Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
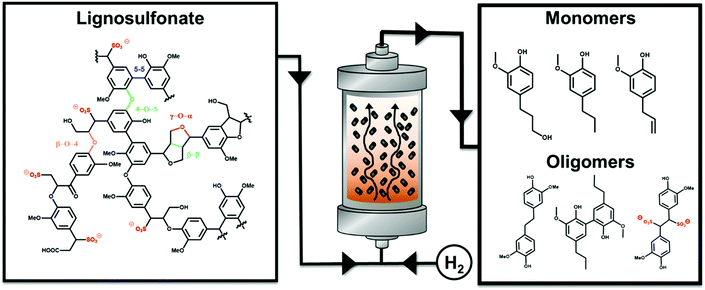
Controlled lignosulfonate depolymerization via solvothermal fragmentation coupled with catalytic hydrogenolysis/hydrogenation in a continuous flow reactor†
Francesco
Brandi
 ,
Markus
Antonietti
,
Markus
Antonietti
 and
Majd
Al-Naji
and
Majd
Al-Naji
 *
*
Max Planck Institute of Colloids and Interfaces, Colloid Chemistry Department, Am Mühlenberg 1, 14476 Potsdam, Germany. E-mail: majd.al-naji@mpikg.mpg.de
First published on 10th September 2021
Abstract
Sodium lignosulfonate (LS) was valorized to low molecular weight (Mw) fractions by combining solvothermal (SF) and catalytic hydrogenolysis/hydrogenation fragmentation (SHF) in a continuous flow system. This was achieved in either alcohol/H2O (EtOH/H2O or MeOH/H2O) or H2O as a solvent and Ni on nitrogen-doped carbon as a catalyst. The tunability according to the temperature of both SF and catalytic SHF of LS has been separately investigated at 150 °C, 200 °C, and 250 °C. In SF, the minimal Mw was 2994 g mol−1 at 250 °C with a dispersity (Đ) of 5.3 using MeOH/H2O. In catalytic SHF using MeOH/H2O, extremely low Mw was found (433 mg gLS−1) with a Đ of 1.2 combined with 34 mg gLS−1. The monomer yield was improved to 42 mg gLS−1 using dual catalytic beds. These results provide direct evidence that lignin is an unstable polymer at elevated temperatures and could be efficiently deconstructed under hydrothermal conditions with and without a catalyst.
Introduction
In the last few centuries, extensive exploitation of fossil resources has led to the current global environmental challenges that we are facing.1–4 A transition toward more sustainable and renewable resources and production processes for energy and commodities is in great demand.5–11 Among the renewable resources, lignocellulosic biomass (LCB) is one of the most promising feedstock since it is abundant, cheap, and intrinsically sustainable as it is produced via photosynthesis by plants.12–14 LCB is a complex solid composite, made of three biopolymers: cellulose, hemicellulose, and lignin.13,15,16 Currently, LCB is industrially processed on the Gt scale per year, and about 4 Gt of wood products are made every year, with about 1 Gt ending in pulp and paper factories.16,17 Within the scope of the current article, it is important to note that only the sugar fractions are valorized, e.g. as pulp. The remaining lignin (which can be isolated nowadays as a by-product) is used in some product streams with lower capacity, but is mostly used for energy and heat generation via combustion.18–22 Moving toward a more sustainable and mature biorefinery is a broadly proclaimed target, and is based on efficient valorization of all LCB components, including lignin.23–28 In 2007, the U.S. Department of Energy (DoE) report posed a milestone for lignin valorization and defined the bio-economy of lignin.29 Accordingly, in the report three major valorization scenarios for lignin were defined with respect to their usage in the future: 1st combustion for energy purposes (short term), 2nd application of lignin as a macromolecule (middle term), and 3rd application of lignin as a source of aromatic monomers and oligomers (long term).Lignin is a complex three-dimensional network of cross-linked phenylpropanoid units connected through different types of ether bonds, i.e., β–O–4, 4–O–5, γ–O–α, and carbon–carbon bonds, e.g., 5–5 and β–β linkages, viz.Fig. 1.15,30–34 Some of the big challenges in lignin valorization are its heterogeneity, recalcitrance against treatments, and the sensitivity of lignin fragments for follow-up condensation reactions.12,30 Currently, four main technologies are used to separate lignin from the other biomass components, i.e., kraft, soda, organosolv and sulfite processes.21,30,35,36 These processes rely on lignin separation from the LCB matrix via solubilization by applying harsh conditions with pH = 1–5 for sulfite and organosolv or pH = 11–13 for soda and kraft, as well as high temperatures (140–220 °C).21,37 The resulting lignin is named technical lignin and is indeed different from the primary plant product.21,37 In the DoE Report, the technical lignin is proposed as the most probable future source of lignin since it is abundant and already available from industrial processes, such as the pulping process.29 Nowadays, the 1st scenario is still the major application of technical lignin and every year, from the principal 250 Mt, about ∼70 Mt of technical lignin is separated, of which an estimated 95% is burned to produce energy.38,39 Of that amount, approximately 2 Mt per y are produced only by the sulfite process.21,38
In the sulfite pulping, debarked wood chips are treated with sulfite and bisulfite salt solution (Na is the major counter-ion to bisulfite) in acidic aqueous media (pH 1–5) at temperatures ranging from 125 to 190 °C.21,40 In this process, the ether bonds in lignin are partially hydrolyzed followed by the sulfitization step (introducing –SO3− functionalities), or oxidative coupling to form parasitic C–C bonds.21,29 Consequently, the resulting technical lignin is named sodium lignosulfonate (LS). It possesses a wide range of molecular weight (Mw) from 1000 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, the obtained Mw values depend on process severity.21 LS contains a large amount of sulfur (3 to 8 wt%) and a reduced number of C–O bonds (Fig. 1) compared to the native lignin in wood.30 Uniquely, LS is highly water-soluble due to the presence of SO3− groups. Sulfonate endows LS with surfactant properties and it is used in current materials applications as a macromolecule (2nd scenario), e.g., as superplasticizer in cements and clays,41 and as dispersing agents for polymeric foams,42,43 dyes44 and colloidal dispersions.45,46 Additionally, LS has been used to create completely new lignin-based materials, e.g., bio-composites for fire-retardant thermal insulators,47,48 or S-doped carbonaceous materials for high-performance electrochemical devices such as batteries and supercapacitors.47,49,50 All the abovementioned applications have been reported to depend strongly on the molecular weight distribution (Mw) of the obtained LS.20,48,51
000 g mol−1, the obtained Mw values depend on process severity.21 LS contains a large amount of sulfur (3 to 8 wt%) and a reduced number of C–O bonds (Fig. 1) compared to the native lignin in wood.30 Uniquely, LS is highly water-soluble due to the presence of SO3− groups. Sulfonate endows LS with surfactant properties and it is used in current materials applications as a macromolecule (2nd scenario), e.g., as superplasticizer in cements and clays,41 and as dispersing agents for polymeric foams,42,43 dyes44 and colloidal dispersions.45,46 Additionally, LS has been used to create completely new lignin-based materials, e.g., bio-composites for fire-retardant thermal insulators,47,48 or S-doped carbonaceous materials for high-performance electrochemical devices such as batteries and supercapacitors.47,49,50 All the abovementioned applications have been reported to depend strongly on the molecular weight distribution (Mw) of the obtained LS.20,48,51
As LS has a high phenolic content, it also shows great potential for the production of bio-based aromatics via oxidative or reductive catalytic fractionation (O or RCF) and is considered the 3rd scenario.45,52–56 In the last decade, RCF, pioneered by the Sels group, has been reported as one of the methods with the highest potential for lignin valorization towards phenolic single units, mostly starting directly from wood.12,23,28,57–62 Based on this process, an approach for the valorisation of lignin toward both phenolic monomers and phenolic oligomers has been proposed to scale up such a process to industry.23,63 The simultaneous valorization of monomers and dimers in such an approach has the potential to provide a further step toward the complete usage of lignin in the biorefinery.
Despite the potential of RCF, few studies have been reported using LS solutions. To this end, Shu et al.53 reported LS hydrogenolysis using a Pt on a carbon catalyst and CrCl3 homogeneous co-catalyst at 280 °C, 3 MPa of H2, in batch systems. Additionally, the effect of a Ni and Mo bimetallic catalyst on Al2O3 was studied in ethylene glycol and supercritical ethanol at 310 °C and 26 bar of H2 in batch systems.64,65 This approach led to LS fragmentation mostly towards dimers and oligomers resulting in an optimized 88 wt% oil yield, while the minor fraction of monomers was only qualitatively studied.64,65 All these studies were performed in batch systems, which present disadvantages when compared to continuous flow systems, e.g., complex product–catalyst separation, time and energy-consumption in discontinuous steps and cost efficiency.10,12,59,66,67 Horáček et al.52 reported only the LS fragmentation in a continuous flow system at a high reaction temperature (320 °C) over a bimetallic Ni and Mo catalyst supported on Al2O3. In this study and due to over hydrogenation, guaiacol was found as the major product of LS catalytic fragmentation (yield higher than 1.8 wt%).
Herein, we investigate the LS depolymerization in a continuous flow system under mild reaction conditions using the water/alcohol solvent system. In addition, the effect of catalyst-free solvothermal fragmentation (SF) of LS to low Mw fractions and phenolic monomers was explored. Furthermore, the latter step was coupled to hydrogenolysis/hydrogenation fragmentation (SHF) in a continuous flow system using the water/alcohol solvent and 35 wt% Ni supported on NDC as a catalyst.68,69 Finally, the advantage of operating the catalytic depolymerization of LS in a continuous flow system is highlighted with respect to the batch system.
Experimental section
Materials
All the materials, including sodium lignosulfonate (LS), were utilized as received without further purification. The complete list of chemicals used, suppliers, and purities can be found in section S1 of the ESI.†Catalyst synthesis and characterization
A pelletized catalyst of 35 wt% Ni deposited on nitrogen-doped carbon (35Ni/NDC) has been synthesized and characterized following the “kitchen lab” approach, as previously reported by our group, cf. sections S2 and S3 in the ESI.†68 The catalyst was characterized via combustion elemental analysis (EA), inductively coupled plasma (ICP) optical emission spectroscopy elemental analysis, N2 physisorption, powder X-ray diffraction (XRD), thermogravimetric analysis (TGA), high-resolution scanning transmission electron microscopy (HR-STEM), and energy-dispersive X-ray spectrometry (EDS) and CO-chemisorption. All procedures of catalyst characterization are described in detail in section S3 in the ESI.†Continuous flow setup
Liquid-phase LS depolymerization using water/alcohol solvent mixtures was conducted in a continuous flow fixed bed reactor, similar to our previously described system (Fig. S2 in the ESI†).68–70This system consists of: (A) an HPLC pump equipped with a pressure sensor (Knauer Azura P 4.1S Series), (B) a mass-flow controller to supply a specific H2 flow (Model SLA58050 from Brooks), (C) a T-piece for the gas–liquid mixer (Swagelok SS-400-30) to mix the supplied H2 with the reaction solution before reaching the pre-heating unit and the catalyst bed, (D) a two-side open heating unit equipped with a heat controller (Model # 4848 from Parr Instrument Company), and (E) a sampling unit equipped with proportional relief valves also used as a pressure regulator (Swagelok SS-RL4M8F8-EP), cf. Fig. S2 in the ESI.† To ensure efficient heating, a cylindrical aluminium adapter with three boreholes was matched inside the heating unit. One bore is used for the preheating unit for the reactant before it comes in contact with the catalyst, another hole for the thermocouple (Model # A472E5 Parr Instrument Company) and the third one for the tubular reactor (Fig. S3 in the ESI†). The reactions were performed in a stainless steel tubular reactor (inner diameter = 21 mm, outer diameter = 25 mm, length = 280 mm), viz. Fig. S4 in the ESI.† Finally, an experiment using two consecutive fixed bed reactors filled with 10 g each of 35Ni/NDC was conducted. To perform these experiments two fixed bed reactors (D1–D2) were coupled in a setup similar to the one described above (Fig. S5 in the ESI†).
Catalytic experiments
Prior to each experiment, the LS solution (cLS = 1.0 wt% or 2.5 wt%) was filtered through filter paper (Whatman™ grade 40, 8 μm) to remove any solid residues that could cause clogging of the continuous flow system. In a typical experiment, the solution of LS was fed by the HPLC pump at 1.0 cm3 min−1, then mixed with H2 (20 cm3 min−1) and passed through the preheating unit and the tubular reactor. The temperature and pressure were kept constant (25 °C and atmospheric pressure) for 30 minutes at this steady state. Afterwards, the system was pressurized to 7.0 MPa to ensure the presence of the water/alcohol solvent system in the liquid state. Later, the system was heated to the desired reaction temperature (150 °C, 200 °C and 250 °C). In each catalytic experiment, 10 g of catalyst mass was used. Samples (30 cm3) were collected once the steady-state was reached (ca. 60 min). For the time on stream (TOS) experiment, samples were collected after 1 h, 3 h, 6 h, and 9 h of TOS. In the case of the two coupled reactors, a second, similarly filled reactor was mounted after the first reactor, and the same conditions were used as in the single reactor experiments. Samples were collected once both systems reached the steady-state (ca. 120 min).The collected samples were injected into a size exclusion chromatography (SEC) system without further processing, while the product analysis procedure was established to separate the residual LS from the reaction mixture, and therefore analyze the separated mixtures by GC-MS, GC-FID, 2D HSQC NMR, FTIR, elemental analysis and EDS. The established product analysis procedure is described in detail in the ESI in section S5.†
The batch experiment was conducted in a stainless steel autoclave equipped with PTFE liners and a magnetic stirrer from Berghof (Model: BR-100). The catalytic experiments were performed at 250 °C using 30 cm3 of LS solution in a MeOH/H2O solvent mixture (cLS = 2.5 wt%) with 1.0 g of 35Ni/NDC and 7.0 MPa of external H2 pressure for 1 and 3 h. Prior to heating to 250 °C, air was purged out using 1.0 MPa of N2 three times. The analysis of the reaction products followed the same protocol that was established for the continuous flow experiments.
Results and discussion
Composition analysis of sodium lignosulfonate
Initially, the chemical composition of the utilized commercially available sodium lignosulfonate (LS) was investigated via elemental analysis, (Table 1). The LS was found to contain 7.0 wt% of sulfur (Table 1). This S content is comparable to the reported industrial sulfite process by Borregaard.21,43 This indicates a high sulfonation degree and consequentially a high content of counter ions. In this context, Na was found to be the major sulfonate counter ion with 10 wt%, combined with the presence of Ca and Mg traces (Table 1). The presence of these Ca and Mg ions points to the usage of mixed sulfite salts containing Na, Ca and Mg in the industrial isolation process.21 Moreover, TGA analysis for LS showed a relatively high residual mass at 700 °C of 56 wt%, confirming the high sulfonation degree, cf. Fig. S7 in the ESI.† Finally, the moisture content was found to be 4.5 wt%, as determined by TGA analysis (see Table 1 and Fig. S7 in the ESI†).The SEC analysis of an aqueous LS solution (1 wt%) showed a weight average molecular weight (Mw) of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 g mol−1 with a dispersity (Đ) of 9.1, viz. Fig. S8 in the ESI.† This high Đ value indicates that LS presents a polydisperse distribution of the molecular weight. The 2D HSQC NMR spectra of the LS showed the absence of syringyl units (S), and only guaiacyl units (G) have been found in the typical aromatic region (δC/δH 100–150/6.2–7.5), viz. Fig. S9 in the ESI.†71,72 This indicates that the utilized LS in this work has been produced from softwood, in which the S/G molar ratio is zero. Generally, lignin rich in G units is favorable for certain applications such as additives for polymers and resins. This is due to the reactive ortho-position of the phenolic rings, which promotes radical-initiated crosslinking.73 2D HSQC NMR of LS showed the presence of sulfo-groups in the α position (α-Sα,β,γ) at δC/δH of 66–68/4.5–4.7, 79–82/4.7–5.1 and 60–62/3.9–4.0, respectively. These are typical for LS, viz. Fig. S9 in the ESI.†71,72 Moreover as expected, FTIR analysis showed the typical O–H stretching bond at around 3400 cm−1, the asymmetric and symmetric O
390 g mol−1 with a dispersity (Đ) of 9.1, viz. Fig. S8 in the ESI.† This high Đ value indicates that LS presents a polydisperse distribution of the molecular weight. The 2D HSQC NMR spectra of the LS showed the absence of syringyl units (S), and only guaiacyl units (G) have been found in the typical aromatic region (δC/δH 100–150/6.2–7.5), viz. Fig. S9 in the ESI.†71,72 This indicates that the utilized LS in this work has been produced from softwood, in which the S/G molar ratio is zero. Generally, lignin rich in G units is favorable for certain applications such as additives for polymers and resins. This is due to the reactive ortho-position of the phenolic rings, which promotes radical-initiated crosslinking.73 2D HSQC NMR of LS showed the presence of sulfo-groups in the α position (α-Sα,β,γ) at δC/δH of 66–68/4.5–4.7, 79–82/4.7–5.1 and 60–62/3.9–4.0, respectively. These are typical for LS, viz. Fig. S9 in the ESI.†71,72 Moreover as expected, FTIR analysis showed the typical O–H stretching bond at around 3400 cm−1, the asymmetric and symmetric O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching at 1150–1200 cm−1 and 1036 cm−1 and the typical signals of the aromatic ring vibration at around 1400 cm−1respectively, viz. Fig. S10 and Table S1 in the ESI.†74
O stretching at 1150–1200 cm−1 and 1036 cm−1 and the typical signals of the aromatic ring vibration at around 1400 cm−1respectively, viz. Fig. S10 and Table S1 in the ESI.†74
Solvothermal fragmentation (SF) of sodium lignosulfonate
The usage of short chain bio-derivable alcohol such as ethanol (EtOH) and methanol (MeOH), as well as their mixture with water, has been reported as efficient solvent mixtures for both lignin extraction and fractionation.57,75–78 Due to the low solubility of LS in organic solvents, an aqueous solvent mixture, i.e., MeOH/H2O and EtOH/H2O, is necessary.39 Therefore, the efficiency of water/alcohol mixtures (MeOH/H2O and EtOH/H2O with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio) and of pure H2O as a solvent was studied for the catalyst-free solvothermal fragmentation (SF) of LS.
1 weight ratio) and of pure H2O as a solvent was studied for the catalyst-free solvothermal fragmentation (SF) of LS.
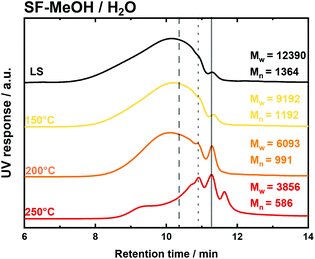
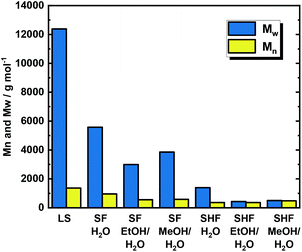
The solvothermal fragmentation reactions (SF) were performed at 150 °C, 200 °C, and 250 °C in the absence of the catalyst. In all cases, i.e., using MeOH/H2O and EtOH/H2O mixtures and H2O as the solvent, the SEC elugrams exhibited the successive disappearance of the peak at low retention times (<10 min), corresponding to the high molecular weight fraction, cf.Fig. 2 and Fig. S11 in the ESI.† For the MeOH/H2O solvent mixture, the derived mass average molecular weight (Mw) was decreased from 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 g mol−1 for untreated LS to 9132 g mol−1, 6093 g mol−1 and 3856 g mol−1 following the increase of reaction temperature to 150 °C, 200 °C and 250 °C, respectively (Fig. 2, Table S2 – entries 2 to 4 and Fig. S12 in the ESI†). Similarly, the EtOH/H2O solvent mixture showed a decrease of Mw with the increase of reaction temperatures, i.e., from 150 °C to 200 °C and to 250 °C, giving the lowest Mw value of 3000 g mol−1 at 250 °C, viz. Fig. 3, Table S2 – entries 5 to 7 and Fig. S12 in the ESI.† Also using pure H2O as a solvent, the Mw was found to be following the same behaviour, i.e. decreasing with the temperature increase, with the lowest Mw of 5578 g mol−1 at 250 °C (Fig. 3, Table S2 – entries 8 to 10 and Fig. S12 in the ESI†). Analogously to Mw, the number averaged molecular weight distribution (Mn) decreased with the increase of the reaction temperatures, giving the minimum at 250 °C, i.e., Mn = 586 g mol−1, 556 g mol−1 and 957 g mol−1 for MeOH/H2O, EtOH/H2O and H2O respectively, cf. Fig. S12 and Table S2 – entries 4, 7, and 10 in the ESI.†
390 g mol−1 for untreated LS to 9132 g mol−1, 6093 g mol−1 and 3856 g mol−1 following the increase of reaction temperature to 150 °C, 200 °C and 250 °C, respectively (Fig. 2, Table S2 – entries 2 to 4 and Fig. S12 in the ESI†). Similarly, the EtOH/H2O solvent mixture showed a decrease of Mw with the increase of reaction temperatures, i.e., from 150 °C to 200 °C and to 250 °C, giving the lowest Mw value of 3000 g mol−1 at 250 °C, viz. Fig. 3, Table S2 – entries 5 to 7 and Fig. S12 in the ESI.† Also using pure H2O as a solvent, the Mw was found to be following the same behaviour, i.e. decreasing with the temperature increase, with the lowest Mw of 5578 g mol−1 at 250 °C (Fig. 3, Table S2 – entries 8 to 10 and Fig. S12 in the ESI†). Analogously to Mw, the number averaged molecular weight distribution (Mn) decreased with the increase of the reaction temperatures, giving the minimum at 250 °C, i.e., Mn = 586 g mol−1, 556 g mol−1 and 957 g mol−1 for MeOH/H2O, EtOH/H2O and H2O respectively, cf. Fig. S12 and Table S2 – entries 4, 7, and 10 in the ESI.†
The significant decrease in the molecular weight in all cases indicates that the cleavage of the weaker linkages of LS (mostly phenyl ether bonds) has already occurred at the lower reaction temperatures, i.e., 150 °C and 200 °C. Rather expectedly, this fragmentation is becoming most effective at the highest applied temperature, i.e., 250 °C. However, the molecular weight dispersity (Đ) was found to be higher than 5.0 at all of the investigated temperatures using all solvent systems (Table S2 – entries 2 to 10 – in the ESI†). This indicates that fragmentation occurred only partially, resulting in a heterogeneous mixture with different sizes of oligomers, mostly tri-, tetra- and pentamers. An additional explanation for such a heterogeneous molar mass distribution could be attributed to in situ re-polymerization (side reactions) of the cleaved intermediates or the occurrence of radical polymerization.79 Nonetheless, the water/alcohol mixtures exhibited lower molar mass distributions over all the investigated temperatures. This result is attributed to the better stabilization of the intermediates against re-condensation by water/alcohols mixtures. Based on this, MeOH/H2O and EtOH/H2O samples were selected to qualitatively analyse the monomers using GC-MS, viz. Fig. S13 in the ESI.† From this chromatogram, a wide range of monomers (16 compounds) has been identified and reported in Table S3 in the ESI.† Moreover, the GC-MS chromatogram exhibited peaks in the high retention time region (>20 min), which can be attributed to dimers. However, these dimers were not identified and recognized by the compound database of the GC device. Among the identified compounds, 4-propyl guaiacol (G1), 4-ethyl guaiacol (G2), homovanillyl alcohol (G3), dihydroconyferyl alcohol (G4), guaiacol (G5) creosol (G6), eugenol (G7) and isoeugenol (G8) were quantified using GC-FID, and cumulative monomer yields of 8.7 mg gLS−1 and 2.9 mg gLS−1 were calculated for MeOH/H2O and EtOH/H2O, respectively (Table 2 and Fig. S14 in the ESI†). These higher cumulative monomer yields are attributed to better monomer stabilization by the MeOH/H2O solvent mixtures. It is noteworthy to be mentioned here that SF in continuous flow systems produced partially depolymerized LS. In this simple approach, the molecular weight distribution has been found to depend directly on the reaction temperature. This finding can be attributed to the thermal elimination of single phenol units from LS. This is a typical behaviour of a thermodynamically unstable polymer that undergoes reversible addition–fragmentation reactions. Moreover, the derived dispersity (Đ = 6.5 at 250 °C using MeOH/H2O) indicates that this equilibrium is perturbed by side reactions such as condensation of the cleaved fragments. This phenomenon provides clear evidence of the presence of a ceiling temperature, above which the polymer is spontaneously depolymerized into smaller units. Interestingly, the presence of a ceiling temperature is a general phenomenon in polymer science but was mostly not reported in the community of lignin biorefinery.80
| Sample | c LS/wt% | T/°C | G1/mg gLS−1 | G2/mg gLS−1 | G3/mg gLS−1 | G4/mg gLS−1 | G5/mg gLS−1 | G6/mg gLS−1 | G7/mg gLS−1 | G8/mg gLS−1 | H1/mg gLS−1 | Y CM/mg gLS−1 | STYCM/mg h−1 gNi−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n.a. = not applicable. Note: The reaction conditions of these experiments can be found in the captions of Fig. 2, 3, 4, 5, 6 and S11 at ESI.† | |||||||||||||
| SF-MeOH/H2O | 1 | 250 | 1.2 | 0.48 | 1.5 | 0.94 | 0.80 | 0.04 | 3.4 | 0.35 | n.a. | 8.7 | n.a. |
| SF-EtOH/H2O | 1 | 250 | 0.89 | 0.01 | n.a. | 0.94 | 0.93 | 0.05 | n.a. | 0.10. | n.a. | 2.9 | n.a. |
| SHF-MeOH/H2O | 1 | 150 | 6.7 | 0.22 | n.a. | 0.72 | 0.80 | 0.46 | n.a. | n.a. | 0.19 | 9.1 | 1.6 |
| 1 | 200 | 6.6 | 0.35 | 2.9 | 5.1 | 0.89 | 0.04 | n.a. | n.a. | 0.37 | 16 | 2.7 | |
| 1 | 250 | 16 | 2.7 | 3.1 | 8.2 | 2.2 | 0.22 | n.a. | n.a. | 0.83 | 34 | 5.8 | |
| SHF-EtOH/H2O | 1 | 150 | n.a. | n.a. | n.a. | 0.72 | 1.0 | n.a. | n.a. | n.a. | 0.04 | 1.7 | 0.29 |
| 1 | 200 | 2.4 | 0.12 | 0.6 | 2.6 | 0.61 | 0.01 | n.a. | n.a. | 0.04 | 6.3 | 1.1 | |
| 1 | 250 | 17 | 0.72 | 2.4 | 9.7 | 0.78 | 0.10 | n.a. | n.a. | 0.53 | 31 | 5.3 | |
| Flow 2.5 wt% | 2.5 | 250 | 16 | 4.9 | 0.71 | 5.6 | 3.7 | 1.6 | n.a. | n.a. | 0.1 | 33 | 14 |
| Dual column | 2.5 | 250 | 20 | 5.7 | 0.94 | 9.6 | 3.4 | 1.6 | n.a. | n.a. | 0.2 | 42 | 9.0 |
| Batch 1h | 2.5 | 250 | 12 | 0.70 | 2.0 | 1.9 | 1.3 | 0.17 | 0.53 | 0.48 | n.a. | 19 | n.a. |
Solvothermally assisted catalytic hydrogenolysis/hydrogenation fragmentation (SHF) of sodium lignosulfonate
Primarily, the utilized 35Ni/NDC in pellet form was synthesized using our “kitchen lab” approach that was previously reported.64–66 The N2 sorption isotherms of the parent carbonized NDC and 35Ni/NDC represent type IV isotherms (Fig. S15 in the ESI†), which is characteristic of mesoporous materials. NDC and 35Ni/NDC exhibited high specific surface areas of 755 m2 g−1 and 578 m2 g−1 (Table S4 in the ESI†), respectively. This combined with a C/N ratio of 22 indicates relatively high N doping (Table S4 in the ESI†). XRD of the 35Ni/NDC catalyst showed the typical reflection of Ni0 at 44° and 51°, indicating the complete reduction of NiO formed in the calcination step, viz. Fig. S16 in the ESI.† The HR-TEM images also showed Ni nanoparticles of the expected size, i.e. 20–40 nm, viz. Fig. S17 in the ESI.† These findings are in agreement with the crystalline size from CO-TPD of 21 nm, which is correlated to the Ni surface area of 21 m2 g−1, cf. Table S4 in the ESI.†In this part, the influence of coupling a catalytic hydrogenolysis/hydrogenation step with the abovementioned solvothermal treatment on LS depolymerization was investigated. Herein, the presence of a redox catalyst (35Ni/NDC) facilitates LS depolymerization through hydrogenolysis of ether bonds of LS fragments, mostly β–O–4, and 4–O–5 (viz. Fig. S18 in the ESI†). Simultaneously, the unsaturated and unstable fragments formed solvothermally are catalytically hydrogenated, preventing their recondensation (viz. Fig. S18 in the ESI†).59,81 For this purpose, a set of experiments was performed at three different reaction temperatures, i.e., 150 °C, 200 °C and 250 °C, using water/alcohol with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, i.e., EtOH/H2O and MeOH/H2O, as well as using only H2O as a solvent. These experiments are noted as follows: SHF-MeOH/H2O, SHF-EtOH/H2O, and SHF-H2O.
1, i.e., EtOH/H2O and MeOH/H2O, as well as using only H2O as a solvent. These experiments are noted as follows: SHF-MeOH/H2O, SHF-EtOH/H2O, and SHF-H2O.
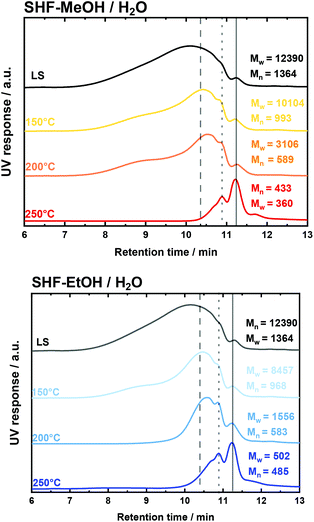
The dependence of the molecular weight (from SEC) on the reaction temperature in these experiments is shown in Fig. 4 and Fig. S19.† By increasing the reaction temperature from 150 °C to 200 °C and to 250 °C, the SEC elugrams showed a disappearance of the peak at low retention time (RT < 9 min), which corresponds to the high molecular weight fraction, in all solvent systems. Similarly, Mw and Mn exhibited significant decay when the temperature was increased from 150 °C to 200 °C and to 250 °C for SHF-MeOH/H2O, SHF-MeOH/H2O and SHF-H2O, viz.Fig. 3, Fig. S19, and S20 in the ESI.† The Mw was found to have decreased from 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 g mol−1 (before reaction) to 433 g mol−1, 502 g mol−1 and 1393 g mol−1 at 250 °C for SHF-MeOH/H2O, SHF-EtOH/H2O, and SHF-H2O, respectively (Fig. 4 and Table S2 – entries 13 and 16 in the ESI†). All these values are smaller than those for SF (Table S2 – entries 4, 7 and 10 – in the ESI†).
390 g mol−1 (before reaction) to 433 g mol−1, 502 g mol−1 and 1393 g mol−1 at 250 °C for SHF-MeOH/H2O, SHF-EtOH/H2O, and SHF-H2O, respectively (Fig. 4 and Table S2 – entries 13 and 16 in the ESI†). All these values are smaller than those for SF (Table S2 – entries 4, 7 and 10 – in the ESI†).
Nevertheless, using pure H2O as a solvent resulted in a higher molecular weight at all investigated temperatures compared to its water/alcohol counterparts. This observation is in agreement with the SF experiments, wherein using pure water as a solvent lead to the formation of oligomers and dimers.77,82 Moreover, Đ has substantially decreased from 6.5 to 1.2 for SHF-MEOH/H2O and SHF-EtOH/H2O, and to 3.8 for SHF-H2O i.e., from highly dispersed to relatively monodisperse (Table S2 – entries 4 and 13 in the ESI†). This decrease of both Đ and Mw in SHF clearly indicates a controlled and efficient LS depolymerization in the presence of the catalyst with respect to SF. These observations are in good agreement with the proposed stabilization of the formed monomers via hydrogenolysis/hydrogenation due to the presence of both H2 and the redox catalyst. Thus, this step is required to prevent recondensation of the fragmented fraction.59,81
For detailed insights into the structure of the sample taken from SHF-MeOH/H2O at 250 °C, characterization of the freeze dried solid residue (denoted as SHF-LS) was performed via elemental analysis (EA) including analysis of Na, Ca, and Mg via ICP-OES, FTIR and 2D HSQC NMR. The EA showed a decrease of the S content from 7.0 wt% to 5.0 wt%, while the O content decreased slightly from 36 wt% to 34 wt%, cf.Table 1. Differently, the Na, Ca and Mg amounts in the freeze-dried solid residue remained constant before and after the experiment. The difference in the S content is attributed to the elimination of sodium sulfate and sodium sulfide as a result of sulfonate hydrolysis and reduction. We also find a slightly higher acidity of the product solution (pH = 5.5) when compared to the reactant solution (pH = 6.3). It is important to note that at this pH, the water soluble phenolates are not formed without subsequent loss of aromatics in the water phase. The 2D HSQC NMR spectra for SHF-LS showed the disappearance of the sulfo-group signals (α-Sα,β,γ at δC/δH 66–68/4.5–4.7, 79–82/4.7–5.1 and 60–62/3.9–4.0), which is an indication of the sulfonate group removal from LS (Fig. S21 in the ESI†). In addition, low intensity of Ni2S3 with respect to Ni0 was found in the XRD pattern of the spent catalyst indicating a small amount of S2− reacting on the catalyst surface (Fig. S22 in the ESI†), which however does not influence the catalyst performance, as proven by SEC and monomers yield. In addition, FTIR analysis of SHF samples showed a decrease of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands at 1036 cm−1 and at around 1200 cm−1, when compared to the original FTIR spectrum of LS, viz. Fig. S9 and Table S1 in the ESI.† Moreover, FTIR showed that the C–H stretching modes at 2954 and 2937 cm−1 exhibited a higher peak intensity in SHF samples than in the original LS. This suggests a higher concentration of alkyl chains in the SHF sample due to the LS hydrogenolysis/hydrogenation.
O stretching bands at 1036 cm−1 and at around 1200 cm−1, when compared to the original FTIR spectrum of LS, viz. Fig. S9 and Table S1 in the ESI.† Moreover, FTIR showed that the C–H stretching modes at 2954 and 2937 cm−1 exhibited a higher peak intensity in SHF samples than in the original LS. This suggests a higher concentration of alkyl chains in the SHF sample due to the LS hydrogenolysis/hydrogenation.
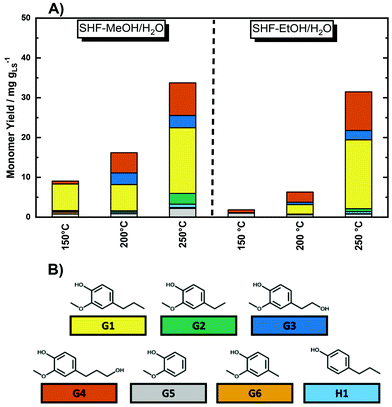
In order to evaluate the depolymerization efficiency at different temperatures, the SHF product has been qualitatively and quantitatively analyzed using GC-MS and GC-FID, respectively. By GC-MS it was possible to identify a wide range of monomers (18 compounds), as reported in Table S5 in the ESI.† Among the identified compounds, 4-propyl guaiacol (G1), 4-ethyl guaiacol (G2), homovanillyl alcohol (G3), dihydroconyferyl alcohol (G4), guaiacol (G5), creosol (G6), and 4-propyl phenol (H1) were the predominant ones and have been quantified from GC-FID chromatograms (Table 2, and Fig. S13 and S22 in the ESI†). Interestingly, the compounds with unsaturated alkene tails, i.e., eugenol (G7) and isoeugenol (G8), viz. Fig. S14 in the ESI,† were found only in traces, indicating the efficient hydrogenation of the double bonds at the alkene tails using 35Ni/NDC. At a reaction temperature of 150 °C, the cumulative monomers yield (YCM) was found to be 9.1 mg gLS−1, 1.7 mg gLS−1 and 0.1 mg gLS−1 with MeOH/H2O, EtOH/H2O and H2O as the solvent, respectively (Fig. 5, Table 2, and Fig. S24 and S25 in the ESI†). These compounds have boiling points between 200 °C and 300 °C, which allows them to be separated from the product mixture for further chemo-catalytic upgrading.28,83 Alternatively, separation based on solvent extraction can be performed.28,63,83,84 Once separated, the monomer can be valorised toward phenols and alkenes via hydroprocessing and dealkylation, while the oligomers can be used for materials applications.23,63
Increasing the temperature from 150 °C to 200 °C and 250 °C corresponded to an increase in the YCM from 9.1 mg gLS−1 to 16 mg gLS−1 and to 34 mg gLS−1 for SHF-MeOH/H2O mixtures and from 1.7 mg gLS−1 to 6.3 mg gLS−1 and to 31 mg gLS−1 for SHF-EtOH/H2O, which correspond to the space–time cumulative monomer yield (STYCM) of 1.6, 2.7, and 5.8 mg h−1 gNi−1 for MeOH/H2O and 0.29, 1.1, and 5.3 mg h−1 gNi−1 for EtOH/H2O. In contrast, SHF-H2O exhibited a minimal YCM increase from 0.1 mg gLS−1 to 0.7 mg gLS−1 and to 1.7 mg gLS−1 at 150 °C, 200 °C, and 250 °C, respectively, viz. Fig. S25 in the ESI.† This lower YCM using pure H2O instead of the water/alcohol mixture is in agreement with the previous study conducted with lignin77,82 In this context, low YCM is attributed to the poor solubility of the formed monomers in water, which lead to their adsorption on the catalyst surface, which subsequently led to low YCM with respect to the water/alcohol solvent mixture.82 Moreover, the hydrogenation of phenols in pure H2O can lead to over-hydrogenation toward hexanols.85 This is confirmed by the presence of only four monomers among the previously identified compounds, i.e.G2, G3G5, and H, viz. Fig. S25 in the ESI.† The low YCM combined with the high Mw of the SHF-H2O experiment indicates the necessity of using the water/alcohol mixture. In all the cases using alcohol/water mixtures, except EtOH/H2O at 150 °C, the monomers were similarly distributed with 4-propyl guaiacol (G1) and dihydroconyferyl alcohol (G4) as the major products, viz.Fig. 5. However, the dominant distribution of phenolic monomers with reduced tail, such as G1–G6 and H1, indicates the effective hydrogenolysis/hydrogenation of LS fragments in the SHF process by the 35Ni/NDC catalyst. Nevertheless, it is important to underline that the selected compounds do not represent the entire amount of phenolic monomers, and for this reason, the cumulative monomer yield remains under-estimated. The values of monomer yield in both water/alcohol solvent systems at low temperature, i.e., at 150 °C and 200 °C, are in-line with the maximum theoretical yield (∼10 mg gLS−1) that Rinaldi et al. reported only for the β–O–4 linkages of LS, indicating the cleavage of these linkage types.30 However, at 250 °C, the cumulative monomer yield was found to be three times higher than the maximum theoretical yield, indicating the cleavage of other linkages in addition to β–O–4. These observations indicate that at 150 °C and 200 °C, depolymerization occurs mostly via hydrogenolysis of ether bonds. Whereas at 250 °C, in addition to β–O–4, a wide number of linkages, e.g., 4–O–5 and 5–5 bonds, are cleaved solvothermally followed by the stabilization of the unsaturated bonds, viz in Fig. S26 in the ESI.† This process is within the typical lignin fragmentation at high temperatures (250–400 °C).79,86 The solvothermal cleavage of bonds produces unstable intermediates, such as radicals and double bonds, which are hydrogenated using 35Ni/NDC, viz. Fig. S26 in the ESI.† This proposed scheme would confirm the protective action against recondensation of the 35Ni/NDC catalyst. For the catalytic hydrogenolysis of β–O–4 and 4–Ov5 ether bonds, we proposed that these steps are following a classical hydrogenation mechanism. This mechanism based on dissociative H2 adsorption on Ni followed by LS adsorption and bond cleavage, is analogous to those of studies in the literature conducted with model compounds, viz Fig. S27.†87–89
In the studied system, the SHF-MeOH/H2O solvent mixture showed a higher monomer yield over all temperature ranges than SHF-EtOH/H2O. The higher activity of MeOH/H2O confirms the results of the LS experiments and is attributed to the high polarity of this mixture that allows better solubility of LS, which led to high diffusion of LS through the 35Ni/NDC catalyst and higher LS hydrogenolysis/hydrogenation rates. These findings are in strong agreement with other studies conducted with MeOH on catalytic lignin depolymerization reported by the Sels group.76,90 This demonstrated the crucial role of a polar solvent in lignin solubilization which could lead to improved process efficiency. Therefore, MeOH/H2O has been selected for further investigations as the solvent mixture, due to its higher YCM when compared to EtOH/H2O or pure H2O.
Process optimization
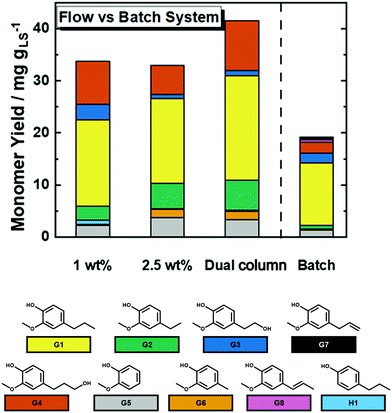
In order to maximize the efficiency of LS depolymerization toward monomers, an experiment has been performed with a higher LS concentration (2.5 wt%) than in previous SF and SHF (1.0 wt%) experiments at 250 °C. It is important to mention that 2.5 wt% was chosen as the highest concentration, i.e., it is the highest limit of LS solubility in the used solvent mixture (MeOH/H2O) at room temperature.In terms of weight hourly space velocity (WHSV), increasing the concentration from 1 to 2.5 wt% corresponded to an increase from 0.17 to 0.43 gLS h−1 gNi−1. Herein, Mw and Mn of 430 and 391 g mol−1 with Đ of 1.1, respectively, were found (see Table 3). These values are comparable to the one obtained from the experiment conducted using 1.0 wt% LS solution (Table 3). Also, the cumulative monomer yield was found to be similar in both cases. These results show no significant influence of the higher concentration (from 1.0 wt% to 2.5 wt%) of LS on the catalyst performance. Accordingly, increasing the concentration from 1 to 2.5 wt% corresponded to an increase of STYCM from 5.8 to 14 mg h−1 gNi−1. Because of the higher weight productivity, the 2.5 wt% concentration was selected to investigate the catalyst stability over time on stream (TOS), viz. Fig. S28 in the ESI.† Exposing the 35Ni/NDC catalyst to the reaction conditions for 9 h, did not affect the YCM, which was found to be constant (ca. 30 mg gLS−1). However, the N2 physisorption of 35Ni/NDC-TOS exhibited a loss in specific surface area from 578 to 272 m2 g−1 (Fig. S29 in the ESI†). This decrease in the surface area indicates product deposition onto the catalysts, due to the affinity between aromatics and porous carbon, which is in agreement with a similar study conducted by our group for vanillin hydrogenation.68 The XRD diffraction patterns of the spent catalyst after 9 h of TOS (35Ni/NDC-TOS) showed similar peaks to those of the spent catalyst after 1 h (Fig. S22 in the ESI†), with mainly Ni0 reflections and the presence of Ni(OH)2 and Ni3S2 phases. This remarkable resistance of Ni0 for such a long time under harsh conditions (250 °C and the presence of S), is attributed to the establishment of a Mott–Schottky stabilization effect between the Ni0 and the N of the carbon support.68,91,92
| Sample | M w /g mol−1 | M n /g mol−1 | Đ /— | Y CM /mg gLS−1 | STYCMb/mg h−1gNi−1 |
|---|---|---|---|---|---|
| n.a. = not applicable.a Calculated by SEC.b Calculated from GC-FID results.c Not determined as the Mw is high with respect to 1 h. | |||||
| LS | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390 |
1364 | 9.1 | n.a. | n.a |
| SHF-MeOH/H2O | 433 | 360 | 1.2 | 34 | 5.8 |
| Flow 2.5 wt% | 430 | 391 | 1.1 | 33 | 14 |
| Dual column | 393 | 337 | 1.1 | 42 | 9.0 |
| Batch 1 h | 1615 | 491 | 3.3 | 19 | n.a. |
| Batch 3 h | 6942 | 684 | 10 | n.d.c | n.a |
One of the advantages of operating in continuous flow systems is the possibility to couple multiple reactors together.67 Herein, two identical packed reactors with 35Ni/NDC have been coupled in series, in order to prolong the contact time between the LS solution and the catalyst and consequently optimize the monomer yield (Fig. 6 and Fig. S5 in the ESI†). Moreover, coupling two flow reactors in series aims to mimic an industrial scale up of such process. Therein, the concentrated 2.5 wt% LS solution has been continuously fed through two coupled reactors packed with 35Ni/NDC. Accordingly, the double amount of the catalyst is used, resulting in two-times lower WHSV (from 0.43 to 0.21 gLS h−1 gNi−1). The molecular weight distributions in this experiment showed a slight decrease when compared with the sole reactor experiment using 2.5 wt% (Table 3). In contrast, the cumulative monomer yield increased from 33 to 42 mg gLS−1 when compared to the single column experiment (Fig. 6 and Table 2), i.e. prolonging the residence time resulted in an improved monomer yield. In addition, this monomer yield (42 mg gLS−1) is four times higher than the maximum theoretical monomer yield (∼10 mg gLS−1) reported by Rinaldi et al. based on the cleavage of β–O–4 linkages in LS.30 However, the benefits of using two columns in terms of YCM are combined with STY decreasing from 14 to 9.0 mg h−1 gNi−1, viz.Table 3. This indicates that an excess amount of the catalyst is used in the dual columns experiment, with a non-optimized WHSV.
Comparison between batch and flow
To identify the assumed advantages of continuous flow over batch systems, catalytic LS depolymerization was performed in a batch system using the MeOH/H2O solvent system. 1 h reaction time has been set to be comparable to the one in the flow system, yielding a Mw of 1615 g mol−1 and a Đ of 3.3 (Table 3). These values are considerably higher than the ones in the flow system experiment. In addition, the cumulative monomer yield was found to be 19 mg gLS−1, which is lower than that for the continuous flow system (33 mg gLS−1 using 2.5 wt% LS solution), viz. Fig. 6 and Table 2. Interestingly, the batch systems showed the presence of monomer with an unsaturated alkene tail, i.e., eugenol and isoeugenol, which could be a sign of the beginning of catalyst deactivation. Moreover, increasing the reaction time from 1 h to 3 h, corresponded to an increase of Mw from 1615 g mol−1 to 6942 g mol−1 as a result of catalyst deactivation, combined with an increase of Đ from 3.3 to 10. These findings are attributed to the fragment re-polymerization in a batch system that also leads to catalyst deactivation due to longer contact times between reactants and products.93Conclusions
Industrial sodium lignosulfonate (LS) was successfully valorized using solvothermal fragmentation independently and combined with catalytic hydrogenolysis/hydrogenation in continuous flow systems. The depolymerization was found to occur thermally in the absence of the catalyst, rather independent of the solvent mixture. The decrease of molecular weight has been found to depend on the reaction temperature, i.e., LS at those temperatures is an unstable polymer and shows ceiling behaviour. This allows direct and simple tuning of molecular weights of the formed fractions based on reaction temperatures. To obtain monomers and lower molecular weight fractions, the solvothermal fragmentation should be coupled with a catalytic hydrogenolysis/hydrogenation step. An optimum cumulative monomer yield of 34 mg gLS−1 (STY of 5.8 mg h−1 gNi−1) has been found at 250 °C using MeOH/H2O as a solvent, while indeed most of the fragments are in the dimer and trimer range, which is more exciting from a polymer perspective. Moreover, the presence of a hydrogenation catalyst allowed a higher degree of depolymerization even at a lower temperature. This clearly showed that 35Ni/NDC plays a vital role in this approach, as it is responsible for further fragmentation through hydrogenolysis/hydrogenation steps.Extending the residence time by coupling two reactors yielded an even higher cumulative monomer yield of 42 mg gLS−1 with respect to the experiment in a single tubular reactor. However, the decreased STY of this approach (9.0 mg h−1 gNi−1) pointed out the need for higher weight productivity in view of further possible improvements.
The obtained monomer yield is relatively low, but also expected and well-known for technical lignin.30 This is due to the high sulfonate (and counter-ion) content, as well as the low amount of C–O bonds of LS when compared to native lignin.30 This is the intrinsic limitation of using technical lignin as the starting feedstock for lignin depolymerization. Nevertheless, in our contribution, we reported on a high cumulative monomer yield when it is compared to other work presented in the literature using similar lignin.52,53
In addition to the monomers, the SHF process simultaneously produces lignin oligomers, which can be also applied in a wide range of applications. Based on this, we believe that our approach has the potential to use both the monomer and the oligomers fractions, moreover the weight of the product solutions can be tuned according to the temperature.
We also have to underline that all these data were obtained using an industrial LS waste, i.e., containing 7.0 wt% sulfur and 10 wt% sodium, rather than impurity-free lignin. Certainly, other lignin types could allow high targeted monomer yields. Nevertheless, giving value to industrial waste will increase the sustainability of already established biorefinery processes and accelerate the transition toward a sustainable circular bio-economy.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful to the Max Planck Society for the financial support. Paolo Giusto, Aleksander Savateev, Alessandro Vincenzo Cataldo, Enrico Lepre and Jose Alirio Mendoza Mesa are greatly acknowledged for scientific discussion, feedback, assistance and suggestions. The authors are grateful to Irina Shekova for the support in catalyst synthesis. Jessica Brandt, Antje Völkel, Marlies Gräwert and Bolortuya Badamdorj from the Max Planck Institute of Colloids and Interfaces are acknowledged for ICP, EA, SEC and EDS analyses, respectively. Thanks are also extended to Klaus Bienert, Michael Born, Marco Bott and Tobias Schmidt from the electrical and mechanical workshop at the Max Planck Institute of Colloids and Interfaces. Joris Snaet is greatly acknowledged for the design of the cover artwork image. Open Access funding was provided by the Max Planck Society.References
- A. Katelhon, R. Meys, S. Deutz, S. Suh and A. Bardow, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11187–11194 CrossRef CAS PubMed.
- M. Al-Naji, J. Van Aelst, Y. Liao, M. d'Hullian, Z. Tian, C. Wang, R. Gläser and B. F. Sels, Green Chem., 2020, 22, 1171–1181 RSC.
- M. Al-Naji, A. Yepez, A. M. Balu, A. A. Romero, Z. Chen, N. Wilde, H. Li, K. Shih, R. Gläser and R. Luque, J. Mol. Catal. A: Chem., 2016, 417, 145–152 CrossRef CAS.
- M. Al-Naji, M. Popova, Z. Chen, N. Wilde and R. Gläser, ACS Sustainable Chem. Eng., 2019, 8, 393–402 CrossRef.
- C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695–699 CrossRef CAS PubMed.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2017, 6, 32–48 CrossRef.
- S. Pacala and R. Socolow, Science, 2004, 305, 968–972 CrossRef CAS PubMed.
- B. Kumru, J. A. Mendoza Mesa, M. Antonietti and M. Al-Naji, ACS Sustainable Chem. Eng., 2019, 7, 17574–17579 CrossRef CAS.
- M. Al-Naji, H. Schlaad and M. Antonietti, Macromol. Rapid Commun., 2020, 2000485, DOI:10.1002/marc.202000485.
- J. A. Mendoza Mesa, F. Brandi, I. Shekova, M. Antonietti and M. Al-Naji, Green Chem., 2020, 22, 7398–7405 RSC.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- M. M. Abu-Omar, K. Barta, G. T. Beckham, J. Luterbacher, J. Ralph, R. Rinaldi, Y. Roman-Leshkov, J. Samec, B. Sels and F. Wang, Energy Environ. Sci., 2021, 14, 262–292 RSC.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 CrossRef CAS PubMed.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- D. Esposito and M. Antonietti, Chem. Soc. Rev., 2015, 44, 5821–5835 RSC.
- J. Martin and M. Haggith, Enviromental Paper Network: The State of Global Paper Industry https://www.environmentalpaper.org, (accessed 27 January 2021).
- C. E. Wyman, B. E. Dale, R. T. Elander, M. Holtzapple, M. R. Ladisch and Y. Lee, Bioresour. Technol., 2005, 96, 1959–1966 CrossRef CAS PubMed.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 1246843–1246843 CrossRef PubMed.
- H. Sadeghifar and A. Ragauskas, ACS Sustainable Chem. Eng., 2020, 8, 8086–8101 CrossRef CAS.
- T. Aro and P. Fatehi, ChemSusChem, 2017, 10, 1861–1877 CrossRef CAS PubMed.
- P. C. A. Bruijnincx, R. Rinaldi and B. M. Weckhuysen, Green Chem., 2015, 17, 4860–4861 RSC.
- Y. Liao, S.-F. Koelewijn, G. Van den Bossche, J. Van Aelst, S. Van den Bosch, T. Renders, K. Navare, T. Nicolaï, K. Van Aelst, M. Maesen and B. Sels, Science, 2020, 367, 1385–1390 CrossRef CAS PubMed.
- E. Paone, T. Tabanelli and F. Mauriello, Curr. Opin. Green Sustainable Chem., 2020, 24, 1–6 CrossRef.
- S. S. Hassan, G. A. Williams and A. K. Jaiswal, Renewable Sustainable Energy Rev., 2019, 101, 590–599 CrossRef CAS.
- P. C. Bruijnincx, R. Rinaldi and B. M. Weckhuysen, Green Chem., 2015, 17, 4860–4861 RSC.
- Z. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef CAS PubMed.
- W. Schutyser, T. Renders, S. Van den Bosch, S. F. Koelewijn, G. T. Beckham and B. F. Sels, Chem. Soc. Rev., 2018, 47, 852–908 RSC.
- J. E. Holladay, J. F. White, J. J. Bozell and D. Johnson, Top value-added chemicals from biomass-Volume II—Results of screening for potential candidates from biorefinery lignin, Pacific Northwest National Lab.(PNNL), Richland, WA (United States), 2007 Search PubMed.
- R. Rinaldi, R. Jastrzebski, M. T. Clough, J. Ralph, M. Kennema, P. C. A. Bruijnincx and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2016, 55, 8164–8215 CrossRef CAS PubMed.
- J. Zakzeski, P. C. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- H. Kobayashi, H. Ohta and A. Fukuoka, in Catalytic Hydrogenation for Biomass Valorization, ed. R. Rinaldi, RCS Energy and Environment Series No. 13, 2015, pp. 52–71 Search PubMed.
- A. Llevot, P. K. Dannecker, M. von Czapiewski, L. C. Over, Z. Soyler and M. A. Meier, Chem. – Eur. J., 2016, 22, 11510–11521 CrossRef CAS PubMed.
- A. Llevot, E. Grau, S. Carlotti, S. Grelier and H. Cramail, Macromol. Rapid. Commun., 2016, 37, 9–28 CrossRef CAS PubMed.
- Z. Strassberger, S. Tanase and G. Rothenberg, RSC Adv., 2014, 4, 25310–25318 RSC.
- S. Laurichesse and L. Avérous, Prog. Polym. Sci., 2014, 39, 1266–1290 CrossRef CAS.
- Z. Sun, J. Cheng, D. Wang, T. Q. Yuan, G. Song and K. Barta, ChemSusChem, 2020, 13(19), 5199–5212 CrossRef CAS PubMed.
- I. F. Demuner, J. L. Colodette, A. J. Demuner and C. M. Jardim, Bioresour. Technol., 2019, 14(3), 7543–7581 Search PubMed.
- J. Ruwoldt, Surfaces, 2020, 3, 622–648 CrossRef CAS.
- P. Fatehi and Y. Ni, in Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass, ed. B. Zhu, ACS Symposium Series; American Chemical Society, Washington, DC, 2011 Search PubMed.
- M. Y. A. Mollah, W. Yu, R. Schennach and D. L. Cocke, Cem. Concr. Res., 2000, 267–273, DOI:10.1016/S0008-8846(99)00243-4.
- L. Hu, Y. Zhou, M. Zhang and R. Liu, BioResources, 2012, 7, 554–564 CAS.
- Borregaard, Potential applications for different lignin sources based on experience from Borregaard, https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwit0eSf7KruAhURahQKHRtFCFoQFjAAegQIBxAC&url=http%3A%2F%2Fwww.lth.se%2Ffileadmin%2Fenergiportalen%2FEnergy_Portal%2FFiles%2FPres_Rodsrud.pdf&usg=AOvVaw2jH3uyvQBINJ-PiQySZp9l, (accessed 27 January 2021).
- Y. Qin, D. Yang and X. Qiu, ACS Sustainable Chem. Eng., 2015, 3, 3239–3244 CrossRef CAS.
- S. Gao, Z. Cheng, X. Zhou, Y. Liu, R. Chen, J. Wang, C. Wang, F. Chu, F. Xu and D. Zhang, Int. J. Biol. Macromol., 2020, 161, 755–762 CrossRef CAS PubMed.
- B. V. K. J. Schmidt, V. Molinari, D. Esposito, K. Tauer and M. Antonietti, Polymer, 2017, 112, 418–426 CrossRef CAS.
- C. Che, M. Vagin, U. Ail, V. Gueskine, J. Phopase, R. Brooke, R. Gabrielsson, M. P. Jonsson, W. C. Mak, M. Berggren and X. Crispin, Adv. Sustainable Syst., 2019, 3, 1900039 CrossRef CAS.
- R. Guterman, V. Molinari and E. Josef, Angew. Chem., Int. Ed., 2019, 58, 13044–13050 CrossRef CAS PubMed.
- X. Zhang, A. L. Licon, T. I. Harris, P. F. Oliveira, B. J. McFarland, B. E. Taurone, B. J. Walsh, B. E. Bell, C. T. Walker, R. V. Lewis and J. A. Jones, ACS Omega, 2019, 4, 4832–4838 CrossRef CAS PubMed.
- J. Pang, W. Zhang, H. Zhang, J. Zhang, H. Zhang, G. Cao, M. Han and Y. Yang, Carbon, 2018, 132, 280–293 CrossRef CAS.
- V. Molinari and M. Antonietti, Lignin-based composite materials and methods for its preparation, European patent office Patent NoEP 3 266 810 A1, 2018 Search PubMed.
- J. Horáček, J.-P. Mikkola, A. Samikannu, G. Št’ávová, W. Larsson, L. Hora and D. Kubička, Top. Catal., 2013, 56, 794–799 CrossRef.
- R. Shu, Y. Xu, L. Ma, Q. Zhang, T. Wang, P. Chen and Q. Wu, RSC Adv., 2016, 6, 88788–88796 RSC.
- Q. Song, F. Wang and J. Xu, Chem. Commun., 2012, 48, 7019–7021 RSC.
- P. Cui, H.-X. Fang, C. Qian and M.-H. Cheng, Chromatographia, 2019, 83, 87–93 CrossRef.
- M. Fache, B. Boutevin and S. Caillol, ACS Sustainable Chem. Eng., 2015, 4, 35–46 CrossRef.
- K. Van Aelst, E. Van Sinay, T. Vangeel, E. Cooreman, G. Van den Bossche, T. Renders, J. Van Aelst, S. Van den Bosch and B. F. Sels, Chem. Sci., 2020, 11, 11498–11508 RSC.
- S. Van den Bosch, S. F. Koelewijn, T. Renders, G. Van den Bossche, T. Vangeel, W. Schutyser and B. Sels, in Lignin Chemistry, ed. L. Serrano, R. Luque and B. Sels, Springer Nature Switzerland, Cham, 2020, pp. 129–168 Search PubMed.
- T. Renders, G. Van den Bossche, T. Vangeel, K. Van Aelst and B. Sels, Curr. Opin. Biotechnol, 2019, 56, 193–201 CrossRef CAS PubMed.
- S. Van den Bosch, S. F. Koelewijn, T. Renders, G. Van den Bossche, T. Vangeel, W. Schutyser and B. F. Sels, in Topics Curr Chem, 2018, vol. 376 Search PubMed.
- T. Renders, S. Van den Bosch, S. F. Koelewijn, W. Schutyser and B. F. Sels, Energy Environ. Sci., 2017, 10, 1551–1557 RSC.
- E. Cooreman, T. Vangeel, K. Van Aelst, J. Van Aelst, J. Lauwaert, J. W. Thybaut, S. Van den Bosch and B. F. Sels, Ind. Eng. Chem. Res., 2020, 59, 17035–17045 CrossRef CAS.
- Y. Liao, S. Van den Bosch, J. Van Aelst and B. Sels, Catalytic funneling of phenolics, US Patent No17083154, 2021 Search PubMed.
- S. G. Parto, J. M. Christensen, L. S. Pedersen, A. B. Hansen, F. Tjosås, C. Spiga, C. D. Damsgaard, D. B. Larsen, J. Ø. Duus and A. D. Jensen, Energy Fuels, 2019, 33, 1196–1209 CrossRef CAS.
- S. Ghafarnejad Parto, J. Munkholt Christensen, L. Saaby Pedersen, F. Tjosås and A. D. Jensen, Catalysts, 2018, 8, 502 CrossRef.
- M. L. Stone, E. M. Anderson, K. M. Meek, M. Reed, R. Katahira, F. Chen, R. A. Dixon, G. T. Beckham and Y. Román-Leshkov, ACS Sustainable Chem. Eng., 2018, 6, 11211–11218 CrossRef CAS.
- F. Brandi, I. Khalil, M. Antonietti and M. Al-Naji, ACS Sustainable Chem. Eng., 2021, 9, 927–935 CrossRef CAS.
- F. Brandi, M. Bäumel, V. Molinari, I. Shekova, I. Lauermann, T. Heil, M. Antonietti and M. Al-Naji, Green Chem., 2020, 22, 2755–2766 RSC.
- F. Brandi, M. Bäumel, I. Shekova, V. Molinari and M. Al-Naji, Sustainable Chem., 2020, 1, 106–115 CrossRef.
- M. Al-Naji, B. Puertolas, B. Kumru, D. Cruz, M. Baumel, B. Schmidt, N. V. Tarakina and J. Perez-Ramirez, ChemSusChem, 2019, 12(12), 2628–2636 CrossRef CAS PubMed.
- O. Musl, I. Sulaeva, M. Bacher, A. K. Mahler, T. Rosenau and A. Potthast, ChemSusChem, 2020, 13, 4595–4604 CrossRef CAS PubMed.
- B. F. Lutnaes, B. O. Myrvold, R. A. Lauten and M. M. Endeshaw, Magn. Reson. Chem., 2008, 46, 299–305 CrossRef CAS PubMed.
- M. Alonso, M. Oliet, F. Rodriguez, G. Astarloa and J. Echeverría, J. Appl. Polym. Sci., 2004, 94, 643–650 CrossRef CAS.
- K. Fukuhara, K. Nakajima, M. Kitano, S. Hayashi and M. Hara, Phys. Chem. Chem. Phys., 2013, 15, 9343–9350 RSC.
- E. M. Anderson, M. L. Stone, R. Katahira, M. Reed, G. T. Beckham and Y. Román-Leshkov, Joule, 2017, 1, 613–622 CrossRef CAS.
- W. Schutyser, S. Van den Bosch, T. Renders, T. De Boe, S. F. Koelewijn, A. Dewaele, T. Ennaert, O. Verkinderen, B. Goderis, C. M. Courtin and B. F. Sels, Green Chem., 2015, 17, 5035–5045 RSC.
- T. Renders, S. Van den Bosch, T. Vangeel, T. Ennaert, S.-F. Koelewijn, G. Van den Bossche, C. M. Courtin, W. Schutyser and B. F. Sels, ACS Sustainable Chem. Eng., 2016, 4, 6894–6904 CrossRef CAS.
- X. Wang and R. Rinaldi, ChemSusChem, 2012, 5, 1455–1466 CrossRef CAS PubMed.
- T. Watanabe, H. Kawamoto and S. Saka, Holzforschung, 2009, 63, 424–430 CAS.
- B. D. Mar, H. W. Qi, F. Liu and H. J. Kulik, J. Phys. Chem. A, 2015, 119, 6551–6562 CrossRef CAS PubMed.
- Y. M. Questell-Santiago, M. V. Galkin, K. Barta and J. S. Luterbacher, Nat. Rev. Chem., 2020, 4, 311–330 CrossRef CAS.
- T. Renders, E. Cooreman, S. Van den Bosch, W. Schutyser, S. F. Koelewijn, T. Vangeel, A. Deneyer, G. Van den Bossche, C. M. Courtin and B. F. Sels, Green Chem., 2018, 20, 4607–4619 RSC.
- O. D. Mante, D. C. Dayton and M. Soukri, RSC Adv., 2016, 6, 94247–94255 RSC.
- J. S. Kim, Bioresour. Technol., 2015, 178, 90–98 CrossRef CAS PubMed.
- J. He, C. Zhao and J. A. Lercher, J. Catal., 2014, 309, 362–375 CrossRef CAS.
- X. Zhou, L. J. Broadbelt and R. Vinu, in Thermochemical Process Engineering, 2016, pp. 95–198, DOI:10.1016/bs.ache.2016.09.002.
- Y. Li, S. D. Karlen, B. Demir, H. Kim, J. Luterbacher, J. A. Dumesic, S. S. Stahl and J. Ralph, ChemSusChem, 2020, 13, 4487–4494 CrossRef CAS PubMed.
- J. He, C. Zhao, D. Mei and J. A. Lercher, J. Catal., 2014, 309, 280–290 CrossRef CAS.
- J. He, C. Zhao and J. A. Lercher, J. Am. Chem. Soc., 2012, 134, 20768–20775 CrossRef CAS PubMed.
- J. H. Li, C. F. Lin, W. Qin, X. B. Xiao and L. Wei, Acta Phys. – Chim. Sin., 2016, 32, 2717–2723 CAS.
- Z. H. Xue, J. T. Han, W. J. Feng, Q. Y. Yu, X. H. Li, M. Antonietti and J. S. Chen, Angew. Chem., Int. Ed., 2018, 57, 2697–2701 CrossRef CAS PubMed.
- X. H. Li and M. Antonietti, Chem. Soc. Rev., 2013, 42, 6593–6604 RSC.
- S. M. G. Lama, J. Pampel, T.-P. Fellinger, V. P. Beškoski, L. Slavković-Beškoski, M. Antonietti and V. Molinari, ACS Sustainable Chem. Eng., 2017, 5, 2415–2420 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc01714d |
| This journal is © The Royal Society of Chemistry 2021 |