Open Access Article
Open Access ArticleHigh proton conductivity of NaMg1−xLixHx (PO3)3·yH2O with a three-dimensional open framework in the intermediate temperature range†
Naoya
Ueda
a,
Jun
Nakajima
a,
Daisuke
Mori
b,
Sou
Taminato
 b,
Nobuyuki
Imanishi
b,
Shinya
Higashimoto
a and
Yasuaki
Matsuda
b,
Nobuyuki
Imanishi
b,
Shinya
Higashimoto
a and
Yasuaki
Matsuda
 *a
*a
aDepartment of Applied Chemistry, Faculty of Engineering, Osaka Institute of Technology, 5-16-1 Ohmiya, Asahi-Ku, Osaka, 535-8585, Japan. E-mail: yasuaki.matsuda@oit.ac.jp
bDepartment of Chemistry for Materials, Graduate School of Engineering, Mie University, Tsu, Mie 514-8507, Japan
First published on 9th August 2021
Abstract
Proton solid electrolytes, which exhibit high proton conductivity and thermal stability in a wide range of intermediate temperatures, are desirable for operating fuel cells at a temperature suitable for applications such as automobiles and cogeneration systems. A new proton conductor NaMg1−xLixHx(PO3)3·yH2O was synthesised by a coprecipitation method. Powder X-ray structure refinement reveals that the three-dimensional tunnel framework is formed by the sharing of corner oxygens of zigzag PO4 tetrahedral chains and face-shared (NaO6)(MgO6) chains. In the three-dimensional tunnel, the oxygen sites of water for crystallisation were observed. In Fourier transform infrared spectroscopy (FTIR) measurement, an increase in absorption peaks corresponding to the modes of O–H and P–O–H bonds for x in NaMg1−xLixHx(PO3)3·yH2O was observed, which reflects the introduction of protons to the crystal structure. Although a multi-step weight loss due to desorption of water of crystallisation was observed for thermogravimetry (TG) curves, NaMg1−xLixHx(PO3)3·yH2O retained the framework up to 800 °C. The proton conductivity shows a positive tendency for x. NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibited a high proton conductivity of over 10−2 S cm−1 in the temperature range of 150–500 °C, and the value reached 2.4 × 10−2 S cm−1 at 225 °C under a non-humidified atmosphere. The proton conductivity of NaMg0.8Li0.2H0.2(PO3)3·yH2O changed with the amount of crystalline water. In a humidified atmosphere (pH2O = 4 kPa), NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibited a conductivity of over 10−3 S cm−1 from room temperature to 500 °C and reached 2.6 × 10−2 S cm−1 at 225 °C. After keeping the dehydrated sample in the humidified atmosphere at room temperature for 2 h, NaMg0.8Li0.2H0.2(PO3)3·yH2O showed a proton conductivity of over 10−2 S cm−1 again in the intermediate temperature range.
Introduction
Proton conduction is a common phenomenon observed in various materials, including liquids and organic and inorganic solids,1–6 and is essential for the metabolism of biological systems7–9 and the operation of fuel cells.10–12 In particular, proton conductors are important for practical applications as electrolytes that determine the operating temperature of fuel cells. Since the chemical reaction rate is thermally excited and the selectivity of the reactions is excellent at a low temperature, the operating temperature of the fuel cells is desirable in the temperature range of 100–500 °C. Acids, liquid-impregnated polymers,1 metal–organic framework (MOF) systems,2,12–14 and inorganic solids such as CsHSO4 and CsH2PO43,15–17 exhibit high proton conductivity below low-intermediate temperatures. In-doped SnP2O7 exhibits a high proton conductivity up to 350 °C.4,11 Perovskites exhibit excellent thermal stability and high proton conductivity above 300 °C.5,6,18,19 Although all of them are excellent proton conductors, they have a common issue that they exhibit high proton conductivity in a limited temperature range.To develop materials that exhibit thermal stability and high proton conductivity, it is necessary to find a new structure with a proton diffusion pathway in the inorganic framework. In developing such materials, the material design that divides the structure into inorganic blocks and hydrogen bond networks observed in MOF-based materials is considered attractive. However, the use of non-combustible inorganic blocks is not sufficient, and a framework formed only by strong bonds, such as covalent and ionic bonds like perovskites, is needed to achieve thermal stability above 500 °C. Furthermore, to develop high proton conductivity, it needs to form a hydrogen bond network that results in the proton conduction pathway by controlling the arrangement of molecules, and retention of these molecules up to intermediate temperatures.
Mixed cation phosphates are attractive materials that exhibit high proton conductivity in a wide temperature range. AMg1−xH2x(PO3)3·yH2O (A: Rb+ and K+) has a one-dimensional tunnel framework that is composed of face-shared (AO6)(MgO6) chains and PO4 chains.20,21 Water molecules were arranged along the PO4 chains via hydrogen bonds in the tunnel and retained by the connection via a coordination bond with a large alkali cation that forms the framework. Although a phase transition occurs at around 300 °C, these tunnel phosphates exhibit a high proton conductivity of over 10−3 S cm−1 from room temperature to 250 °C. Benitoite-type phosphate, which is the high-temperature phase of KMg1−xH2x(PO3)3·yH2O, has a layered framework formed by stacking the layers of the edge-shared (AO6)(MgO6) octahedral units and those of the P3O9 ring in the c-axis direction, and exhibits a high proton conductivity up to 500 °C.22
Mixed cation phosphates form various open frameworks by combining cations with different valences, sizes, and synthesis conditions.20–25 These open frameworks are formed by a rigid connection between the octahedral cations and PO4 tetrahedra, which results in the thermal stability of the proton conductors. Since the PO4 tetrahedra tend to form hydrogen bonds, the connection of the PO4 tetrahedra provides an adjacent arrangement of water molecules in the tunnel framework, which participates as a proton conduction pathway. Water molecules that exist in the tunnel are not only connected to the PO4 tetrahedra via hydrogen bonds, but their oxygen also coordinates with large alkaline cations.21 Furthermore, with mixed cation phosphate, proton conductivity is expected to improve with the introduction of protons as charge compensation for the formation of cation deficiency and the substitution of aliovalent cations. The phase transition of KMg1−xH2x(PO3)3·yH2O is thought to occur to extend the distance between the alternately arranged K+ and Mg+. Although it is desirable to include a large cation for the formation of coordination bonds with oxygen in water molecules, it is necessary to select cations smaller than K+ to prevent the phase transition. In the present study, we focus on NaMg(PO3)3, which has a three-dimensional open framework with PO4 chains.26 Improvement of proton conductivity was attempted by introducing protons into NaMg1−xLixHx(PO3)3·yH2O. To introduce protons as charge compensation of aliovalent substitution, NaMg1−xLixHx(PO3)3·yH2O was synthesised with a coprecipitation method and revealed the exhibition of a proton conductivity exceeding 10−2 S cm−1 in a wide temperature range of 150–500 °C without large humidification. This paper discusses the relationship among the composition, structure, and proton conductivity of NaMg1−xLixHx (PO3)3·yH2O.
Experimental
The NaMg1−xLixHx(PO3)3·yH2O series was synthesised by the coprecipitation method. Appropriate molar ratios of Na2CO3 (Nacalai Tesque, >99.5%), Li2CO3 (Wako) and (MgCO3)4Mg(OH)2·xH2O (Nacalai Tesque) were weighed and dissolved in 30 mL of phosphoric acid solution (0.5 mol L−1, Kanto Chemical Lab. Co.). The solution was heated at 120 °C in air for a day, and a white-coloured residual powder was obtained. The sample powder obtained was pressed into a pellet (10 mm diameter, 1–2 mm thick) and then heated in air at 400 °C for 12 h on an alumina boat with an intermediate grinding step. X-ray diffraction (XRD) patterns for the powder samples were obtained using a diffractometer (Rigaku RINT2000) with CuKα radiation in the 2θ range from 10° to 90° with 0.02 step widths. Thermogravimetry/differential thermal analysis (TG/DTA; Rigaku Thermo Plus EVO TG 8120) was performed at a heating rate of 10 °C min−1 under a N2 gas flow. The structures of the samples were refined by X-ray Rietveld analysis using the Z-Rietveld programme.27 The activation energy was estimated using the soft VB programme.28 Fourier transform infrared (FTIR) absorption spectra of the samples were recorded with an IR Affinity 1S spectrometer (Shimadzu Inc.) with a spectral range of 600–4000 cm−1, 50 scans and a resolution of 0.5 cm−1.The ionic conductivities were measured by the AC impedance method in the temperature range from room temperature to 500 °C under an N2 gas flow over the frequency range of 10 Hz to 1 MHz using an LCR metre (Hioki MI3536) as a frequency response analyser. Measurements were performed using a sample pellet (10 mm diameter, 1–2 mm thick) with gold electrodes on each side. The relative density of the measured pellets was approximately 80%.
Results and discussion
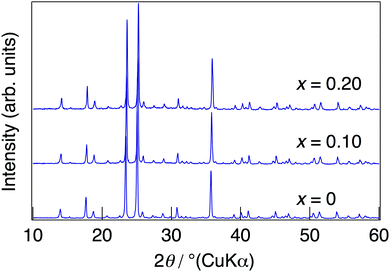
NaMg1−xLixHx(PO3)3·yH2O was synthesised in order to introduce protons into the crystal as charge compensation by partial substitution of Mg2+ with Li+. Fig. 1 shows the XRD patterns of NaMg1−xLixHx(PO3)3·yH2O synthesised in air at 400 °C for 12 h. Diffraction patterns of NaMg1−xLixHx(PO3)3·yH2O were similar to those of NaMg(PO3)3 and all diffraction peaks could be assigned to the space group Pbca.26 The structural parameters of NaMg1−xLixHx(PO3)3·yH2O were refined by XRD Rietveld refinement analysis using the structural model (space group Pbca).26 We considered a model in which lithium occupies only Mg1 or Mg2 sites and a model in which lithium occupies both of these sites. The best result was obtained by refinement with the third model. In this model, the lithium occupancy was fixed to the charged composition, and the analysis proceeded. Five oxygen sites in the water of crystallization were newly determined using the differential Fourier synthesis method. Fig. 2 displays the observed and calculated XRD patterns and their difference for NaMg1−xLixHx(PO3)3·yH2O (x = 0.20) as representative data. Structural parameters obtained from the final refinement cycle are listed in Table 1. The profile fitting provided good agreement factors; Rwp of 10.76%, Rp of 8.31%, RB of 4.92%, RF of 4.88%, and S = 1.45.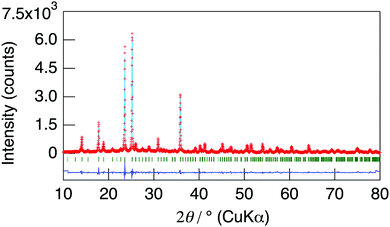 | ||
| Fig. 2 The observed and calculated XRD patterns and their difference for NaMg1−xLixHx(PO3)3·yH2O (x = 0.20). | ||
| Atom | Site | g | x | y | z | B/Å2 |
|---|---|---|---|---|---|---|
| Space group: Pbca (61), a = 14.239 (3) Å, b = 14.210 (3) Å, c = 14.199 (3) Å, S: 1.45, Rwp(%): 10.76, Rp(%): 8.31, Re(%): 7.40, RB(%): 4.92, RF(%): 4.88. | ||||||
| P1 | 8c | 1.0 | 0.020(14) | 0.245(15) | 0.473(16) | 0.5 |
| P2 | 8c | 1.0 | 0.218(13) | 0.225(18) | 0.489(16) | 0.5 |
| P3 | 8c | 1.0 | 0.266(13) | 0.024(2) | 0.481(19) | 0.5 |
| P4 | 8c | 1.0 | 0.275(14) | −0.007(19) | 0.267(15) | 0.5 |
| P5 | 8c | 1.0 | 0.474(13) | 0.019(2) | 0.239(16) | 0.5 |
| P6 | 8c | 1.0 | 0.494(15) | 0.233(18) | 0.224(18) | 0.5 |
| Mg1 | 8c | 0.8 | 0.122(2) | 0.121(3) | 0.134(2) | 0.5 |
| Li1 | 8c | 0.2 | =x(Li1) | =y(Li1) | =z(Li1) | 0.5 |
| Mg2 | 8c | 0.8 | 0.376(2) | 0.378(2) | 0.380(3) | 0.5 |
| Li2 | 8c | 0.2 | =x(Li2) | =y(Li2) | =z(Li2) | 0.5 |
| Na1 | 4a | 1.0 | 0 | 1/2 | 1/2 | 0.5 |
| Na2 | 4b | 1.0 | 1/2 | 1/2 | 1/2 | 0.5 |
| Na3 | 8c | 1.0 | 0.247(18) | 0.251(3) | 0.252(2) | 0.5 |
| O1 | 8c | 1.0 | 0.493(3) | 0.263(3) | 0.126(4) | 1.0 |
| O2 | 8c | 1.0 | 0.04(3) | 0.160(3) | 0.023(3) | 1.0 |
| O3 | 8c | 1.0 | 0.467(3) | 0.327(4) | 0.481(4) | 1.0 |
| O4 | 8c | 1.0 | 0.131(3) | 0.175(3) | 0.442(3) | 1.0 |
| O5 | 8c | 1.0 | 0.224(3) | 0.216(4) | 0.081(3) | 1.0 |
| O6 | 8c | 1.0 | 0.279(3) | 0.273(4) | 0.419(4) | 1.0 |
| O7 | 8c | 1.0 | 0.261(3) | 0.376(4) | 0.019(3) | 1.0 |
| O8 | 8c | 1.0 | 0.157(2) | 0.037(3) | 0.022(4) | 1.0 |
| O9 | 8c | 1.0 | 0.329(3) | 0.474(3) | 0.471(3) | 1.0 |
| O10 | 8c | 1.0 | 0.283(2) | 0.033(3) | 0.356(3) | 1.0 |
| O11 | 8c | 1.0 | 0.228(3) | 0.076(3) | 0.218(4) | 1.0 |
| O12 | 8c | 1.0 | 0.270(3) | 0.397(3) | 0.288(3) | 1.0 |
| O13 | 8c | 1.0 | 0.111(3) | 0.452(3) | 0.241(4) | 1.0 |
| O14 | 8c | 1.0 | 0.475(3) | 0.471(3) | 0.342(4) | 1.0 |
| O15 | 8c | 1.0 | 0.022(3) | 0.038(3) | 0.173(3) | 1.0 |
| O16 | 8c | 1.0 | 0.429(2) | 0.130(5) | 0.198(3) | 1.0 |
| O17 | 8c | 1.0 | 0.414(3) | 0.276(4) | 0.279(3) | 1.0 |
| O18 | 8c | 1.0 | 0.086(2) | 0.203(3) | 0.234(3) | 1.0 |
| O19 | 8c | 0.60(5) | 0.444(4) | 0.637(5) | 0.769(4) | 1.5 |
| O20 | 8c | 0.41(4) | 0.876(7) | 0.624(8) | 0.882(9) | 1.5 |
| O21 | 8c | 0.56(5) | 0.56(5) | 0.392(4) | 0.905(5) | 1.5 |
| O22 | 8c | 0.23(4) | 0.144(10) | 0.808(13) | 0.202(13) | 1.5 |
| O23 | 8c | 0.54(4) | 0.166(5) | 0.877(4) | −0.032(5) | 1.5 |
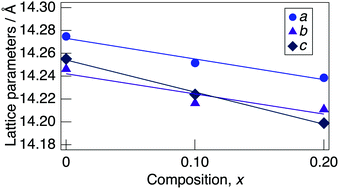
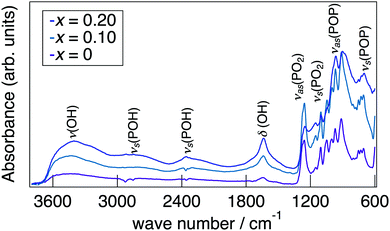
Fig. 3 presents a change in the refined lattice parameter as a function of x in NaMg1−xLixHx(PO3)3·yH2O. The a, b, and c axes contracted for x, suggesting the formation of the solid solution in the range of x = 0–0.20. It is thought that the shrinkage of the lattice by the replacement of Li+ (0.74 Å) with an ionic radius larger than Mg2+ (0.72 Å) is caused by the contraction between the oxygens due to the formation of hydrogen bonds with the introduced protons. Fig. 4 depicts the FTIR spectra for NaMg1−xLixHx(PO3)3·yH2O. To remove the effect of adsorbed water, the samples were dried at 120 °C before measurement. The strong absorbance peaks at 743 cm−1 and 970 cm−1 can be assigned to the symmetric and asymmetric vibration modes of νs(P–O–P) and νas(P–O–P) stretching vibrations.23,29 This corresponds to the formation of PO4 tetrahedral chains. The vibration bands at around 1095 and 1253 cm−1 are due to the symmetric and asymmetric PO2 vibration modes of νs(PO2), and νas(PO2). The broad absorbance peaks at 2300 and 2750 cm−1 are attributed to the vibration modes of νs(P–O–H). The νs(P–O–H) and νas(P–O–P) modes in polyphosphates at 684 cm−1 and 950 cm−1, respectively, are enhanced by the connection of protons to the PO4 unit.29 The bands at approximately 1600 cm−1 and 3500 cm−1 are assigned to δ (OH) and ν (OH), reflecting the presence of water of crystallisation. These vibration modes reveal strong absorbance peaks with increasing x, which suggests the introduction of H+ in the crystal structure.
Fig. 5 illustrates the TG/DTA curves for NaMg1−xLixHx(PO3)3·yH2O with x = 0 and x = 0.20 measured under an N2 gas flow with a heating rate of 10 °C min−1. The XRD patterns for NaMg1−xLixHx(PO3)3·yH2O (x = 0 and 0.20) measured after holding at different temperatures for 6 h are shown in Fig. S1(a) and (b) (ESI†). For x = 0, a three-step weight loss due to dehydration reactions occurred at room temperature, 150 °C, and 600 °C. For x = 0.20, dehydration reactions were observed at room temperature and 150 °C. The amount of weight loss for the temperature of the samples with x = 0.20 decreased from 250 °C, and a dehydration reaction proceeded up to 800 °C. No impurity peaks were observed for XRD patterns for the samples with x = 0–0.20 and the framework was retained up to 800 °C. It is considered that the water of crystallisation desorbed at 600 °C for the sample with x = 0 and desorbed at 250 °C due to the introduction of H+ for the samples with x = 0.20. The oxygens of water molecules coordinate with cations using their lone pairs; therefore, the formation of H3O+ due to the connection of H+ and lone pairs weakens the coordination bonding of water molecules. The initial weight loss corresponds to the loss of adsorbed water and water of crystallisation, and the second step corresponds to the loss of only water of crystallisation. Water contents of NaMg1−xLixHx(PO3)3·yH2O, as calculated from the weight loss in the range of 30–800 °C, are summarised in Table 2. The water content of the samples tended to increase with x and varied from 0.7 to 1.2 per unit formula.
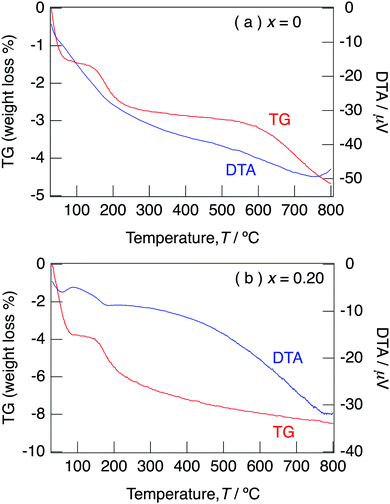 | ||
| Fig. 5 TG/DTA curves for NaMg1−xLixHx(PO3)3·yH2O with x = 0 (a) and x = 0.20 (b) measured under an N2 gas flow with a heating rate of 10 °C min−1. | ||
| x | 0 | 0.10 | 0.20 |
|---|---|---|---|
| Water content | 0.7 | 1.1 | 1.2 |
Fig. 6 presents the crystal structure of NaMg0.8Li0.2H0.2(PO3)3·yH2O viewed along the [001] direction (a) and PO4 chains along the a-axis direction with oxygens of water molecules adjacent to PO4 tetrahedra (b). The averaged bond length and bond angles of NaMg1−xLixHx(PO3)3·yH2O are summarised in Table S1 (ESI†). Sodium ions are shown in light blue spheres, magnesium ions are yellow ones, and PO4 tetrahedra are displayed in dark gray in the figure. The newly detected oxygens of the water molecule are indicated by dark blue spheres. The PO4 tetrahedra are connected by the sharing of corner oxygens to form a zigzag chain along the a-axis direction. Face-shared (NaO6)(MgO6) octahedral chains are linked along the [111] direction, and crystallographically equivalent octahedral chains are connected by corner-sharing with the zigzag PO4 chains to form a three-dimensional open framework. Two of the oxygens in the PO4 tetrahedron are shared by the NaO6 and MgO6 octahedra, and the others connect with the adjacent PO4 tetrahedra. One of the P–O bonds shared by adjacent PO4 was longer than the others, and these values varied in the range of 1.39–1.96 Å. The bond angles of O–P–O ranged from 92–130° and were distorted from the ideal tetrahedral bond angle of 109.5°. No systematic changes in the bond length or bond angle for compositional changes were observed for cations composing the framework.
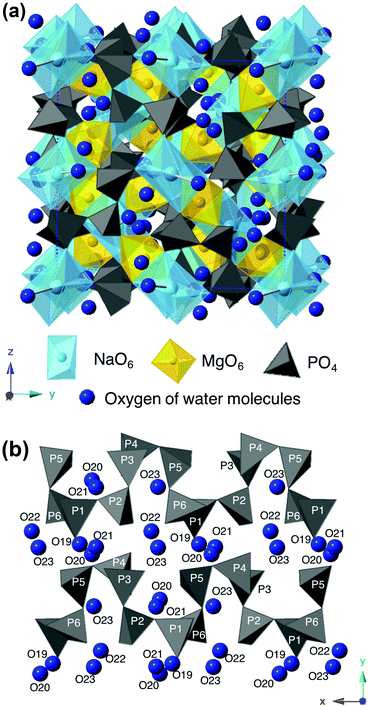 | ||
| Fig. 6 The crystal structure of NaMg0.8Li0.2H0.2(PO3)3·yH2O viewed along the [001] direction (a) and PO4 chains along the a-axis direction with oxygens of water molecules (b). | ||
Five oxygens of the water molecules exist in the three-dimensional tunnel along with PO4 tetrahedral chains. The enhancement of the P–O–H and P–O–P absorption peaks for x in the FTIR spectra reflects the formation of O–H bonds between protons and oxygens of the PO4 tetrahedra. Since water molecules are connected to the PO4 tetrahedron via hydrogen bonds, they are thought to be arranged adjacent to the PO4 tetrahedral chain. Three oxygens, O21–23, form bonds with Na+ in the range of 1.9–2.45 Å and exist as coordination water molecules, which would be due to the preference of large cations for a large coordination number of anions.21
The rigid framework formed by covalent and/or ionic bonds without the connection of hydrogen bonds with molecules results in the retention of the structure up to 800 °C, even if the desorption of water of crystallisation occurs. The weight loss due to the stepwise dehydration reaction on the TG curves comes from the different coordination environments of the water molecules in the tunnel. Coordinated water molecules connected with Na+ should be maintained up to high temperatures.21 It is thought that the formation of H3O+ would weaken the contact between Na+ and water molecules; however, a TG curve of x = 0.20 suggests residual water of crystallisation above 500 °C. This material has the potential for exhibiting high proton conductivity up to intermediate temperatures due to the proton diffusion accompanied by the hopping of water molecules in the tunnel along PO4 chains.20,21
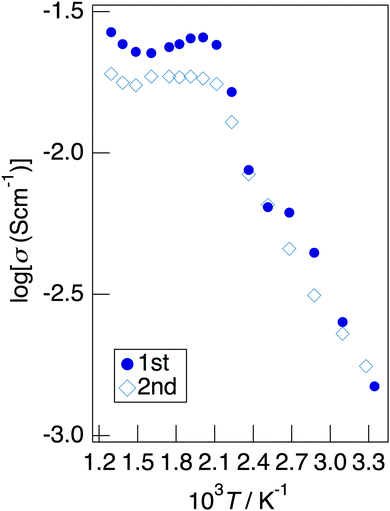
Fig. 7 depicts the Cole–Cole plots for the sample with x = 0.20 in NaMg1−xLixHx(PO3)3·yH2O measured at different temperatures under a non-humidified N2 gas flow. The plots for the samples with x = 0.20 showed a spike due to the electrode contribution, which is a typical behaviour for materials with high ionic conductivity. The total resistivity was obtained from the real axis intercepts of the spike caused by the electrode contribution. The conductivity increased up to 225 °C and then decreased, which would be due to the dehydration. Fig. 8 shows the temperature dependence of the proton conductivity of NaMg1−xLixHx(PO3)3·yH2O measured under a non-humidified N2 gas flow. The proton conductivity increased with x, reflecting the introduction of protons. The conductivity of x = 0 increased with the temperature from room temperature to 125 °C, then less increase in conductivity with temperature was observed at 150 °C, at which point the second stage dehydration reaction occurred.
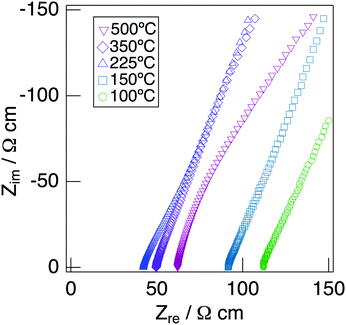 | ||
| Fig. 7 Cole–Cole plots for the sample with x = 0.20 in NaMg1−xLixHx(PO3)3·yH2O measured at different temperatures under a non-humidified N2 gas flow. | ||
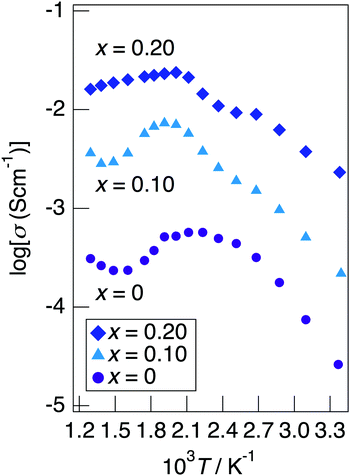 | ||
| Fig. 8 The temperature dependence of the proton conductivity of NaMg1−xLixHx(PO3)3·yH2O measured under a non-humidified N2 gas flow. | ||
It decreased from 200 to 300 °C as the dehydration reaction progressed and then increased again above 300 °C. The change in conductivity for the temperature of the sample with x = 0.10 exhibited an intermediate behaviour between x = 0 and x = 0.20. The sample with x = 0.20 exhibited a high proton conductivity of 2.3 × 10−3 S cm−1 at 23 °C, and the highest conductivity value of 2.4 × 10−2 S cm−1 was obtained. The apparent activation energy of x = 0.20 in the range of 23–225 °C was 13.6 kJ mol−1. This sample exhibited a value of over 10−2 S cm−1 in a wide temperature range of 150–500 °C. It is thought that the conductivity of the sample with x = 0 improves for temperature because the amount of water of crystallisation becomes almost constant at 300–600 °C, whereas the conductivity of the sample with x = 0.20 decreases gradually due to the desorption of water of crystallisation.
Fig. 9 presents the change in proton conductivity for the water content of NaMg0.8Li0.2H0.2(PO3)3·yH2O, measured at 150 °C under a non-humidified atmosphere. Samples with low water contents were obtained by heating to a high temperature in the impedance measurement cell under a non-humidified atmosphere. The water content at the increased temperature calculated based on the TG curve was used as the water content of the sample. The conductivity of the sample containing almost no water of crystallisation was below the lower limit of measurement of the apparatus. The conductivity of NaMg0.8Li0.2H0.2(PO3)3·yH2O showed a positive tendency for the water content, which suggests proton diffusion associated with the water of crystallisation. Unlike protons, the diffusion of Na+ and Li+ needs the formation of defects at normal positions and the movement of interstitial sites for diffusion in the crystal structure. Since Li+ partially occupies the Mg sites, it requires the diffusion of Mg2+ to diffuse in the crystal. The activation energy of Mg2+ estimated using the soft VB programme28 is as high as 2.4 eV, which suggests that diffusion of Li+ hardly occurs in this structure due to the obstruction by Mg2+. The energy barrier for Na+ diffusion also shows a high value of over 0.7 eV. It is considered that the diffusion of these cations that form the framework barely occurs in this structure. Even if the diffusion of Na+ and Li+ contributes to the conductivity, its value is considered to be less than 4.3 × 10−5 S cm−1 of the sample with the lowest water content because the movement of these cations is independent of water content. Therefore, the conductivity derived from the diffusion of Na+ and Li+ must be lower than 4.3 × 10−5 S cm−1 of the sample with the lowest water content. This value is 0.4% of the sample, exhibiting a value of 1.1 × 10−2 S cm−1, and the high conductivity of this material is considered to be due to proton diffusion through water molecules.
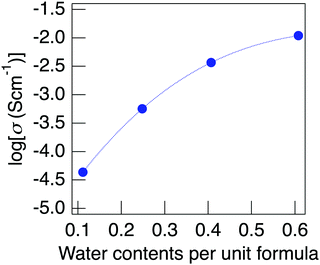 | ||
| Fig. 9 The change in the proton conductivity for the water content of NaMg0.8Li0.2H0.2(PO3)3·yH2O, measured at 150 °C under a non-humidified atmosphere. | ||
Fig. 10 shows the temperature dependence of the proton conductivity of NaMg0.8Li0.2H0.2(PO3)3·yH2O under a humidified atmosphere (pH2O = 4 kPa). At an initial heating step, the conductivity of NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibited a high proton conductivity of over 10−3 S cm−1 from room temperature, and it reached 2.6 × 10−2 S cm−1 at 225 °C. Although the conductivity decreased slightly from 250 °C to 350 °C due to dehydration, NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibited a high proton conductivity of over 2 × 10−2 S cm−1. Since the desorption of water of crystallisation is relieved under a humidified atmosphere rather than under a non-humidified atmosphere, the conductivity would increase again above 350 °C. After the first heating step, the sample was held for 2 h in an N2 gas flow saturated at room temperature, and then the conductivity was measured again. NaMg0.8Li0.2H0.2(PO3)3·yH2O showed a proton conductivity of over 10−3 S cm−1 from room temperature, and the value reached 1.8 × 10−2 S cm−1 at 225 °C in the second heating step. The sample held for 2 h at room temperature under a humidified atmosphere contained water molecules of 1.0 H2O that was calculated from the weight loss of 30–800 °C on the TG curve, which is over 80% of the water of crystallization of the sample before the initial heating step. Since desorption and absorption of water of crystallization occur reversibly in this structure, it is considered that the high proton conductivity corresponding to the amount of water of crystallization is exhibited again under a humidified atmosphere.
Fig. 11 presents the temperature dependence of the proton conductivity of NaMg0.8Li0.2H0.2(PO3)3·yH2O together with those of previously reported proton conductors. NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibits high proton conductivity over a wide temperature range from room temperature to 500 °C, and the values exceed 10−2 S cm−1 in the range of 150–500 °C. Materials impregnated with liquids, such as Nafion membranes, exhibit high proton conductivity at low temperatures with a large amount of humidification.1 However, it is difficult to maintain high proton conductivity up to the intermediate temperature due to water volatilisation. Inorganic solids containing water molecules and polyoxometalates also exhibit high proton conductivity at low temperatures.30,31 Many MOF materials, which have a non-combustible inorganic block and a hydrogen bond network of molecules that provide a proton diffusion pathway, exhibit a high proton conductivity below 150 °C.2,13,14 Solid acids such as CsHSO4 and CsH2PO4 exhibit high proton conductivity above the temperature at which the phase transition occurs.15,16 The structure of MOF-based materials and these solid acids become thermally unstable because of the dissociation of hydrogen bonds, contrary to showing fast proton diffusion in the low-intermediate temperature range. Inorganic solid acids expand the temperature range in which high proton conductivity is exhibited as composites with other ceramics.32 In-doped SnP2O7 exhibits a high proton conductivity up to 350 °C11; however, the conductivity has changed by five orders of magnitude by synthesis methods and research, which has caused controversy over whether the origin of proton diffusion is intercrystalline or a grain boundary.29,33–36 Perovskites with rigid frameworks formed by only ionic/covalent bonds show excellent thermal stability and exhibit high proton conductivity at around 400 °C by the material design based on the point defects chemistry. However, an achievement of fast proton diffusion via the Grotthouss mechanism below 300 °C is still difficult due to the high charge density on the surface of protons.5,6
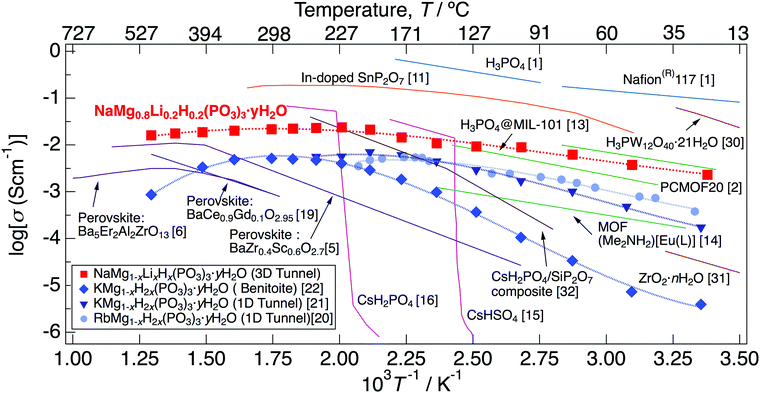 | ||
| Fig. 11 The temperature dependence of the proton conductivity of NaMg0.8Li0.2H0.2(PO3)3·yH2O together with those of previously reported proton conductors. | ||
The three-dimensional tunnel framework of NaMg0.8Li0.2H0.2(PO3)3·yH2O is formed by the connection of (NaO6)(MgO6) octahedral chains and PO4 tetrahedral chains without hydrogen bonding with water molecules. The rigid framework connected by only covalent/ionic bonds of NaMg0.8Li0.2H0.2(PO3)3·yH2O results in thermal stability up to 800 °C. Since the framework is formed without water molecules, the structure is maintained even if the dehydration reaction occurs. Because the framework was retained after dehydration at a high temperature, it exhibited high proton conductivity again after incorporating water of crystallisation. Mixed cation phosphates, AMg1−xH2x(PO3)3·yH2O (A: K+ and Rb+), have water molecule chains that provide a proton diffusion pathway in a one-dimensional tunnel framework formed by (AO6)(MgO6) octahedral chains and PO4 tetrahedral chains and exhibit high proton conductivity from room temperature to 250 °C.20,21 Although these phosphates have a rigid framework as well as NaMg0.8Li0.2H0.2(PO3)3·yH2O, this change to the high-temperature phase allows longer distances between the A−site cation and Mg2+. Benitoite-type phosphate, which is the high-temperature phase of the 1D tunnel-type KMg1−xH2x(PO3)3·yH2O, also shows thermal stability and exhibits high proton conductivity up to 500 °C.22 Due to the smaller cation size of Na+ compared to K+ and Rb+, NaMg0.8Li0.2H0.2(PO3)3·yH2O would maintain enough distance between Na+ and Mg2+ in the octahedral chain and no phase transition occurs up to the melting point. NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibited higher proton conductivity over a wider temperature range than other mixed cation phosphates.
The conductivity change for the water content suggests the proton diffusion associated with the water of crystallisation in NaMg0.8Li0.2H0.2(PO3)3·yH2O. In the three-dimensional tunnel framework of NaMg0.8Li0.2H0.2(PO3)3·yH2O, oxygen sites of water molecules were confirmed. The connection of PO4 tetrahedra and H2O/H3O+via hydrogen bonds is observed from the FTIR spectra. The connection of PO4 tetrahedra induces the adjacent hopping sites of water molecules in the tunnel framework of mixed cation phosphates.20,21 A part of the water molecules in NaMg0.8Li0.2H0.2(PO3)3·yH2O is coordinated to Na+ that forms the framework. The large cation in the mixed cation phosphates tends to form coordination bonding with the oxygen of water molecules, and it enhances the retention ability of water molecules in the structure due to the stronger connection than that of the hydrogen bonds.21 The strong connection of water molecules by the coordination bond causes a slightly lower proton conductivity of NaMg0.8Li0.2H0.2(PO3)3·yH2O at low temperatures than other water-contained systems; however, the adjacent arrangement of water molecules in the tunnel framework should result in high proton conductivity over 10−3 S cm−1 from room temperature. The increase in conductivity reached a plateau at around 200 °C due to the desorption of water of crystallisation; however, it exhibited a high proton conductivity of over 10−2 S cm−1 up to 500 °C due to the retention of water molecules by the strong connection with the rigid framework.
Conclusions
Mixed cation phosphate, NaMg1−xLixHx(PO3)3·yH2O, with a three-dimensional open framework was investigated as a fast proton conductor. NaMg0.8Li0.2H0.2(PO3)3·yH2O was synthesised by the coprecipitation method. A solid solution was formed in the composition range of x = 0–0.20. From the thermal measurement, a stepwise elimination reaction of water of crystallisation was observed. In FTIR measurements, an increase in absorption peaks corresponding to the modes of O–H and P–O–H bonds for the composition change in NaMg1−xLixHx(PO3)3·yH2O was observed. The three-dimensional tunnel framework of NaMg0.8Li0.2H0.2(PO3)3·yH2O was formed by the connection of (NaO6)(MgO6) octahedral chains and PO4 tetrahedral chains. In the three-dimensional tunnel framework of NaMg0.8Li0.2H0.2(PO3)3·yH2O, the oxygen sites of water molecules were confirmed. A multi-step weight loss due to desorption of water of crystallisation was observed for the TG curves. The framework was retained up to 800 °C. The proton conductivity improved with increasing x and NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibited a high proton conductivity of over 10−2 S cm−1 in the temperature range of 150–500 °C, and the value reached 2.4 × 10−2 S cm−1 at 225 °C under a non-humidified atmosphere. The proton conductivity of NaMg0.8Li0.2H0.2(PO3)3·yH2O depends on the amount of crystalline water. Under a humidified atmosphere (pH2O = 4 kPa), NaMg0.8Li0.2H0.2(PO3)3·yH2O exhibited a conductivity of over 10−3 S cm−1 from room temperature to 500 °C and reached 2.6 × 10−2 S cm−1 at 225 °C. After keeping the dehydrated sample in the humidified atmosphere at room temperature for 2 h, NaMg0.8Li0.2H0.2(PO3)3·yH2O showed a proton conductivity of over 10−2 S cm−1 again in the intermediate temperature range.Author contributions
Y. M. conceived and designed the experiments. N. U., J. N. and A. T. prepared the samples and carried out XRD and impedance measurements. N. U. carried out FTIR measurements. Y. M. and N. U. performed TG/DTA measurements. N. U. and Y. M. refined the crystal structure. Y. M., D. M., S. T., N. I., and S. H. discussed the results and analysed the data. Y. M. and N. U. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by JSPS KAKENHI Grant Number 19H05793 for D. M. and 21K05246 for Y. M.Notes and references
- K. D. Kreuer, Proton Conductivity: Materials and Applications, Chem. Mater., 1996, 8, 610–641 CrossRef CAS.
- P. Ramaswamy, N. E. Wong, B. S. Gelfand and G. K. H. Shimizu, A Water Stable Magnesium MOF that Conducts Protons over 10−2 S cm−1, J. Am. Chem. Soc., 2015, 137, 7640–7643 CrossRef CAS PubMed.
- I. Baranov, V. P. Khiznichenko, V. A. Sandler and L. A. Shuvalov, Frequency Dielectric Dispersion in the Ferroelectric and Superionic Phases of CsH2PO4, Ferroelectrics, 1988, 81, 183–186 CrossRef.
- M. Nagao, T. Kamiya, P. Heo, A. Tomita, T. Hibino and M. Sano, Proton Conduction in In3+-Doped SnP2O7 at Intermediate Temperatures, J. Electrochem. Soc., 2006, 153, A1604–A1609 CrossRef CAS.
- J. Hyodo, K. Kitabayashi, K. Hoshino, Y. Okuyama and Y. Yamazaki, Fast and Stable Proton Conduction in Heavily Scandium-Doped Polycrystalline Barium Zirconate at Intermediate Temperatures, Adv. Energy Mater., 2020, 10, 200213 Search PubMed.
- T. Murakami, J. R. Hester and M. Yashima, High Proton Conductivity in Ba5Er2Al2ZrO13, a Hexagonal Perovskite-Related Oxide with Intrinsically Oxygen-Deficient Layers, J. Am. Chem. Soc., 2020, 142, 11653–11657 CrossRef CAS PubMed.
- H. Luecke, B. Schobert, H. T. Richter, J. P. Cartailler and J. K. Lanyi, Structural Changes in Bacteriorhodopsin During Ion Transport at 2 Angstrom Resolution, Science, 1999, 286, 255–260 CrossRef CAS PubMed.
- W. Gu and V. Helms, Tightly Connected Water Wires Facilitate Fast Proton Uptake at the Proton Entrance of Proton Pumping Proteins, J. Am. Chem. Soc., 2009, 131, 2080–2081 CrossRef CAS PubMed.
- S. Iwata, C. Ostermeier, B. Ludwig and H. Michel, Structure at 2.8 Å Resolution of Cytochrome c Oxidase from Paracoccus Denitrificans, Nature, 1995, 376, 660–669 CrossRef CAS PubMed.
- D. A. Boysen, T. Uda, C. R. I. Chisholm and S. M. Haile, High-Performance Solid Acid Fuel Cells Through Humidity Stabilization, Science, 2004, 303, 68–70 CrossRef CAS PubMed.
- T. Hibino, Intermediate-Temperature Proton Conductors and their Applications to Energy and Environmental Devices, J. Ceram. Soc. Jpn., 2011, 119, 677–686 CrossRef CAS.
- M. Inukai, S. Horike, T. Itakura, R. Shinozaki, N. Ogiwara, D. Umeyama, S. Nagarkar, Y. Nishiyama, M. Malon, A. Hayashi, T. Ohhara, R. Kiyanagi and S. Kitagawa, Encapsulating Mobile Proton Carriers into Structural Defects in Coordination Polymer Crystals: High Anhydrous Proton Conduction and Fuel Cell Application, J. Am. Chem. Soc., 2016, 138, 8505–8511 CrossRef CAS PubMed.
- V. G. Ponomareva, K. A. Kovalenko, A. P. Chupakhin, D. N. Dybtsev, E. S. Shutova and V. P. Fedin, Imparting High Proton Conductivity to a Metal–Organic Framework Material by Controlled Acid Impregnation, J. Am. Chem. Soc., 2012, 134, 15640–15643 CrossRef CAS PubMed.
- Y. S. Wei, X. P. Hu and Z. Han, X, Y, Dong, S. Q. Zang and T. C. W. Mak, Unique Proton Dynamics in an Efficient MOF-Based Proton Conductor, J. Am. Chem. Soc., 2017, 139, 3505–3512 CrossRef CAS PubMed.
- A. I. Baranov, L. A. Shuvalov and N. M. Schagina, Superion Conductivity and Phase Transitions in CsHSO4 and CsHSeO4 crystals, JETP Lett., 1982, 36, 459–462 Search PubMed.
- S. M. Haile, C. R. I. Chisholm, K. Sasaki, D. A. Boysen and T. Uda, Solid Acid Proton Conductors: From Laboratory Curiosities to Fuel Cell Electrolytes, Faraday Discuss., 2007, 134, 17–39 RSC.
- A. I. Baranov, V. P. Khiznichenko, V. A. Sandler and L. A. Shuvalov, Frequency Dielectric Dispersion in the Ferroelectric and Superionic Phases of CsH2PO4, Ferroelectrics, 1988, 81, 183–186 CrossRef.
- H. Iwahara, T. Esaka, H. Uchida and N. Maeda, Proton Conduction in Sintered Oxides and its Application to Steam Electrolysis for Hydrogen Production, Solid State Ionics, 1981, 3–4, 359–363 CrossRef CAS.
- N. Bonanos, B. Ellis, K. S. Knight and M. N. Mahmood, Ionic Conductivity of Gadolinium-doped Barium Cerate Perovskites, Solid State Ionics, 1989, 35, 179–188 CrossRef.
- Y. Matsuda, M. Yonemura, H. Koga, C. Pitteloud, M. Nagao, M. Hirayama and R. Kanno, Synthesis, crystal structure, and ionic conductivity of tunnel structure phosphates, RbMg1−xH2x(PO3)3·y(H2O), J. Mater. Chem. A, 2013, 1, 15544–15551 RSC.
- Y. Matsuda, K. Funakoshi, R. Sebe, G. Kobayashi, M. Yonemura, N. Imanishi, D. Mori and S. Higashimoto, Arrangement of Water Molecules and High Proton Conductivity of Tunnel Structure Phosphates, KMg1−xH2x(PO3)3·yH2O, RSC Adv., 2020, 10, 7803–7811 RSC.
- Y. Matsuda, N. Ueda, K. Funakoshi, J. Nakajima, D. Mori, S. Taminato and S. Higashimoto, Proton Conductivity in Mixed Phosphate, KMg1−xH2x(PO3)3·yH2O with a Layered Structure at Low-Intermediate Temperatures, Dalton Trans., 2021, 50, 7678–7685 RSC.
- A. A. Kapshuk, P. G. Nagorny and O. V. Petrenko, Synthesis, IR Spectra, and Structures of Double Metaphosphates, MNi(PO3)3 (M = Na or K), Crystallogr. Rep., 2000, 45(2), 206–209 CrossRef.
- A. Rene, D. Rene, D. Andre, P. Marie Therese and Q. D. Tran, Preparation and Crystallographic Study of KM(PO3)3 Type Trimetaphosphates, C. R. Seances Acad. Sci., Ser. B, 1966, 262(B(10)), 718–721 Search PubMed.
- A. Maierhaba, P. Xiaobo, H. Shujuan, R. Yilimiranmu, Y. Zhihua, W. Hongping and P. Shilie, MIMIIP3O9 (MI = Rb, MII = Cd, Mg, Ca; MI = Cs, MII = Pb, Sr; MI = K, MII = Mg): Cation Substitution Application in Cyclophosphate Family and Nonlinear Optical Properties, Inorg. Chem., 2018, 57, 7372–7379 CrossRef PubMed.
- I. Abrahams, A. Ahmed, C. J. Groombridge, G. E. Hawkes and T. G. Nunes, Cation Distribution in Cubic NaM(PO3)3 (M = Mg or Zn) Using X-ray Powder Diffraction and Solid State NMR, J. Chem. Soc., Dalton Trans., 2000, 24, 4479–4701 Search PubMed.
- R. Oishi, M. Yonemura, Y. Nishimaki, S. Torii, A. Hoshikawa, T. Ishigaki, T. Morishima, K. Mori and T. Kamiyama, Rietveld Analysis Software for J-PARC, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, 600, 94–96 CrossRef CAS.
- H. Chen, L. L. Wong and S. Adams, SoftBV—A software tool for screening the materials genome of inorganic fast ion conductors, Acta Crystallogr., 2019, B75, 18–33 Search PubMed.
- C. R. Kreller, H. H. Pham, M. S. Wilson, R. Mukundan, N. Henson, M. Sykora, M. Hartl, L. Daemen and F. H. Garzon, Intragranular Phase Proton Conduction in Crystalline Sn1−xInxP2O7 (x = 0 and 0.1), Phys. Chem. C, 2017, 121(43), 23896–23905 CrossRef CAS.
- A. Hardwick, P. G. Dickens and R. C. T. Slade, Investigation of H+ Motion in the 21-Hydrates of 12-Tungstophosphoric and 12-Molybdophosphoric Acids by AC Conductivity and Pulsed 1H NMR Measurements, Solid State Ionics, 1984, 13, 345–350 CrossRef CAS.
- W. A. England, M. G. Cross, A. Hamnett, P. J. Wisemam and J. B. Goodenough, Fast Proton Conduction in Inorganic Ion Exchange Compounds, Solid State Ionics, 1980, 1, 231–249 CrossRef CAS.
- V. Ponomareva and G. Lavrova, Controlling the Proton Transport Properties of Solid Acids via Structural and Microstructural Modification, J. Solid State Chem., 2011, 15, 213–221 CAS.
- S. R. Phadke, C. R. Bowers, E. D. Wachsman and J. C. Nino, Proton Conduction in Acceptor Doped SnP2O7, Solid State Ionics, 2011, 183, 26–31 CrossRef CAS.
- S. Tao, Conductivity of SnP2O7 and In-Doped SnP2O7 Prepared by an Aqueous Solution Method, Solid State Ionics, 2009, 180, 148–153 CrossRef CAS.
- X. Xu, S. Tao, P. Wormald and J. T. S. Irvine, Intermediate Temperature Stable Proton Conductors Based Upon SnP2O7, Including Additional H3PO4, J. Mater. Chem., 2010, 20, 7827–7833 RSC.
- H. Wang, J. Xiao, Z. Zhou, F. Zhang, H. Zhang and G. Ma, Ionic Conduction in Undoped SnP2O7 at Intermediate Temperatures, Solid State Ionics, 2010, 181, 1521–1524 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma00592h |
| This journal is © The Royal Society of Chemistry 2021 |