Lab-on-a-chip technology for in situ combined observations in oceanography
Tatsuhiro
Fukuba
 *ab and
Teruo
Fujii
*ab and
Teruo
Fujii
 b
b
aInstitute for Marine-Earth Exploration and Engineering, Japan Agency for Marine-Earth Science and Technology, Natsushima-cho 2-15, Yokosuka, Kanagawa 237-0061, Japan. E-mail: bafuk@jamstec.go.jp
bInstitute of Industrial Science, The University of Tokyo, Komaba 4-6-1, Meguro-ku, Tokyo, 153-8505, Japan
First published on 10th December 2020
Abstract
The oceans sustain the global environment and diverse ecosystems through a variety of biogeochemical processes and their complex interactions. In order to understand the dynamism of the local or global marine environments, multimodal combined observations must be carried out in situ. On the other hand, instrumentation of in situ measurement techniques enabling biological and/or biochemical combined observations is challenging in aquatic environments, including the ocean, because biochemical flow analyses require a more complex configuration than physicochemical electrode sensors. Despite this technical hurdle, in situ analyzers have been developed to measure the concentrations of seawater contents such as nutrients, trace metals, and biological components. These technologies have been used for cutting-edge ocean observations to elucidate the biogeochemical properties of water mass with a high spatiotemporal resolution. In this context, the contribution of lab-on-a-chip (LoC) technology toward the miniaturization and functional integration of in situ analyzers has been gaining momentum. Due to their mountability, in situ LoC technologies provide ideal instrumentation for underwater analyzers, especially for miniaturized underwater observation platforms. Consequently, the appropriate combination of reliable LoC and underwater technologies is essential to realize practical in situ LoC analyzers suitable for underwater environments, including the deep sea. Moreover, the development of fundamental LoC technologies for underwater analyzers, which operate stably in extreme environments, should also contribute to in situ measurements for public or industrial purposes in harsh environments as well as the exploration of the extraterrestrial frontier.
1. Introduction
Oceans, which involve a complex circulation of huge amounts of seawater, are the most macroscopic fluid systems on the planet. They include processes on numerous spatiotemporal scales such as global heat cycles, mass cycles, and global environmental regulation. These phenomena are attributed to the general circulation of seawater, the formation of water masses via vertical mixing due to local and complex ocean currents, seawater density changes, and the resultant formation of characteristic weather.1 Understanding the intricate structure and diversity of the Earth's unique ecosystems as well as the dynamism of climate change, which are formed by the complex interrelationships of these processes, is essential for the sustainable development of human society.Dissolved macronutrients, including phosphates, nitrates, and silicates, are supplied to the ocean environment naturally and from human activities. Their behavior is dictated by the circulation of seawater.1,2 The dynamics and availability of macronutrients are factors that regulate the growth and decay of planktonic primary producers that support biological production.3 On the other hand, excessive nutrient input to the ocean is a major factor of harmful algal blooms (HAB). However, the complex relationships between the timing of nutrient supply, HAB species composition, and other environmental factors have yet to be fully elucidated.4,5 In addition, with rising atmospheric carbon dioxide (CO2) concentrations and the resultant concerns about global warming, ocean CO2 uptake and its role in the global carbon cycle are matters of serious scientific interest.6 In particular, ocean acidification due to rising oceanic CO2 concentrations may seriously impact the marine ecosystem.7,8 The marine environment is the richest repository of biological and genetic diversity, and efforts to understand, protect, and equitably distribute its natural resources have become more important.9
In recent years, remote sensing utilizing satellites and airplanes has made global, wide-area observations of the marine environment and biological parameters available.10,11 However, direct observations by research vessels and other underwater or surface observation platforms are still necessary to understand details about the ocean's interior. Therefore, reliable and portable in situ measurement technologies continue to be an essential component of modern oceanography.
Ocean observations and the development of innovative tools have continually progressed.12–14 For conventional measurements in the marine environment, observation platforms related to the marine industry or marine science such as in situ measurements and water sampling suites represented by CTD (conductivity, temperature, and depth) profilers or CTD-RMS (rosette multi sampler) have been deployed using wirelines from a research vessel,12,15 manned submersibles,16 and recently unmanned underwater vehicles.17–19 Previous underwater sensors or analyzers, which weighed between a few kilograms and several tens of kilograms in water and ranged in size from several tens of centimeters to several meters, have been mounted on these relatively large underwater observation platforms. However, there has been a trend toward miniaturization in ocean measurements. Instead of heavy-duty work class ROVs operated with large support vessels and winches, which were originally developed for the oil industry and underwater salvage, new platforms and small vessels such as ultra-compact, low-cost, tabletop-sized small ROVs or underwater drones20,21 and micro AUVs22 are used for underwater observations. Furthermore, advanced platforms such as underwater gliders23–25 that use a buoyancy control mechanism to glide through the ocean and wave gliders26,27 that can convert wave power into propulsion have been put into full-scale operation.
Among the practical small undersea observation platforms, Argo floats28,29 are the most widely deployed for scientific purposes. Today, thousands of autonomous Argo floats are dispersed across the oceans for observations of global ocean dynamism and long-term monitoring of variations in physical and chemical parameters.28,29 To be mounted on these small underwater platforms, in situ sensors and analyzers should be miniaturized to have a weight in water less than one kilogram but as close to zero kilograms as possible and a few centimeters to a few dozen centimeters in size due to the limited payload capacity. Additionally, further miniaturization of the sensors is necessary to simultaneously measure multiple environmental parameters to enable combined observations. To date, the Biogeochemical Argo30 has been deployed with sensors that measure dissolved oxygen, nitrate, pH, chlorophyll, and suspended particles as well as CTD profiling sensors. On the other hand, the analysis of dissolved components in seawater, especially colorimetric and luminescence analysis based on chemical reactions, depends on collecting seawater samples and subsequently analyzing them in shipboard or land-based laboratories. Consequently, the spatiotemporal resolution of the analysis for dissolved components is limited and the obtained data are sparse compared to the parameters that can be measured using in situ sensors such as temperature, salinity, and depth. Therefore, episodic phenomena that occur within a shorter period of time than the sampling frequency may be missed due to the sparse sampling resolution.31 Moreover, reproducible analysis requires skilled technicians. The breakthrough technology for this situation is the in situ underwater analysis. LoC technologies, which enable the automation of sophisticated liquid handling using microfluidic devices (MFDs), are indispensable to promote the on-going trends toward the miniaturization and weight reduction of in situ analyzers. To date, a variety of MFDs or LoC analyzers for environmental analysis have been successfully demonstrated in the ocean.32–34 When considering their applicability to smaller underwater platforms, the contribution of LoC technologies goes beyond the reduction of material consumption and the application of microfluidic phenomena. It also includes extreme miniaturization of the physical dimensions of the analyzers. To miniaturize the analysis system, not only the microfluidics, but everything, including the fluid delivery system, detection system, electrical system, and exterior, should be significantly downsized.
This review overviews the contributions of LoC technologies for in situ measurements to elucidate the biogeochemical processes in the ocean, which is the largest fluidic system on the Earth. The review focuses on field-proven LoC-based in situ analyzers for oceanography. The novel microTAS-related technical elements that will contribute to measurements in aquatic environments in the future are also discussed.
2. Requirements for LoC analysis systems for underwater applications
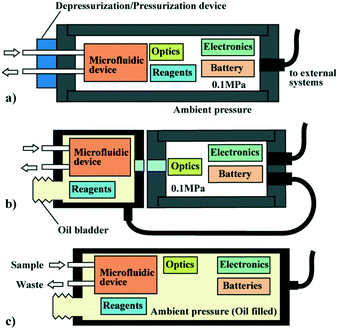
LoC systems used in aquatic environments, including oceans, have specific requirements due to the harsh operating conditions. In contrast to equipment utilized on land, the electrical systems for LoC systems must operate in an environment surrounded by water and, in some cases, must be able to withstand high hydrostatic pressure. There are limitations in the selection of materials and analysis or operating principles for LoC analyzers to be used in the presence of highly corrosive seawater and high hydrostatic pressure. A high degree of automation is also required to enable unmanned operation in remote environments. These essential requirements for underwater LoC systems are reviewed below.First, an underwater system must be waterproofed for electrical insulation. For example, in deep water measurements, where large hydrostatic pressures are applied to the system, the most common approach is to enclose the core elements in a rugged, pressure vessel (Fig. 1). The pressure vessel can maintain a near-atmospheric pressure environment inside it, even against an elevated outside hydrostatic pressure (approx. 0.1 MPa increase every 10 m of descent). This approach allows equipment that can be used in a tabletop environment to be placed directly into the water. However, conventional pressure vessels made of metal, glass,35 or ceramics36 tend to have thicker walls and heavier weight to resist high hydrostatic pressure. Hence, the pressure vessel tends to account for a larger percentage of the total volume and weight of the in situ analyzer. Therefore, the core analysis elements housed in the pressure vessel should be miniaturized as much as possible to minimize the size and weight of the pressure vessel. At the same time, the pressure vessel needs to be cylindrical or spherical in shape because it is more resistant to external pressure. Consequently, the geometry of the core analysis elements is limited.
Second, to introduce a liquid sample such as seawater into a pressure-resistant vessel from a high hydrostatic pressure environment, a depressurization mechanism is necessary. For example, in the case of the environmental sample processor (ESP) developed for in situ molecular biology analysis,37–42 the deep water sampling module (DWSM)38,43 is used as an add-on to the ESP to depressurize the sample during deep water operations at depths of up to 800 m.44 Conversely, if excess sample or analytical waste needs to be drained from the pressure vessel, it must be discharged from the low-pressure side to the high-pressure side using a powerful pumping device. With this approach, analysis elements that operate at atmospheric pressure can be brought into an underwater field without any modifications. However, this requires additional mechanisms for sample decompression and waste drainage, which increase the complexity of the analyzer.
There is a different approach for waterproofing the core elements used underwater, which is the adoption of a pressure-balanced structure by liquid immersion. Instead of storing the core elements in a pressure vessel maintained at atmospheric pressure, they are immersed in an insulating fluid such as oil and stored in a sealed container to provide electrical insulation (Fig. 1b and c). A pressure-balanced container must be sufficiently flexible to be deformed by the contraction of the insulating fluid as the hydrostatic pressure increases or it must have a soft diaphragm or bladder. Since a pressure-balanced housing does not need to be pressure-resistant, the shape is not restricted and it can be made of standard plastic sealed containers used in a laboratory. Additionally, small micropumps can be employed to introduce samples or reagents to the analysis elements and drain wastes because there is not a large pressure difference to overcome. However, the analysis elements stored in the pressure-balanced housing are directly exposed to the ambient hydrostatic pressure. Therefore, the analysis elements themselves and the structure must have a sufficient pressure tolerance.
In reality, a hybrid system is the most common approach. In such a system, only microfluidic elements and reagents are exposed to the environmental pressure to equalize the pressure, while fragile elements such as control electronics and optical systems are stored in a pressure-resistant container (Fig. 1b).
For materials commonly used for LoC devices such as glass, silicon, plastics, and elastomers, the change in the material volume itself due to elevated hydrostatic pressure is typically not a limiting factor. For example, even PDMS (polydimethylsiloxane), which has one of the lowest bulk moduli (2.20–4.95 MPa)45 among LoC device materials, can be used in deep-sea environments at depths of 1000 m.46,47 By supplying organic solvent vapors to substrates with microgrooves formed by machining against PMMA (polymethylmethacrylate) and COC (cyclo olefin copolymer) substrates, the fabrication of devices for optical applications has been achieved simultaneously by bonding paired pieces and smoothing out the microchannel surface.48 LoC devices fabricated using solvent vapor bonding are suitable for a marine environment as described in this review. Another method, the solvent vapor-assisted thermal fusion bonding method, also achieves bonding of strong PMMA substrates suitable for marine environments.49 Although these top-down machining dependent fabrication methods are not suitable to realize fine patterns on the micrometer or nanometer scale, they provide an economical and practical way to form LoC devices without photomasks or photofabrication equipment.
In the case of a structure where the gas phase remains as voids in the microstructure, compression and deformation occur due to hydrostatic pressure and it must be avoided. Relatedly, it is almost impossible to use gas pressure as the driving force for the pumping of liquid or pneumatic microvalves.
A high degree of automation is necessary for standalone equipment used in the offshore field. In the case of analyzers operating at large water depths or in remote locations with limited human access, the sample collection, the analytical sequence, the fluid control, and the data acquisition must be fully automated. Therefore, analytical operations performed in a LoC system must be simple and practical without complex fluid control elements. For this reason, most successful underwater in situ analysis systems are based on devices that employ simple and reliable flow analysis methods, including flow injection (FIA),50–53 continuous flow analysis (CFA) and its derivatives,54–69 stopped-flow analysis (SFA),70–77 and reverse FIA (rFIA).78–81
3. Essential technologies for in situ LoC analyzers
With the advances in LoC technologies in the last few decades, in situ underwater analyzers have become a reality. A key component to realize truly miniaturized and practical in situ LoC analyzers is a compact and reliable pumping device.33,82 When applying commercially available pumping technology to in situ analyzers, appropriate pumps must be chosen from 1) plunger (syringe) pumps, 2) solenoid pumps, 3) peristaltic pumps, 4) gear pumps, 5) piezo pumps, and 6) other pumps.Due to the precision of the pumping volume and the flow rate without flow-rate sensors, the higher pumping pressure, and the simpler structure, the most widely used in situ pumping device for LoC analyzers is a plunger pump.38,42,43,46,70–77,83–85 A typical plunger pump cannot, in principle, complete continuous flow on its own. It requires periodic refilling of the cylinder with fluid. To overcome this limitation, alternating actuation of multiple syringes in parallel allows for continuous pumping.86
Solenoid pumps, which use a solenoid-driven plunger or diaphragm driven by the plunger to drive a liquid, are used for in situ LoC analyzers as well as the diaphragm pump.79–81,87,88 The pumping of a solution by a solenoid pump is based on the intermittent actuation of the solenoid to discharge the entire volume of the solution in one stroke. Using solenoid pumps with a sufficiently small discharge volume, CFA becomes possible with MFDs. In addition, compared to stepper motors, which are commonly used to drive plunger pumps, actuation of a solenoid requires a simpler driver circuitry for operation. Thus, using them is advantageous to realize a reliable in situ LoC analyzer with lower complexity on its configuration.
Peristaltic pumps use an eccentric cam driven by a DC or stepper motor to create an intermittent continuous flow by squeezing an elastic tube without valving devices. In addition to applications to continuous flow colorimetric LoC analyzers,89,90 miniaturized peristaltic pumps have been applied to biological in situ LoC analyzers due to their low contamination risk since the fluid is in contact with only the tubing materials (Fig. 2).69 It should be noted that when a peristaltic pump is used in an oil immersion, the oil resistance of the tubing material, which is typically a silicone elastomer, rubber, or vinyl, must be considered.
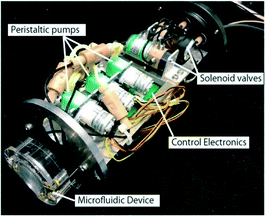 | ||
| Fig. 2 Analysis module for the in situ ATP analyzer. Three peristaltic pumps and three solenoid-actuated three-way valves are employed as the pumping device. All the components shown in this photo, including the PMMA MFD, are immersed in oil for underwater operations. Reprinted from T. Fukuba et al., 2018.69 | ||
Gear pumps can discharge the fluid continuously by rotating a plastic or metallic gear driven by a DC or stepper motor in the pump chamber. Because it can pump continuously with a smaller flow pulsation compared to solenoid or peristaltic pumps, it is utilized for in situ analyzers, which require a stable liquid flow for fluid manipulations.47,91 The flow rate is roughly proportional to the speed of the gears under no load. However, in practice, the flow rate depends on the flow resistance. If accurate flow rates must be maintained, a combination of flow sensors is needed.47
Most of the pumps based on electric actuators such as motors or solenoids can be used in an oil-immersion under high pressure without substantial modification. It must be noted that some holes must be carefully opened on the sealed housing to entirely fill the oil inside the actuator.
The most compact commercially available pumps with continuous pumping capability are mechanical micropumps built with micromachine technology. These are typified by piezoelectric driven pumps, which have extremely small dead volumes, inner volumes, and electric power consumption.92 Similar to a gear pump, the flow-rate of piezo pumps is influenced by flow-resistance, and a flow-rate sensor is needed for precise flow-rate control. Although MEMS flow-rate sensors based on heat transfer phenomena have been developed93 and one was used in a deep-sea application,47 the pulsations produced by piezo pumps reduce the measurement accuracy of the flow sensors.94,95 Piezo-driven micropumps can also be used in an oil-immersion under high pressure with minimum modifications. One study developed an underwater system where such a micropump was used for sample collection because strict accuracy of the flow rate is not required.96
As an application of pumps without electric power consumption, osmotic pumps,97,98 which are generally used for drug infusion by implantation in the medical or biological field, were utilized for LoC analyzers for long-term deployment.60,61 An elastomeric balloon pump,99 a practical medical device for drug infusion, is another example of a pump that does not require electric power. It has been applied to LoC systems.100–102 The advantages of these pumps are that they do not require a power source and realize smooth continuous pumping. However, they require careful evaluation and appropriate setting of the flow rate in advance of in situ deployments. Therefore, they are also useful in applications that do not require strict flow control such as periodic cleaning of fluidic lines or housekeeping applications for systems such as biofouling prevention during long-term in situ operations.
For fluid switching, solenoid-actuated valves are used in most in situ LoC analyzers. Well miniaturized solenoid valves are available as off-the-shelf items, and their use or functional integration103 with MFDs is a commonly accepted strategy to realize portable analyzers. The miniaturization and integration of valve functions is recognized as a major challenge for LoC systems. On-chip microvalves with a variety of valving principles are reported elsewhere.104 However, most require external actuators or pressure sources to drive the microvalves integrated into the MFD. Compared to discrete valves, the major contributions of on-chip microvalves are the reduction in dead volume, higher switching speed, and precise metering of a small liquid volume in a device. Consequently, the contribution to the total miniaturization is limited. However, the impact on the overall miniaturization of the analyzer can be significant when smaller pressure sources such as EOPs105 are utilized.47,106 For in situ LoC analyzers where miniaturization and integration as well as long-term reliability are important, non-actuator-based valving mechanisms such as frozen plug valves107,108 are a promising solution.
Photodetection devices are essential for many in situ LoC analyzers. A light source and a photodetector are needed for colorimetric analysis, while a highly sensitive photodetector like a photomultiplier tube (PMT) is required for photometric analysis. The conventional approach is to enclose them in a pressure vessel. However, most solid-state silicon semiconductor devices such as LED light sources and photodiodes are usable under elevated hydrostatic pressure.109–111 Since the PMT devices for highly sensitive photometric analysis generally have a vacuum tube with thin glass walls, it is difficult to expose them to hydrostatic pressure. On the other hand, novel photodetectors such as micro PMTs112 or a silicon PMT (or a multi-pixel photon counter: MPPC)113,114 have become available in recent years. Although the hydrostatic resistance of these novel photodetectors needs to be further evaluated, it may be possible to use them outside the pressure vessel if they are electrically insulated (e.g., by immersing them in oil or a transparent resin). Hence, they may greatly contribute to the miniaturization of in situ LoC analyzers for colorimetric or photometric analysis in future.
4. Sample collection and LoC
Generally, in the marine environments, samples containing the targets to be analyzed by in situ LoC analyzers are seawater, which is abundant in the vicinity of the analyzers. Consequently, there is no need to introduce samples into the MFDs using devices like micro pipettes. Instead, a sample can be introduced by a pump to bring the surrounding fluid into the analyzer. In addition, for time-consuming and high-resolution analysis with samples collected with a highly resolved spatiotemporal resolution, temporal storage of a small amount of samples in the analyzer is another promising application of LoC technologies.115–117 On the other hand, most molecular biological analysis of marine microorganisms or eDNA requires the capability to collect and concentrate samples. Because the previously described example of an in situ genetic analyzer using a LoC system46 does not have a specific system for sample preconcentration, the sample volume that can be processed is limited to a few milliliters at most. In addition, due to the low throughput of the DNA extraction process using microfluidics, even the microbial lysis, DNA concentration and the purification process for a small amount of seawater samples take tens of minutes.46 Hence, the challenge for LoC analyzers is system adaptability to rarefied samples in nature.As widely accepted, LoC systems often have fundamental difficulties processing large quantities of samples (e.g., tens of milliliters or liters). One possible solution is parallelization of the analysis. For example, practical technologies for the generation of droplets or emulsions and their applications in biological and biochemical analysis are expected.118,119 Unlike instruments for tabletop or industrial applications, which target samples with an approximately known size range and concentration and can be manually supported as needed, examples of such technologies for underwater in situ analysis that requires fully automated processing of a broad range of particles have yet to be reported. On the other hand, there are ways to overcome this challenge by a combination with a “macrofluidic” system. That is, a filter concentration mechanism is reasonable when analysis of particles dispersed in space is needed. To monitor airborne microorganisms using a tabletop device, a combination of a dust collection mechanism to collect airborne microbes and subsequently transferring the concentrated samples to MFDs has been used.120,121 In an effort to realize underwater in situ analysis, MBARI's ESP provides a successful example.37–39,43
Several automatic sample filtration and concentration systems have been proposed for use in the ocean,122 and some, including ESP, are commercially available. In one study, a moored ESP platform with an automated sample collection and fixation capability archived RNA samples for a metatranscriptome analysis of marine microbes.123 In another study on metatranscriptomic analysis of the daily changes in the microbial flora in surface seawater, an ESP tethered to the seafloor at a depth of about 200 m offshore of Santa Catalina Island, California, USA archived samples.124 In addition, deep-ESP, which can be operated in the deep sea, collected microbes in the hydrothermal system, and metatranscriptomic analysis was subsequently performed.124 A different study, which occurred over four days on the deep seafloor simultaneously collected one liter of sample, filtered it, and fixed RNA using an RNAlater solution.125
In the case of eDNA analysis, the required sample volume ranges from tens of milliliters to several liters. Although the contribution of the LoC system alone is a challenge, combining a LoC system with a useful field deployment platform and sample collection system may support studies requiring a higher sensitivity such as the estimation of spawning grounds or monitoring for endangered species represented by eels.126,127 In addition, the collection of marine microplastics requires larger quantities of seawater128 and a combination with macrofluidic systems will be essential for future in situ LoC analyzers.
Three-dimensional printer technology will be useful to realize the aforementioned macro-to-micro interface. In particular, stereolithography and material-jet 3D printers can fabricate various MFDs.129,130 Moreover, 3D printer technology can integrate various functional elements such as 3D channel shapes and integrally molded valves,131 which are difficult to obtain with conventional photofabrication methods. Hence, its usefulness will increase in the future. Furthermore, 3D printer technology can simultaneously mold micro- and macro-structures such as fluidic manifolds, filter holders, reagent reservoirs, and fluidic connectors. Therefore, it is one of the most efficient methods to integrate microfluidic structures for analysis and macrofluidic structures for preconcentration that require relatively large throughputs. For example, we have applied material-jet 3D printer technology to realize a 3D flow cell for underwater adenosine triphosphate (ATP) quantification132 and realization of a novel in situ totally integrated genetic analyzer with multi-scale fluidic manipulation capability.
5. Colorimetric analysis
Colorimetry is the analytical process to quantify dissolved chemical elements or compounds based on the amount of color change due to the formation of dye molecules by adding chemical reagents to an aquatic sample to be analyzed. In the field of oceanography, flow analysis based on colorimetric chemistry is common in the analysis of sub-micromolar to nanomolar macronutrients133–136 and sub-micromolar to sub-nanomolar trace metals.134,136 In addition, the seawater pH is frequently measured using colorimetry with an accuracy in excess of ±0.01 pH unit.137,138 The major factors that determine the performance of colorimetric analysis are high sensitivity and accurate measurement of absorbance following efficient mixing of reagents and samples for colorimetric reactions. The realization of a portable optofluidic device that integrates these essential functions and the completion of the total in situ analyzer are major contributions of LoC technologies in oceanography. The most successful deployment of LoC technology in oceanography has been its application to miniaturized colorimetric nutrient analyzers, due to the continued and significant demand for it and relatively simple system configuration. These advanced technologies have already been applied to practical in situ measurement.32–345.1 Nutrient analysis
Among the marine parameters frequently measured by the colorimetric method, those closely related to biological activities are macronutrients, which include nitrate, phosphate, and silicate. The in situ concentrations of nutrients essential for the growth of marine plankton, especially in the surface zone, are found on the nM level of low concentration.135 Consequently, instrumentation of highly sensitive analyzers based on the colorimetric method is essential for oceanography applications. Even before LoC technologies became practical, miniaturization techniques using a top-down approach82 were applied to develop in situ nutrient analyzers such as nitrite,51 nitrate and nitrite,53,78 and silicate and sulfide.57 For a review, see Mills and Fones (2012).139 For example, SCANNER, a pioneering colorimetric analyzer, which was mounted on a manned submersible vehicle together with dissolved oxygen (DO) and temperature sensors in the vicinity of hydrothermal systems at depths greater than 2500 m, detected rapid changes in the concentrations of silicate and sulfide around hydrothermal vents.57 On the other hand, there is a strong demand for miniaturization and functional integration of fluidic components for nutrient analyzers that require multiple pumps and valves. To date, LoC devices with functions for reagent mixing and reduction of analytes using a solid catalyst have been developed and integrated into in situ analyzers as reviewed below.An essential element for colorimetric analysis is an optofluidic flow cell to measure the absorbance. Typically, transparent plastic materials are used due to their easy microfluidic formation and lower manufacturing cost compared to glass. Among them, PMMA and COC have high transparencies, and various reliable microfabrication methods have been developed such as mechanical cutting and hot embossing for microchannel or flow-cell formation. One of the earliest successes is the development of an autonomous nutrient analyzer in situ (ANAIS), which is based on PMMA MFDs for nitrate and phosphate, and PEEK devices for silicate analysis. ANAIS acquired vertical profiles of nitrate up to a depth of 1000 m in the Mediterranean Sea.79 More recently, further miniaturized and functionally integrated analyzers for in situ measurements of nitrite,70 nitrite and nitrate (Fig. 3),71 and phosphate72,76 based on plastic MFDs with an optofluidic cell140 fabricated by the solvent vapor bonding method48 have been developed. Tinted PMMA shows a superior performance as a material for the absorbance cell compared to transparent substrates because it suppresses stray light.140,141 In addition, month-long continuous observations in subtropical oligotrophic areas76 were achieved using in situ phosphate analyzers based on the solvent vapor-bonding technology and molybdenum blue batch method.142 A miniaturized in situ analyzer with an in situ calibration capability demonstrated not only vertical profiling of nitrate and nitrite but also mooring operations at elevated water depths offshore in Western Africa.143 Moreover, a highly automated LoC system for colorimetric analysis has been mounted on an underwater glider, which is an autonomous observation platform, as a payload sensor. Deployment in the Central Celtic Sea confirmed the practical performance of continuous vertical profiling of nitrate + nitrite (∑NOx) (Fig. 4).84 The concentrations of ∑NOx measured using the LoC colorimetric analyzer family introduced above are in excellent agreement with those measured by the conventional tabletop flow analysis method. The combined measurement uncertainty associated with the data produced from multiple LoC ∑NOx analyzers is <5%, demonstrating the feasibility of parallel operations of multiple analyzers to cover a wider area.144
 | ||
| Fig. 3 Tinted PMMA MFD for in situ analysis of nitrate and nitrite (left), and the MFD integrated with fluidic components for in situ operations (right). Reprinted with permission from Beaton et al., 2012.71 Copyright (2012) American Chemical Society. | ||
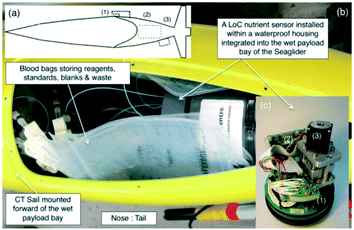 | ||
| Fig. 4 (a) Mounting locations of sensors deployed on an underwater glider (Seaglider, Kongsberg, Norway). (1) Conductivity and temperature (CT) sensor, (2) LoC ∑NOx analyzer, and (3) optical back scatter sensor. (b) LoC ∑NOx analyzer placed into the payload bay of a Seaglider. (c) LoC ∑NOx analyzer consisting of (1) tinted PMMA MFD, (2) electronics, and (3) custom-made syringe pump assembly. Reprinted from Marine Chemistry vol. 205, 20, A. G. Vincent et al., Nitrate drawdown during a shelf sea spring bloom revealed using a novel microfluidic in situ chemical sensor deployed within an autonomous underwater glider, pages 29–36,84 Copyright (2018), with permission from Elsevier. | ||
To obtain data sets with a higher spatiotemporal resolution using mobile platforms such as vertically casted CTDs and underwater gliders, the measurement frequency must be improved. A multiplexed stop-flow analysis method using multiple syringe pumps can enhance the temporal response of LoC analyzers.86
Similar miniaturized in situ analyzers made of tinted PMMA devices, including a multi-step pretreatment process for silicate measurements, have been evaluated.85 Sequential mixing of multiple chemicals for colorimetric measurements of silicate based on the molybdenum blue method can be performed via a continuous flow format using the MFD with multiple static mixers.85
Solid catalysts are sometimes used in colorimetric analysis for the reduction of the analytes. For example, a generic approach for nitrate analysis is the use of off-chip copperized cadmium catalysts.71 Because the integration of the catalyst into the MFD minimizes the dead volume, it can improve the response of colorimetric measurements. As an example, a solid cadmium catalyst disk with an engraved microfluidic channel was embedded to a PMMA device for nitrate analysis.79 Integration of the catalyst and other fluidic components to minimize the dead volume is essential especially for an analysis device with a very low flow-rate. In the case of a pioneering nitrate analyzer with osmotic pumps60,61 as the pumping component, a disk-shaped solid catalyst was embedded on a PVC plate with microfluidic channels formed by embossing.60 Since the sample and reagent flow rates were 12 and 1 μl h−1, respectively,60 elimination of the dead volume is critical to enhance the response and temporal resolution of the analyzer.
As an alternative to the widely utilized Griess assay for nitrate determination,145 instrumentation involving the chromotropic acid reagent method has been examined because it does not require a catalytic reduction step.89,90 Although it has yet to be applied to real field measurements, it seems to be a promising method for nitrate determination by a simpler analyzer.
5.2 Trace metal analysis
Hydrothermal activities supply large quantities of trace metal elements such as iron and manganese to the ocean.58,146 Hydrothermal activities are some of the most dynamic geological phenomena on the seafloor, and are useful for mapping hydrothermal fluid diffusion,58 estimating the dynamism of hydrothermal activity,147 and searching for new hydrothermal sites.148 These trace metal elements can be quantitatively analyzed by colorimetric methods. Hence, automated measurements have been conducted using various in situ colorimetric analyzers.58,63,64 The manganese concentrations in the coastal waters of Scotland's fjords were continuously mapped using a compact in situ analyzer installed on an autonomous underwater vehicle (AUV).62,149 A flow analysis device, CHEMINI,52 which can measure dissolved iron and total dissolved sulfide in the deep sea up to 6000 m, was installed to the miniaturized seafloor observation platform TempoMINI together with a video camera for biological analysis. The variation of the iron concentration in the hydrothermal system was continuously measured for 3.8 months.150 Although these in situ trace metal analyzers were successfully deployed in real environments, the flow elements were composed of discrete pumps, valves, and tubes, demonstrating that further miniaturization is possible.Recently, a LoC analyzer incorporating a tinted PMMA-based microfluidic colorimetric device140 developed by a solvent vapor bonding procedure48 was installed in a CTD profiler to demonstrate manganese and iron measurements on the tens of nM levels.74 During a nine-day deployment at the sea surface in the Baltic Sea, an improved LoC analyzer with a low-nM to several-μM measurement range was used for vertical profiling of iron.75 To improve the dynamic measurement range, the device was sequentially connected three optofluidic cells, three sets of LED light sources, and a photodetector for absorbance measurements.75
Most colorimetric analyzers proposed to date use SFA instead of CFA because reciprocating syringe pumps are used for the reagent and sample supply. These systems use static mixing by diffusion of the reagent/sample components in a simple microfluidic channel or mixing that relies on injection and transfer of the sample plugged into the intermittent carrier flow.70–73,79,86 On the other hand, fast mixing of reagents and samples by employing 2D or 3D passive mixing structures151,152 or active mixers153 should improve the time resolution of the analysis, especially for fully continuous flow analysis. To enhance mixing with lower fabrication costs, an in situ manganese quantification device was developed using 3D printing technology.154 A 3D-printed PMMA mixing device based on a multilaminar concept realized a high flow rate, fast mixing, and fast coloration for in situ manganese analysis with a high spatiotemporal resolution.154
Electric power consumption limits the continuous operating period of in situ analyzers in long-term measurements. For a continuous measurement system, pumping devices often consume a considerable portion of the electricity. As a practical solution, a LoC system using osmotic pumps (Fig. 5) was applied for year-long monitoring of iron concentrations, which appear to be linked to the hydrothermal activity on the seafloor.61 Two osmotic pumps sequentially added ascorbic acid and ferrozine to a seawater sample for colored complex formation. The osmotic pumps were modified by replacing the volume of the working fluid (saturated NaCl) with seawater for sample introduction. Standard and blank samples were periodically injected into the stream using solenoid pumps for system calibration. After a year-long deployment at a 1500 m deep hydrothermal vent on the Juan de Fuca Ridge, the short (<1 day) and long-term (>1 month) variabilities of the iron concentration were elucidated in the range from sub-μM to tens of μM.
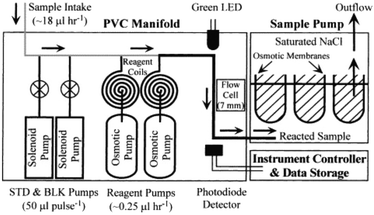 | ||
| Fig. 5 Schematic diagram of an in situ Fe analyzer using an osmotic pump. Reprinted from Analytica Chimica Acta vol. 463, T. P. Chapin et al., In situ analyzer for the year-long continuous determination of Fe in hydrothermal systems, pages 265–274,61 Copyright (2002), with permission from Elsevier. | ||
Osmotic pumps have also been applied to develop a continuous water sampler, OsmoSampler, for hydrothermal fluid collection for bench-top analysis.155 Although applying osmotic pumps for short-term analysis is challenging because flow-rate stabilization under in situ water temperature and pressure conditions is time-consuming (over 2 weeks),61 it is advantageous for long-term continuous analysis.
5.3 pH measurement
One of the most important parameters, especially when ocean acidification is recognized as a serious environmental issue, is seawater pH.7,8 The decrease in seawater pH due to ocean acidification in the ocean surface mixing layer is close to 0.015 units per decade.8 Therefore, in situ pH measurements to monitor ocean acidification require an extremely high precision and accuracy. Along with nutrient and trace metal analysis, perhaps one of the most widely known colorimetric methods is pH measurement using colorimetric pH indicators.137,138,156 In the field of oceanographic observations, thymol blue and metacresol purple are standard pH indicators for surface seawaters and surface to deep ocean waters, respectively.156,157Compared to potentiometric in situ glass pH electrodes158,159 and semiconductor pH sensors,160–162 which are common for in situ pH measurements, a colorimetric pH analyzer requires a flow system. This makes the colorimetric analyzer configuration more complex. Therefore, it may be necessary to develop a highly reliable device for long-term measurements for extreme and remote environments such as the polar regions.163 On the other hand, although simple pH measurements using electrodes will continue to be a standard method for in situ pH measurements, colorimetric pH measurements are superior in terms of pH measurement accuracy when using carefully purified indicator dyes.164 In addition, the colorimetric method does not require frequent calibration which is needed for glass electrodes to compensate for the considerable output drift.164
There are several examples of the development and field deployment of colorimetric pH analyzers65,80,81,83 and pH/pCO2 analyzers.66,165 The tinted PMMA MFD with a static mixer and a 10 mm absorbance cell was developed and integrated with syringe pumps, a LED light source, and a linear array photodiode spectrophotometer for colorimetric pH measurement using thymol blue.73,83 The estimated short term precision of the system was 0.001 pH unit and the accuracy was within the accuracy of a certified pH standard solution (0.004 pH unit). The completed LoC pH measurement system was evaluated by continuously measuring seawater pH in an onboard laboratory for one month and more than 5000 continuous pH measurements were demonstrated in European shelf waters.73 Towards the improvement of the mixing of reagents and samples as well as further miniaturization of the analyzer, MFDs have been applied to in situ pH analyzers.73,83 Some studies have proposed extending the pH measurement range by mixing multiple dyes. An autonomous chemical sensing platform with a tinted PMMA MFD capable of pH measurements between pH 4 and 9 has been proposed.166 A PDMS MFD with folding micropatterns for reagent mixing and multiple absorbance cells has been developed.151 Because the device has a small geometry similar to a microscope glass and simple absorbance cells that fit to LED light sources and photodetectors, it can be integrated with an electrical printed circuit board (PCB) to realize an ultra-compact colorimetric pH analyzer in the future. Because of the existence of pH electrodes as robust, miniaturized, and commercialized sensing devices, the application of the LoC technology for pH measurement is still limited compared to the other colorimetric analysis. On the other hand, highly reliable in situ pH measurements based on the use of the well-established and scientifically accepted pH-indicating dyes are essential for accurate pH measurements needed for monitoring of ocean acidification, and LoC has great potential for its field instrumentation, so further developments can be expected in the future.
