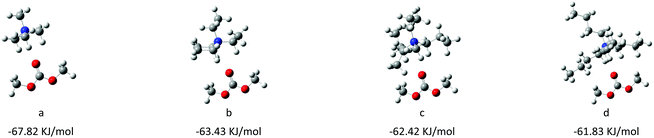Synthesis of bio-based polycarbonate via one-step melt polycondensation of isosorbide and dimethyl carbonate by dual site-functionalized ionic liquid catalysts†
Zifeng
Yang
 ab,
Xue
Li
a,
Fei
Xu
ab,
Xue
Li
a,
Fei
Xu
 *ac,
Weiwei
Wang
a,
Yongqing
Shi
a,
Zhencai
Zhang
*ac,
Weiwei
Wang
a,
Yongqing
Shi
a,
Zhencai
Zhang
 a,
Wenjuan
Fang
a,
Wenjuan
Fang
 a,
Lei
Liu
a and
Suojiang
Zhang
a,
Lei
Liu
a and
Suojiang
Zhang
 *ab
*ab
aBeijing Key Laboratory of Ionic Liquids Clean Process, Key Laboratory of Green Process and Engineering, State Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P.R. China. E-mail: sjzhang@ipe.ac.cn
bSchool of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing 100049, P.R. China
cZhongke Langfang Institute of Process Engineering, CAS, 065000, P.R. China
First published on 19th November 2020
Abstract
The synthesis of green products using renewable bio-monomers has become a research trend with the requirement of sustainable development. In this work, we reported a green pathway to synthesize poly(isosorbide carbonate) (PIC) via one-step melt polycondensation of biomass-derived isosorbide (ISB) and CO2-derived dimethyl carbonate (DMC) catalyzed by eco-friendly dual site-functionalized ionic liquids; a series of these catalysts were synthesized for the first time to the best of our knowledge and their effects on the molecular weight and terminal groups of PIC were systematically investigated. The results showed that the steric structure of cations and anions of catalysts could significantly influence their catalytic performance in polymer synthesis reactions. Meanwhile, it was found that the ability of catalysts to activate the reaction substrate gradually increased with decreasing steric hindrance of cations and anions. Among our exploited ionic liquid catalysts, the selectivity of carboxymethylated products was increased significantly by using bis-tetraethylammonium hydroquinone ([N2222]2 [HQ]), and a PIC with a weight average molecular weight (Mw) of 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 along with an ISB conversion up to 99.0% was obtained. As far as we know, the catalyst of [N2222]2 [HQ] was the most efficient catalyst for PIC one-step synthesis compared with the existing traditional ionic liquid catalysts. Besides, according to the detected intermediates of the reaction process and the results of various analytical means, a possible mechanism for the synergetic catalysis of cations–anions promoting the chain growth was proposed.
600 along with an ISB conversion up to 99.0% was obtained. As far as we know, the catalyst of [N2222]2 [HQ] was the most efficient catalyst for PIC one-step synthesis compared with the existing traditional ionic liquid catalysts. Besides, according to the detected intermediates of the reaction process and the results of various analytical means, a possible mechanism for the synergetic catalysis of cations–anions promoting the chain growth was proposed.
Introduction
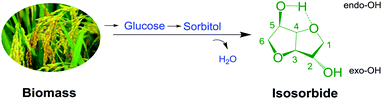
Polycarbonate (PC) as an engineering plastic has been widely applied in all walks of life, but with the requirements of green chemistry and sustainable development, the traditional PC synthesis technology from phosgene and petroleum-derived bisphenol-A (BPA) is gradually eliminated due to the safety problems of technological processes and health concerns of raw materials.1–3 Therefore, it is urgent to develop a novel and green chemical technology for PC synthesis by utilizing renewable resources.4–6In the past few years, bio-derived PC has attracted widespread attention as a green sustainable material.7–12 Notably, poly(isosorbide carbonate) (PIC) as a kind of bio-derived PC material compared with BPA-based PC is not only green and renewable, but also has superior thermal and optical properties, which has become a hot topic.11–14 As a raw material of PIC, isosorbide (ISB) derived from biomass is a renewable resource (Scheme 1), and has a comparable structural rigidity to BPA.15–18 Therefore, ISB has been gradually considered as an ideal choice to replace traditional BPA to synthesize PC.19–21
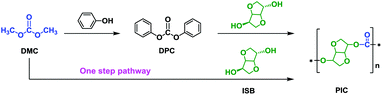
At present, there are two main synthetic routes to PIC by using ISB as shown in Scheme 2. Most of the reported works have focused on the traditional route using ISB and diphenyl carbonate (DPC), while few research works to date have concentrated on the synthesis of PIC using ISB and dimethyl carbonate (DMC) via a one-step pathway (Scheme 2). However, CO2-derived DMC, the cornerstone of the green chemical industry in the 21st century, is an essential intermediate for the synthesis of DPC.22–25 Additionally, the low-boiling methanol, a byproduct of DMC during melt polycondensation, is more easy to remove than phenol, a byproduct of DPC.26–28 Therefore, the synthesis of PIC using ISB with DMC by a one-step pathway is the most green and cost-effective.29–31
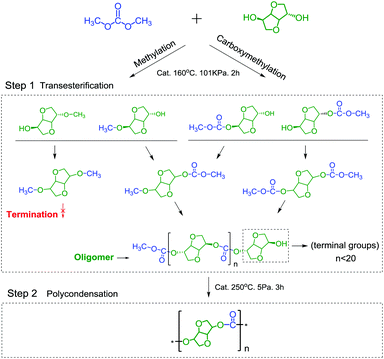
Although the one-step pathway for synthesizing PIC is simplified and eco-friendly, to synthesize PIC with a high molecular weight via this method is still a daunting challenge due to the weak reactivity of the intramolecular hydroxyl (endo-OH) groups of ISB32,33 and the methylation reaction of DMC.34,35 In our and other previous studies, it has been found that the formation of intramolecular hydrogen bonds would decrease the reactivity of ISB and retard the chain growth (Scheme 1).27–30 On the other hand, DMC would undergo a methylation reaction compared with DPC,26,35 and the methylation products can inhibit chain growth as shown in Scheme 3. Therefore, it is necessary to develop a kind of high-efficiency catalyst and to further regulate and control the reaction for synthesis of high molecular weight PIC.
