Molecular engineering of 1,3,5-triaryl-2-pyrazoline fluorescent logic systems responsive to acidity and oxidisability and attachment to polymer beads†
Nicole
Zerafa
,
Miriam
Cini
and
David C.
Magri
 *
*
Department of Chemistry, Faculty of Science, University of Malta, Msida, MSD 2080, Malta. E-mail: david.magri@um.edu.mt
First published on 23rd November 2020
Abstract
1,3,5-Triaryl-2-pyrazolines were designed, synthesised and covalently immobilised onto submillimetre TentaGel® polystyrene beads (functionalised with amino-terminated polyethyleneglycol ligands) by peptide bond formation. The molecules are modularly designed based on photoinduced electron transfer (PET) according to electron-donor–spacer–fluorophore–receptor and receptor1–spacer–fluorophore–receptor formats with ferrocene and N,N-dimethylaniline as the electron donor and receptor1. A reference fluorophore–receptor compound, associated with an internal charge transfer (ICT) mechanism, is included for comparison. A carboxylate moiety at the para-position of the 1-phenyl ring assists the molecules with aqueous solubility and serves as the site for covalent attachment to the polystyrene beads. The H+, Fe3+-driven INHIBIT, H+-driven off–on–off and H+-driven NOT logic systems are demonstrated in aqueous methanol and attached onto heterogeneous polymer submillimeter beads. The INHIBIT gate was tested with the stronger oxidant, ammonium persulfate, which resulted in a greater fluorescence quantum yield (Φf = 0.192). A double-tagged polymer bead integrating INHIBIT and off–on–off multi-valued logic was also prepared.
Design, System, ApplicationMolecules with electron-donor–spacer–fluorophore–receptor and receptor1–spacer–fluorophore–receptor systems are demonstrated as molecular logic gates using chemical inputs and a fluorescent light output. A carboxylate moiety at the para-position of the 1-phenyl ring of the 1,3,5-triaryl-2-pyrazoline fluorophore assists the molecules with aqueous solubility and serves as the site for covalent attachment to the polystyrene beads. An application is the discrete labelling of object population sets from proteins and cells to passports and banknotes. |
Introduction
Engineering is the study of using scientific principles to design and build machines and structures, typically associated with large macroscopic constructions such as planes, bridges and towers.1 Chemistry is another discipline associated with building structures,2 typically at sub-nanometre dimensions by covalent3 and/or non-covalent4 bond formation.5 Living organisms synthesise proteins in cells by combining amino acid building blocks to form peptide bonds by a reverse hydrolysis reaction.6 The current knowledge-based digital revolution is built on the foundations of mathematics, logic, electronics and engineering principles.7 The modern field of molecular logic-based computation,8 the subject of this paper, combines the design, synthesis and testing of molecular prototypes in the pursuit of functional intelligent systems.9–15 Expressions of ingenuity from labs around the world have contributed new concepts towards information security,16 drug delivery,17 and multi-analyte sensing.18Molecular-logic computation applies Boolean algebra principles to analogue analytical response curves. Customarily, the sigmoidal response is digitally represented by input–output truth tables and electronic symbols.8 For example, YES logic is represented by modulation in an off–on manner, while NOT logic is represented by on–off switching. H+ and OH− have served as convenient chemical inputs for colorimetric and fluorescent pH indicators for over a century.19 During the past three decades other chemical species (i.e. metal cations, anions, organic substrates, enzymes) have been targeted alone, or in various two-input and three-input combinations according to gate functions such as AND, NAND, OR, NOR and INHIBIT.8
A sensible application of this logic diversity using optical outputs is the labelling of population sets,20 similar to barcodes on items in a grocery store. However, this approach is done more discretely as the fluorescent labels are not visible to the unassisted naked eye without the aid of a light source. The name given to this invention is molecular computational identification (MCID).21 The prototypical labelled objects are near-identical polymer beads chemically modified with a fluorescent logic tag.22–27 In practice, the objects could also be proteins or cells,28 or valuable documents such as passports and banknotes.29
Up to now the logic functions explicitly emulated on a polymer bead with a fluorescence output entail anthracene-based H+-driven PASS 1 and YES,21 Na+, H+-driven AND21 and H+-driven ternary off–on–off;22 pyrene-based Na+, K+-driven OR;23 4-amino-1,8-naphthalimide-based H+-driven PASS 1, YES and double-tagged PASS 1 and YES,24 and azaBODIPY (diazaborondipyrromethene)-based H+-driven PASS 1, YES26 and double-tagged 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 multi-value logic tags.25 We have contributed 4-amino-1,8-naphthalimide PASS 1; Fe3+, H+-driven TRANSFER; and Fe3+, H+-driven AND logic gates with two distinguishable optical output channels responsive to acid–base and redox chemistry.27 More complex logic functions can be derived from integrating two or more logic gate types together. For example, off–on–off multi-valued logic is an integration of YES and NOT functions;30 INHIBIT logic is an integration of AND and NOT functions.31
2 multi-value logic tags.25 We have contributed 4-amino-1,8-naphthalimide PASS 1; Fe3+, H+-driven TRANSFER; and Fe3+, H+-driven AND logic gates with two distinguishable optical output channels responsive to acid–base and redox chemistry.27 More complex logic functions can be derived from integrating two or more logic gate types together. For example, off–on–off multi-valued logic is an integration of YES and NOT functions;30 INHIBIT logic is an integration of AND and NOT functions.31
In Fig. 1 we present 1,3,5-triaryl-2-pyrazoline logic gates 1–3 along with their design layouts. Compounds 1 and 2 are conceived on the premise of electron-donor–spacer–fluorophore32 and receptor–spacer–fluorophore33 formats with a photoinduced electron transfer (PET) switching mechanism. Intrinsic to the pyrazoline is the N2 nitrogen atom and an internal charge transfer (ICT) excited state, which is deactivated with high H+ on protonation34 (or Hg2+ on complexation).351–3 are substituted with ferrocene (electron donor) 1, N,N-dimethylaniline (receptor1) 2 or phenyl (devoid of a PET mechanism) 3, respectively, at the 5-position separated by a heterocyclic aliphatic bridge (CH2CH spacer) from the pyrazoline fluorophore. Hence we have engineered molecules with electron-donor–spacer–fluorophore–receptor,36receptor1–spacer–fluorophore–receptor37 and fluorophore–receptor formats. We demonstrate 1 is a Fe3+, H+-driven INHIBIT gate, 2 is a H+-driven off–on–off gate and 3 is a H+-driven NOT gate.
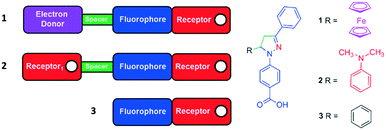 | ||
| Fig. 1 The colour-coded modular constructs and molecular structures of the 1,3,5-triaryl-2-pyrazoline logic gates 1–3. | ||
Molecules 1–3 are strategically endowed with a carboxylic acid moiety at the para position of the pyrazoline 1-phenyl moiety for covalent immobilisation onto TentaGel® MB-NH2 polymer beads to give 4–7 by an amide coupling reaction (analogous to protein synthesis).6 The linker is a polyethyleneglycol (PEG) chain.21 The H+, Fe3+-driven polymer INHIBIT gate 4 has an electron-donor–spacer–fluorophore(–linker–bead)–receptor format (Fig. 2, top left). Solid tag 5 has a receptor1–spacer–fluorophore(–linker–bead)–receptor format (Fig. 2, bottom left). Polymer tag 6 has a simpler fluorophore(–linker–bead)–receptor format (Fig. 2, bottom right). Additionally, we include a H+, Fe3+-driven double-tagged polymer bead 7 labelled with INHIBIT gate 1 and off–on–off logic gate 2.
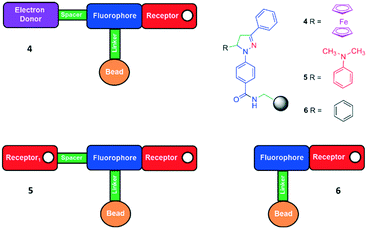 | ||
| Fig. 2 The designs of the 1,3,5-triaryl-2-pyrazolines 1–3 covalently attached to Tentagel MB-NH2 tagged logic gates 4–6. | ||
Results and discussion
The 1,3,5-triaryl-2-pyrazolines 1–3 were synthesised by a two-step synthetic procedure.36,37 Ferrocenecarboxaldehyde, 4-aminobenzaldehyde or benzaldehyde was reacted with acetophenone in the presence of ethanol or methanol and aqueous sodium hydroxide to provide the α,β-unsaturated ketone, which was refluxed with 4-hydrazinobenzoic acid in acidic methanol. Covalent attachment of 1–3 onto the TentaGel® MB-NH2 beads to yield the single-tagged logic beads 4–6 and double-tagged bead 7 were carried out in DMF with N,N′-dicyclohexylcarbodiimide (DCC) and tert-butyl alcohol.22 The double-tagged bead 7 consists of an equal ratio of 1 and 2. The tagged beads were recovered by vacuum filtration and washed sequentially with DMF, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) DMF/MeOH and MeOH. A synthetic scheme is shown in Fig. 3.
1 (v/v) DMF/MeOH and MeOH. A synthetic scheme is shown in Fig. 3.
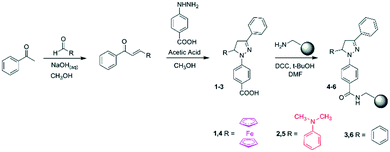 | ||
| Fig. 3 The synthesis of logic gates 1–3 and subsequent covalent attachment to give the polymer tagged beads 4–6. | ||
The UV-visible absorption spectra of 1–3 were measured in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) MeOH/H2O (Table 1). Under basic conditions, broad peaks appear at 367 nm, 356 nm and 368 nm for 1–3, respectively. The large molar extinction coefficients (log
1 (v/v) MeOH/H2O (Table 1). Under basic conditions, broad peaks appear at 367 nm, 356 nm and 368 nm for 1–3, respectively. The large molar extinction coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε ∼ 4.5) are confirmation of the strong π → π* transitions associated with the 2-pyrazoline chromophore. Isosbestic points at 325 nm and 396 nm are attributed to the ICT character of carboxypyrazolines and protonation of the imide and/or carboxy functionalities. Titration of 1–3 from 10−7 M to 1 M acid resulted in only slight changes in the UV–visible absorption spectra (Fig. 4).
ε ∼ 4.5) are confirmation of the strong π → π* transitions associated with the 2-pyrazoline chromophore. Isosbestic points at 325 nm and 396 nm are attributed to the ICT character of carboxypyrazolines and protonation of the imide and/or carboxy functionalities. Titration of 1–3 from 10−7 M to 1 M acid resulted in only slight changes in the UV–visible absorption spectra (Fig. 4).
| Solvent | λ abs | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) εc εc |
λ em | pKad | FEHe | |
|---|---|---|---|---|---|---|
a 10−6 M.
b Units nm−1.
c Units mol L−1 cm−1.
d Titrations with 1 M CH3SO3H and 0.1 M NaOH solution and analysis with equation log[(Imax − I)/(I − Imin)] = pKa + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βH+.
e Fluorescence enhancement (FE). Φf in 1 βH+.
e Fluorescence enhancement (FE). Φf in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) MeOH/H2O are 1Φf = 0.02 (10−2 M H+, 120 μM Fe3+), 2Φf = 0.38 (10−2 M H+) and 3Φf = 0.17 versus anthracene in aerated ethanol (ΦF = 0.27) excited at 367 nm. Literature 3Φf = 0.19 A. Dorlars et al.47
f nd = not determined. 1 (v/v) MeOH/H2O are 1Φf = 0.02 (10−2 M H+, 120 μM Fe3+), 2Φf = 0.38 (10−2 M H+) and 3Φf = 0.17 versus anthracene in aerated ethanol (ΦF = 0.27) excited at 367 nm. Literature 3Φf = 0.19 A. Dorlars et al.47
f nd = not determined.
|
||||||
| 1 | MeOH | 367 | 4.12 | 448 | 1.5 | 72 |
| 1 | MeOH/H2O | 367 | 4.53 | 448 | 1.7 | 28 |
| 1 | THF | 368 | 3.66 | 432 | ndf | 60 |
| 2 | MeOH/H2O | 356 | 4.57 | 451 | 0.7, 4.0 | 20 |
| 3 | MeOH/H2O | 368 | 4.51 | 470 | 0.7 | 5 |
The fluorescence spectra are broad mirror images of the S0 → S1 UV transition with λem at 448 nm, 451 nm and 470 nm in the on state for 1–3, respectively. Stokes shifts of ca. 80 nm eliminate the probability of an inner filter effect. In the presence of 10−2 M H+ and 70 μM Fe3+, 1 displays a 28-fold fluorescence enhancement (FE).36 In MeOH and THF, 1 has FEs of 72 and 60. At the same 10−2 M H+ level, 2 and 3 have more modest switching ratios of 20 and 5.
Binding constants for H+ and Fe3+ were determined by fluorimetric titrations according to the Henderson–Hasselbalch eqn (1)
log[(Imax − I)/(I − Imin)] = −log[M+] + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βM+ βM+ | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) MeOH/H2O gave N2 receptor pKas of 1.5, 0.7 and 0.7 for 1, 2 and 3 (Table 1). The apparent Fe3+ binding constant pβFe3+ for 1 is 4.2 and the pKa of the N,N-dimethylaniline for 2 is 4.0.37
1 (v/v) MeOH/H2O gave N2 receptor pKas of 1.5, 0.7 and 0.7 for 1, 2 and 3 (Table 1). The apparent Fe3+ binding constant pβFe3+ for 1 is 4.2 and the pKa of the N,N-dimethylaniline for 2 is 4.0.37
Testing the molecular logic gates 1–3
By traditional PET design standards, 1 is a Fe3+-driven redox-fluorescent switch.11 However, H+ acts as a disabling input at high levels.36,37 Therefore, 1 is a H+, Fe3+-driven INHIBIT logic gate. The optimal high and low acid input levels are 101 M H+ and 10−3 M H+, respectively, and the optimal oxidant levels are 120 μM Fe3+ and no oxidant added. The H+ domain range is of significance as the solubility of Fe3+ may be compromised at pH > 4. Fe3+ forms insoluble hydroxide salts at higher pHs resulting in a turbid solution. In this study we explored the use of ammonium persulfate (APS) as an alternative oxidant (vide infra).Fig. 5 illustrates a pictorial representation of the four logic states of 1 after excitation with light. In the absence of both inputs, PET from ferrocene to the excited state pyrazoline quenches the fluorescence (entry A). The PET reaction is strong enough to overcome the weak negative electrostatic charge of the carboxylate. However, in the presence of oxidant the ferrocene is oxidised to ferrocenium, which lowers the HOMO energy level of the electron donor. PET is no longer thermodynamically feasible and a blue fluorescence is observed from this charge neutral excited state (entry B). At 101 M H+ protonation occurs at the carboxylate and the N2 atom of the pyrazoline invoking both the ICT and PET quenching pathways (entry C). The positively charged fluorophore at the N2 atom contributes a positive electric field, which assists to further encourage PET. In the final case, the oxidised ferrocenium state, ICT is the dominant quenching pathway in acid (entry D). In summary, optimal brightness is only observed with high oxidant and low H+ (entry B).
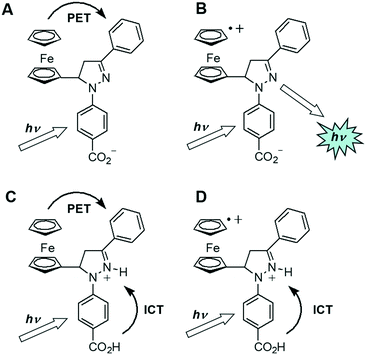 | ||
| Fig. 5 Pictorial representation of the truth table showing the four states of INHIBIT logic gate 1 and the deactivation pathways. The letter designations A-D correspond to the labels in Table 2. | ||
Although 1 has a high fluorescence enhancement of 28-fold in MeOH/H2O, the fluorescence quantum yield (Φf) is only 2% in the presence of 120 μM of Fe3+. Higher Fe3+ concentrations quench the fluorescence due to an inner filter effect, and possibly due to weak coordination at the N2 atom.34,35 To circumvent these issues, we tested ammonium persulfate (NH4)2S2O8 (E° = +2.1 V). It is a stronger oxidant than Fe3+ (E° = +0.77 V) and readily dissolves in water across the pH scale, as well as being transparent and colourless in the UV-visible region. It transfers two mole equivalents of electrons versus only one mole equivalent for Fe3+ and the product of the oxidation is sulfate, a relatively innocent anion.
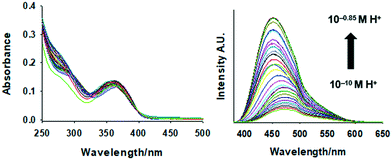
The emission spectra and truth table for the INHIBIT logic function of 1 with APS as the oxidant are given in Fig. 7 and Table 2, respectively. Addition of 5.0 mM persulfate with no added H+ gave a 100-plus fluorescence enhancement with a bright quantum yield (Φf = 0.192). Fluorimetric titration of 1 in the presence of 4.0 mM APS between pH 0.85 and 10.0 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) methanol/water yields a blue-shift from 470 nm to 450 nm. At high persulfate and low H+, fluorescence is observed, while high H+ in the presence of the oxidant disables most of the fluorescence. These observation are consistent with the attributes of an oxidant-enabled, H+-disabled INHIBIT logic gate.36
1 (v/v) methanol/water yields a blue-shift from 470 nm to 450 nm. At high persulfate and low H+, fluorescence is observed, while high H+ in the presence of the oxidant disables most of the fluorescence. These observation are consistent with the attributes of an oxidant-enabled, H+-disabled INHIBIT logic gate.36
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH/H2Oa
1 MeOH/H2Oa
| Label Fig. 5 | Input1b (H+) | Input2c (APS) | Output emissiond (ΦF) |
|---|---|---|---|
| a 10 μM 1 excited at 365 nm. b High input level 101 M H+ added as methanesulfonic acid. Low input level 10−6 M H+. c High input level 4.0 mM (NH4)2S2O8. Low input level with no (NH4)2S2O8 added. d Relative quantum yields measured with quinine sulfate in 0.1 M H2SO4 aerated water (ΦF = 0.55). Output level high when ΦF > 0.10. | |||
| A | 0 (low) | 0 (low) | 0 (low, 0.001) |
| B | 0 (low) | 1 (high) | 1 (low, 0.192) |
| C | 1 (high) | 0 (low) | 0 (low, 0.001) |
| D | 1 (high) | 1 (high) | 0 (low, 0.030) |
Logic gate 2 is a H+-driven off–on–off ternary logic gate as shown in Fig. 6 and summarised in Table 3. At 10−3 M H+, maximum fluorescence is observed with a Φf of 0.38 as a result of the PET quenching mechanism being arrested due to protonation of the N,N-dimethylaniline moiety (pKa = 4.0). At extreme high H+ concentration, protonation of the N2 atom of the heterocycle (pKa = 0.7) turns the fluorescence off by the ICT mechanism. The proximity of the two pKas provides an optimal ΔpKa of 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log units and a Gaussian-shaped pH-intensity profile.37 Protonation of the carboxylate to the carboxylic acid is also anticipated (pKa = 2.5) and enhances the electron-withdrawing character of the fluorophore (vide infra). Logic gate 3 with a phenyl at the 5-position is devoid of a PET mechanism. It is brightly fluorescent over most of the pH range with a Φf of 0.17 except at high acidic conditions (pKa = 0.7) with a minimum Φf of 0.034 near pH 0.
log units and a Gaussian-shaped pH-intensity profile.37 Protonation of the carboxylate to the carboxylic acid is also anticipated (pKa = 2.5) and enhances the electron-withdrawing character of the fluorophore (vide infra). Logic gate 3 with a phenyl at the 5-position is devoid of a PET mechanism. It is brightly fluorescent over most of the pH range with a Φf of 0.17 except at high acidic conditions (pKa = 0.7) with a minimum Φf of 0.034 near pH 0.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH/H2Oa
1 MeOH/H2Oa
| Label Fig. 6 | Inputb (H+) | Output emissionc (ΦF) |
|---|---|---|
| a 4 μM 1 excited at 357 nm. b H+ added as methanesulfonic acid. c Relative quantum yields measured with reference to anthracene in aerated water (ΦF = 0.27). Output level high when ΦF > 0.10. Data from ref. 37. | ||
| A | 0 (low, 10−7 M) | 0 (low, 0.019) |
| B | 1 (high, 10−3 M) | 1 (low, 0.38) |
| C | 2 (low, 10−1 M) | 0 (low, 0.025) |
The thermodynamics for the PET driving forces are calculated using the Weller equation, eqn (2), based on electrochemical and photophysical data.11,36,37 The oxidation potentials (Eox) of ferrocene and N,N-dimethylaniline are identical (+0.45 eV vs. SCE) so the same PET driving forces are expected for 1 and 2. The reduction potential (Ered) and the excited state energy (Es) of 5-carboxyphenyl pyrazolines are not directly known. However, fortuitously the Hammett parameter σp for hydrogen and carboxylate are both 0.00.43 The Ered and Es values for unsubstituted 1-phenyl pyrazolines are −2.34 eV and 2.29 eV,11 respectively, and the coulombic term (e2/εr) contributes 0.1 eV. From eqn (2), ΔGPET = −0.10 eV, meaning PET for 1 and 2 from ferrocene or N,N-dimethylaniline to the excited carboxypyrazoline is readily feasible.36 The ground state ET reactions from Fe3+ and S2O82− to ferrocene are −0.32 eV and −1.65 eV, respectively.
| ΔGPET = Eox − Ered − Es − e2/εr | (2) |
Despite the similar fluorescence quantum yields, they do differ to some degree in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) MeOH/H2O. The Φf of 1 is 0.192 and for 2 is 0.38. The difference is due to the type of chemical equilibria. Proton transfer to N,N-dimethylaniline forms a covalent bond, which ties up the electron lone pair to yield a stable cationic species. Electron transfer from oxidation of ferrocene by Fe3+ or S2O82− results in the ferrocenium radical cation and Fe2+or SO42−.
1 (v/v) MeOH/H2O. The Φf of 1 is 0.192 and for 2 is 0.38. The difference is due to the type of chemical equilibria. Proton transfer to N,N-dimethylaniline forms a covalent bond, which ties up the electron lone pair to yield a stable cationic species. Electron transfer from oxidation of ferrocene by Fe3+ or S2O82− results in the ferrocenium radical cation and Fe2+or SO42−.
Testing the logic tagged beads 4–7
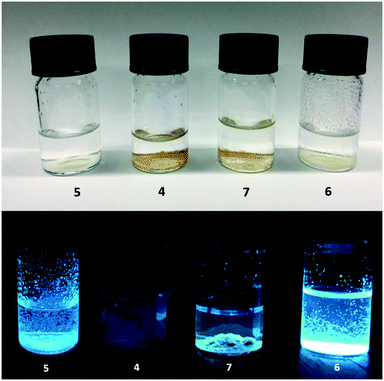
The heterogeneous polymer tagged beads 4–7 are not soluble in methanol, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) methanol/water (or water) as shown in Fig. 8. Naphthalimide-based logic beads are also insoluble in these solvents.24,27 However, the physicochemical properties of the two types of beads are notably different. The ferrocene-substituted pyrazoline beads 4 and 7 have a tint of brown colour, while 5 and 6 are pale yellow. In methanolic solution, 4 and 5 tend to aggregate and float to the surface and/or adhere to the side of the glass vial. In water, the beads tend to settle to the bottom of the vial. Beads 6 and 7 adhere to the vial in both methanol and water. These observations are attributed to the intrinsic hydrophobicity of the fluorophore despite the bead surface being functionalised with PEG ligands. Log
1 (v/v) methanol/water (or water) as shown in Fig. 8. Naphthalimide-based logic beads are also insoluble in these solvents.24,27 However, the physicochemical properties of the two types of beads are notably different. The ferrocene-substituted pyrazoline beads 4 and 7 have a tint of brown colour, while 5 and 6 are pale yellow. In methanolic solution, 4 and 5 tend to aggregate and float to the surface and/or adhere to the side of the glass vial. In water, the beads tend to settle to the bottom of the vial. Beads 6 and 7 adhere to the vial in both methanol and water. These observations are attributed to the intrinsic hydrophobicity of the fluorophore despite the bead surface being functionalised with PEG ligands. Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of 4.64, 4.16, 4.35 and 3.32 for 2, 2H+, 3 and 3− from Chemdraw give a fair measure of the degree of lipophilicity.‡
P values of 4.64, 4.16, 4.35 and 3.32 for 2, 2H+, 3 and 3− from Chemdraw give a fair measure of the degree of lipophilicity.‡
On irradiation of the beads 4–7 in the absence of inputs with 365 nm UV light, various shades of blue fluorescence are observed. In methanol, the fluorophore–receptor logic tag 6 emits a bright blue fluorescence, logic bead 5 has a weaker fluorescence, while 4 is not fluorescent due to intramolecular PET from ferrocene to the pyrazoline. The weak fluorescence of 5 is attributable to partial protonation of the dimethylaniline moiety in the semi-hydrophobic polymer interface.
The logic beads 4–7 were tested systematically in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) methanol/water at pH 1.4, 4.0 and 9.3. At pH ≤ 4.0, Fe3+ is stable and readily soluble in solution, but at pH 9.3 it forms insoluble hydroxide species and is ineffective as an input. The solid bead 4 (INHIBIT logic gate ‘Pourbaix sensor’)38–42 exhibits a low fluorescence unless the enabling oxidant input is present and the H+ level remains low as observed similarly with 1 (state B in Fig. 5). Very low fluorescence is observed at high acidic conditions due to ICT from the N2 pyrazoline atom and ferrocene quenching by PET (state C). In either case, the beads display relatively no fluorescence. Polymer beads 5 and 6 function as expected as H+-driven off–on–off and NOT logic gates (analogous to Fig. 6).
1 (v/v) methanol/water at pH 1.4, 4.0 and 9.3. At pH ≤ 4.0, Fe3+ is stable and readily soluble in solution, but at pH 9.3 it forms insoluble hydroxide species and is ineffective as an input. The solid bead 4 (INHIBIT logic gate ‘Pourbaix sensor’)38–42 exhibits a low fluorescence unless the enabling oxidant input is present and the H+ level remains low as observed similarly with 1 (state B in Fig. 5). Very low fluorescence is observed at high acidic conditions due to ICT from the N2 pyrazoline atom and ferrocene quenching by PET (state C). In either case, the beads display relatively no fluorescence. Polymer beads 5 and 6 function as expected as H+-driven off–on–off and NOT logic gates (analogous to Fig. 6).
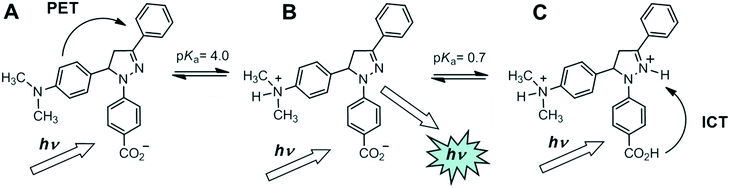 | ||
| Fig. 6 Pictorial representation showing the three states of off–on–off ternary logic gate 2. The truth table data are provided as Table 3. | ||
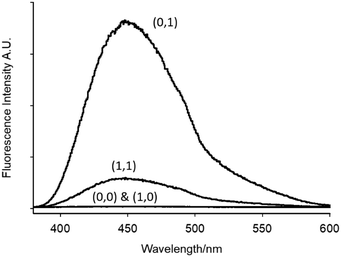 | ||
| Fig. 7 Emission spectra of INHIBIT logic gate 1. The numbering designations correspond to the input conditions given in Table 2. | ||
The attachment of molecules 1–3 onto the polymer bead converts the carboxylic acid moiety to an amide group within 4–7. Contrary to our past study where the point of attachment was isolated from the 4-amino-1,8-naphthalimide fluorophore by an alkyl spacer,27 attachment in this study is directly to the pyrazoline fluorophore, which contributes a synergistic inductive effect. The Hammett parameters σp for carboxylate (COO−), carboxylic acid (COOH), and amide (NHCOMe) are 0.00, 0.45 and 0.36 for 1, 1H+ and 4, respectively.43 Protonation of the carboxylate to the carboxylic acid, and coupling with the amino-terminated polymer beads to form an amide bond increases the σp value, and enhances the electron-withdrawing character of the pyrazoline. Thus, attachment of molecules 1–3 onto the polymer beads via the amino terminal PEG ligands lowers the pyrazoline LUMO energy level within 4–7 compared to the negatively charged carboxypyrazoline states of 1–3. Hence, PET from the ferrocene and N,N-dimethylaniline moieties should be even more thermodynamically feasible on the beads with ΔGPET < −0.10 eV for 4 and 5. This is an important feature as the lower polarity media of the polymer may retard PET and provide less stabilisation to the radical cation and anion intermediates.24
The non-polar microenvironment of the PEG chains at the polymer bead interface influences the excited state pKa* and the FE.24 In this past study with H+-driven anthracene and 4-amino-1,8-naphthalimide YES gate polymer beads, pKas* were measured to be 1.4 and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log units less than the molecular models. The lower pKa* associated with the beads is due to a repulsive electric field effect generated from protonation of the untagged terminal amines on the polymer particles. In the same study, the FEs were found to be 64% and 48% lower for the anthracene and 4-amino-1,8-naphthalimide tagged beads than with the molecular versions in 1
log units less than the molecular models. The lower pKa* associated with the beads is due to a repulsive electric field effect generated from protonation of the untagged terminal amines on the polymer particles. In the same study, the FEs were found to be 64% and 48% lower for the anthracene and 4-amino-1,8-naphthalimide tagged beads than with the molecular versions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) MeOH/water. Aprotic solvents tend to cause significant quenching to pyrazolines due to strengthening of the hydrogen bond between the N2 atom and protic solvent of the ICT excited state.44 Indeed, in MeOH and THF 1 has higher FEs than in MeOH/H2O (Table 1). It might be expected that the less polar microenvironment of the polymer beads contributes to greater emission brightness.45
1 (v/v) MeOH/water. Aprotic solvents tend to cause significant quenching to pyrazolines due to strengthening of the hydrogen bond between the N2 atom and protic solvent of the ICT excited state.44 Indeed, in MeOH and THF 1 has higher FEs than in MeOH/H2O (Table 1). It might be expected that the less polar microenvironment of the polymer beads contributes to greater emission brightness.45
Confocal fluorescence microscopy images of 4–7 were examined on a glass microscope slide in order to demonstrate the identity of individual beads within a mixed population set upon exposure to various inputs as reported by de Silva21,25 and us in our previous study with 4-amino-1,8-naphthalimides.27 Unfortunately, the shortest laser wavelength available was 457 nm, which is beyond the pyrazoline π–π* transition (see Table 1, Fig. 4 UV spectra). We attempted excitation at 457 nm, nonetheless, and observed green rather than vibrant blue emission. Given that at this longer wavelength the absorbance is weak, and that any transitions could be associated with forbidden n–π* transitions, we concluded our study.
Experimental
Synthetic procedures
The synthesis of 1 and 2 was previously reported.36,37 Pyrazoline 3 gave agreeable analytical data.46 Covalent attachment of 1–3 onto the TentaGel® MB-NH2 polymer beads to yield 4–7 was performed using literature procedures.22Conclusions
1,3,5-Triaryl-2-pyrazolines exhibiting H+, oxidant-driven INHIBIT 1, H+-driven off–on–off2 and H+-driven NOT 3 logic are reported. Fluorescent studies on logic gate 1 using ammonium persulfate as an alternative oxidant to Fe3+ resulted in a greater fluorescence enhancement. Attachment of the molecular logic gates onto TentaGel® MB-NH2 beads was achieved by DCC coupling via an amide bond to give micro-diameter logic tags 4–6. A double-tagged bead 7 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 1 and 2 was also demonstrated. To the best of our knowledge, 4 is the first example of a pyrazoline-based INHIBIT logic gate on a solid polymer support. This study introduces a new fluorophore to the MCID library responsive to acidity and/or oxidisability. Fine tuning of the substituents on the versatile pyrazoline platform should allow for tagged polymer beads with improved photophysical properties (i.e. longer wavelength, brighter fluorescence),45 and more complex logic functions.8
1 ratio of 1 and 2 was also demonstrated. To the best of our knowledge, 4 is the first example of a pyrazoline-based INHIBIT logic gate on a solid polymer support. This study introduces a new fluorophore to the MCID library responsive to acidity and/or oxidisability. Fine tuning of the substituents on the versatile pyrazoline platform should allow for tagged polymer beads with improved photophysical properties (i.e. longer wavelength, brighter fluorescence),45 and more complex logic functions.8
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The University of Malta is thanked for financial support. Prof. Robert M. Borg is acknowledged for assistance with NMR spectra acquisition. The Department of Metallurgy and Materials is thanked for access to the fluorescent confocal microscope.Notes and references
- Cambridge Advanced Learner's Dictionary & Thesaurus, Cambridge University Press, 2020 Search PubMed.
- (a) Molecular and Supramolecular Information Processing: From Molecular Switches to Logic Systems, ed. E. Katz, Wiley-VCH Verlag, Weinheim, 2012 Search PubMed; (b) Biomolecular Information Processing: From Logic Systems to Smart Sensors and Actuators, ed. E. Katz, Wiley-VCH Verlag, Weinheim, 2012 Search PubMed; (c) J.-H. Fuhrhop and G. Li, Organic Synthesis, Wiley-VCH, Weinheim, 2003 Search PubMed.
- For example: (a) P. L. Anelli, P. R. Ashton, D. Philp, M. Pietraszkiewicz, M. V. Reddington, N. Spencer, J. F. Stoddart, C. Vicent, R. Ballardini, V. Balzani, M. T. Gandolfi, L. Prodi, M. Delgado, T. T. Goodnow, A. E. Kaifer, A. M. Z. Slawin and D. J. Williams, J. Am. Chem. Soc., 1992, 114, 193 CrossRef CAS; (b) S. J. Cantrill, A. R. Pease and J. F. Stoddart, The paper discusses the concept of a molecular Meccano kit with building blocks based on non-covalent interactions, J. Chem. Soc., Dalton Trans., 2000, 3715 RSC.
- For example: (a) G. R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier, Amsterdam, 1989 Search PubMed; (b) G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311 CrossRef CAS; (c) J. Simon and P. Bassoul, Design of Molecular Materials: Supramolecular Engineering, Wiley-VCH, Weinhein, 2000 Search PubMed; (d) G. R. Desiraju, J. Am. Chem. Soc., 2013, 135, 9952 CrossRef CAS.
- R. A. Bissell, A. P. de Silva, H. Q. N. Gunaratne, P. L. M. Lynch, G. E. M. Maguire and K. R. A. S. Sandanayake, Chem. Soc. Rev., 1992, 21, 187 RSC.
- E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606 RSC.
- K. Szaciłowski, Chem. Rev., 2008, 108, 3481 CrossRef.
- A. P. de Silva, Molecular Logic-based Computation, RSC, Cambridge, 2013 Search PubMed.
- K. Szaciłowski, Infochemistry, Wiley-VCH, Chichester, UK, 2012 Search PubMed.
- (a) E. Katz, Enzyme-Based Computing Systems, Wiley-VCH, Weinheim, Germany, 2019 Search PubMed; (b) E. Katz and V. Privman, Chem. Soc. Rev., 2010, 39, 1835 RSC.
- C.-Y. Yao, H.-Y. Lin, H. S. N. Cory and A. P. de Silva, Mol. Syst. Des. Eng., 2020, 5, 1325 RSC.
- S. Erbas-Cakmak, T. Gunnlaugsson, S. Kolemen, T. D. James, A. C. Sedgwick, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2266 RSC.
- J. Andréasson and U. Pischel, Chem. Soc. Rev., 2015, 44, 1053 RSC.
- J. Ling, B. Daly, V. A. D. Silverson and A. P. de Silva, Chem. Commun., 2015, 51, 8403 RSC.
- (a) B. Daly, T. S. Moody, A. J. M. Huxley, C.-Y. Yao, B. Schazmann, A. Alves-Areias, J. F. Malone, H. Q. N. Gunaratne, P. Nockemann and A. P. de Silva, Nat. Commun., 2019, 10, 49 CrossRef; (b) A. P. de Silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399 CrossRef CAS; (c) C. Yao, S. Uchiyama and A. P. de Silva, Polymer, 2019, 11, 1351 CrossRef CAS.
- (a) T. Sarkar, K. Selvakumar, L. Motiei and D. Margulies, Nat. Commun., 2016, 7, 11374 CrossRef CAS; (b) O. Lustgarten, R. Carmieli, L. Motiei and D. Margulies, Angew. Chem., Int. Ed., 2019, 58, 184 CrossRef CAS; (c) J. Andréasson and U. Pischel, Chem. Soc. Rev., 2018, 47, 2228 RSC; (d) O. Lustgarten, L. Motiei and D. Margulies, ChemPhysChem, 2017, 18, 1678 CrossRef CAS; (e) D. Margulies, C. E. Felder, G. Melman and A. Shanzer, J. Am. Chem. Soc., 2007, 129, 347 CrossRef CAS.
- (a) U. G. Reddy, J. Axthelm, P. Hoffmann, N. Taye, S. Glaser, H. Gorls, S. L. Hopkins, W. Plass, U. Neugebauer, S. Bonnet and A. Schiller, J. Am. Chem. Soc., 2017, 139, 4991 CrossRef; (b) I. S. Turan, G. Gunaydin, S. Ayan and E. U. Akkaya, Nat. Commun., 2018, 9, 805 CrossRef.
- (a) G. J. Scerri, J. C. Spiteri, C. J. Mallia and D. C. Magri, Chem. Commun., 2019, 55, 4961 RSC; (b) D. C. Magri, M. Camilleri Fava and C. J. Mallia, Chem. Commun., 2014, 50, 1009 RSC; (c) D. C. Magri, G. J. Brown, G. D. McClean and A. P. de Silva, J. Am. Chem. Soc., 2006, 128, 4950 CrossRef CAS; (d) M. Schmittel and H.-W. Lin, Angew. Chem., Int. Ed., 2007, 46, 893 CrossRef CAS; (e) B. Rout, L. Unger, G. Armony, M. A. Iron and D. Margulies, Angew. Chem., Int. Ed., 2012, 51, 12477 CrossRef CAS; (f) G. D. Wright, C.-Y. Yao, T. S. Moody and A. P. de Silva, Chem. Commun., 2020, 56, 6838 RSC.
- J. M. Ottaway, in Indicators, ed. E. Bishop, Pergamon, London, 1972, p. 469 Search PubMed.
- B. J. Egner, S. Rana, H. Smith, N. Bouloc, J. G. Frey, W. S. Brocklesby and M. Bradley, Chem. Commun., 1997, 735 RSC.
- A. P. de Silva, M. R. James, B. F. McKinney, D. A. Pears and S. M. Weir, Nat. Mater., 2006, 5, 787 CrossRef.
- G. J. Brown, A. P. de Silva, M. R. James, B. O. F. McKinney, D. A. Pears and S. M. Weir, Tetrahedron, 2008, 64, 8301 CrossRef CAS.
- S. Nath and U. Maitra, Org. Lett., 2006, 8, 3239 CrossRef CAS.
- B. O. F. McKinney, B. Daly, C. Yao, M. Schroeder and A. P. de Silva, ChemPhysChem, 2017, 18, 1760 CrossRef CAS.
- C. Yao, J. Ling, L. Chen and A. P. de Silva, Chem. Sci., 2019, 10, 2272 RSC.
- A. Palma, M. Tasior, D. O. Frimannsson, T. T. Vu, R. Méallet-Renault and D. F. O'Shea, Org. Lett., 2009, 11, 3638 CrossRef CAS.
- (a) M. Vella Refalo, J. C. Spiteri and D. C. Magri, New J. Chem., 2018, 42, 16474 RSC; (b) M. Vella Refalo, N. V. Farrugia, A. D. Johnson, S. Klejna, K. Szaciłowski and D. C. Magri, J. Mater. Chem. C, 2019, 7, 15225 RSC.
- M. Nyan Win and C. D. Smolke, Science, 2008, 322, 456 CrossRef.
- (a) K. Jiang, L. Zhang, J. Lu, C. Xu, C. Cai and H. Lin, Angew. Chem., Int. Ed., 2016, 55, 7231 CrossRef CAS; (b) Gemini™ with SPARK® Security through design and combined technologies, De La Rue, 2017 Search PubMed.
- A. P. de Silva, H. Q. N. Gunaratne and C. P. McCoy, Chem. Commun., 1996, 2399 RSC.
- T. Gunnlaugsson, D. A. Mac Dónail and D. Parker, Chem. Commun., 2000, 93 RSC.
- D. C. Magri, Analyst, 2015, 140, 7487 RSC; D. C. Magri, Analyst, 2017, 142, 676 RSC.
- A. P. de Silva, T. S. Moody and G. D. Wright, Analyst, 2009, 134, 2385 RSC.
- M. T. Morgan, P. Bagchi and C. J. Fahrni, Dalton Trans., 2013, 42, 3240 RSC.
- A. P. de Silva, I. M. Dixon, H. Q. N. Gunaratne, T. Gunnlaugsson, P. R. S. Maxwell and T. E. Rice, J. Am. Chem. Soc., 1999, 121, 1393 CrossRef CAS.
- G. J. Scerri, M. Cini, J. S. Schembri, P. F. da Costa, A. D. Johnson and D. C. Magri, ChemPhysChem, 2017, 18, 1742 CrossRef CAS.
- R. Zammit, M. Pappova, E. Zammit, J. Gabarretta and D. C. Magri, Can. J. Chem., 2015, 93, 199 CrossRef CAS.
- T. J. Farrugia and D. C. Magri, New J. Chem., 2013, 37, 148 RSC.
- J. C. Spiteri, J. S. Schembri and D. C. Magri, New J. Chem., 2015, 39, 3349 RSC.
- A. D. Johnson, K. A. Paterson, J. C. Spiteri, S. A. Denisov, G. Jonusauskas, A. Tron, N. D. McClenaghan and D. C. Magri, New J. Chem., 2016, 40, 9917 RSC.
- J. C. Spiteri, S. A. Denisov, G. Jonusauskas, S. Klejna, K. Szaciłowski, N. D. McClenaghan and D. C. Magri, Org. Biomol. Chem., 2018, 16, 6195 RSC.
- J. C. Spiteri, A. D. Johnson, S. A. Denisov, G. Jonusauskas, N. D. McClenaghan and D. C. Magri, Dyes Pigm., 2018, 157, 278 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165 CrossRef CAS.
- M. D. P. de Costa, A. P. de Silva and S. T. Pathirana, Can. J. Chem., 1987, 65, 1416 CrossRef CAS.
- (a) D. E. Rivett, J. Rosevear and J. F. K. Wilshire, Aust. J. Chem., 1979, 32, 1601 CrossRef CAS; (b) D. E. Rivett, J. Rosevear and J. F. K. Wilshire, Aust. J. Chem., 1983, 36, 1649 CrossRef CAS.
- A. S. Morkovnik and O. Y. Okhlobystin, Chem. Heterocycl. Compd., 1985, 21, 461–464 CrossRef.
- A. Dorlars, C.-W. Schellhammer and J. Schroeder, Angew. Chem., Int. Ed. Engl., 1975, 14, 665 CrossRef.
Footnotes |
| † “Molecular Engineering” is the title of an influential paper by A. R. von Hippel, Science, 1956, 123, 315–317. |
‡ Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values were obtained from Chemdraw Ultra version 12.0.2.1076. P values were obtained from Chemdraw Ultra version 12.0.2.1076. |
| This journal is © The Royal Society of Chemistry 2021 |