6. Chemiluminescence/bioluminescence analysis
Chemiluminescence or bioluminescence analysis employs similar instrumentation to colorimetric analysis. It uses microfluidic mixers, flow cells, and photodetectors. In principle, these methods measure the concentration of components by assessing the intensity of chemiluminescence or bioluminescence emitted due to a reaction between the reagent and the component to be measured. Typically, the light source, which is essential for colorimetric analysis and increases noise on measurement, is unnecessary in the analysis. Hence, such analyses have a higher sensitivity due to the improved signal-to-noise ratio and a wider linear dynamic range of quantification compared to spectroscopic or colorimetric analysis.134 On the other hand, an extremely sensitive photodetector such as a PMT is needed to measure the intensity of weak luminescence light.6.1 Trace metal analysis with chemiluminescence
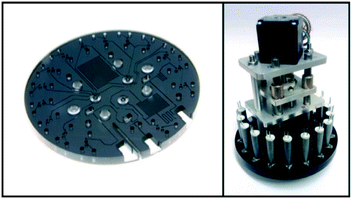
In addition to macronutrients, trace metals, which are called micronutrients due to their very low concentration in nature, are also essential for the growth of marine organisms. In the field of chemical oceanography, the concentrations and distributions of iron,167–170 manganese,171,172 copper,173 and cobalt174 have been elucidated using automated flow analyzers that can perform luminol-based chemiluminescence analysis. Instead of sampling and shipboard analysis with limited spatiotemporal resolutions, practical in situ analyzers developed via a top-down approach have been utilized for in situ continuous analysis based on the chemiluminescence.67,68,175 Mapping of the manganese concentration using an AUV with a submersible in situ Mn analyzer (GAMOS) revealed anomalies around an undersea volcanic crater in the Sagami Bay, Japan.176Although the reports are limited compared to the applications of colorimetric analyzers, LoC technologies have also been applied to in situ chemiluminescence analysis. For example, a PDMS MFD with an integrated chelate-resin column for Fe removal for in situ Mn determination with a CFA format by the luminol chemiluminescence reaction has been reported.177Fig. 6 shows an in situ manganese analyzer using a PDMS MFD with integrated valves and flow-rate regulators.47 Hydraulic pressure was used as the driving force for the microvalves and flow-rate regulator. Methanol pumped by electro-osmotic pumps (EOP)105 as the hydraulic pressure source has been used as the working fluid to drive them.106 This device has a Tesla mixer structure178 to mix the reagent and sample prior to supplying to the serpentine flow cell for chemiluminescence detection. The reagent and seawater sample are pumped using a miniature annular gear pump connected to the outlet of the PDMS device. By monitoring the flow rate of the CL reagent and the total flow rate using off-the-shelf miniature thermal flow-rate sensors, the flow-rate ratio between the sample and CL reagent can be kept constant using an integrated flow-rate regulator driven by an electroosmotic pump. This pumping format allows the sample seawater into the PDMS device with a minimal dead volume. The use of a single pump is also advantageous to realize a simple and lower power consumption analyzer. Fig. 7 shows a pumping device and the completed in situ manganese analyzer mounted on an ROV for combined measurement, which contributed to the discovery of a novel underwater hydrothermal site in the Okinawa Trough, Japan. The analyzer detected an anomaly in the manganese concentration along with lower pH and higher temperature values.47
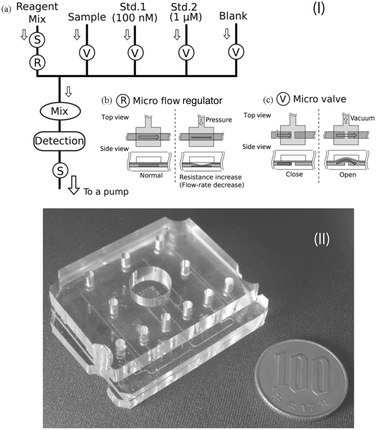 | ||
| Fig. 6 (I) (a) Schematic diagram of an in situ Mn analyzer with (b) a flow-rate regulator and (c) microvalves. (II) Photo of a PDMS MFD for an in situ Mn analyzer. © [2013] IEEE. Reprinted, with permission, from C. Provin et al., 2013.47 | ||
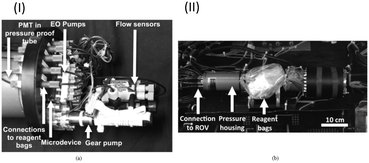 | ||
| Fig. 7 (I) MFD connected to a gear pump, flow-rate sensors, and electroosmotic pumps as a pressure source for actuation of a flow-rate regulator and micro valves. (II) View of the whole in situ LOC analyzer with a pressure vessel for the connected photodetector and reagent bags. © [2013] IEEE. Reprinted, with permission, from C. Provin et al., 2013.47 | ||
Since luminescence analysis generally requires extremely weak luminescence intensities to be measured, the photodetectors must be more sensitive than those for spectroscopic or colorimetric analysis. Typically, PMTs capable of photon counting are used in pressure vessels. The pressure vessels for photometry purposes consist of thick walls to withstand hydrostatic pressure and a thick transparent pressure-resistant window to let light in. This setup increases the overall size of the analyzer. On the other hand, advanced microfabrication technology can produce ultra-small PMTs with a high sensitivity, which are available commercially.112,179 Using these modern, compact photodetectors, the capacity of the pressure vessel can be minimized. Moreover, attempts have been made to achieve photometric analyzers without pressure vessels to further miniaturize LoC analyzers.101,132
7. Biological analysis
To study the amount, distribution, diversity, and dynamic responses to changes in environmental conditions, traditional studies involve sample collection and conventional microscopic observations of prokaryotic and eukaryotic marine microorganisms as well as viruses. However, times have changed. In situ molecular biological analysis targeting biomolecules, including DNA and RNA, is now a feasible methodology supporting the expansion of modern marine microbiology. Towards the realization of in situ underwater molecular biological analyzers, advanced LoC technologies have been applied. For example, a completely automated and parallel fluid control approach holds promise.7.1 In situ flow cytometry
High-throughput automated particle measurement methods, which apply flow cytometry and their derivative techniques,180 have been used to identify and quantify prokaryotic181–184 and eukaryotic183–185 plankton and viruses186 living in the ocean. These techniques have realized labor savings in marine microbiology-related studies. The realization of a miniature particle counting device based on the formation of stable laminar sheath flow by microfluidic technology and its application to marine plankton analysis are a promising and practical solution. For example, in situ flow cytometers have been mounted on surface buoys and automatically counted phytoplankton and transmitted measured data every two hours in an outdoor water basin.185 The reported in situ cytometer can detect 0.5 μm of latex beads or 1 to 2 μm of cyanobacterial cells in a ribbon-shaped core fluid (0.4 to 4 m s−1 of flow velocity) in a square quartz flow channel with 1 mm width connected to a gear pump. Other devices have also combined miniaturized optical187 and electrical188 detection methods. One study distinguished several species of eukaryotic and prokaryotic phytoplankton cells using a PDMS MFD with chevron patterns on the top and bottom of the channel for the precise control of the sheath fluid to compress the sample stream in the channel.187 Simultaneous detection of two fluorescence signals from chlorophyll and phycoerythrin and side scatter allows the species analyzed to be distinguished.187Another combined microfluidic resistance pulse sensor (RPS) technology and flow-through fluorescence analysis with MFDs to analyze the size spectrum of marine microalgae.189 The PDMS MFD, which has a narrowed spot to detect the increase of flow resistance when the particles pass through it, successfully detected cells of microalgae as small as 3 μm.189
Recently, utilization of imaging cytometry based on high throughput video observation of flowing particles became available for practical taxonomy of marine plankton.190–192 Some instruments are operable in situ for real-time biological observation.193,194 The application of LoC technology for imaging cytometers has been demonstrated in the field of cell biology, primarily for biomedical applications.195–197 On the other hand, due to the extremely wide size spectrum of marine plankton cells compared to the cultured cells targeted in cell biology, the realization of a LoC system based on MFDs that can handle the analysis of natural samples is extremely challenging. Hashemi et al. reported an imaging cytometer based on an MFD capable of processing 1 to 80 μm of phytoplankton particles by designing a wide (390 μm wide) microfluidic inlet on the PDMS MFD to prevent the destruction of larger plankton cells.198 The chevron patterns187 properly worked here again to focus the core flow from the wider sample inlet.198
Flow cytometry instruments have the advantage of being able to analyze a large number of particles in a short period of time. In particular, imaging cytometers need to handle large amounts of data, and therefore, they need real-time processing of the acquired data to enable continuous analysis for a long period of time. Considering the limited capacity of the data storage and data transmission throughput in remote environments, application of real-time in situ image recognition technology based on deep learning is essential for practical deployment at sea.199,200
7.2 Biomass assay
Some of the biologically relevant molecules can be detected using luminescence analysis. For example, the luciferin luciferase (LL) assay with a modified firefly luciferase can quantitatively analyze adenosine triphosphate (ATP), which is the energy currency of living organisms and a proxy of biomass. The detection and quantitative analysis of ATP via an LL assay are widely used for cleanliness testing in the fields of public health and food production due to its high sensitivity and simple protocol.201 In the field of marine microbiology, ATP values are recognized as a proxy for the biomass of invisible marine microbes.202A LoC analyzer for in situ ATP analysis with a PDMS MFD for the LL assay in the SFA format has been evaluated at a hydrothermal vent in shallow water.77 A two-layer PDMS device was developed with serpentine microchannels for cell lysis and a spiral channel for bioluminescence detection due to the LL reaction. The PDMS channel was bonded with a glass substrate. The device had transparent heaters and a resistance temperature device (RTD) as a temperature sensor made of indium tin oxide (ITO) to maintain the optimum temperature (25 °C) for the LL assay. The MFD was connected to pumping devices, which consisted of miniature syringe pumps and solenoid valves. All the components for analysis were immersed in oil and operated at ambient pressure, except for the electronics and a PMT as a photodetector. This analyzer measured the total ATP (dissolved + intracellular) concentration as a semiquantitative indicator of the biological activity. As a field trial, the ATP analyzer was suspended to the seafloor from the surface vessel and placed near the hydrothermal vent by a human diver. The measured in situ ATP concentration from the diver-collected sample was clearly lower than the desktop value, implying that in situ measurements of the non-conservative biological component such as ATP are important to determine the real in situ concentration.77 Recently, the improved CFA version of the in situ ATP analyzer has been evaluated in deep-sea environments.69
Fig. 8 shows the PMMA MFD for the LL assay, which has a microchannel with a serpentine pattern for cell lysis and a photometry flow cell on its center. Syringe pumps are replaced with miniature peristaltic pumps for continuous pumping of reagents and samples. This was deployed at a deep-sea hydrothermal area, reaching a depth of 200 m at the Oomuro Hole, Japan. As a result of in situ measurement during the ROV dive, in situ ATP values of 2.0–3.0 × 10−11 M were successfully obtained.69
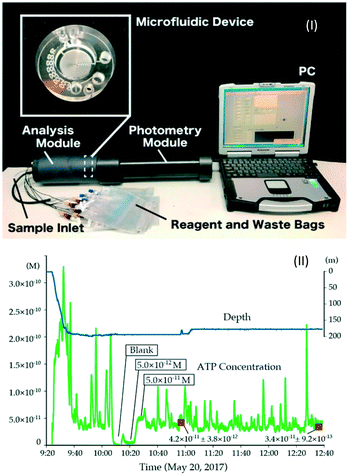 | ||
| Fig. 8 (I) In situ ATP analyzer and (II) result of an in situ ATP measurement at deep sea. Reprinted from T. Fukuba et al., 2018.69 | ||
7.3 Genetic analysis
For modern environmental microbiology based on the detection of genetic biomarkers or metagenomic analysis,203 molecular biological assays targeting genetic materials such as DNA and RNA are an essential tool. Metatranscriptomic analysis, which targets microbial RNA, can elucidate the response of microbiota under diverse environmental conditions. Furthermore, numerous unknown transcripts can be detected.204 In recent years, the scientific and industrial interest applying environmental DNA (eDNA) analysis to detect and estimate the dynamics of marine organisms, including large animals such as fish,205,206 has been increasing. There is even a demand to develop practical instruments for in situ analysis of eDNA.Molecular biological analysis is one of the most promising targets for LoC systems. On the other hand, factors such as the need for precise manipulations of micro-scale solutions and the complexity of analytical procedures such as nucleic acid extraction, purification, concentration, and amplification have hindered the development of in situ underwater genetic analyzers that adopt LoC technologies. For example, the Autonomous Microbial Genosensor (AMG), which is an underwater in situ analyzer, can collect samples, extract RNA, determine its concentration, and conduct real-time NASBA (nucleic acid sequence-based amplification).207 A hand-held thermal-regulating fluorometer for AMG has a simple reaction chamber made of black acrylonitrile butadiene styrene (ABS), with fluorescence detection optics208 and portable pumping devices. RNA templates can be prepared in the analyzer, and real-time NASBA, which targets marine dinoflagellate, can be autonomously performed.207
MBARI (Monterey Bay Aquarium Research Institute, USA) developed and deployed a family of autonomous in situ genetic analyzers, or genosensors, ESP (environmental sample processor).37,39,43 ESPs can collect samples and concentrate them onto a filter unit called a “Puck.” DNA or RNA is extracted from the sample particles. Then sandwich hybridization assays (SHA)209 detect the sequences using nucleic acid fragments blotted onto the membrane.37 ESPs with SHA capabilities have detected toxic algal blooms during deployments for practical missions.39,40 Pioneering attempts have also been made to use ESPs for direct in situ detection of HAB-produced toxin, domoic acid, using competitive ELISA and simultaneous detection of HAB algae by SHA.41
More recently, an MFD for real-time PCR has been integrated into an ESP to improve the sensitivity. Fig. 9 shows the microfluidic block for PCR integrated into an ESP,42 which consists of a perfluoropropylene-co-tetrafluoroethylene (FEP) tube with a temperature control mechanism composed of an etched silicon resistive heater and optics with LEDs and solid-state detectors.210 Real-time PCR can be performed in an air-segmented droplet at the center of the heated zone by the silicon resistive heater.210 This simple MFD can conduct quantitative PCR analysis of DNA extracted from samples autonomously collected and concentrated by an ESP. Measurements can be performed repeatedly and serially without cross-contamination. An ESP with PCR capabilities was deployed in the field to monitor the blooms and declines of marine bacterioplankton at a high resolution on time scales of years to months.211 The drifting version of an ESP with LoC qPCR capability measured the variation of marine microbiota with a much higher temporal resolution by in situ detection of nitrogen-fixation-associated genes every 16 hours for 10 days.212 Moreover, social implementation of ESP technology is being attempted by applying it to the early detection of fecal indicator bacteria (FIB) and red tide-causing algae, which have a significant industrial and social impact.213
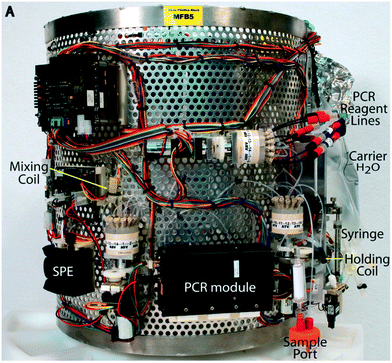 | ||
| Fig. 9 Microfluidic block for an ESP with the miniature PCR thermal cycler. Reprinted from C. M. Preston et al., 2011.42 | ||
LoC technologies have utilized an all-in-one MFD to realize a much smaller in situ genetic analyzer. A flow-through PCR MFD consisting of PDMS and glass214 was updated by integrating the functions for chemical lysis of biological cells, solid-phase extraction and concentration of DNA using glass beads, and optical detection of amplified PCR products.46 The glass substrate has six pairs of patterned platinum film heaters and RTDs to form a segmented temperature distribution for flow-through PCR. For in situ deployment, the MFD is connected to a pumping device, which consists of miniature plunger pumps, solenoid valves, and reagent reservoirs. For deep-sea operations, the entire analysis element is enclosed in a pressure balanced (oil-immersed) container to complete the integrated in situ analyzer for genetic exploration (IISA-Gene). By mounting IISA-Gene on an ROV, we demonstrated that IISA-Gene can amplify the microbial universal 16S rRNA gene and detect fluorescence by flow-through SYBR-Green PCR in the vicinity of a deep-sea hydrothermal vent in the Okinawa Trough, Japan.46
To overcome the current barriers in the not so distant future, more complex molecular biology analysis methods will be applicable to the underwater fields as higher-level automation of advanced fluid manipulation with the latest LOC technologies. A promising technology is a combination of continuous generation and reproducible manipulation of water–oil droplets, and analysis methods based on such technologies, including droplet PCR.215 Massive parallel processing powered by microdroplet technology will realize real-time in situ processing of natural samples for spatiotemporally resolved elucidation of the ecosystem's dynamism with unprecedented throughput. For example, a LoC analyzer for in situ measurements of nitrate and nitrite by continuous flow colorimetric analysis using microdroplet technology can conduct measurements in a river environment at a faster rate (0.1 Hz) than the non-droplet system.216 On the other hand, when targeting rare members of marine ecosystems or environmental DNA, which are rarely found in the environment for analysis using LOC analyzers, samples larger than a liter may need to be concentrated. Consequently, appropriate combinations with macrofluidic systems such as those implemented on ESPs are needed. These are discussed in section 4.
