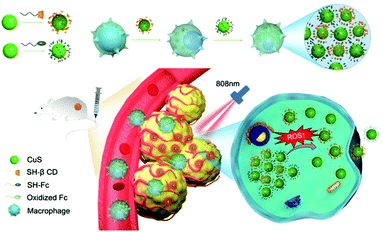
Macrophage-hitchhiking supramolecular aggregates of CuS nanoparticles for enhanced tumor deposition and photothermal therapy†
Junyan
Li‡
a,
Qian
Cheng‡
a,
Ludan
Yue‡
 a,
Cheng
Gao
a,
Cheng
Gao
 a,
Jianwen
Wei
a,
Yuanfu
Ding
a,
Yitao
Wang
a,
Ying
Zheng
a,
Jianwen
Wei
a,
Yuanfu
Ding
a,
Yitao
Wang
a,
Ying
Zheng
 ab and
Ruibing
Wang
ab and
Ruibing
Wang
 *ab
*ab
aState Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau, China. E-mail: rwang@um.edu.mo
bMoE Frontiers Science Center for Precision Oncology, University of Macau, Macau, China
First published on 1st September 2021
Abstract
In this design, small CuS nanoparticles (NPs) were intracellularly self-assembled into large supramolecular aggregates via host–guest interactions between sequentially internalized β-cyclodextrin-capped CuS NPs and ferrocene-capped CuS NPs inside macrophages, thus the efflux of CuS NPs was significantly inhibited during the macrophage-hitchhiking delivery. Biodistribution studies in mice confirmed the dramatically enhanced deposition of CuS NPs in the tumor tissue of mice injected with macrophages carrying intracellular CuS aggregates, in comparison to that of mice treated with macrophages carrying CuS NPs. In response to the inflammatory tumor microenvironment, the oxidation of ferrocene would dissociate the β-cyclodextrin–ferrocene host–guest pair, driving disassembly of the CuS aggregates and release of small CuS NPs for deep tissue penetration and enhanced photothermal therapy. This precisely controlled intracellular self-assembly and disassembly of the nanomedicine inside macrophages provides a novel cell-hitchhiking delivery strategy that not only minimizes premature leakage of the nanomedicine but also greatly improves the delivery efficiency and tumor penetration for safe, effective tumor therapy.
New conceptsCells have recently emerged as promising carriers of drugs/nanomedicines (via cellular internalization) to improve the delivery efficiency to the targeted tissue. However, excessive drug/nanomedicine efflux and exocytosis during systemic circulation has presented a key challenge for cell-based carriers. Herein, we developed an unprecedented macrophage-hitchhiking delivery system based on supramolecularly self-assembled/aggregated nanomedicines inside macrophages, to significantly reduce premature loss during systemic delivery, and specifically release the nanomedicine in the tumor in response to the inflammatory environment that may disassemble the supramolecular aggregates. This design provides a novel cell-hitchhiking delivery strategy that not only minimizes premature leakage of the nanomedicine but also greatly improves the delivery efficiency and tumor penetration for safe, effective tumor therapy. |
Cancer is one of the leading causes of death in humans and much effort has been devoted to the development of new cancer therapeutic technology.1,2 Recently, photothermal therapy has attracted increasing attention as a minimally invasive, yet effective cancer treatment modality.3–5 Among a variety of photosensitizers, small-sized copper sulfide nanoparticles (CuS NPs) have been extensively studied due to their high photothermal conversion efficiency, low cost, and ease of preparation and functionalization.6,7 However, CuS NPs are often captured by the endothelial reticular system (RES) or quickly cleared via the kidneys, leading to poor accumulation in the tumor.8–10 Therefore, new delivery systems or strategies are urgently needed to transport the functional nanomaterials to the tumor with high efficiency.
Tumor tissues, due to their inflammatory nature, often attract immune cells, thus various immune cells have been used as “Trojan Horses”, with internalized medicines, for tumor-specific drug/nanomedicine delivery.11–13 Among them, macrophages have exhibited great potential as cell-based drug carriers attributed to their high phagocytosis rate, tolerance to drugs/nanoparticles, and plasticity in response to tumors.14,15 For instance, Zhang et al. employed macrophages for delivering doxorubicin to 4T1 tumors in a specific manner, which effectively inhibited tumor invasion.16 Tan et al. designed apoptotic body-encapsulated nanomedicines for specific phagocytosis by inflammatory monocytes and macrophages, which subsequently carried the nanomedicine to the tumor tissue due to their tumor-homing effects.17 Similarly, Wang et al. reported that macrophages carried internalized nanomedicines efficiently to inflammatory atherosclerotic plaques, due to inflammatory tropism.15 However, excessive drug/nanomedicine loss via efflux and exocytosis during systemic delivery remains one of the great challenges for cell-based drug carriers.18
Herein, we developed a novel macrophage-hitchhiking delivery system based on supramolecularly self-aggregated nanomedicines inside macrophages, to significantly reduce premature loss during systemic delivery, and specifically release nanomedicines in the tumor in response to the inflammatory environment that may disassemble the supramolecular aggregates. In this work, β-cyclodextrin (β-CD) capped CuS NPs (CD-CuS) and ferrocene (Fc) capped CuS NPs (Fc-CuS) were phagocytosed by macrophages sequentially and formed large supramolecular aggregates via multiple, strong β-CD–Fc host–guest interactions,19 thereby inhibiting efflux from the macrophage. In the inflammatory tumor environment,20 the oxidation of Fc dissociated the host–guest pair21,22 and disassembled the aggregates, leading to tumor-specific release of small-sized NPs for deep penetration into the tumor tissue and enhanced photothermal therapy (Scheme 1).
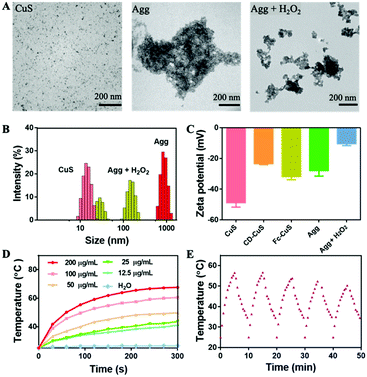
First, CuS NPs were prepared according to a literature method,23 which exhibited a spherical nanostructure with an average diameter of 10 nm under TEM (Fig. 1A, left). The respective modification of thiolated β-CD and Fc onto the surface of the CuS NPs was verified using 1H NMR analysis (Fig. S1 and S2, ESI†). The quantity of β-CD and Fc anchored onto the CuS NPs was 0.5711 and 0.3671 μmol mg−1, respectively. When the β-CD and Fc capped CuS NPs were mixed in an aqueous solution, obvious large aggregates (Agg) were observed, leading to supramolecular clusters of about 1 μm, as shown by the TEM and DLS results (Fig. 1A middle and B), that were approximately 100-fold larger than the individual CuS NPs. The CuS aggregates incubated under different pH conditions remained stable (Fig. S3, ESI†). Interestingly, incubation of the supramolecular aggregates in 1 mM H2O2 led to significant size reduction, due to the dissociation of oxidized Fc from β-CD (Fig. 1A right, B and Fig. S4, ESI†). The increased zeta potential after adding H2O2 also suggested the successful oxidation of Fc into ferrocenium (Fc+) (Fig. 1C).24 The photothermal potency of the CuS NPs was demonstrated by the significant temperature increase, in a concentration-dependent manner (up to 40 °C rise for 200 μg mL−1 CuS NPs), when irradiated with an 808 nm laser (1 W cm−2) for 5 min (Fig. 1D). The repeatable cycles of photothermal conversion indicated a reasonably good photothermal stability (Fig. 1E). Besides, the CuS NPs and CuS aggregates exhibited comparable photothermal capabilities (Fig. S5, ESI†), with photothermal conversion efficiencies of 28.51% and 28.43%, respectively (Fig. S6, ESI†).
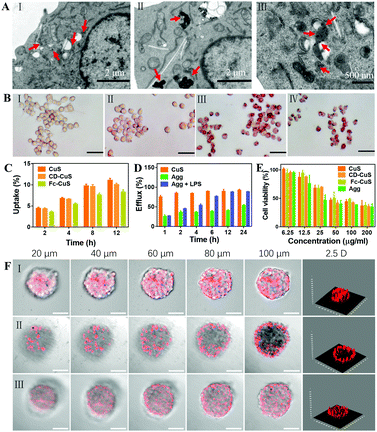
Next, the intracellular supramolecular self-assembly of CD-CuS and Fc-CuS was clearly shown using cryo-TEM analysis of a cross section of the macrophages (Fig. 2A). In addition, CD-CuS and Fc-CuS were co-located in the cytoplasm with a high co-localization coefficient (Fig. S7 and S8, ESI†). The amount of intracellular CD-CuS and Fc-CuS was quantified using ICP-MS (Fig. S9a, ESI†). Obvious large aggregations (Agg) were observed in the TEM images (Fig. S9b, ESI†) when CD-CuS and Fc-CuS were mixed at a proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, the same as their intracellular proportions. Besides, as shown in Fig. 2A, there were obvious aggregates of about 1 μm in the macrophages sequentially incubated with CD-CuS and Fc-CuS. In contrast, individual NPs were observed in macrophages treated with CuS NPs under cryo-TEM. When the macrophages were activated by lipopolysaccharides (LPS) to generate ROS intracellularly (Fig. S10 and S11, ESI†), few aggregates of NPs were observed in the macrophages, likely due to the dissociation of oxidized Fc from β-CD. Moreover, the LPS-activation and ROS-induced oxidation of Fc was verified using Perls Prussian blue staining of the macrophages as shown in Fig. 2B.25 Subsequently, the cellular uptake and efflux behaviors of the macrophages treated with different CuS NPs were examined via ICP-MS. As shown in Fig. 2C, macrophages exhibited strong phagocytosis towards all of these CuS NPs in a time-dependent manner over an incubation period of 8 h (saturation point). Macrophages were incubated with native CuS NPs for 8 h, or CD-CuS for 4 h and Fc-CuS for another 4 h sequentially, to obtain CuS NP loaded macrophages and aggregate loaded macrophages, respectively. A nearly 80.0% efflux of CuS NPs was observed in the macrophages internalized with CuS NPs within 1 h, in dramatic contrast to only a 25.9% efflux rate of macrophages sequentially treated with CD-CuS and Fc-CuS NPs (Agg). Even after 24 h, there was still ∼50% of the CuS NPs remaining in the macrophages of the Agg group, as shown in Fig. 2D, suggesting that the supramolecular aggregation of CuS NPs obviously reduced the efflux rate from macrophages, which would minimize premature loss of nanoparticles during systemic delivery and improve the delivery efficiency to the targeted tissue. Very interestingly, upon the addition of LPS to activate macrophages loaded with CuS aggregates, a significantly improved efflux of CuS NPs (∼80% at 6 h) was observed, implying that macrophage activation may disassemble the supramolecular aggregates of the NPs intracellularly and specifically release NPs in the inflammatory tissue. Additionally, the tumor-tropic migration ability of the CuS-loaded macrophages was evaluated using a transwell experiment. The migration of cargo-loaded macrophages was comparable to that of the unloaded ones (Fig. S12, ESI†), suggesting that the loading of the CuS NPs and their intracellular aggregation had a negligible influence on the tumor-homing property of the macrophages. Importantly, CuS, CD-CuS, Fc-CuS and Agg all exhibited high biocompatibility toward both B16 cells and macrophages (Fig. S13, ESI†) via MTT assays. Under laser irradiation for 5 min, the viability of B16 cells and macrophages dramatically decreased in a dose-dependent manner (Fig. 2E and Fig. S13, ESI†), and there was no noticeable difference in the phototoxicity of the CuS NPs with or without CD and Fc modification or supramolecular aggregation (Fig. 2E and Fig. S14, ESI†). Moreover, we established 3D tumor spheroid models and studied the tissue penetration of our system ex vivo. As shown in Fig. 2F and Fig. S15 (ESI†), Agg showed rather weak penetration into B16 tumor spheroids without H2O2 treatment, and the fluorescence signal was mainly distributed in the periphery of the spheroids. After incubation with H2O2, the penetration depth significantly increased, demonstrating that oxidation by ROS could enhance deep tumor penetration.
0.8, the same as their intracellular proportions. Besides, as shown in Fig. 2A, there were obvious aggregates of about 1 μm in the macrophages sequentially incubated with CD-CuS and Fc-CuS. In contrast, individual NPs were observed in macrophages treated with CuS NPs under cryo-TEM. When the macrophages were activated by lipopolysaccharides (LPS) to generate ROS intracellularly (Fig. S10 and S11, ESI†), few aggregates of NPs were observed in the macrophages, likely due to the dissociation of oxidized Fc from β-CD. Moreover, the LPS-activation and ROS-induced oxidation of Fc was verified using Perls Prussian blue staining of the macrophages as shown in Fig. 2B.25 Subsequently, the cellular uptake and efflux behaviors of the macrophages treated with different CuS NPs were examined via ICP-MS. As shown in Fig. 2C, macrophages exhibited strong phagocytosis towards all of these CuS NPs in a time-dependent manner over an incubation period of 8 h (saturation point). Macrophages were incubated with native CuS NPs for 8 h, or CD-CuS for 4 h and Fc-CuS for another 4 h sequentially, to obtain CuS NP loaded macrophages and aggregate loaded macrophages, respectively. A nearly 80.0% efflux of CuS NPs was observed in the macrophages internalized with CuS NPs within 1 h, in dramatic contrast to only a 25.9% efflux rate of macrophages sequentially treated with CD-CuS and Fc-CuS NPs (Agg). Even after 24 h, there was still ∼50% of the CuS NPs remaining in the macrophages of the Agg group, as shown in Fig. 2D, suggesting that the supramolecular aggregation of CuS NPs obviously reduced the efflux rate from macrophages, which would minimize premature loss of nanoparticles during systemic delivery and improve the delivery efficiency to the targeted tissue. Very interestingly, upon the addition of LPS to activate macrophages loaded with CuS aggregates, a significantly improved efflux of CuS NPs (∼80% at 6 h) was observed, implying that macrophage activation may disassemble the supramolecular aggregates of the NPs intracellularly and specifically release NPs in the inflammatory tissue. Additionally, the tumor-tropic migration ability of the CuS-loaded macrophages was evaluated using a transwell experiment. The migration of cargo-loaded macrophages was comparable to that of the unloaded ones (Fig. S12, ESI†), suggesting that the loading of the CuS NPs and their intracellular aggregation had a negligible influence on the tumor-homing property of the macrophages. Importantly, CuS, CD-CuS, Fc-CuS and Agg all exhibited high biocompatibility toward both B16 cells and macrophages (Fig. S13, ESI†) via MTT assays. Under laser irradiation for 5 min, the viability of B16 cells and macrophages dramatically decreased in a dose-dependent manner (Fig. 2E and Fig. S13, ESI†), and there was no noticeable difference in the phototoxicity of the CuS NPs with or without CD and Fc modification or supramolecular aggregation (Fig. 2E and Fig. S14, ESI†). Moreover, we established 3D tumor spheroid models and studied the tissue penetration of our system ex vivo. As shown in Fig. 2F and Fig. S15 (ESI†), Agg showed rather weak penetration into B16 tumor spheroids without H2O2 treatment, and the fluorescence signal was mainly distributed in the periphery of the spheroids. After incubation with H2O2, the penetration depth significantly increased, demonstrating that oxidation by ROS could enhance deep tumor penetration.
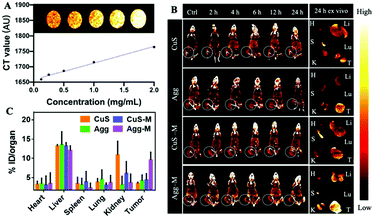
Biosafety assessments were carried out to assess the in vivo toxicity of our formulations. As shown in Fig. S16 (ESI†), no significant changes in the blood biochemistry (including the liver and kidney function biomarkers) and hematological parameters were detected. Further hematoxylin and eosin (H&E) staining (Fig. S17, ESI†) analysis also confirmed that there was no obvious damage in these organs. Next, the pharmacokinetic profile was evaluated using the copper content in the blood from healthy mice at different time points after intravenous injection of our formulations. The plasma concentration–time curve shown in Fig. S18 (ESI†) revealed that Agg-M had much a longer systemic circulation when compared with the other formulations, which is an essential requirement for enhanced accumulation in the tumor. The CT signal of the CuS NPs in vitro exhibited a dose-dependent manner, suggesting the significant potential of the CuS NPs for CT bioimaging (Fig. 3A). Thus, the biodistribution of the CuS NPs, CuS NPs internalized macrophages (CuS-M), supramolecular aggregates of CuS NPs (Agg) and supramolecular aggregates of the CuS NPs internalized macrophages (Agg-M) was investigated in melanoma tumor-bearing mice via CT imaging. As shown in Fig. 3B and Fig. S19 (ESI†), there was no obvious CT signal in the tumor tissues from the mice i.v. treated directly with the CuS NPs. In dramatic contrast, starting from 2 h after i.v. injection of Agg-M and CuS-M, the CT signal (of Cu) gradually increased in the tumor, suggesting the macrophage-hitchhiking delivery of the materials to the tumor. Comparatively, Agg-M showed significantly more accumulation in the tumor, suggesting minimal premature loss of the CuS NPs during systemic delivery, which is attributed to the supramolecular aggregation of the NPs intracellularly that inhibited cellular efflux. At 24 h after administration, all the major organs (heart, liver, spleen, lungs and kidneys) and tumors were harvested for ex vivo CT imaging (Fig. 3B) and quantitative analysis of the Cu content via ICP-MS (Fig. 3C) indicated that the tumors of the mice treated with Agg-M exhibited the most copper accumulation, and most other organs showed low copper accumulation. Of note, the high accumulation of CuS in the kidneys of mice administered with CuS NPs suggested the fast renal clearance of the small NPs. Large sized Agg was likely cleared out via the RES system.8 Therefore, targeted delivery of small-sized NPs to the tumor is of paramount importance in order to improve tumor accumulation and retention. Moreover, in order to confirm the macrophage-hitchhiking delivery into the tumor tissue, DiI (a dye staining cell membrane) stained Agg-M and CuS-M (where the CuS NPs were labelled with Cy5.5) were employed to indicate the localization of the macrophages (green channel) and NPs (red channel) in the various organs and tumors. As shown in Fig. S19c (ESI†), mainly green fluorescence was observed in the tumors of mice treated with CuS-M, suggesting that only macrophage carriers were delivered into the tumor with limited CuS NPs due to premature release during delivery. This was also confirmed by the separated red and green fluorescence observed in the liver and kidney tissues. In dramatic contrast, green and red fluorescence was observed to overlap in the organs and tumors of mice treated with Agg-M. Taken together, these results demonstrated that the supramolecular aggregates of NP internalized macrophages (Agg-M) efficiently delivered CuS NPs to the tumor in a highly targeted manner, with minimal premature loss during the systemic delivery.
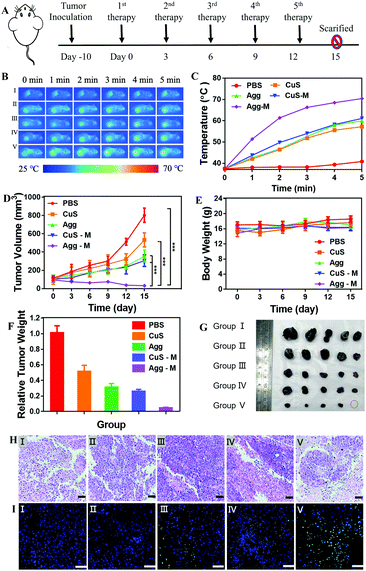
Finally, the in vivo photothermal anti-tumor efficacy of CuS NPs self-assembled in the macrophages for cell-hitchhiking delivery and tumor therapy was investigated. Melanoma tumor-bearing mice were randomly divided into five groups when the tumor volume reached approximately 100 mm3. PBS, CuS NPs, CuS-M, Agg and Agg-M were respectively i.v. injected into the tail veins of mice once every 3 days, for 5 times in total in each group of treatment. The tumors were exposed to laser irradiation for 5 min at 4 h after each injection at a density of 1 W cm−2 (Fig. 4A) and the temperature increase was monitored (Fig. 4B and C), which showed a localized temperature increase in the photothermia level with minimal influence on the healthy surrounding tissues. The tumor volume and bodyweight of the mice were measured daily for 15 days. As shown in Fig. 4D, the tumor volumes of the PBS and CuS treated mice increased rapidly during the follow-up, whereas the tumor volumes of mice treated with CuS-M and Agg experienced a much slower growth when compared with that of the PBS and CuS groups, suggesting that macrophage-hitchhiking and large sized aggregates may improve targeted delivery into the tumor, via inflammatory tropism and reduced renal clearance, respectively. Accordingly, the tumors of mice treated with Agg-M showed a dramatic suppression of tumor growth (Fig. 4D). Meanwhile, the mice of all groups showed similar weight growth trends (Fig. 4E), suggesting that the formulations of the CuS NPs were generally biocompatible. Very significantly, the final harvested tumors of mice in the Agg-M treatment group were nearly eliminated by the end of 15 days (Fig. 4F and G), in dramatic contrast to the other treatment groups. Hematoxylin and eosin (H&E) staining (Fig. 4H) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (Fig. 4I) showed that there was nearly no tumor injury in the control group, in comparison to the abundant cell apoptosis observed in the Agg-M group. In addition, the H&E and TUNEL staining also showed no obvious damage in the major organs (Fig. S20 and S21, ESI†), confirming the excellent biocompatibility of these formulations of CuS NPs. Immunostaining (F4/80+, green channel) of the tumor tissues collected from mice treated with CuS-M and Agg-M (DiI labelled macrophage carriers before delivery) were used to indicate the macrophage accumulation in the tumors. As shown in Fig. S22 (ESI†), the strong green fluorescence indicated the presence of a large amount of macrophages (endogenous and carriers), and the red fluorescence in both groups indicated that the carrier macrophages in the CuS-M and Agg-M groups both efficiently accumulated in the tumor. Approximately 18% of the total macrophages were exogenous carrier macrophages, regardless of what they carried. Collectively, these results all demonstrated that the highly desirable therapeutic effects of Agg-M were achieved via the intracellular self-assembly of the CuS NPs inside the macrophages for targeted hitchhiking delivery to the tumor.
Conclusions
In summary, we demonstrated the novel, intracellularly controlled supramolecular self-assembly/disassembly of CuS NPs inside macrophages for significantly improved macrophage-hitchhiking delivery of the NPs specifically to the tumor tissues, due to the inflammatory tropism of macrophages. In this design, small CuS NPs were intracellularly self-assembled into large supramolecular aggregates via multiple β-cyclodextrin–ferrocene host–guest interactions between sequentially internalized β-cyclodextrin-capped CuS NPs and ferrocene-capped CuS NPs inside the macrophages, which inhibited efflux and premature loss of the CuS NPs from the cell-based carriers. Oxidation of ferrocene in response to the inflammatory tumor microenvironment dissociated the β-cyclodextrin–ferrocene host–guest pairs, leading to disassembly of the CuS aggregates inside the macrophages for tumor-specific release. As a result, a significantly enhanced tumor deposition of CuS NPs in the mice injected with macrophages carrying intracellularly self-assembled CuS NPs was observed via CT bioimaging. More importantly, this unprecedented macrophage-hitchhiking delivery of supramolecular aggregates of CuS NPs greatly suppressed tumor growth in mice via photothermal therapy, with excellent biocompatibility. The precisely controlled intracellular self-assembly and disassembly of the nanomedicine inside live cells not only provides a novel cell-hitchhiking delivery strategy for specific cancer therapy, but also offers important new insights into the design and development of new cell-based medicine carriers to meet the needs of diverse biomedical applications.Methods/experimental
All experimental details for this study are available in the ESI.†All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the University of Macau and were approved by the Animal Ethics Committee, University of Macau.
Author contributions
Junyan Li: conceptualization, investigation, data curation, writing – original draft. Qian Cheng: investigation. Ludan Yue: investigation. Cheng Gao: conceptualization, data curation. Jianwen Wei: investigation. Yuanfu Ding: investigation. Yitao Wang: investigation. Ying Zheng: writing – review and editing. Ruibing Wang: conceptualization, data curation, writing – review and editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The SKL-QRCM Open Fund by the Science and Technology Development Fund (FDCT), Macau SAR (SKL-QRCM(UM)-2020-2022), and the National Natural Science Foundation of China (Grant no. 21871301 and 22071275) are gratefully acknowledged for providing financial support to this work. CG is supported by the UM Macao Postdoctoral Fellowship.References
- M. M. Koo, R. Swann, S. McPhail, G. A. Abel, L. Elliss-Brookes, G. P. Rubin and G. Lyratzopoulos, Lancet Oncol., 2020, 21, 73–79 CrossRef PubMed.
- C. de Martel, D. Georges, F. Bray, J. Ferlay and G. M. Clifford, Lancet Glob. Health, 2020, 8, e180–e190 CrossRef PubMed.
- H. Wang, J. Chang, M. Shi, W. Pan, N. Li and B. Tang, Angew. Chem. Int. Ed., 2019, 58, 1057–1061 CrossRef CAS PubMed.
- D. Yang, G. Yang, P. Yang, R. Lv, S. Gai, C. Li, F. He and J. Lin, Adv. Funct. Mater., 2017, 27, 1700371 CrossRef.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- J. Yang, D. Dai, X. Lou, L. Ma, B. Wang and Y.-W. Yang, Theranostics, 2020, 10, 615–629 CrossRef CAS PubMed.
- S. Goel, F. Chen and W. Cai, Small, 2014, 10, 631–645 CrossRef CAS PubMed.
- J. Bourquin, A. Milosevic, D. Hauser, R. Lehner, F. Blank, A. Petri-Fink and B. Rothen-Rutishauser, Adv. Mater., 2018, 30, 1704307 CrossRef PubMed.
- H. Kobayashi, R. Watanabe and P. L. Choyke, Theranostics, 2013, 4, 81–89 CrossRef PubMed.
- F. Chen, H. Hong, S. Goel, S. A. Graves, H. Orbay, E. B. Ehlerding, S. Shi, C. P. Theuer, R. J. Nickles and W. Cai, ACS Nano, 2015, 9, 3926–3934 CrossRef CAS PubMed.
- J. Xue, Z. Zhao, L. Zhang, L. Xue, S. Shen, Y. Wen, Z. Wei, L. Wang, L. Kong, H. Sun, Q. Ping, R. Mo and C. Zhang, Nat. Nanotechnol., 2017, 12, 692–700 CrossRef CAS PubMed.
- D. Chu, X. Dong, X. Shi, C. Zhang and Z. Wang, Adv. Mater., 2018, 30, 1706245 CrossRef PubMed.
- Y. Xia, L. Rao, H. Yao, Z. Wang, P. Ning and X. Chen, Adv. Mater., 2020, 32, 2002054 CrossRef CAS PubMed.
- P. Sun, Q. Deng, L. Kang, Y. Sun, J. Ren and X. Qu, ACS Nano, 2020, 14, 13894–13904 CrossRef CAS PubMed.
- C. Gao, Q. Huang, C. Liu, C. H. T. Kwong, L. Yue, J.-B. Wan, S. M. Y. Lee and R. Wang, Nat. Commun., 2020, 11, 2622 CrossRef CAS PubMed.
- J. Fu, D. Wang, D. Mei, H. Zhang, Z. Wang, B. He, W. Dai, H. Zhang, X. Wang and Q. Zhang, J. Control. Release, 2015, 204, 11–19 CrossRef CAS PubMed.
- L. Zheng, X. Hu, H. Wu, L. Mo, S. Xie, J. Li, C. Peng, S. Xu, L. Qiu and W. Tan, J. Am. Chem. Soc., 2020, 142, 382–391 CrossRef CAS PubMed.
- Z. Li, H. Huang, S. Tang, Y. Li, X.-F. Yu, H. Wang, P. Li, Z. Sun, H. Zhang, C. Liu and P. K. Chu, Biomaterials, 2016, 74, 144–154 CrossRef CAS PubMed.
- Y.-M. Zhang, Y.-H. Liu and Y. Liu, Adv. Mater., 2020, 32, 1806158 CrossRef CAS PubMed.
- Z. Yang, Y. Dai, C. Yin, Q. Fan, W. Zhang, J. Song, G. Yu, W. Tang, W. Fan, B. C. Yung, J. Li, X. Li, X. Li, Y. Tang, W. Huang, J. Song and X. Chen, Adv. Mater., 2018, 30, 1707509 CrossRef PubMed.
- J.-G. Cheng, Y.-M. Zhang and Y. Liu, ChemNanoMat, 2018, 4, 758–763 CrossRef CAS.
- L. Yang, A. Gomez-Casado, J. F. Young, H. D. Nguyen, J. Cabanas-Danés, J. Huskens, L. Brunsveld and P. Jonkheijm, J. Am. Chem. Soc., 2012, 134, 19199–19206 CrossRef CAS PubMed.
- M. Zhou, R. Zhang, M. Huang, W. Lu, S. Song, M. P. Melancon, M. Tian, D. Liang and C. Li, J. Am. Chem. Soc., 2010, 132, 15351–15358 CrossRef CAS PubMed.
- X. Yang, Y. Wang, W. Qi, R. Su and Z. He, Nanoscale, 2017, 9, 15323–15331 RSC.
- P. Zhang, Y. Hou, J. Zeng, Y. Li, Z. Wang, R. Zhu, T. Ma and M. Gao, Angew. Chem. Int. Ed., 2019, 58, 11088–11096 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: All experimental details and associated figures. See DOI: 10.1039/d1nh00291k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |