Open Access Article
Open Access ArticleGas-phase CO2 electroreduction over Sn–Cu hollow fibers†
Xiao
Dong
 a,
Guihua
Li
a,
Guihua
Li
 a,
Wei
Chen
a,
Wei
Chen
 *a,
Chang
Zhu
a,
Tong
Li
a,
Yanfang
Song
*a,
Chang
Zhu
a,
Tong
Li
a,
Yanfang
Song
 a,
Nannan
Sun
a,
Nannan
Sun
 a and
Wei
Wei
*ab
a and
Wei
Wei
*ab
aCAS Key Laboratory of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, P. R. China. E-mail: chenw@sari.ac.cn; weiwei@sari.ac.cn
bSchool of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, P. R. China
First published on 27th November 2020
Abstract
CO2 electroreduction to value-added chemicals by virtue of renewable electricity is significant for carbon emission abatement and renewable energy conversion/storage. Conventional CO2 electroreduction occurring in aqueous solution or ionic liquid suffers from insufficient CO2 solubility and a separation dilemma of the soluble products. In this work, we report that when using Sn–Cu hollow fiber electrodes, gas-phase CO2 can be directly electroreduced into various multicarbon oxygenates. The faradaic efficiencies of acetaldehyde and acetone over a 0.3 wt% Sn–Cu hollow fiber electrode are 10 and 12% at the cell voltage of −1.4 V. The presence of an appropriate amount of SnO2 nanoparticles decorated on a Cu hollow fiber surface not only facilitates the reaction kinetics with elevated current densities, but also improves the C–C coupling of intermediates, promoting the formation of multicarbon oxygenates.
The continuously elevating atmospheric CO2 concentration which has arisen from tremendous fossil fuel consumption severely threatens the ecological environment.1,2 Utilizing CO2 as a safe, economic and sustainable C1 resource via modern techniques to obtain value-added chemicals could contribute to carbon emission abatement, which is of great importance.3–6 Comparing with the thermocatalytic conversion of CO2, which requires harsh conditions and a massive amount of energy, the CO2 electroreduction conducted under mild conditions exhibits great application potentials.7,8 Besides, the rapid-developing renewable electricity from wind and solar power would cause critical impacts on the transmission grid due to its randomness, intermittency and volatility, while CO2 electroreduction could take in this low-grade renewable electricity in CO2 conversion to obtain valuable chemicals.
Although CO2 electrocatalysis has been widely studied for decades, the limited reaction kinetics constrained by the insufficient CO2 solubility in liquid electrolyte (both aqueous solutions and ionic liquids),8–13 and the separation dilemma of soluble products from liquid electrolyte (since the energy required to recover the products is higher than the energy stored in the produced molecules),7 are still the two key issues. In spite of the many attempts of adopting gas-diffusion electrodes to alleviate the former issue by Sargent and other researchers, which could yield ethylene with a faradaic efficiency (FE) of 70%14 and a 62% C2+ product selectivity with a current density of 653 mA cm−2,15 for example, the complicated fabrication procedures and multi-component configuration (active component|hydrophobic binder|conductive additions) of such gas-diffusion electrodes still hinder the industrial applications of CO2 electrocatalysis.
In contrast, the gas-phase CO2 electrocatalysis would allow easy product separation, and could get rid of the CO2 solubility problems at the same time. Centi et al. first came up with the idea of gas-phase CO2 electroreduction and the possibility to obtain long-chain hydrocarbon products with Pt/C catalysts and solid electrolyte Nafion®.16 Kriescher et al. reported a Cu felt electrode with Fumapem membrane, obtaining mainly methane with FEs no more than 0.12%.17 Recent studies from Gutiérrez-Guerra et al. used polybenzimidazoles (PBI) and Sterion® as the solid electrolyte for gas-phase CO2 electroreduction at 90 °C, obtaining methanol and acetaldehyde with yields of 40 and 110 μmol gcat−1 h−1 over Cu catalysts.18–20 However, these studies are all based on conventional types of Cu-based electrodes, with unsatisfying overall efficiencies and insufficient value-added multicarbon products for the electroreduction of gas-phase CO2.
The porous hollow fiber (HF) electrode stands out as an alternative to the above-mentioned issues, which is fabricated via a combined phase-inversion and sintering process using commercial metal powder. This kind of self-supported gas-diffusion electrode can be adopted in electrochemical reaction systems without any binder. Its hierarchical pore structure, good stability and conductivity, have shown unique advantages in electrocatalysis applications,21–23 and could be a suitable platform to study the gas-phase CO2 electroreduction process.
On the other hand, Sn-based catalysts have been frequently used in CO2 electroreduction due its intrinsic high activity. Lei et al. reported confined Sn in graphene which exhibited high CO2 electroreduction activities with 85% HCOOH FE at −0.48 V overpotential.24 Bai et al. found that alloying Sn with Pd reached nearly 100% HCOOH FE via an optimal surface Pd–Sn–O configuration at the lowest overpotential of −0.26 V.25 An et al. revealed that Sn2+/Sn4+ species can reduce the overpotential and improve the HCOOH selectivity.26 Liu et al. reported SnO2 quantum wires with enhanced current density and improved HCOOH FE of over 80%.27 Geng et al. found the Sn2+ node in ZIF-8 can accelerate HCOOH formation with a FE of 74% and a total current density of 27 mA cm−2.28 These findings indicate the great promotion of HCOOH formation by introducing Sn species in aqueous CO2 electroreduction. However, during gas-phase CO2 electroreduction, SnO2 nanoparticles on the HF surface would play more important roles beyond facilitating the production of HCOOH species.
Here, a novel gas-phase CO2 electrochemical reactor is developed based on Sn–Cu HF electrodes as illustrated in Scheme 1. The porous Cu HF electrode is applied as a self-supported gas-diffusion electrode, while its surface is modified by Sn species via wet chemistry reduction with sodium citrate as the stabilizing agent and sodium borohydride (NaBH4) as the reductive agent (see the ESI† for details). The Sn-based catalysts always help the formation of formate,24–28 while Cu-based catalysts are famous for the potential to promote C–C coupling and the formation of C2 products such as ethylene and ethanol.8,29–31 The loadings of Sn element in Sn–Cu HFs are controlled by varying the molar ratio between the SnCl2 precursor and Cu HF. The obtained samples are denoted as xSn–Cu HFs, in which the x represents the loading of Sn element determined by ICP-OES (inductively coupled plasma-optical emission spectroscopy). Cu HF is also studied as the counterpart for comparison purposes.
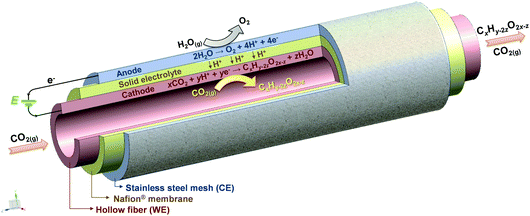 | ||
| Scheme 1 Schematic diagrams of a gas-phase CO2 electrochemical reaction system based on Sn–Cu hollow fibers as the working electrode. | ||
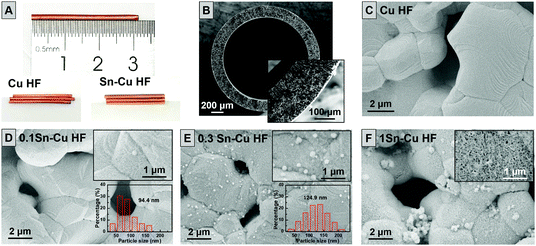
As shown in Fig. 1A, the typical length of Cu HFs used in this work is about 3 cm with a color of light orange. The cross-section and surface scanning electron microscopy (SEM) images of Cu HF in Fig. 1B and C suggest the hollow structure of Cu HFs with an outer diameter of ∼2 mm, as well as the porous fiber wall with a thickness of ∼200 μm. The characteristic finger-like pores are dispersed in the fiber wall without any visible macrovoids, while abundant pores are formed with an average size of 2–5 μm on the smooth fiber surface. The porous structure of Cu HFs benefits the diffusion of gaseous reactants and products by providing plenty of pathways for mass transfer.21,22 While the metallic nature of Cu HF guarantees sufficient electron conductivity, which would enlarge the three-phase boundary of reaction interfaces and facilitate the kinetics of CO2 electroreduction.21,22 After the wet chemistry reduction preparation, no obvious appearance change could be observed for Sn–Cu HFs in Fig. 1A. While the SEM images in Fig. 1D–F clearly indicate the formation of SnO2 nanoparticles or layer (see the following surface structure characterization for details) on the surface of Sn–Cu HFs. With the elevated loading of Sn component, not only the amount of SnO2 nanoparticles increases, but also the average particle size enlarges from ∼94 nm for 0.1Sn–Cu HF (Fig. 1D) to ∼125 nm for 0.3Sn–Cu HF (Fig. 1E). For 1Sn–Cu HF, even a fabric-like SnO2 layer is formed, composed of dense SnO2 nanosheets vertically packed on the Cu HF surface (as shown in Fig. 1F).32 While for all Sn–Cu HF samples, the abundant pore structure of Cu HFs is still well-preserved, similar to other reported works.23
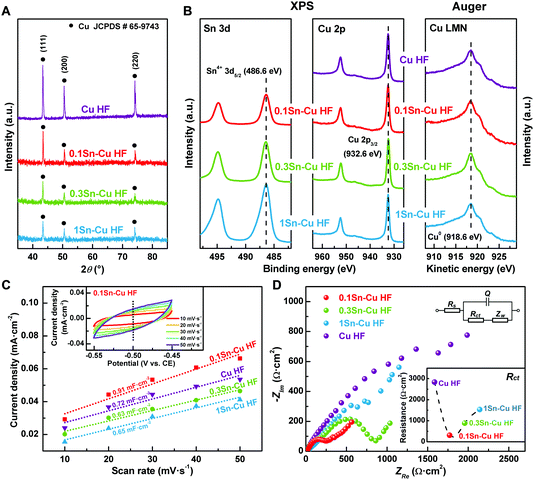
Fig. 2A shows the bulk structure of each HF sample characterized via X-ray diffraction (XRD) measurements. Peaks appearing at 43.3, 50.4 and 74.1° in all samples could be assigned to the characteristic peaks for (111), (200) and (220) planes of metallic Cu (JCPDS no. 65-9743), indicating that the main component of Cu HF and Sn–Cu HFs is metallic Cu.33,34 On the other hand, no diffraction peaks of all Sn–Cu electrodes corresponding to SnO2, SnOx or other tin species23,25,26 could be observed, suggesting the nanoparticles are amorphous with insufficient loadings.35
In contrast to the bulk composition, the surface electronic states of catalysts can exert significant influence on the adsorption/desorption capacity as well as the catalytic performance. Fig. 2B shows the surface electronic states of Sn–Cu HFs characterized via X-ray photoelectron spectroscopy (XPS). For Cu 2p spectra in the binding energy (BE) range of 925–965 eV, a Cu 2p3/2 peak at 932.6 eV could be observed for each sample. Since the Cu 2p3/2 peaks for both Cu0 and Cu+ would appear around this binding energy range (while the Cu2+ 2p3/2 peak locates at ∼942 eV), it is hard to distinguish the accurate electronic states of Cu with XPS results only.33,36 Thus the Auger curves of Cu LMM in the kinetic energy (KE) range of 908–929 eV (i.e. 558–579 eV for BE) were collected. The Cu LMM peak of each sample appears at 918.6 eV, while that of Cu+ locates at 916.8 eV, suggesting that the Cu0 species exists on the surface of all HF samples.36 For Sn 3d spectra in the BE range of 482–498 eV, Sn 3d5/2 peaks at 486.6 eV could be observed for all Sn–Cu HF samples, which can be assigned to Sn4+ species rather than Sn2+ and Sn0 species with peaks at around 486.0 and 485.2 eV, respectively.23,25,26 Due to the oxophilic intrinsic property of the tin element, the Sn component existed in the SnO2 state via the wet chemistry preparation route, as evidenced by the XPS results (Fig. 2B). This indicates the presence of SnO2 nanoparticles or layer on the Cu HF surface as observed in the SEM images. Tin oxide can be formed rapidly when metallic Sn is exposed to air due to the oxophilicity of Sn,25 while the relatively lower oxygen affinity of Cu would keep it from being oxidized.23 Meanwhile, the intensity of Sn 3d5/2 peaks increased with the elevated Sn component loading, indicating the growing amount of Sn4+ species on the surface of Sn–Cu HFs.
The enrichment of SnO2 on the Sn–Cu HF surface could further be confirmed via the surface atomic ratio extracted from quantified XPS results, and the average atomic ratio in each Sn–Cu HF sample obtained from ICP analysis.35 Table S1 (ESI†) presents the comparison between the surface atomic Sn/Cu ratio detected by XPS, (Sn/Cu)XPS, and the average atomic Sn/Cu ratio from ICP analysis, (Sn/Cu)ICP, for Sn–Cu HF samples. It is clear that the (Sn/Cu)XPS numbers are a hundred times that of (Sn/Cu)ICP, demonstrating that the SnO2 deposited via wet chemistry reduction preparation is enriched at the surface of Sn–Cu HFs. It is also reported that SnOx with a valence of +4 can play an essential role in the CO2 electroreduction process.37 This abundance of oxide species existing on the Cu electrode surface would affect the CO2 electroreduction significantly.
The gas-phase CO2 electrochemical reaction system based on a hollow fiber electrode is shown in Scheme 1 (see the ESI† for details). A single hollow fiber is used as the working electrode (WE) and support. A Nafion® membrane is covered on the surface of the hollow fiber as the solid electrolyte and diaphragm between the anode and cathode. Stainless steel mesh (SSM) is then deployed on the surface of Nafion®, functioning as the counter electrode (CE). Both electrochemical characterization and reaction performance tests were conducted on this reaction system.
The electrochemical active surface areas (ECSAs) of Sn–Cu HF electrodes are determined based on their double-layer capacitances via a cyclic voltammetry (CV) method. All CV profiles (Fig. S2, ESI† and the inset in Fig. 2C) exhibit nearly symmetrical shapes in the range of −0.45 to −0.55 V vs. CE, indicating the capacitive behaviors of double-layer capacitance for Sn–Cu HF electrodes. The cathodic and anodic current densities are obtained from the double layer charge/discharge CV curves at −0.5 V vs. CE. The double layer capacitance (Cdl), which is directly proportional to the ECSA, is then calculated by averaging the absolute values of cathodic and anodic current density slopes via linear fits.22 The obtained Cdl values are 0.72, 0.91, 0.63 and 0.65 mF cm−2 for Cu HF, 0.1Sn–Cu HF, 0.3Sn–Cu HF and 1Sn–Cu HF, respectively (as shown in Fig. 2C). This implies that the ECSA of 0.1Sn–Cu HF is the largest, while the ECSAs of 0.3Sn–Cu HF and 1Sn–Cu HF are quite similar to each other.
The electrochemical impedance spectra (EIS) of Sn–Cu HF electrodes at −1.4 V vs. CE, as well as the corresponding charge transfer resistances (Rct) are shown in Fig. 2D, which are obtained via the equivalent circuit shown in the inset. The Rct of Cu HF is 2.8 kΩ cm2, which is much larger than those of Sn–Cu HFs. The Rct increases monotonically from 0.3 kΩ cm2 for 0.1Sn–Cu HF, to 0.9 kΩ cm2 for 0.3Sn–Cu HF and 1.5 kΩ cm2 for 1Sn–Cu HF with the increase of SnO2 content. This phenomenon indicates that the presence of few SnO2 nanoparticles significantly promotes the reaction kinetics for their high activities, leading to smaller Rct corresponding to electrochemical reaction resistance. SnO2 has been reported for its efficient, selective, and durable performance for CO2 electroreduction.24–28 On the other hand, although SnO2 is known as a p-type semiconductor with relatively fast electron transportation, the electronic conductivity of SnO2 is still far below that of the metallic conductor. Therefore, the higher amount of SnO2 nanoparticles and even SnO2 layer on the Cu HF surface would hamper the charge transfer steps of electrochemical reaction due to the semiconductive nature of SnO2, leading to increased Rct.25
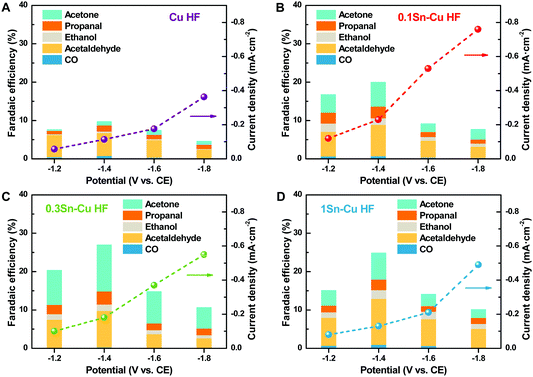
Fig. 3 shows the gas-phase CO2 electroreduction performance of hollow fiber electrodes at different potentials. No reduction products are observed under open circuit voltage (OCV), i.e., with no current applied, while a large variety of products is obtained under polarization conditions. Acetaldehyde could be observed as one of the main products for all electrodes with several other C1–C3 products, while no H2 from the competitive H2 evolution reaction could be detected in the examined conditions, which is consistent with the results of former gas-phase CO2 electroreduction studies.16–20 The gas-phase electrocatalytic reduction of CO2 is a complex multistep reaction involving shared intermediates and multiple reaction pathways.19,20 The formation of the different products can be described by a general reaction as shown in Scheme 1 for the cathodic reaction of CO2 and H+ with e−.
For the Cu HF electrode, the current densities are −0.06, −0.11, −0.18 and −0.36 mA cm−2 respectively, at working potentials of −1.2, −1.4, −1.6 and −1.8 V vs. CE. Acetaldehyde is the main reduction product at all conditions, which is in agreement with previous studies of gas-phase CO2 electroreduction.18–20 At the potential of −1.4 V vs. CE, the total FE for all products is about 10%, while ∼25 nmol cm−2 h−1 acetaldehyde is formed with a FE of around 6%. Table S2 (ESI†) summarizes the gas-phase CO2 electroreduction performances on Cu-based catalysts reported in the literature. Although Gutiérrez-Guerra et al. obtained 55 nmol cm−2 h−1 acetaldehyde production (110 μmol gcat−1 h−1 with a catalyst loading of 0.5 mg cm−2) at a higher temperature and potential (90 °C, −2.75 V, −1.6 mA cm−2), the FE of acetaldehyde is only 1%.18 This phenomenon indicates that the gas-phase CO2 electroreduction system based on HF electrodes could be more efficient, and would be a better platform for developing novel electrocatalysts.
For Sn–Cu HF electrodes, a greater amount of C3 products such as propanal and acetone could be observed. The current densities for 0.1Sn–Cu HF are greatly improved to −0.12, −0.23, −0.53 and −0.76 mA cm−2, respectively, at each working potential. The small amount of SnO2 nanoparticles deposited on the Cu HF surface facilitates the kinetics of gas-phase CO2 electroreduction, leading to the elevated current densities and the reduced reaction resistance (Fig. 2D). Besides, the total FE for all reduction products is about 20%, among which 34 nmol cm−2 h−1 acetone is formed with a FE of around 6% at −1.4 V vs. CE. Although the proportion of acetone in all products has been increased, acetaldehyde is still the main product with a FE of ∼8%. The yield of acetaldehyde leaps to 70 nmol cm−2 h−1, which is higher than those reported in the literature as shown in Table S2 (ESI†),18–20 indicating the promoted activities for both C2 and C3 products with few deposited SnO2 nanoparticles on the Cu HF electrode surface (0.1 wt%).
The current densities for 0.3Sn–Cu HF and 1Sn–Cu HF are not further increased. At a working potential of −1.2, −1.4, −1.6 and −1.8 V vs. CE, the current densities for 0.3Sn–Cu HF drop to −0.10, −0.18, −0.37 and −0.55 mA cm−2 respectively, while those for 1Sn–Cu HF keep decreasing to −0.08, −0.13, −0.21 and −0.49 mA cm−2, but are still higher than those of Cu HF. The current density changes are consistent with the trend of Rct in Fig. 2D. The larger amount of SnO2 nanoparticles and even SnO2 layer on the Cu HF surface with its semiconductive property hinders the charge transfer steps. However, the total FEs for all products on both electrodes are elevated to about 27% and 24%. The increased SnO2 nanoparticle deposition (0.3 wt%) further promotes the production of acetone and restrains the formation of acetaldehyde. For 0.3Sn–Cu HF, the production of acetaldehyde reduces to 64 nmol cm−2 h−1 with a FE of 10% at −1.4 V vs. CE, while in contrast that of acetone rises to 51 nmol cm−2 h−1 with a FE of 12%, indicating that the highly dispersed SnO2 nanoparticles would facilitate further C–C coupling of C2 intermediates on the Cu surface. While for 1Sn–Cu HF the acetaldehyde production rate keeps reducing to 58 nmol cm−2 h−1 with a FE of 12%, and that of acetone also drops back to 18 nmol cm−2 h−1 with a FE of 6%. The excessive SnO2 loading (1 wt%) leads to the formation of a poorly conductive SnO2 layer on the Cu HF surface, hindering the charge transfer procedures, resulting in the downward yields for both acetaldehyde and acetone.
It is believed that the sites with SnO2 particles located on the Cu HF surface are the active centers. As illustrated in Fig. 3, although the Cu HF electrode itself shows some electrochemical activity for gas-phase CO2 reduction to multicarbon products, the current density and their faradaic efficiencies are much lower compared to the Sn-modified electrodes. Introducing Sn species into aqueous CO2 electroreduction usually promotes the formation of HCOOH,24–28 while during gas-phase CO2 electroreduction, SnO2 nanoparticles on the Cu surface play more important roles. The HCOOH species facilitated by virtue of SnO2 are further converted into *CHO intermediates at the SnO2/Cu interface, which subsequently dimerize into C2 intermediate glyoxal (CHO)2.38,39 The next steps include the formation of *COCHO and *CHCOH to obtain acetaldehyde and ethanol,38,39 while the adsorbed *CHCOH could further undergo intermolecular C–C coupling with an adjacent *CHO to form propanal and acetone.39,40 Thus, the CO2 activation and subsequent coupling of the intermediates are enhanced by virtue of the decoration of SnO2 on Cu surfaces, resulting in the improvement of the multicarbon product faradaic efficiencies. However, excess amount of SnO2 hinders the performance greatly on the 1Sn–Cu HF electrode, implying a slowing of charge transfer due to the dielectric property of the SnO2 layer. Therefore, the formation of multicarbon products with relatively high efficiency on the appropriate 0.3Sn–Cu HF electrode can be attributed to the synergistic effect between SnO2 particles and Cu surface, which favour the HCOOH intermediate formation and the subsequent coupling of the key intermediate *CHO.
Conclusions
In summary, a novel gas-phase CO2 eletroreduction system is developed based on the Sn–Cu HF electrode in this work, taking the unique advantages of HFs such as large surface area, adjustable pore structure, good chemical stability and conductivity. The presence of an appropriate amount of SnO2 nanoparticles decorated on the Cu HF surface via NaBH4 reduction preparation could optimize the surface active structure for the gas-phase CO2 electroreduction, which not only facilitates the reaction kinetics to increase the current densities of CO2 electroreduction, but also improves the C–C coupling of intermediates on the Cu HF surface, promoting the formation of multicarbon oxygenates, especially acetaldehyde and acetone. At a cell voltage of −1.4 V, the yields of acetaldehyde and acetone over the 0.3Sn–Cu HF electrode are 64 and 51 nmol cm−2 h−1 with FEs of 10 and 12%, respectively. These results highlight the advantages of Sn–Cu HF electrodes in efficient gas-phase CO2 electroreduction to obtain multicarbon oxygenate products, and inspire the design and development of novel electrocatalytic systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 91745114 and 21802160), the Ministry of Science and Technology of China (no. 2016YFA0202800 and 2018YFB0604700), the Shanghai Sailing Program (no. 18YF1425700), the Hundred Talents Program of Chinese Academy of Sciences and the Shanghai Functional Platform for Innovation Low Carbon Technology.References
- M. Christophe and E. Paul, Nature, 2015, 517, 187–190 CrossRef PubMed.
- J. Tollefson, Nature, 2015, 520, 14–15 CrossRef CAS PubMed.
- G. A. Olah, G. K. S. Prakash and G. Alain, J. Am. Chem. Soc., 2011, 133, 12881–12898 CrossRef CAS PubMed.
- W. I. A. Sadat and L. A. Archer, Sci. Adv., 2016, 2, e1600968 CrossRef PubMed.
- Z. Huang, J. G. Uranga, S. Zhou, H. Jia, Z. Fei, Y. Wang, F. D. Bobbink, Q. Lu and P. J. Dyson, J. Mater. Chem. A, 2018, 6, 20916–20925 RSC.
- G. Xu, H. Zhang, J. Wei, H. X. Zhang, X. Wu, Y. Li, C. Li, J. Zhang and J. Ye, ACS Nano, 2018, 12, 5333–5340 CrossRef CAS PubMed.
- E. V. Kondratenko, G. Mul, J. Baltrusaitis, G. O. Larrazabal and J. Perez-Ramirez, Energy Environ. Sci., 2013, 6, 3112–3135 RSC.
- D. D. Zhu, J. L. Liu and S. Z. Qiao, Adv. Mater., 2016, 28, 3423–3452 CrossRef CAS PubMed.
- Z. Sun, T. Ma, H. Tao, Q. Fan and B. Han, Chemistry, 2017, 3, 560–587 CrossRef CAS.
- S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Nørskov, T. F. Jaramillo and I. Chorkendorff, Chem. Rev., 2019, 119, 7610–7672 CrossRef CAS PubMed.
- M. B. Ross, P. De Luna, Y. Li, C.-T. Dinh, D. Kim, P. Yang and E. H. Sargent, Nat. Catal., 2019, 2, 648–658 CrossRef CAS.
- N. Hollingsworth, S. F. R. Taylor, M. T. Galante, J. Jacquemin, C. Longo, K. B. Holt, N. H. D. Leeuw and C. Hardacre, Angew. Chem., Int. Ed., 2015, 54, 14164–14168 CrossRef CAS PubMed.
- M. Alvarez-Guerra, J. Albo, E. Alvarez-Guerra and Á. Irabien, Energy Environ. Sci., 2015, 8, 2574–2599 RSC.
- C.-T. Dinh, T. Burdyny, M. G. Kibria, A. Seifitokaldani, C. M. Gabardo, F. P. García de Arquer, A. Kiani, J. P. Edwards, P. De Luna, O. S. Bushuyev, C. Zou, R. Quintero-Bermudez, Y. Pang, D. Sinton and E. H. Sargent, Science, 2018, 360, 783–787 CrossRef CAS PubMed.
- J.-J. Lv, M. Jouny, W. Luc, W. Zhu, J.-J. Zhu and F. Jiao, Adv. Mater., 2018, 30, 1803111 CrossRef PubMed.
- G. Centi, S. Perathoner, G. Win and M. Gangeri, Green Chem., 2007, 9, 671–678 RSC.
- S. M. A. Kriescher, K. Kugler, S. S. Hosseiny, Y. Gendel and M. Wessling, Electrochem. Commun., 2015, 50, 64–68 CrossRef CAS.
- N. Gutiérrez-Guerra, L. Moreno-López, J. C. Serrano-Ruiz, J. L. Valverde and A. de Lucas-Consuegra, Appl. Catal., B, 2016, 188, 272–282 CrossRef.
- N. Gutiérrez-Guerra, J. L. Valverde, A. Romero, J. C. Serrano-Ruiz and A. de Lucas-Consuegra, Electrochem. Commun., 2017, 81, 128–131 CrossRef.
- N. Gutiérrez-Guerra, J. A. González, J. C. Serrano-Ruiz, E. López-Fernández, J. L. Valverde and A. de Lucas-Consuegra, J. Energy Chem., 2019, 31, 46–53 CrossRef.
- R. Kas, K. K. Hummadi, R. Kortlever, P. de Wit, A. Milbrat, M. W. J. Luiten-Olieman, N. E. Benes, M. T. M. Koper and G. Mul, Nat. Commun., 2016, 7, 10748–10754 CrossRef CAS PubMed.
- Z. Guo, W. Chen, Y. Song, X. Dong, G. Li, W. Wei and Y. Sun, Chin. J. Catal., 2020, 41, 1067–1072 CrossRef CAS.
- H. Rabiee, X. Zhang, L. Ge, S. Hu, M. Li, S. Smart, Z. Zhu and Z. Yuan, ACS Appl. Mater. Interfaces, 2020, 12, 21670–21681 CrossRef CAS PubMed.
- F. Lei, W. Liu, Y. Sun, J. Xu, K. Liu, L. Liang, T. Yao, B. Pan, S. Wei and Y. Xie, Nat. Commun., 2016, 7, 12697–12705 CrossRef CAS PubMed.
- X. Bai, W. Chen, C. Zhao, S. Li, Y. Song, R. Ge, W. Wei and Y. Sun, Angew. Chem., Int. Ed., 2017, 56, 12219–12223 CrossRef CAS PubMed.
- X. An, S. Li, A. Yoshida, Z. Wang, X. Hao, A. Abudula and G. Guan, ACS Sustainable Chem. Eng., 2019, 7, 9360–9368 CrossRef CAS.
- S. Liu, J. Xiao, X. F. Lu, J. Wang, X. Wang and X. W. Lou, Angew. Chem., Int. Ed., 2019, 58, 8499–8503 CrossRef CAS PubMed.
- W. Geng, W. Chen, G. Li, X. Dong, Y. Song, W. Wei and Y. Sun, ChemSusChem, 2020, 13, 4035–4040 CrossRef CAS PubMed.
- D. Ren, Y. Deng, A. D. Handoko, C. S. Chen, S. Malkhandi and B. S. Yeo, ACS Catal., 2015, 5, 2814–2821 CrossRef CAS.
- Q. Li, W. Zhu, J. Fu, H. Zhang, G. Wu and S. Sun, Nano Energy, 2016, 24, 1–9 CrossRef CAS.
- Y. Song, R. Peng, D. K. Hensley, P. V. Bonnesen, L. Liang, Z. Wu, H. M. Meyer, M. Chi, C. Ma, B. G. Sumpter and A. J. Rondinone, ChemistrySelect, 2016, 1, 6055–6061 CrossRef CAS.
- F. Li, L. Chen, G. P. Knowles, D. R. MacFarlane and J. Zhang, Angew. Chem., Int. Ed., 2017, 56, 505–509 CrossRef CAS PubMed.
- S. Rasul, D. H. Anjum, A. Jedidi, Y. Minenkov, L. Cavallo and K. Takanabe, Angew. Chem., Int. Ed., 2015, 127, 2174–2178 CrossRef.
- M. Wu, C. Zhu, K. Wang, G. Li, X. Dong, Y. Song, J. Xue, W. Chen, W. Wei and Y. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 11562–11569 CrossRef CAS PubMed.
- X. Dong, P. Zheng, A. G. Zheng, H. F. Li, G. F. Xia, M. F. Li, R. Y. Zheng and B. Q. Xu, Catal. Today, 2018, 316, 162–170 CrossRef CAS.
- T. Yano, M. Ebizuka, S. Shibata and M. Yamane, J. Electron Spectrosc. Relat. Phenom., 2003, 131–132, 133–144 CrossRef.
- Y. Li, J. Qiao, X. Zhang, T. Lei, A. Girma, Y. Liu and J. Zhang, ChemElectroChem, 2016, 3, 1618–1628 CrossRef CAS.
- A. J. Garza, A. T. Bell and M. Head-Gordon, ACS Catal., 2018, 8, 1490–1499 CrossRef CAS.
- L. Wang, W. Chen, D. Zhang, Y. Du, R. Amal, S. Qiao, J. Wu and Z. Yin, Chem. Soc. Rev., 2019, 48, 5310–5349 RSC.
- S. Munir, A. R. Varzeghani and S. Kaya, Sustainable Energy Fuels, 2018, 2, 2532–2541 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00851f |
| This journal is © The Royal Society of Chemistry 2021 |