Dual site-functionalized ionic liquids have demonstrated vast superiority over conventional ionic liquids in academia during the past few years, especially in the aspect of catalysis. Moreover, the catalytic effect and economic efficiency can be improved by designing dual functionalized catalytic active sites.36–40 In this study, a series of eco-friendly dual site-functionalized ionic liquid catalysts were developed for the first time to improve the reactivity of endo-OH of ISB and the selectivity of carboxymethylation reaction of DMC for high molecular weight PIC. Under the optimal reaction conditions, bis-tetraethylammonium hydroquinone ([N2222]2 [HQ]) showed the highest catalytic reactivity compared with the existing traditional ionic liquid catalyst. It turned out that a PIC with the highest molecular weight of 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 and an ISB conversion up to 99.0% was obtained.41 In addition, the effects of the steric structure of cations and anions on the terminal group of PIC and catalytic synthesis mechanism in the process were in-depth investigated by density functional theory (DFT) calculations and nuclear magnetic resonance (NMR) spectroscopy. Moreover, the intermediates of the transesterification and polycondensation steps were captured and detected using mass spectrometry (MS) technology, and a possible synergetic mechanism of cations–anions was further proposed. The process reported here offered a direction to develop catalysts for synthesizing higher molecular weights PIC, and provided a cost-effective and green industrial synthetic route to PIC.
600 and an ISB conversion up to 99.0% was obtained.41 In addition, the effects of the steric structure of cations and anions on the terminal group of PIC and catalytic synthesis mechanism in the process were in-depth investigated by density functional theory (DFT) calculations and nuclear magnetic resonance (NMR) spectroscopy. Moreover, the intermediates of the transesterification and polycondensation steps were captured and detected using mass spectrometry (MS) technology, and a possible synergetic mechanism of cations–anions was further proposed. The process reported here offered a direction to develop catalysts for synthesizing higher molecular weights PIC, and provided a cost-effective and green industrial synthetic route to PIC.
Results and discussion
Structural characterization of PIC
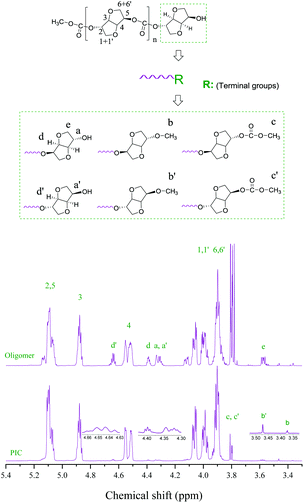
The chemical structures and terminal groups of the oligomer and PIC were characterized by 1H and 13C NMR as shown in Fig. 1 and Fig. S16.† All proton signals were found at the expected position.27–30 The protons at δ3.87–4.09, 4.55, 4.88 and 5.05–5.12 ppm were marked as the H atoms of the PIC repeating units, and assigned to H-(6-6′,1-1′), H-4, H-3 and H-(5-2), respectively. The proton signals of the ISB moiety in the PIC terminal group at 4.64, 4.39 and 3.57 ppm were recorded as d′, d and e, respectively. The signals of a, b, and c and a′, b′, and c′ were marked as the protons the of ISB terminal group including terminal exo-OH, exo-OCH3, and exo-COOCH3 and endo-OH, endo-OCH3, and endo-COOCH3, respectively. As shown in Fig. S16,† the signals in the 13C NMR spectrum were in good correspondence with those of carbons in the PIC molecular chain.27–30 The peaks of the ISB in the PIC repeating unit at δ153.5, 85.8, 81.6, 81.1, 77.3, 73.3 and 70.7 ppm were marked as C-7, C-3, C-4, C-5, C-2, C-1 and C-6, respectively.Screening of catalysts
The process for synthesizing PIC with ISB and DMC via a one-pot pathway includes two stages, transesterification and polycondensation. ISB was converted into oligomers in the transesterification stage, and oligomers were further linked together to obtain high molecular weight PIC during the polycondensation stage. As shown in Table 1, a series of dual site-functionalized ionic liquids were designed and synthesized for the first time, and their catalytic performance for synthesizing PIC was screened (specific data are summarized in Tables S1–S4†).| Entry | Catalyst | Transesterification stage | Polycondensation stage | |||||
|---|---|---|---|---|---|---|---|---|
| ISB Con.c (%) | Molecular weightd | Molecular weightd | ||||||
| M n | M w | PDI | M n | M w | PDI | |||
a (1) Transesterification: 101 kPa, (160 °C, 60 min)–(180 °C, 60 min). (2) Polycondensation: 5 Pa, 230 °C, 60 min, n(DMC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(ISB) = 7 n(ISB) = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and catalyst amount of 8 × 10−3 mol based on ISB.
b Catalyst amount of 16 × 10−3 mol based on ISB.
c Determined by GC.
d Determined by DMF-GPC using polystyrene standards (RI detector). The average of data was obtained by three measurements to ensure the accuracy of the results. 1, and catalyst amount of 8 × 10−3 mol based on ISB.
b Catalyst amount of 16 × 10−3 mol based on ISB.
c Determined by GC.
d Determined by DMF-GPC using polystyrene standards (RI detector). The average of data was obtained by three measurements to ensure the accuracy of the results.
|
||||||||
| 1 | [N1111]2 [PY] | 91 | 2700 | 4100 | 1.52 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.63 |
| 2 | [N1111]2 [RE] | 93 | 2600 | 3900 | 1.50 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.66 |
| 3 | [N1111]2 [HQ] | 98 | 3100 | 4800 | 1.55 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.63 |
| 4 | [N2222]2 [HQ] | 98 | 3400 | 5000 | 1.47 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.64 |
| 5 | [N3333]2 [HQ] | 91 | 2800 | 4400 | 1.57 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.66 |
| 6 | [N4444]2 [HQ] | 86 | 2700 | 4200 | 1.56 | 9100 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.59 |
| 7 | [N1111] [PH] | 78 | 2200 | 3800 | 1.73 | 7400 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.84 |
| 8b | [N1111] [PH] | 91 | 2800 | 4500 | 1.61 | 9600 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.75 |
The conversion of ISB and molecular weight of PIC
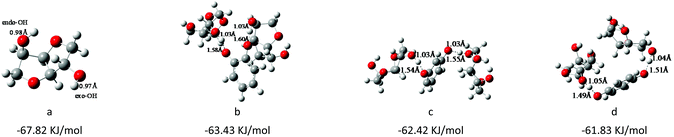
As we have previously reported,28,45,46 the interaction energy of cations and anions of the catalyst can significantly influence the catalytic performance, and as is well known, there are many factors that affect the interaction energy between cations and anions in ionic liquid catalysts, such as the species and steric structure of cations and anions, and so on.36–38 In this work, we focused on studying how the steric structure of cations and anions can affect the catalytic activity, and the results of catalyst screening are summarized in Table 1. Firstly, we changed the substituted positions of two O− anions on the benzene ring from ortho-, meta-, to para-, and investigated the effect of steric hindrance of anions on the catalytic activity. The order of the catalytic activity of anions was found to be HQ > RE > PY (Table 1, entries 1–3); the results indicated that the steric hindrance of the catalyst could significantly influence its catalytic performance. In order to further verify this conjecture, as shown in Table 2, DFT calculations were employed to further investigate the reasons for the different catalytic activity (using the Gaussian 09 program; the details are described in the Characterization section). It was found that when the steric hindrance of active sites decreased from ortho- to para-, the interaction energy of cations and anions of the catalyst turned to be weakened (Table 2, entries a–c); however, the conversion rate of ISB gradually increased from 91% to 98%. The reason may be that the weak interaction energy of cations and anions of the catalyst facilitated the separation of cations and anions, which was conducive to the activation of functional groups of corresponding substrates.In addition, the results of DFT calculations in Table 3 further illustrated the interactions between ISB and different anions, the same as other studies,11,28 the OH of ISB was activated and new hydrogen bonds formed between the OH of ISB and O− of different anions. The order of the hydrogen bond lengths was PY > RE > HQ, which showed that the ability to activate the ISB increased with decreasing the steric hindrance of anions. The interaction between HQ and OH of ISB was strongest, which was strikingly consistent with catalytic experiments. Therefore, the small steric hindrance of anions was advantageous for ionic liquid catalysis in polymer synthesis reactions.28
Secondly, we selected the HQ as the optimal anion, and changed the cations of ionic liquid catalysts by gradually increasing the alkyl chain length to further investigate the effect of the steric structure of cations on the catalytic performance. It was found that the activity of catalysts was gradually decreased from 98% to 86% when the chain length of cations increased from [N1111] to [N4444] (Table 1, entries 3–6). According to previous studies,27–29 DMC could be activated by electrophilic cations, so we also employed the DFT calculation to investigate the interaction between DMC and cations of catalysts. The results showed that the interaction energy decreased regularly with increasing the alkyl chain length of cations (Table 4), which illustrated that the ability to activate the DMC gradually increased with decreasing the steric hindrance of cations; the DFT results were consistent with the experimental results. Finally, we also synthesized [N1111] [PH] with only a single active site for comparison; the result showed that the activity of the catalyst was significantly reduced when the single-site catalyst dosage was the same as the dual-site IL catalyst (Table 1, entry 7 vs. 3). Moreover, the results showed that the catalytic activity did not depend on the simple superposition of the number of active sites, which further verified the advantage of dual active site-functionalized ionic liquid catalysts (Table 1, entry 8 vs. 3).
The terminal groups of the oligomers and PIC
The chemical structures and terminal groups of the oligomer and PIC were investigated to further seek the reasons for the differences in the activities of different catalysts. Firstly, we characterized the chemical structures and terminal groups of the oligomer and PIC by 1H and 13C NMR, as represented in Fig. 1 and S16.† It was found that the ratio of endo-CH3/exo-CH3 was less than 1 in the oligomers, and the ratio of endo-OH/exo-OH was more than 1; the results revealed that the reactivity of endo-OH of ISB was lower (Table 5, entries 1–6).23,24 Moreover, the content of the methylation product decreased when the chain length of cations increased from [N1111] to [N4444]; especially, a PIC with the highest Mw of 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 was obtained by the [N2222]2 [HQ] catalyst, and the content of the methylation product was significantly lower than that of the other catalysts, which further indicated that the methylation side reaction of ISB could significantly inhibit the chain growth (Table S3,† entries 1–4).10,28,29
300 was obtained by the [N2222]2 [HQ] catalyst, and the content of the methylation product was significantly lower than that of the other catalysts, which further indicated that the methylation side reaction of ISB could significantly inhibit the chain growth (Table S3,† entries 1–4).10,28,29
| Entry | Catalyst | Oligomer terminal groupsb | PIC terminal groupsb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Methylation | endo-CH3/exo-CH3 | –OH | endo-OH/exo-OH | Methylation | endo-CH3/exo-CH3 | –OH | endo-OH/exo-OH | ||
a (1) Transesterification: 101 kPa, (160 °C, 60 min)–(180 °C, 60 min). (2) Polycondensation: 5 Pa, 230 °C, 60 min, n(DMC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(ISB) = 7 n(ISB) = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and catalyst amount of 8 × 10−3 mol based on ISB.
b The peak integration of the proton from ISB in the repeating unit (peak 3 at δ 4.88 ppm in Fig. 1) was normalized to be 1, and the contents of other terminal groups were calculated on this basis. 1, and catalyst amount of 8 × 10−3 mol based on ISB.
b The peak integration of the proton from ISB in the repeating unit (peak 3 at δ 4.88 ppm in Fig. 1) was normalized to be 1, and the contents of other terminal groups were calculated on this basis.
|
|||||||||
| 1 | [N1111]2 [PY] | 0.332 | 0.96 | 0.119 | 1.29 | 0.267 | 1.21 | 0.035 | 1.33 |
| 2 | [N1111]2 [RE] | 0.256 | 0.90 | 0.073 | 1.28 | 0.249 | 1.11 | 0.026 | 1.17 |
| 3 | [N1111]2 [HQ] | 0.197 | 0.96 | 0.060 | 1.22 | 0.235 | 1.53 | 0.077 | 1.41 |
| 4 | [N2222]2 [HQ] | 0.057 | 0.84 | 0.186 | 1.33 | 0.018 | 1.57 | 0.071 | 1.29 |
| 5 | [N3333]2 [HQ] | 0.129 | 0.71 | 0.207 | 1.39 | 0.047 | 1.47 | 0.050 | 1.38 |
| 6 | [N4444]2 [HQ] | 0.154 | 0.75 | 0.242 | 1.35 | 0.078 | 1.84 | 0.077 | 1.41 |
In view of the excellent catalytic performance of [N2222]2 [HQ], we employed in situ NMR spectroscopy for full-scale and in-depth investigation of the process of melt polycondensation of ISB and DMC (Table 6). The results showed that the methylation product was synthesized in the transesterification stage, and the content of methylation products gradually decreased and tended to be stable when the reaction continued to the polycondensation stage, which may be the reason that some of the small molecular methylation products were extracted in the vacuum during the polycondensation reaction.
| Entry | Catalyst | Stage | Time (h) | Terminal groupsb | |||
|---|---|---|---|---|---|---|---|
| Methylation | endo-CH3/exo-CH3 | –OH | endo-OH/exo-OH | ||||
a (1) Transesterification: 101 kPa, 160–180 °C. (2) Polycondensation: 5 Pa, 230 °C, n(DMC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(ISB) = 7 n(ISB) = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and catalyst amount of 8 × 10−3 mol based on ISB.
b The peak integration of the proton from isosorbide in the repeating unit (peak 3 at δ 4.88 ppm in Fig. 1) was normalized to be 1, and the contents of other terminal groups were calculated on this basis. 1, and catalyst amount of 8 × 10−3 mol based on ISB.
b The peak integration of the proton from isosorbide in the repeating unit (peak 3 at δ 4.88 ppm in Fig. 1) was normalized to be 1, and the contents of other terminal groups were calculated on this basis.
|
|||||||
| 1 |
|
Transesterification | 1 | 0.080 | 0.51 | 0.659 | 1.12 |
| 2 | 2 | 0.057 | 0.84 | 0.186 | 1.33 | ||
| 3 | Polycondensation | 1 | 0.018 | 1.57 | 0.071 | 1.29 | |
| 4 | 2 | 0.015 | 1.50 | 0.045 | 1.65 | ||
| 5 | 3 | 0.016 | 1.60 | 0.035 | 1.50 | ||
Effect of reaction parameters
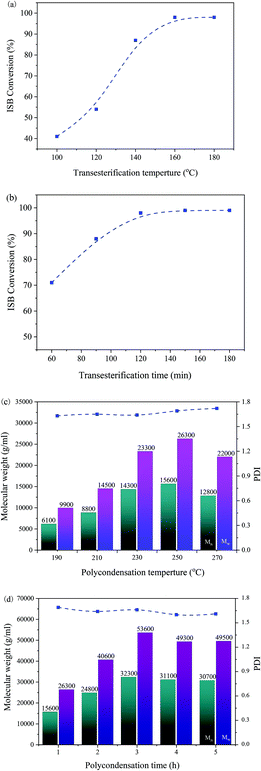
We selected the [N2222]2 [HQ] with a better catalytic activity as a catalyst to further find the optimal conditions of the reaction. As shown in Fig. 2, the influence of reaction conditions including time and temperature of the two reaction stages was investigated.The effect of the temperature on the transesterification reaction stage is depicted in Fig. 2(a); the results showed that the temperature had an outstanding influence on the reaction. The ISB conversion was improved from 41% to nearly 99% when the temperature increased from 100 to 180 °C. When the reaction temperature was increased to 160 °C, the conversion curve of ISB tended to be stable, possibly because trace amounts of ISB were challenging to convert; thus, the optimal transesterification reaction temperature should be 160 °C.
As illustrated in Fig. 2(b), the dependence of the ISB conversion on the reaction time of the transesterification reaction stage was explored. The results showed that the reaction time had a pronounced positive effect; the ISB conversion rate was fast in the initial stage, and remained almost invariant after 120 min. Thereby, the optimum reaction time was 120 min in the transesterification reaction stage.
As shown in Fig. 2(c), the reaction temperature of the polycondensation stage was also evaluated, and the results showed that the Mw of the PIC reached up to 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 when the polycondensation temperature was up to 250 °C; this could be explained that higher temperature facilitated the removal of small molecules and reduced the viscosity of the polymer, which would accelerate the polymerization reaction.41 However, the Mw of PIC declined a little when the reaction temperature continued to increase up to 270 °C; the reason should be that high temperature accelerated the thermal degradation of PIC.29,41 Therefore, the optimum reaction temperature of the polycondensation stage was 250 °C for PIC synthesis.
300 when the polycondensation temperature was up to 250 °C; this could be explained that higher temperature facilitated the removal of small molecules and reduced the viscosity of the polymer, which would accelerate the polymerization reaction.41 However, the Mw of PIC declined a little when the reaction temperature continued to increase up to 270 °C; the reason should be that high temperature accelerated the thermal degradation of PIC.29,41 Therefore, the optimum reaction temperature of the polycondensation stage was 250 °C for PIC synthesis.
Finally, the effect of reaction time on the polymerization stage was investigated, as shown in Fig. 2(d). The polymerization reaction time showed a positive impact on the Mw values of PIC, which increased quickly with the polymerization time increasing from 1 to 3 h; the maximum Mw value of 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 could be achieved when the time was 3 h. However, when the time was further increased from 3 to 5 h, the Mw value of PIC slightly decreased, which may be owing to that high melt viscosity hindered the polymer chain growth and the ongoing competing reaction of the thermal degradation of PIC.10,22 Therefore, the reaction time of 3 h was the optimum time in the polycondensation stage.
600 could be achieved when the time was 3 h. However, when the time was further increased from 3 to 5 h, the Mw value of PIC slightly decreased, which may be owing to that high melt viscosity hindered the polymer chain growth and the ongoing competing reaction of the thermal degradation of PIC.10,22 Therefore, the reaction time of 3 h was the optimum time in the polycondensation stage.
Possible reaction mechanism
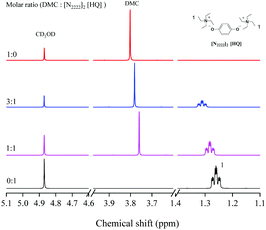
As shown in Scheme 5, according to the previous reports and the results of various analysis means including NMR spectroscopy, DFT calculations and MS, a possible mechanism for the synergetic catalysis of cations–anions was proposed.27–29,41 Taking into account the complexity of the structure of the dual site-functionalized ionic liquid catalyst, we used a single anion and cation to simplify the catalyst and described the catalytic mechanism according to other literature studies.36–38The interaction between the DMC and the [N2222]2 [HQ] catalyst was captured by 1H NMR as shown in Fig. 3; the proton signals of [HQ] and DMC distinctly shifted when increasing the molar ratio of DMC to [N2222]2 [HQ] from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 3
0 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating that the cation of the catalyst could activate DMC. As similarly mentioned in other literature studies,26–28 the cation of [N2222] could activate the carbonyl group of DMC and form a weaker interaction with the C
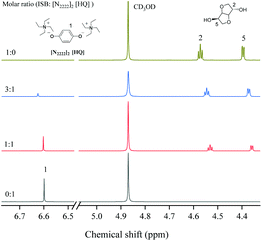
1, indicating that the cation of the catalyst could activate DMC. As similarly mentioned in other literature studies,26–28 the cation of [N2222] could activate the carbonyl group of DMC and form a weaker interaction with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of DMC (step 1). Moreover, the results of DFT calculation illustrated that DMC could be activated by different cations of the catalyst, and the interaction energy changed regularly with the increase of the alkyl chain length of cations (Table 4). In addition, the O atom of the [HQ] anion was suggested to activate the OH of ISB through an intermolecular hydrogen bond (step 1);29,41 the results of DFT calculation in Table 3 have illustrated hydrogen bond formation between the OH of ISB and anion, and the 1H NMR chemical shift of the ISB and catalyst mixture further verified the inference (Fig. 4).
O group of DMC (step 1). Moreover, the results of DFT calculation illustrated that DMC could be activated by different cations of the catalyst, and the interaction energy changed regularly with the increase of the alkyl chain length of cations (Table 4). In addition, the O atom of the [HQ] anion was suggested to activate the OH of ISB through an intermolecular hydrogen bond (step 1);29,41 the results of DFT calculation in Table 3 have illustrated hydrogen bond formation between the OH of ISB and anion, and the 1H NMR chemical shift of the ISB and catalyst mixture further verified the inference (Fig. 4).
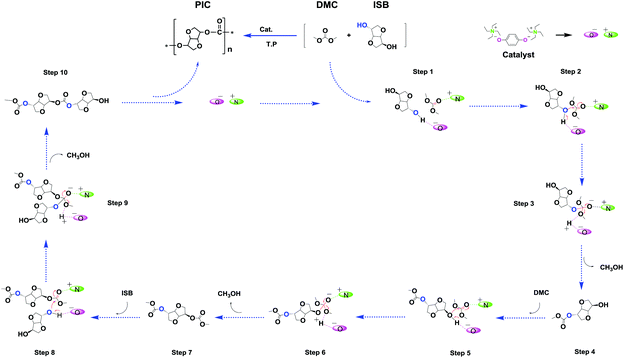
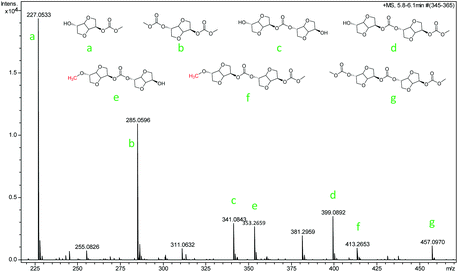
The ISB and DMC were activated by the anion and cation of the catalyst respectively (step 1); thereafter, along with the transfer of electrons, the transesterification took place, and formed an unstable complex intermediate state (steps 2 and, 3). Consequently, the mono-carboxymethyl ISB intermediates and methanol were synthesized by the reaction of ISB with DMC (step 4), similarly to that reported in other literature studies.28–30,41 As shown in Fig. 5, the key intermediates in the transesterification stage were detected by using ESI-MS, and the results could further verify the above conjecture and pointed out the direction for the next reaction. Meanwhile, the content of the structures e and f was less than that of a, b, c, d and g; the trend might manifest that the ISB methylation reaction was suppressed and the remaining hydroxyl groups of ISB were more prone to carboxymethylation; therefore, this suggested that the next steps of steps 5 and 6 and steps 8 and 9 were supposed to preferentially repeat the steps 2 and 3;28,41 especially, the structures b (step 7), d (step 10) and g were detected to further verify this deduction.
The small molecules including methylation and methanol products were continuously removed in the polycondensation vacuum stage, and as the reaction repeated, a high molecular weight PIC was obtained. In order to further obtain the detailed information of the polymer terminal groups, some intermediate oligomer structure was detected by MALDI-TOF-Ms in this work. As shown in Fig. S17,† the results showed that the content of carboxymethylation product a and intermediate c was much more than that of methylation product b. This may be the reason that the methylation reaction inhibited the chain growth, which was consistent with the experimental results that the content of carboxymethylation products was much larger than that of methylation products.
Conclusions
In summary, a series of dual site-functionalized ionic liquid catalysts were designed and synthesized for the first time, which exhibited excellent catalytic activity for bio-based polycarbonate synthesis via one-step direct melt polycondensation of ISB and DMC. Noteworthily, the steric structure of the catalyst could significantly influence the catalytic performance in polymer synthesis reactions; the ability of the catalyst to activate the reaction substrate gradually increased with decreasing steric hindrance of cations and anions. Moreover, based on NMR analysis and DFT calculations, we discovered that the OH of ISB was activated and new hydrogen bonds formed between the OH of ISB and O− of different anions, and the ability to activate the ISB increased with decreasing the steric hindrance of anions, especially for the catalyst of [N2222]2 [HQ], which showed the most brilliant catalytic efficiency. In addition, a possible reaction mechanism for the synergetic effect of cations–anions of ionic liquid catalysts was proposed, which provides guidance for developing other dual site ionic liquid catalysts to synthesize higher molecular weight polycarbonates.Experimental section
Materials
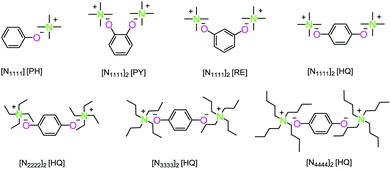
Dimethyl carbonate (99%), isosorbide (98%), phenol (PH, 99.5%), pyrocatechol (PY, 99%), resorcinol (RE, 99%), hydroquinone (HQ, 99%), tetramethylammonium hydroxide solution ([N1111]OH, 25 wt% in H2O), tetraethylammonium hydroxide solution ([N2222]OH, 25 wt% in H2O), tetrapropylammonium hydroxide solution ([N3333]OH, 25 wt% in H2O), and tetrabutylammonium hydroxide solution ([N4444]OH, 40 wt% in MeOH) were bought from Aladdin Co. Ltd. Isosorbide was purified by recrystallization four times in acetone, and the other chemicals were used without any further purification.11,12Synthesis of dual site-functionalized ionic liquid catalysts
The dual site-functionalized ionic liquid catalysts were designed and synthesized through the acid–base neutralization reaction (Scheme 4).42–44 A typical synthetic procedure of dual site-functionalized ionic liquids was performed as follows. Taking bis-tetraethylammonium hydroquinone ([N2222]2 [HQ]) as an example, the hydroquinone (1.32 g, 0.012 mol) was dissolved in 25 mL ethyl alcohol, and then tetraethylammonium hydroxide solution (25 wt% in H2O) (11.76 g, 0.02 mol) was dropped into the hydroquinone solution and stirred at room temperature for 24 h under a nitrogen atmosphere. After removing water and ethyl alcohol by distillation, the ionic liquid [N2222]2 [HQ] was obtained and dried under vacuum for 48 h at 50 °C.The synthetic method for bis-tetramethylammonium pyrocatechol ([N1111]2 [PY]), bis-tetramethylammonium resorcinol ([N1111]2 [RE]), bis-tetramethylammonium hydroquinone ([N1111]2 [HQ]), bis-tetrapropylammonium hydroquinone ([N3333]2 [HQ]), bis-tetrabutylammonium hydroquinone ([N4444]2 [HQ]), and tetramethylammonium phenol ([N1111] [PH]) was the same as that of [N2222]2 [HQ]. The characterization results of all the ionic liquids are shown in Fig. S1–S14,† and the TGA curves of ionic liquids are shown in Fig. S15.† All of them exhibited good thermal stability.
Preparation of poly(isosorbide carbonate)
The PIC was synthesized via a one-pot melt polycondensation method in a 250 mL four-necked flask with a mechanical stirrer, a reflux condenser, and a nitrogen inlet. The first transesterification step was proceeded under a nitrogen atmosphere. ISB (14.6 g, 0.1 mol), DMC (63.0 g, 0.7 mol), and catalyst (8 × 10−4 mol) were heated at 160 °C for 60 minutes. The temperature of the distillation head was controlled at about 35 °C by adjusting the cooling water to prevent DMC volatilization. Then, the temperature was increased to 180 °C to distill off the residual DMC and the generated methyl alcohol for 60 min. The second stage is polycondensation; the temperature was gradually increased to 230 °C and kept constant for 60 minutes under a vacuum atmosphere. Finally, the PIC samples were dissolved in dichloromethane, precipitated in methanol, and then vacuum-dried at 70 °C for 24 h for further characterization.10–12 Similar to other articles,9–13 the catalyst was not separated and recycled.Characterization
1H NMR and 13C NMR spectra were recorded in deuterated methanol (CD3OD) and deuterated water (D2O) with tetramethylsilane as the internal standard on a Bruker AVANCE III 600 MHz. The weight average molecular weight (MW), number-average molecular weight (Mn) and their distribution (PDI = Mw/Mn) were determined by Gel Permeation Chromatography (GPC) performed on an Agilent PL-GPC 50 device. Dimethyl formamide (DMF) was used as the eluent with a flow rate of 1.0 mL min−1 at 40 °C and polystyrene was used as the standard to establish a calibration curve. MS analysis was performed on an AXIMA-Performance matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF MS) (Shimadzu, Japan) fitted with a pulsed nitrogen laser (337 nm) and 2,5-dihydroxybenzoic acid solution was used as the MALDI matrix, and electron spray ionization mass spectrometry was performed with a Bruker micro TOF-QII spectrometer (Germany). Thermo gravimetric analysis was conducted with a DTG-60 (Shimadzu, Japan) from 30 to 600 °C at a rate of 10 °C min−1 under a nitrogen atmosphere. All DFT calculations were performed by using the Gaussian 09 program Revision D.01. The geometry optimizations were carried with the B3LYP functional in conjunction with the 6-31G* basis set.47,48Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21878316), Science Fund for Creative Research Groups of the National Natural Science Foundation of China (21921005), Strategic Foundation of Chinese Academy of Sciences (CXJJ-19-B01), “Transformational Technologies for Clean Energy and Demonstration”, Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA21031000), and International Partnership Program of Chinese Academy of Sciences (No. 122111KYSB20190029).Notes and references
- J. B. Zimmerman and P. T. Anastas, Science, 2015, 347, 1198–1199 CrossRef CAS.
- X.-Y. Song, W.-Y. Hu, W.-W. Huang, H. Wang, S. Yan, S. Yu and F.-S. Liu, Chem. Eng. J., 2020, 388, 124324 CrossRef.
- J.-L. Song, H.-L. Fan, J. Ma and B.-X. Han, Green Chem., 2013, 15, 2619–2635 RSC.
- M. Selva, A. Perosa, D. Rodríguez-Padrón and R. Luque, ACS Sustainable Chem. Eng., 2019, 7, 6471–6479 CrossRef CAS.
- Z.-R. Zhang, J.-L. Song and B.-X. Han, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS.
- O. Hauenstein, M. Reiter, S. Agarwal, B. Rieger and A. Greiner, Green Chem., 2016, 18, 760–770 RSC.
- M. Winkler, C. Romain, M. A. R. Meier and C. K. Williams, Green Chem., 2014, 17, 300–306 RSC.
- F. H. Isikgora and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- M. Zhang, W.-Q. Lai, L. Su and G.-Z. Wu, Ind. Eng. Chem. Res., 2018, 57, 4824–4831 CrossRef CAS.
- Q. Li, W.-X. Zhu, C.-C. Li, G.-H. Guan, D. Zhang, Y. Xiao and L.-C. Zheng, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1387–1397 CrossRef CAS.
- Z.-C. Zhang, F. Xu, Z.-X. Li and S.-J. Zhang, Green Chem., 2019, 21, 3891–3901 RSC.
- C.-K. Ma, F. Xu, W.-G. Cheng, X. Tan, Q. Su and S.-J. Zhang, ACS Sustainable Chem. Eng., 2018, 6, 2684–2693 CrossRef CAS.
- W.-Q. Lai, L. Su, M. Zhang, J. Yan and G.-Z. Wu, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 1670–1681 CrossRef CAS.
- Y.-R. Ma, X.-Z. He, Y.-H. Cong, B. Zhang, Z. Liu, J.-W. Wang and Y.-G. Jia, Liq. Cryst., 2019, 4, 1–13 Search PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2011, 13, 520–540 RSC.
- R. M. Almeida, J. Li, C. Nederlof, P. O'Connor, M. Makkee and J. A. Moulijn, ChemSusChem, 2010, 3, 325–328 CrossRef.
- J. A. Galbis, M. de G. G. Martín and E. Galbis, Chem. Rev., 2015, 116, 1600–1636 CrossRef.
- X.-L. Shen, Z.-Q. Wang, Q.-Y. Wang and G.-Y. Wang, Chin. J. Polym. Sci., 2018, 36, 1027–1035 CrossRef CAS.
- Y. S. Eo, H. W. Rhee and S. Shin, J. Ind. Eng. Chem., 2016, 37, 42–46 CrossRef CAS.
- W. Sun, F. Xu, W.-G. Cheng, J. Sun, G. Ning and S.-J. Zhang, Chin. J. Catal., 2017, 38, 908–917 CrossRef CAS.
- G. Fiorani, A. Perosa and M. Selva, Green Chem., 2018, 20, 288–322 RSC.
- F. Aricò, S. Evaristo and P. Tundo, Green Chem., 2015, 17, 1176–1185 RSC.
- P. Tundo and M. Selva, Acc. Chem. Res., 2002, 35, 706–716 CrossRef CAS.
- A. Caretto and A. Perosa, ACS Sustainable Chem. Eng., 2013, 1, 989–994 CrossRef CAS.
- H.-Z. An, Z.-F. Yang, K.-L. Bi, F. Xu, C.-H. Li, W.-J. Fang, Z.-C. Zhang and S.-J. Zhang, Ind. Eng. Chem. Res., 2020, 59, 13948–13955 CrossRef CAS.
- Z.-C. Zhang, F. Xu, Y.-Q. Zhang, C.-H. Li, H.-Y. He, Z.-F. Yang and Z.-X. Li, Green Chem., 2020, 22, 2534–2542 RSC.
- Z.-F. Yang, L. Liu, H.-Z. An, F. Xu and S.-J. Zhang, ACS Sustainable Chem. Eng., 2020, 8, 9968–9979 CrossRef CAS.
- W. Qian, L. Liu, Z.-L. Zhang, Q. Su, W.-G. Cheng and S.-J. Zhang, Green Chem., 2020, 22, 2488–2497 RSC.
- W.-J. Fang, Z.-C. Zhang, Z.-F. Yang, F. Xu and S.-J. Zhang, Green Chem., 2020, 22, 4550–4560 RSC.
- F. Aricò and P. Tundo, Beilstein J. Org. Chem., 2016, 12, 2256–2266 CrossRef.
- F. Arick, A. S. Aldoshin and P. Tundo, ChemSusChem, 2017, 10, 53–57 CrossRef.
- M. Rose and R. Palkovits, ChemSusChem, 2012, 5, 167–176 CrossRef CAS.
- P. Tundo, F. Aric, G. Gauthier, L. Rossi, R. L. Sievertand and C. P. Newman, ChemSusChem, 2010, 3, 566–570 CrossRef CAS.
- W. Qian, Q. Su, W.-G. Cheng, F. Xu and S.-J. Zhang, ChemSusChem, 2019, 12, 1169–1178 CrossRef CAS.
- G.-K. Cui, J.-J. Wang and S.-J. Zhang, Chem. Soc. Rev., 2016, 45, 4307–4339 RSC.
- W.-J. Qian, J. Texter and F. Yan, Chem. Soc. Rev., 2017, 46, 1124–1159 RSC.
- Z.-R. Zhang, J.-L. Song and B.-X. Han, Chem. Rev., 2016, 117, 6834–6880 CrossRef.
- R. Hayes, G. G. Warr and R. Atkin, Chem. Rev., 2015, 115, 6357–6426 CrossRef CAS.
- G. Chatel, J. F. B. Pereira, V. Debbeti, H. Wang and R. D. Rogers, Green Chem., 2014, 16, 2051–2083 RSC.
- W. Qian, X.-F. Ma, L. Liu, L. Deng, R.-B. Bai, Z.-L. Zhang, W.-G. Cheng and F. Xu, Green Chem., 2020, 22, 5357–5368 RSC.
- B. Yu, H. Zhang, Y. Zhao, S. Chen, J. Xu, L. Hao and Z. Liu, ACS Catal., 2013, 3, 2076–2082 CrossRef CAS.
- K. Fukumoto, M. Yoshizawa and H. Ohno, J. Am. Chem. Soc., 2005, 127, 2398–2399 CrossRef CAS.
- C.-M. Wang, H. Luo, H.-R. Li, X. Zhu, B. Yu and S. Dai, Chem. – Eur. J., 2012, 18, 2153–2160 CrossRef CAS.
- M. E. Wilhelm, M. H. Anthofer, M. Cokoja, I. I. E. Markovits, W. A. Herrmann and F. E. Kühn, ChemSusChem, 2014, 7, 1357–1360 CrossRef CAS.
- V. Calo, A. Nacci, A. Monopoli and A. Fanizzi, Org. Lett., 2002, 4, 2561–2563 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- T. Clark, J. Chandrasekhar, G. W. Spitznagel and P. V. R. Schleyer, Autumn (Fall), 1983, 294–301 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The structures properties of oligomer and PIC; detailed characterizations; the 1H and 13C NMR spectrum of different catalysts; TGA curves of ionic liquids, etc. See DOI: 10.1039/d0gc03247f |
| This journal is © The Royal Society of Chemistry 2021 |