Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Graphene based electrochemical immunosensor for the ultra-sensitive label free detection of Alzheimer's beta amyloid peptides Aβ(1–42)†
Hina. Y.
Abbasi
 *ab,
Zari
Tehrani
*ab,
Zari
Tehrani
 ab,
Anitha
Devadoss
ab,
Muhammad Munem
Ali
ab,
Anitha
Devadoss
ab,
Muhammad Munem
Ali
 a,
Soraya
Moradi-Bachiller
a,
Soraya
Moradi-Bachiller
 c,
Diego
Albani
c and
Owen. J.
Guy
*ab
c,
Diego
Albani
c and
Owen. J.
Guy
*ab
aCentre for NanoHealth, College of Engineering, Swansea University, Swansea, SA2 8PP, UK. E-mail: h.y.abbasi@swansea.ac.uk; o.j.guy@swansea.ac.uk; Tel: +44 (0) 1792 606475 Tel: +44 (0) 1792 513181
bDepartment of Chemistry, College of Science, Swansea University, Swansea, SA2 8PP, UK
cDepartment of Neuroscience, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Via La Masa 19, 20156, Milan, Italy
First published on 20th February 2021
Abstract
An immunosensor capable of high sensitivity detection of beta-amyloid peptides, shown to be a reliable biomarker for Alzheimer's disease, has been developed using screen printed graphene electrodes (SPGEs) modified with ultra-thin layers of polymerised 1,5-diaminonaphthalene (pDAN). Electropolymerization of 1,5-diaminonaphthalene (DAN) was performed to coat the graphene screen printed electrodes in a continuous polymer layer with controlled thickness. The surface characteristics of pristine graphene and polymer modified graphene electrodes were examined using Raman and X-ray photoelectron spectroscopy. The effects of polymer thickness on the electron transfer rates were investigated. An immunosensor for selective detection of beta amyloid peptides Aβ(1–42) was developed via biofunctionalization of the pDAN modified SPGE with the anti-beta amyloid antibody used as the peptide bioreceptor. The immunosensor has been used for specific detection of Aβ(1–42) with a linear range of 1 pg mL−1 to 1000 pg mL−1 and showed 1.4 pg mL−1 and 4.25 pg mL−1 detection and quantification limit, respectively. The biosensor was further validated for the analysis of spiked human plasma. The immunosensor enables rapid, accurate, precise, reproducible and highly sensitive detection of Aβ(1–42) using a low-cost SPGE platform, which opens the possibilities for diagnostic ex vivo applications and research-based real time studies.
1. Introduction
Alzheimer's Disease (AD) is the most common cause of dementia that influences millions of people across the world and becomes more prevalent with aging. AD is a neurodegenerative disease triggered by extracellular accumulation of amyloid β peptide (Aβ), intracellular appearance of neurofibrillary tangles and neuronal loss.1,2 This degeneration leads to changes in behaviour, personality and functional capacity, which deter the daily life of the patient. In 2020, an estimated 35 million people suffered from AD globally and it is predicted to affect 115 million individuals by 2050.3 The existing investigation methods of AD are complicated and are usually made when the disease is already in an advanced stage. In addition, there are no treatments available to avoid this condition4 and current therapies only slow the advancement of the disease.4 Thus, there is an intense need for the development of easy analytical tools for the rapid detection of AD biomarkers for early-stage point-of-care diagnosis.5–7 Aβ(1–42) peptide is the key element of the senile plaques present in AD.8 Other pathological characteristics of AD consist of intraneuronal inclusions of hyperphosphorylated tau protein in neurofibrillary tangles, together with downstream processes such as inflammation and oxidative stress. Additionally, a certain isoform of apolipoprotein, ApoE4, is the foremost genetic risk factor for AD, because it also leads to excess of amyloid formation in the brain.9 All these components cause the loss of synaptic integrity, progressive neurodegeneration and effective neural network connectivity.3,6,10Aβ(1–42) is usually expressed in cerebrospinal fluid (CSF) and plasma and it is found that the CSF levels of Aβ(1–42) are lower in AD patients than in normal controls, which indicates amyloid pathology. Gagni et al. reported the detection of low CSF Aβ(1–42) levels at preclinical disease stages that predicted future cognitive decline and neurodegeneration.11 An Aβ(1–42) concentration of <500 pg mL−1 (0.1 nM) indicates that Aβ(1–42) is accumulating in the brain and not circulating in the CSF.11 However, more recently Emadi et al. reported that Aβ(1–42) concentration levels in peripheral blood serum samples at the preclinical disease stage is 3.41 ± 2.17 pg mL−1 in aged people and 3.34 ± 1.95 pg mL−1 in young people.12 They stated that in female AD patients with increasing age, more amounts of Aβ(1–42) remain in brain and thus appears less in the patient's serum, whereas in male AD patients the amount of Aβ(1–42) that remains in the brain is smaller than that of Aβ(1–40). It was concluded that female AD patients would show more adverse cognitive decline than male patients with increasing age because the toxicity of Aβ(1–42) has been shown to be far more than that of Aβ(1–40).12 It is therefore suggested that detection of Aβ(1–42) in physiological fluids such as CSF, serum and plasma at lower levels can be used for both screening of AD at an early stage and for monitoring disease progression.
Several methods have been introduced to detect amyloid beta using different sensing platforms.13–19 An enzyme linked immunosorbent assay (ELISA), which is evaluated by western or dot blot analysis, is currently used for its clinical detection. Although these tests are reliable, they are labour intensive, time consuming and require complicated instruments to perform measurements. Also, the sensitivity of these tests is not adequate to detect the ultra-low levels of the disease biomarkers at the early stages of disease onset.
In 2013 Jeseung et al. reported a CNT film-based biosensor with a metal semiconductor field effect transistor structure (CNT-MESFET) for the real time detection of amyloid-β (Aβ) in human serum. Their sensor showed an LOD of 1 pg mL−1 within the linear range of 10−12 to 10−9 g mL−1. Nevertheless, the fabrication of this sensor is complex, costly and labour-intensive.15
Electrochemical biosensors offer a rapid, cost-effective, easy and sensitive testing technique.10,14,16,20,21 Furthermore, label-free electrochemical immunosensors reduce sample complexity because of the exclusion of potentially confounding molecular labels.22,23 They provide a promising approach for both sensitive and selective analysis due to their high compatibility and repeatability, rapidness, simple instrumentation, low power requirements and easy signal quantification.24–26 Label-free sensors on the other hand can also be integrated into lab-on-a-chip platforms and have the benefit of using small volumes for rapid and inexpensive measurements as opposed to the label-based technologies which are often more costly and time-consuming.27 Pedro and co-workers used a modified gold electrode surface for the sensitive detection of Aβ(1–42). This sensor showed good accuracy and reproducibility with a limit of detection of 5.2 pg mL−1. However, the selected antibody was also able to recognize Aβ(1–40), limiting the specificity of the technique.16 Furthermore, Troung et al presented a label-free impedimetric immunosensor using carbon disposable electrochemical printed chips modified with AuNPs, for the detection of amyloid beta. They bound protein G to the antibody for its controlled immobilization which in turn lowered the LOD to 0.57 nM.21
It has been reported that coating polymeric films on the electrode surface may well improve the behaviour and performance of electrochemically modified electrodes.28,29 Furthermore, such modifications may increase the reaction rate, enhance the electrocatalytic properties of the substrates, and the reproducibility and stability of the electrodes.30 Poly(1,5diaminonaphthalene) (pDAN) is a conducting polymer that is obtained from the polymerization of aromatic monomers comprising of two amino groups.31 Thus, there are free amine groups present in the polymer structure that aid in binding the biomolecules to the electrode surface. The unique properties of pDAN make it an outstanding material for electrochemical electrode modification. Several studies have used pDAN in electrochemical sensors for detection of, for example, H2O2, cholesterol, H2O, dopamine and lactose.32–36
In this work we have used a graphene based electrode, due to its high conductivity, large surface to volume ratio, low cost and low environmental impact, in the fabrication of sensors and biosensor-based devices.37,38 Graphene biosensors offer the advantages of high sensitivity, lower detection limits and high throughput detection when compared to other methods such as ELISA, Polymerase Chain Reaction (PCR) and fluorescence assays. Myung-Sic et al. used oxygen-plasma-treated rGO surfaces as reactive interfaces for the electrical detection of Aβ peptides, which in turn improved the antibody immobilization on electrodes and yielded improved sensing performance due to the enhancement in surface functionality. This was confirmed by measuring the changes in the electrode's electrical characteristics, with a 3.33-fold steeper slope for electrical responses versus the analyte concentration curve of the oxygen plasma treated sample compared to the untreated one.39 More recently a graphene/rGO dual layer SPE was reported by Sethi and co-workers for the detection of Aβ(1–42). The proposed sensor showed a detection limit of 2.398 pM with high selectivity.40
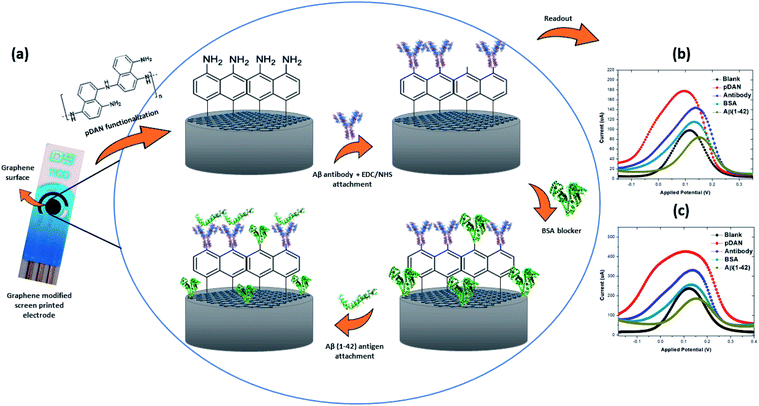
Here, we report the development of a label-free, electrochemical immunosensor, using graphene modified screen printed electrodes (SPGEs) for high sensitivity detection of beta amyloid peptides, isoform 42. The graphene surface was modified with amine functional groups using a 1,5-diaminonaphthalene (DAN) electropolymerization process with optimised amine surface coverage,41 whilst maintaining minimal thickness for better charge transfer from the electrolyte solution to the electrode surface. Polymer DAN (pDAN) modification provided controlled amount of amines without even compromising the actual graphene properties. Subsequent attachment of the anti-beta amyloid antibody on to the sensor was performed and the influence of antibody concentrations with respect to Aβ(1–42) peptide sensing efficiency has been analysed in detail. BSA was used as the blocking agent. The implications of this work towards developing a commercially viable, robust and sensitive immunosensor for Alzheimer's disease by using biomarker Aβ(1–42) are presented.
2. Materials and methods
2.1. Materials
Graphene modified screen printed electrodes (SPGEs) were purchased from Metrohm Ltd; 1,5-diaminonaphthalene (DAN) (97%), 1-ethyl-3-(3-(dimethylamino)-propyl) carbodiimide hydrochloride (EDC) (>98%), N-hydroxysuccinimide (NHS) (98%), ferrocene carboxylic acid (FeCOOH) (97%), potassium hexacyanoferrate(III) (>99%), potassium hexacyanoferrate(II) trihydrate (98.5–102%) and phosphate buffered saline (PBS) tablets were supplied by Sigma-Aldrich and used as received. Anti-beta amyloid antibody and beta-amyloid peptide (1–42) human were obtained from Abcam Ltd.2.2. Electropolymerization
Graphene electrode surfaces were functionalized with ultra-thin polymer layers of 1,5-diaminonaphthalene (DAN) via an electropolymerization technique mentioned in detail in our previous work.41 Typically, the polymer films were deposited from 10 mM DAN in 0.25 M H2SO4 using standard graphene modified screen printed electrodes (SPGEs), where graphene was used as the working electrode, carbon as the counter electrode and Ag/AgCl as the reference electrode. Electropolymerization of the monomer DAN results in the deposition of ultra-thin polymer DAN (pDAN) layers on the graphene electrode surface, with the resulting pDAN layer containing NH2 functional groups which can subsequently be used for the attachment of “bioreceptor” antibodies to the graphene surface. Following pDAN deposition, the electrodes were rinsed with deionized (DI) water and dried under nitrogen.2.3. Bio-functionalization
Bio-functionalization protocols were optimised for improved sensor performance. Typically, anti-beta amyloid antibody was ‘activated’ in a solution containing 5 mM EDC/NHS for 40 minutes. Once activated, the antibody solution was added to the sensor surface and incubated for 30 minutes at 4 °C. The antibody incubation time was optimised to achieve the highest sensitivity for the proposed biosensor (Fig. S1†). The antibody functionalised SPGE was then rinsed thoroughly with deionised water, to wash away any non-specifically bound probe on the surface. Blocking of unbound NH2 surfaces was performed by drop-casting 1% BSA onto the electrode surface and incubating for 30 minutes at 4 °C, subsequently removing any unbound BSA by rinsing with DI water and drying with N2. In this work 0.1 mM concentration of PBS was used in all experiments in order to increase the Debye length to approximately 7.3 nm and hence to achieve better signal amplification.422.4. Amyloid beta (1–42) pre-treatment method
Aβ(1–42) was received in the lyophilized form. It was first dissolved in 10 mM sodium hydroxide, followed by gentle vortexing for less than 1 minute to make a homogeneous solution. It is reported that under these highly alkaline conditions the peptide is fully dissolved and exists only as monomers.43 The stock solution was then aliquoted and stored at −20 °C until further use.2.5. Human blood collection and plasma preparation
Human plasma samples were received from IRCCS – Istituto di Ricerche Farmacologiche “Mario Negri” Via La Masa 19, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156 Milan – Italy. Fasting blood samples (3 mL) from AD patients were collected by the venipuncture method. The aliquot was centrifuged at 2000 rpm for 15 minutes at 4 °C to separate the plasma fraction. After that the plasma was transferred to a fresh tube, aliquoted and immediately frozen at −80 °C till further use. Informed consent was obtained from all human subjects. Besides, all of the investigation protocols in this study have been approved by relevant local ethics committee for clinical research (Milan, Italy).
156 Milan – Italy. Fasting blood samples (3 mL) from AD patients were collected by the venipuncture method. The aliquot was centrifuged at 2000 rpm for 15 minutes at 4 °C to separate the plasma fraction. After that the plasma was transferred to a fresh tube, aliquoted and immediately frozen at −80 °C till further use. Informed consent was obtained from all human subjects. Besides, all of the investigation protocols in this study have been approved by relevant local ethics committee for clinical research (Milan, Italy).
2.6. Characterization
The electrode layers were characterized for their thickness using Raman spectroscopy and X-ray photoelectron spectroscopy to check the quality of the electrode.X-ray photoelectron spectroscopic (XPS) measurements were performed using a Kratos Axis Supra, with an Al Kα monochromatic X-ray source, running at an emission current of 15 mA. Raman mapping measurements were performed using a Renishaw system, with a 532 nm excitation laser and 2 mW power, before and after pDAN modification on the electrode surface.
Electrochemical analysis was done using an advanced potentiostat (PGSTAT-302N, Metrohm AutoLab, Runcorn, UK). Square wave voltammetry (SWV) and differential pulse voltammetry (DPV) were used as characterization techniques with the scanning voltage in the range of −0.2 V to 0.5 V for evaluating the electrochemical performance of blank and modified electrodes. 5 mM K3[Fe(CN)6]/K4[Fe(CN)6] in 0.1 mM PBS solution (pH = 7.4) was used as an electrolyte.
3. Results and discussion
3.1. Grafting pDAN layers on SPGEs
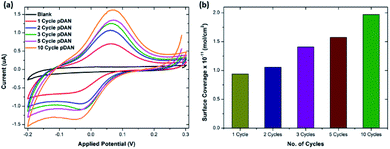
To enable biofunctionalization, SPGE surfaces were modified with poly-1,5-diamino naphthalene (pDAN) layers using electropolymerization as described in Section 2.2. Fig. S2† shows the voltammetric response of SPGEs during the electropolymerization process. The dominant peak at 0.55 V (vs. Ag/AgCl) corresponds to the oxidation of the monomer DAN species, which reduces subsequently with increase in the number of scan cycles, indicating that DAN monomers are being oxidised and converted into polymers. Also, a new peak at 0.3 V originating during the second cycle is attributed to polymer layer formation and this peak increases with increase in the scan cycles. Polymer layer thickness was controlled via controlling the number of scan cycles. In order to achieve a greater number of surface amine groups for biofunctionalization, SPGE electrodes were modified with pDAN layers of different thicknesses.413.2. Mechanism of action of pDAN/graphene interfaces
| Ip = (2.69 × 105)n3/2ACD1/2υ1/2 | (1) |
| Γ = Q/nFA | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 s A mol−1) and A is the geometrical area of the electrode (0.1256 cm2). Fig. 1b shows the surface coverage dependence on the number of scan cycles. As anticipated, it is found that the amine surface coverage increases with increase in pDAN layer thickness (scan cycles), which is also attested by the increase in the capacitive current. Although the higher pDAN layer thickness produces large amine density on the surface, it is found that the charge transfer characteristics decrease after reaching a certain thickness (Section 3.2.1). Thus, it is critical to find a fair balance between increased amine surface coverage as well as charge transfer characteristics. For our experiments – 3 cycles of pDAN layers (3-pDAN) were chosen to be the optimal layer thickness for further electrochemical sensor development.
485 s A mol−1) and A is the geometrical area of the electrode (0.1256 cm2). Fig. 1b shows the surface coverage dependence on the number of scan cycles. As anticipated, it is found that the amine surface coverage increases with increase in pDAN layer thickness (scan cycles), which is also attested by the increase in the capacitive current. Although the higher pDAN layer thickness produces large amine density on the surface, it is found that the charge transfer characteristics decrease after reaching a certain thickness (Section 3.2.1). Thus, it is critical to find a fair balance between increased amine surface coverage as well as charge transfer characteristics. For our experiments – 3 cycles of pDAN layers (3-pDAN) were chosen to be the optimal layer thickness for further electrochemical sensor development.
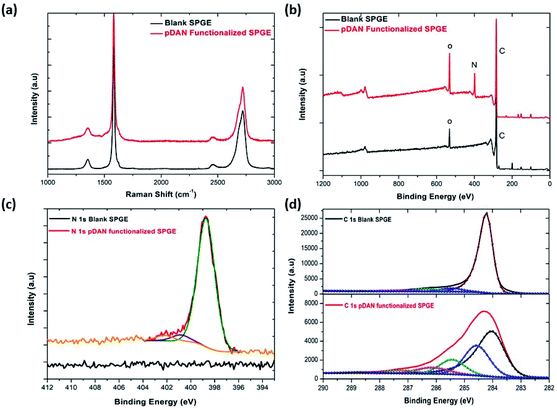
X-ray photoelectron spectroscopy is a tool used to investigate the chemical environment of graphene modified with foreign dopants.41,45 To study the influence of electropolymerization on SPGEs, XPS measurements were carried out on blank and pDAN modified SPGE surfaces. Fig. 2b–d show XPS graphs of the wide spectrum (b), N 1s spectrum (c) and C 1s spectrum (d) of pristine graphene and pDAN modified SPGEs. The wide scan acquisition of pristine and pDAN modified SPGE shows a typical C 1s peak at ∼284 eV. Unlike pristine graphene, the pDAN modified SPGE shows a clear peak at ∼400 eV in the wide spectra (Fig. 2b), confirming that nitrogen-based moieties were introduced after electropolymerization. The atomic concentrations of C, O and N present were calculated from the XPS spectra and are listed in Table S2.† It is found that the atomic percentage of N increases drastically upon electropolymerization and additional components appear in the C spectra (Fig. 2d), which can be ascribed to the carbon and nitrogen species present in DAN. To further analyse the nature of C, O, and N species, the N 1s (Fig. 2c) and C 1s (Fig. 2d) peaks were fitted with Gaussian/Lorentzian peaks to analyse the distribution of carbon and nitrogen bonding. The C 1s peak of pDAN modified graphene was deconvoluted into six carbon peaks, which are ascribed to Caromatic (∼284.04 eV), Caliphatic (∼284.56 eV), C–N (∼285.44 eV), C–O (∼286.14), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (∼287.20 eV) and O–C
N (∼287.20 eV) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N–C
O/N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (∼288.76 eV). The N 1s peak of pDAN modified graphene is observed at 400.16 eV, indicating that nitrogen compounds are present upon successful electropolymerization. Furthermore, the three fitted peaks at ∼400.12 eV, 402.26 eV and ∼403.59 eV correspond to C–N, C–NH and N–H groups respectively, confirming that the pDAN electropolymerization process generates –NH2 groups on the graphene surface.46
O (∼288.76 eV). The N 1s peak of pDAN modified graphene is observed at 400.16 eV, indicating that nitrogen compounds are present upon successful electropolymerization. Furthermore, the three fitted peaks at ∼400.12 eV, 402.26 eV and ∼403.59 eV correspond to C–N, C–NH and N–H groups respectively, confirming that the pDAN electropolymerization process generates –NH2 groups on the graphene surface.46
3.3. Mechanism of antibody/pDAN/graphene interfaces
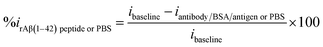 | (3) |
3.4. Evaluating the electrochemical immunosensor performance
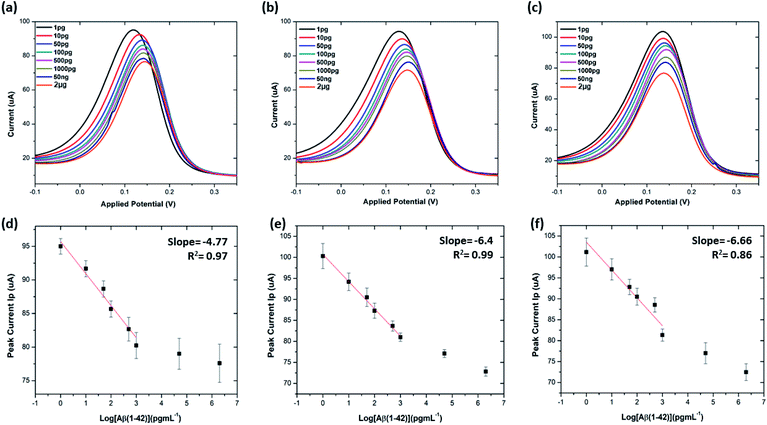
The limit of detection (LOD) and limit of quantitation (LOQ) for the precise detection of amyloid β peptide (1–42) were calculated within the linear range of 1 pg mL−1 to 1000 pg mL−1, utilising the standard deviation of the intercepts and the average of slopes of the straight lines from the analytical curves, using the following equations:16,40,52,53
| LOD = 3.3(SD of the lowest concentration/SLOPE) | (4) |
| LOQ = 10(SD of the lowest concentration/SLOPE) | (5) |
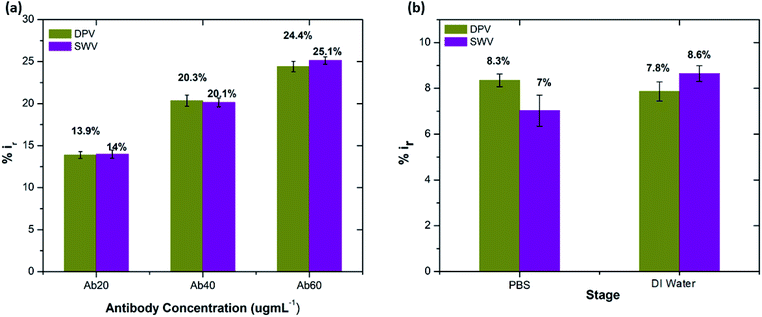
Table S3† shows the list of LODs and LOQs obtained for the electrodes functionalised with different concentrations of antibody. Sensors functionalised with 20 μg mL−1 antibody show the lowest LOD, 1.4 pg mL−1 and 4.25 pg mL−1 LOQ. It is observed that the LOD increased with increase in the concentration of the surface bound antibodies, indicating the significance of achieving an optimal antibody loading onto the sensor surface. Our sensor shows higher sensitivity over the other existing label-free biosensors (Table 1).
| Electrode materials | Receptor system | Detection techniques | Limit of detection (pg mL−1) | Ref. |
|---|---|---|---|---|
| a *Values were converted from pM and nM to pg mL−1. **SC-D17, NT-11H3, NT-8G7, Cov-4G8 and Cov-12F4. | ||||
| Au | Anti-Aβ(1–42)/AuNPs/MPA/Au electrode | EIS, SWV | 5.2 | 16 |
| SiO2 | Anti-Aβ(1–16)/glutaraldehyde/PVP-CHO/3-aminopropyl triethoxysilane | Electrical impedance | — | 50 |
| Graphene/rGO SPE | Anti Aβ(1–42) antibody (H31L21)/Pyr-NHS/graphene-rGO SPE | DPV | 10.8* | 40 |
| Si/SiO2 | (Range of antibodies**)/poly(DMA-co-NAS-co-MAPS)/silicon microarrays | Fluorescence | 73 | 11 |
| Carbon ink electrode of DEP chip | Monoclonal Aβ antibody/protein G/MHDA SAM/AuNPs/carbon DEP chip | EIS | 2573.02* | 21 |
| ICE-Au | Anti-Aβ antibody (MOAB-2)/EDC-NHS/MHA SAM/ICE | EIS | 70, 100 | 51 |
| Graphene SPE | Anti Aβ(1–42) antibody (mOC64)/pDAN/graphene SPE | DPV | 1.4 | This work |
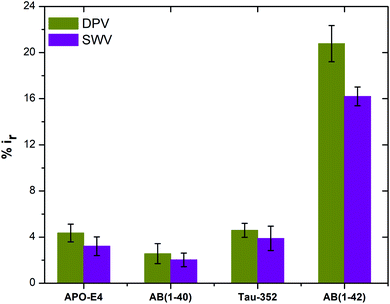 | ||
| Fig. 7 Peak current reduction %ir obtained from DPV and SWV plots for non-specific testing using APO-E4, Aβ(1–40), Tau-352 and Aβ(1–42) (n = 3). | ||
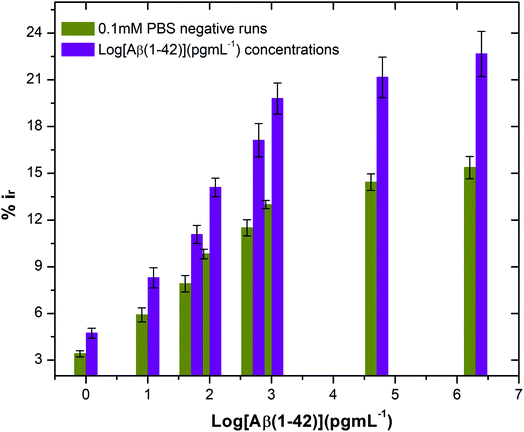
The stability of the immunosensor was also examined by storing the antibody modified SPGEs in a refrigerator at 4 °C for 1 week. The current response of the prepared immunosensor decreased by 22% after eight days, thus representing an acceptable stability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
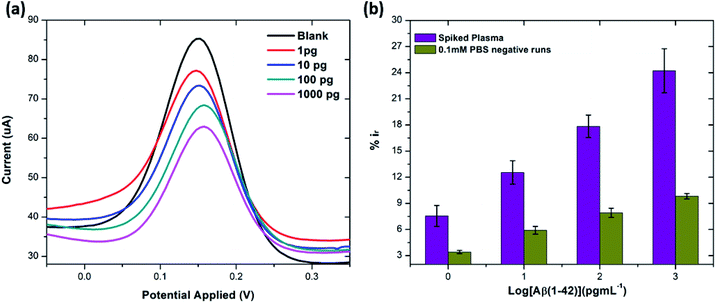
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and later spiked with known concentrations of beta amyloid peptide Aβ(1–42) including 1, 10, 100 and 1000 pg mL−1 respectively. The percentage reduction in peak current (%ir) vs. log of peptide concentrations (pg mL−1) is shown in Fig. 8. The peak current reduction (%ir) with exposure to the 0.1 mM PBS was also replotted here to show a comparison with the human plasma results. A significant reduction of DPV signal (up to 27%) in spiked samples was observed as compared to the PBS only samples (up to 9.8%). These results validate the applicability of the proposed method in clinical sample analysis.
100 and later spiked with known concentrations of beta amyloid peptide Aβ(1–42) including 1, 10, 100 and 1000 pg mL−1 respectively. The percentage reduction in peak current (%ir) vs. log of peptide concentrations (pg mL−1) is shown in Fig. 8. The peak current reduction (%ir) with exposure to the 0.1 mM PBS was also replotted here to show a comparison with the human plasma results. A significant reduction of DPV signal (up to 27%) in spiked samples was observed as compared to the PBS only samples (up to 9.8%). These results validate the applicability of the proposed method in clinical sample analysis.
4. Conclusions
In summary, a simple and rapid label-free electrochemical biosensor, based on a modified graphene screen printed electrode, has been developed for highly sensitive and selective detection of Aβ(1–42). Under the optimized conditions, our proposed immunosensor exhibited excellent analytical performance for Aβ(1–42) with a wide linear range and low detection limit. Antibodies targeted against Aβ(1–42) were immobilised onto the amine surface using EDC/NHS chemistry to obtain preferential antibody orientation attachment. Furthermore, three different concentrations of anti-beta amyloid antibody were used in order to understand the effects that each concentration had on Aβ(1–42) detection sensitivity. The sensor showed negligible response to the non-specific interactions with Tau-352, Aβ(1–40) and APO-E4 proteins. It also showed excellent sensing performance for spiked human plasma samples. Our proposed immunosensor modification method can certainly be used for the detection of other proteins, with optimisation of detection platforms enabling tuning of the sensitivity range. With all these characteristics, the main limitation of our biosensor is its sensitivity towards the water and PBS only samples. Therefore, for future work we are aiming to develop a biosensor with negligible sensitivity towards these fluids.Ethical approval
Ethical approval was provided by the local ethics committee for clinical research (Milan, Italy).Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
H. A greatly acknowledges the financial support of Horizon 2020 Marie Skłodowska-Curie Actions-ITN-ETN BBDiag project under grant No. 721281. Z. T and A. D also acknowledge the joint-financial support from Welsh Government and European Commission under European Regional Development Funds (ERDF) through Sêr Cymru II Fellowships (project number: 80761-SU-100) and (project number: 663830-SU-077).References
- K. Blennow, M. J. de Leon and H. Zetterberg, Lancet, 2006, 368, 387–403 CrossRef CAS.
- J. L. Cummings and G. Cole, Jama, 2002, 287, 2335–2338 CrossRef CAS PubMed.
- J. L. Cummings, Alzheimer's Dementia, 2011, 7, e13–e44 CrossRef CAS PubMed.
- A. s. Association, Alzheimer's Dementia, 2019, 15, 321–387 CrossRef.
- D.-Y. Kang, J.-H. Lee, B.-K. Oh and J.-W. Choi, Biosens. Bioelectron., 2009, 24, 1431–1436 CrossRef CAS PubMed.
- H. Hampel, Y. Shen, D. M. Walsh, P. Aisen, L. M. Shaw, H. Zetterberg, J. Q. Trojanowski and K. Blennow, Exp. Neurol., 2010, 223, 334–346 CrossRef CAS.
- R. Craig-Schapiro, A. M. Fagan and D. M. Holtzman, Neurobiol. Dis., 2009, 35, 128–140 CrossRef CAS PubMed.
- J. Hardy and D. J. Selkoe, science, 2002, 297, 353–356 CrossRef CAS PubMed.
- T. Polvikoski, R. Sulkava, M. Haltia, K. Kainulainen, A. Vuorio, A. Verkkoniemi, L. Niinistö, P. Halonen and K. Kontula, N. Engl. J. Med., 1995, 333, 1242–1248 CrossRef CAS PubMed.
- H. Zetterberg, K. Blennow and E. Hanse, Exp. Gerontol., 2010, 45, 23–29 CrossRef CAS PubMed.
- P. Gagni, L. Sola, M. Cretich and M. Chiari, Biosens. Bioelectron., 2013, 47, 490–495 CrossRef CAS.
- S. Emadi, L. Aghababaee, M. Maghbooli, R. Veeramachaneni and P. S. Indurkar, Int J Res Med Sci., 2016, 4, 421–427 CrossRef.
- M. d. Vestergaard, K. Kerman, M. Saito, N. Nagatani, Y. Takamura and E. Tamiya, J. Am. Chem. Soc., 2005, 127, 11892–11893 CrossRef CAS.
- J. V. Rushworth, A. Ahmed, H. H. Griffiths, N. M. Pollock, N. M. Hooper and P. A. Millner, Biosens. Bioelectron., 2014, 56, 83–90 CrossRef CAS PubMed.
- J. Oh, G. Yoo, Y. W. Chang, H. J. Kim, J. Jose, E. Kim, J.-C. Pyun and K.-H. Yoo, Biosens. Bioelectron., 2013, 50, 345–350 CrossRef CAS PubMed.
- P. Carneiro, J. Loureiro, C. Delerue-Matos, S. Morais and M. do Carmo Pereira, Sens. Actuators, B, 2017, 239, 157–165 CrossRef CAS.
- E. S. Oh, M. M. Mielke, P. B. Rosenberg, A. Jain, N. S. Fedarko, C. G. Lyketsos and P. D. Mehta, J. Alzheimer's Dis., 2010, 21, 769 CAS.
- K. A. Bruggink, W. Jongbloed, E. A. Biemans, R. Veerhuis, J. A. Claassen, H. B. Kuiperij and M. M. Verbeek, Anal. Biochem., 2013, 433, 112–120 CrossRef CAS.
- H. Englund, D. Sehlin, A. S. Johansson, L. N. Nilsson, P. Gellerfors, S. Paulie, L. Lannfelt and F. E. Pettersson, J. Neurochem., 2007, 103, 334–345 CAS.
- L. Liu, Q. He, F. Zhao, N. Xia, H. Liu, S. Li, R. Liu and H. Zhang, Biosens. Bioelectron., 2014, 51, 208–212 CrossRef CAS PubMed.
- T. T. N. Lien, Y. Takamura, E. Tamiya and M. d. C. Vestergaard, Anal. Chim. Acta, 2015, 892, 69–76 CrossRef CAS PubMed.
- M. Z. Mousavi, H.-Y. Chen, H.-S. Hou, C.-Y.-Y. Chang, S. Roffler, P.-K. Wei and J.-Y. Cheng, Biosensors, 2015, 5, 98–117 CrossRef PubMed.
- A. C. H. de Castro, L. M. Alves, A. C. S. Siquieroli, J. M. Madurro and A. G. Brito-Madurro, Microchem. J., 2020, 155, 104746 CrossRef.
- Y.-S. Fang, H.-Y. Wang, L.-S. Wang and J.-F. Wang, Biosens. Bioelectron., 2014, 51, 310–316 CrossRef CAS PubMed.
- X. Zhang, Y. Shen, Y. Zhang, G. Shen, H. Xiang and X. Long, Talanta, 2017, 164, 483–489 CrossRef CAS.
- Y. Zeng, J. Bao, Y. Zhao, D. Huo, M. Chen, M. Yang, H. Fa and C. Hou, Talanta, 2018, 178, 122–128 CrossRef CAS PubMed.
- Z. O. Uygun, L. Yeniay and F. G. r. n. Sağın, Anal. Chim. Acta, 2020, 1121, 35–41 CrossRef CAS PubMed.
- M. Pournaghi-Azar and R. Ojani, Talanta, 1995, 42, 657–662 CrossRef CAS PubMed.
- R. Ojani, J. B. Raoof and S. R. H. Zavvarmahalleh, J. Solid State Electrochem., 2009, 13, 1605–1611 CrossRef CAS.
- K. Hassan, A. Hathoot, W. Ashour and M. Abdel-Azzem, J. Solid State Electrochem., 2015, 19, 1063–1072 CrossRef CAS.
- K. Jackowska, M. Skompska and E. Przyłuska, J. Electroanal. Chem., 1996, 418, 35–39 CrossRef CAS.
- J. Vidal, E. Garcıa and J. Castillo, Sens. Actuators, B, 1999, 57, 219–226 CrossRef CAS.
- J. C. Vidal, E. Garcia-Ruiz, J. Espuelas, T. Aramendia and J. R. Castillo, Anal. Bioanal. Chem., 2003, 377, 273–280 CrossRef CAS PubMed.
- M. A. Rahman, M.-S. Won, N.-H. Kwon, J.-H. Yoon, D.-S. Park and Y.-B. Shim, Anal. Chem., 2008, 80, 5307–5311 CrossRef CAS PubMed.
- A. A. Abdelwahab, H.-M. Lee and Y.-B. Shim, Anal. Chim. Acta, 2009, 650, 247–253 CrossRef CAS PubMed.
- B. H. Nguyen, B. T. Nguyen, H. Van Vu, C. Van Nguyen, D. T. Nguyen, L. T. Nguyen, T. T. Vu and L. Dai Tran, Curr. Appl. Phys., 2016, 16, 135–140 CrossRef.
- A. K. Geim and K. S. Novoselov, in Nanoscience and Technology: A Collection of Reviews from Nature Journals, World Scientific, 2010, pp. 11–19 Search PubMed.
- Z. Tehrani, G. Burwell, M. M. Azmi, A. Castaing, R. Rickman, J. Almarashi, P. Dunstan, A. M. Beigi, S. Doak and O. Guy, 2D Materials, 2014, 1, 025004 CrossRef CAS.
- M.-S. Chae, J. Kim, D. Jeong, Y. Kim, J. H. Roh, S. M. Lee, Y. Heo, J. Y. Kang, J. H. Lee and D. S. Yoon, Biosens. Bioelectron., 2017, 92, 610–617 CrossRef CAS PubMed.
- J. Sethi, M. Van Bulck, A. Suhail, M. Safarzadeh, A. Perez-Castillo and G. Pan, Microchim. Acta, 2020, 187, 1–10 CrossRef PubMed.
- A. Devadoss, R. Forsyth, R. Bigham, H. Abbasi, M. Ali, Z. Tehrani, Y. Liu and J. O. Guy, Biosensors, 2019, 9, 16 CrossRef CAS PubMed.
- G. Wu, X. Tang, Z. Lin, M. Meyyappan and K. W. C. Lai, 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), IEEE, pp. 242–245 Search PubMed.
- E. House, J. Collingwood, A. Khan, O. Korchazkina, G. Berthon and C. Exley, J. Alzheim. Dis., 2004, 6, 291–301 CAS.
- E. B. Barros, N. S. Demir, A. G. Souza Filho, J. Mendes Filho, A. Jorio, G. Dresselhaus and M. S. Dresselhaus, Phys. Rev. B, 2005, 71, 165422 CrossRef.
- Z. Tehrani, S. P. Whelan, B. Mostert, J. V. Paulin, M. M. Ali, E. D. Ahmadi, C. F. de Oliveira Graeff, O. J. Guy and D. Gethin, 2D Materials, 2020 Search PubMed.
- D. M. Eby, K. Artyushkova, A. K. Paravastu and G. R. Johnson, J. Mater. Chem., 2012, 22, 9875–9883 RSC.
- M. A. Booth, K. Kannappan, A. Hosseini and A. Partridge, Langmuir, 2015, 31, 8033–8041 CrossRef CAS PubMed.
- W. Wang, L. Ge, X. Sun, T. Hou and F. Li, ACS Appl. Mater. Interfaces, 2015, 7, 28566–28575 CrossRef CAS PubMed.
- F. Islam, M. H. Haque, S. Yadav, M. N. Islam, V. Gopalan, N.-T. Nguyen, A. K. Lam and M. J. A. Shiddiky, Sci. Rep., 2017, 7, 133 CrossRef PubMed.
- Y. K. Yoo, J. Kim, G. Kim, Y. S. Kim, H. Y. Kim, S. Lee, W. W. Cho, S. Kim, S.-M. Lee and B. C. Lee, Sci. Rep., 2017, 7, 1–10 CrossRef PubMed.
- H. T. N. Le, J. Park, S. R. Chinnadayyala and S. Cho, Biosens. Bioelectron., 2019, 144, 111694 CrossRef PubMed.
- A. S. Lorenzetti, T. Sierra, C. E. Domini, A. G. Lista, A. G. Crevillen and A. Escarpa, Sensors, 2020, 20, 76 CrossRef CAS PubMed.
- G. Palla, K. Malecka, W. Dehaen, J. Radecki and H. Radecka, Bioelectrochemistry, 2021, 137, 107643 CrossRef CAS PubMed.
- H. Kim, J. U. Lee, S. Kim, S. Song and S. J. Sim, ACS Sens., 2019, 4, 595–602 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0na00801j |
| This journal is © The Royal Society of Chemistry 2021 |