8. Applications to in situ electrochemical measurements
As described above, LoC technologies offer numerous advantages to realize compact in situ analyzers when utilized as a core element. In addition, the combination of LoC technologies and electrodes for advanced in situ electrochemical measurements holds promise. Today, various in situ electrode sensors are combined with MFDs for environmental monitoring. In addition to conventional glass electrodes, semiconductor electrode devices such as ion-sensitive field-effect transistors (ISFETs) have been developed and utilized in the marine environment as novel tools for in situ chemical measurements and such devices have steadily progressed.160–162Electrode-based sensors have a fundamental advantage over flow analyzers as they are naturally smaller. In addition, their response is significantly faster than that of flow analyzers. Due to these advantages, attempts have been made to realize multidimensional in situ chemical measurements with a high spatiotemporal resolution by mounting electrode-based sensors on mobile ocean observation platforms.160,217,218 However, one drawback of electrode devices is drift, especially when continuous measurements are made over a long period. Drift is attributed to many factors such as dilution and degradation of the internal solution of the glass electrode, blockage of the liquid junction, changes in reference electrode characteristics over time, and biofouling.219 In the case of electrodes in a deep-sea environment, there are additional factors such as pressure and temperature that largely differ from those in an atmospheric environment. Moreover, when electrodes are mounted on a moving underwater platform, the environmental parameters change repeatedly and rapidly, which should exacerbate drift problems. Recently, careful sensor operating guidelines have been proposed to reduce the drift itself or its impact on measurements.219
Another way to solve the drift problem is to integrate the sensor with fluidic systems. For example, if the sensor is calibrated by periodically supplying standard solutions and the degree of sensor drift can be ascertained in situ, the sensor output or the measured data can be adjusted after the deployment to virtually compensate for the effects of sensor drift. Continuous fluid flow supply is also effective against biofouling.219 To avoid sacrificing the portability of the electrode sensor, a miniature in situ calibration device has been proposed in which a PDMS MFD is integrated into a pH-ISFET chip and connected to a miniature pumping unit to provide two pH standards to the pH-ISFET in situ.91 The major feature of the MFD is minimizing the dead volume from the intake of the sample seawater to the ion-sensitive part of the pH-ISFET to maintains the fast response of electrode sensors. Instead of using a solenoid valve for ceasing the flow of sample seawater during the calibration using standard solutions, a narrow channel was placed between the sample intake and the flow cell to increase the flow resistance. Although both seawater and the standard solution are supplied into the flow cell when one of the valves for the standard is opened, only the standard solution can flow-through the ion-sensitive part of the pH-ISFET due to laminar flow formation. Although the effect of hydrostatic pressure and other factors on the pH values of standard solutions needs to be evaluated quantitatively, the possibility of providing precise fluid control for in situ calibrations of electrode sensors with occasional drift in deep-sea environments has been demonstrated by this study.91
Applications of gas permeation using MFDs with thin films have also been reported. For example, an integrated electrode device with an MFD and a PDMS membrane measured dissolved inorganic carbon.116,117 Specifically, micro electrodes measure the decrease in conductivity of the NaOH solution as a sensing solution due to an increased CO2 concentration, which is caused by gas diffusion through a thin PDMS film from the seawater stored in microchannels. To realize more sensitive in situ analysis of ammonia than colorimetric analysis, an in situ device combining a gas diffusion cell and electrodes for conductivity measurements has been proposed to separate and concentrate ammonia.87 The gas diffusion cell has a simple structure with a thin Teflon tape sandwiched between polysulfone blocks with a serpentine microchannel pattern.
9. Future perspectives
LoC technologies will be critical in the development of next-generation underwater observation systems, which require miniaturization while simultaneously operating at a low power consumption over a long period. LoC technologies along with advances in underwater observation platform technologies will support next-generation underwater observation systems. Further miniaturization of flow analyzers will allow LoC technologies to be mounted on smaller platforms such as Argo floats,28,29 which have been deployed around the globe. By constructing a real-time measurement network with a higher spatiotemporal density in various aquatic environments such as rivers, lakes, and oceans, it will become possible to promote significant innovation toward elucidating the dynamism of the global environmental changes and visualizing the influence of human activities. Moreover, fundamental technologies, which are necessary to realize in situ LoC analyzers that can be operated in harsh marine environments, should be applicable to diverse fields, including water quality management or pathogen detection in public or industrial facilities, water supply and sewerage systems, and public facilities such as swimming pools.To date, efforts to explore extraterrestrial water environments mainly target Mars and a group of outer planet satellites.220 Since the payloads of the landers and exploration robots used in these expeditions are extremely limited, the miniaturization of the onboard chemical and biochemical analyzers is essential. The major contributions of LoC technologies to planetary science are the realization of capillary electrophoresis and its sample preparation.221–226 Moreover, a variety of pioneering devices such as a miniaturized CTD sensor and particle sampler,227 cell counter,228 life-marker detection device by immunoassay,229 and electrochemical sensing system with microfluidics230 have been proposed, developed, and evaluated towards pilot testing and mounting on exploration landers or robots operated in the extraterrestrial world. In the future, we will proceed to analyze not only the surface of planets and satellites, but also terrestrial fluids such as water and hydrocarbons that exist on the surface and underground.227
Microfluidics should also be integrated with in situ electrochemical sensors for conductivity and pH regulation towards life detection in extraterrestrial oceans.231 NASA's Ames research center has developed a microfluidic Sample Processor for Life on Icy Worlds (SPLIce) for autonomous detection of signatures of life in an ocean world on icy satellites such as Europa (Fig. 10).232 SPLIce has multiple functions, including sample retrieval, dehydrated reagent storage and rehydration, physicochemical sensing (pressure, pH, and conductivity), particle filtration, sample preparation (concentration adjustments and degassing), and precise pumping for these operations. Considering the high impact kinetic penetration to the underwater world from the orbit, MFDs must survive the impact. For such extreme missions, hydraulic actuated microvalves and optical components for laser-induced fluorescence (LIF) analysis to detect organic compounds have been developed. They can survive under more than 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g of acceleration.233 Well miniaturized analysis systems powered by LoC technologies can be mounted on underwater vehicles or ice penetrators for exploitation of the ocean world under the ice, providing precious knowledge about extraterrestrial environments. The in situ analyzers for such extreme missions must be sufficiently compact and at the same time fully automated with higher levels of redundancy and reliability. Current and future LoC technologies for underwater in situ analysis can be an essential milestone for establishing the fundamental technologies to withstand such severe demands for the expeditions at the frontier of life.
000g of acceleration.233 Well miniaturized analysis systems powered by LoC technologies can be mounted on underwater vehicles or ice penetrators for exploitation of the ocean world under the ice, providing precious knowledge about extraterrestrial environments. The in situ analyzers for such extreme missions must be sufficiently compact and at the same time fully automated with higher levels of redundancy and reliability. Current and future LoC technologies for underwater in situ analysis can be an essential milestone for establishing the fundamental technologies to withstand such severe demands for the expeditions at the frontier of life.
 | ||
| Fig. 10 Sample processor for life in icy worlds (SPLIce) developed and evaluated for extraterrestrial missions. Chinn et al., 2017.232 Reproduced with permission from the Chemical and Biological Microsystems Society (CBMS). Copyright 2017 CBMS. | ||
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to acknowledge funding from the Council for Science, Technology and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion Program (SIP) “Next-generation technology for ocean resources exploration”, Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (B) 18H01651, the Ocean Resource Use Promotion Technology Development Program from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and the Strategic International Collaborative Research Program (SICORP) from the Japan Science and Technology Agency (JST).References
- T. S. Garrison, in Oceanography: an invitation to marine science, Cengage Learning, 9th edn, 2012, ch. Circulation of the Ocean, pp. 249–281 Search PubMed.
- R. G. Williams and M. J. Follows, in Ocean biogeochemistry, Springer, 2003, ch. Physical transort of nutrients and the maintenance of biological production, pp. 19–51 Search PubMed.
- T. S. Garrison, in Oceanography: an invitation to marine science, Cengage Learning, 9th edn., 2012, ch. Circulation of the Ocean, pp. 393–414 Search PubMed.
- P. M. Glibert, S. Seitzinger, C. A. Heil, J. M. Burkholder, M. W. Parrow, L. A. Codispoti and V. Kelly, Oceanography, 2005, 18, 198 CrossRef.
- P. M. Glibert and M. A. Burford, Oceanography, 2017, 30, 58–69 CrossRef.
- A. J. Watson and J. C. Orr, in Ocean Biogeochemistry, Springer, 2003, ch. Carbon dioxide fluxes in the global ocean, pp. 123–143 Search PubMed.
- S. C. Doney, V. J. Fabry, R. A. Feely and J. A. Kleypas, Annu. Rev. Mar. Sci., 2009, 1, 169–192 CrossRef.
- P. M. Haugan and H. Drange, Energy Convers. Manage., 1996, 37, 1019–1022 CrossRef CAS.
- R. Blasiak, R. Wynberg, K. Grorud-Colvert, S. Thambisetty, N. M. Bandarra, A. V. M. Canário, J. da Silva, C. M. Duarte, M. Jaspars and A. Rogers, Nat. Sustain., 2020, 1–9 Search PubMed.
- I. S. Robinson, in Discovering the Ocean from Space: The unique applications of satellite oceanography, Springer Science & Business Media, 2010, ch. Ocean biology from space Search PubMed.
- W. Emery and A. Camps, in Introduction to satellite remote sensing: atmosphere, ocean, land and cryosphere applications, Elsevier, 2017, ch. Ocean Applications Search PubMed.
- A. J. Williams, presented in part at the 2011 International Symposium on Ocean Electronics, Kochi, India, 2011, pp. 3–17 Search PubMed.
- T. S. Garrison, in Oceanography: an invitation to marine science, Cengage Learning, 9th edn, 2012, ch. A History of Marine Science, pp. 25–57 Search PubMed.
- T. S. Moore, K. M. Mullaugh, R. R. Holyoke, A. S. Madison, M. Yücel and G. W. Luther Iii, Annu. Rev. Mar. Sci., 2009, 1, 91–115 CrossRef.
- A. J. Williams, in Encyclopedia of Ocean Sciences, ed. J. H. Steele, Academic Press, 2nd edn, 2009, ch. CTD (Conductivity, Temerature, Depth) Profiler, pp. 708–717 Search PubMed.
- W. Kohnen, Mar. Technol. Soc. J., 2018, 52, 125–151 CrossRef.
- U. K. Verfuss, A. S. Aniceto, D. V. Harris, D. Gillespie, S. Fielding, G. Jiménez, P. Johnston, R. R. Sinclair, A. Sivertsen and S. A. Solbø, Mar. Pollut. Bull., 2019, 140, 17–29 CrossRef CAS.
- R. Bogue, Ind. Rob., 2015, 42(3), 186–191 CrossRef.
- R. Bogue, Ind. Rob., 2019, 47(1), 1–6 CrossRef.
- K. Poore, C. Kitts, G. Wheat and W. Kirkwood, presented in part at the OCEANS’06 MTS/IEEE, Monterey, USA, 2016 Search PubMed.
- A. Underwood and C. Murphy, presented in part at the OCEANS 2017 MTS/IEEE, Aberdeen, Scotland, 2017 Search PubMed.
- O. A. Viquez, E. M. Fischell, N. R. Rypkema and H. Schmidt, presented in part at the 2016 IEEE/OES Autonomous Underwater Vehicles (AUV), Tokyo, Japan, 2016, pp. 151–155 Search PubMed.
- J. Sherman, R. E. Davis, W. B. Owens and J. Valdes, IEEE J. Oceanic Eng., 2001, 26, 437–446 CrossRef.
- C. C. Eriksen, T. J. Osse, R. D. Light, T. Wen, T. W. Lehman, P. L. Sabin, J. W. Ballard and A. M. Chiodi, IEEE J. Oceanic Eng., 2001, 26, 424–436 CrossRef.
- D. C. Webb, P. J. Simonetti and C. P. Jones, IEEE J. Oceanic Eng., 2001, 26, 447–452 CrossRef.
- R. Hine, S. Willcox, G. Hine and T. Richardson, presented in part at the OCEANS'09 MTS/IEEE, Biloxi, USA, 2009 Search PubMed.
- J. E. Manley, R. Carlon and G. Hine, presented in part at the OCEANS 2017 MTS/IEEE, Anchorage, USA, 2017 Search PubMed.
- D. Roemmich, G. C. Johnson, S. Riser, R. Davis, J. Gilson, W. B. Owens, S. L. Garzoli, C. Schmid and M. Ignaszewski, Oceanography, 2009, 22, 34–43 CrossRef.
- S. C. Riser, H. J. Freeland, D. Roemmich, S. Wijffels, A. Troisi, M. Belbéoch, D. Gilbert, J. Xu, S. Pouliquen and A. Thresher, Nat. Clim. Change, 2016, 6, 145–153 CrossRef.
- H. Claustre, K. S. Johnson and Y. Takeshita, Annu. Rev. Mar. Sci., 2020, 12, 23–48 CrossRef.
- R. D. Prien, Mar. Chem., 2007, 107, 422–432 CrossRef CAS.
- C. D. M. Campos and J. A. F. da Silva, RSC Adv., 2013, 3, 18216–18227 RSC.
- A. M. Nightingale, A. D. Beaton and M. C. Mowlem, Sens. Actuators, B, 2015, 221, 1398–1405 CrossRef CAS.
- F. Wang, J. Zhu, L. Chen, Y. Zuo, X. Hu and Y. Yang, Micromachines, 2020, 11, 69 CrossRef.
- S. Raymond, presented in part at the OCEAN 75 Conference, San Diego, USA, 1975, pp. 537–544 Search PubMed.
- K. Asakawa, T. Hyakudome, M. Yoshida, N. Okubo, M. Ito and I. Terada, IEEE J. Oceanic Eng., 2012, 37, 756–763 Search PubMed.
- D. I. Greenfield, R. Marin Iii, S. Jensen, E. Massion, B. Roman, J. Feldman and C. A. Scholin, Limnol. Oceanogr.: Methods, 2006, 4, 426–435 CrossRef.
- C. Scholin, S. Jensen, B. Roman, E. Massion, R. Marin, C. Preston, D. Greenfield, W. Jones and K. Wheeler, presented in part at the OCEANS 2006 MTS/IEEE, Boston, USA, 2006 Search PubMed.
- D. I. Greenfield, R. Marin Iii, G. J. Doucette, C. Mikulski, K. Jones, S. Jensen, B. Roman, N. Alvarado, J. Feldman and C. Scholin, Limnol. Oceanogr.: Methods, 2008, 6, 667–679 CrossRef CAS.
- C. Scholin, G. Doucette, S. Jensen, B. Roman, D. Pargett, R. Marin Iii, C. Preston, W. Jones, J. Feldman and C. Everlove, Oceanography, 2009, 22, 158–167 CrossRef.
- G. J. Doucette, C. M. Mikulski, K. L. Jones, K. L. King, D. I. Greenfield, R. Marin Iii, S. Jensen, B. Roman, C. T. Elliott and C. A. Scholin, Harmful Algae, 2009, 8, 880–888 CrossRef CAS.
- C. M. Preston, A. Harris, J. P. Ryan, B. Roman, R. Marin Iii, S. Jensen, C. Everlove, J. Birch, J. M. Dzenitis and D. Pargett, PLoS One, 2011, 6, e22522 CrossRef CAS.
- C. A. Scholin, J. Birch, S. Jensen, R. Marin Iii, E. Massion, D. Pargett, C. Preston, B. Roman and W. Ussler Iii, Oceanography, 2017, 30, 100–113 CrossRef.
- W. Ussler Iii, C. Preston, P. Tavormina, D. Pargett, S. Jensen, B. Roman, R. Marin Iii, S. R. Shah, P. R. Girguis and J. M. Birch, Environ. Sci. Technol., 2013, 47, 9339–9346 CrossRef.
- I. D. Johnston, D. K. McCluskey, C. K. L. Tan and M. C. Tracey, J. Micromech. Microeng., 2014, 24, 035017 CrossRef.
- T. Fukuba, A. Miyaji, T. Okamoto, T. Yamamoto, S. Kaneda and T. Fujii, RSC Adv., 2011, 1, 1567–1573 RSC.
- C. Provin, T. Fukuba, K. Okamura and T. Fujii, IEEE J. Oceanic Eng., 2012, 38, 178–185 Search PubMed.
- I. R. G. Ogilvie, V. J. Sieben, C. F. A. Floquet, R. Zmijan, M. C. Mowlem and H. Morgan, J. Micromech. Microeng., 2010, 20, 065016 CrossRef.
- D. Sun, M. Tweedie, D. R. Gajula, B. Ward and P. D. Maguire, Microfluid. Nanofluid., 2015, 19, 913–922 CrossRef CAS.
- N. Le Bris, P. M. Sarradin, D. Birot and A. M. Alayse-Danet, Mar. Chem., 2000, 72, 1–15 CrossRef CAS.
- D. A. Weeks and K. S. Johnson, Anal. Chem., 1996, 68, 2717–2719 CrossRef CAS.
- R. Vuillemin, D. Le Roux, P. Dorval, K. Bucas, J. P. Sudreau, M. Hamon, C. Le Gall and P. M. Sarradin, Deep Sea Res., Part I, 2009, 56, 1391–1399 CrossRef CAS.
- A. Daniel, D. Birot, S. Blain, P. Tréguer, B. Leïldé and E. Menut, Mar. Chem., 1995, 51, 67–77 CrossRef CAS.
- C. Crandell, J. Autom. Chem., 1985, 7(3), 145–148 CrossRef CAS.
- L. I. Gordon, J. C. Jennings Jr, A. A. Ross and J. M. Krest, WOCE Hydrographic Program Office, Methods Manual WHPO, 1993, pp. 1–52 Search PubMed.
- C. M. Sakamoto-Arnold, K. S. Johnson and C. L. Beehler, Limnol. Oceanogr., 1986, 31, 894–900 CrossRef CAS.
- K. S. Johnson, C. L. Beehler and C. M. Sakamoto-Arnold, Anal. Chim. Acta, 1986, 179, 245–257 CrossRef CAS.
- K. H. Coale, C. S. Chin, G. J. Massoth, K. S. Johnson and E. T. Baker, Nature, 1991, 352, 325–328 CrossRef CAS.
- T. Gamo, H. Sakai, E. Nakayama, K. Ishida and H. Kimoto, Anal. Sci., 1994, 10, 843–848 CrossRef CAS.
- H. W. Jannasch, K. S. Johnson and C. M. Sakamoto, Anal. Chem., 1994, 66, 3352–3361 CrossRef CAS.
- T. P. Chapin, H. W. Jannasch and K. S. Johnson, Anal. Chim. Acta, 2002, 463, 265–274 CrossRef CAS.
- P. J. Statham, D. P. Connelly, C. R. German, E. Bulukin, N. Millard, S. McPhail, M. Pebody, J. Perrett, M. Squires and P. Stevenson, Underwater Technology, 2003, 25, 129–134 CrossRef.
- C. S. Chin, K. S. Johnson and K. H. Coale, Mar. Chem., 1992, 37, 65–82 CrossRef CAS.
- C. S. Chin, K. H. Coale, V. A. Elrod, K. S. Johnson, G. J. Massoth and E. T. Baker, J. Geophys. Res.: Solid Earth, 1994, 99, 4969–4984 CrossRef CAS.
- X. Liu, Z. A. Wang, R. H. Byrne, E. A. Kaltenbacher and R. E. Bernstein, Environ. Sci. Technol., 2006, 40, 5036–5044 CrossRef CAS.
- Y. Nakano, H. Kimoto, S. Watanabe, K. Harada and Y. W. Watanabe, J. Oceanogr., 2006, 62, 71–81 CrossRef CAS.
- K. Okamura, H. Kimoto, K. Saeki, J. Ishibashi, H. Obata, M. Maruo, T. Gamo, E. Nakayama and Y. Nozaki, Mar. Chem., 2001, 76, 17–26 CrossRef CAS.
- K. Okamura, H. Hatanaka, H. Kimoto, M. Suzuki, Y. Sohrin, E. Nakayama, T. Gamo and J.-i. Ishibashi, Geochem. J., 2004, 38, 635–642 CrossRef CAS.
- T. Fukuba, T. Noguchi, K. Okamura and T. Fujii, Micromachines, 2018, 9, 370 CrossRef.
- A. D. Beaton, V. J. Sieben, C. F. A. Floquet, E. M. Waugh, S. A. K. Bey, I. R. G. Ogilvie, M. C. Mowlem and H. Morgan, Sens. Actuators, B, 2011, 156, 1009–1014 CrossRef CAS.
- A. D. Beaton, C. L. Cardwell, R. S. Thomas, V. J. Sieben, F.-E. Legiret, E. M. Waugh, P. J. Statham, M. C. Mowlem and H. Morgan, Environ. Sci. Technol., 2012, 46, 9548–9556 CrossRef CAS.
- F.-E. Legiret, V. J. Sieben, E. M. S. Woodward, S. K. A. K. Bey, M. C. Mowlem, D. P. Connelly and E. P. Achterberg, Talanta, 2013, 116, 382–387 CrossRef CAS.
- V. M. C. Rérolle, C. F. A. Floquet, A. J. K. Harris, M. C. Mowlem, R. R. G. J. Bellerby and E. P. Achterberg, Anal. Chim. Acta, 2013, 786, 124–131 CrossRef.
- A. Milani, P. J. Statham, M. C. Mowlem and D. P. Connelly, Talanta, 2015, 136, 15–22 CrossRef CAS.
- F. Geißler, E. P. Achterberg, A. D. Beaton, M. J. Hopwood, J. S. Clarke, A. Mutzberg, M. C. Mowlem and D. P. Connelly, Front. Mar. Sci., 2017, 4, 322 CrossRef.
- M. M. Grand, G. S. Clinton-Bailey, A. D. Beaton, A. M. Schaap, T. H. Johengen, M. N. Tamburri, D. P. Connelly, M. C. Mowlem and E. P. Achterberg, Front. Mar. Sci., 2017, 4, 255 CrossRef.
- T. Fukuba, Y. Aoki, N. Fukuzawa, T. Yamamoto, M. Kyo and T. Fujii, Lab Chip, 2011, 11, 3508–3515 RSC.
- K. S. Johnson and R. L. Petty, Limnol. Oceanogr., 1983, 28, 1260–1266 CrossRef CAS.
- D. Thouron, R. Vuillemin, X. Philippon, A. Lourenço, C. Provost, A. Cruzado and V. Garçon, Anal. Chem., 2003, 75, 2601–2609 CrossRef CAS.
- T. R. Martz, J. J. Carr, C. R. French and M. D. DeGrandpre, Anal. Chem., 2003, 75, 1844–1850 CrossRef CAS.
- M. P. Seidel, M. D. DeGrandpre and A. G. Dickson, Mar. Chem., 2008, 109, 18–28 CrossRef CAS.
- S. Blain, H. W. Jannasch and K. Johnson, in Chemical sensors in oceanography, ed. M. S. Varney, Gordon and Breach Science Publishers, ch. In situ Chemical Analysis with Colorimetric Detection, 2000, pp. 49–70 Search PubMed.
- V. M. C. Rérolle, C. F. A. Floquet, M. C. Mowlem, D. P. Connelly, E. P. Achterberg and R. R. G. J. Bellerby, TrAC, Trends Anal. Chem., 2012, 40, 146–157 CrossRef.
- A. G. Vincent, R. W. Pascal, A. D. Beaton, J. Walk, J. E. Hopkins, E. M. S. Woodward, M. Mowlem and M. C. Lohan, Mar. Chem., 2018, 205, 29–36 CrossRef CAS.
- X. Cao, S. W. Zhang, D. Z. Chu, N. Wu, H. K. Ma and Y. Liu, presented in a part of the 3rd International Conference on Water Resource and Environment (WRE 2017), Qingdao, China, 2017.
- I. R. G. Ogilvie, V. J. Sieben, M. C. Mowlem and H. Morgan, Anal. Chem., 2011, 83, 4814–4821 CrossRef CAS.
- J. N. Plant, K. S. Johnson, J. A. Needoba and L. J. Coletti, Limnol. Oceanogr.: Methods, 2009, 7, 144–156 CrossRef CAS.
- J. Cleary, D. Maher, C. Slater and D. Diamond, presented in part at the 2010 IEEE Sensors Applications Symposium (SAS), 2010 Search PubMed.
- D. Cogan, J. Cleary, T. Phelan, E. McNamara, M. Bowkett and D. Diamond, Anal. Methods, 2013, 5, 4798–4804 RSC.
- D. Cogan, C. Fay, D. Boyle, C. Osborne, N. Kent, J. Cleary and D. Diamond, Anal. Methods, 2015, 7, 5396–5405 RSC.
- T. Fukuba, Y. Tamai, M. Kyo, K. Shitashima, Y. Koike and T. Fujii, presented in part at the 12th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2008), San Diego, USA, 2008.
- E. A. Sideris and H. C. de Lange, Sens. Actuators, A, 2020, 111915 CrossRef CAS.
- J. T. W. Kuo, L. Yu and E. Meng, Micromachines, 2012, 3, 550–573 CrossRef.
- O. Woitschach, C. Sosna, W. Lang and J. Uckelmann, presented in part at the IEEE SENSORS, 2010 Conference, Waikoloa, USA, 2010, pp. 2472–2477 Search PubMed.
- C. Jenke, J. Pallejà Rubio, S. Kibler, J. Häfner, M. Richter and C. Kutter, Sensors, 2017, 17, 755 CrossRef.
- K. Okamura, T. Noguchi, M. Hatta, M. Sunamura, T. Suzue, H. Kimoto, T. Fukuba and T. Fujii, Methods in Oceanography, 2013, 8, 75–90 CrossRef.
- F. Theeuwes and S. I. Yum, Ann. Biomed. Eng., 1976, 4, 343–353 CrossRef CAS.
- S. Herrlich, S. Spieth, S. Messner and R. Zengerle, Adv. Drug Delivery Rev., 2012, 64, 1617–1627 CrossRef CAS.
- S. Ozcelik, in Encyclopedia of Medical Devices and Instrumentation, ed. J. G. Webster, Wiley Online Library, 2006, ch. Drug infusion systems, pp. 495–508 Search PubMed.
- P. Thurgood, J. Y. Zhu, N. Nguyen, S. Nahavandi, A. R. Jex, E. Pirogova, S. Baratchi and K. Khoshmanesh, Lab Chip, 2018, 18, 2730–2740 RSC.
- T. Fukuba, Y. Sano, H. Yamamoto, T. Miwa and T. Fujii, presented in part at the 2019 IEEE Underwater Technology (UT), Kaohsiung, Taiwan, 2019 Search PubMed.
- T. Fukuba, A. Nakasa, O. Tsukada and T. Fujii, presented in part at the The 22nd International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2018), Kaohsiung, Taiwan, 2018, pp. 2281–2283 Search PubMed.
- S. E. Hulme, S. S. Shevkoplyas and G. M. Whitesides, Lab Chip, 2009, 9, 79–86 RSC.
- J.-Y. Qian, C.-W. Hou, X.-J. Li and Z.-J. Jin, Micromachines, 2020, 11, 172 CrossRef.
- X. Wang, C. Cheng, S. Wang and S. Liu, Microfluid. Nanofluid., 2009, 6, 145–162 CrossRef CAS.
- H. Kinoshita, T. Atsumi, T. Fukuba and T. Fujii, presented in part at the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2010), Groningen, The Netherlands, 2010, pp. 390–392 Search PubMed.
- H. W. Jannasch, P. R. McGill, M. Zdeblick and J. Erickson, presented in part at the The 5th Symposium on Micro Total Analysis Systems (MicroTAS 2001), Monterey, USA, 2001, pp. 529–530 Search PubMed.
- J. C. Anderson and R. P. Welle, JALA, 2008, 13, 65–72 CAS.
- K. R. Hardy, M. S. Olsson, B. P. Lakin, K. A. Steeves, J. R. Sanderson, J. E. Simmons and P. E. Weber, Recent Advances in Deep Sea LEDs for the Offshore Industry, https://ocean-innovations.net/companies/deepsea/knowledgebase/recent-advances-deep-sea-leds-offshore-industry/, (accessed June 14, 2020) Search PubMed.
- P. Kampmann, J. Lemburg, H. Hanff and F. Kirchner, presented in part at the OCEANS’12 MTS/IEEE, Hampton Roads, USA, 2012 Search PubMed.
- J. Sutton, presented in part at the OCEANS'79, San Diego, USA, 1979, pp. 460–469 Search PubMed.
- T. Szczęśniak, M. Grodzicka, M. Moszyński, M. Szawłowski and J. Baszak, IEEE Trans. Nucl. Sci., 2014, 61, 2687–2693 Search PubMed.
- B. Dolgoshein, V. Balagura, P. Buzhan, M. Danilov, L. Filatov, E. Garutti, M. Groll, A. Ilyin, V. Kantserov and V. Kaplin, Nucl. Instrum. Methods Phys. Res., Sect. A, 2006, 563, 368–376 CrossRef CAS.
- K. Yamamoto, K. Yamamura, K. Sato, T. Ota, H. Suzuki and S. Ohsuka, presented in part at the 2006 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC 2006), San Diego, USA, 2006, pp. 1094–1097 Search PubMed.
- M. Díaz-González, A. Baldi and C. Fernández-Sánchez, Sens. Actuators, B, 2017, 251, 93–98 CrossRef.
- M. Tweedie, D. Sun, B. Ward and P. D. Maguire, Lab Chip, 2019, 19, 1287–1295 RSC.
- M. Tweedie, A. Macquart, J. Almeida, B. Ward and P. Maguire, Meas. Sci. Technol., 2020, 31, 065104 CrossRef CAS.
- G. T. Vladisavljević, N. Khalid, M. A. Neves, T. Kuroiwa, M. Nakajima, K. Uemura, S. Ichikawa and I. Kobayashi, Adv. Drug Delivery Rev., 2013, 65, 1626–1663 CrossRef.
- H.-H. Jeong, D. Issadore and D. Lee, Korean J. Chem. Eng., 2016, 33, 1757–1766 CrossRef CAS.
- B. J. Hindson, M. T. McBride, A. J. Makarewicz, B. D. Henderer, U. S. Setlur, S. M. Smith, D. M. Gutierrez, T. R. Metz, S. L. Nasarabadi and K. S. Venkateswaran, Anal. Chem., 2005, 77, 284–289 CrossRef CAS.
- J. F. Regan, A. J. Makarewicz, B. J. Hindson, T. R. Metz, D. M. Gutierrez, T. H. Corzett, D. R. Hadley, R. C. Mahnke, B. D. Henderer and J. W. Breneman Iv, Anal. Chem., 2008, 80, 7422–7429 CrossRef CAS.
- J. S. McQuillan and J. C. Robidart, Curr. Opin. Biotechnol., 2017, 45, 43–50 CrossRef CAS.
- E. A. Ottesen, R. Marin, C. M. Preston, C. R. Young, J. P. Ryan, C. A. Scholin and E. F. DeLong, ISME J., 2011, 5, 1881–1895 CrossRef CAS.
- D. M. Needham, E. B. Fichot, E. Wang, L. Berdjeb, J. A. Cram, C. G. Fichot and J. A. Fuhrman, ISME J., 2018, 12, 2417–2432 CrossRef CAS.
- H. C. Olins, D. R. Rogers, C. Preston, W. Ussler Iii, D. Pargett, S. Jensen, B. Roman, J. M. Birch, C. A. Scholin and M. F. Haroon, Front Microbiol, 2017, 8, 1042 CrossRef.
- A. Takeuchi, S. Watanabe, S. Yamamoto, M. J. Miller, T. Fukuba, T. Miwa, T. Okino, T. Minamoto and K. Tsukamoto, Mar. Ecol.: Prog. Ser., 2019, 609, 187–196 CrossRef CAS.
- J. B. Cardás, D. Deconinck, I. Márquez, P. P. Torre, E. Garcia-Vazquez and G. Machado-Schiaffino, Biol. Conserv., 2020, 250, 108750 CrossRef.
- V. Hidalgo-Ruz, L. Gutow, R. C. Thompson and M. Thiel, Environ. Sci. Technol., 2012, 46, 3060–3075 CrossRef CAS.
- A. K. Au, W. Huynh, L. F. Horowitz and A. Folch, Angew. Chem., Int. Ed., 2016, 55, 3862–3881 CrossRef CAS.
- A. V. Nielsen, M. J. Beauchamp, G. P. Nordin and A. T. Woolley, Annu. Rev. Anal. Chem., 2019, 13, 45–65 CrossRef.
- A. K. Au, N. Bhattacharjee, L. F. Horowitz, T. C. Chang and A. Folch, Lab Chip, 2015, 15, 1934–1941 RSC.
- T. Fukuba and Y. Sano, presented in part at the The 23rd International Conference on Miniaturized Systems for Chemistry and Life Sciences (microTAS 2019), Basel, Switzerland, 2019, pp. 1158–1159 Search PubMed.
- M. D. Patey, M. J. A. Rijkenberg, P. J. Statham, M. C. Stinchcombe, E. P. Achterberg and M. Mowlem, TrAC, Trends Anal. Chem., 2008, 27, 169–182 CrossRef CAS.
- P. J. Worsfold, R. Clough, M. C. Lohan, P. Monbet, P. S. Ellis, C. R. Quétel, G. H. Floor and I. D. McKelvie, Anal. Chim. Acta, 2013, 803, 15–40 CrossRef CAS.
- J. Ma, L. Adornato, R. H. Byrne and D. Yuan, TrAC, Trends Anal. Chem., 2014, 60, 1–15 CrossRef CAS.
- J. Ma, D. Yuan, K. Lin, S. Feng, T. Zhou and Q. Li, Trends Environ. Anal. Chem., 2016, 10, 1–10 CrossRef CAS.
- D. W. King and D. R. Kester, Mar. Chem., 1989, 26, 5–20 CrossRef CAS.
- R. H. Byrne and J. A. Breland, Deep-Sea Res., Part A, 1989, 36, 803–810 CrossRef CAS.
- G. Mills and G. Fones, Sens. Rev., 2012, 32(1), 17–28 CrossRef.
- C. F. A. Floquet, V. J. Sieben, A. Milani, E. P. Joly, I. R. G. Ogilvie, H. Morgan and M. C. Mowlem, Talanta, 2011, 84, 235–239 CrossRef CAS.
- V. J. Sieben, C. F. A. Floquet, I. R. G. Ogilvie, M. C. Mowlem and H. Morgan, Anal. Methods, 2010, 2, 484–491 RSC.
- J. Murphy and J. P. Riley, Anal. Chim. Acta, 1962, 27, 31–36 CrossRef CAS.
- M. Yücel, A. D. Beaton, M. Dengler, M. C. Mowlem, F. Sohl and S. Sommer, PLoS One, 2015, 10, e0132785 CrossRef.
- A. J. Birchill, G. Clinton-Bailey, R. Hanz, E. Mawji, T. Cariou, C. White, S. J. Ussher, P. J. Worsfold, E. P. Achterberg and M. Mowlem, Talanta, 2019, 200, 228–235 CrossRef CAS.
- M. J. Moorcroft, J. Davis and R. G. Compton, Talanta, 2001, 54, 785–803 CrossRef CAS.
- J. M. Edmond, K. L. Von Damm, R. E. McDuff and C. I. Measures, Nature, 1982, 297, 187–191 CrossRef CAS.
- G. J. Massoth, E. T. Baker, R. A. Feely, J. E. Lupton, R. W. Collier, J. F. Gendron, K. K. Roe, S. M. Maenner and J. A. Resing, Deep Sea Res., Part II, 1998, 45, 2683–2712 CrossRef CAS.
- G. Klinkhammer, P. Rona, M. Greaves and H. Elderfield, Nature, 1985, 314, 727–731 CrossRef CAS.
- P. J. Statham, D. P. Connelly, C. R. German, T. Brand, J. O. Overnell, E. Bulukin, N. Millard, S. McPhail, M. Pebody and J. Perrett, Environ. Sci. Technol., 2005, 39, 9440–9445 CrossRef CAS.
- J. Sarrazin, D. Cuvelier, L. Peton, P. Legendre and P.-M. Sarradin, Deep Sea Res., Part I, 2014, 90, 62–75 CrossRef.
- V. C. Pinto, C. F. Araújo, P. J. Sousa, L. M. Gonçalves and G. Minas, Sens. Actuators, B, 2019, 290, 285–292 CrossRef CAS.
- C.-Y. Lee, W.-T. Wang, C.-C. Liu and L.-M. Fu, Chem. Eng. J., 2016, 288, 146–160 CrossRef CAS.
- G. Cai, L. Xue, H. Zhang and J. Lin, Micromachines, 2017, 8, 274 CrossRef.
- D. Meyer, R. D. Prien, O. Dellwig, J. J. Waniek, I. Schuffenhauer, J. Donath, S. Krüger, M. Pallentin and D. E. Schulz-Bull, Sensors, 2016, 16, 2027 CrossRef.
- C. G. Wheat, H. W. Jannasch, J. N. Plant, C. L. Moyer, F. J. Sansone and G. M. McMurtry, J. Geophys. Res.: Solid Earth, 2000, 105, 19353–19367 CrossRef CAS.
- T. D. Clayton and R. H. Byrne, Deep Sea Res., Part I, 1993, 40, 2115–2129 CrossRef CAS.
- R. H. Byrne, Anal. Chem., 1987, 59, 1479–1481 CrossRef CAS.
- N. Le Bris, P.-M. Sarradin and S. Pennec, Deep Sea Res., Part I, 2001, 48, 1941–1951 CrossRef CAS.
- K. Okamura, T. Noguchi, M. Hatta and J.-i. Ishibashi, presented in part at the Techno-Ocean 2016, Kobe, Japan, 2016, pp. 410–414 Search PubMed.
- K. Shitashima, presented in part at the MTS/IEEE OCEANS'10, Seattle, USA, 2010 Search PubMed.
- T. R. Martz, J. G. Connery and K. S. Johnson, Limnol. Oceanogr.: Methods, 2010, 8, 172–184 CrossRef CAS.
- K. S. Johnson, H. W. Jannasch, L. J. Coletti, V. A. Elrod, T. R. Martz, Y. Takeshita, R. J. Carlson and J. G. Connery, Anal. Chem., 2016, 88, 3249–3256 CrossRef CAS.
- V. Rérolle, D. Ruiz-Pino, M. Rafizadeh, S. Loucaides, S. Papadimitriou, M. Mowlem and J. Chen, Methods in Oceanography, 2016, 17, 32–49 CrossRef.
- R. A. Easley and R. H. Byrne, Environ. Sci. Technol., 2012, 46, 5018–5024 CrossRef CAS.
- Y. Nakano, H. Kimoto, T. Miwa and H. Yoshida, presented in part at the EGU General Assembly 2013, Vienna, Austria, 2013, EGU2013-6745 Search PubMed.
- I. M. P. de Vargas Sansalvador, C. D. Fay, J. Cleary, A. M. Nightingale, M. C. Mowlem and D. Diamond, Sens. Actuators, B, 2016, 225, 369–376 CrossRef.
- V. A. Elrod, K. S. Johnson and K. H. Coale, Anal. Chem., 1991, 63, 893–898 CrossRef CAS.
- H. Obata, H. Karatani and E. Nakayama, Anal. Chem., 1993, 65, 1524–1528 CrossRef CAS.
- R. T. Powell, D. W. King and W. M. Landing, Mar. Chem., 1995, 50, 13–20 CrossRef CAS.
- A. R. Bowie, E. P. Achterberg, R. F. C. Mantoura and P. J. Worsfold, Anal. Chim. Acta, 1998, 361, 189–200 CrossRef CAS.
- T. P. Chapin, K. S. Johnson and K. H. Coale, Anal. Chim. Acta, 1991, 249, 469–478 CrossRef CAS.
- E. Nakayama, K. Isshiki, Y. Sohrin and H. Karatani, Anal. Chem., 1989, 61, 1392–1396 CrossRef CAS.
- K. H. Coale, K. S. Johnson, P. M. Stout and C. M. Sakamoto, Anal. Chim. Acta, 1992, 266, 345–351 CrossRef CAS.
- C. M. Sakamoto-Arnold and K. S. Johnson, Anal. Chem., 1987, 59, 1789–1794 CrossRef CAS.
- C. E. Holm, Z. Chase, H. W. Jannasch and K. S. Johnson, Limnol. Oceanogr.: Methods, 2008, 6, 336–346 CrossRef CAS.
- T. Doi, M. Takano, K. Okamura, T. Ura and T. Gamo, J. Oceanogr., 2008, 64, 471–477 CrossRef CAS.
- T. Fukuba, C. Provin, K. Okamura and T. Fujii, IEEJ Transactions on Sensors and Micromachines, 2009, 129, 69–72 CrossRef.
- C.-C. Hong, J.-W. Choi and C. H. Ahn, Lab Chip, 2004, 4, 109–113 RSC.
- https://www.hamamatsu.com/us/en/product/optical-sensors/pmt/micro-pmt/index.html, (accessed July 7, 2020).
- J. S. Erickson, N. Hashemi, J. M. Sullivan, A. D. Weidemann and F. S. Ligler, Anal. Chem., 2012, 84, 839–850 CrossRef CAS.
- R. J. Olson, D. Vaulot and S. W. Chisholm, Deep-Sea Res., Part A, 1985, 32, 1273–1280 CrossRef.
- B. R. Robertson and D. K. Button, Cytometry, 1989, 10, 70–76 CrossRef CAS.
- G. B. J. Dubelaar, A. C. Groenewegen, W. Stokdijk, G. J. Van Den Engh and J. W. M. Visser, Cytometry, 1989, 10, 529–539 CrossRef CAS.
- J. C. H. Peeters, G. B. J. Dubelaar, J. Ringelberg and J. W. M. Visser, Cytometry, 1989, 10, 522–528 CrossRef CAS.
- G. B. J. Dubelaar, P. L. Gerritzen, A. E. R. Beeker, R. R. Jonker and K. Tangen, Cytometry, 1999, 37, 247–254 CrossRef CAS.
- D. Marie, C. P. D. Brussaard, R. Thyrhaug, G. Bratbak and D. Vaulot, Appl. Environ. Microbiol., 1999, 65, 45–52 CrossRef CAS.
- N. Hashemi, J. S. Erickson, J. P. Golden and F. S. Ligler, Biomicrofluidics, 2011, 5, 032009 CrossRef.
- M. M. Maw, J. Wang, F. Li, J. Jiang, Y. Song and X. Pan, Int. J. Mol. Sci., 2015, 16, 25560–25575 CrossRef CAS.
- J. Wang, Y. Song, M. M. Maw, Y. Song, X. Pan, Y. Sun and D. Li, J. Exp. Mar. Biol. Ecol., 2015, 473, 129–137 CrossRef.
- S. Dunker, Cytometry, Part A, 2019, 95, 854–868 CrossRef.
- S. Dunker, Cytometry, Part A, 2020, 97(7), 727–736 CrossRef CAS.
- V. Dashkova, D. Malashenkov, N. Poulton, I. Vorobjev and N. S. Barteneva, Methods, 2017, 112, 188–200 CrossRef CAS.
- R. J. Olson and H. M. Sosik, Limnol. Oceanogr.: Methods, 2007, 5, 195–203 CrossRef.
- H. M. Sosik and R. J. Olson, Limnol. Oceanogr.: Methods, 2007, 5, 204–216 CrossRef.
- Y. J. Heo, D. Lee, J. Kang, K. Lee and W. K. Chung, Sci. Rep., 2017, 7, 1–9 CrossRef.
- M. J. Kim, S. C. Lee, S. Pal, E. Han and J. M. Song, Lab Chip, 2011, 11, 104–114 RSC.
- S. Stavrakis, G. Holzner, J. Choo and A. DeMello, Curr. Opin. Biotechnol., 2019, 55, 36–43 CrossRef CAS.
- N. Hashemi, J. S. Erickson, J. P. Golden, K. M. Jackson and F. S. Ligler, Biosens. Bioelectron., 2011, 26, 4263–4269 CrossRef CAS.
- S. Dunker, D. Boho, J. Wäldchen and P. Mäder, BMC Ecol., 2018, 18, 51 CrossRef.
- Z. Göröcs, M. Tamamitsu, V. Bianco, P. Wolf, S. Roy, K. Shindo, K. Yanny, Y. Wu, H. C. Koydemir and Y. Rivenson, Light: Sci. Appl., 2018, 7, 1–12 CrossRef.
- J.-M. Hawronskyj and J. Holah, Trends Food Sci. Technol., 1997, 8, 79–84 CrossRef CAS.
- D. M. Karl, Microbiol. Rev., 1980, 44, 739 CrossRef CAS.
- E. F. DeLong, C. M. Preston, T. Mincer, V. Rich, S. J. Hallam, N.-U. Frigaard, A. Martinez, M. B. Sullivan, R. Edwards and B. R. Brito, Science, 2006, 311, 496–503 CrossRef CAS.
- J. A. Gilbert, D. Field, Y. Huang, R. Edwards, W. Li, P. Gilna and I. Joint, PLoS One, 2008, 3, e3042 CrossRef.
- P. F. Thomsen, J. Kielgast, L. L. Iversen, P. R. Møller, M. Rasmussen and E. Willerslev, PLoS One, 2012, 7, e41732 CrossRef CAS.
- M. Miya, Y. Sato, T. Fukunaga, T. Sado, J. Y. Poulsen, K. Sato, T. Minamoto, S. Yamamoto, H. Yamanaka and H. Araki, R. Soc. Open Sci., 2015, 2, 150088 CrossRef CAS.
- D. Fries, J. Paul, M. Smith, A. Farmer, E. Casper and J. Wilson, Microsc. Microanal., 2007, 13, 514–515 CrossRef.
- A. S. Farmer, D. P. Fries, W. Flannery and J. Massini, Rev. Sci. Instrum., 2005, 76, 115102 CrossRef.
- C. A. Scholin, R. Marin Iii, P. E. Miller, G. J. Doucette, C. L. Powell, P. Haydock, J. Howard and J. Ray, J. Phycol., 1999, 35, 1356–1367 CrossRef CAS.
- P. Belgrader, C. J. Elkin, S. B. Brown, S. N. Nasarabadi, R. G. Langlois, F. P. Milanovich, B. W. Colston and G. D. Marshall, Anal. Chem., 2003, 75, 3446–3450 CrossRef.
- J. C. Robidart, C. M. Preston, R. W. Paerl, K. A. Turk, A. C. Mosier, C. A. Francis, C. A. Scholin and J. P. Zehr, ISME J., 2012, 6, 513–523 CrossRef CAS.
- J. C. Robidart, M. J. Church, J. P. Ryan, F. Ascani, S. T. Wilson, D. Bombar, R. Marin, K. J. Richards, D. M. Karl and C. A. Scholin, ISME J., 2014, 8, 1175–1185 CrossRef CAS.
- K. M. Yamahara, E. Demir-Hilton, C. M. Preston, R. Marin Iii, D. Pargett, B. Roman, S. Jensen, J. M. Birch, A. B. Boehm and C. A. Scholin, Lett. Appl. Microbiol., 2015, 61, 130–138 CrossRef CAS.
- T. Fukuba, T. Yamamoto, T. Naganuma and T. Fujii, Chem. Eng. J., 2004, 101, 151–156 CrossRef CAS.
- Y. Zhang and H.-R. Jiang, Anal. Chim. Acta, 2016, 914, 7–16 CrossRef CAS.
- A. M. Nightingale, S.-u. Hassan, B. M. Warren, K. Makris, G. W. H. Evans, E. Papadopoulou, S. Coleman and X. Niu, Environ. Sci. Technol., 2019, 53, 9677–9685 CrossRef CAS.
- M. P. Hemming, J. Kaiser, K. J. Heywood, D. C. E. Bakker, J. Boutin, K. Shitashima, G. Lee, O. Legge and R. Onken, Ocean Sci., 2017, 13, 427–442 CrossRef CAS.
- G. Saba, E. K. Wright-Fairbanks, B. Chen, W.-J. Cai, A. Barnard, C. Jones, C. W. Branham, K. Wang and T. Miles, Front. Mar. Sci., 2019, 6, 664 CrossRef.
- P. J. Bresnahan Jr, T. R. Martz, Y. Takeshita, K. S. Johnson and M. LaShomb, Methods in Oceanography, 2014, 9, 44–60 CrossRef.
- K. B. Clark, presented in part at the 2007 IEEE Aerospace Conference, Big City, USA, 2007 Search PubMed.
- L. D. Hutt, D. P. Glavin, J. L. Bada and R. A. Mathies, Anal. Chem., 1999, 71, 4000–4006 CrossRef CAS.
- M. F. Mora, F. Greer, A. M. Stockton, S. Bryant and P. A. Willis, Anal. Chem., 2011, 83, 8636–8641 CrossRef CAS.
- M. F. Mora, A. M. Stockton and P. A. Willis, Electrophoresis, 2012, 33, 2624–2638 CrossRef CAS.
- J. Kim, E. C. Jensen, A. M. Stockton and R. A. Mathies, Anal. Chem., 2013, 85, 7682–7688 CrossRef CAS.
- Z. A. Duca, G. K. Tan, T. Cantrell, M. Van Enige, M. Dorn, M. Cato, N. C. Speller, A. Pital, R. A. Mathies and A. M. Stockton, Sens. Actuators, B, 2018, 272, 229–235 CrossRef CAS.
- A. M. Skelley, J. R. Scherer, A. D. Aubrey, W. H. Grover, R. H. C. Ivester, P. Ehrenfreund, F. J. Grunthaner, J. L. Bada and R. A. Mathies, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 1041–1046 CrossRef CAS.
- J. Jonsson, J. Sundqvist, H. Nguyen, M. Berglund, S. Ogden, K. Palmer, K. Smedfors, L. Johansson, K. Hjort and G. Thornell, Acta Astronaut., 2012, 79, 203–211 CrossRef.
- N. C. Speller, M. Cato, J. L. McNeice, M. R. Meister, A. D. Mullen, D. Dichek, B. E. Schmidt and A. M. Stockton, presented in part at the Astrobiology Science Conference (AbSciCon 2019), Bellevue, USA, 2019 Search PubMed.
- A. Nascetti, M. Mirasoli, E. Marchegiani, M. Zangheri, F. Costantini, A. Porchetta, L. Iannascoli, N. Lovecchio, D. Caputo and G. de Cesare, Biosens. Bioelectron., 2019, 123, 195–203 CrossRef CAS.
- A. C. Noell, E. Jaramillo, S. P. Kounaves, M. H. Hecht, D. J. Harrison, R. C. Quinn, J. E. Koehne, J. Forgione and A. J. Ricco, presented in part at the 2019 Astrobiology Science Conference (AbSciCon 2019), Bellevue, USA, 2019 Search PubMed.
- S. D. Thomson, R. C. Quinn, A. J. Ricco and J. E. Koehne, ChemElectroChem, 2020, 7, 614–623 CrossRef CAS.
- T. N. Chinn, A. K. Lee, T. D. Boone, M. X. Tan, M. M. Chin, G. C. McCutcheon, M. F. Horne, M. R. Padgen, J. T. Blaich and J. B. Forgione, presented in part at the 21st International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2017), Savannah, USA, 2017, pp. 1479–1480 Search PubMed.
- T. P. Cantrell, M. Cato, Z. Duca, J. Muller, D. Eichstedt, B. Schmidt, S. Foreman, J. Kim, P. Putman, H. Jiao, E. Jensen, H. Mehrabani and A. Stockton, presented in part at the 21st International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2017), Savannah, USA, 2017, pp. 1437–1438 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |