang
and
Fengxia
Bian
*
Chongqing Key Laboratory of Catalysis and New Environmental Materials, College of Environmental and Resources, National Base of International Science and Technology Cooperation for Intelligent Manufacturing Service, Chongqing Technology and Business University, Chongqing 400067, China. E-mail: orgjiang@163.com; bianqian1201@163.com
First published on 5th November 2020
Abstract
Heterogeneous chemoselective or stereoselective C–C coupling reactions remain extremely challenging in traditional organic synthesis. Here, we constructed a AuPd@N-rich carbon nitride (NRCN) photocatalyst through simple ammonia solution heat treatment of carbon nitride and then AuPd NP loading. AuPd@NRCN exhibited extraordinary light color promoted catalytic performance in C–C bond formation under visible light in air. Surprisingly, both high chemoselectivity to unsymmetrical Ullmann biaryl products and satisfactory stereoselectivity to Z-type Heck reaction products could be achieved by changing the light source color. Various substrates exhibited great potential for the economical synthesis of unsymmetrical biaryl products and Z-type olefins. Efficient visible light promoted C–I bond activation accompanied with improved photocatalytic coupling reaction efficiency over AuPd@NRCN was verified firstly by in situ DRIFTS. Considering that the Ullmann cross-coupling reaction is a multi-photon reaction, the improved photocatalytic performance in the Ullmann cross-coupling reaction using a combination of light sources with different colors might be due to the activation of different substrates and/or steps requiring different energies, and the combination of the two energy sources was beneficial for improving the activation efficiency of different substrates and/or steps. The activation of iodobenzene and styrene in the Heck reaction with light was also beneficial to the formation of the stilbene product. The light color promoted chemoselectivity and stereoselectivity are expected to have profound impact on organic synthetic methodology improvement.
1. Introduction
C–C coupling reactions are key steps in the total synthesis of a large number of natural products, biologically active compounds, and many organic building blocks. The formation of C–C bonds is mainly achieved using various named reactions such as Ullmann, Suzuki–Miyaura, Heck, Sonogashira, Stille, and Hiyama reactions, which generally employ transition metals, including Pd, Rh, Cu, Fe, Ni and Co, etc., as catalysts.1–4 Many conventional thermocatalytical systems could obtain C–C bond coupling products with high yields, but these reactions could almost specifically lead to thermodynamically more favorable target products and were generally carried out under harsh conditions such as rather high temperature.5–7 Moreover, organohalides or pseudohalides (triflates or nonaflates) along with organo-metallics or metalloids (organo-boron, -magnesium, -tin, or -zinc species) are often indispensable for C–C cross-coupling reactions to form more valuable unsymmetrical compounds for the above venerable named reactions.8–10 Therefore, it is necessary and rather challenging to develop effective strategies to generate unsymmetrical aryl–aryl bonds and thermodynamically unfavorable coupling products.Photocatalytic technology could convert the easily available light energy into the energy required for chemical reactions, and has been widely applied in the fields of energy, environmental catalysis and chemical synthesis. Photocatalysis was believed to have attractive potential to achieve many organic transformations that traditional thermal catalysis could not, despite limited successful reports in photocatalytic organic transformations in recent years.11–14 In 2013, two research groups almost simultaneously found that AuPd bimetallic nanostructures for the photocatalytic Suzuki cross-coupling reaction could obtain excellent activity.15,16 Inspired researchers have discovered some other systems for highly efficient photocatalytic C–C bond synthesis.17–20 In addition to the Suzuki reaction, there are limited reports on other C–C coupling reactions. Moreover, taking into account the special substrate adsorption configuration requirement on the catalytically active center, the selective chemical bond activation brought out by visible light under relatively mild conditions as well as the difficulties in exclusion of unwanted side reactions, light-induced heterogeneous chemoselective or stereoselective C–C coupling reactions are extremely challenging.
Polymer carbon nitride (CN), with a suitable energy band gap of about 2.75 eV, has received widespread attention for its excellent chemical stability, visible light capture capability, and easy availability.21–28 In addition, due to their metal-free nature and particularly stable highly dense frame, environment-friendly CN based photocatalysts have been proven to have superior catalytic activity in different applications with visible light, such as photocatalytic hydrogen evolution, organic pollutant photodegradation, photocatalytic CO2 reduction, photoinduced organic synthesis, etc.21–28 Recently, Ghosh et al.29 successfully employed mesoporous graphitic carbon nitride (mpg-CN) as a competent photocatalyst for a series of new insertion reactions and tricomponent coupling to functionalize arenes and heteroarenes with more than 20 diverse functional groups. It is worth mentioning that mpg-CN remained stable in the presence of many destructive free radical intermediates, and the use of a semiconductor as a photocatalyst for organic synthesis presented the opportunity to access distinctive modes of reactivity. The photocatalytic efficiency of CN is not only related to its crystal structure and porosity, but also closely related to the N content of the CN framework. It was found that N-rich carbon nitride could effectively improve the electron–hole separation efficiency, electrical conductivity and catalytic performance. The photocatalytic performance improvement in hydrogen production and CO2 photoreduction with N-rich CN catalysts has been successively reported in recent years. Similarly, Antil et al.30 prepared an N-rich holey carbon nitride through a direct thermal polymerization method, and the N-rich CN was found to be very good in the photocatalytic H2 evolution reaction. Mo et al.31 prepared porous nitrogen-rich C3N4 nanotubes by supramolecular self-assembly, which obviously improved CO2 photoreduction performance.
In this paper, we prepared novel N-rich carbon nitride (NRCN) by simple heat treatment of carbon nitride with ammonia solution, which had a smaller size and more amino groups than CN. A AuPd@NRCN photocatalyst was then prepared by supporting AuPd alloy nanoparticles on NRCN. With the combination of the NRCN and AuPd nanoparticles, the photocatalytic activity of the Ullmann reaction and Heck reaction was significantly promoted with the salient features of ligand-free reaction conditions (ambient temperature and air atmosphere) as well as good recyclability. Strikingly, high chemoselective unsymmetrical biaryl products in Ullmann cross-coupling products could be achieved with different visible light irradiation combinations in air. For the first time, good to excellent stereoselectivity to Z-type Heck reaction products was realized with different visible wavelength irradiation in air. A good substrate scope which tolerated a variety of functional groups provided a great possibility for large scale and more atom-economical synthetic methods to obtain various unsymmetrical biaryl products and Z-type Heck reaction products. Preliminary mechanistic insights into AuPd@NRCN photocatalysis was obtained with in situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS), and a possible reaction mechanism was proposed.
2. Results and discussion
N-Rich carbon nitride, generally prepared with the assistance of ammonium salt30–34 or hydrazine hydrate,35 is known to possess the ability to improve the electron–hole separation efficiency, electrical conductivity and catalytic performance. Different from these previous reports, small size N-rich carbon nitride was prepared with ammonia solution treatment of bulk carbon nitride in this study. During the ammonia heat treatment, bulk carbon nitride turned into the state of melem hydrate, and the free ammonia in the ammonia solvent would further connect to the surface of the carbon nitride to form a plurality of terminal amino groups. Therefore, the N-rich carbon nitride could be successfully synthesized in the above heat treatment process. AuPd NPs were supported on NRCN powder to form photocatalysts (for the detailed method, see the Experimental section in the ESI†). Pure Au and Pd NPs on the NRCN support (Au@NRCN and Pd@NRCN) were also prepared under synthetic conditions similar to AuPd@NRCN. Scanning electron microscopy (SEM) revealed that the morphology of CN to NRCN obviously changed during the ammonia solution treatment. As shown in Fig. S1A,† the pure CN sample was mainly composed of large blocks. Smaller irregular sheets in the NRCN sample (Fig. S1B†) indicated that the ammonia solution heat treatment could drastically reduce the size of the particles. Additionally, energy-dispersive X-ray spectroscopy (EDS) revealed the coexistence of C, N, Au, and Pd elements in AuPd@NRCN (Fig. S1C†). To investigate the elemental distribution of the as-prepared photocatalyst, EDS elemental mappings of the AuPd@NRCN catalyst were performed (Fig. S1D–G†). EDS elemental mapping of the SEM image demonstrated that C, N, Au and Pd were homogeneously distributed throughout the sample. The small-sized sheets were also observed in the transmission electron microscopy (TEM) image of the NRCN supported AuPd NPs (Fig. S2A†). At the same time, it was found that the AuPd NPs were uniformly distributed on the NRCN surface, and the average diameter of the AuPd NPs was about 3 nm (Fig. S2B†). The high resolution TEM (HRTEM) image showed the lattice spacing of the AuPd alloy NPs (Fig. S2C and D†). The lattice fringe spacing of 0.236 nm corresponded to the AuPd alloy (111) plane distance (Fig. S2D†).17,35In order to study the effect of ammonia solution heat treatment on the CN structure, the obtained samples were subjected to X-ray diffraction (XRD) (Fig. 1A) and Fourier transform infrared (FT-IR) (Fig. 1B). Characteristic peaks at 13.0° and 27.5° were found in all samples, which were respectively (100) and (002) of carbon nitride (Fig. 1A). Consistent with previous reports36,37 the (110), (220), (202) and (550) characteristic peaks corresponding to melem hydrate appeared significantly in all NRCN samples. The characteristic peaks of Pd (111) and Au (111) were found in both Pd@NRCN and Au@NRCN (Fig. 1A c and d), and a weak characteristic peak between Au(111) and Pd(111) appeared in AuPd@NRCN, indicating the formation of the AuPd alloy (Fig. 1A e). FT-IR spectroscopy (Fig. 1B) was employed to check the maintenance of the conjugated CN heterocycles as well as the change of amino groups in NRCN samples. Compared with CN, all NRCN samples showed similar absorptions in the wavenumber range of 1200–1600 cm−1 and 810 cm−1, which were the stretching vibrations of conjugated CN rings and the characteristic breathing mode of s-triazine, respectively. The peak at 3000–3500 cm−1 was attributed to the presence of N–H in CN rings. After the ammonia solution heat treatment, the relative intensity of N–H absorption in NRCN was significantly enhanced, which indicated that the amino group on the CN rings of NRCN was increased. At the same time, the relative strength of CN rings and s-triazine of NRCN was also obviously enhanced due to the formation of melem hydrate after ammonia solution treatment. After the metal loading, the relative intensity of N–H, CN heterocycle and s-triazine showed a significant decrease due to the coordination between the metal and the N atom.38,39
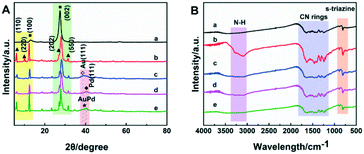 | ||
| Fig. 1 (A) XRD patterns and (B) FT-IR spectra of the photocatalysts: (a) CN, (b) NRCN, (c) Au@NRCN, (d) Pd@NRCN, and (e) AuPd@NRCN. | ||
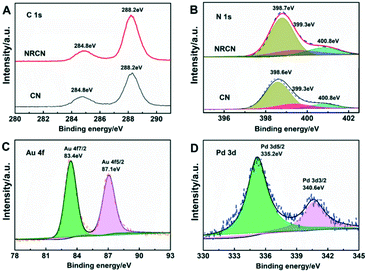
The content of C and N and the chemical valence of Au and Pd in the AuPd@NRCN sample were analyzed by X-ray photoelectron spectroscopy (XPS). Fig. 2A depicts the XPS peaks of C 1s, and CN and NRCN had a similar C 1s spectrum, which could be assigned to the graphite C–C bonds at 284.8 eV and the C species in N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds at 288.2 eV, accordingly. In comparison, there was no significant change in the binding energy of C 1s core electrons, indicating that the chemical state of NRCN was the same as that of CN. The N 1s XPS binding energy could be deconvoluted into three peaks located at 398.7, 399.3 and 400.8 eV, which represented N atoms in the C
N bonds at 288.2 eV, accordingly. In comparison, there was no significant change in the binding energy of C 1s core electrons, indicating that the chemical state of NRCN was the same as that of CN. The N 1s XPS binding energy could be deconvoluted into three peaks located at 398.7, 399.3 and 400.8 eV, which represented N atoms in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C, N–(C)3 and C–N–H functional groups in the NRCN structure. Compared with CN, the position of these binding energies were consistent, and the content of the element N was increased from 45.1% to 50.7%, while the proportion of the element C was almost maintained. Among them, the relative content of the peaks representing C–N–H significantly increased (Fig. 2B). It is reasonably suggested that the ammonia solution heat treatment increased the number of amino groups in NRCN samples. As shown in Fig. 2C, binding energies of 83.4 and 87.1 eV belong to the Au 4f7/2 and Au 4f5/2 peaks, respectively, slightly lower than that of Au0 in the literature.40 This negative shift was caused by electron build-up on Au as Au is more electronegative than Pd. In addition, the peak of Pd 3d5/2 and Pd 3d3/2 in the Pd 3d spectrum of AuPd@NRCN was at 335.2 eV and 340.6 eV, respectively, corresponding to the AuPd alloy.41,42
N–C, N–(C)3 and C–N–H functional groups in the NRCN structure. Compared with CN, the position of these binding energies were consistent, and the content of the element N was increased from 45.1% to 50.7%, while the proportion of the element C was almost maintained. Among them, the relative content of the peaks representing C–N–H significantly increased (Fig. 2B). It is reasonably suggested that the ammonia solution heat treatment increased the number of amino groups in NRCN samples. As shown in Fig. 2C, binding energies of 83.4 and 87.1 eV belong to the Au 4f7/2 and Au 4f5/2 peaks, respectively, slightly lower than that of Au0 in the literature.40 This negative shift was caused by electron build-up on Au as Au is more electronegative than Pd. In addition, the peak of Pd 3d5/2 and Pd 3d3/2 in the Pd 3d spectrum of AuPd@NRCN was at 335.2 eV and 340.6 eV, respectively, corresponding to the AuPd alloy.41,42
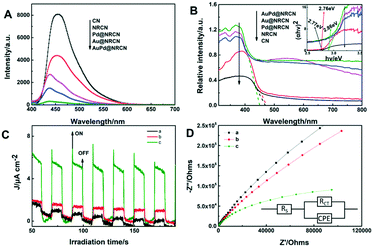
The separation and recombination rates of photogenerated electrons and holes in the samples were investigated by photoluminescence (PL) emission spectroscopy. The PL emission spectrum of these samples (Fig. 3A) was excited at 365 nm. The PL intensity of NRCN was significantly lower than that of pure CN, indicating that the charge recombination was greatly inhibited after the NRCN ammonia solution heat treatment, so that there were more electron–hole pairs in the NRCN material. After loading the metal NPs, the PL intensity of the catalytic samples continued to decrease, and AuPd@NRCN had the lowest PL intensity, which was consistent with our photocatalytic experimental results. In addition, both CN and NRCN exhibited strong fluorescence emission peaks around 455 nm, and the peaks of these samples after loading the NPs showed a blue shift (top to bottom signals at 438 nm, 436 nm and 436 nm for Pd@NRCN, Au@NRCN and AuPd@NRCN, respectively), which was consistent with the band gap energy change caused by the quantum size effect.43 The lifetime of photoinduced carriers in the time-resolved fluorescence spectra at 450 nm was further investigated under 360 nm laser irradiation at room temperature to disclose the charge-transfer dynamics of CN and AuPd@NRCN (Fig. S3†). The curves fit well to a biexponential decay function and all the fitting parameters are summarized in Table S1.† The short lifetime (τ1) was 1.3473 and 1.6236 ns and the long lifetime (τ2) was 5.4881 and 7.8085 ns for CN and AuPd@NRCN, respectively. Compared with CN, the lifetime of the charge carriers in AuPd@NRCN was clearly prolonged, indicating that NRCN formation along with AuPd loading could improve the charge transfer and separation in AuPd@NRCN. The prolonged lifetime of the charge carriers increased their probability to participate in photocatalytic reactions before recombination.
The UV-vis diffuse reflectance absorption spectra (UV-vis DRS) of all samples and the band gap energies were derived by the Kubelka–Munk method, as displayed in Fig. 3B. Compared to the pure CN sample, the absorption edge of the NRCN sample had a red shift phenomenon, while the absorption in the visible light region was slightly enhanced. After the introduction of AuPd NPs, the absorption edge of the sample showed a blue shift, while the AuPd@NRCN sample was significantly enhanced in UV-vis DRS absorption compared to CN and NRCN, which was consistent with the quantum size effect in PL results (Fig. 3A). The Au@NRCN sample presented slight light absorption at 550 nm, probably due to the surface plasmon resonance (SPR) effect of the Au NPs, while the other samples did not show a clear SPR signal in the absorption spectrum. The Tauc plots indicated that the band gap energy of pure CN was 2.77 eV and the band gap energy of NRCN was 2.76 eV. The higher band gap energy of 2.86 eV for AuPd@NRCN sample should be attributed to the quantum size effect.
To better confirm and understand the charge separation of all the samples, photoelectrochemical measurements were recorded. Thus, photocurrent response measurements and electrochemical impedance spectroscopy (EIS) were used to investigate the performance of the as-prepared samples. Fig. 3C indicates the transient photocurrent response of the catalysts supported on the indium tin oxide (ITO) electrode under visible light illumination. The photocurrent was mainly formed by photoelectron–hole pairs separating and diffusing from the internal structure of the photocatalyst to the surface and the free charge acceptor in the electrolyte.44 The photocurrent density of NRCN heat treated with ammonia solution was stronger than that of pure CN, indicating that the enrichment of amino groups could improve the charge separation efficiency. Among all the samples, AuPd@NRCN had the highest photocurrent density, indicating that photoelectron–hole pairs characterized less recombination and longer lifetime. In addition, we performed EIS under dark conditions to study the ability to shuttle and transport carriers to the target reaction site, as shown in Fig. 3D. The relevant parameters of the equivalent circuit are shown in Table S2.† Obviously, AuPd@NRCN had the smallest arc radius in comparison with other samples, which indicated that the electron transfer resistance of AuPd@NRCN was much lower than that of NRCN and CN, so it was more conducive for charge transfer.45 In combination with photoluminescence spectroscopy, time-resolved fluorescence spectroscopy, photocurrent response and electrochemical impedance spectroscopy analyses, NRCN formation and AuPd loading strategies could promote the transfer and separation of charge carriers, thus enhance the photocatalytic performance of AuPd@NRCN.
Biaryl moieties and olefins, which could be produced by the Ullmann reaction and Heck reaction, are widely used in medicine, materials and natural products. We used iodobenzene as the aryl halide substrate to study the photocatalytic condition control in Ullmann homo-coupling and Heck reactions (Fig. 4A), which were carried out under the conditions described in the Experimental section in the ESI.† Without the photocatalyst, no products were detected in the Ullmann and Heck reactions. When there was no base, the photocatalytic activity of Ullmann homo-coupling and Heck reactions was sharply reduced, and the conversion rates for Ullmann and Heck reactions were just 0.6% and 1%, respectively. In order to eliminate the influence of the photothermal heat, we carried out the Ullmann homo-coupling and Heck reactions at 50 °C and 80 °C without light irradiation. There were 22% conversion of the Ullmann homo-coupling product and just 7% low conversion in the Heck reaction. Without changing the experimental conditions, the conversion of the photocatalytic Ullmann homo-coupling and Heck reactions was up to 99% and 97%, respectively. The above results indicated the important role of the catalyst, base and light irradiation in photocatalytic reactions. In particular, an extremely large conversion difference was observed in the Heck reaction between light irradiation and dark conditions with AuPd@NRCN as the photocatalyst. Keeping the other reaction conditions consistent, when using Au@NRCN, Pd@NRCN or AuPd@CN as a photocatalyst, the conversion of the photocatalytic Ullmann homo-coupling reaction was as low as 4%, 2% and 16%, respectively; and the conversion was just 6%, 52% and 3% for the photocatalytic Heck reaction, respectively. These results indicated that the combination of NRCN and AuPd NPs could greatly enhance the photocatalytic activity, which was consistent with the previous optical characterization analysis of the samples (Fig. 3). Additionally, just 5% Ullmann reaction conversion and 1% Heck reaction conversion were detected for AuPd@PVP (Table S3,† entry 10), which demonstrated that carbon nitride played an important role in the photocatalytic C–C bond formation. In order to study the stability of AuPd@NRCN, Ullmann and Heck reaction cycle experiments were performed under the same reaction conditions, and the catalytic activity and selectivity were maintained after four cycle experiments (Fig. 4A and Table S9†). No obvious change was detected in the XRD patterns and FT-IR spectra of the recovered catalyst (Fig. S4 and S5†).
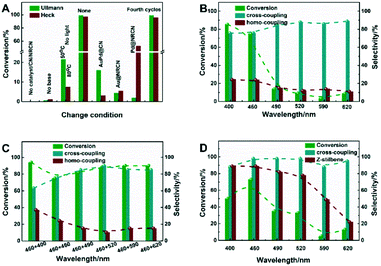 | ||
| Fig. 4 (A) Condition control of Ullmann and Heck reactions (Ullmann reaction: iodobenzene as the reactant, Heck reaction: iodobenzene and styrene as reactants, detailed data see Table S3†); (B) effect of monochromatic light on the Ullmann cross-coupling reaction (iodobenzene and p-iodotoluene as reactants, detailed data see Table S6†); (C) effect of two-color light on the Ullmann cross-coupling reaction (iodobenzene and p-iodotoluene as reactants, detailed data see Table S7†); (D) effect of monochromatic light on the Heck cross-coupling reaction (iodobenzene and styrene as reactants, detailed data see Table S8†). | ||
As we all know, solvents and bases have strong influence on Pd-catalyzed coupling reactions, which would affect the rate and selectivity of the reaction.46,47 Here, we investigated the effect of different bases and solvents on the photocatalytic Ullmann and Heck reactions with the AuPd@NRCN photocatalyst (Table S4 and S5 in the ESI†). It was found that the best catalytic performance was obtained when NaOH was used as the base and methanol was used as the solvent in the Ullmann reaction. 99% conversion of iodobenzene and 99% chemoselectivity to biphenyl could be achieved (Table S4,† entry 3). In aprotic solvents, however, only very low conversion was observed (Table S4,† entries 1 and 4). In the Heck reaction, the results revealed that the catalytic performance of DMF as the solvent was rather good with base additive Et3N. The 98% conversion of iodobenzene and as high as 98% chemoselectivity to stilbene could be achieved with visible light (Table S5,† entry 1). It was worth noting that there was a large amount of aryl halide homo-coupling products when protic solvent methanol was used in the Heck reaction (Table S5,† entries 3, 4).
As an important structural motif in natural products and catalytic synthesis, many symmetric biaryl compounds could be successfully obtained by the Ullmann reaction. However, the unsymmetrical biaryl synthesis through classical methods was complicated, which often requires the participation of organometallic reagents (Zn, Mg, etc.).48,49 It is necessary to develop mild and simple methods for unsymmetrical biaryls synthesis. Herein, a AuPd@NRCN photocatalyst was designed for the photocatalytic Ullmann cross-coupling reaction with different color LEDs in air. Firstly, we examined the photocatalytic Ullmann cross-coupling under different monochromatic light sources (Fig. 4B). p-Iodotoluene and iodobenzene were chosen as substrates, and high p-iodotoluene conversion and cross-coupling chemoselectivity were achieved with UV light (λ = 400 nm) irradiation. With the increase of the irradiation light wavelength to 460 nm (blue LED), the cross-coupling chemoselectivity increased with a slight conversion decrease. However, a further increase of the irradiation light wavelength led to a sharp decrease in photocatalytic activity. It could be explained that the light irradiation energy decreased with the wavelength increase, and the light energy decrease would result in difficulty in exciting the photo-generated electron and hole pairs in the catalyst. With the efficient photocatalytic performance of visible blue LED light, another color of light was further introduced to investigate the effect of different light color combinations on the Ullmann cross-coupling reaction (Fig. 4C). When UV light and blue light were combined, p-iodotoluene could reach 94% conversion with a p-methylbiphenyl chemoselectivity as low as 63%. With the second light wavelength increasing from 460 nm to 520 nm, the cross-coupling selectivity gradually increased. When the light source combination was 460 nm and 520 nm, the chemoselectivity of the cross-coupling product p-methylbiphenyl could reach as high as 90% with 88% p-iodotoluene conversion in air. As the wavelength further increases, the cross-coupling selectivity slightly decreased. Therefore, we chose the 460 nm and 520 nm combination as the reaction light source for more Ullmann reaction substrate extension. Moreover, the Ullman cross-coupling between iodobenzene and p-iodotoluene under optimized conditions and a N2 atmosphere achieved a lower photocatalytic efficiency (79% conversion and 90% cross-coupling chemoselectivity in 24 h) than the efficiency in air as shown in Fig. 4C.
The effect of light intensity with blue and green LEDs on the photocatalytic performance has also been studied. Firstly, the blue LED was fixed at 0.15 W cm−2 (Fig. S6A†), Ullmann cross-coupling reaction activity and chemoselectivity with iodobenzene and p-iodotoluene showed a volcano shape. When the light intensity of the green LED increased to 0.15 W cm−2, Ullmann cross-coupling reaction activity and chemoselectivity reached the maximum. Then, the light intensity of the green LED was fixed at 0.15 W cm−2 (Fig. S6B†), the introduction of the blue LED obviously improved the Ullmann cross-coupling reaction activity. Interestingly, Ullmann cross-coupling reaction chemoselectivity could reach a rather high level with just the green LED. With the improvement of Ullmann cross-coupling reaction activity after the introduction of the blue LED, Ullmann cross-coupling reaction chemoselectivity showed a tendency to slightly decline initially and then recover. When both the blue LED and green LED were at 0.15 W cm−2, the Ullmann cross-coupling reaction activity and chemoselectivity both reached the optimal value.
The action spectrum has been provided to give insight into the Ullmann cross-coupling and Heck reaction activity over AuPd@NRCN as shown in Fig. S7.† From Fig. S7A,† it could be seen that the Ullmann cross-coupling reaction, undergoing the C–I bond photoactivation step, was significantly affected by the wavelength of the irradiation light. UV and blue LEDs could efficiently activate the Ullmann cross-coupling reaction, and the Ullmann cross-coupling activation efficiency was greatly decreased by increasing the irradiation light wavelength. From Fig. S7B,† it could be found that the photocatalytic Heck reaction action spectrum in the visible light part was similar to the Ullmann cross-coupling reaction. UV light exhibited an anomalous phenomenon in the photocatalytic Heck reaction activation efficiency, which might be due to the high energy UV light being unfavorable for the olefin substrate adsorption and activation on the AuPd@NRCN catalyst.
The Heck reaction catalyzed under traditional heating conditions often leads to E-olefin products, due to the Z-olefins being thermodynamically unfavorable target products.50,51 Obviously, there were some challenges in obtaining high Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E stereoselectivity for the catalytic Heck reaction. Different monochromatic light sources were used to investigate the effect of light wavelength on the Heck reaction. As shown in Fig. 4D, iodobenzene under 460 nm irradiation could reach 73% conversion accompanied with 98% stilbene chemoselectivity, and as high as 90% stereoselectivity to Z-stilbene in 24 h. Either a decrease or an increase in the light source wavelength led to a catalytic activity decrease and the stilbene chemoselectivity was not significantly affected by the wavelength change. With UV light, the stereoselectivity to Z-stilbene was almost the same as that with blue light, however, the Z-stilbene stereoselectivity gradually decreased with the increase of the light source wavelength, and just 22% of Z-stilbene was detected at 620 nm. In summary, the wavelength of monochromatic light could significantly affect the Z
E stereoselectivity for the catalytic Heck reaction. Different monochromatic light sources were used to investigate the effect of light wavelength on the Heck reaction. As shown in Fig. 4D, iodobenzene under 460 nm irradiation could reach 73% conversion accompanied with 98% stilbene chemoselectivity, and as high as 90% stereoselectivity to Z-stilbene in 24 h. Either a decrease or an increase in the light source wavelength led to a catalytic activity decrease and the stilbene chemoselectivity was not significantly affected by the wavelength change. With UV light, the stereoselectivity to Z-stilbene was almost the same as that with blue light, however, the Z-stilbene stereoselectivity gradually decreased with the increase of the light source wavelength, and just 22% of Z-stilbene was detected at 620 nm. In summary, the wavelength of monochromatic light could significantly affect the Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E product stereoselectivity in the photocatalytic Heck reaction.
E product stereoselectivity in the photocatalytic Heck reaction.
The filtration test was further performed to clarify the characteristics of the photocatalytic reaction process. 12% conversion could be achieved in 5 h for the Ullmann cross-coupling reaction between iodobenzene and p-iodotoluene; however, no further conversion change was detected with an additional 5 h of reaction after the catalyst was filtered off with high-speed centrifugation. Similarly, 10% conversion could be achieved in the initial 0.5 h for the Heck reaction between iodobenzene and styrene; however, no further conversion change was detected with an additional 5 h of reaction after the catalyst was filtered off with high-speed centrifugation. All the filtration results above strongly support the proposal that the photocatalytic C–C bond formation reactions proceed under heterogeneous catalysis.
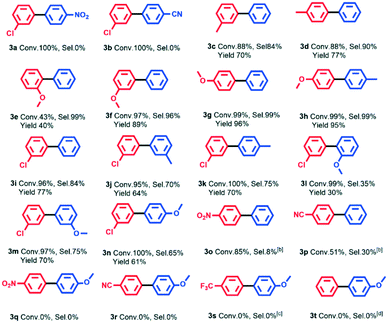
In order to investigate the substrate applicability of the AuPd@NRCN photocatalyst in the Ullmann and Heck reactions, different substrates were tested. We firstly studied the AuPd@NRCN photocatalytic Ullmann homo-coupling reaction, as shown in Table S10.† The Ullmann homo-coupling reaction demonstrated good activity in most aryl iodides (Table S10,† entries 1–7), and no coupling products were observed in some strong electron-withdrawing group substrates (Table S10,† entries 8 and 9). In particular, bromobenzene and chlorobenzene could also reach satisfactory conversion after adjusting the light irradiation intensity and reaction time (Table S10,† entries 10–12). Furthermore, we examined the substrate scope of the Ullmann cross-coupling reaction catalyzed by AuPd@NRCN under a combination of blue and green light (Scheme 1). The Ullmann reaction scope included (i) pull–pull or push–push systems where both substituents were either EWG or EDG; and (ii) push–pull systems, with one aryl group substituted with an electron donating group (EDG) and the other with an electron withdrawing group (EWG). Similar to a previous report,52 no cross-coupling products were observed in the pull–pull system (Scheme 1, 3a, 3b) and only the m-chloroiodobenzene self-coupling product was detected. The strong electron-withdrawing effects of –NO2 and –CN were thought as unfavorable for the elimination of corresponding products.53,54 For most push–push systems, good photocatalytic activity and chemoselectivity to cross-coupled products were achieved (Scheme 1, 3c, 3d, 3f–3h), which could be attributed to the fact that the moderate EDG was more favorable for the aryl iodide insertion to form the Ar–AuPd–I intermediate. As for the combination of o-methoxyiodobenzene and iodobenzene, only 43% conversion was observed due to the steric effect of o-methoxyiodobenzene (Scheme 1, 3e). For push–pull systems with the moderate EWG (i.e., m-chloroiodobenzene), the coupling reaction activity was almost maintained under the conditions studied (Scheme 1, 3i–3n). In comparison with methyl group substrates, methoxy group substrates with a stronger electron donating effect was generally unfavorable for the cross-coupling selectivity (Scheme 1, 3l–3nvs.3j–3k). Meanwhile, steric hindrance in the electron donating substrates was also important to cross-coupling selectivity (Scheme 1, 3jvs.3k, 3lvs.3mvs.3n). Other substrates with EWG substituents such as –NO2 and –CN were also tested, numerous homo-coupling products of EDG-substrates were detected (Scheme 1, 3o, 3p). –p-CN or –p-NO2 substituted substrates could not react with the –p-OCH3 substrate (Scheme 1, 3q, 3r). Therefore, both electronic and steric effects in substrates had an obvious influence on the visible light induced catalytic activity and chemoselectivity, and it's rather interesting that good catalytic performance was achieved with push–push systems in air. Additionally, aryl bromides were also tested in this photocatalytic system (Scheme 1, 3s, 3t), but no photocatalytic activity was detected.
Additionally, we investigated the substrate scope of the Heck reaction (Scheme 2). Good to excellent conversion, chemoselectivity and stereoselectivity could be obtained for most substrates (Scheme 2). When the olefin part was styrene, the substituent on the aryl iodide was not conducive to the improvement of the photocatalytic Heck reaction activity, and the position of the substituent had a significant effect on the product stereoselectivity. When the EDG was located at the para position of the aryl iodide, more Z-olefin products were formed (Scheme 2, 7c, 7f). When the EDG was located at other positions of the aryl iodide (Scheme 2, 7b, 7d, 7e), the stereoselectivity of Z-olefin was severely reduced due to the steric hindrance. p-Iodoanisole and styrene could be completely converted in 48 hour, the chemoselectivity of the cross-coupling product could reach 97%, and the Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E stereoselectivity could reach 96
E stereoselectivity could reach 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Scheme 2, 7f). High Z-olefin stereoselectivity could also be obtained when the EWG substituted aryl iodide was the substrate (Scheme 2, 7g). When ethyl acrylate was used as the olefin substrate, high Z-olefin stereoselectivity could be obtained. After 34 hour of reaction between iodobenzene and ethyl acrylate under blue LED light irradiation, the product had a Z
4 (Scheme 2, 7f). High Z-olefin stereoselectivity could also be obtained when the EWG substituted aryl iodide was the substrate (Scheme 2, 7g). When ethyl acrylate was used as the olefin substrate, high Z-olefin stereoselectivity could be obtained. After 34 hour of reaction between iodobenzene and ethyl acrylate under blue LED light irradiation, the product had a Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E stereoselectivity up to 99
E stereoselectivity up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 2, 7h). Additionally, aryl bromides were also tested (Scheme 2, 7j, 7k), and the photocatalytic activity was sharply decreased. In short, the reaction products of various substituted aryl iodides with styrene or ethyl acrylate mainly achieved Z-olefins under the blue LED irradiation. This study provided a new way to obtain Z-olefins with high stereoselectivity based on a one-pot heterogeneous photocatalytic reaction under mild conditions and light irradiation.
1 (Scheme 2, 7h). Additionally, aryl bromides were also tested (Scheme 2, 7j, 7k), and the photocatalytic activity was sharply decreased. In short, the reaction products of various substituted aryl iodides with styrene or ethyl acrylate mainly achieved Z-olefins under the blue LED irradiation. This study provided a new way to obtain Z-olefins with high stereoselectivity based on a one-pot heterogeneous photocatalytic reaction under mild conditions and light irradiation.
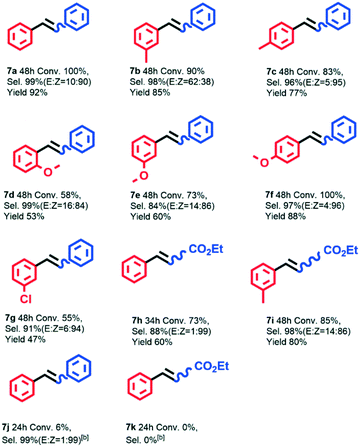 | ||
| Scheme 2 Photocatalytic Heck reaction of aryl halides with alkenes over AuPd@NRCN.[a] [a] Red as a limiting reagent. [b] Bromobenzene instead of iodobenzene and bromobenzene as a limiting reagent. | ||
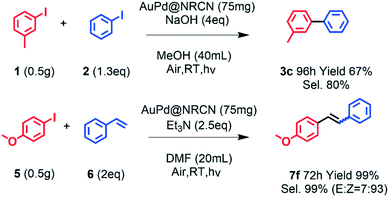
To demonstrate the synthetic potential of this photocatalytic C–C bond formation methodology, the chemoselective C–C bond formation to the unsymmetrical biaryl product with Ullmann cross-coupling and the stereoselective C–C bond formation to the Z-type Heck reaction product could be scaled up to gram quantities (Scheme 3). Unsymmetrical biaryl product 3c could reach 67% yield accompanied with 80% chemoselectivity in 96 h. Z-Type Heck reaction product 7f could be achieved with 100% yield accompanied with 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 of Z
7 of Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E stereoselectivity in 72 h.
E stereoselectivity in 72 h.
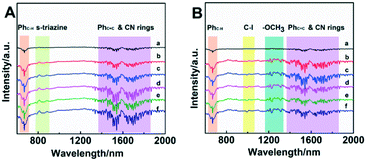
In Ullmann and Heck reactions, the activation of the C–X bond (X = I as an example) was the key step for the C–C bond formation.55,56 In order to study the C–I bond activation in the AuPd@NRCN photocatalytic system, in situ DRIFTS measurements were conducted to compare the difference in C–I bond activation in the dark at 50 °C or under light irradiation at room temperature under simulated catalytic conditions (Fig. 5). All infrared spectra represent relative changes in the catalytic system because the initial state of the background spectrum was subtracted from the sample. Fig. 5A shows the in situ DRIFTS of the sample in the dark at 50 °C. The negative peaks between 600 and 750 cm−1 were ascribed to the C–H vibration of the benzene ring from p-iodoanisole, and the peaks at 1400–1800 cm−1 could be attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeleton vibration of the benzene ring and the stretching vibrations of CN rings from NRCN, which could also be observed under light (Fig. 5B). In Fig. 5A, the positive peaks at 806 cm−1 and 880 cm−1, which was gradually increased with time, should be ascribed to the characteristic breathing mode of s-triazine in NRCN. However, these two peaks were not observed under light conditions, which indicated that heating had a more significant influence on the NRCN s-triazine structure. Due to the efficient cleavage of the C–I bond under light conditions, the negative peak at 994 cm−1 was observed and enhanced as the illumination time increased.57 Meanwhile, the negative peaks between 1200 and 1350 cm−1 under light conditions were attributed to the –OCH3 vibration of p-iodoanisole, which was related to the change caused by C–I cleavage. The above results indicated that the light irradiation was conducive to the stabilization of NRCN and the C–I bond activation, thereby promoting the photocatalytic coupling reaction.
C skeleton vibration of the benzene ring and the stretching vibrations of CN rings from NRCN, which could also be observed under light (Fig. 5B). In Fig. 5A, the positive peaks at 806 cm−1 and 880 cm−1, which was gradually increased with time, should be ascribed to the characteristic breathing mode of s-triazine in NRCN. However, these two peaks were not observed under light conditions, which indicated that heating had a more significant influence on the NRCN s-triazine structure. Due to the efficient cleavage of the C–I bond under light conditions, the negative peak at 994 cm−1 was observed and enhanced as the illumination time increased.57 Meanwhile, the negative peaks between 1200 and 1350 cm−1 under light conditions were attributed to the –OCH3 vibration of p-iodoanisole, which was related to the change caused by C–I cleavage. The above results indicated that the light irradiation was conducive to the stabilization of NRCN and the C–I bond activation, thereby promoting the photocatalytic coupling reaction.
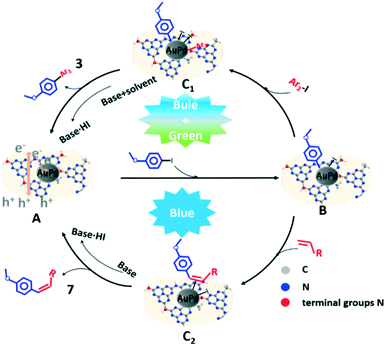
To gain deep understanding of the role of light in the photocatalytic process, light activation of different substrates in the Ullmann cross-coupling reaction and Heck reaction was studied as shown in Tables S13 and S14.† In Table S13,† iodobenzene without photoactivation had no conversion (Table S13,† entry 1); iodobenzene had obvious self-coupling during 2 h photoactivation (Table S13,† entry 2); for comparison, photoactivated p-iodoanisole had no self-coupling products (Table S13,† entry 3). The photoactivated iodobenzene and photoactivated p-iodoanisole mixture could generate cross-coupling products under dark conditions (Table S13,† entry 4), however, photoactivated p-iodoanisole could not react with non-photoactivated iodobenzene (Table S13,† entry 5). The result in Table S13† indicated that the Ullmann cross-coupling reaction was a multi-photon reaction, and the activation of the two components by light was necessary for the Ullmann cross-coupling reaction. On the other hand, the obvious self-coupling of photoactivated iodobenzene was also observed in the photocatalytic Heck stereoselective coupling reaction (Table S14† entry 1). The photoactivated iodobenzene and photoactivated styrene mixture under dark conditions would obviously proceed to stilbene (Table S14† entry 4). The photoactivated iodobenzene and non-photoactivated styrene mixture mainly generated the iodobenzene self-coupling product under dark conditions (Table S14† entry 5). The result of these Heck reactions indicated that the activation of iodobenzene and styrene with light was beneficial to the formation of the stilbene product. We had proposed a possible mechanism by using p-iodoanisole as an example (Scheme 4). The coupling reactions involved two steps: the C–I bond cleavage in aryl iodide and the coupling partner activation. In this study, AuPd@NRCN firstly generated electron–hole pairs under light irradiation. The generated electrons were transferred from the conduction band (CB) to the AuPd NP surface. The p-iodoanisole adsorbed on the AuPd surface was electronically activated and cleaved the C–I bond (B). In the Ullmann cross-coupling reaction, another aryl iodide oxidative addition and the substrate reductive elimination determined the chemoselectivity of 3, and the transformation had the following situations: the intermediate (B) which coordinated with a strong EDG was more favorable to oxidative addition with another weaker electron donor aryl iodide (such as –OCH3vs. –CH3, –CH3vs. –H) to form the intermediate (C1); when the second aryl iodide was an EWG (such as –Cl) substrate, the product reductive elimination slowed down, leading to the decrease of 3 chemoselectivity. The base and the proton in the solvent promoted the iodine leaving from the AuPd surface to form C1 and paved the catalyst revival procedure to obtain A. The activation of the two components in the Ullmann cross-coupling reaction using a combination of light sources with different colors was helpful for the cross-coupling efficiency (Tables S6 and S7†). The improved photocatalytic performance in the multi-photon Ullmann cross-coupling reaction might be due to the activation of different substrates and/or steps58,59 requiring different energies, and the combination of the two energy sources was beneficial to improve the activation efficiency of different substrates and/or steps. In the Heck reaction, olefin molecules rapidly interacted with B to form the intermediate C2. The formation of the stereoselective Z-olefin should be attributed to the special interaction mode between the reaction substrates and the catalytic material under visible light irradiation. In addition, the electronic state difference on the catalytic material surface caused by the photon energy of different light colors also significantly affected the stereoselectivity of product 7. Product 7 could be obtained by reductive elimination, while the base assisted iodine to leave the AuPd surface for maintaining the activity of AuPd@NRCN.
3. Conclusion
In summary, a novel AuPd@NRCN photocatalyst was constructed through simple ammonia solution heat treatment of carbon nitride and then AuPd alloy nanoparticle loading. AuPd@NRCN showed rather good photocatalytic performance in challenging chemoselective or stereoselective C–C coupling under visible light irradiation, and the ammonia solution treatment process obviously improved the photocatalytic performance. Unprecedentedly, up to 99% unsymmetrical chemoselective biaryl products through one-step Ullmann cross-coupling could be achieved with different visible light irradiation color combinations in air; and as high as 99% Z-type stereoselective Heck reaction products could also be realized directly with different monochromatic visible light irradiation in air. The application of various substrates provided a great possibility for the economical synthesis of unsymmetrical biaryl products and Z-type olefins. This work established a highly efficient way to realize both unsymmetrical biaryl products and Z-type Heck reaction products via novel NRCN material preparation and successful AuPd alloy nanoparticle loading on NRCN, as well as light color promoted chemoselectivity and stereoselectivity improvement. The efficient visible light promoted C–I bond activation accompanied with improved photocatalytic coupling reaction efficiency over AuPd@NRCN was verified firstly by in situ DRIFTS. Additionally, the activation of the two components in the Ullmann cross-coupling reaction using a combination of light sources with different colors was helpful for the cross-coupling efficiency. The improved photocatalytic performance in the multi-photon Ullmann cross-coupling reaction might be due to the activation of different substrates and/or steps requiring different energies, and the combination of the two energy sources was beneficial for improving the activation efficiency of different substrates and/or steps; the activation of iodobenzene and styrene in the Heck reaction with light was also beneficial to the formation of the stilbene product. The light color promoted chemoselectivity and stereoselectivity in C–C bond formation reactions are expected to have profound impact on organic synthetic chemistry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation Project of CQ (no. cstc2018jcyjAX0735, cstc2019jcyj-msxmX0641), the National Natural Science Foundation of China (no. 21201184), the Ministry of Education of Chongqing (no. KJQN201900811), the Chongqing Technology and Business University (950119090) and Chongqing Key Laboratory of Catalysis, New Environmental Materials (KFJJ2019082), and the Key Disciplines of Chemical Engineering and Technology in Chongqing Colleges and Universities during the 13th Five Year Plan and Innovation Group of New Technologies for Industrial Pollution Control of Chongqing Education Commission (CXQT19023).Notes and references
- F. Han, Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: a remarkable advance from palladium to nickel catalysts, Chem. Soc. Rev., 2013, 42, 5270–5298 RSC.
- P. Riente and T. Noel, Application of metal oxide semiconductors in light-driven organic transformations, Catal. Sci. Technol., 2019, 9, 5186–5232 RSC.
- G. Yue, K. Lei, H. Hirao and J. Zhou, Palladium-catalyzed asymmetric reductive Heck reaction of aryl halides, Angew. Chem., Int. Ed., 2015, 54, 6531–6535 CrossRef CAS.
- R. L. Oliveira, J. Kerstien, R. Schomacker and A. Thomas, Pd nanoparticles confined in mesoporous N-doped carbon silica supports: a synergistic effect between catalyst and support, Catal. Sci. Technol., 2020, 10, 1385–1394 RSC.
- J. I. Ayogu and E. A. Onoabedje, Recent advances in transition metal-catalysed cross-coupling of (hetero)aryl halides and analogues under ligand-free conditions, Catal. Sci. Technol., 2019, 9, 5233–5255 RSC.
- M. Gopiraman, R. Karvembu and I. S. Kim, Highly active, selective, and reusable RuO2/SWCNT catalyst for Heck olefination of aryl halides, ACS Catal., 2014, 4, 2118–2129 CrossRef CAS.
- J. Xia, Y. Fu, G. He, X. Sun and X. Wang, Core-shell-like Ni-Pd nanoparticles supported on carbon black as a magnetically separable catalyst for green Suzuki-Miyaura coupling reactions, Appl. Catal., B, 2017, 200, 39–46 CrossRef CAS.
- F. Khan, M. Dlugosch, X. Liu and M. G. Banwell, The palladium-catalyzed Ullmann cross-coupling reaction: a modern variant on a time-honored process, Acc. Chem. Res., 2018, 51, 1784–1795 CrossRef CAS.
- H. J. Schäfer, Unsymmetrical biaryl compounds: metal- and reagent free electrochemical couplings are on the advance, Angew. Chem., Int. Ed., 2017, 56, 15502–15503 CrossRef.
- B. Karimi, H. Barzegara and H. Vali, Au–Pd bimetallic nanoparticles supported on a high nitrogen-rich ordered mesoporous carbon as an efficient catalyst for room temperature Ullmann coupling of aryl chlorides in aqueous media, Chem. Commun., 2018, 54, 7155–7158 RSC.
- D. G. Ma, A. Liu, S. H. Li, C. C. Lu and C. C. Chen, TiO2 photocatalysis for C-C bond formation, Catal. Sci. Technol., 2018, 8, 2030–2045 RSC.
- G. Chen, G. I. N. Waterhouse, R. Shi, J. Zhao, Z. Li, L. Z. Wu, C. H. Tung and T. Zhang, From solar energy to fuels: recent advances in light-driven C1 chemistry, Angew. Chem., Int. Ed., 2019, 58, 17528–17551 CrossRef CAS.
- Q. H. Jia, S. F. Zhang, X. X. Jia, X. Y. Dong, Z. W. Gao and Q. Gu, Photocatalytic coupled redox cycle for two organic transformations over Pd/carbon nitride composites, Catal. Sci. Technol., 2019, 9, 5077–5089 RSC.
- A. Yamamoto, T. Ohara and H. Yoshida, Visible-light-induced photocatalytic benzene/cyclohexane cross-coupling utilizing a ligand-to-metal charge transfer benzene complex adsorbed on titanium oxides, Catal. Sci. Technol., 2018, 8, 2046–2050 RSC.
- X. Huang, Y. Li, Y. Chen, H. Zhou, X. Duan and Y. Huang, Plasmonic and catalytic AuPd nanowheels for the efficient conversion of light into chemical energy, Angew. Chem., Int. Ed., 2013, 52, 6063–6067 CrossRef CAS.
- F. Wang, C. Li, H. Chen, R. Jiang, L. D. Sun, Q. Li, J. Wang, J. C. Yu and C. H. Yan, Plasmonic harvesting of light energy for Suzuki coupling reactions, J. Am. Chem. Soc., 2013, 135, 5588–5601 CrossRef CAS.
- Q. Xiao, S. Sarina, A. Bo, J. Jia, H. Liu, D. P. Arnold, Y. Huang, H. Wu and H. Zhu, Visible light-driven cross-coupling reactions at lower temperatures using a photocatalyst of palladium and gold alloy nanoparticles, ACS Catal., 2014, 4, 1725–1734 CrossRef CAS.
- S. Zhang, C. R. Chang, Z. Q. Huang, Y. Ma, W. Gao, J. Li and Y. Qu, Visible-light-activated Suzuki-Miyaura coupling reactions of aryl chlorides over the multifunctional Pd/Au/porous nanorods of CeO2 catalysts, ACS Catal., 2015, 5, 6481–6488 CrossRef CAS.
- H. H. Shin, E. Kang, H. Park, T. Han, C. H. Lee and D. K. Lim, Pd-nanodot decorated MoS2 nanosheets as a highly efficient photocatalyst for the visible-lightinduced Suzuki–Miyaura coupling reaction, J. Mater. Chem. A, 2017, 5, 24965–24971 RSC.
- H. H. Shin, T. Han, W. Yang and D. K. Lim, Pd2+-conjugated reduced graphene oxide as a highly efficient visible-light-induced photocatalytic Suzuki-Miyaura coupling, Carbon, 2019, 143, 362–370 CrossRef CAS.
- M. Q. Zhang, K. Zhu, L. X. Qin, S. Z. Kang and X. Q. Li, Enhanced electron transfer and photocatalytic hydrogen production over the carbon nitride/porphyrin nanohybrid finely bridged by special copper, Catal. Sci. Technol., 2020, 10, 1640–1649 RSC.
- H. Jiang, C. Zang, Y. Zhang, W. Wang, C. Yang, B. Sun, Y. Shen and F. Bian, 2D MXene-derived Nb2O5/C/Nb2C/g-C3N4 heterojunctions for efficient nitrogen photofixation, Catal. Sci. Technol., 2019, 9, 6938–6945 RSC.
- H. L. Dou, Y. M. Qin, F. Pan, D. Long, X. Rao, G. Q. Xu and Y. P. Zhang, Core-shell g-C3N4/Pt/TiO2 nanowires for simultaneous photocatalytic H2 evolution and RhB degradation under visible light irradiation, Catal. Sci. Technol., 2019, 9, 4898–4908 RSC.
- L. L. Wang, M. Liu, W. Y. Zha, Y. C. Wei, X. F. Ma, C. W. Xu, C. G. Lu, N. F. Qin, L. Gao, W. Z. Qiu, R. J. Sa, X. Z. Fu and R. S. Yuan, Mechanistic study of visible light-driven CdS or g-C3N4-catalyzed C-H direct trifluoromethylation of (hetero)arenes using CF3SO2Na as the trifluoromethyl source, J. Catal., 2020, 389, 533–543 CrossRef CAS.
- S. Barata-Vallejo and A. Postigo, New visible-light-triggered photocatalytic trifluoromethylation reactions of carbon-carbon multiple bonds and (hetero)aromatic compounds, Chem. – Eur. J., 2020, 26, 11065–11084 CrossRef CAS.
- S. Gisbertz, S. Reischauer and B. Pieber, Overcoming limitations in dual photoredox/nickel-catalysed C-N cross-couplings due to catalyst deactivation, Nat. Catal., 2020, 3, 611–620 CrossRef CAS.
- J. Khamrai, I. Ghosh, A. Savateev, M. Antonietti and B. Konig, Photo-Ni-dual-catalytic C(sp(2))-C(sp(3)) cross-coupling reactions with mesoporous graphitic carbon nitride as a heterogeneous organic semiconductor photocatalyst, ACS Catal., 2020, 10, 3526–3532 CrossRef CAS.
- C. Yang, R. Li, K. A. I. Zhang, W. Lin, K. Landfester and X. C. Wang, Heterogeneous photoredox flow chemistry for the scalable organosynthesis of fine chemicals, Nat. Commun., 2020, 11, 1239 CrossRef CAS.
- I. Ghosh, J. Khamrai, A. Savateev, N. Shlapakov, M. Antonietti and B. König, Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes, Science, 2019, 365, 360–366 CrossRef CAS.
- B. Antil, L. Kumar, K. P. Reddy, C. S. Gopinath and S. Deka, Direct thermal polymerization approach to N-rich holey carbon nitride nanosheets and their promising photocatalytic H2 evolution and charge-storage activities, ACS Sustainable Chem. Eng., 2019, 7, 9428–9438 CrossRef CAS.
- Z. Mo, X. Zhu, Z. Jiang, Y. Song, D. Liu, H. Li, X. Yang, Y. She, Y. Lei, S. Yuan, H. Li, L. Song, Q. Yan and H. Xu, Porous nitrogen-rich g-C3N4 nanotubes for efficient photocatalytic CO2 Reduction, Appl. Catal., B, 2019, 256, 117854 CrossRef CAS.
- X. Wu, D. Gao, P. Wang, H. Yu and J. Yu, NH4Cl-induced low-temperature formation of nitrogen-rich g-C3N4 nanosheets with improved photocatalytic hydrogen evolution, Carbon, 2019, 153, 757–766 CrossRef CAS.
- J. Yan, C. Zhou, P. Li, B. Chen, S. Zhang, X. Dong, F. Xi and J. Liu, Nitrogen-rich graphitic carbon nitride: Controllable nanosheet-likemorphology, enhanced visible light absorption and superiorphotocatalytic performance, Colloids Surf., A, 2016, 508, 257–264 CrossRef CAS.
- L. Luo, M. Zhang, P. Wang, Y. Wang and F. Wang, Nitrogen rich carbon nitride synthesized by copolymerization with enhanced visible light photocatalytic hydrogen evolution, New J. Chem., 2018, 42, 1087–1091 RSC.
- Q. Xiao, Z. Liu, A. Bo, S. Zavahir, S. Sarina, S. Bottle, J. D. Riches and H. Zhu, Catalytic transformation of aliphatic alcohols to corresponding esters in O2 under neutral conditions using visible-light irradiation, J. Am. Chem. Soc., 2015, 5, 1956–1966 CrossRef.
- N. Jiang, H. Wang, Y. Luo, S. Yu, A. Liu, W. Zou, F. Gao and L. Dong, Facile two-step treatment of carbon nitride for preparation of highly efficient visible-light photocatalyst, Appl. Catal., B, 2018, 227, 541–547 CrossRef CAS.
- J. Xu, S. Zhang, X. Liu, F. Bian and H. Jiang, Rh/polymeric carbon nitride porous tubular catalyst: visible light enhanced chlorophenol hydrodechlorination in base-free aqueous medium, Catal. Sci. Technol., 2019, 9, 6938–6945 RSC.
- F. Huo, Y. Liu, Y. Tang, Y. Cao, C. Tan, F. Yang and X. Yang, Aggregation induced emission of amino-thiol capped gold nanoparticles (GNPs) through metal-amino-coordination, Colloids Surf., B, 2019, 183, 110335 CrossRef CAS.
- A. Y. Robin and K. M. Fromm, Coordination polymer networks with O- and N-donors: What they are, why and how they are made, Coord. Chem. Rev., 2006, 250, 2127–2157 CrossRef CAS.
- S. Jiang, C. Xiong, S. Song and B. Cheng, Plasmonic graphene-like Au/C3N4 nanosheets with barrier-free interface for photocatalytically sustainable evolution of active oxygen species, ACS Sustainable Chem. Eng., 2019, 7, 2018–2026 CrossRef CAS.
- M. Muzzio, C. Yu, H. Lin, T. Yom, D. A. Boga, Z. Xi, N. Li, Z. Yin, J. Li, J. A. Dunn and S. Sun, Reductive amination of ethyl levulinate to pyrrolidones over AuPd nanoparticles at ambient hydrogen pressure, Green Chem., 2019, 21, 1895–1899 RSC.
- M. Wissing and A. Studer, Tuning the selectivity of AuPd nanoalloys towards selective dehydrogenative alkyne silylation, Chem. – Eur. J., 2019, 25, 5870–5874 CrossRef CAS.
- Y. Zeng, X. Liu, C. Liu, L. Wang, Y. Xia, S. Zhang, S. Luo and Y. Pei, Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity, Appl. Catal., B, 2018, 224, 1–9 CrossRef CAS.
- S. Zhang, J. Xu, H. Cheng, C. Zang, F. Bian, B. Sun, Y. Shen and H. Jiang, Photocatalytic H2 evolution from ammonia borane: improvement of charge separation and directional charge transmission, ChemSusChem, 2020, 13, 5264–5272 CrossRef CAS.
- M. Z. Rahman, J. Ran, Y. Tang, M. Jaroniec and S. Z. Qiao, Surface activated carbon nitride nanosheets with optimized electro-optical properties for highly efficient photocatalytic hydrogen production, J. Mater. Chem. A, 2016, 4, 2445–2452 RSC.
- J. Sherwood, J. H. Clark, I. J. S. Fairlamb and J. M. Slattery, Solvent effects in palladium catalyzed cross-coupling reactions, Green Chem., 2019, 21, 2164–2213 RSC.
- H. Cheng, X. Long, F. Bian, C. Yang, X. Liu and H. Jiang, Efficient photocatalytic one-pot hydrogenation and N-alkylation of nitrobenzenes/benzonitriles with alcohols over Pd/MOFs: Effect of the crystal morphology & “quasi-MOF” structure, J. Catal., 2020, 389, 121–131 CrossRef CAS.
- T. D. Bluemke, W. Clegg, P. García-Alvarez, A. R. Kennedy, K. Koszinowski, M. D. McCall, L. Russob and E. Hevia, Structural and reactivity insights in Mg–Zn hybrid chemistry: Zn–I exchange and Pd-catalysed crosscoupling applications of aromatic substrates, Chem. Sci., 2014, 5, 3552–3562 RSC.
- L. K. G. Ackerman, M. M. Lovell and D. J. Weix, Multimetallic catalysed cross-coupling of aryl bromides with aryl triflates, Nature, 2015, 524, 454–457 CrossRef CAS.
- H. M. Song, B. A. Moosa and N. M. Khash, Water-dispersable hybrid Au–Pd nanoparticles as catalysts in ethanol oxidation, aqueous phase Suzuki–Miyaura and Heck reactions, J. Mater. Chem., 2012, 22, 15953–15959 RSC.
- M. Nasrollahzadeh and A. Banaei, Hybrid Au/Pd nanoparticles as reusable catalysts for Heck coupling reactions in water under aerobic conditions, Tetrahedron Lett., 2015, 56, 500–503 CrossRef CAS.
- N. Marina, A. E. Lanterna and J. C. Scaiano, Expanding the color space in the two-color heterogeneous photocatalysis of Ullmann C-C coupling reactions, ACS Catal., 2018, 8, 7593–7597 CrossRef CAS.
- Q. Jia, S. Zhang and Q. Gu, C-C formation mediated by photoinduced electrons from crystallized carbon nitride nanobelts under visible light irradiation, J. Energy Chem., 2019, 30, 152–161 CrossRef.
- S. Nadri, E. Azadi, A. Ataei, M. Joshaghani and E. Rafiee, Investigation of the catalytic activity of a Pd/biphenyl-based phosphine system in the Ullmann homocoupling of aryl bromides, J. Organomet. Chem., 2011, 696, 2966–2970 CrossRef CAS.
- D. Sun and Z. Li, Double-solvent method to Pd nanoclusters encapsulated inside the cavity of NH2−Uio-66(Zr) for efficient visible-light-promoted Suzuki coupling reaction, J. Phys. Chem. C, 2016, 120, 19744–19750 CrossRef CAS.
- G. B. Smith, G. C. Dezeny, D. L. Hughes, A. O. King and T. R. Verhoeven, Mechanistic studies of the Suzuki cross-coupling reaction, J. Org. Chem., 1994, 59, 8151–8156 CrossRef CAS.
- M. Li, X. Shi, J. Zhu and C. Ma, In situ fourier infrared spectroscopy studies on electrochemical deiodination reaction of 2-iodobenzoic acid, Fenxi Huaxue, 2012, 5, 791–795 CrossRef.
- G. M. Torres, Y. Liu and B. A. Arndtsen, A dual light-driven palladium catalyst: Breaking the barriers in carbonylation reactions, Science, 2020, 368, 318–323 CrossRef CAS.
- A. Rakshit, P. Kumar, T. Alam, H. Dhara and B. K. Patel, Visible-light-accelerated Pd-catalyzed cascade addition/cyclization of arylboronic acids to γ- and β-ketodinitriles for the construction of 3-cyanopyridines and 3-cyanopyrrole analogues, J. Org. Chem., 2020, 85, 12482–12504 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cy01881c |
| This journal is © The Royal Society of Chemistry 2021 |