Open Access Article
Open Access ArticleA new strategy for large-scale synthesis of Na0.5Bi0.5TiO3 nanowires and their application in piezocatalytic degradation†
Rui
Huang
,
Jiang
Wu
 *,
Enzhu
Lin
*,
Enzhu
Lin
 ,
Zihan
Kang
,
Zihan
Kang
 ,
Ni
Qin
,
Ni
Qin
 * and
Dinghua
Bao
* and
Dinghua
Bao

State Key Laboratory of Optoelectronic Materials and Technologies, School of Materials Science and Engineering, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: wujiang1990@126.com; qinni2@mail.sysu.edu.cn
First published on 1st April 2021
Abstract
Developing new techniques that can synthesize one-dimensional piezoelectric materials on a large scale is of great significance for boosting piezocatalytic applications. In this work, we proposed a high-efficiency template hydrothermal method for large-scale synthesis of piezoelectric Na0.5Bi0.5TiO3 (NBT) nanowires. By ion-exchange with Bi3+, Na2Ti3O7 template nanowires can be easily and entirely transformed to NBT. The piezocatalytic activity of the NBT nanowires was thoroughly investigated with respect to their capability to degrade typical organic pollutants, including Rhodamine B, methylene blue, methyl orange, tetracycline hydrochloride, phenol, and bisphenol A. The NBT nanowires exhibited the highest efficiency in piezocatalytic degradation of Rhodamine B, which was completely decomposed within 80 min (rate constant ∼0.0575 min−1). The electron spin resonance spin-trapping technique and active species capture experiments were employed to characterize free radicals. The present work is advantageous for the high yield of NBT nanowires and the excellent piezocatalytic performance. The reported template hydrothermal method can potentially be extended to the synthesis of other perovskite nanowires.
Introduction
With the rapid development of industrialization, environmental water contamination has become increasingly prominent.1,2 Various advanced oxidation processes (AOPs)3 such as the Fenton reaction,4 photolysis,5 photocatalysis6 and processes based on ozone7 have been developed to remove organic compounds from wastewater. As a novel AOP technology with cost-competitive and environmentally friendly properties, piezocatalysis has received widespread attention.8 A typical piezocatalytic process usually adopts a piezoelectric material in powder form as the piezocatalyst. When a varying mechanical force is applied to the piezocatalyst, a polarization field will be generated in the piezocatalyst. The polarization field can lead to separation of free charges and production of reactive oxygen species (ROS) through redox reactions.9Representative piezocatalysts include BaTiO3,10 Pb(Zr0.52Ti0.48)O3,11 Pb(Mg1/3Nb2/3)O3–xPbTiO3,12 Bi2WO6,13 KNbO3,14 and NaNbO3,15 which have been proved to be effective in degrading various organic pollutants. Unfortunately, most of the reported piezocatalysts suffer from low efficiency in actual applications. Much effort has been devoted to improving the functional performance of piezocatalysts. It is generally believed that the large piezo-electric coefficient, highly flexible structure and abundant active sites of piezocatalysts are beneficial. More recently, electrical conductivity was regarded as another vital factor that influenced the piezocatalytic performance.16–19 It was suggested that the high electrical conductivity helped to accelerate charge transfer in the piezocatalytic process and thus enhanced the piezocatalytic activity.20
Na0.5Bi0.5TiO3 (NBT) is an attractive piezoelectric material because of its large piezoelectric coefficient (d33 value of ∼58 pC N−1)21,22 and high Curie temperature (340 °C).23 In addition, it is reported that the relatively high electrical conductivity (∼10−5 S m−1) of NBT may be favorable for piezocatalytic applications.20 Recently, research studies on the piezocatalytic performance of NBT have been carried out.24–26 It is worth noting that most of the reported NBT piezocatalysts are microparticles24 or multi-crystalline nanofibers.25 The piezocatalytic performance of NBT single-crystalline nanowires has never been reported. It is highly expected that single-crystalline nanowires may have higher piezocatalytic activity because of their higher flexibility and larger specific surface area.
In this work, NBT single-crystalline nanowires with uniform morphology were prepared via a template hydrothermal method using Na2Ti3O7 nanowires as the template. The piezocatalytic activity of the NBT nanowires was evaluated from the degradation efficiency for different kinds of organic pollutants. Piezocatalytic mechanisms were also systematically investigated through quenching experiments and ESR characterization. This study may open up a promising approach to design and synthesize piezoelectric nanowires for environmental purification.
Experimental section
Preparation of Na0.5Bi0.5TiO3 nanowires
NBT nanowires were synthesized based on a template hydrothermal reaction. The first step in the reaction is to synthesize Na2Ti3O7 nanowires,27 which act as the titanium precursor for the NBT nanowires. First, 1.5974 g of TiO2 was dispersed in 70 mL of 10 mol L−1 NaOH solution. The mixed solution was then sealed in a 100 mL Teflon-lined stainless-steel autoclave and heated at 240 °C for 24 h. Following this reaction, the resulting Na2Ti3O7 nanowires were washed by repeated centrifugation with DI water, until the pH reached around 7. Finally, the product was dried at 70 °C for 12 h.The NBT nanowires were subsequently synthesized through a hydrothermal reaction, in which the prepared Na2Ti3O7 nanowires were employed as a Ti4+ source and Bi(NO3)3·5H2O was employed as a Bi3+ source. First, 0.4851 g of Bi(NO3)3·5H2O and 0.2010 g of Na2Ti3O7 were dispersed in NaOH solution. The mixed solution was then sonicated for 20 min and stirred for 30 min to form a uniform suspension. The final mixture was hydrothermally treated at 200–240 °C for 6–24 h in a 100 mL Teflon-lined stainless-steel autoclave with a 70% filling factor. The reaction temperature, reaction time, and mineralizer concentration for the synthesis of pure NBT nanowires were investigated in detail. After the reaction, the formed white precipitate was collected by centrifugation, washed several times with distilled water and 0.2 mol L−1 acetic acid, and finally dried at 60 °C for 12 h.
Characterization
X-ray diffraction (XRD, Rigaku D/MAX 2200 VPC) with Cu Kα1 radiation was employed to investigate the structure of the crystalline samples. The morphology of the samples was observed by field-emission scanning electron microscopy (SEM, Hitachi SU8010). Energy dispersive spectroscopy mapping (EDS-mapping) was carried out using a Zeiss G500 equipped with an energy spectrum analyzer. High resolution transmission electron microscopy (HRTEM) investigations were performed using a JEOL JEM-2100 TEM (with an acceleration voltage of 200 kV). X-ray photoelectron spectroscopy (XPS) was carried out using a high-performance electron spectrometer (Thermo Scientific ESCALAB 250Xi) with an Al Kα X-ray source. All binding energies were calibrated using the C 1s peak with a fixed value of 284.8 eV.Piezocatalytic activity test
The piezocatalytic ability of the prepared NBT samples was systematically evaluated from the degradation of Rhodamine B (5 mg L−1) under ultrasonic vibration (80 W, ∼40 kHz). In a typical piezocatalytic experiment, 0.1 g NBT sample was added into a 100 mL solution of organic pollutants. Prior to the ultrasonic vibration, the suspension was magnetically stirred in the dark for 30 min to reach the adsorption–desorption equilibrium between NBT and the pollutants. The temperature of the piezocatalytic reaction system was kept at room temperature (25 °C) by using a water cooling circulation system. During the whole degradation process, 4 mL of the suspension was collected and centrifuged to remove the catalysts. The ultraviolet-visible (UV-vis) absorption spectrum of the supernatant was analyzed using a Shimadzu UV-3600 spectrophotometer. Furthermore, methylene orange (MO, 5 mg L−1), methylene blue (MB, 5 mg L−1), bisphenol A (BPA, 5 mg L−1), tetracycline hydrochloride (TH, 5 mg L−1), and phenol (5 mg L−1) were also employed to evaluate the piezocatalytic activity of NBT nanowires.Detection of free radicals
In situ capture experiments were conducted to investigate the active species generated in the piezocatalytic process. Specifically, 2 mmol L−1 disodium ethylene diamine tetra-acetate dehydrate (EDTA-2Na), 10 mmol L−1tert-butyl alcohol (TBA), and 0.15 mmol L−1 benzoquinone (BQ) were employed as scavengers for holes (h+), hydroxyl radicals (·OH) and superoxides (·O2−), respectively.Furthermore, the electron spin resonance (ESR) technique was also employed to detect active free radicals such as radical ·OH and radical ·O2− with the assistance of 5,5-dimethyl-1-pyrroline N-oxide (DMPO). ESR investigations were conducted on a Bruker A300-10-12 spectrometer. Specifically, for the detection of radical ·OH, the as-prepared sample was dispersed in deionized water, and for the detection of radical ·O2−, the as-prepared sample was dispersed in dimethyl sulphoxide (DMSO).
Results and discussion
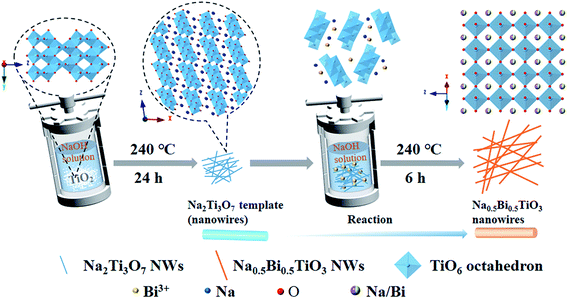
In hydrothermal synthesis, the properties of precursors have a major effect on the morphology of the products. Commonly used Ti precursors, including tetrabutyl titanate,28 titanium dioxide,29 and titanium tetrachloride,30 often lead to the formation of coarse agglomerated particles because of their low solubility or hydrolysis properties.31 The layered titanate Na2Ti3O7 emerged as a great substitute for these precursors. The crystal structure of Na2Ti3O7 is composed of TiO6 octahedra, which are joined by corners and edges to form a zig–zag structure, with rectangular tunnels along the z-axis. The open-layered structure of Na2Ti3O7 is favorable for topochemical transformation.32 In our previous work,33 Na2Ti3O7 was employed as the template for growing bismuth titanate under hydrothermal conditions. A similar process was adopted in this work to synthesize NBT nanowires.Fig. 1 schematically depicts the processing steps for growing NBT nanowires. In the first step, Na2Ti3O7 nanowires were prepared via a simple hydrothermal method. In the following step, Na2Ti3O7 and Bi(NO3)3·5H2O were added to NaOH solution. During the reaction, Bi3+ ions will reduce the stability of the layered titanate Na2Ti3O7 and break the connection between the TiO6 octahedra.34 The dissociated TiO6 octahedra will then react with Bi3+ and Na+ ions to form perovskite-type NBT.
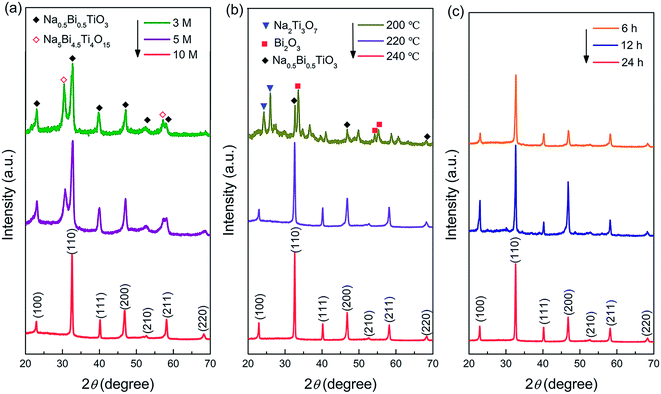
The XRD pattern of the synthesized Na2Ti3O7 nanowires is shown in Fig. S1,† which demonstrates that pure-phase Na2Ti3O7 (JCPDS No. 31–1329) has been formed. Fig. 2a–c show the XRD patterns of the NBT samples prepared under different reaction conditions. As shown in Fig. 2a, a composed phase structure was obtained in the samples synthesized at lower NaOH concentrations (3–5 mol L−1). The major phase can be identified as NBT with a monoclinic or pseudo hexagonal structure (JCPDS No. 46-0001), and the additional peaks can be ascribed to Na5Bi4.5Ti4O15. When increasing the NaOH concentration to 10 mol L−1, the secondary phase disappeared completely, and pure-phase NBT was obtained. Fig. 2b illustrates the influence of reaction temperature (200–240 °C) on the phase constitution of the product. The NaOH concentration was 10 mol L−1. In the synthesis at 200 °C for 24 h, the NBT phase was formed along with Na2Ti3O7 and Bi2O3 secondary phases. The pure-phase product was obtained by increasing the reaction temperature to over 220 °C. Based on these results, one may conclude that the high mineralizer concentration and high reaction temperature are beneficial for the hydrothermal synthesis of NBT. We found that the reaction time (6 h to 24 h) has little influence on the phase constitution of the hydrothermal product (Fig. 2c). For the NBT samples synthesized after 12 hours (NBT-12), the peak intensity of (110) and (220) was slightly enhanced, which may be due to the fact that the NBT-12 samples were a mixture of nanobelts and nanoparticles (Fig. S2†). The larger area of nanobelts results in the (100) and (200) facets being more easily scanned by X-ray. The NBT samples obtained from the 24 h-synthesis are crystallized into micro-scale particles (Fig. S3†). Pure-phase NBT nanowires were finally obtained by reducing the hydrothermal reaction time to 6 h.
The morphology of the pure-phase NBT nanowires can be clearly observed by SEM. Fig. 3a shows the SEM image of the Na2Ti3O7 template nanowires, which are straight and smooth with an average lateral dimension of ∼250 nm and a longitude larger than 5 μm. After reacting with Bi(NO3)3 solution, the Na2Ti3O7 template nanowires were entirely transformed to uniform and slender NBT nanowires (Fig. 3b). The lateral dimension of the nanowires is about 100 nm. The distribution of elements in the NBT nanowires was analyzed by the EDX spectroscopy elemental mapping technique. As shown in Fig. 3c, all the involved elements (Bi, Na, Ti, and O) are homogeneously distributed and well-matched with each other. The analytical composition of the obtained Na0.5Bi0.5TiO3 was characterized using EDS spectra (Fig. S4†), in which the ratio of Na, Bi, and Ti in NBT nanowires is 22.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22.8
22.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55. The TEM images (Fig. 3d–e) illustrate the straight and smooth surface of the NBT nanowires, indicating the high-quality of the present material. The amplified HRTEM image (Fig. 3f) taken from the selected area of Fig. 3e shows clear lattice fringes of (113) planes (d = 1.78 Å), which are oriented along the extension direction of the nanowire.
55. The TEM images (Fig. 3d–e) illustrate the straight and smooth surface of the NBT nanowires, indicating the high-quality of the present material. The amplified HRTEM image (Fig. 3f) taken from the selected area of Fig. 3e shows clear lattice fringes of (113) planes (d = 1.78 Å), which are oriented along the extension direction of the nanowire.
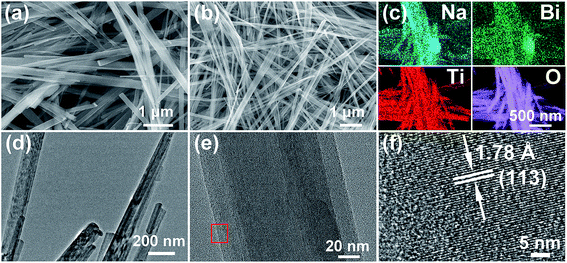 | ||
| Fig. 3 SEM images of (a) Na2Ti3O7 and (b) Na0.5Bi0.5TiO3 nanowires; (c) EDS mapping, (d and e) TEM, and (f) HRTEM of Na0.5Bi0.5TiO3 nanowires. | ||
XPS investigation was carried out to elucidate the valence state of the as-prepared NBT nanowires. The full survey XPS spectrum in Fig. S5† clearly shows the existence of Na, Bi, Ti and O elements in the sample. The high-resolution XPS spectrum of Na 1s (Fig. 4a) shows a peak at 1070.75 eV. Fig. 4b presents two main peaks at 158.75 and 164.05 eV, which correspond to Bi 4f7/2 and Bi 4f5/2, respectively. Fig. 4c shows the high-resolution XPS spectrum of Ti 2p. The Ti 2p3/2 and Ti 2p1/2 peaks are located at 457.9 eV and 464 eV, respectively, in accordance with the data of Ti4+. The XPS spectrum of O 1s in Fig. 4d is deconvoluted into three peaks, which can be assigned to lattice oxygen (OL, 529.2 eV), defective oxygen (OV, 531.1 eV) and surface adsorbed oxygen (OC, 533.8 eV), respectively.35 The presence of oxygen vacancies could efficiently promote the adsorption and activation of O2 on the surface of the piezocatalyst and consequently enhance piezocatalytic activity.36 Therefore, it is speculated that the oxygen vacancy defects in NBT may contribute to its excellent piezocatalytic performance. However, the effect of oxygen defects on the energy band structure and the mechanism of improving the piezocatalytic performance need to be further studied.
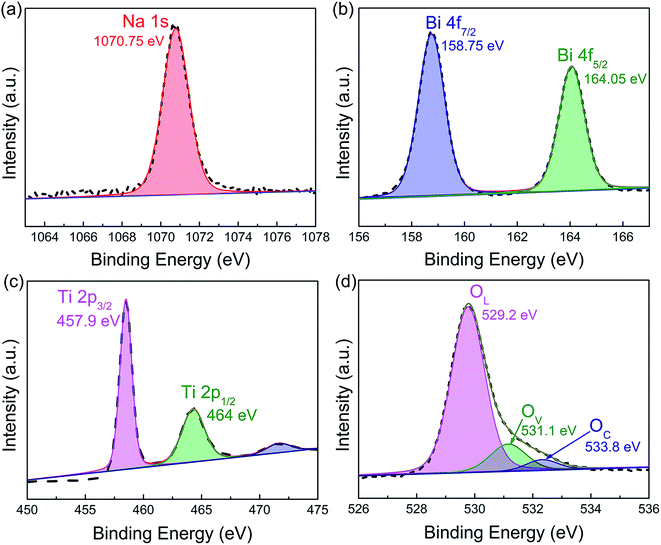 | ||
| Fig. 4 High-resolution XPS spectra of the as-prepared Na0.5Bi0.5TiO3 nanowires: (a) Na 1s, (b) Bi 4f, (c) Ti 2p, and (d) O 1s. | ||
PFM testing was employed to characterize the piezoelectricity of the prepared NBT nanowires. The experimental setup of PFM is illustrated in the inset of Fig. 5a. By applying a tip bias of ±3 V, localized point-to-point piezo-responses of the samples were probed. The PFM amplitude–voltage butterfly curve and phase–voltage hysteresis loop are given in Fig. 5. The butterfly-shaped curve appears symmetrical, indicating the certain piezoelectric properties. On the other hand, the phase hysteresis loop presents about 180° domain switching at ±3 V (Fig. 5b).
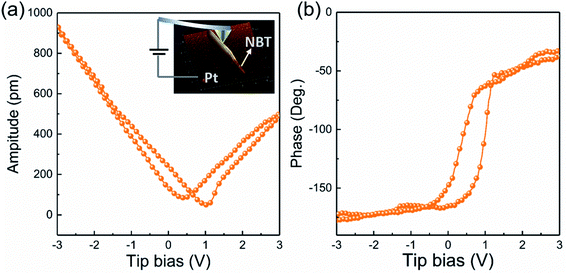 | ||
| Fig. 5 (a) Amplitude butterfly curve (the inset: experimental setup of PFM) and (b) phase hysteresis of NBT NWs. | ||
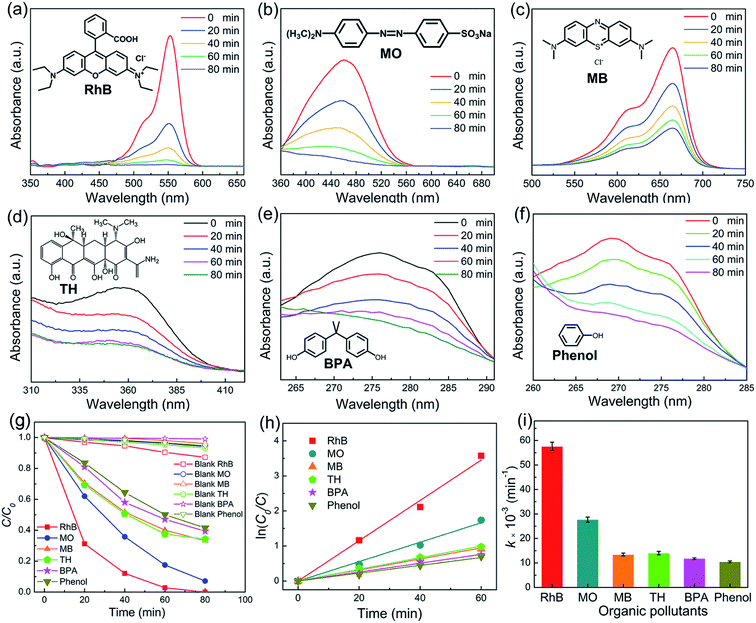
To study the piezocatalytic performance of NBT nanowires, diverse industrial pollutants, including anionic dyes (RhB and MB), a cationic dye (MO), and neutral stubborn pollutants (TH, BPA and phenol), are used as target contaminants. Fig. 6a–f display the typical absorption spectra of the aqueous solutions of these pollutants. Piezocatalytic experiments without using NBT were carried out. It was clear that the pollutants could hardly be degraded without NBT under ultrasonic vibration. After being treated with ultrasonic vibration for 80 min, RhB, MO, MB, TH, BPA and phenol were removed 100%, 92.8%, 66.7%, 65.7%, 60.6% and 58.5% (Fig. 6g), respectively. The results demonstrated that the NBT nanowires are effective in degrading various kinds of organic pollutants. The first-order rate constants for the piezocatalytic degradation of the target pollutants were evaluated by refinement of the ln(C0/C) − t plot (Fig. 6h). The results are presented in Fig. 6i. The highest rate constant (0.0575 min−1) was obtained for RhB. The piezocatalytic performance of NBT samples in this work was compared with that of other reported NBT materials. As shown in Table 1, the NBT nanowires prepared by the template hydrothermal method show the highest piezocatalytic performance.
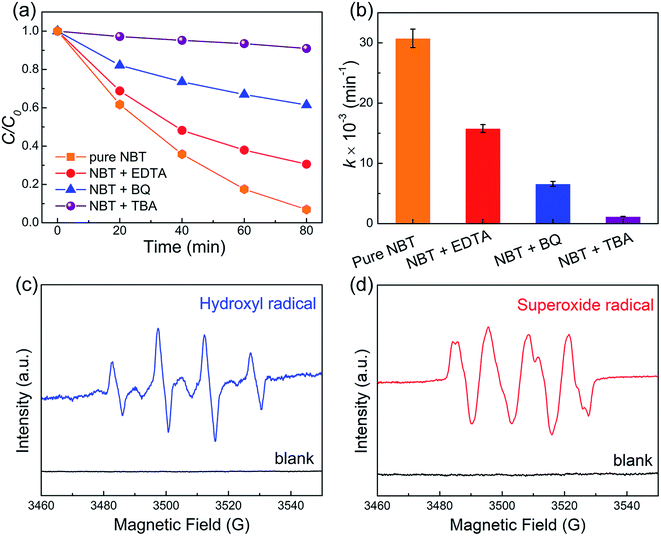
To distinguish the role of active radicals in the piezocatalytic degradation of MO over NBT, trapping experiments were conducted using TBA, BQ and EDTA–2Na as radical scavengers. As revealed in Fig. 7a and b, the piezocatalytic performance was inhibited greatly upon introducing TBA, which indicated that ·OH was the primary active species. Furthermore, the piezocatalytic oxidation ability of NBT was also restrained by the addition of BQ and EDTA, indicating that ·O2− and h+ played an auxiliary role in the piezocatalytic degradation of MO over NBT. ESR characterization was carried out to confirm the existence of ·OH and ·O2−. The nitrone spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was introduced into the aqueous and dimethyl sulphoxide (DMSO) dispersions of the NBT catalyst for probing hydroxyl radicals (·OH) and superoxide radicals (·O2−), respectively. Well-defined signals (Fig. 7c and d) of the specific DMPO–·OH and DMPO–·O2− adducts were observed after being treated with ultrasound for 5 minutes. These results confirmed the generation of ·OH and ·O2− radicals in the piezocatalytic process.
Conclusions
In summary, a novel template hydrothermal reaction for the large-scale synthesis of NBT nanowires has been developed. The as-prepared NBT nanowires exhibited superior piezocatalytic performance for the degradation of various organic pollutants. Particularly, the highest degradation rate was obtained for RhB (100% in 80 min). The active species capture experiments and ESR measurements reveal that ·OH, ·O2− and h+ play important roles in the piezocatalytic process in the order of ·OH > ·O2− > h+. In view of the superior piezocatalytic performance of NBT nanowires, the template hydrothermal method developed here has great potential for large scale synthesis of other piezoelectric perovskite ATiO3 nanowire nanostructures.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (2020M672959), National Natural Science Foundation of China (No. 51872335 and 51202298), and Natural Science Foundation of Guangdong Province, China (No. 2015A030311019).References
- F. Rudroff, M. D. Mihovilovic, H. Gröger, R. Snajdrova, H. Iding and U. T. Bornscheuer, Nat. Catal., 2018, 1, 12–22 CrossRef.
- H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- J. J. Pignatello, E. Oliveros and A. MacKay, Crit. Rev. Environ. Sci. Technol., 2006, 36, 1–84 CrossRef CAS.
- L. Gomathi Devi, S. Girish Kumar, K. Mohan Reddy and C. Munikrishnappa, J. Hazard. Mater., 2009, 164, 459–467 CrossRef CAS PubMed.
- E. J. Rosenfeldt and K. G. Linden, Environ. Sci. Technol., 2004, 38, 5476–5483 CrossRef CAS PubMed.
- D. Chatterjee and S. Dasgupta, J. Photochem. Photobiol., C, 2005, 6, 186–205 CrossRef CAS.
- L. Bilińska, K. Blus, M. Gmurek and S. Ledakowicz, Chem. Eng. J., 2019, 358, 992–1001 CrossRef.
- H. You, Z. Wu, L. Zhang, Y. Ying, Y. Liu, L. Fei, X. Chen, Y. Jia, Y. Wang, F. Wang, S. Ju, J. Qiao, C.-H. Lam and H. Huang, Angew. Chem., Int. Ed., 2019, 58, 11779–11784 CrossRef CAS PubMed.
- S. Tu, Y. Guo, Y. Zhang, C. Hu, T. Zhang, T. Ma and H. Huang, Adv. Funct. Mater., 2020, 30, 2005158 CrossRef CAS.
- J. Wu, Q. Xu, E. Lin, B. Yuan, N. Qin, S. K. Thatikonda and D. Bao, ACS Appl. Mater. Interfaces, 2018, 10, 17842–17849 CrossRef CAS PubMed.
- T. P. Pham Thi, Z. Yan, G. Nick, K. Hamideh, H. D. Nguyen Phuc, Z. Xuefan, Z. Dou, Z. Kechao, D. Steve and B. Chris, Iscience, 2020, 23, 101095 CrossRef PubMed.
- B. Yuan, J. Wu, N. Qin, E. Lin, Z. Kang and D. Bao, Applied Materials Today, 2019, 17, 183–192 CrossRef.
- Z. Kang, N. Qin, E. Lin, J. Wu, B. Yuan and D. Bao, J. Cleaner Prod., 2020, 261, 121125 CrossRef CAS.
- D. Yu, Z. Liu, J. Zhang, S. Li, Z. Zhao, L. Zhu, W. Liu, Y. Lin, H. Liu and Z. Zhang, Nano Energy, 2019, 58, 695–705 CrossRef CAS.
- S. Singh and N. Khare, Nano Energy, 2017, 38, 335–341 CrossRef CAS.
- L. Guo, C. Wang, X. Luo, G. Cui and H. Li, Chem. Commun., 2010, 46, 5960–5962 RSC.
- O. Y. Podyacheva and Z. R. Ismagilov, Catal. Today, 2015, 249, 12–22 CrossRef CAS.
- Y. Zhang, T. Mori, J. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294–6295 CrossRef CAS PubMed.
- D. Voiry, J. Yang and M. Chhowalla, Adv. Mater., 2016, 28, 6197–6206 CrossRef CAS PubMed.
- Z. Zhao, L. Wei, S. Li, L. Zhu, Y. Su, Y. Liu, Y. Bu, Y. Lin, W. Liu and Z. Zhang, J. Mater. Chem. A, 2020, 8, 16238–16245 RSC.
- B. K. Barick, R. N. P. Choudhary and D. K. Pradhan, Mater. Chem. Phys., 2012, 132, 1007–1014 CrossRef CAS.
- X. Liu and X. Tan, Adv. Mater., 2016, 28, 574–578 CrossRef CAS PubMed.
- P. Marchet, E. Boucher, V. Dorcet and J. P. Mercurio, J. Eur. Ceram. Soc., 2006, 26, 3037–3041 CrossRef CAS.
- R. Zhang, X. Wu, Y. Li, W. Shao, Y. Zhang, Z. Liu, J. Nie, J. Tan and W. Ye, RSC Adv., 2020, 10, 7443–7451 RSC.
- D. Liu, Y. Song, Z. Xin, G. Liu, C. Jin and F. Shan, Nano Energy, 2019, 65, 104024 CrossRef CAS.
- G. Cilaveni, K. V. Ashok Kumar, S. S. K. Raavi, C. Subrahmanyam and S. Asthana, J. Alloys Compd., 2019, 798, 540–552 CrossRef CAS.
- Y. Zhang, W. Fu, H. Yang, M. Li, Y. Li, W. Zhao, P. Sun, M. Yuan, D. Ma, B. Liu and G. Zou, Sens. Actuators, B, 2008, 135, 317–321 CrossRef CAS.
- Y. Liu, Y. Lu and S. Dai, J. Alloys Compd., 2009, 484, 801–805 CrossRef CAS.
- H. Zhang, L. Liu, M. Zhu, Y. Hou, R. Wang and H. Yan, Int. J. Appl. Ceram. Technol., 2016, 13, 569–578 CrossRef CAS.
- H. Xu, L. Gao and J. Guo, J. Am. Ceram. Soc., 2002, 85, 727–729 CrossRef CAS.
- N. Bao, L. Shen, G. Srinivasan, K. Yanagisawa and A. Gupta, J. Phys. Chem. C, 2008, 112, 8634–8642 CrossRef CAS.
- A. Rudola, K. Saravanan, C. W. Mason and P. Balaya, J. Mater. Chem. A, 2013, 1, 2653–2662 RSC.
- J. Wu, N. Qin, E. Lin, B. Yuan, Z. Kang and D. Bao, Nanoscale, 2019, 11, 21128–21136 RSC.
- G. Xu, Y. Yang, H. Bai, J. Wang, H. Tian, R. Zhao, X. Wei, X. Yang and G. Han, CrystEngComm, 2016, 18, 2268–2274 RSC.
- K. K. Bharathi, B. Moorthy, H. K. Dara, L. Durai and D. K. Kim, J. Mater. Sci., 2019, 54, 13236–13246 CrossRef CAS.
- P. L. Wang, X. Y. Li, S. Y. Fan, X. Chen, M. C. Qin, D. Long, M. O. Tad and S. M. Liu, Appl. Catal., B, 2020, 279, 119340 CrossRef CAS.
- R. Zhang, X. Wu, Y. Li, W. Shao, Y. Zhang, Z. Liu, J. Nie, J. Tan and W. Ye, RSC Adv., 2020, 10, 7443–7451 RSC.
- D. Liu, Y. Song, Z. Xin, G. Liu, C. Jin and F. Shan, Nano Energy, 2019, 65, 104024 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00024a |
| This journal is © The Royal Society of Chemistry 2021 |