A new purification method based on a thiol silica column for high precision antimony isotope measurements†
Shuyang
Li
ab,
Yanli
Deng
a,
Hongtao
Zheng
ab,
Xing
Liu
a,
Peidong
Tang
c,
Jianwei
Zhou
c and
Zhenli
Zhu
 *a
*a
aState Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences, Wuhan, 430074, China. E-mail: zhuzl03@gmail.com; zlzhu@cug.edu.cn; Fax: +86 27 67883456; Tel: +86 27 67883452
bFaculty of Material Science and Chemistry, China University of Geosciences, Wuhan, 430074, China
cSchool of Environmental Studies, China University of Geosciences, Wuhan, 430074, China
First published on 2nd November 2020
Abstract
In this study, a new purification procedure for high precision Sb isotopic analysis was developed by using a thiol silica gel column and is applied to determine Sb isotope composition in natural water samples affected by mining activities. In our proposed procedure, Sb(V) was firstly pre-reduced with 0.5% (w/v) KI–ascorbic acid mixed solution and then Sb(III) in the solution could be separated and purified simply by elution with hydrochloric acid. With the developed procedure, the recovery of antimony was greater than 95.2% and blank values were <0.1 ng. An Apex Ω desolvating sample introduction system was used to improve the sensitivity during Sb isotopic measurement by MC-ICP-MS, and the instrumental mass discrimination was corrected using the sample–standard bracketing (SSB) method combined with the In doping technique. Isobaric effects, matrix effects, and concentration effects were all investigated in detail. 123Sb/121Sb ratios could be measured with a precision of 0.04‰ (2SD). To verify the reliability of the new method, both matrix containing mixed interference elements and a simulated matrix prepared by Sb standard reference material BHVO-2 doping with Spex Sb standard solution were determined. No measurable Sb isotope fractionation was observed with the developed thiol silica gel column procedure. Finally, natural water samples collected from Lengshuijiang were analysed, and significant Sb isotope variations (−0.20 to 0.27‰) were observed, which suggests that Sb isotopes may provide a promising tool to study antimony migration and pollution.
1. Introduction
Antimony (Sb) is one of the ten major non-ferrous metal elements and has been widely used in the production of flame retardants, semiconductors, ceramics, catalysts, alloys, plastics etc. Unfortunately, antimony, a metalloid, is toxic and carcinogenic and has become a global pollutant as a result of its extensive use and corresponding Sb-mining and smelting activities.1–4 Large quantities of Sb have been released into the environment resulting in serious Sb contamination, and very high levels of Sb pollution in natural water have also been detected.5,6 The presence of high Sb concentration in the environment poses significant health risks to humans and it is highly desirable that effective techniques to curb Sb pollution are developed. With the development of the MC-ICP-MS technique, Sb isotopes in environmental systems have also been studied and received increasing attention due to their potential in identifying sources and/or elucidating environmental and biogeochemical processes.7–12Antimony has two stable isotopes, 123Sb and 121Sb, with an abundance of 57.213% and 42.787%.13 In recent years, significant variations of Sb isotopic composition have been reported in various geological and environmental samples.7–9,14–16 It was found that biological processes,10 reduction8,10 and adsorption processes,12 as well as evaporation and precipitation7 in environmental systems could produce significant isotope fractionation. These observations imply that Sb isotopes might be a useful environmental tracer to reveal the source, transformation, migration and geochemical processes of Sb in the environment. Currently, high precision Sb isotope ratio measurement is mostly achieved by using multi-collector inductively coupled plasma-mass spectrometry (MC-ICP-MS).7,8,11,14–17 However, during the measurement, the interference of isobars such as Te can affect the accuracy. In addition, non-spectral interference arising from geological matrices could also affect the precision of Sb isotopic analysis. Thus, it is necessary to separate and purify Sb in the pre-treatment process to reduce or eliminate potential interferents contained in natural samples before isotopic analysis.18
Recent studies have reported preconcentration and separation methods for Sb mainly employing thiol cotton fiber (TCF) based methods and ion exchange resin methods.8–11,14–17 Many studies applied TCF to separate As, Sb, Sn, Se, Hg, etc.9,19–21 In 2003, Rouxel et al.8 first developed a method by using TCF and AG50-X8 cation exchange resin to purify Sb for isotopic analysis. Later, Asaoka et al.9 reported a modified TCF procedure and bypassed the cation exchange resin step. In 2015, Resongles et al.11 further improved the method using thiol cotton powder (TCP) and found that TCP was more efficient in purifying Sb. TCF/TCP offers several excellent properties at a low price including strong hydrophilicity, a large number of absorbable elements, and good selectivity.21 However, the preparation of TCF/TCP is typically time-consuming and may introduce impurities because of the use of different organic reagents.22 Moreover, it is hard to ensure good reproducibility between and within batches. To solve these problems, Lobo et al.14–17 developed a Sb separation method using an ion exchange resin, where they employed AG50-X8 cation exchange resin and IRA 743 anion exchange resin to separate Sb from matrix elements. They successfully measured Sb isotopic compositions in Roman glass and stibnite samples. Compared with TCF/TCP based methods using an ion-exchange resin seems to be simpler and faster for pre-concentration and separation of antimony. However, it should be noted that the recovery of Sb is typically low, in particular when using an anion exchange resin,23 which may hinder its wide application. Additionally, the use of a significant amount of HF creates an operational risk which would be better avoided if possible. More recently, Liu et al.24 developed a method for purifying antimony (Sb) from geological samples by using two successive chromatographic columns loaded with AG 1-X4 and AG 50W-X8 resin, which resulted in a better recovery compared to previous studies. It is still of interest to develop alternative separation methods for high precision Sb isotope analysis. In this regard it is of note that thiol resins are widely used to enrich and separate elements including Sb, Se, Cd, Sn, Hg, and As.25,26 Chang et al.22 have developed a preconcentration and separation method for Se isotopic analysis using thiol resins, which is fast, efficient and reproducible for Se purification from seawater.
In the present study, we present a new method to separate Sb from geological samples for precise isotopic measurement by MC-ICP-MS. The method, based on a thiol silica-gel column, is very simple and the entire separation process only requires MQ water and hydrochloric acid. Additionally, high precision Sb isotopic measurements using a membrane desolvation system are demonstrated. Isobaric and matrix problems along with concentration effects were investigated in detail. The accuracy of the method was demonstrated by the analysis of artificial matrix containing samples. Finally, the proposed purification method was successfully applied to the analysis of Sb isotopes in natural water samples affected by mining activities.
2. Experimental
2.1 Instrumentation
123Sb/121Sb isotope ratios were measured using a Nu Plasma II MC-ICP-MS instrument (Nu instruments, UK) which is able to measure high-precision isotope ratios of elements in the mass range of 3–300 amu. Its detection system consists of 16 Faraday cups and 5 ion counters. Table 1 provides the instrument settings and acquisition parameters used for isotopic analysis. Samples were introduced into the plasma source by means of an Apex Ω membrane desolvation system (Elemental Scientific, USA) with the concentric nebulizer working at a 100 μL min−1 sample uptake rate. Elemental concentrations were measured using an ICP-MS Agilent 7700× ICP-MS instrument (Agilent Technologies, USA) with an uptake rate of 0.96 mL min−1 using a MicroMist glass nebulizer (GE, Australia), and all solution samples were acidified and diluted in 2% nitric acid for chemical analysis.| Instrument | Component | Parameters |
|---|---|---|
| MC-ICP-MS | Sampling/skimmer cone | Nickel |
| RF power | 1300 W | |
| Sample uptake rate (μL min−1) | 100 | |
| Coolant gas (L min−1) | 13.00 | |
| Auxiliary gas (L min−1) | 0.85 | |
| Scan times | 2 × 20 | |
| Integration time | 10 s | |
| Membrane desolvator | Nebulization temperature | 140 °C |
| Cooler temperature | 3 °C | |
| Sweep gas (L min−1) | 2.90 |
| Cup configuration | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cup | H7 | H6 | H5 | H4 | H3 | Ax | L2 | L4 | L5 |
| Isotopes | 125Te | 123Sb | 122Sn | 121Sb | 120Sn | 117Sn | 115In | 113In | 111Cd |
The combination of sample–standard bracketing (SSB) and the In doping technique was used to correct the instrument mass discrimination, which enabled the determination of accurate and precise Sb isotope ratios.
Variations in the isotopic composition of Sb samples were expressed as δ123Sb (eqn (1)), relative to Spex standard Sb solution.
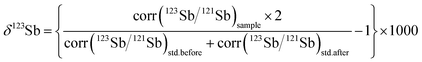 | (1) |
In eqn (1), std.before is the Spex standard solution tested before the sample while std.after refers to the following solution.
2.2 Materials, reagents and standards
All acids used in this work were twice-purified with a Savillex's DST-1000 sub-distillation system before use. Ultrapure water (18.2 MΩ cm) used for all the experiments was produced from a water purification system (Millipore Company, America). Argon (99.999%) was obtained from Heyuanqingsheng Gas Co. (Wuhan, China). Stock solutions of inorganic In, Sn, Cd, Te, and As single element standard solutions (1000 μg mL−1) were obtained from the National Analysis Center for Iron and Steel (Beijing, China). Potassium iodide and ascorbic acid (Analytical Reagent, National Drug Reagent Co. Ltd, Shanghai, China) were used to pre-reduce samples. A commercialized column packed with thiol chemically bonded silica (Cleaner SH, Tianjin Bonna-Agela Technologies Inc., China, pore size: 60 Å, grain size: 40 μm, specification: 0.25 g/6 mL, length: 60 mm, internal diameter: 10 mm; surface area: 490 m2 g−1) was employed for Sb separation. Spex Sb standard solution (Lot #: CL8-06SBY, Spex CertiPrep Company, America) was used as a “δ-zero” isotopic standard solution to calculate the δ123Sb value. The basalt standard reference material BHVO-2 obtained from USGS was used to prepare a matrix solution for the validation of the proposed purification method.2.3 Sb purification procedure
An antimony preconcentration and purification procedure using the thiol silica column was modified referring to the work of Asaoka et al.;9 and the optimized procedure is summarized in Fig. S1.† The columns were firstly prewashed with 6 M HCl (5 mL), and 2.5 M HCl (5 mL) sequentially, and then conditioned with 0.5 M HCl (5 mL). Prior to sample loading, Sb in samples adjusted with 0.5 M HCl was pre-reduced to Sb(III) by using a 10% (w/v) KI–ascorbic acid mixed solution to make a final concentration of 0.5% (w/v) for 5 h. Subsequently, 5 mL of the sample solution containing 1000–2000 ng Sb was loaded onto the column. Sb(III) in the solution was retained on the resin, whereas most of the matrix elements passed through the column. To remove the residual matrix (such as Cd, In, As, Sn, etc.), the columns were rinsed with 0.5 M HCl (5 mL) and 2.5 M HCl (5 mL), sequentially. Finally, Sb was eluted from the columns by passing 5 mL of 6 M HCl. The final eluate was kept in a PTFE beaker on a hotplate, after evaporation to near dryness at 90 °C, and then concentrated HNO3 (1 mL) and H2O2 (0.2 mL) were added. The residues were heated for more than 8 hours at 100 °C for secondary digestion to remove impurities such as fillers and other organic matter that might be picked up during the column separation process. After digestion, it was finally diluted to 75–100 ng mL−1 with 2% HNO3 for the MC-ICP-MS test. The yield of the extraction procedure was tested by ICP-MS.2.4 Sample collection and digestion
Natural water samples were collected around the Xikuangshan antimony mine in Lengshuijiang City, Hunan Province, China. The Xikuangshan mine is the world's largest reservoir of antimony which is stibnite buried in a Devonian limestone formation.27 The water samples were collected from different locations, which include rain water near tailings, river water and groundwater near mining areas. Samples for Sb isotopic analysis were collected in 500 mL high-density polyethylene (HDPE) bottles after filtering through 0.45 μm membranes (Millipore). Filtered samples for analysis were acidified to a pH value of less than 2.0 in the field with ultrapure HNO3.(1) Water sample digestion procedure
The digestion procedure was performed on a hot plate in several steps. First, a volume of water containing 1000–2000 ng Sb was weighed into a PTFE beaker, and then evaporated to near dryness at 90 °C. A mixture of 2 mL concentrated HNO3 and 0.4 mL H2O2 was added to the vessel which was sealed and held at 100 °C for 12 h to remove organic material. Subsequently, the vessel was opened and the mixture was evaporated to almost dryness at 90 °C. The residue was then redissolved in 4.5 mL of 0.5 M HCl. After digestion, the elemental concentrations of Sb were measured by ICP-MS.
(2) BHVO-2 digestion procedure
For the BHVO-2 basalt standard sample, 100 mg of sample powder was precisely weighed into a PTFE-lined stainless steel bomb, and then 1 mL HNO3 and 1 mL HF were slowly added. Then bomb was sealed and heated in an oven at 190 °C for 48 hours to ensure complete sample digestion. After cooling, the bomb was opened and placed on a hotplate at 120 °C to evaporate the sample solutions. Then, 1 mL HNO3 was added and evaporated to dryness at 120 °C in order to remove HF. After that, 3 mL 30% HNO3 was added, and the bomb was sealed and heated in an oven at 190 °C for 12 hours. Subsequently, the sample solution was evaporated to dryness and redissolved in 5 mL of 0.5 mol L−1 HCl. Finally, 1 mL of this BHVO digested solution and 2 mL of Spex Sb solution (1000 ng mL−1) were mixed and evaporated to dryness and then redissolved in 5 mL of 0.5 mol L−1 HCl prior to the column separation.
3. Results and discussion
3.1 Development of the Sb purification procedure using the thiol silica gel column
The determination of Sb isotope ratios by MC-ICP-MS is susceptible to isobaric (123Te on 123Sb) and polyatomic ion (122SnH+, 120SnH+, 120TeH+, etc.) interference.8 In this study, indium was selected as the internal standard element to correct for the instrumental mass bias during Sb measurements but this also requires the assessment of interference that may affect the In (115In and 113In) measurement such as 115Sn, 75As40Ar+, 113Cd, 75As38Ar+, 112SnH+, etc.Table 2 illustrates the main spectral interference affecting Sb isotopic analysis, indicating the need for efficient separation of Te, Sn, As, and Cd. In addition, matrix effects, caused by major cations present in samples (such as Ca, K, Na, Mg, etc.), may potentially impact Sb isotope determination. Therefore, it was also necessary to remove these interfering elements as much as possible in the process of separation before Sb isotopic measurement by MC-ICP-MS.| 111Cd | 113In | 115In | 117Sn | 121Sb | 123Sb | 125Te |
|---|---|---|---|---|---|---|
| Ga40Ar+ | 113Cd | 115Sn | 116SnH+ | 120SnH+ | 123Te | 124SnH+ |
| 73Ge38Ar+ | 73Ge40Ar+ | 75As40Ar+ | 77Se40Ar+ | 120TeH+ | 122SnH+ | |
| 75As36Ar+ | 75As38Ar+ | 82Se35Cl+ | 83Kr38Ar+ | |||
| 77Se36Ar+ | 80Se37Cl+ | |||||
| 112SnH+ | ||||||
| 75As38Ar+ |
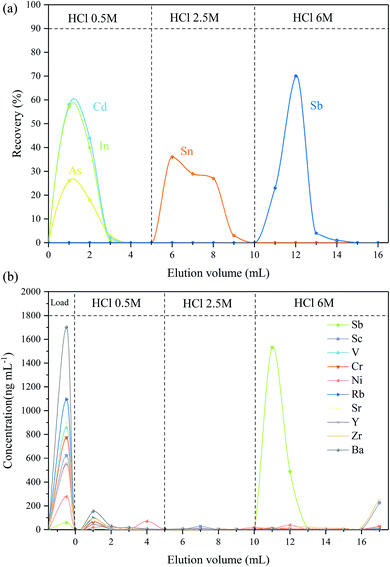
The required concentration of HCl for the effective separation of Sb from other interfering elements (As, Cd, In, Sn and Te) was first investigated using the thiol silica gel column. It was found that As, Cd and In could be eluted efficiently with 0.5 M HCl, whereas Sn could be separated from the Sb fraction by using a 2.5 M or stronger HCl solution. This result is quite similar to a previous TCF study.9 It was also observed that Sb(III) was collected completely by using 6 M HCl. Thus, after optimization, 5 mL of 0.5 M HCl and 5 mL of 2.5 M HCl were employed to separate the interfering elements before Sb collection; thereafter, Sb was collected by use of 6 M HCl (6 mL).
In the next stage of this study a mixed solution containing Sb, Sn, Te, In, Cd and As was used to test the efficiency of matrix separation. It was observed that Sb and other elements with high adsorption capability (e.g. Te, Sn, and As) were retained on the column while major cations directly passed through the column during the sample loading steps, which agrees with other reported studies using TCF.8,11 From the leaching profile shown in Fig. 1a, it is clear that As, In and Cd were completely eluted from the column with the first 3 mL of 0.5 M HCl elution, and then Sn was efficiently removed in the 2.5 M HCl fraction, and finally Sb was completely collected with 6 M HCl. It was also found that Te did not elute during the whole separation process. During rinsing, the droplet flow rate was controlled by pressurization at a droplet rate of about one every 10 s, and the separation and enrichment process typically can be achieved within 5 hours. The recovery of Sb in the separation process was greater than 95% in repeated trials; the blank of the process was <0.1 ng. The concentration of Sn, Te, In, Cd and As in the collected Sb fraction was below the detection limit. The results indicated that the proposed method employing the thiol silica column could achieve efficient separation of Sb from the interfering elements, and the presence of these elements did not affect Sb elution.
In addition to the noted isobaric interference, the effect of other matrix elements in geological samples was also accessed using the BHVO-2 basalt standard sample as a representative sample (Sb concentration, 0.1034 μg g−1). BHVO-2 was digested and then repeatedly dried and re-diluted with 2% HNO3 to a final dilution factor of 250. Then, 2 mL of Spex Sb solution (1000 ng mL−1) was added to the BHVO solution. It should be noted here that the added Sb (2000 ng) is more than 5000 times the Sb in BHVO solution, and thus it can be assumed that the Sb isotopic composition in the final solution is equal to that of the Spex Sb standard (δ = 0). This synthesized Sb sample was then processed using the same pre-reduction and leaching procedure previously outlined, and the leaching profile is displayed in Fig. 1b. It was found that Sb was quantitatively retained on the resin, whereas alkaline earth metals (Sr and Ba), transition metals (V, Cr, and Ni), and main group elements (In and Tl) passed through the column during the sample loading steps. Only a few residual ions remained after 0.5 M HCl elution. Sb was also efficiently separated from potentially interfering elements, and the recovery of Sb was greater than 95.2%. Although a small amount of Zr and Sc was also eluted by 6 M HCl, Zr and Sc do not cause spectral interference with Sb; thus, the separation of Sb from Zr and Sc was not considered necessary.
The above experiments clearly demonstrated that the optimized procedure can remove almost all interfering elements and typical matrix elements in natural samples will not significantly affect the retention and elution of Sb. To determine whether our method can reduce the matrix elements to acceptable levels for Sb isotopic analysis, we assessed the influence of these elements on the measurement of 121Sb/123Sb isotope ratios.
3.2 Evaluation of isobaric and matrix interference
The determination of Sb isotopes, as for any other isotopic measurement by MC-ICP-MS, is susceptible to isobaric interference and matrix effects. Although most of these interferents may be largely removed by the separation procedure, any residual coexisting ions may still be present at a level that affects the accuracy and precision of Sb analysis. The interference of isobaric ions (Te, Sn, Cd, etc.) and some matrix elements (K, Ca, Na, Mg, etc.) was evaluated in detail in this study.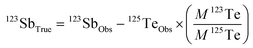 | (2) |
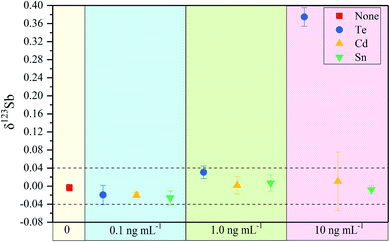
In eqn (2), 123SbTrue represents the true Sb isotope value; Obs is the observed value; M is the natural abundance. From eqn (2), the interference of the trace elements in samples such as Sn, Te, and Cd, which may remain after the purification process, can be corrected. However, this correction method is typically only limited for low concentrations of the interfering elements. We investigate here how effective the correction method is for various interferents by doping Te, Sn and Cd into Spex Sb standard solution (Fig. 2).
The 123Sb/121Sb isotope ratio of Spex Sb standard solutions (100 ng mL−1) doped with different amounts of Te, Cd and Sn (i.e. 0.1 ng mL−1, 1 ng mL−1 and 10.0 ng mL−1) was determined using 75 ng mL−1 In as an internal standard. As shown in Fig. 2, the effect of Te on δ123Sb can be neglected when the concentration of Te is below 1.0 ng mL−1 even after Te correction. However, when the concentration of Te reached 10 ng mL−1, the accuracy of the test decreased greatly with a deviation of +0.40‰ observed on the 123Sb/121Sb value. In contrast, no significant effect occurred in the presence of Sn at levels as high as 10 ng mL−1, and an external reproducibility could be maintained within 0.04‰. In the case of Cd, it was found that Cd did not affect the 123Sb/121Sb value significantly even at a concentration of 10 ng mL−1, though the precision of the measurement surprisingly deteriorated, especially for Cd at 10 ng mL−1. From these results, we can conclude that the determination of Sb isotopes should be performed with Te/Sb < 0.01, Sn/Sb < 0.1 and Cd/Sb < 0.01. After our separation and enrichment process, Te/Sb, Sn/Sb and Cd/Sb can be reduced to below 0.001, and thus the influence of Te, Cd and Sn on Sb isotopic determination can be neglected. The purification method presented in this study can remove these critical interfering elements to acceptable levels for the Sb isotopic analysis.
3.3 The precision and accuracy for Sb isotopic measurement
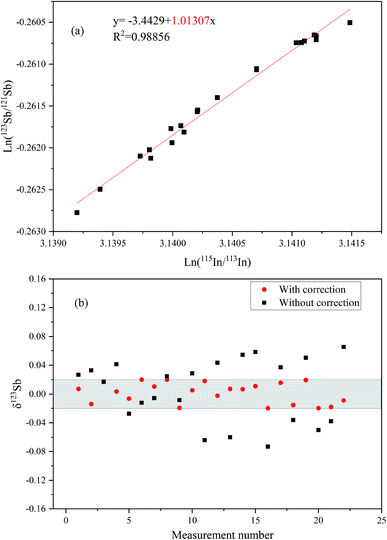
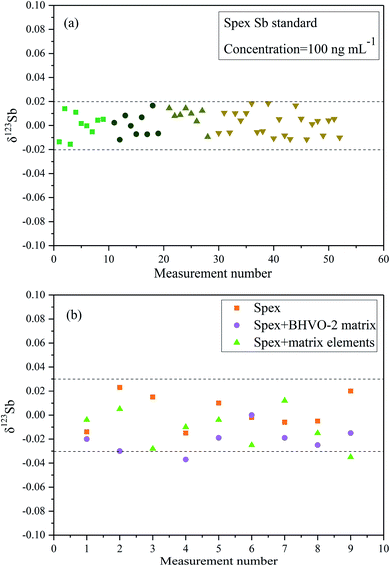
Fig. 4a presents the ln–ln plots of 115In/113In and 123Sb/121Sb of Spex Sb standard solution based on long-term determinations over three months (after SSB correction). The slope of the line is about 1.013 close to the theoretic value (ln(mass115In/mass113In)/ln(mass123Sb/mass121Sb) = 1.07) predicted by the exponential law, which indicates that In and Sb behave similarly in the MC-ICP-MS measurement and In can be used as an effective internal standard to correct mass discrimination in the determination of Sb isotope ratios.16 We further compared the values of δ123Sb of the corrected data using the Indium-internal standard method and uncorrected data simply by SSB in this study (Fig. 4b). The results clearly demonstrated that using In as an internal standard can better correct instrumental mass discrimination and significantly improve precision and accuracy, where the external precision of measuring Spex standard solution was 0.04‰ (2SD).Fig. 5a shows the multiple Sb isotope measurements from April to May 2019. The ion beam intensity of In (75 ng mL−1) is about 1.5 V and 36 V of 113In and 115In, respectively. The sensitivity of 121Sb and 123Sb can reach 13.5 and 10.5 V by using an Apex Ω membrane desolvation system. The long-term external precision of the standard solution was 0.04‰ (2SD, n = 52), which is comparable with those in other previously reported studies.8,9,14–16
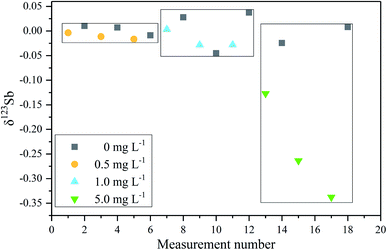
In order to verify the efficiency of the purification method based on the thiol silica gel column and its possible effect on the Sb isotope measurement, Spex Sb standard samples doped with different matrix elements were measured. Both the synthetic matrix containing Sn, Cd, In, As, and Te and a matrix from the reference material BHVO-2 were employed. As shown in Fig. 5b, it is clearly observed that there was no observable difference in the measurement accuracy between doped solutions (both synthetic matrix and BHVO matrix) and pure Spex Sb solution. The precision of the Spex solution after elution with the column is about 0.06‰ (2SD), slightly worse than the 0.04‰ (2SD) of the pure Spex standard solution, which is sufficient to investigate Sb isotopes in environmental samples. These results further demonstrated that the efficient separation of the matrix elements and the good recovery of Sb of the proposed thiol silica gel column procedure give confidence in high precision Sb isotopic determination.
