Determination of bismuth by optical emission spectrometry with liquid anode/cathode atmospheric pressure glow discharge†
Monika
Gorska
 *,
Krzysztof
Greda
*,
Krzysztof
Greda
 and
Pawel
Pohl
and
Pawel
Pohl

Wroclaw University of Science and Technology, Faculty of Chemistry, Division of Analytical Chemistry and Chemical Metallurgy, Wybrzeze Stanislawa Wyspianskiego 27, 50-370 Wroclaw, Poland. E-mail: monika.gorska@pwr.edu.pl
First published on 23rd November 2020
Abstract
Novel atmospheric pressure glow discharge (APGD) microplasma systems, sustained between a miniaturized flowing liquid anode (FLA) or cathode (FLC) and a He nozzle jet were investigated for the determination of Bi with the aid of optical emission spectrometry (OES). The most influential working conditions, i.e., the acid type, the acid concentration, the discharge current, the He flow rate, the sample flow rate, and the discharge gap, were optimized for both studied methods. Furthermore, the effect of the addition of low molecular weight organic compounds (LMWOCs) into FLA/FLC solutions on the signal intensity of Bi was investigated. It was found that the addition of formic acid (5%) into the FLC solution enhanced the signal intensity 10 times. Under the optimized conditions, detection limits (DLs, assessed on the basis of the 3σ criterion) reached 33 μg L−1 for the FLC-APGD system and 0.34 μg L−1 in the case of the FLA-APGD system. The DL of Bi offered by the FLA-APGD-OES method was better than those reported for other microplasma techniques. The latter method was successfully applied for a quantitative determination of Bi in spiked water samples. The influence of concomitant ions on the signal intensity of Bi was thoroughly studied and the recoveries of Bi added to these water samples (at a concentration of 100 μg L−1) were within the range of 86–101%, confirming the good accuracy and usefulness of the developed FLA-APGD-OES method.
1. Introduction
Bismuth is considered as an environmentally significant element, due to numerous applications of its compounds in different areas, which include: semiconductors, cosmetics and medicine production as well as chemical and metallurgical industries. The increasing use of Bi in the abovementioned areas augments its environmental distribution and the organism exposure to this element.1,2 Additionally, a great number of toxic effects related to Bi compounds have been reported.3 For all of the aforesaid reasons, Bi is widely determined in medicines,4,5 human hair,6,7 urine,8,9 soil,10,11 sediments,12,13 different water samples,14,15 and food samples.16,17Several spectrometric methods have been applied to Bi determination, e.g., atomic absorption spectrometry (AAS),6,12 hydride generation atomic absorption spectrometry (HG-AAS),11,18 hydride generation atomic fluorescence spectrometry (HG-AFS),2,8 inductively coupled plasma optical emission spectrometry (ICP-OES),19,20 hydride generation inductively coupled plasma optical emission spectrometry (HG-ICP-OES),21,22 hydride generation microwave induced plasma optical emission spectrometry (HG-MIP-OES),23,24 and inductively coupled plasma mass spectrometry (ICP-MS).10,25 The aforesaid commercially available techniques are valued for providing good selectivity, sensitivity, low detection limits (DLs), high precision and trueness as well as the possibility of performing multi-element analysis. However, a number of disadvantages have been recognized in regard to the application of these methods, e.g., bulky and complex instrumentation, and high power and gas consumption, resulting in high operating costs.
To overcome these disadvantages, the attention of many researchers has been directed to the development of miniaturized excitation sources which would provide a similar or better analytical performance at low costs and reduced power and gas consumption, concurrently requiring a simplified device design.26,27 Among numerous systems proposed for the time being,27–32 discharges generated in contact with flowing solutions enjoy a special interest. They provide a simple design, and operation at a low power (<100 W) and often in an open to air atmosphere (no discharge gas is required) as well as the transport of analytes directly from a sample solution, which eliminates the need for nebulizers or spray chamber application. All of this results in notably reduced operation costs, contemporaneously offering sensitivity and detectability comparable to or better than those obtained with conventionally used bulky instruments.33–35
Surprisingly, there are only a few reports of such microplasma systems being applied in the quantitative determination of Bi. They include the following techniques: hydride generation dielectric barrier discharge atomic fluorescence spectrometry (HG-DBD-AFS),36 hydride generation dielectric barrier discharge atomic absorption spectrometry (HG-DBD-AAS),37 hydride generation point discharge optical emission spectrometry (HG-PD-OES),38 solution anode glow discharge atomic emission spectrometry (SAGD-AES),39 and hydride generation flowing liquid anode atmospheric pressure glow discharge optical emission spectrometry (HG-FLA-APGD-OES).40 It is noteworthy that the majority of the aforesaid techniques employ the HG technique. Although the application of the HG reaction enhances the sensitivity and improves the transport efficiency of analytes, it is well known that this method suffers from interference in the liquid phase coming from the sample matrix and significant memory effects. In addition, it requires the use of a toxic and unstable reducing agent and the pre-reduction step is often needed in real sample analysis to obtain lower oxidation states of elements.41–43
The last from the above listed techniques, i.e., SAGD, known also as flowing liquid anode atmospheric pressure glow discharge (FLA-APGD),44 was repeatedly proven to be a promising tool for highly sensitive determination of Ag, Bi, Cd, Hg, In, Pb, Tl, and Zn.39,44–46 It offers high precision, wide linearity ranges, good trueness, and DLs of the above-mentioned analytes improved up to 3 orders of magnitude in comparison with those achievable with other microplasma techniques as well as commercially available instruments, such as ICP-OES. Very recently, Bi was successfully determined by two independent research groups using the FLA-APGD excitation source combined with OES detection. In the first case,40 the obtained DL of Bi was 0.8 μg L−1; however, in this approach the HG technique was applied. On the other hand, Yuan et al.39 proposed a simplified design for the SAGD device but the DL obtained in this work was over two times higher, i.e., 2.0 μg L−1.
Therefore, the aim of this work was to improve the sensitivity and detectability of Bi determination by the FLA-APGD-OES method, but without applying the HG technique. To reach this goal, the optimization of crucial working parameters was performed at first. Afterwards, the influence of the addition of low molecular weight organic compounds (LMWOCs) on the signal intensity of Bi was investigated. Under optimal operating conditions, the analytical performance of the FLA-APGD-OES method (in terms of the DL of Bi, the linearity range of the calibration curve, and the precision of measurements) was evaluated. Moreover, the effect of the selected foreign ions on the signal intensity of Bi was evaluated and the analysis of the selected water samples was carried out. Additionally, the optimization, the evaluation of the analytical performance and the study of the effect of the addition of LMWOCs were also conducted for flowing liquid cathode atmospheric pressure glow discharge (FLC-APGD) sustained and operated in the discharge system similar to FLA-APGD. The results attained for both methods were compared and discussed comprehensively.
2. Experimental
2.1. Instrumentation
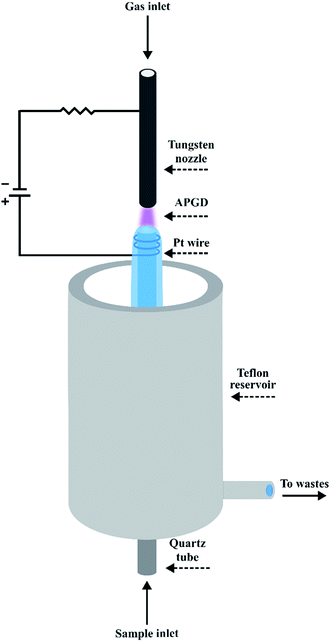
A schematic drawing of the FLA-APGD system is presented in Fig. 1. The FLC-APGD system was almost the same but differed from the FLA-APGD system only in electrode polarization and a sample inlet tube construction. In both cases, the discharge was sustained in an open-to-air chamber between a tungsten nozzle (OD/ID 3/1 mm, length 50 mm) and a vertically oriented quartz tube (OD/ID 4/2 mm). In the case of the FLA-APGD system, the electrical contact was provided directly to the tungsten nozzle and with the aid of a platinum spiral wrapped around the quartz tube. In the case of the FLC-APGD system, a graphite tube (OD/ID 6/4 mm, length 20 mm) was inserted into the quartz tube to provide appropriate electrical contact and stabilize solution surface formation. The sample solutions acidified with HCl or HNO3, which served as either the anode or the cathode, were pumped through the quartz tube by means of a 3-channel REGLO ICC peristaltic pump (Ismatec, USA) at a flow rate up to 4.5 mL min−1. As the solution reached the top of the quartz tube it flowed down to a PTFE reservoir, from which it was pumped out. The tungsten nozzle was fed with He at a flow rate up to 350 mL min−1, which was controlled using an FC-2900 flow controller and an RO-28 digital flow meter (Tylan General, USA). The distance between the solution surface and the anode/cathode nozzle was set in the 1–5 mm range.A voltage of 800–1200 V was applied to both electrodes by a HV DC power supply (model DP50H-024PH, DSC-Electronics, Germany). Its exact value depended on the applied He flow rate, the discharge current, and the acid concentration. To stabilize the discharge, a 2.2 kΩ (FLA-APGD) or 6.9 kΩ (FLC-APGD) ballast resistor was connected into the circuit.
The radiation emitted by APGD was imaged (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on the entrance slit (10 μm) of a Shamrock 500i imaging spectrometer (Andor, UK), using an achromatic quartz lens (f = 80). The spectrometer was equipped with a holographic grating (1800 lines per mm) and a Newton DU-920P-OE UV-Vis CCD camera (Andor, UK). The integration time was 10 s during all experiments and the intensities of atomic emission lines of Bi were background corrected.
1) on the entrance slit (10 μm) of a Shamrock 500i imaging spectrometer (Andor, UK), using an achromatic quartz lens (f = 80). The spectrometer was equipped with a holographic grating (1800 lines per mm) and a Newton DU-920P-OE UV-Vis CCD camera (Andor, UK). The integration time was 10 s during all experiments and the intensities of atomic emission lines of Bi were background corrected.
2.2. Reagents and sample preparation
Deionized water (18.2 MΩ cm−1) from an Easypure water purification system (Thermolyne Corp., USA) was used throughout the study. All chemicals were at least of analytical grade. He of 99.999% purity was supplied by Air Products (Poland). Stock standard solutions of Ag, Bi, Ca, Cu, Fe, Hg, K, Mg, Mn, Na, Pb, Sn, and Zn (1000 mg L−1), obtained from Sigma-Aldrich (Germany), were utilized to prepare all working standard solutions. Solutions of methanol (100%), ethanol (96%), and formic acid (85%), applied for the investigation of the influence of organic compounds on the signal intensity of Bi, were provided by Avantor Performance Materials (Poland). To acidify the FLA/FLC solutions, concentrated HNO3 (65% m m−1) or HCl (37% m m−1) solutions (Merck, Germany) were employed.Mineral water was purchased from a local store. Bystrzyca river water (sampled in the city of Swidnica, Poland) and municipal tap water (Wroclaw, Poland) were collected into pre-cleaned 1 L PE bottles. River water was initially filtered through cellulose filter paper (grade 595) and subsequently acidified with concentrated HNO3 to reach its final concentration of 0.015 mol L−1.
A portion of 10.0 g of each water sample was transferred into a twist cup container. The samples of mineral and tap waters were then acidified with concentrated HNO3, spiked with the stock standard of Bi, and subsequently filled with deionized water to the final mass of 30.0 g. The resulting concentrations of HNO3 and Bi were 0.005 mol L−1 and 100 μg L−1, respectively. Such prepared sample solutions were divided into three separate parts and the standard addition was performed on the last two of them. All the abovementioned water samples were prepared in triplicate; besides, appropriate procedural blanks were prepared and considered in the final results.
3. Results and discussion
3.1. Identification of the emission lines of Bi
Initially, in order to identify the emission lines of Bi, a solution containing 10 mg L−1 of this element, acidified with 0.01 (FLA-APGD) or 0.1 (FLC-APGD) mol L−1 HNO3, was prepared. The measurement conditions were as follows: a discharge current of 50 mA, a He flow rate of 300 (FLA-APGD) or 50 (FLC-APGD) mL min−1, and a sample flow rate of 3.5 mL min−1. The emission spectrum was recorded in the 200–280 nm range, for both the investigated discharge systems (see Fig. SI-1†).Table 1 presents the values of the signal to background ratio (SBR) for all acquired atomic emission lines. Although 8 of such lines were identified in the FLA-APGD spectrum, the sensitivity of most of them was rather poor (except for Bi 223.1 nm). In the case of FLC-APGD, a weak emission from the resonance line of Bi (223.1 nm) as well as three other lines at 202.1 nm, 206.2 nm, and 262.8 nm was noted. The obtained results are in line with the outcomes reported in other studies, concerning FLA- and FLC-APGD systems, indicating that only a few most prominent atomic lines are observed for most of the elements.44,47 Based on the received signal intensities of Bi for the studied discharge systems, it could be expected that FLA-APGD would provide much better excitation conditions for Bi (because of lower saturation of the discharge gas with water vapor), as compared to FLC-APGD. The enhanced transport of Bi can contribute to its improved analytical response as well; the efficiency of the volatile species generation of Bi in the case of FLA-APGD is much higher than the transport of this element through the solution sputtering in the case of FLC-APGD. Previously, such a difference was reported for other elements, specifically Ag, Cd, Pb, Tl, and Zn.44 It is also noteworthy that the background intensity in the whole studied spectral range was perceptibly lower (up to 21 times) for the FLC-APGD system. A similar observation was also previously made in both above cited studies. This is likely due to increased water evaporation into the FLC-APGD system (by about 3–4 times, as compared to FLA-APGD),44,48 leading to a limited diffusion of the surrounding air into the discharge phase. As a result, the emission from molecular bands such as NO in the investigated spectral range was lowered.
As the SBR of the resonance line of Bi (223.1 nm) was 3–69 times higher than that for the other lines in both APGD systems, this atomic line was considered in all subsequent experiments.
3.2. Optimization of working parameters
In order to successfully use the proposed APGD excitation sources for Bi determination by OES, it was necessary to optimize the most influential operating parameters. The following ones were identified to have a significant impact on the emission from Bi atoms: the acid type (HCl, and HNO3), the acid concentration (0.001–0.01 mol L−1 for FLA-APGD and 0.01–0.1 mol L−1 for FLC-APGD), the discharge current (30–70 mA), the He flow rate (50–350 mL min−1), the sample flow rate (1.0–4.5 mL min−1), and the discharge gap (1–5 mm). Optimization was performed using either a Bi standard solution of 300 μg L−1 (for FLA-APGD) or a Bi standard solution of 10 mg L−1 (for FLC-APGD). If not stated otherwise, the measurement conditions were as follows: a discharge current of 50 mA, a He flow rate of 350 mL min−1, a sample flow rate of 3.5 mL min−1, and the discharge gap set to approximately 1 mm.As for the FLA-APGD system, the influence of the HNO3 concentration was studied in the range of 0.001–0.01 mol L−1. It was established that when the lower acidification of the introduced solutions was employed, poorer discharge stability was observed. As the acid concentration was equal to 0.001 mol L−1, it was impossible to ignite the discharge; likely due to the insufficient solution conductivity. In the case of the FLC-APGD system, it is expected that the acid concentration in the analyzed solution needs to be higher than this for the FLA-APGD system.44,49 Hence, for FLC-APGD, the investigated HNO3 concentrations ranged between 0.01 and 0.1 mol L−1 (lower and higher acid concentrations than given destabilized the discharge).
The highest emission from Bi was observed at HNO3 concentrations of 0.005 mol L−1 and 0.1 mol L−1, respectively for the FLA-APGD and FLC-APGD systems (see Comment SI-2†). For both studied systems, as the acid concentration increased, the background intensity slightly dropped (less than 10% over the whole studied ranges), hence the fluctuations in SBRs resulted predominantly from the changes in the Bi signal intensities (see Fig. SI-2 and SI-3†). As a result, the maximum SBRs were obtained at 0.005 mol L−1 and 0.1 mol L−1, for FLA- and FLC-APGD, respectively (see Fig. 2).
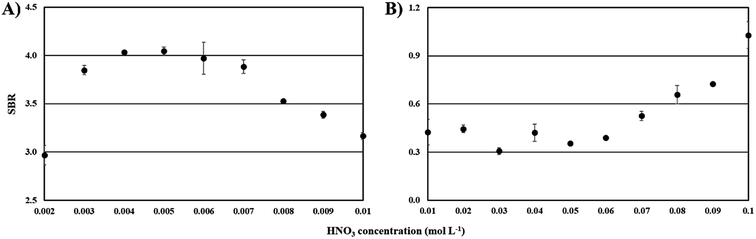 | ||
| Fig. 2 The effect of the acid concentration on the SBR for Bi in the case of the FLA-APGD (A) and FLC-APGD (B) systems. | ||
The results obtained for the FLC-APGD system are in good agreement with other literature data,50,51 reported for that system, indicating that the signal intensities of different elements decreased with lowering sample acidification. For this system, it was proved52,53 that at a high acid concentration, the cathode voltage drop is reduced, leading to the enhanced recombination of the sputtered element species with the electrons and hence, increasing their transport efficiency into the discharge.
Concerning the results obtained for the FLA-APGD system, their clarification is more troublesome due to a much smaller number of studies devoted to this excitation source. Similar observations regarding the changes in the signal intensity of the analytes with the increasing acid concentration were made by Liu et al. for Cd and Zn (regardless of the kind of acid used, i.e., HCl or HNO3)45 and Greda et al. for Ag, Cd, Pb, Tl, and Zn (in HNO3 media).54 A supposed reason for the decrease of the Bi signal intensity (with the increase of the acid concentration) was likely the scavenging of the solvated electrons by H3O+ ions present in the FLA solution (see Comment SI-3†).48
Regardless of the specific reason for the observed effect of HNO3, acid concentrations of 0.005 mol L−1 (for FLA-APGD) and 0.1 mol L−1 (for FLC-APGD) offered the highest SBRs, and hence they were applied in further experiments.
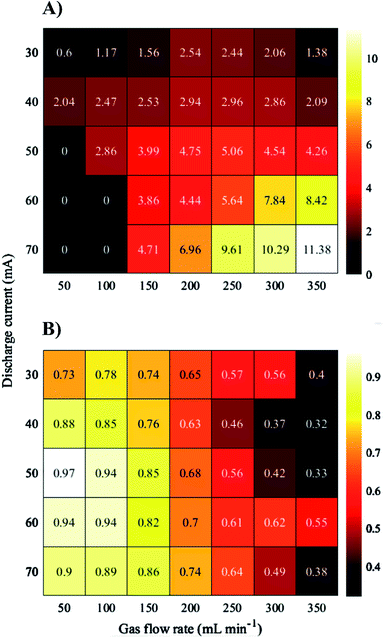
It was repeatedly shown in the literature44,45,54–57 that the increase of the discharge current leads to a linear enhancement of the signal intensity of analytes in the case of both FLA- and FLC-APGD systems. This signal intensity enhancement of elements is usually associated with a more efficient introduction of the analytes into the discharge.54 That being so, unsurprisingly, an enhancement of the Bi signal intensity (over the whole studied range) was observed in this work as well, for both investigated systems. A linear rise in the background intensity was observed as the discharge current increased (for both the FLC- and FLA-APGD system). The only small exception occurred for the FLA-APGD system operated at He flow rates ≤100 mL min−1 (see Comment SI-4†). In regard to the effect of the He flow rate in the case of FLA-APGD, the higher the gas flow rate, the higher the analytical signal observed. This signal enhancement was especially remarkable for the discharge system operating at currents exceeding 50 mA. In contrast to FLA-APGD, for FLC-APGD the maximum response from Bi was noted at lower He flow rates, i.e., 100–150 mL min−1. These observations are in agreement with the previous ones,58 and may result from a hindered diffusion of the analyte atoms into the discharge phase at a stronger He atom flux used.
As for the background intensity, it was increasing over the whole range of the He flow rates, regardless of the discharge current applied, in both the studied systems. Noteworthily, the background intensity enhancement for FLA-APGD was remarkably higher as compared to that observed for FLC-APGD (see Comment SI-5†). All of this resulted in receiving the SBR patterns presented in Fig. 3 (see Comment SI-6†). In the case of the FLC-APGD system, the discharge current and the He flow rate corresponding to the highest SBR obtained were 50 mA and 50 mL min−1, respectively. These settings were used in subsequent experiments. As for FLA-APGD, the best results were obtained at a discharge current of 70 mA. However, at 60 mA the discharge seemed to be more stable and for that reason the discharge current of 60 mA and a He flow rate of 350 mL min−1 were applied in the following experiments.
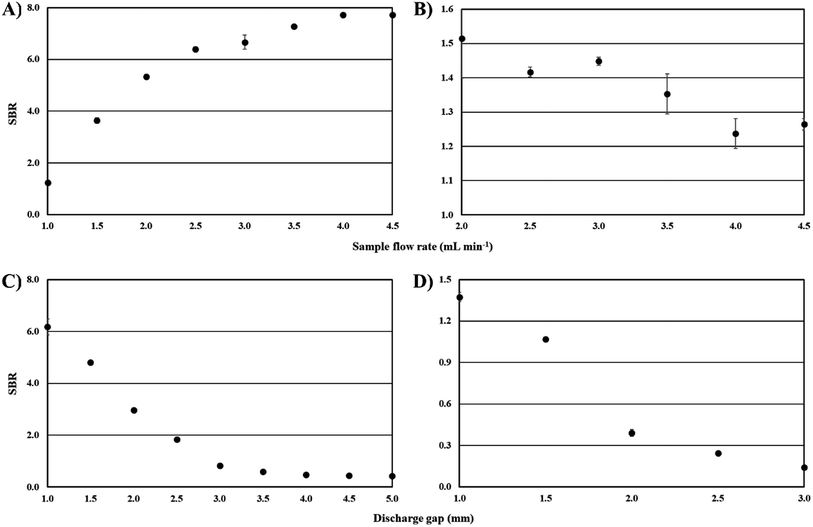
Considering the FLA-APGD system, the signal intensity of Bi was increasing as the solution flow rate was enhanced up to 3.5 mL min−1 and then reached a plateau. A quite similar observation was made by other researchers dealing with FLA-APGD.55 It is likely that by increasing the solution flow rate the amount of Bi volatile species released from the analyzed sample increased. Moreover, it was found that by increasing the solution flow rate (in the range of 2.0–4.5 mL min−1) the water evaporation rate declined from 26% to 9%. As a result, the saturation of the discharge gas phase with water vapor is lower, yielding improved excitation conditions. In regard to FLC-APGD, the signal intensity of Bi reduced by 29% as the sample flow rate increased from 2.0 up to 4.5 mL min−1. The received outcomes are in good agreement with the results obtained previously for other elements, such as Ca, Cd, Cu, Mg, and Zn.47,59,60 The reliable explanation for these observations has not been given up to now, and a more detailed study is needed.
Regarding the background intensity, its apparent drop was noted for both the studied discharge systems, i.e., 24% (for FLA-APGD) and 17% (for FLC-APGD), when the solution flow rate increased from the lowest studied value up to 4.5 mL min−1.
The abovementioned changes in the signal and background intensities are charted in Fig. SI-2 and SI-3† and resulted in obtaining the SBRs given in Fig. 4A and B. In the case of the FLA-APGD system, although the highest SBR was obtained for a solution flow rate of 4.5 mL min−1, the differences in the SBR values obtained between 3.5 and 4.5 mL min−1 were negligible. Considering the need for lowering the sample consumption, a sample flow rate of 3.5 mL min−1 was used in all subsequent experiments. In regard to the FLC-APGD system, although the highest signal intensity of Bi was acquired at a sample flow rate of 2.0 mL min−1, the discharge stability and the measurement precision were poor under these conditions. Therefore, the sample flow rate of 3.0 mL min−1 was applied in further experiments.
Considering the outcomes for the Bi emission line, their explanation is much more troublesome. It seems unlikely that the increasing discharge gap deteriorated the efficiency of the Bi volatile species generation. In that case, the only suspected reason for the observed suppression in the Bi emission (with the increasing discharge gap) is impaired atomization/excitation conditions. There are only residual data on the effect of the discharge gap on the microplasma performance (both in the case of FLA- and FLC-APGD), and what is worse, they unanimously indicate that with the discharge gap expansion, the spectroscopic temperatures increase,54,56,59 which rather improves the atomization/excitation conditions. Undoubtedly, to make a plausible hypothesis explaining the influence of the discharge gap on the signal intensity of Bi, a more-in-depth study would be necessary.
Nevertheless, the SBRs were highest at a discharge gap of 1 mm (see Fig. 4C and D), regardless of the studied system, which in both cases was a consequence of the rapid growth of the background intensity with increasing gap. Therefore, the discharge gap of 1 mm was chosen for further experiments.
3.3. The effect of foreign anions
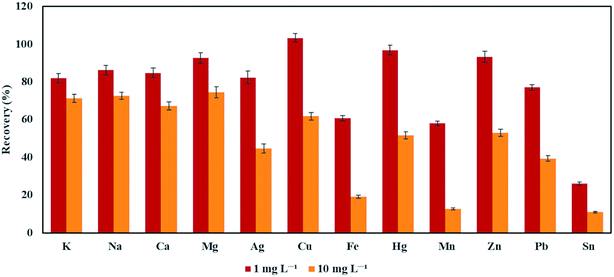
In order to investigate the endurance of the FLA-APGD-OES method to the interference from concomitant elements, the effect of the presence of Ag, Ca, Cu, Fe, Hg, K, Mg, Mn, Na, Pb, Sn, and Zn on the signal intensity of Bi was assessed. Each of the potentially interfering elements was added separately to a single-element solution of Bi. The concentration of Bi was equal to 0.5 mg L−1, whereas the concentration of the concomitant elements was either 1 or 10 mg L−1. The same effect for the FLC-APGD-OES method was not studied due to the low signal intensity of Bi at its concentration of 10 mg L−1.The results are presented in Fig. 5. It was found that the addition of Fe, Mn, Pb, and Sn at a concentration being only two times higher than the analyte content (i.e., 1 mg L−1), strongly lowered the recovery of Bi (ranging from 26% in the case of Sn to 77% in the case of Pb). The impact of the remaining metals (at the same concentration) was less significant and the recoveries obtained in their presence ranged between 81% for K to 103% for Cu. As for the concomitant elements at a concentration of 10 mg L−1, their negative influence was apparently stronger; the obtained recoveries of Bi ranged from 11% (in the presence of Sn) to 75% (for Mg). Surprisingly, alkali and alkaline earth metals seemed to have a minor impact on the signal intensity of Bi, as compared to the remaining metals at a concentration of 10 mg L−1. It was previously shown that the presence of Ca, Mg, Na, and K at a concentration similar to the one investigated herein influenced the recoveries of other analytes, i.e., Ag, Cd, Hg, In, Pb, Tl, and Zn, in a greater degree than other studied interferents, e.g., Al, Cu, Cd, Pb, Fe, and Mn.54,55,61 Very recently, Greda et al.61 proved that matrix effects in the FLA-APGD system results from the decreased efficiency of the volatile species generation (in the presence of foreign ions). Moreover, they noticed that the highest ionic radius of the concomitant element showed the greater interfering effect. Herein, a similar correlation between the degree of matrix effects and the ionic radius of the interfering element was not observed. The elements that affected the response of Bi to the greatest extent were not alkali and alkaline earth metals but Sn, Mn, and Fe. The determined matrix effects for Bi were quite similar to those reported in chemical vapor generation (CVG), e.g., in the reaction with sodium tetrahydroborate (THB), where transition metals are considered to have a more adverse effect as compared to alkali metals.62 This observation indicates that the mechanism of Bi volatile species generation (assisted by FLA-APGD) likely differs from that of Ag, Cd, Hg, Pb, Tl, and Zn.
Nevertheless, it was revealed that the presence of different elements in a sample solution is a serious limitation for the investigated method. Therefore, the standard addition method or selected separation techniques should be applied in order to eliminate the matrix effects.
3.4. The effect of the addition of low molecular weight organic compounds
Low molecular weight organic compounds (LMWOCs), such as methanol, ethanol, formic acid, and acetic acid, are known to intensify the signals of the analytes and lower their DLs in the FLC-APGD-OES method.34 As for the FLA-APGD system, the effect of LMWOCs was investigated only twice.46,48 Greda et al.46 found that the presence of methanol and ethanol remarkably enhanced the signal intensity of In, over 30 times at an alcohol concentration of 0.5%. However, in the case of other elements, i.e., Ag, Cd, Hg, Pb, Tl, and Zn, the addition of the studied alcohols either did not result in apparent signal intensity improvement or even suppressed the response (Ag and Pb). Also Swiderski et al.48 investigated the influence of LMWOCs on the signals intensity of Ag, Cd, and Pb in FLA-APGD-OES, and they found that under the optimized conditions it was possible to boost the emission from Ag, Cd, and Pb up to 5-fold.Neither in FLA- nor in FLC-APGD systems, the influence of LMWOCs on the response of Bi has been investigated so far. Therefore, the effect of the addition of methanol, ethanol, and formic acid, in the concentration range of 0.1–10%, was investigated herein in both the studied systems. The concentration of Bi in the solution was 0.5 mg L−1 (FLA) and 10 mg L−1 (FLC). Table 2 shows the relative intensities of the Bi signal in the presence of the studied organic media (calculated in reference to the signal obtained for the solution without any organic additives). In the case of FLA-APGD, it was found that the presence of all studied organic media significantly reduced the signal intensity of Bi (around 1 order of magnitude). The highest intensity drop occurred as the concentration of LMWOCs increased in the range of 0–1%, and above 1%; the analytical response was approximately constant. Concurrently, the background intensity in the presence of LMWOCs was roughly constant. Small exceptions occurred in the case of methanol and ethanol; as their concentrations increased in the range of 1–10%, the background intensity increased by 11% and 48%, respectively. Considering the FLC-APGD system, the signal intensity of Bi was significantly higher in the presence of the studied LMWOCs (especially formic acid). For the investigated alcohols, the maximum analytical response occurred as the concentrations of methanol and ethanol were 1% and 5%, respectively. In both cases, the factor of intensity enhancement was roughly 1.8. Even higher improvement of the analytical response, i.e., 10-fold, was observed as formic acid was added in the concentration ranging from 3% to 10%. The impact of LMWOCs on the background intensity was similar to the one observed for the FLA-APGD system. In the presence of methanol and ethanol, the background intensity did not significantly change up to their concentration of 1% and afterward, it increased by 20% or 2-fold for methanol and ethanol, respectively. In the case of formic acid, it remained constant over the whole examined concentration range.
| Organic compound | Concentration (%) | Relative intensity (a.u.) | |
|---|---|---|---|
| FLA-APGD | FLC-APGD | ||
| a Not measured due to the discharge instability. | |||
| Methanol | 0.1 | 0.30 | 1.30 |
| 0.3 | 0.14 | 1.39 | |
| 0.5 | 0.07 | 1.50 | |
| 1 | 0.07 | 1.75 | |
| 3 | 0.06 | 1.18 | |
| 5 | 0.07 | 0.74 | |
| 10 | 0.09 | —a | |
| Ethanol | 0.1 | 0.33 | 1.07 |
| 0.3 | 0.16 | 1.17 | |
| 0.5 | 0.11 | 1.26 | |
| 1 | 0.09 | 1.34 | |
| 3 | 0.09 | 1.47 | |
| 5 | 0.10 | 1.81 | |
| 10 | 0.10 | —a | |
| Formic acid | 0.1 | 0.11 | 0.96 |
| 0.3 | 0.03 | 1.47 | |
| 0.5 | 0.10 | 2.65 | |
| 1 | 0.18 | 6.98 | |
| 3 | 0.15 | 10.2 | |
| 5 | 0.13 | 10.2 | |
| 10 | 0.09 | 9.74 | |
Additionally, in the spectra of both discharges, the CO bands (the fourth positive A1Π → X1Σ system) and the CO+ bands (the first negative B2Π → 2Σ) were observed (see Fig. SI-4†), which proves that the LMWOCs were decomposed and transported into the discharge in both the systems. As can be seen from this figure, although the intensity of these bands was roughly equal, they were much more noticeable in the FLC-APGD spectrum. This is due to a significantly lower emission from the NO molecules for this discharge system. Thus, in the vicinity of the Bi atomic emission line there were mainly the CO bands (FLC-APGD) and the CO bands overlapped by the NO bands (FLA-APGD). Therefore, the increase of the background intensity for the FLC-APGD system in the case of the increased alcohol concentration was directly related to the increase in the emission from the CO and CO+ bands.
Considering the impact of the studied organic additives on the response of the analytes, it was repeatedly suggested that their signal intensity enhancement, observed for the FLC-APGD systems, could be a result of a change in the boiling point, the surface tension and/or the viscosity of the FLC solutions.58,63,64 However, in such a case, a similar effect would also be expected in the case of the FLA-APGD system, which apparently was not a case herein. Moreover, it was previously established that the boiling point changes of water mixtures with methanol, ethanol, and formic acid (at concentrations similar to those used in this work) are negligible.65
The observed differences in the influence of the LMWOCs on the signal intensities of Bi in both the studied systems could be clarified regarding the mechanisms of the analyte transport in these discharges. As for FLA-APGD, it is believed that it takes place by analyte reduction with the solvated electrons in the liquid phase, leading to form some kind of volatile species, which are then transported into the discharge.44,48 In such a case, a likely reason for lowering the Bi signal intensity in the presence of the LMWOCs could be the scavenging of solvated electrons by these compounds in the liquid phase, yielding the lowered efficiency of the volatile species generation.48 In the case of the FLC-APGD, the analytes are transported to the plasma phase most likely by cathode sputtering. It is suspected that in the presence of LMWOCs, organic radicals (e.g., ˙CO2−, ˙CO2H, and ˙CH2OH) are additionally formed, and they could improve the transport efficiency of Bi by the formation of some volatile species. The confirmation of this theory could be the results obtained by Doroski et al.,63 indicating that the addition of LMWOCs enhances the signal intensity of elements forming the volatile species (e.g., Ag, Hg, Pb, and Se) to a higher degree. Although it would then be expected to observe the same effect for FLA-APGD, the scavenging of the solvated electrons by the added LMWOCs could be the predominant effect. Bearing all this in mind, the addition of LMWOCs was no longer used in further studies on the FLA-APGD system.
3.5. Analytical performance and sample analysis
The analytical performance of the studied systems, i.e., FLA- and FLC-APGD, on the determination of Bi by OES detection was evaluated under the optimized conditions, as summarized in Table 3. Additionally, the analytical performance with the addition of 5% formic acid for the FLC-APGD system was also established. For this purpose, the DLs of Bi, the extent of linearity of the calibration curves, and the precision were determined. Instrumental DLs were calculated to be 3σ/a, where “3σ” stands for 3 times the standard deviation of 30 consecutive measurements of an appropriate blank solution, and “a” stands for the sensitivity of a corresponding calibration curve. The linearity extents were examined using 9 (for FLA-APGD-OES) or 7 (for FLC-APGD-OES) single-element standard solutions in concentration ranges of 0.01–1 mg L−1 (FLA-APGD), 10–100 mg L−1 (FLC-APGD without formic acid) or 1–10 mg L−1 (FLC with the addition of 5% formic acid). The precision was expressed as the relative standard deviation (RSD) for 10 consecutive measurements of the standard solutions. The RSD was measured 3 times for each method and the averaged results were given. The concentration of Bi for the precision measurements was as follows: 30 μg L−1 for FLA-APGD, 10 mg L−1 for FLC-APGD without the addition of formic acid, and 1 and 10 mg L−1 for FLC-APGD with the presence of 5% formic acid in the FLC solution.| Method | Acid type | Acid concentration (mol L−1) | Discharge current (mA) | Gas flow rate (mL min−1) | Sample flow rate (mL min−1) | Discharge gap (mm) |
|---|---|---|---|---|---|---|
| FLA-APGD | HNO3 | 0.005 | 60 | 350 | 3.5 | 1 |
| FLC-APGD | 0.1 | 50 | 50 | 3.0 |
Table 4 exhibits the abovementioned figures of merit achieved by the proposed methods. It is noteworthy that the addition of 5% formic acid allowed lowering the DL of Bi over 8 times, in the case of the FLC-APGD system. It was established that the presence of formic acid did not influence the background fluctuations and their level, and therefore the lowered DL of the element was the result of the improved sensitivity. However, the DL obtained with the aid of the FLA-APGD system was incomparably better, being almost 100 times lower than the one obtained for the FLC-APGD system when formic acid was added to the solution and over 800 times better, as compared to conditions without the additive of the organic media. The achieved DLs of Bi were also compared to those reported for other spectrometric methods, constituting both miniaturized plasma excitation sources and conventionally employed bulky plasmas (see Table 5). As can be noticed from this table, each of the presented methods provided significantly better DLs of Bi than that with FLC-APGD. On the other hand, the proposed FLA-APGD excitation source with OES detection assured a better DL than the majority of techniques in which bulky20,37 and miniaturized plasma sources38–40 were used. Moreover, the obtained DL of Bi for FLA-APGD-OES was also improved, as compared to DLs of this element achievable with the methods combined with the HG technique,37,38 including the HG-FLA-APGD-OES method, previously studied by our group.40 Although, a few of the cited studies21,23,36 reported better DLs of Bi than the one obtained in this work, the applied methods were premised upon bulky instruments21,23 and/or required the HG technique.21,23,36 It is also worth mentioning that – to the best of our knowledge – the microplasma techniques cited in the table are the only ones by which Bi has ever been determined.
| Method | Instrumental detection limit (μg L−1) | Linearity rangea (mg L−1) | R 2 | Sensitivity (a.u. per μg per L) | RSD (%) |
|---|---|---|---|---|---|
| a Given as the range between the quantification limit and the highest standard concentration measured in this work (yielding a linear response). b With the addition of 5% formic acid to the solution. c For Bi concentrations of 1 and 10 mg L−1, respectively. | |||||
| FLA-APGD | 0.34 | 0.001–1 | 0.9968 | 1301.7 | 3.76 |
| FLC-APGD | 274 | 0.9–100 | 0.9988 | 0.56 | 2.22 |
| FLC-APGDb | 33 | 0.1–10 | 0.9963 | 3.60 | 1.99/0.52c |
| Method | Detection limit (μg L−1) | Reference |
|---|---|---|
| a Without and with the addition of 5% formic acid, respectively. FLA – flowing liquid anode, APGD – atmospheric pressure glow discharge, FLC – flowing liquid cathode; SAGD – solution anode glow discharge, AES – atomic emission spectrometry, HG – hydride generation, PD – point discharge, OES – optical emission spectrometry, ICP – inductively coupled plasma, MIP – microwave induced plasma, DBD – dielectric barrier discharge, AFS – atomic fluorescence spectrometry, and AAS – atomic absorption spectrometry. | ||
| FLA-APGD | 0.34 | This work |
| FLC-APGD | 274/33a | This work |
| SAGD-AES | 1.98 | 39 |
| HG-FLA-APGD | 0.85 | 40 |
| HG-PD-OES | 1.0 | 38 |
| ICP-OES | 0.78 | 20 |
| HG-MIP-OES | 0.09 | 23 |
| HG-ICP-OES | 0.16 | 21 |
| HG-DBD-AFS | 0.07 | 36 |
| HG-DBD-AAS | 1.1 | 37 |
As for the other analytical figures of merit, the precision was established to be <4% for both the studied methods, being slightly better for the FLC-APGD-OES method. Nonetheless, the observed differences in the precision between FLA- and FLC-APGD excitation sources was likely related to the Bi concentrations in the solutions, as the SBRs for Bi were 0.71, 1.09, and 8.52 for FLA-APGD, FLC-APGD and FLC-APGD with the presence of 5% formic acid in the solution, respectively. The calibration curves for the atomic emission line of Bi were linear (R2 > 0.995) over the whole studied concentration ranges. The linearity ranges of the calibration curves covered at least 3 orders of magnitude for the FLA-APGD and at least 2 orders of magnitude for the FLC-APGD systems (higher concentrations were not studied).
Based on these findings, it was concluded that the proposed FLA-APGD-OES method is a promising alternative to the competitive ones (especially, in terms of the DL). As for the FLC-APGD-OES method, although it provides a much worse DL of Bi, it could be used for the determination of Bi in samples containing higher abundances of this element, e.g., drugs or wastes, especially with the addition of formic acid as a matrix modifier.
To demonstrate the trueness of the proposed FLA-APGD-OES method, an attempt was made to determine Bi in river, tap, and mineral waters. Due to the matrix effects from the concomitant ions, the standard addition method was applied. The reliability of the method was checked by the spike and recovery test. It was established, that the matrix of the analyzed samples lowered the response of Bi by similar values, i.e., 64%, 66%, and 58% for river, tap, and mineral water, respectively (as compared to the solution not containing any matrix). As it had been expected that the primary minerals present in all water samples would be alkali and alkaline earth metals, the quantification of Ca, K, Mg, and Na was performed using an Agilent ICP-OES instrument, model 5110. It was revealed that the total amount of these elements was equal to 46.7, 116.5, and 123.3 mg L−1 in river, tap, and mineral water, respectively. Although the content of the studied alkali and alkaline earth metals was over 2 times lower in river water (as compared to tap and mineral waters), apparently it was high enough to cause a serious reduction of the Bi response and a further increase of the interfering elements did not lead to the reduction of the Bi signal intensity. With regard to the DLs of Bi in the analyzed water samples they covered the range of 0.7–1.1 μg L−1, and were in good agreement with the observed suppression in the Bi signal intensity.
The obtained recovery values ranged between 86 and 101% (see Table 6), which confirms the good trueness of the developed method. The precision of the real sample analysis was slightly worse and fell within the range of 6–10%, which was a consequence of a reduced signal intensity. The trueness of the FLC-APGD-OES method in reference to the determination of Bi in the collected water samples was not studied because it would require their spiking with Bi in an amount of the order of several mg L−1, which is rather not found in naturally occurring and drinking water.
4. Conclusions
The use of FLA- and FLC-APGD systems for Bi determination by OES was studied and the results were compared. It was established that the FLA-APGD excitation source provides a much better analytical performance (as compared to FLC-APGD) in terms of the DL of Bi and sensitivity. Although the matrix effects from the concomitant ions became a serious limitation, water sample analysis could be performed with the standard addition method. The developed method seems to be a reliable alternative to methods involving the conventionally employed large-scale spectrometric instruments, especially for the analysis of samples containing lower abundances of foreign ions, i.e., drugs. It offers the advantages of a small size, a stable operation at a relatively low discharge current, reduced gas consumption, and no requirement for the use of the HG technique.Despite the fact that the FLC-APGD-OES method is a well-known and efficient tool for the sensitive determination of a wide group of elements, in the case of Bi determination it seems to be less useful than the FLA-APGD-OES method. Nevertheless, it was demonstrated that the addition of formic acid improved the DL of Bi by almost one order of magnitude. Moreover, this method offers a very good precision, an admittable linearity range, and all the abovementioned advantages of the microplasma system, and therefore it could be still applied for the determination of higher concentrations of Bi.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Science Centre (Poland) based on decision no. DEC-2017/24/C/ST4/00325.References
- A. K. Das, R. Chakraborty, M. L. Cervera and M. de La Guardia, Analytical techniques for the determination of bismuth in solid environmental samples, TrAC, Trends Anal. Chem., 2006, 25, 599–608 CrossRef CAS.
- W. N. L. dos Santos, A. de Freitas Santos Junior, L. O. B. Silva, B. R. da Silva Santos and D. L. F. da Silva, Multivariate optimization of a digestion procedure for bismuth determination in urine using continuous flow hydride generation and atomic fluorescence spectrometry, Microchem. J., 2017, 130, 147–152 CrossRef.
- A. Slikkerveer and F. A. de Wolff, Pharmacokinetics and toxicity of bismuth compounds, Med. Toxicol. Adverse Drug Exper., 1989, 4, 303–323 CrossRef CAS.
- P. C. A. Jeronimo, A. N. Araujo, M. C. B. S. M. Montenegro, D. Satinsky and P. Solich, Colorimetric bismuth determination in pharmaceuticals using a xylenol orange sol–gel sensor coupled to a multicommutated flow system, Anal. Chim. Acta, 2004, 504, 235–241 CrossRef CAS.
- A. P. Argekar and A. K. Shetty, Extraction and spectrophotometric determination of bismuth(III) with Cyanex 301, Analyst, 1995, 120, 1819–1822 RSC.
- H. Ashkenani and M. A. Taher, Application of a new ion-imprinted polymer for solid-phase extraction of bismuth from various samples and its determination by ETAAS, Int. J. Environ. Anal. Chem., 2013, 93, 1132–1145 CrossRef CAS.
- A. S. Amin, Cloud-Point Extraction and Spectrophotometric Determination of Trace Quantities of Bismuth in Environmental Water and Biological Samples, Spectrosc. Lett., 2011, 44, 424–431 CrossRef CAS.
- H. Wu, B. Du and C. Fang, Flow Injection On-line Preconcentration Coupled with Hydride Generation Atomic Fluorescence Spectrometry for Ultra-Trace Amounts of Bismuth Determination in Biological and Environmental Water Samples, Anal. Lett., 2007, 40, 2772–2782 CrossRef CAS.
- S. Moyano, R. G. Wuilloud, R. A. Olsina, J. A. Gasquez and L. D. Martinez, On-line preconcentration system for bismuth determination in urine by flow injection hydride generation inductively coupled plasma atomic emission spectrometry, Talanta, 2001, 54, 211–219 CrossRef CAS.
- T. Tokumaru, H. Ozaki, S. Onwona-Agyeman, J. Ofosu-Anim and I. Watanabe, Determination of the Extent of Trace Metals Pollution in Soils, Sediments and Human Hair at e-Waste Recycling Site in Ghana, Arch. Environ. Contam. Toxicol., 2017, 73, 377–390 CrossRef CAS.
- M. C. Saha, R. Baskey, S. Lahiri and S. Dutta, Quantitative determination of antimony, bismuth, and tellurium in geological samples by inductively coupled plasma mass spectrometry and flow injection hydride generation atomic absorption technique, At. Spectrosc., 2017, 38, 7–11 CrossRef CAS.
- R. Dobrowolski, J. Dobrzynska and B. Gawronska, Determination of bismuth in environmental samples by slurry sampling graphite furnace atomic absorption spectrometry using combined chemical modifiers, Environ. Monit. Assess., 2015, 187, 4125 CrossRef.
- M. A. Taher and B. K. Puri, Differential Pulse Polarographic Determination of Trace Amount of Bismuth in Various Complex Samples After Preconcentration of Its 1-(2-Pyridylazo)-2-naphthol Complex by Column and Microcrystalline Naphthalene Methods, Electroanalysis, 1999, 11, 899–904 CrossRef CAS.
- C. Calderilla, J. Avivar, L. O. Leal and V. Cerda, Multivariate optimisation of a rapid and simple automated method for bismuth determination in well water samples exploiting long path length spectrophotometry, Int. J. Environ. Anal. Chem., 2016, 96, 653–666 CrossRef CAS.
- A. S. Bashammakh, Extractive Spectrophotometric Determination of Bismuth(III) in Water Using Some Ion Pairing Reagents, E-J. Chem., 2011, 8, 1462–1471 CrossRef CAS.
- J. F. Ventura-Gayete, E. Rodenas-Torralba, A. Morales-Rubio, S. Garrigues and M. de La Guardia, A Multicommutated Flow System for Determination of Bismuth in Milk Shakes by Hydride Generation Atomic Fluorescence Spectrometry Incorporating On-Line Neutralization of Waste Effluent, J. AOAC Int., 2004, 87, 1252–1259 CrossRef CAS.
- R. J. Medeiros, L. M. G. dos Santos, A. S. Freire, R. E. Santelli, A. M. C. B. Braga, T. M. Krauss and S. d. C. Jacob, Determination of inorganic trace elements in edible marine fish from Rio de Janeiro State, Brazil, Food Control, 2012, 23, 535–541 CrossRef CAS.
- C. Moscoso-Perez, J. Moreda-Pineiro, P. Lopez-Mahia, S. Muniategui-Lorenzo, E. Fernandez-Fernandez and D. Prada-Rodrigez, Bismuth determination in environmental samples by hydride generation-electrothermal atomic absorption spectrometry, Talanta, 2003, 61, 633–642 CrossRef CAS.
- D. D. Afonso, S. Baytak and Z. Arslan, Simultaneous generation of hydrides of bismuth, lead and tin in the presence of ferricyanide and application to determination in biominerals by ICP-AES, J. Anal. At. Spectrom., 2010, 25, 726–729 RSC.
- T. Sahraeian, H. Sereshti and A. Rohanifar, Simultaneous Determination of Bismuth, Lead, and Iron in Water Samples by Optimization of USAEME and ICP–OES via Experimental Design, J. Anal. Test., 2018, 2, 98–105 CrossRef.
- E. Kilinc and F. Aydin, Optimization of Continuous Flow Hydride Generation Inductively Coupled Plasma Optical Emission Spectrometry for Sensitivity Improvement of Bismuth, Anal. Lett., 2012, 45, 2623–2636 CrossRef CAS.
- M. Welna, A. Szymczycha-Madeja and P. Pohl, Critical evaluation of strategies for single and simultaneous determinations of As, Bi, Sb and Se by hydride generation inductively coupled plasma optical emission spectrometry, Talanta, 2017, 167, 217–226 CrossRef CAS.
- R. C. Machado, C. D. B. Amaral, J. A. Nobrega and A. R. Araujo Nogueira, Multielemental Determination of As, Bi, Ge, Sb, and Sn in Agricultural Samples Using Hydride Generation Coupled to Microwave-Induced Plasma Optical Emission Spectrometry, J. Agric. Food Chem., 2017, 65, 4839–4842 CrossRef CAS.
- A. Matsumoto and T. Nakahara, Determination of Some Trace Elements in Steels by High Power Nitrogen Microwave Induced Plasma Atomic Emission Spectrometry Coupled with Hydride Generation Technique, Tetsu to Hagane, 2003, 89, 881–889 CrossRef.
- P. Schramel, I. Wendler and J. Angerer, The determination of metals (antimony, bismuth, lead, cadmium, mercury, palladium, platinum, tellurium, thallium, tin and tungsten) in urine samples by inductively coupled plasma-mass spectrometry, Int. Arch. Occup. Environ. Health, 1997, 69, 219–223 CrossRef CAS.
- Z. Wang, R. Gai, L. Zhou and Z. Zhang, Design modification of a solution-cathode glow discharge-atomic emission spectrometer for the determination of trace metals in titanium dioxide, J. Anal. At. Spectrom., 2014, 29, 2042–2049 RSC.
- J. A. C. Broekaert, Plasma bubbles detect elements, Nature, 2008, 455, 1185–1186 CrossRef CAS.
- S. Weagant and V. Karanassios, Helium-hydrogen microplasma device (MPD) on postage-stamp-size plastic-quartz chips, Anal. Bioanal. Chem., 2009, 395, 577–589 CrossRef CAS.
- M. Miclea, K. Kunze, G. Musa, J. Franzke and K. Niemax, The dielectric barrier discharge—a powerful microchip plasma for diode laser spectrometry, Spectrochim. Acta, Part B, 2001, 56, 37–43 CrossRef.
- A. Kitano, A. Iiduka, T. Yamamoto, Y. Ukita, E. Tamiya and Y. Takamura, Highly sensitive elemental analysis for Cd and Pb by liquid electrode plasma atomic emission spectrometry with quartz glass chip and sample flow, Anal. Chem., 2011, 83, 9424–9430 CrossRef CAS.
- W. C. Davis and R. K. Marcus, Role of powering geometries and sheath gas composition on operation characteristics and the optical emission in the liquid sampling-atmospheric pressure glow discharge, Spectrochim. Acta, Part B, 2002, 57, 1473–1486 CrossRef.
- J. C. T. Eijkel, H. Stoeri and A. Manz, A Molecular Emission Detector on a Chip Employing a Direct Current Microplasma, Anal. Chem., 1999, 71, 2600–2606 CrossRef CAS.
- A. J. Schwartz, K. L. Williams, G. M. Hieftje and J. T. Shelley, Atmospheric-pressure solution-cathode glow discharge: a versatile ion source for atomic and molecular mass spectrometry, Anal. Chim. Acta, 2017, 950, 119–128 CrossRef CAS.
- P. Pohl, P. Jamroz, K. Swiderski, A. Dzimitrowicz and A. Lesniewicz, Critical evaluation of recent achievements in low power glow discharge generated at atmospheric pressure between a flowing liquid cathode and a metallic anode for element analysis by optical emission spectrometry, TrAC, Trends Anal. Chem., 2017, 88, 119–133 CrossRef CAS.
- K. Swiderski, P. Pohl and P. Jamroz, A miniaturized atmospheric pressure glow microdischarge system generated in contact with a hanging drop electrode – a new approach to spectrochemical analysis of liquid microsamples, J. Anal. At. Spectrom., 2019, 34, 1287–1293 RSC.
- Z. Xing, J. Wang, S. Zhang and X. Zhang, Determination of bismuth in solid samples by hydride generation atomic fluorescence spectrometry with a dielectric barrier discharge atomizer, Talanta, 2009, 80, 139–142 CrossRef CAS.
- J. Kratzer, J. Bousek, R. E. Sturgeon, Z. Mester and J. Dedina, Determination of bismuth by dielectric barrier discharge atomic absorption spectrometry coupled with hydride generation: method optimization and evaluation of analytical performance, Anal. Chem., 2014, 86, 9620–9625 CrossRef CAS.
- M. Li, Y. Deng, C. Zheng, X. Jiang and X. Hou, Hydride generation-point discharge microplasma-optical emission spectrometry for the determination of trace As, Bi, Sb and Sn, J. Anal. At. Spectrom., 2016, 31, 2427–2433 RSC.
- M. Yuan, X. Peng, F. Ge, Q. Li, K. Wang, D.-G. Yu and Z. Wang, Simplified design for solution anode glow discharge atomic emission spectrometry device for highly sensitive detection of Ag, Bi, Cd, Hg, Pb, Tl, and Zn, Microchem. J., 2020, 155, 104785 CrossRef CAS.
- M. Gorska, K. Greda and P. Pohl, On the coupling of hydride generation (HG) with flowing liquid anode atmospheric pressure glow discharge (FLA-APGD) for simultaneous determination of traces of As, Bi, Hg, Sb and Se by optical emission spectrometry (OES), Talanta, 2021, 222, 121510 CrossRef CAS.
- Y. Gao, R. Liu and L. Yang, Application of chemical vapor generation in ICP-MS: a review, Chin. Sci. Bull., 2013, 58, 1980–1991 CrossRef CAS.
- X. Liu, Z. Zhu, Z. Bao, D. He, H. Zheng, Z. Liu and S. Hu, Simultaneous Sensitive Determination of Selenium, Silver, Antimony, Lead, and Bismuth in Microsamples Based on Liquid Spray Dielectric Barrier Discharge Plasma-Induced Vapor Generation, Anal. Chem., 2019, 91, 928–934 CrossRef CAS.
- P. Pohl and P. Jamroz, Recent achievements in chemical hydride generation inductively coupled and microwave induced plasmas with optical emission spectrometry detection, J. Anal. At. Spectrom., 2011, 26, 1317–1337 RSC.
- K. Greda, K. Swiderski, P. Jamroz and P. Pohl, Flowing Liquid Anode Atmospheric Pressure Glow Discharge as an Excitation Source for Optical Emission Spectrometry with the Improved Detectability of Ag, Cd, Hg, Pb, Tl, and Zn, Anal. Chem., 2016, 88, 8812–8820 CrossRef CAS.
- X. Liu, Z. Zhu, D. He, H. Zheng, Y. Gan, N. Stanley Belshaw, S. Hu and Y. Wang, Highly sensitive elemental analysis of Cd and Zn by solution anode glow discharge atomic emission spectrometry, J. Anal. At. Spectrom., 2016, 31, 1089–1096 RSC.
- K. Greda, S. Burhenn, P. Pohl and J. Franzke, Enhancement of emission from indium in flowing liquid anode atmospheric pressure glow discharge using organic media, Talanta, 2019, 204, 304–309 CrossRef CAS.
- P. Jamroz, P. Pohl and W. Zyrnicki, An analytical performance of atmospheric pressure glow discharge generated in contact with flowing small size liquid cathode, J. Anal. At. Spectrom., 2012, 27, 1032–1037 RSC.
- K. Swiderski, A. Dzimitrowicz, P. Jamroz and P. Pohl, Influence of pH and low-molecular weight organic compounds in solution on selected spectroscopic and analytical parameters of flowing liquid anode atmospheric pressure glow discharge (FLA-APGD) for the optical emission spectrometric (OES) determination of Ag, Cd, and Pb, J. Anal. At. Spectrom., 2018, 33, 437–451 RSC.
- X. Liu, Z. Zhu, P. Xing, H. Zheng and S. Hu, Plasma induced chemical vapor generation for atomic spectrometry: a review, Spectrochim. Acta, Part B, 2020, 167, 105822 CrossRef CAS.
- Y. S. Park, S. H. Ku, S. H. Hong, H. J. Kim and E. H. Piepmeier, Fundamental studies of electrolyte-as-cathode glow discharge-atomic emission spectrometry for the determination of trace metals in flowing water, Spectrochim. Acta, Part B, 1998, 53, 1167–1179 CrossRef.
- M. A. Mottaleb, Y.-A. Woo and H.-J. Kim, Evaluation of open-air type electrolyte-as-cathode glow discharge-atomic emission spectrometry for determination of trace heavy metals in liquid samples, Microchem. J., 2001, 69, 219–230 CrossRef CAS.
- P. Mezei, T. Cserfalvi and M. Janossy, Pressure Dependence of the Atmospheric Electrolyte Cathode Glow Discharge Spectrum, J. Anal. At. Spectrom., 1997, 12, 1203–1208 RSC.
- P. Mezei, T. Cserfalvi, H. J. Kim and M. A. Mottaleb, The influence of chlorine on the intensity of metal atomic lines emitted by an electrolyte cathode atmospheric glow discharge, Analyst, 2001, 126, 712–714 RSC.
- K. Greda, M. Gorska, M. Welna, P. Jamroz and P. Pohl, In-situ generation of Ag, Cd, Hg, In, Pb, Tl and Zn volatile species by flowing liquid anode atmospheric pressure glow discharge operated in gaseous jet mode - Evaluation of excitation processes and analytical performance, Talanta, 2019, 199, 107–115 CrossRef CAS.
- X. Liu, Z. Liu, Z. Zhu, D. He, S. Yao, H. Zheng and S. Hu, Generation of Volatile Cadmium and Zinc Species Based on Solution Anode Glow Discharge Induced Plasma Electrochemical Processes, Anal. Chem., 2017, 89, 3739–3746 CrossRef CAS.
- M. R. Webb, F. J. Andrade, G. Gamez, R. McCrindle and G. M. Hieftje, Spectroscopic and electrical studies of a solution-cathode glow discharge, J. Anal. At. Spectrom., 2005, 20, 1218–1225 RSC.
- Q. He, Z. Zhu, S. Hu and L. Jin, Solution cathode glow discharge induced vapor generation of mercury and its application to mercury speciation by high performance liquid chromatography-atomic fluorescence spectrometry, J. Chromatogr. A, 2011, 1218, 4462–4467 CrossRef CAS.
- K. Greda, P. Jamroz and P. Pohl, Comparison of the performance of direct current atmospheric pressure glow microdischarges operated between a small sized flowing liquid cathode and miniature argon or helium flow microjets, J. Anal. At. Spectrom., 2013, 28, 1233–1241 RSC.
- P. Jamroz and W. Zyrnicki, Spectroscopic Characterization of Miniaturized Atmospheric-Pressure dc Glow Discharge Generated in Contact with Flowing Small Size Liquid Cathode, Plasma Chem. Plasma Process., 2011, 31, 681–696 CrossRef CAS.
- A. Shaltout, Micro Plasma Generation Using Liquid Sampling-Atmospheric Pressure Glow Discharge, Microchim. Acta, 2006, 155, 447–452 CrossRef CAS.
- K. Greda, A. Szymczycha-Madeja and P. Pohl, Study and reduction of matrix effects in flowing liquid anode - atmospheric pressure glow discharge - optical emission spectrometry, Anal. Chim. Acta, 2020, 1123, 81–90 CrossRef CAS.
- A. R. Kumar and P. Riyazuddin, Chemical interferences in hydride-generation atomic spectrometry, TrAC, Trends Anal. Chem., 2010, 29, 166–176 CrossRef CAS.
- T. A. Doroski and M. R. Webb, Signal enhancement in solution-cathode glow discharge—optical emission spectrometry via low molecular weight organic compounds, Spectrochim. Acta, Part B, 2013, 88, 40–45 CrossRef CAS.
- R. Shekhar, Improvement of sensitivity of electrolyte cathode discharge atomic emission spectrometry (ELCAD-AES) for mercury using acetic acid medium, Talanta, 2012, 93, 32–36 CrossRef CAS.
- C. G. Decker and M. R. Webb, Measurement of sample and plasma properties in solution-cathode glow discharge and effects of organic additives on these properties, J. Anal. At. Spectrom., 2016, 31, 311–318 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ja00401d |
| This journal is © The Royal Society of Chemistry 2021 |