Open Access Article
Open Access ArticleHigh heat resistance and good melt spinnability of a polyamide 66 containing benzene structure
Yuanyuan
Ma†
ab,
Jingnan
Zhang†
 a,
Xinyu
Cao
a,
Xinyu
Cao
 a,
Pengfei
Wu
c,
Gang
Ye
a,
Yang
Fu
d,
Yafang
Zhuang
a,
Aiqing
Zhang
b,
Kun
Zheng
a,
Pengfei
Wu
c,
Gang
Ye
a,
Yang
Fu
d,
Yafang
Zhuang
a,
Aiqing
Zhang
b,
Kun
Zheng
 *a and
Yongmei
Ma
*a and
Yongmei
Ma
 *ae
*ae
aInstitute of Chemistry, Chinese Academy of Science, Beijing 100190, China. E-mail: maym@iccas.ac.cn; zhengkunwd@iccas.ac.cn
bCollege of Chemical and Materials Engineering, South-Centrol University for Nationalities, Wuhan 430074, Hubei, China
cState Key Laboratory of Biobased Fiber Manufacturing Technology, China Textile Academy, Beijing 100025, China
dHigh & New Technology Research Center of Henan Academy of Sciences, Zhengzhou, Henan 450002, China
eBeijing National Laboratory for Molecular Sciences (BNLMS), Beijing, 100190, China
First published on 30th August 2021
Abstract
A polymer based bag filter with high temperature resistance plays a key role in dust and acid gas treatment in the power industry. Currently, the polymers used for preparing filters with improved heat resistance include those with a high content of aromatic units. But the aromatic structure inevitably leads to a high melting point, which is disadvantageous for melt spinning. Here, we report a polyamide 66 and polyamide 6T copolymer (PA66-co-PA6T) fiber with both high thermal stability and good spinnability. The retention rates of heat resistance of fibers after heat treatment at 185 °C and 200 °C were higher than 97.8% and 88.4%, respectively. This high thermal stability is ascribed to the high thermal degradation activation energy (E), which was confirmed by DSC, TG, and XRD characterization studies and fitting of kinetic data. PA66-co-PA6T with good melt spinnability, high heat resistance strength and low cost is expected to be applied in high-temperature resistance filtration equipment such as bag filters, expanding the application range of PA66 materials.
Introduction
According to British Petroleum's Statistical World Energy Yearbook 2019, global electricity demand takes up to 50% of primary energy consumption. Coal remains the main fuel for electric power generation. However, coal fired power plants produce dust particles and acid gases, which are the main sources of air contamination. At present, the most competitive dust removal equipment is the bag filter.1 A bag filter is made of textile filter cloth or non-textile felt, which is used to filter the dust–gas mixture. When the dust–gas mixture enters the bag filter, the large size dust particles fall into the ash hopper due to the action of gravity. When the fine dust–gas mixture passes through the filter material, the dust is retained, so that the gas is purified. Furthermore, the bag filter should meet the requirements of high temperature resistance (generally above 200 °C), corrosion resistance, high mechanical strength, long service life and low cost.2 Common bag filter materials are polyphenylene sulfide (PPS), polyimide (PI), polytetrafluoroethylene (PTFE) and fiberglass.3–6 However, the raw materials of most fibers are costly and the preparation processes are complex. Therefore, there is an urgent need to develop high-temperature resistant fibers at low cost and with easy processing.7–9Polyamide 66 (PA66) fiber is an important fiber material with excellent chemical corrosion resistance, mechanical properties and abrasion resistance.10–14 However, PA66 belongs to a class of aliphatic polymers with low glass transition temperature (Tg) and melting point (Tm), which greatly limit its use in the high-temperature fields. Compared with aliphatic polyamides, semi-aromatic polyamides have better rigidity, heat resistance and dimensional stability.15–18 For example, PA6T is one of the important semi-aromatic polyamides, which is widely applied in fields requiring high temperature resistance, especially in automobile manufacturing, electronics and electrical industry.19,20 But, PA6T cannot be melted for spinning because its melting point exceeds its thermal decomposition temperature, causing difficulty in the fabrication of high-temperature resistant fabric.21
For combining the properties of various polymers, a copolymerization or blending method is generally used.22–24 For example, Wang et al. synthesized PA6T/6 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) copolymers with high content of rigid aromatic units, which show improved thermal stability and mechanical properties.25 Qu et al. obtained PA6T/2T copolymer and found that the melting temperature decreased from 368 °C for PA6T to 347 °C for PA6T/2T copolymer. This is because the structure and regularity of the repeated units of PA2T and PA6T are greatly different and cannot form a cocrystal, resulting in the defective crystal structure of the copolymer.26 Nevertheless, rigid the aromatic structure leads to a high melting temperature of the copolymer, which makes melt spinning difficult.27–30 As far as we know, few studies have been reported on PA66 fibers with low content of benzene by melt spinning.
50) copolymers with high content of rigid aromatic units, which show improved thermal stability and mechanical properties.25 Qu et al. obtained PA6T/2T copolymer and found that the melting temperature decreased from 368 °C for PA6T to 347 °C for PA6T/2T copolymer. This is because the structure and regularity of the repeated units of PA2T and PA6T are greatly different and cannot form a cocrystal, resulting in the defective crystal structure of the copolymer.26 Nevertheless, rigid the aromatic structure leads to a high melting temperature of the copolymer, which makes melt spinning difficult.27–30 As far as we know, few studies have been reported on PA66 fibers with low content of benzene by melt spinning.
In this paper, PA66 with a low benzene content was obtained via the copolymerization method. By copolymerizing a small amount of PA6T (less than 20 wt%) with PA66, the obtained PA66-co-6T copolymers had both high heat resistance and good spinnability. The heat resistance and mechanical properties of the copolymer fibers were evaluated by melting and crystallization behavior of copolymers and thermal degradation kinetics. This study widens the application field of PA66 materials, especially in today's increasingly deteriorating environment, and it is of great significance to apply PA66 materials in high-temperature resistant filtration equipment of coal-fired power generation.31,32
Methods
Materials
PA66 salt and 1,6-diaminohexane were purchased from Pingdingshan Shenma Engineering Plastics Co. Ltd. Benzoic acid was obtained from Sinopharm Chemical Reagent Co. Ltd. Terephthalic acid was obtained from Luoyang Petrochemical Engineering Corporation Ltd/SINOPEC. Hypophosphite was obtained from Beijing Hengye Zhongyuan Chemical Co. Ltd. All of them were of commercial grade and were used as received without further purification.Preparation
Characterization
Result and discussion
FTIR and 1H-NMR characterization studies
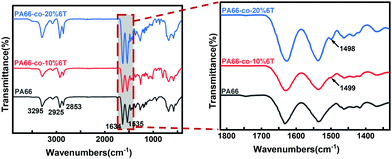
Fig. 1 shows the IR results for copolymers with different contents of 6T. The vibration absorption peak of benzene appears around 1500 cm−1, and the strength of this peak becomes stronger with the increase in the contents of benzene. The position located at 3295 cm−1 is assigned to the characteristic group peak of C–N stretching vibration in amide, and 2925 cm−1 and 2853 cm−1 correspond to the stretching vibration absorption peak of C–H of methylene. The peaks at 1634 cm−1and 1535 cm−1 are the stretching vibration peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in amide and C–N–H vibration, respectively.
O in amide and C–N–H vibration, respectively.
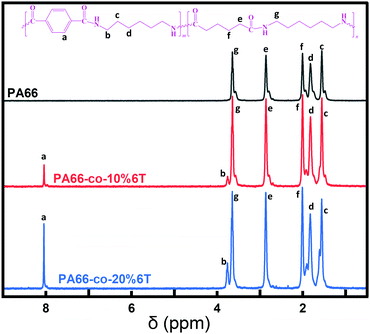
To further clarify the structure of the copolymers, 1H-NMR was carried out and the corresponding spectra are shown in Fig. 2. According to the different chemical environments, the H atoms in the copolymers can be roughly divided into 7 categories. Namely, the hydrogen atoms on the benzene ring, the hydrogen atoms on the carbon atoms behind the amide bond formed by the acid containing benzene ring and the aliphatic group, and the hydrogen atoms on the binary amine. Each type of hydrogen atom is assigned, and the ratio of the integral area of each part corresponds to the ratio of the number of each type of hydrogen atom.