3.4 Isotopic determination of natural water samples
Sb isotopes of water samples collected from different locations near Xikuangshan in Hunan Province were measured. The rising concentration of antimony in aquifers presents a significant threat to the local water supply. Antimony isotopic data may provide powerful tools for tracing Sb cycling and mobilization, but the Sb isotopic composition of these samples has not been determined to the best of our knowledge. In this study, water samples were collected from different locations in the Xikuangshan mine area, including rivers near mining pits, groundwater, leachate and spring water. The Sb isotopic compositions of water samples obtained in this study are listed in Table 3 and also shown in Fig. S2.† Each sample was tested three times. It should be noted that all the determined Sb isotope values for each sample are comparable, further demonstrating the reliability of our proposed method.| Sample | Sample type | Sb concentration (mg L−1) | Recovery | δ 123Sb | 2SD (N = 3) |
|---|---|---|---|---|---|
| S1 | River water | 1.00 | 97.3% | 0.19 | 0.02 |
| S2 | River water | 1.87 | 96.5% | 0.22 | 0.04 |
| S3 | River water | 1.70 | 96.9% | 0.19 | 0.03 |
| R1 | Rain water | 0.03 | 97.1% | 0.17 | 0.02 |
| L1 | Waste mineral water/slag pile leachate | 29.8 | 99.2% | 0.27 | 0.04 |
| M1 | Groundwater | 5.73 | 95.8% | −0.20 | 0.04 |
| M2 | Groundwater | 4.32 | 95.3% | −0.11 | 0.02 |
| W1 | Spring water/well water | 16.7 | 98.7% | −0.09 | 0.03 |
Among them, S1, S2 and S3 are samples of Feishuiyanxi stream, M1 and M2 are groundwater samples near mining pits, R1 is a sample of rain water near tailings, W1 is a sample of well water and L1 is a leachate sample near the waste slag. It is observed that all the collected water samples are heavily contaminated with Sb, with the Sb concentration from river, groundwater, leachate and well water all >1 mg L−1. The Sb in the rain water also exceeds China's and WHO's guidelines for drinking water of 0.01 mg L−1. The antimony isotopic composition (δ123Sb) of the mine-affected water ranged from −0.11‰ to 0.27‰, which demonstrates that natural Sb isotopic variations are significant in water samples from different sources in this area. The external reproducibility of our test method was 0.04‰ (2SD), which can meet the requirements of Sb isotopic measurement of natural water samples.
By comparing the results of the groundwater samples (M1 and M2) and river water samples (S1, S2 and S3), we observed a significant increase of the δ123Sb signature (from −0.20‰ and −0.11‰ to about +0.2‰) and a decrease of Sb concentration (from 4.32–5.73 mg L−1 to 1.00–1.87 mg L−1). W1 is a well water sample with a high Sb concentration and its isotopic composition of δ123Sb was −0.09 ± 0.03‰ (n = 3). All these data show a trend of enrichment of light isotopes in ground water, which is likely related to the interaction of groundwater with host aquifer rocks in the mining area. The Sb isotopic composition of the rain water sample R1 with a low Sb concentration is 0.17 ± 0.02‰ (n = 3), which is difficult to interpret but it is speculated that waste gas or dust pollution in the process of metal melting may have an effect on local rainfall. The Sb concentration of L1 is especially high with a value of 29.8 mg L−1, and the isotopic composition also gives the highest value. Although the fractionation mechanism is not clear, the Sb isotopic data indicate that the leachate might be a source of pollution in the mine-affected watershed. In summary, the data showed that significant variation of Sb isotopes occurs in the mining-affected area, and it holds promise to be used as a proxy for source tracing of Sb pollution.
4. Conclusion
In this study, we developed a new simple purification method for the precise determination of Sb isotopes in natural water samples through preconcentration by using thiol resin. In the presented procedure, potassium iodide and ascorbic acid were added to pre-reduce the sample, and then a thiol silica gel column was employed to separate Sb from other matrix elements. Using this new method, isobaric ions and major matrix elements could be clearly removed. The recovery of antimony was greater than 95.2% and that of blank values was <0.1 ng. The thiol resin preconcentration and separation procedure was reproducible and had a high separation efficiency and short sample preparation time. In addition, the method developed for the preconcentration of Sb caused no isotope fractionation. 123Sb/121Sb ratios could be measured with a precision of 0.04‰ (2SD) and instrumental mass discrimination was corrected for using the SSB method combined with an In internal standard. The proposed method was validated by the analysis of a Spex Sb standard solution doped with different matrix elements. In addition, different types of water samples collected from the Xikuangshan antimony mine of Hunan Province were successfully measured in this study. Significant Sb isotopic fractionation was observed among these natural water samples, indicating that the stable Sb isotopes could be an effective tool for tracing Sb pollution and related processes in the environment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (no. 21822405, 41673014, 41572344, and 41521001), National Key Research and Development Program (2017YFD0801202), and Natural Science Foundation of Hubei Province (2016CFA038).References
- M. Filella, N. Belzile and Y. W. Chen, Earth-Sci. Rev., 2002, 57, 125–176 CrossRef CAS.
- S. Sundar and J. Chakravarty, Int. J. Environ. Res. Public Health, 2010, 7, 4267 CrossRef CAS.
- M. Filella and P. A. Williams, Geochemistry, 2012, 72, 49–65 CrossRef CAS.
- C. Casiot, M. Ujevic, M. Munoz, J. L. Seidel and F. Elbaz-Poulichet, Appl. Geochem., 2007, 22, 788–798 CrossRef CAS.
- X. Wang, M. He, J. Xie, J. Xi and X. Lu, J. Soils Sediments, 2010, 10, 827–837 CrossRef CAS.
- J. Li, B. Zheng, Y. He, Y. Zhou, X. Chen, S. Ruan, Y. Yang, C. Dai and L. Tang, Ecotoxicol. Environ. Saf., 2018, 156, 125–134 CrossRef CAS.
- M. Tanimizu, Y. Araki, S. Asaka and Y. Takahashi, Geochem. J., 2011, 45, 27–32 CrossRef CAS.
- O. Rouxel, J. Ludden and Y. Fouquet, Chem. Geol., 2003, 200, 25–40 CrossRef CAS.
- S. Asaoka, Y. Takahashi, Y. Araki and M. Tanimizu, Anal. Sci., 2011, 27, 25–28 CrossRef CAS.
- S. Wehmeier, R. Ellam and J. Feldmann, J. Anal. At. Spectrom., 2003, 18, 1001–1007 RSC.
- E. Resongles, R. Freydier, C. Casiot, J. Viers, J. Chmeleff and F. Elbaz-Poulichet, Talanta, 2015, 144, 851–861 CrossRef CAS.
- Y. Araki, M. Tanimizu and Y. Takahashi, Geochim. Cosmochim. Acta, 2009, 73(13), A49 Search PubMed.
- V. I. Zherebchevsky, I. E. Alekseev, K. A. Gridnev, E. B. Krymov, T. V. Lazareva, N. A. Maltsev, R. B. Panin, N. A. Prokofyev, S. Y. Torilov and A. I. Shtamburg, Bull. Russ. Acad. Sci.: Phys., 2016, 80, 888–893 CAS.
- L. Lobo, P. Degryse, A. Shortland, K. Eremin and F. Vanhaecke, J. Anal. At. Spectrom., 2014, 29, 58–64 RSC.
- L. Lobo, P. Degryse, A. Shortland and F. Vanhaecke, J. Anal. At. Spectrom., 2013, 28, 1213–1219 RSC.
- L. Lobo, V. Devulder, P. Degryse and F. Vanhaecke, J. Anal. At. Spectrom., 2012, 27, 1304–1310 RSC.
- P. Degryse, L. Lobo, A. Shortand, F. Vanhaecke, A. Blomme, J. Painter, D. Gimeno, K. Eremin, J. Greene, S. Kirk and M. Walton, J. Archaeol. Sci., 2015, 62, 153–160 CrossRef CAS.
- J. Barling and D. Weis, J. Anal. At. Spectrom., 2008, 23, 1017–1025 RSC.
- N. Elwaer and H. Hintelmann, J. Anal. At. Spectrom., 2008, 23, 733–743 RSC.
- S. Z. Zhang, A. X. Lu, F. Han and X. Q. Shan, Anal. Sci., 2005, 21, 651–654 CrossRef CAS.
- M. Q. Yu, D. W. Sun, W. Tian, G. P. Wang, W. B. Shen and N. Xu, Anal. Chim. Acta, 2002, 456, 147–155 CrossRef CAS.
- Y. Chang, J. Zhang, J. Q. Qu and Y. Xue, Chem. Geol., 2017, 471, 65–73 CrossRef CAS.
- B. Wen, J. W. Zhou, A. G. Zhou, C. F. Liu and L. G. Li, Int. Biodeterior. Biodegrad., 2018, 128, 109–116 CrossRef CAS.
- J. F. Liu, J. B. Chen, T. Zhang, Y. N. Wang, W. Yuan, Y. C. Lang, C. L. Tu, L. Z. Liu and J. L. Birck, J. Anal. At. Spectrom., 2020, 35, 1360–1367 RSC.
- M. Biver and W. Shotyk, Geochim. Cosmochim. Acta, 2012, 79, 127–139 CrossRef CAS.
- Y. Xu and M. A. A. Schoonen, Am. Mineral., 2000, 85, 543–556 CrossRef CAS.
- J. T. Peng, R. Z. Hu, H. L. Deng and W. C. Su, Geochimica, 2001,(03), 248–256 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ja00367k |
| This journal is © The Royal Society of Chemistry 2021 |