Room temperature synthesis of low-dimensional rubidium copper halide colloidal nanocrystals with near unity photoluminescence quantum yield†
Parth
Vashishtha
 *a,
Thomas J. N.
Hooper
b,
Yanan
Fang
*a,
Thomas J. N.
Hooper
b,
Yanan
Fang
 a,
Deviana
Kathleen
a,
David
Giovanni
a,
Deviana
Kathleen
a,
David
Giovanni
 c,
Maciej
Klein
cd,
Tze Chien
Sum
c,
Maciej
Klein
cd,
Tze Chien
Sum
 c,
Subodh G.
Mhaisalkar
c,
Subodh G.
Mhaisalkar
 ad,
Nripan
Mathews
ad,
Nripan
Mathews
 *ad and
Tim
White
*a
*ad and
Tim
White
*a
aSchool of Materials Science and Engineering, Nanyang Technological University (NTU), 50 Nanyang Avenue, Singapore 639798, Republic of Singapore. E-mail: pvashishtha@ntu.edu.sg
bHigh Field NMR Facility, School of Physics and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371, Republic of Singapore
cDivision of Physics and Applied Physics, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371, Republic of Singapore
dEnergy Research Institute @NTU (ERI@N), Research Techno Plaza, X-Frontier Block, Level 5, 50 Nanyang Drive, Singapore 637553, Republic of Singapore
First published on 10th December 2020
Abstract
Metal lead halide perovskite nanocrystals have emerged as promising candidates for optoelectronic applications. However, the inclusion of toxic lead is a major concern for the commercial viability of these materials. Herein, we introduce a new family of non-toxic reduced dimension Rb2CuX3 (X = Br, Cl) colloidal nanocrystals with one-dimensional crystal structure consisting [CuX4]3− ribbons isolated by Rb+ cations. These nanocrystals were synthesised using a room-temperature method under ambient conditions, which makes them cost effective and scalable. Phase purity quantification was confirmed by Rietveld refinement of powder X-ray diffraction and corroborated by 87Rb MAS NMR technique. Both samples also exhibited high thermal stability up to 500 °C, which is essential for optoelectronic applications. Rb2CuBr3 and Rb2CuCl3 display PL emission peaks at 387 nm and 400 nm with high PLQYs of ∼100% and ∼49%, respectively. Lastly, the first colloidal synthesis of quantum-confined rubidium copper halide-based nanocrystals opens up a new avenue to exploit their optical properties in lighting technology as well as water sterilisation and air purification.
Introduction
Semiconductor nanocrystals offer several advantages over their bulk counterparts due to quantum confinement which results in improved photoluminescence quantum yield (PLQY), narrow emission line-width, surface functionality, and size tunable emission wavelength.1–4 In the last decade, significant efforts have been made to not only improve the synthesis of nanocrystals but also to find novel nanocrystals with desirable optical properties.5–10 Among them, metal halide perovskite nanocrystals, such as CsPbX3, MAPbX3, FAPbX3 (FA = formamidinium, MA = methylammonium, X = Cl, Br, I), have emerged as a new class of most promising candidates for optoelectronic applications.9,11–15 In particular, due to the pure inorganic structure of CsPbX3 nanocrystals, high thermal stability can be achieved alongside their characteristic advantages of near unity PLQY and low processing cost.12,16–18 Therefore, research into other possible inorganic perovskite structures, such as Cs4PbBr6 and RbyCs1−yPbBr3, has intensified.19,20 However, the commercial viability of these materials are limited due to the toxicity of lead, resulting in the pursuit of lead-free nanocrystals. The most obvious alternative was stannous based perovskite nanocrystals (CsSnX3), due to the favoured transformation of Sn2+ to Sn4+, these materials were found to be too unstable.21 Similarly, many other nanocrystals such as Cs3Sb2X9,22 Cs3Bi2I9,23 Cs2AgInCl6,24 Cs2AgSbCl6,25 and Cs2AgBiCl6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 remain ambiguous for lighting applications.
25 remain ambiguous for lighting applications.
Reduced-dimensional Cs3Cu2I5 lead-free metal halide crystal structures consists isolated octahedra which causes high exciton binding energies (∼490 eV) as a result of exciton confinement within each octahedra.26 Thus, higher exciton binding energies pave the way for high PLQYs in this class of materials.26 Recently, nanocrystals of Cs3CuCl5 (PL = 521 nm), Cs3CuBr5 (PL = 458 nm), Cs3CuI5 (PL = 453 nm), and CsCu2I3 (PL = 570 nm) with high PLQY and room temperature phase stability were obtained in our previous reports.27,28 These materials demonstrated pure white emission as a result of colour tunable emission wavelengths and reduced halide segregation- an effect which is unavoidable in lead-based perovskite materials.27 After the development of cesium copper halide nanocrystals, research towards developing similar copper based metal halide nanocrystals have garnered tremendous attention.29 Another alternate could be rubidium copper halide (Rb2CuX3), which exhibits large band gaps and emission in the UVA spectral region (380–400 nm).30 Rb2CuX3 has one dimensional crystal structures demonstrating [CuX4]3− ribbons isolated by Rb+ cations. Yang et al. were the first to report the bulk crystals of Rb2CuBr3, synthesised using an acidic medium at high temperature for application in X-ray scintillators.31 Later, Creason et al. used a solid state method to synthesize bulk pellets of Rb2CuX3 (X = Cl, Br) for up-conversion.30 Both samples displayed high PL quantum yield (>60%) with PL peak position at 386 nm and 400 nm for Rb2CuBr3 and Rb2CuCl3, respectively.30 These materials also exhibited a large stoke shift of 85 nm for Rb2CuBr3 and 93 nm for Rb2CuCl3, indicating potential applications in solid-state or phosphor-based lighting.7 However, the synthesis of Rb2CuX3 in the form of colloidal nanocrystals has not been developed, despite the fact that colloidal nanocrystals offer several advantages over bulk crystals in term of easier solution processability, and superior optical structural properties, as described earlier.28,31 To date, there is no report of solution processed room temperature syntheses of Rb2CuX3 colloidal nanocrystals.
This work describes the first synthesis of colloidal Rb2CuX3 (X = Cl, Br) nanocrystals using a room-temperature ligand assisted re-precipitation (LARP) method. A detailed quantitative structural analysis was performed via powder X-ray diffraction followed by Rietveld refinement. Additionally, solid-state NMR was utilized to investigate the local structure and surface properties, while indirectly validating that Cu is formed in the Cu(I) oxidation state in the bulk of each nanocrystal. Thermogravimetric analysis (TGA) measurements were performed to probe the thermal stability of these materials for optoelectronic applications. Optical properties of the colloidal nanocrystal solutions was analysed using static-state absorption and photoluminescence spectroscopy followed by analysis of PLQY.
Results and discussion
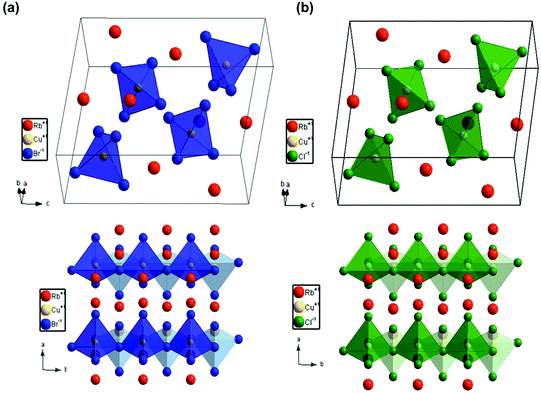
Colloidal nanocrystals of Rb2CuX3 were synthesized via a room temperature ligand assisted re-precipitation (LARP) method. In this method, the halide precursor salts of RbX (X = Cl, Br), and CuX were firstly dissolved in dimethyl sulfoxide solvent. After that, the precursor solution was added dropwise to a solution of toluene and oleic acid ligands, which resulted in the formation of the nanocrystals. After 5 minutes, the reaction was stopped, the nanocrystals were purified and finally dissolved in iso-propanol (IPA) to prepare a colloidal solution (see Experimental section for further details).Nanocrystals were characterized using powder XRD to analyse the crystallographic properties. Fig. 1(a and b) depict the diffraction pattern of Rb2CuBr3 and Rb2CuCl3 nanocrystals. Rietveld refinement using TOPAS confirmed the orthorhombic crystal structure of Rb2CuBr3 (Pnma) and Rb2CuCl3 (Pnma) with lattice parameters of a = 13.0786 Å, b = 4.4518 Å, c = 13.6465 Å and a = 12.5084 Å, b = 4.2741 Å, c = 13.0085 Å, respectively. The cell parameters are listed in Tables S1 and S2.† Expected peak broadening due to nanocrystallinity was not completely observed as the samples were prepared by vacuum drying nanocrystal powders to clearly observe all of the reflection for phase identification, which is also consistent with XRD patterns of other reduced dimensional copper halide colloidal nanocrystals.27,28,32–34 Additionally, Rietveld refinement revealed some phase impurity of RbBr (0.4%), and RbCu2Br3 (4.4%) in the Rb2CuBr3 sample and RbCl (6.4%), and RbCu2Cl3 (2.4%) in the Rb2CuCl3 sample. However, the total amount of impurity is less than 9% in both samples. A diagram identifying each peak individually is presented in Fig. S1.† A phase degradation study over 6 days under ambient conditions using XRD on these nanocrystals explained that the Cu+ slowly oxidizes to Cu2+. It was found that within 6 days 77% of the Rb2CuCl3 had slowly degraded to RbCuCl3 (42%) and RbCl (35%); in contrast, only 17% of Rb2CuBr3 had degraded to CuBr2 (13%) and RbBr (4%) over 6 days (Fig. S2† and Table 1). The faster degradation of Rb2CuCl3, in comparison to Rb2CuBr3, can be attributed to the higher hygroscopicity of RbCl than that of RbBr. Therefore, the degradation of these materials could be due to the absorption of moisture, which leads to the oxidation of Cu+ structures to Cu2+ structures and also the decomposition to RbBr/RbCl.30 Moreover, X-ray Photoelectron Spectroscopy (XPS) study of Rb2CuBr3 reveals that copper core-level spectrum consists of a 2p doublet exhibiting binding energies of 931.7 eV (2p3/2) and 951.5 eV (2p1/2) with a separation of 19.7 eV, which is consistent with Cu+ (Fig. S4a†).35,36 Similarly, Cu 2p XPS spectrum for Rb2CuCl3 shows 2p doublet with the binding energies of 934.6 eV (2p3/2) and 954.3 eV (2p1/2) with a separation of 19.7 eV, corresponding to Cu+.31,35 However, Cu 2p XPS spectrum of Rb2CuCl3 also shows strong Cu2+ satelite peak, which indiactes the surface oxidation in Rb2CuCl3 nanocrystals.36 A noticable color change of the nanocrystal powders from white to green/yellow was observed after a few hours under ambient conditions. Despite being susceptible to degradation in powder form, the colloidal solution of these nanocrystals was found to be stable over a week (white colored transparent liquid). Fig. 2 shows the crystal structure of both samples. In the typical crystal structure, the Cu atom is surrounded by four bromine/chlorine atoms as a [CuX4]3− tetrahedron (blue or yellow colour). As depicted in the bottom panel of Fig. 2, these tetrahedrons form corner-sharing one dimensional chain along the 〈010〉 plane separated by Rb+ cations. The synthesis of Rb2Cu(Br/Cl)3 was also attempted, however the yield was small with many side products formed (Fig. S1c†), suggesting further research is required to optimize the mixed-halide phase.
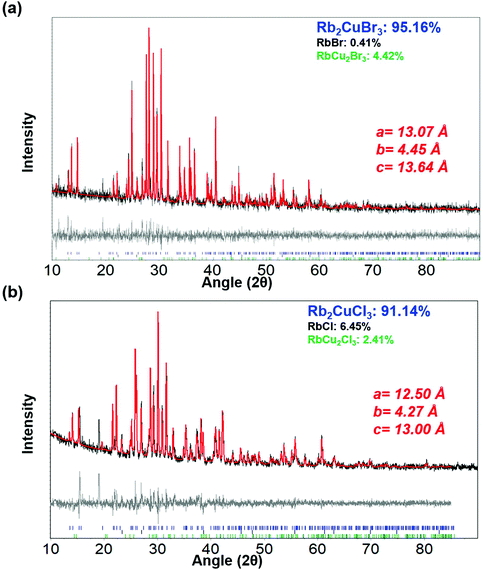 | ||
| Fig. 1 Powder XRD pattern (black lines) of (a) Rb2CuBr3 and (b) Rb2CuCl3 nanocrystals with Rietveld refinement fits (red lines) using TOPAS and residual maps of both graphs (gray lines). | ||
| Time (days) | Rb2CuBr3 (wt%) | RbCu2Br3 (wt%) | RbBr (wt%) | CuBr2 (wt%) |
|---|---|---|---|---|
| Rb 2 CuBr 3 nanocrystals | ||||
| 0 days | 95.16 | 4.42 | 0.41 | — |
| 3 days | 87.17 | — | 3.46 | 9.37 |
| 6 days | 83.13 | — | 4.01 | 12.87 |
| Time (days) | Rb2CuCl3 (wt%) | RbCu2Cl3 (wt%) | RbCl (wt%) | RbCuCl3 (wt%) |
|---|---|---|---|---|
| Rb 2 CuCl 3 nanocrystals | ||||
| 0 days | 91.14 | 2.41 | 6.45 | — |
| 3 days | 88.78 | — | 5.31 | 5.91 |
| 6 days | 23.43 | — | 34.40 | 42.17 |
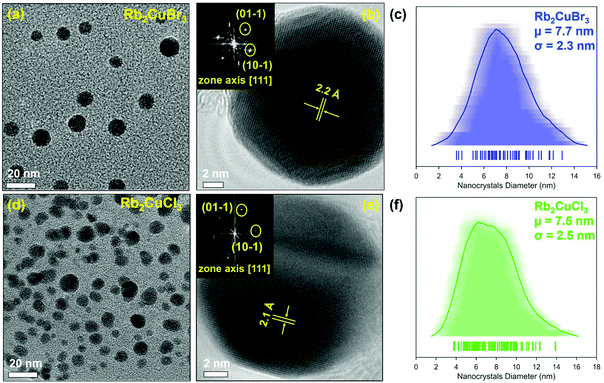
The nanocrystals were examined using transmission electron microscopy (TEM) to analyse the morphology and size. Fig. 3 depicts the TEM micrographs of Rb2Cu2Br3 and Rb2Cu2Cl3 nanocrystals. Both samples showed nanoplate-like faceted morphology with an average size of ∼7.7 nm for Rb2CuBr3 and ∼7.5 nm for Rb2CuCl3 (Fig. 3 and S3†). An average shifted histogram (Fig. 3c and f) and a standard histogram (Fig. S3c and f†) are plotted for the illustration of particle size distribution. High-resolution TEM and fast Fourier transform (FFT) (Fig. 3b and e) gives lattice spacings of 2.2 Å and 2.1 Å for Rb2CuBr3 and Rb2CuCl3 nanocrystals, respectively. Matching of FFT with corresponding diffraction patterns plane of both samples reverify the formation of Rb2CuX3 crystal structure. Elemental analysis via energy dispersive X-ray spectroscopy (EDXS) revealed atomic ratios of 1.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00
1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.01 for Rb2CuBr3 and 1.96
3.01 for Rb2CuBr3 and 1.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00
1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.95 Rb2CuCl3 nanocrystals (Fig. S3g, S3h and Table S3†).
2.95 Rb2CuCl3 nanocrystals (Fig. S3g, S3h and Table S3†).
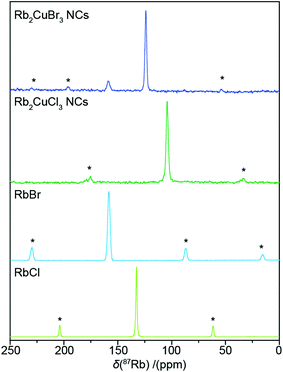
Solid state NMR was also utilized to help further characterise the nanocrystal powders. 87Rb MAS NMR of both nanocrystal samples is shown in Fig. 4, alongside the spectra of the halide precursor salts RbBr and RbCl. The Rb2CuBr3 NCs spectrum presents with a dominant resonance at 124 ppm assigned to the Rb2CuBr3 phase. A smaller resonance at 159 ppm is identical to the resonance given by the pure RbBr powder, and hence can be assigned as the RbBr impurity, detected by XRD. The 87Rb MAS NMR of the Rb2CuCl3 nanocrystal sample presents a singular narrow resonance at 104 ppm. The Rb2CuCl3 resonance is shifted to lower frequency than the corresponding Rb2CuBr3 resonance which is analagous to the shift difference between RbBr and RbCl. The narrowness of the Rb2CuX3 resonances demonstrates the relatively symmetrical environment about the Rb site within the channels created by the 1D [CuX4]3− chains, as no quadrupolar effect is observed. The impurities of RbCl and RbCu2X3 observed in the XRD of the nanocrystal powders are presumed to have a small enough concentration that they cannot be seen above the noise in the 87Rb spectra. In addition, the lack of any observed effect from paramagnetic centres on the 87Rb NMR, as demonstrated by the relatively unchanged 87Rb spin–lattice relaxation times (Table S4†), indirectly confirms that RbCu(II)X3 phases are not present in the fresh powder samples. The 1H MAS NMR of both samples (Fig. S4b†) also reveals that the oleic acid ligands responsible for the nanocrystal formation are still present in the nanocrystal powders.
Collectively, XRD, TEM, EDXS, and NMR have confirmed the formation of Rb2CuX3 nanocrystals with an orthorhombic crystal structure and faceted nanocrystal morphology, with particle sizes less than ∼10 nm.
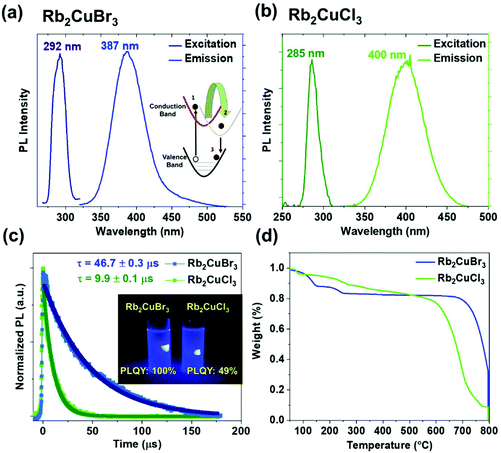
Rb2CuBr3 and Rb2CuCl3 Colloidal solutions exhibit strong absorption at 276 nm and 265 nm respectively, which is ∼20 nm blue shifted compared to the reported absorption profile of the bulk materials and single crystals (Fig. S5a†).30,31 Rb2CuBr3 and Rb2CuCl3 exhibits an excitation peak at 292 nm and 285 nm (Fig. 5a and b), confirming the excitation is due to the excitonic absorption. As depicted in Fig. 5a and b, Rb2CuBr3 shows an emission peak at 387 nm with fwhm of 50 nm, whereas Rb2CuCl3 shows an emission peak at 400 nm with a fwhm of 52 nm. These nanocrystals show extremely bright violet colour under 300 nm UV excitation (Fig. 5c) with PLQY of ∼100% and 49% for Rb2CuBr3 and Rb2CuCl3, respectively. The lower PLQY of Rb2CuCl3 sample can be attributed to structural defects of metal chloride based materials; which has also been observed in chlorine-based perovskite.37,38 In order to confirm there is no emission from mixed phases, excitation dependent PL spectra was measured (Fig. S5b and c†). However no peak shift in emission spectra was observed, confirming the emission source is the main product Rb2CuX3 in both samples. A large stoke shift in both nanocrystals has also been observed in previous studies of bulk materials, which suggests that the emission is not due to band to band emission.30 The excitation and emission spectral features are quite similar, which confirms that the PL originates from the relaxation of the same excited state. Time-resolved PL measurements were conducted to measure the carrier lifetime of these nanocrystals. As depicted in Fig. 5c, Rb2CuBr3 nanocrystals exhibited a long carrier lifetime of 46.7 μs, whereas Rb2CuCl3 exhibits a carrier lifetime of 9.9 μs, which is consistent with their bulk counterparts.30,31 Fig. S7a† shows the linear dependency of PL intensity with excitation power, suggesting the PL does not arise from a permanent defect.33,39 Whereas, the long carrier lifetime is due to self-trapped exciton emission mechanism in these materials.30,31 It should be noted that other copper halide systems such as Cs3Cu2X5 and CsCu2X3 also display similar microsecond carrier lifetimes.28 Moreover, it was found that these materials have very high Huang-Rhys factors, which make them more susceptible for the formation of self-trapped excitons (STEs).31 Under light illumination, copper halide based materials are found to undergo structural reorganization such that the Cu(I)-3d10 forms Cu(II)-3d9 and induces strong Jahn–Teller distortion.26–28 Overall, the energy difference between Cu(II) and Cu(I) causes the large Stokes shift. Similar large stoke shift and STE emission mechanism were observed in other low-dimensional materials such as Cs2AgxNa1−xInCl6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi, Cs3Cu2X5, (C4N2H14X)4SnX6, C4N2H14PbBr4, and CsCu2I3.26–28,40–42 On another note, due to the large stoke shift, there is nearly no overlap between the excitation and emission spectra in these nanocrystals, making them ideal candidates for phosphor-based solid-state lighting application.43
Bi, Cs3Cu2X5, (C4N2H14X)4SnX6, C4N2H14PbBr4, and CsCu2I3.26–28,40–42 On another note, due to the large stoke shift, there is nearly no overlap between the excitation and emission spectra in these nanocrystals, making them ideal candidates for phosphor-based solid-state lighting application.43
Colloidal stability of these nanocrystals was found to be reasonably stable up to 2 days in ambient conditions. Moreover, the colloidal solutions of the Rb2CuBr3 and Rb2CuCl3 nanocrystals displayed up to 13% and 50% reduction in photoluminescence quantum yield, respectively, after storage under ambient conditions (Fig. S6†). The faster PL degradation in Rb2CuCl3 can be attributed to the surface oxidation due to high hygroscopicity of Rb2CuCl3 sample as observed by XPS spectra (Fig. S4a†). These nanocrystals were successfully incorporated in PDMS polymer matrix (Fig. S7b†), which demonstrate their compatibility to form white display devices with YAG yellow phosphor. Materials with potential application in semiconductor devices must also exhibit high thermal stability. The same nanocrystals embedded in a PDMS matrix were utilized for temperature dependent in situ PL measurements. Albeit, the temperature-dependent PL measurement shows the 50% intensity degradation at 100 °C (Fig. S7b†), which could be due to the enhanced non-radiative recombination induced by thermal energy.44 Thermal decomposition stability of the Rb2CuX3 nanocrystal powders using TGA measurements showed some promising results (Fig. 5d). As depicted in Fig. 5d, weight loss up to 250 °C can be attributed to the loss of organic ligands from the surface of the nanocrystals. The TGA curve also reveals that the Rb2CuBr3 exhibits remarkably high thermal stability up to 750 °C, 200 °C higher than the Rb2CuCl3 nanocrystals. Regardless, both samples show high thermal stability up to 550 °C, which is desirable for optoelectronic applications.
Conclusion
This work describes the first synthesis of colloidal Rb2CuBr3 and Rb2CuCl3 nanocrystals using a room-temperature scalable LARP method. Rietveld refinement of the powder XRD diffraction pattern confirms the Pnma orthorhombic phase purity and provides detailed crystallographic information in these nanocrystals. Solid-state NMR was utilized to analyse the local environment and to confirm the presence of oleic acid ligands. Both of these nanocrystals show remarkably high photoluminescence quantum yield in the UVA-violet spectral region with a large difference between excitation and emission spectra, making them suitable for phosphor based lighting applications. More importantly, these nanocrystals exhibit high thermal stability up to 550 °C. Lastly, despite the need of device optimization, we are confident that the application of these nanocrystals can be extended to stable LEDs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. V. acknowledges a Presidential Postdoctoral Fellowship from Nanyang Technological University (NTU), Singapore via grant 04INS000581C150. M. K., N. M., S. G. M., and T. W. acknowledge financial support from the Singapore National Research Foundation, Prime Minister's Office, through the Competitive Research Program (CRP Award No. NRF-CRP14-2014-03). T. C. S. and D. G. acknowledge the financial support from the NRF Investigatorship (NRF-NRFI-2018-04) and the Ministry of Education under its AcRF Tier 1 grant (RG91/19) and Tier 2 grant MOE2019-T2-1-006. The authors would like to acknowledge the Facility for Analysis, Characterization, Testing and Simulation (FACTS) at NTU, Singapore, for use of their electron microscopy and X-ray diffraction facilities. We would also like to acknowledge the NTU Centre of High Field NMR Spectroscopy and Imaging for the use of their NMR facilities. We thank Mr Sai S. H. Dintakurti and Dr Ankit, NTU, Singapore.References
- P. O. Anikeeva, J. E. Halpert, M. G. Bawendi and V. Bulovic, Nano Lett., 2009, 9, 2532–2536 CrossRef CAS PubMed.
- P. O. Anikeeva, J. E. Halpert, M. G. Bawendi and V. Bulović, Nano Lett., 2007, 7, 2196–2200 CrossRef CAS PubMed.
- Q. Sun, Y. A. Wang, L. S. Li, D. Wang, T. Zhu, J. Xu, C. Yang and Y. Li, Nat. Photonics, 2007, 1, 717–722 CrossRef CAS.
- P. Ramasamy, N. Kim, Y.-S. Kang, O. Ramirez and J.-S. Lee, Chem. Mater., 2017, 29, 6893–6899 CrossRef CAS.
- D. W. Ayele, H. M. Chen, W. N. Su, C. J. Pan, L. Y. Chen, H. L. Chou, J. H. Cheng, B. J. Hwang and J. F. Lee, Chem. – Eur. J., 2011, 17, 5737–5744 CrossRef CAS PubMed.
- D. V. Talapin, I. Mekis, S. Götzinger, A. Kornowski, O. Benson and H. Weller, J. Phys. Chem. B, 2004, 108, 18826–18831 CrossRef CAS.
- J. Zhou, M. Zhu, R. Meng, H. Qin and X. Peng, J. Am. Chem. Soc., 2017, 139, 16556–16567 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- J. Butkus, P. Vashishtha, K. Chen, J. K. Gallaher, S. K. Prasad, D. Z. Metin, G. Laufersky, N. Gaston, J. E. Halpert and J. M. Hodgkiss, Chem. Mater., 2017, 29, 3644–3652 CrossRef CAS.
- D. Chen, S. Zhou, F. Tian, H. Ke, N. Jiang, S. Wang, Y. Peng and Y. Liu, Adv. Opt. Mater., 2019, 7, 1901082 CrossRef CAS.
- P. Vashishtha, S. A. Veldhuis, S. S. Dintakurti, N. L. Kelly, B. E. Griffith, A. A. Brown, M. S. Ansari, A. Bruno, N. Mathews and Y. Fang, J. Mater. Chem. C, 2020, 8, 11805–11821 RSC.
- A. A. M. Brown, T. J. N. Hooper, S. A. Veldhuis, X. Y. Chin, A. Bruno, P. Vashishtha, J. N. Tey, L. Jiang, B. Damodaran and S. H. Pu, Nanoscale, 2019, 11, 12370–12380 RSC.
- X. Y. Chin, A. Perumal, A. Bruno, N. Yantara, S. A. Veldhuis, L. Martínez-Sarti, B. Chandran, V. Chirvony, A. S.-Z. Lo and J. So, Energy Environ. Sci., 2018, 11, 1770–1778 RSC.
- Y. Ma, P. Vashishtha, K. Chen, E. L. Peach, D. Ohayon, J. M. Hodgkiss and J. E. Halpert, ChemSusChem, 2017, 10, 2677–2684 CrossRef CAS PubMed.
- P. Vashishtha, S. Bishnoi, C. H. A. Li, M. Jagadeeswararao, T. J. N. Hooper, N. Lohia, S. B. Shivarudraiah, M. S. Ansari, S. N. Sharma and J. E. Halpert, ACS Appl. Electron. Mater., 2020, 2, 3470–3490 CrossRef CAS.
- M. Imran, P. Ijaz, L. Goldoni, D. Maggioni, U. Petralanda, M. Prato, G. Almeida, I. Infante and L. Manna, ACS Energy Lett., 2019, 4, 819–824 CrossRef CAS.
- R. R. Tamming, J. Butkus, M. B. Price, P. Vashishtha, S. K. Prasad, J. E. Halpert, K. Chen and J. M. Hodgkiss, ACS Photonics, 2019, 6, 345–350 CrossRef CAS.
- D. Chen and X. Chen, J. Mater. Chem. C, 2019, 7, 1413–1446 RSC.
- J. W. Xiao, Y. Liang, S. Zhang, Y. Zhao, Y. Li and Q. Chen, Chem. – Eur. J., 2019, 25, 2597–2603 CrossRef CAS PubMed.
- X. Du, G. Wu, J. Cheng, H. Dang, K. Ma, Y.-W. Zhang, P.-F. Tan and S. Chen, RSC Adv., 2017, 7, 10391–10396 RSC.
- T. C. Jellicoe, J. M. Richter, H. F. Glass, M. Tabachnyk, R. Brady, S. N. E. Dutton, A. Rao, R. H. Friend, D. Credgington and N. C. Greenham, J. Am. Chem. Soc., 2016, 138, 2941–2944 CrossRef CAS PubMed.
- J. Zhang, Y. Yang, H. Deng, U. Farooq, X. Yang, J. Khan, J. Tang and H. Song, ACS Nano, 2017, 11, 9294–9302 CrossRef CAS PubMed.
- G. M. Paternò, N. Mishra, A. J. Barker, Z. Dang, G. Lanzani, L. Manna and A. Petrozza, Adv. Funct. Mater., 2019, 29, 1805299 CrossRef.
- F. Locardi, M. Cirignano, D. Baranov, Z. Dang, M. Prato, F. Drago, M. Ferretti, V. Pinchetti, M. Fanciulli and S. Brovelli, J. Am. Chem. Soc., 2018, 140, 12989–12995 CrossRef CAS PubMed.
- A. S. Kshirsagar and A. Nag, J. Chem. Phys., 2019, 151, 161101 CrossRef PubMed.
- T. Jun, K. Sim, S. Iimura, M. Sasase, H. Kamioka, J. Kim and H. Hosono, Adv. Mater., 2018, 30, 1804547 CrossRef PubMed.
- P. Vashishtha, G. V. Nutan, B. E. Griffith, Y. Fang, D. Giovanni, M. Jagadeeswararao, T. C. Sum, N. Mathews, S. G. Mhaisalkar, J. V. Hanna and T. White, Chem. Mater., 2019, 31, 9003–9011 CrossRef CAS.
- Y. Li, P. Vashishtha, Z. Zhou, Z. Li, S. B. Shivarudraiah, C. Ma, J. Liu, K. S. Wong, H. Su and J. E. Halpert, Chem. Mater., 2020, 32, 5515–5524 CrossRef CAS.
- Z. Guo, J. Li, R. Pan, J. Cheng, R. Chen and T. He, Nanoscale, 2020, 12, 15560–15576 RSC.
- T. D. Creason, A. Yangui, R. Roccanova, A. Strom, M. H. Du and B. Saparov, Adv. Opt. Mater., 2020, 8, 1901338 CrossRef CAS.
- B. Yang, L. Yin, G. Niu, J. H. Yuan, K. H. Xue, Z. Tan, X. S. Miao, M. Niu, X. Du and H. Song, Adv. Mater., 2019, 31, 1904711 CrossRef CAS PubMed.
- L. Wang, Z. Shi, Z. Ma, D. Yang, F. Zhang, X. Ji, M. Wang, X. Chen, G. Na and S. Chen, Nano Lett., 2020, 20, 3568–3576 CrossRef CAS PubMed.
- P. Cheng, L. Sun, L. Feng, S. Yang, Y. Yang, D. Zheng, Y. Zhao, Y. Sang, R. Zhang and D. Wei, Angew. Chem., 2019, 131, 16233–16237 CrossRef.
- E. P. Booker, J. T. Griffiths, L. Eyre, C. Ducati, N. C. Greenham and N. J. Davis, J. Phys. Chem. C, 2019, 123, 16951–16956 CrossRef CAS.
- H. Su, Y. Xie, S. Wan, B. Li and Y. Qian, Solid State Ionics, 1999, 123, 319–324 CrossRef CAS.
- X. Jiang, J. Qiao, I. M. Lo, L. Wang, X. Guan, Z. Lu, G. Zhou and C. Xu, J. Hazard. Mater., 2015, 283, 880–887 CrossRef CAS PubMed.
- R. K. Behera, S. Das Adhikari, S. K. Dutta, A. Dutta and N. Pradhan, J. Phys. Chem. Lett., 2018, 9, 6884–6891 CrossRef CAS PubMed.
- J. Kim, C.-H. Chung and K.-H. Hong, Phys. Chem. Chem. Phys., 2016, 18, 27143–27147 RSC.
- E. R. Dohner, A. Jaffe, L. R. Bradshaw and H. I. Karunadasa, J. Am. Chem. Soc., 2014, 136, 13154–13157 CrossRef CAS PubMed.
- J. Luo, X. Wang, S. Li, J. Liu, Y. Guo, G. Niu, L. Yao, Y. Fu, L. Gao and Q. Dong, Nature, 2018, 563, 541–545 CrossRef CAS PubMed.
- Z. Yuan, C. Zhou, Y. Tian, Y. Shu, J. Messier, J. C. Wang, L. J. Van De Burgt, K. Kountouriotis, Y. Xin and E. Holt, Nat. Commun., 2017, 8, 14051 CrossRef CAS PubMed.
- C. Zhou, H. Lin, Y. Tian, Z. Yuan, R. Clark, B. Chen, L. J. van de Burgt, J. C. Wang, Y. Zhou and K. Hanson, Chem. Sci., 2018, 9, 586–593 RSC.
- M. Ghate, H. Dahule, N. T. Kalyani and S. Dhoble, Optik, 2017, 149, 198–205 CrossRef CAS.
- J. P. Freedman, J. H. Leach, E. A. Preble, Z. Sitar, R. F. Davis and J. A. Malen, Sci. Rep., 2013, 3, 1–6 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nr08093d |
| This journal is © The Royal Society of Chemistry 2021 |