Mechanical properties of copolymers
Fig. 3 shows the basic mechanical properties and flowability of the materials. Molecular weight was calculated from viscosity according to the Mark–Houwink equation:| [η] = KMαη | (1) |
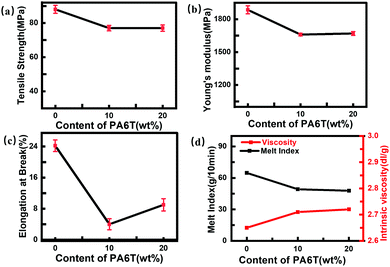 | ||
| Fig. 3 Basic mechanical and flow properties of pure PA66, PA66-co-10%6T and PA66-co-20%6T: (a) tensile strength; (b) Young's modulus; (c) elongation at break; (d) melt index and viscosity. | ||
| Samples | PA66 | PA66-co-10%6T | PA66-co-20%6T |
|---|---|---|---|
| [η]/(dl g−1) | 2.65 | 2.71 | 2.72 |
| M η/(g mol−1) | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652 652 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 138 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 368 368 |
From Fig. 3(a and b), it can be observed that the strength and modulus of copolymers with varying 6T contents were almost the same, only slightly lower than those of the pure PA66. The factors affecting the mechanical performance of the copolymers mainly include molecular weight (the decisive factor), the rigid benzene ring structure added to the main chain and its influence on the crystallinity. It should be noted that the molecular weights of the copolymers with different 6T contents show no major changes therefore, the possible reasons for the difference in the strength and modulus of the polymers are originated from the rigid benzene ring on the main chain of polymerization, which mainly affects the intermolecular chain force and crystallinity. PA66-co-10%6T contains less benzene ring, while it has higher crystallinity, compared with PA66-co-20%6T. Under the combined effects of benzene ring content and crystallinity, these two copolymers show similar strength and modulus. PA66 had a larger elongation at break because it is a flexible molecular chain of aliphatic group. After a benzene ring was added, the rigidity of molecular chain increased, so the elongation of the copolymers at break decreased (Fig. 3c). Among them, PA66-co-10%6T had a high crystallinity, so the elongation at break was the smallest, while PA66-co-20%6T had a low crystallinity, so the elongation at break was larger than that of PA66-co-10%6T.
We also characterized the cross section after tensile fracture (Fig. 4), and it was observed that the semi-crystalline polymer had the characteristics of ductile fracture. A ridge pattern appeared during fracture, and in the amorphous area with plastic deformation appeared tiny fiber bundles. With the increase of benzene content, the rigidity of molecular chain increased and the degree of fracture surface fluctuation decreased.
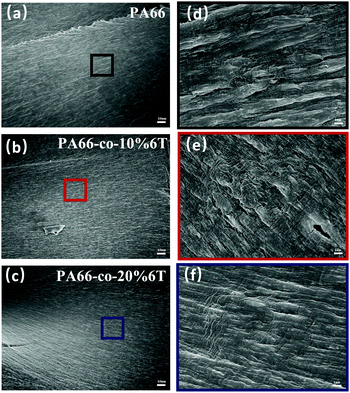 | ||
| Fig. 4 SEM images of PA66 with different amounts of benzene ring: (a) PA66; (b) PA66-co-10%6T; (c) PA66-co-20%6T; (d, e and f) their partial magnifications. | ||
The crystallization properties
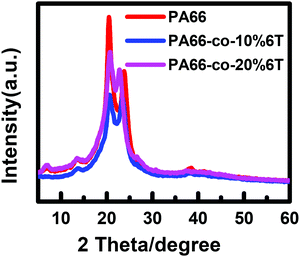
To analyse the crystalline properties of the materials, X-ray diffraction analysis was performed on the copolymers before spinning. Fig. 5 shows the XRD curves and Table 2 shows the summary of crystal parameters after data analysis and fitting. The peak positions of PA66 are 20.3° and 24.1°,33 and the crystal characteristic signal peak positions of PA6T are 18.3° and 23°. The peak positions of 2θ gradually changed from the characteristic peaks of PA66 to that of PA6T after the addition of a benzene ring. The Full Width at Half Maximum (FWHM) of PA66-co-10%6T copolymer at about 20° increased, the peak height decreased, and the grain size of the α1 crystal plane decreased. In addition, the relative peak area occupied by α2 increased, indicating that the content of α2 crystal increased. After adding the benzene ring, the steric hindrance increased, the intermolecular hydrogen bonding decreased, and the van der Waals force between hydrogen bonding surfaces enhanced.| PA66 | PA66-co-10%6T | PA66-co-20%6T | ||||
|---|---|---|---|---|---|---|
| 2θ/° | 20.6 | 23.9 | 20.7 | 23.5 | 20.8 | 22.8 |
| Grain size | 45 | 2 | 28 | 47 | 32 | 49 |
| FWHM | 1.8 | 1.9 | 2.8 | 1.7 | 2.5 | 1.6 |
| Peak height | 1471 | 949 | 753 | 733 | 1100 | 850 |
| Peak area | 232289 | 12794 | 166538 | 117167 | 163105 | 100148 |
| Relative peak area/% | 100.0 | 54.9 | 100.0 | 70.4 | 100.0 | 61.4 |
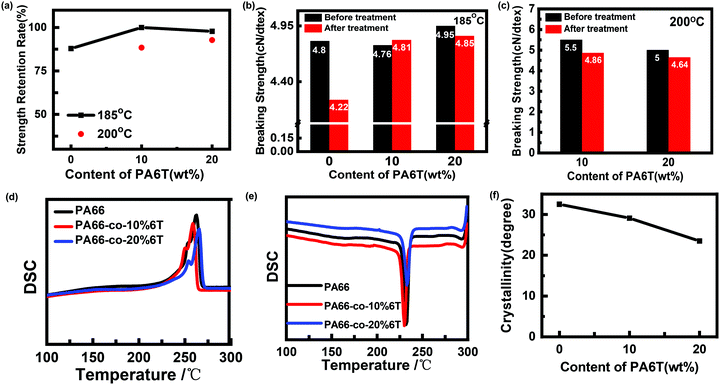
Fig. 6 and Table 3 show the DSC data and the crystallinity of the copolymers. After eliminating the thermal history, the melting point (Tm2) of pure PA66 was 262.2 °C, and the Tm2 of PA66-co-20%6T copolymer was 265.6 °C, slightly higher than that of pure PA66. It is worth noting that the Tm2 of PA66-co-10%6T copolymer was 259.6 °C, lower than that of pure PA66. And the melting temperatures of PA66, PA66-co-10%6T and PA66-co-20%6T ranged from 226 to 275 °C, 219 to 268 °C, and 232 to 277 °C, respectively. Compared with PA66, the initial melting temperature of PA66-co-10%6T was decreased by 7 °C. And the entire melt temperature range moved to a lower temperature direction, which is more conducive to melt spinning. It can also be seen that after adding the benzene ring, the crystallinity of the copolymer decreased. The crystallization properties of the copolymer decreased with the increase in benzene ring content. The crystallinity values of pure PA66, PA66-co-10%6T and PA66-co-20%6T were 29.2%, 28.7% and 25.4%, respectively. Generally, on the one hand, factors that increase intermolecular forces can raise the melting point. On the other hand, increasing the intermolecular rotation resistance and reducing the number of conformation when the polymer melts also increase the melting point. After adding the benzene ring structure, a p–π conjugate is formed, and at the same time the copolymer molecular chain conformation is reduced, leading to a higher melting point of the PA66-co-20%6T copolymer. The melting point of PA66-co-10%6T copolymer was lower than that of pure PA66, which was because the crystallization ability and crystallinity of the copolymer declined, resulting in its melting point decline. However, the melting point decline is more beneficial to melt spinning.34 When the content of benzene ring continued to increase, the crystallinity of the copolymer continued to decline. Meanwhile, the rigid aromatic ring structure increased the intermolecular force, resulting in the melting point of PA66-co-20%6T copolymer being slightly higher than that of pure PA66.35
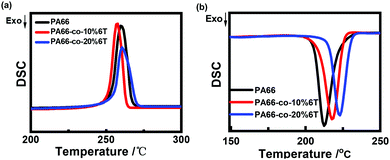 | ||
| Fig. 6 Crystallization and melting behavior of pure PA66 and copolymers with different contents of PA6T: (a) the subsequent heating at a rate of 10 °C min−1; (b) first cooling. | ||
| PA66 | PA66-co-10%6T | PA66-co-20%6T | |
|---|---|---|---|
| T m1 (°C) | 269.4 | 263.6 | 270.4 |
| T m2 (°C) | 262.2 | 259.6 | 265.6 |
| ΔHf1 (J g−1) | 59.7 | 61.9 | 35.1 |
| ΔHf2 (J g−1) | 28.6 | 56.7 | 54.9 |
| Xc (%) | 29.2 | 28.7 | 25.4 |
| Xc-fiber (%) | 32.5 | 29.1 | 23.5 |
| T m-initial | 226 | 219 | 232 |
| T m-termination | 275 | 268 | 277 |
Thermal properties of copolymers
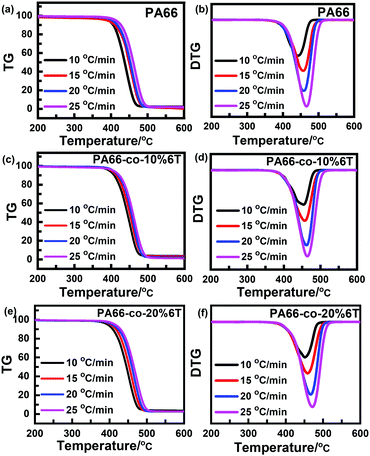
Fig. 7 shows the thermogravimetric curves of the materials before spinning under a N2 atmosphere and at different heating rates.36 It can be seen that the thermal degradation curves are smooth and all the differential Tg curves are single peaks, so the thermal degradation of each material in a N2 atmosphere was a single reaction process. With the increase in heating rate, the curve moved towards high temperature, which was consistent with the random chain breaking mechanism of the polycondensation products.37,38To exclude the influence of the heating rate on thermal degradation temperature, we obtained the relation curves of initial degradation temperature, the temperature of maximum thermal weight loss rate and termination degradation temperature with the heating rate, respectively (as shown in Fig. 8). The characteristic degradation temperatures found by extrapolating the curve to a rate of 0 are shown in Table 4.
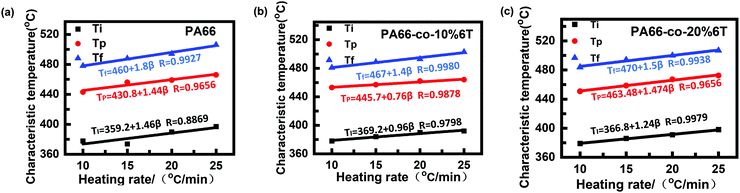 | ||
| Fig. 8 Relation curve between degradation temperature and rate of raw materials before spinning: (a) PA66; (b) PA66-co-10%6T; and (c) PA66-co-20%6T. | ||
| PA66 | PA66-co-10%6T | PA66-co-20%6T | |
|---|---|---|---|
| T 0 i/°C | 359 | 369 | 366 |
| T 0 p/°C | 431 | 446 | 436 |
| T 0 f/°C | 460 | 467 | 470 |
After adding aromatic structure to PA66, the characteristic degradation temperature increased (Table 4). We focus on the initial characteristic degradation temperature T0i and the characteristic degradation temperature T0p at the maximum thermogravimetric rate. The initial characteristic degradation temperature T0i values of pure PA66, PA66-co-10%6T and PA66-co-20%6T were 359 °C, 369 °C and 366 °C, respectively. The characteristic degradation temperature T0p values at the maximum rate of thermal mass loss for pure PA66, PA66-co-10%6T and PA66-co-20%6T were 431 °C, 446 °C and 436 °C, respectively. In all, the PA66-co-10%6T copolymer showed better thermal stability.
To further study the thermal degradation performance of copolymers obtained by adding the aromatic structure to PA66, the thermal degradation kinetics of copolymers was studied. The thermal degradation kinetics curves of the copolymer were obtained by the Flynn–Wall–Ozawa method (Fig. 9).39 After reading the slope of the curve for calculation, the activation energy E value of thermal degradation was obtained, as shown in Fig. 7d.
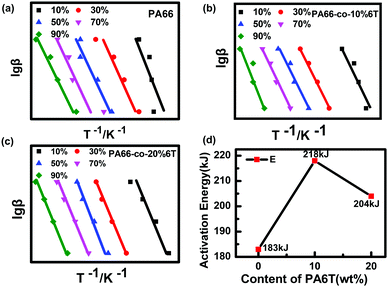 | ||
| Fig. 9 Fitting curve and E value obtained by the Flynn–Wall–Ozawa method of pure PA66, PA66-co-10%6T and PA66-co-20%6T. | ||
The thermal degradation activation energy (E) of the copolymers increased significantly after adding the rigid benzene ring structure, so the thermal stability of the copolymers improved significantly. Its change trend was consistent with the thermal degradation temperature.
Therefore, the thermal degradation activation energy E value of PA66-co-10%6T was the highest and this means it has better thermal stability.40
The properties of fibers
The thermal and mechanical properties of the fibers are the key factors affecting the service life. The fiber strength of the copolymers was measured at 185 °C and 200 °C, respectively, and the crystallinity of polymers was calculated using eqn (2).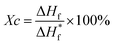 | (2) |
As shown in Fig. 10(a, b and c), the heat resistance retention rate of the benzene-containing copolymer fibers was significantly higher than that of pure PA66 after heat treatment at 185 °C. The breaking strengths of PA66 fiber and PA66-co-10%6T fiber decreased by 12.1% and 2.2%, respectively. It is worth noting that the strength retention rate of PA66-co-10%6T fiber was 100%. In addition, the crystallinity values of pure PA66, PA66-co-10%6T and PA66-co-20%6T fibers were 32.5%, 29.1% and 23.5%, respectively (Fig. 10f). It has been reported that the heat resistance of polymers increased with the increase of crystallinity and the content of rigid components.41 Therefore, the breaking strengths of PA66-co-10%6T fiber and PA66-co-20%6T fiber were higher than that of the pure PA66 after heat treatment, and the breaking strength of PA66-co-20%6T fiber was the highest. In other words, when the benzene ring content is low, it has little influence on the regularity and symmetry of the copolymer fibers,42 so less influence on the crystallization of the copolymers. A small amount of rigid benzene ring also plays a role in heat resistance, resulting in PA66-co-10%6T with a high heat resistance strength retention rate. When the temperature increases up to 200 °C, the copolymer begins to melt (shown in Fig. 10d). It is the rigid benzene ring structure that is responsible for the heat resistance. Therefore, the strength of PA66-co-10%6T decreased, while the heat-resistant strength retention rate of PA66-co-20% 6T fiber was still above 90% at 200 °C.
Conclusion
In this paper, PA66-co-PA6T copolymers with a low content of aromatic units were prepared via copolymerization. The copolymerized fibers exhibited excellent heat resistance. The retention rates of heat resistance of fibers after heat treatment at 185 °C and 200 °C were higher than 97.8% and 88.4%, respectively. By analysing the thermal properties and crystallization properties of the materials, it was found that the thermal degradation temperature and thermal degradation activation energy (E) of the benzene-containing copolymers were significantly increased. The E value of the PA6T-co-10%PA6T copolymer was 218 kJ, significantly higher than that of PA66 (183 kJ). Therefore, PA66-co-PA6T copolymers with a low benzene ring content not only show excellent heat resistance, but can also realize melt spinning; its manufacturing cost is far lower than those of other currently popular high-temperature resistant filtration materials. These prominent properties expand the application range of PA66 materials, making them possible to be used as heat-resistant and high-temperature filtration materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Key R&D Program of China (2016YFB0303000) and the National Natural Science Foundation of China (Grants 51803218, 51373184 and 51373179).References
- L. Bao, M. Musadiq, T. Kijima and K. Kenmochi, Text. Res. J., 2014, 84, 764–771 CrossRef CAS
.
- W. Tanthapanichakoon, M. Furuuchi, K. H. Nitta, M. Hata and Y. Otani, Adv. Powder Technol., 2007, 18, 349–354 CrossRef CAS
.
- Y. R. Lv, H. W. He, F. X. Chen, J. Yu, X. Ning and R. Zhou, Mater. Res. Express, 2019, 6, 075706 CrossRef CAS
.
- H. Xu, W. Y. Jin, F. Wang, C. C. Li, J. Q. Wang, H. L. Zhu and Y. H. Guo, RSC Adv., 2018, 8, 38245–38258 RSC
.
- N. Peng, N. Widjojo, P. Sukitpaneenit, M. M. Teoh, G. G. Lipscomb, T. S. Chung and J. Y. Lai, Prog. Polym. Sci., 2012, 37, 1401–1424 CrossRef CAS
.
- A. Podgorski, A. Balazy and L. Gradon, Chem. Eng. Sci., 2006, 61, 6804–6815 CrossRef CAS
.
- M. H. D. A. Farahani and T.-S. Chung, Chem. Eng. J., 2018, 345, 174–185 CrossRef
.
- P. Marchetti, M. F. J. Solomon, G. Szekely and A. G. Livingston, Chem. Rev., 2014, 114, 10735–10806 CrossRef CAS PubMed
.
- H. Kobayashi, N. Hironaka, K. Iwata, M. Hirano, M. Salvia and Y. Nishi, J. Jpn. Inst. Met., 2008, 72, 249–253 CrossRef CAS
.
- W. Lyu, Y. Cui, X. Zhang, J. Yuan and W. Zhang, Des. Monomers Polym., 2016, 19, 420–428 CrossRef CAS
.
- H. Wang, L. Wang, R. Wang, X. Tian and K. Zheng, Colloid Polym. Sci., 2012, 291, 1001–1007 CrossRef
.
- H. Gharabaghi, M. Rafizadeh and F. A. Taromi, Bull. Mater. Sci., 2016, 39, 935–941 CrossRef CAS
.
- J. F. Mei Huang, J. Wang, X. Zhang, Y. Li and Y. Yan, J. Mater. Sci.: Mater. Med., 2003, 14, 655–660 CrossRef PubMed
.
- W. Peng, Y. Qian, T. Zhou, S. Yang, J. Jin and G. Li, Polymers, 2019, 11, 1735–1743 CrossRef CAS PubMed
.
- S. Haider, A. Kausar and B. Muhammad, Polym.-Plast. Technol. Eng., 2016, 55, 1536–1556 CrossRef CAS
.
- A. Ridhore and J. Jog, J. Appl. Polym. Sci., 2013, 129, 65–72 CrossRef CAS
.
- J. A. Reglero Ruiz, M. Trigo-Lopez, F. C. Garcia and J. M. Garcia, Polymers, 2017, 9, 414–457 CrossRef PubMed
.
- J. M. Garcia, F. C. Garcia, F. Serna and J. L. de la Pena, Prog. Polym. Sci., 2010, 35, 623–686 CrossRef CAS
.
- M. Hajibeygi, H. Jafarzadeh, M. Shabanian and H. Vahabi, J. Therm. Anal. Calorim., 2019, 138, 3949–3959 CrossRef CAS
.
- W.-M. Peng, X. Tong, M.-L. Zhang, X.-J. Wang, G. Zhang, S.-R. Long and J. Yang, J. Appl. Polym. Sci., 2018, 135, 46451–46459 CrossRef
.
- K. Fuktsu, Polym. Degrad. Stab., 2002, 75, 479–484 CrossRef
.
- Q. Zhang, X. L. Luo, M. L. Zhang, X. J. Wang, G. Zhang, S. R. Long and J. Yang, Mater. Sci. Forum, 2015, 815, 503–508 Search PubMed
.
- H. Wang, J. Appl. Polym. Sci., 2001, 80, 2167–2175 CrossRef CAS
.
- X. Wang, Q. Zheng, L. Du and G. Yang, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 201–211 CrossRef CAS
.
- Z. Wang, X. Tong, J.-C. Yang, X.-J. Wang, M.-L. Zhang, G. Zhang, S.-R. Long and J. Yang, Compos. Sci. Technol., 2019, 175, 6–17 CrossRef CAS
.
- L. Qu, S.-R. Long, M.-L. Zhang, G. Zhang, X.-J. Wang and J. Yang, J. Macromol. Sci., Part A: Pure Appl. Chem., 2012, 49, 67–72 CrossRef CAS
.
- J. Seo, H. Takahashi, B. Nazari, A. M. Rhoades, R. P. Schaake and R. H. Colby, Macromolecules, 2018, 51, 4269–4279 CrossRef CAS
.
- J. E. Spruiell, M. D. Danford and J. L. White, J. Appl. Polym. Sci., 1978, 22, 3351–3361 CrossRef
.
- F. Mai, D. Pan, X. Gao, M. Yao, H. Deng, K. Wang, F. Chen and Q. Fu, Polym. Int., 2011, 60, 1646–1654 CrossRef CAS
.
- J. Zhang, X. Gao, X. Zhang, H. Liu, H. Zhang and X. Zhang, J. Mater. Sci., 2019, 54, 11056–11068 CrossRef CAS
.
- Y. Wang, H. Ren, W. Liu, M. Run and H. Zhang, J. Mater. Sci., 2009, 44, 170–178 CrossRef CAS
.
- K. Vijayan and A. Jain, J. Mater. Sci.: Mater. Med., 2002, 37, 2623–2633 CrossRef
.
- J. D. Li, Y. Zuo, X. M. Cheng, W. H. Yang, H. N. Wang and Y. B. Li, J. Mater. Sci.: Mater. Med., 2009, 20, 1031–1038 CrossRef PubMed
.
- Z. Cai, S. Mei, Y. Lu, Y. He, P. Pi, J. Cheng, Y. Qian and X. Wen, Int. J. Mol. Sci., 2013, 14, 20682–20691 CrossRef PubMed
.
- Y. Li and W. A. Goddard III, Macromolecules, 2002, 35, 8440–8455 CrossRef CAS
.
- C. Leer, G. Botelho and P. Gijsman, Mater. Sci. Forum, 2004, 455, 463–466 Search PubMed
.
- X. F. Yang, Q. L. Li, Z. P. Chen, L. Zhang and Y. Zhou, J. Therm. Anal. Calorim., 2013, 112, 567–571 CrossRef CAS
.
- E. Duemichen, U. Braun, R. Kraemer, P. Deglmann and R. Senz, J. Anal. Appl. Pyrolysis, 2015, 115, 288–298 CrossRef CAS
.
- X. F. Yang, Q. L. Li, Z. P. Chen, H. X. Jin and B. Liu, High Perform. Polym., 2013, 25, 502–507 CrossRef
.
- A. Choudhury, A. Balmurulikrishnan and G. Sarkhel, Polym. Adv. Technol., 2008, 19, 1226–1235 CrossRef CAS
.
- M. Trigo-Lopez, A. Miguel-Ortega, S. Vallejos, A. Munoz, D. Izquierdo, A. Colina, F. C. Garcia and J. M. Garcia, Dyes Pigment., 2015, 122, 177–183 CrossRef CAS
.
- J. B. Song, J. X. Liu, Y. H. Zhang, L. H. Chen, Y. M. Zhong and W. B. Yang, J. Compos. Mater., 2015, 49, 415–424 CrossRef CAS
.
Footnote |
| † Yuanyuan Ma and Jingnan Zhang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |