Two-dimensional fence-like Co-doped NiSe2/C nanosheets as an anode for half/full sodium-ion batteries†
Siwei
Fan
,
Guangda
Li
 * and
Jianyu
Liu
* and
Jianyu
Liu
School of Materials Science and Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan 250353, China. E-mail: ligd@qlu.edu.cn
First published on 26th October 2020
Abstract
Two-dimensional fence-like Co-doped NiSe2/C (Co-NiSe2/C) nanosheets are fabricated via a facile solvothermal method followed by a selenization strategy. The Co-NiSe2/C nanosheets are composed of interlaced nanorods, which are made up of small nanoparticles with a size of 20 nm. The surfaces of these small nanoparticles are covered in thin carbon layers. The material displays excellent long-term cycle life (306.1 mA h g−1 after 5000 cycles at 5 A g−1), rate capability (260.1 mA h g−1 at 10 A g−1), and full cell performance (269.1 mA h g−1 after 100 cycles at 0.5 A g−1) as an anode for SIBs. Furthermore, the Na storage mechanism and the reaction kinetics are discussed based on ex situ X-ray diffraction analysis, cyclic voltammetry measurements, a galvanostatic intermittent titration technique, and electrochemical impedance spectra. It is demonstrated that Na+ diffusion in Co-NiSe2/C is a dynamic change process and is accompanied by a phase transition during the discharge/charge processes.
Introduction
Recently, sodium-ion batteries (SIBs) have been researched as a substitute to replace LIBs because sodium and lithium have similar properties and the reserves of sodium are more abundant.1–4 At this stage, the development of cathode materials has been relatively successful, which include layered transition metal oxides and polyanionic compounds, such as FePO4 and Na3V2(PO4)3/reduced graphene oxide.5,6 In addition, electrolytes have also been rapidly developed.7 On the contrary, the development of stable anode materials still faces many difficulties. Due to the large difference in radius between Na+ (0.102 nm) and Li+ (0.076 nm), graphite-based materials which are suitable for LIBs are difficult to use in SIBs because of the smaller interlayer distance of graphite.8 Therefore, the development of anode materials for SIBs has become a challenging task.Metal sulfides and metal selenides are used as the anode materials for SIBs because of their high specific capacity, larger interlayer distance and appropriate working potential.9,10 In 1980, Newman et al. applied TiS2 as an electrode material in SIBs. Since then, researchers have conducted extensive and in-depth research on metal sulfides.11,12 However, metal sulfides are susceptible to pulverization and structural changes during cycles, which is harmful for the repetitive insertion/extraction of Na+.8 In order to improve the performance of metal sulfides, complex preparation methods such as heteroatom doping and carbon coating are usually required, which brings difficulties to the application of these materials.4,13 Metal selenides can be divided into layered structures and non-layered structures. Therein, the layered structure of the metal selenides has attracted people's attention mainly because its stable structure is very suitable for sodium ion deintercalation. In their crystal structure, the metal atom (M) and two nearby selenium atoms are connected by a covalent bond to form a Se–M–Se structure, and further form a layered structure by van der Waals forces. It is beneficial for the insertion of Na+ and accompanying the breaking of the weak van der Waals forces during the insertion process.8,10 Metal selenides have a higher electronic conductivity than metal sulfides due to the higher conductivity of Se than that of S. In addition, M–Se bonds are weaker than M–S bonds, which facilitates Na storage through the conversion reaction.8,10,14,15 These advantages make metal selenide an ideal potential anode material for SIBs. However, the severe volume expansion can give rise to the poor solid electrolyte interface (SEI) stability and structure collapse during the conversion reaction, which also impact the performance of metal selenides materials.
Generally, morphology control is an important means to improve material performance and structural stability. For instance, zero-dimensional (0D) structures, such as nanoparticles, have shortened diffusion paths and a minimum specific surface area, but agglomeration easily occurs in the charge/discharge cycles.16,17 One-dimensional (1D) structures, for example, nanotubes, nanowires and nanorods, possess fast electron transport and short diffusion paths along the 1D direction and radial direction, respectively. However, it is still impossible to effectively avoid agglomeration, pulverization and the resulting capacity fading during long-term cycling. Coating other materials on the surface of 1D materials can make its structure more stable, thereby improving its cycle stability.16–18 For example, Zhou et al. fabricated Co3S4@polyaniline nanotubes by a self-template hydrothermal method, which retained a high discharge capacity of about 170.1 mA h g−1 at 4000 mA g−1 after 400 cycles, while the single Co3S4 nanotubes only showed 75.9 mA h g−1 after 100 cycles at 200 mA g−1.19 Two-dimensional (2D) structures showed a variety of performances compared with their bulk counterparts, including larger specific surface area, mechanical flexibility, rich chemical modification and adjustable electronic characteristics.17 Furthermore, 2D structures are stackable by van der Waals forces, which is beneficial to mitigating the volume change when used as an electrode material.17,20 Liu et al. fabricated NiSe2/NC nanosheets by a facile selenization process, which displayed an excellent electrochemical performance (240 mA h g−1 after 1000 cycles at 5 A g−1).21
Herein, we fabricate 2D fence-like Co-doped NiSe2/C (Co-NiSe2/C) nanosheets though using a facile solvothermal and selenization method. Co-NiSe2/C nanosheets are formed by interlaced nanorods composed of nanoparticles with a size of 20 nm. These nanoparticles are coated by carbon layers, which is beneficial to increase the active sites for Na+ and contact area between the electrolyte and the Co-NiSe2/C nanosheets. Moreover, the existence of carbon layers can improve the conductivity and structure stability. Co-NiSe2/C nanosheets display a high discharge specific capacity of 378.1 mA h g−1 after 1000 cycles at a current density of 1 A g−1, and the discharge specific capacity can reach up to 306.1 mA h g−1 after 5000 cycles even at a current density of 5 A g−1 as the anode in SIBs. The discharge specific capacity can still reach 260.1 mA h g−1 at 10 A g−1, exhibiting an excellent rate performance. We also used the Na3V2(PO4)3 as a cathode and Co-NiSe2/C as an anode to assemble a full cell, which exhibited a discharge specific capacity of 269.1 mA h g−1 after 100 cycles at a current density of 0.5 A g−1. Furthermore, the Na storage mechanism and the reaction kinetics are discussed though ex situ X-ray diffraction analysis (ex-XRD), cyclic voltammetry (CV) measurements, galvanostatic intermittent titration technique (GITT) and electrochemical impedance spectra (EIS). It was demonstrated that the storage of Na+ in Co-NiSe2/C is a conversion reaction during the discharge/charge processes.
Results and discussion
The XRD pattern of the Co-NiSe2/C is shown in Fig. 1a, which demonstrates a cubic crystal structure (space group, Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , JCPDS no. 11-0522). However, all the diffraction peaks of Co-NiSe2/C shift to large angles compared to that of pristine NiSe2, and the deviation of the diffraction peaks at high angles is larger than that at low angles (Fig. S1a†), which is attributed to Co2+ (0.065 nm) having a smaller ionic radius than that of Ni2+ (0.069 nm). According to the Bragg equation (nλ = 2d
, JCPDS no. 11-0522). However, all the diffraction peaks of Co-NiSe2/C shift to large angles compared to that of pristine NiSe2, and the deviation of the diffraction peaks at high angles is larger than that at low angles (Fig. S1a†), which is attributed to Co2+ (0.065 nm) having a smaller ionic radius than that of Ni2+ (0.069 nm). According to the Bragg equation (nλ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Θ), a right shift of the diffraction peak means a reduction in interplanar spacing (Fig. S1b and c†).22,23 The coexistence of C, Co, Ni and Se in the Co-NiSe2/C is shown in the XPS survey spectrum (Fig. 1b). Fig. 1c displays the XPS spectrum of C 1s, the peaks at 284.8, 286.3 and 295.8 eV correspond to the existence of C–C, C–O and C
Θ), a right shift of the diffraction peak means a reduction in interplanar spacing (Fig. S1b and c†).22,23 The coexistence of C, Co, Ni and Se in the Co-NiSe2/C is shown in the XPS survey spectrum (Fig. 1b). Fig. 1c displays the XPS spectrum of C 1s, the peaks at 284.8, 286.3 and 295.8 eV correspond to the existence of C–C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, respectively.24–26 In the Co 2p spectrum (Fig. 1d), the peaks can be divided into two parts, corresponding to Co 2p3/2 and Co 2p1/2, respectively. The peaks situated at 778.3 and 793.3 eV can be ascribed to Co2+ in Co-NiSe2/C, and the peaks located at 780.0 and 796.3 eV correspond to Co3+, which might originate from surface oxidation of Co-NiSe2/C when exposed to air. In addition, the peaks at 783.1, 785.9, 799.3 and 801.8 eV correspond to satellite peaks.8,22 Similarly, in the Ni 2p spectrum (Fig. 1e), Ni 2p3/2 and Ni 2p1/2 split into two peaks, respectively, where those at 853.0 and 870.0 eV correspond to Ni2+, and the peaks with banding energies of 855.1 and 873.0 eV correspond to Ni3+. Moreover, the peaks at 859.1, 863.1, 875.8 and 878.8 eV belong to satellite peaks.22,26 As shown in Fig. 1f, the peaks at 54.7 and 55.5 eV could be owing to Se 3d5/2 and Se 3d3/2, respectively. The peaks at 59.4 eV correspond to SeOx, which might result from oxidization of selenide.8,9,22
C, respectively.24–26 In the Co 2p spectrum (Fig. 1d), the peaks can be divided into two parts, corresponding to Co 2p3/2 and Co 2p1/2, respectively. The peaks situated at 778.3 and 793.3 eV can be ascribed to Co2+ in Co-NiSe2/C, and the peaks located at 780.0 and 796.3 eV correspond to Co3+, which might originate from surface oxidation of Co-NiSe2/C when exposed to air. In addition, the peaks at 783.1, 785.9, 799.3 and 801.8 eV correspond to satellite peaks.8,22 Similarly, in the Ni 2p spectrum (Fig. 1e), Ni 2p3/2 and Ni 2p1/2 split into two peaks, respectively, where those at 853.0 and 870.0 eV correspond to Ni2+, and the peaks with banding energies of 855.1 and 873.0 eV correspond to Ni3+. Moreover, the peaks at 859.1, 863.1, 875.8 and 878.8 eV belong to satellite peaks.22,26 As shown in Fig. 1f, the peaks at 54.7 and 55.5 eV could be owing to Se 3d5/2 and Se 3d3/2, respectively. The peaks at 59.4 eV correspond to SeOx, which might result from oxidization of selenide.8,9,22
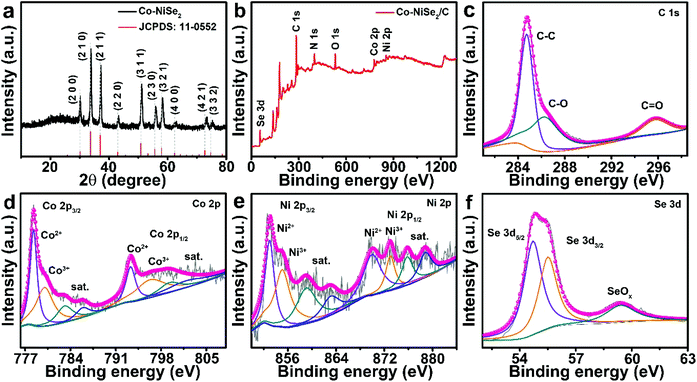 | ||
| Fig. 1 The (a) XRD pattern and (b) full XPS survey spectrum from Co-NiSe2/C. High resolution XPS spectra: (c) C 1s, (d) Co 2p, (e) Ni 2p, and (f) Se 3d. | ||
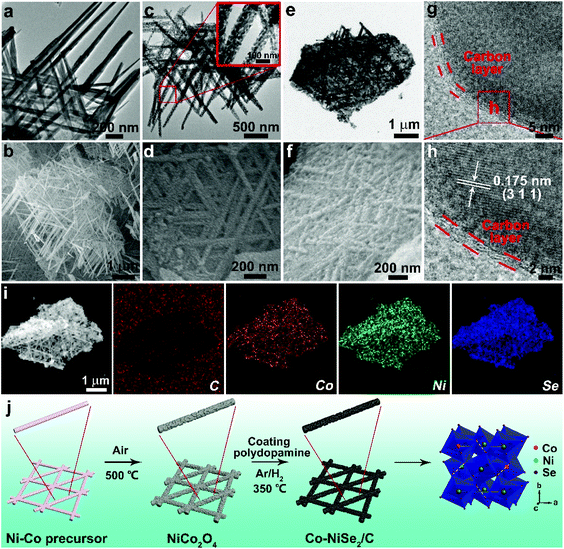
Ni–Co precursor was formed by a solvothermal method. Ni–Co precursors are made of interwoven nanorods, as shown in Fig. 2a and b. Subsequently, NiCo2O4 nanorods can be obtained after a heat treatment at 500 °C (Fig. S2†). According to Fig. 2c and d, it is clearly observed that the NiCo2O4 nanorods are composed of numerous nanoparticles with a size of 20 nm, which might be attributed to the difference in volume shrinkage of the precursors composed of the two metal ions during the heat treatment. Finally, the Co-NiSe2/C is fabricated through coating a polydopamine layer on the surface of the NiCo2O4 nanorods via a typical self-polymerization of dopamine hydrochloride followed by a selenization strategy. During this heat treatment, NiCo2O4 nanorods reacted with Se powder and formed Co-NiSe2 under Ar/H2 atmosphere. At the same time, the polydopamine coating on the NiCo2O4 surface transforms into a carbon layer covering the Co-NiSe2 nanoparticles. As shown in Fig. 2e and f, the interwoven nanorods maintained well after selenization process. The nanorods are intertwined and the surface is covered with a carbon layer. Fig. 2g shows the existence of the carbon layer which is beneficial to improving the stability and conductivity of the material during the charge/discharge processes. The presence of carbon can be further demonstrated by the Raman spectrum (Fig. S3a†). The emerging D peak (amorphous carbon) and G peak (graphitic carbon) located at around 1350.1 cm−1 and 1569.7 cm−1 demonstrate the existence of carbon, and the ratio of ID/IG = 1.0 indicates that the carbon layers on the Co-NiSe2 nanoparticles are mainly amorphous.27 As shown in Fig. S3b,† firstly, a slight decline before 200 °C can be ascribed to the loss of water,27 and next a weight gain can be attributed to the oxidation of NiSe2, generating NiO and SeO2 from 200 to 380 °C.27,28 An abrupt weight loss is mainly due to the volatilization of SeO2 before the temperature reaches 470 °C.27–29 During the second stage, there is an obvious weight loss from 470 to 590 °C, which is attributed to the carbon layer coated on the Co-NiSe2 nanoparticles starting combustion,25,26 and the carbon content was calculated as 13.2%. Finally, Co-NiSe2/C completely decomposed into NiO and SeO2.27 For Co-NiSe2, there is the same trend as with Co-NiSe2/C before 500 °C. The abrupt weight loss is mainly due to the volatilization of SeO2 and generation of NiO from 500 to 700 °C, and there is no platform, which further proves the existence of carbon in Co-NiSe2/C. The HRTEM image of Co-NiSe2/C shows that the lattice fringe spacing is 0.175 nm, which indexed as the (311) plane, and a thin carbon layer of 2 nm covered the surface of the Co-NiSe2 nanoparticle (Fig. 2h). Moreover, elemental mapping (Fig. 2i) further demonstrates the existence and distribution of C, Co, Ni and Se in Co-NiSe2/C. Fig. 2j illustrates the formation process of Co-NiSe2/C.
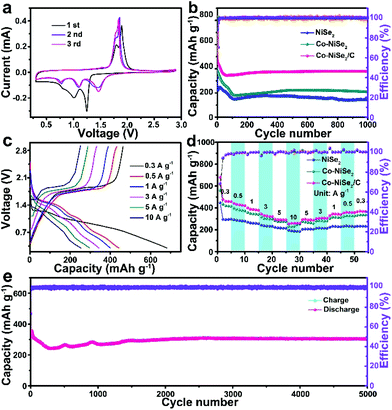
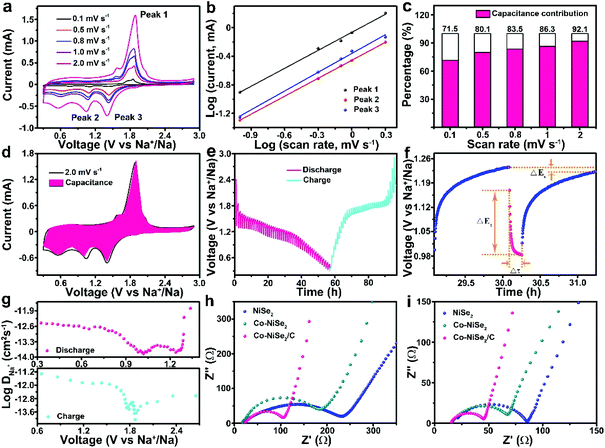
Fig. 3 displays the electrochemical performance of NiSe2, Co-NiSe2 and Co-NiSe2/C electrodes as an anode for SIBs. Fig. 3a shows the CV curves of the Co-NiSe2/C electrode. During the first cathodic scan, there are three reduction peaks located at 1.24 V, 0.99 V and 0.80 V, respectively. It could be put down to the insertion of Na+ into Co-NiSe2/C and the formation of Na2Se and metallic Ni, Co nanocrystals during the conversion reaction.22,30 Two adjacent oxidation peaks are located at 1.89 V and 1.81 V, which could be attributed to the deintercalation of Na+ and the recovery of Co-NiSe2/C during the anodic scan.31,32 Subsequently, the change in the position of the reduction peak may be related to the formation of fine nanocrystals. In addition, there is almost no change in the position of the oxidation peaks, indicating the excellent cycle stability of the Co-NiSe2/C. Moreover, the good overlap of the second and third curves show that the Na+ storage processes are highly reversible. As shown in Fig. 3b, the Co-NiSe2/C electrode delivers an excellent cycle performance. The discharge specific capacity gradually decreases from the initial capacity of 654.8 mA h g−1 to 345.0 mA h g−1 before the 60th cycle, and then the capacity begins to increase slowly to 378.1 mA h g−1 after 200 cycles. The capacity fluctuations in the initial stage may be related to the SEI collapses and the activation and remodeling of the structure.3 After this process, the capacity of Co-NiSe2/C gradually tends to be stabilized during the next discharge/charge processes. And the morphology of the Co-NiSe2/C did not change significantly after 60 and 200 cycles. The fence-like and porous structure is well preserved. After 1000 cycles, although the morphology structure has undergone obvious changes, the porous structure of the Co-NiSe2/C is still maintained, which demonstrates that the electrode structure is stable during the discharge/charge process (Fig. S4†). In contrast, there is a similar tendency for the NiSe2 and Co-NiSe2 electrode during the initial 200 cycles, but the NiSe2 and Co-NiSe2 electrodes display a lower discharge specific capacity of 209 and 147.5 mA h g−1, respectively. It proved that the Co doping and carbon layers are beneficial to improving the electrochemical performance and structure stability of Co-NiSe2/C. Co doping can increase the conductivity and short diffusion path, further accelerating the diffusion of Na+,22,23 and the carbon coating can also increase the structural stability of the Co-NiSe2 nanoparticles and inhibit volume expansion during the insertion/extraction process, so that the material maintains a high capacity. Fig. 3c delivers the charge and discharge voltage curves for the Co-NiSe2/C electrode at different current densities. The coulombic efficiencies are 68.05%, 97.47%, 97.50%, 96.63%, 97.51% and 99.11% at 0.3, 0.5, 1, 3, 5 and 10 A g−1. In the discharge voltage curves, the three discharge platforms are basically consistent with the corresponding three reduction peaks. Fig. 3d shows the rate capability of the NiSe2, Co-NiSe2 and Co-NiSe2/C electrodes. The Co-NiSe2/C electrodes exhibited discharge specific capacities of 458.0, 420.4, 378.4, 320.9 and 290.0 mA h g−1 at corresponding current densities of 0.3, 0.5, 1, 3 and 5 A g−1, respectively. Even at 10 A g−1, the discharge specific capacity reaches up to 259.5 mA h g−1, indicating an excellent rate capability. However, the discharge specific capacities of NiSe2 and Co-NiSe2 are lower than the Co-NiSe2/C electrode at each current density. The discharge specific capacity can maintain itself at 306.1 mA h g−1 after 5000 cycles at a current density of 5 A g−1, exhibiting an excellent long-term cycle life (Fig. 3e).
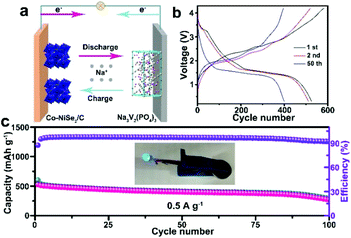
The full cell is assembled by using Na3V2(PO4)3 as a cathode and Co-NiSe2/C as an anode. The synthesis method of Na3V2(PO4)3 is according to a previous report.33 The XRD pattern, TEM images, charge–discharge curves and cycling performance of the Na3V2(PO4)3 cathode are shown in Fig. S5.† The working principles of this full cell are clearly illustrated in Fig. 4a. The Na+ extracts from Na3V2(PO4)3 and inserts into Co-NiSe2/C during the charge process, and in the discharge process, the Na+ returns to Na3V2(PO4)3 from Co-NiSe2/C. Fig. 4b displays the discharge/charge curves at different cycles for this full cell at 0.5 A g−1. It exhibits the first discharge/charge specific capacities of 525.5/600.9 mA h g−1, implying a high coulombic efficiency of 87.45%, and the coulombic efficiency quickly increases to 95.0% or above after the first cycles. Moreover, the full cell shows an excellent cycle performance, which still can maintain a discharge specific capacity of 269.1 mA h g−1 after 100 cycles at 0.5 A g−1 and the coulombic efficiency is higher than 92.3%. The inset image shows that the blue light-emitting diodes (LEDs) can be driven by one assembled cell (Fig. 4c). However, there is a continuous decrease during the cycles, which can be attributed to the following reasons. On the one hand, due to the amount of Na+ being fixed in the full cell, with the occurrence of side reactions and irreversible intercalation/deintercalation processes, no extra Na+ can compensate for the consumption of Na+. On the other hand, although this full cell displays a higher specific capacity using Na3V2(PO4)3 and Co-NiSe2/C, searching for a good cathode material to match Co-NiSe2/C is difficult. The performance of the full cell using selenide as the anode is shown in Table S1.† Our full battery shows good performance in both the capacity and cycle life.
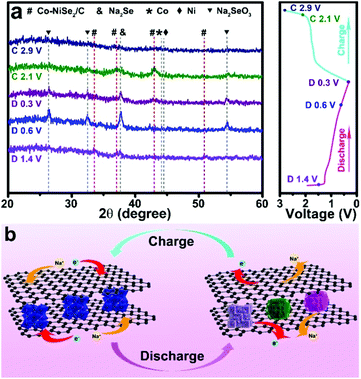
As shown in Fig. 5a, the Na storage mechanism is further investigated by ex situ XRD patterns. There is a coexistence of multiple phases when the electrode was discharged to 1.4 V. The existence of Co-NiSe2, Na2Se and metallic Ni, Co phases indicates that the phase transition has already begun to occur at the beginning of the discharge process, and further proves the fast Na+ migration. The diffraction peaks display an obvious phase change when the electrode discharges to 0.6 V, demonstrating the intercalation and conversion reaction between Na+ and Co-NiSe2 (Co-NiSe2 + 4Na + 4e− → 2Na2Se + 0.5Ni + 0.5Co).22,30 Until the discharge was terminated at 0.3 V, the phases show no significant change. In the charge processes, the diffraction peaks of Co-NiSe2/C reappear when the electrode charges to 2.1 V, which illustrates that the Na+ storage is highly reversible (2 Na2Se + 0.5Ni + 0.5Co → Co-NiSe2 + 4Na+ + 4e−).31,32 The intensity of the diffraction peaks is weak, which can be attributed to the formation of amorphous Co-NiSe2/C nanocrystals during the first cycle. In addition, the diffraction peaks which are not clearly defined can be attributed to the characteristic peaks of Na2SeO3, which can be ascribed to the occurrence of unavoidable side reactions in the conversion reaction. Ex situ XRD demonstrates that the phase transition that occurs during the sodium ion migration has good reversibility. The Na storage mechanism is expounded by the schematic diagram in Fig. 5b.
In order to explore the reaction kinetics of the Co-NiSe2/C electrode, CV measurements, GITT and EIS have been carried out to investigate the reaction kinetics. The CV technique of the Co-NiSe2/C at a series of scan rates (0.1 to 2.0 mV s−1) is used (Fig. 6a). The oxidation and reduction peaks shifted to higher and lower potentials with the scan rate increasing, respectively. It could be attributed to electrode polarization at high current densities.4,8 Based on eqn (1) and (2), the peaks 1, 2 and 3 are selected to do the next calculations. Furthermore, the b value can be calculated and it always showed a linear relationship.
| i = avb | (1) |
log(i) = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v) + log(a) log(v) + log(a) | (2) |
According to previous reports, the b values are 0.5 and 1.0, indicating a diffusion- and capacitive-controlled process, respectively.34,35 The b values of peak 1, 2 and 3 are 0.84, 0.85 and 0.88 (Fig. 6b), respectively, which indicate a mixed contribution from capacitive and diffusion-controlled processes. In addition, the contribution from capacitive and diffusion-controlled processes can be quantified by eqn (3):
| i = k1v + k2v1/2 | (3) |
In this equation, k1v represents the capacitive-controlled processes. Furthermore, it can be transformed to eqn (4):
| i/v1/2 = k1v1/2 + k2. | (4) |
The percentage of the capacitive-controlled process could be attained by calculating the value of b, which is displayed in Fig. 6c. The percentages of the capacitive-controlled process are 71.5%, 80.1%, 83.5%, 86.3% and 92.1% at 0.1, 0.5, 0.8, 1.0 and 2.0 mV s−1, respectively. Fig. 6d delivers the detailed capacitive-controlled fraction at 2.0 mV s−1. The excellent rate performance of the Co-NiSe2/C electrode is derived from a large capacitive-controlled percentage.
The reaction kinetics are further investigated by GITT tests. The GITT pulse duration is 10 min accompanied by a current of 0.1 A g−1, and the cell is allowed to relax for 60 min after each titration step (Fig. 6e).36 The procedure is repeated until a charge/discharge process is finished. The detailed voltage response of the Co-NiSe2/C electrode is shown in Fig. 6f, and the relevant parameters of Δτ, ΔEs and ΔEτ are schematically presented during a single-step GITT measurement. Because of an approximate linear relationship between Eτ and τ1/2, the diffusion coefficient of Na+ (DNa+) is defined by the following eqn (5):37,38
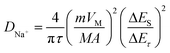 | (5) |
Fig. 6g demonstrates the DNa+ during the first charge/discharge processes. There are two decreases followed by increases from 1.5–2.1 V and 0.6–1.4 V during the charge/discharge processes. In the course of discharge, the first decrease might be due to the gradual generation of NaxCo-NiSe2 with insertion of Na+, and the migration of Na+ needing to surmount the resistance between the Co-NiSe2/C and NaxCo-NiSe2 phase interfaces. The first slight increase might be attributed to the incomplete conversion from Co-NiSe2/C to NaxCo-NiSe2. The second decrease can be ascribed to generation of NaxCo-NiSe2 and Na2Se, and the DNa+ increases secondly with the content of Na2Se gradually increasing. Finally, the Co-NiSe2/C completely converts to Na2Se when the DNa+ reached the highest value as the discharge finished. The change of DNa+ of charge is the same as discharge. In total, migrating ions must overcome the resistance between phase interfaces.8,39
In order to further understand the reaction kinetics, the EIS of the NiSe2, Co-NiSe2 and Co-NiSe2/C electrode before and after 50 cycles at 0.1 A g−1 are shown in Fig. 6h and i. The depressed semicircle and inclined line located at high frequency, middle frequency and low frequency regions composed Nyquist plots are related to the interface resistance (Rs), charge-transfer resistance (Rct), and the Warburg impedance (Zw) of ion diffusion in the electrode.36,40 The EIS spectra comparison results indicate that the impedance values of NiSe2, Co-NiSe2 and Co-NiSe2/C electrodes tend to decrease after 50 cycles. The smaller resistance of the Co-NiSe2/C electrode can be put down to the Co doping and carbon layer, which significantly increase the conduction and reduce the internal resistance of the battery.
Conclusions
Co-NiSe2/C nanosheets are fabricated though using a facile solvothermal and selenization method, and they display excellent long-term cycling performance (306.1 mA h g−1 after 5000 cycles at 5 A g−1), rate performance (260.1 mA h g−1 at 10 A g−1), and full-cell performance (269.1 mA h g−1 after 100 cycles at 0.5 A g−1) as an anode for SIBs. Ex-XRD, CV measurement, GITT, and EIS studies illustrate the Na storage mechanism and the reaction kinetics, which further confirm why the Co-NiSe2/C electrode has excellent electrochemical performance. Therefore, Co-NiSe2/C nanosheets have great potential for application as an anode material for SIBs.Experimental
Synthesis of NiCo2O4 nanorods
0.1524 g Ni(NO3)2·6H2O, 0.2048 g Co(NO3)2·6H2O, 0.2048 g urea and 0.2048 g NH4F were stirred and dissolved in 22 ml deionized water and 11 ml ethylene glycol. Then the mixed solution was poured into a 50 ml Teflon-lined stainless-steel autoclave and heated at 120 °C for 12 h. The precursors were obtained by washing with ethanol and water and drying at 60 °C. Subsequently, the obtained precursors were calcined at 500 °C for 3 h in air.Synthesis of Co-NiSe2/C
0.05 g NiCo2O4 and 0.05 g dopamine hydrochloride were added to 50 ml deionized water with stirring for 24 h, then 0.5 ml NH3·H2O was dropped into the above solution. The obtained solid sample was vacuum-dried overnight at room temperature. The samples and selenium power were mixed together with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and heated at 350 °C for 6 h under Ar/H2 (5 vol% H2) atmosphere. At last, Co-NiSe2/C could be obtained. At the same time, pure Co-NiSe2 or NiSe2 could be obtained without dopamine hydrochloride or Co(NO3)2·6H2O, respectively.
2 and heated at 350 °C for 6 h under Ar/H2 (5 vol% H2) atmosphere. At last, Co-NiSe2/C could be obtained. At the same time, pure Co-NiSe2 or NiSe2 could be obtained without dopamine hydrochloride or Co(NO3)2·6H2O, respectively.
Material characterization
The crystalline structure was measured by X-ray power diffraction (XRD, Bruker D8). The morphology structure of the products was investigated by scanning electron microscopy (SEM, Zeiss G500), transmission electron microscopy (TEM, JEM-1400Flash), high-resolution transmission electron microscopy (HRTEM, JEOL 2100). X-ray photoelectron spectroscopy (XPS, VGESCALABMK) was used to measure the physical and chemical properties.Electrochemical measurements
The 70 wt% active material, 20 wt% super P and 10 wt% polyvinylidene fluoride (PVDF) were dispersed in NMP and then painted onto copper foil. Further, it was tailored to a plate with a diameter of 12 mm. Glass fiber filter paper (Grade GF/D) as the separator, 1.0 M sodium trifluoromethanesulfonate (NaCF3SO3) in diethyleneglycol dimethylether (DEGDME) as the electrolyte, and sodium metal as the counter electrode were assembled in an argon-filled glovebox. The CV curves were determined by an electrochemical workstation (CHI 760E), the charge/discharge tests and rate performance were achieved by a Land battery test system (CT 2001A), and EIS were conducted by Modulab XM ECS. In the full cells, the Co-NiSe2/C anode and Na3V2(PO4)3 cathode coupled under the capacity ratio of anode/cathode was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.36.
1.36.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by the National Natural Science Foundation of China (grant number 11774188), the Natural Science Foundation of Shandong Province (grant number ZR2018QB003), and the Youth Innovation and Technology Support Plan of Shandong Province (No. 2019KJA006).Notes and references
- Y. L. Cao, L. F. Xiao, W. Wang, D. Choi, Z. Nie, J. G. Yu, L. V. Saraf, Z. G. Yang and J. Liu, Reversible sodium ion insertion in single crystalline manganese oxide nanowires with long cycle life, Adv. Mater., 2011, 23, 3155 CrossRef CAS.
- G. D. Li, L. Q. Xu, Y. J. Zhai and Y. P. Hou, Fabrication of hierarchical porous MnCo2O4 and CoMn2O4 microspheres composed of polyhedral nanoparticles as promising anodes for long-life LIBs, J. Mater. Chem. A, 2015, 3, 14298 RSC.
- H. L. Pan, Y. S. Hu and L. Q. Chen, Room-temperature stationary sodium-ion batteries for large-scale electric energy storage, Energy Environ. Sci., 2013, 6, 2338 RSC.
- F. B. Wang, G. D. Li, X. G. Meng, Y. X. Li, Q. F. Gao, Y. Q. Xu and W. F. Cui, FeS2 nanosheets encapsulated in 3D porous carbon spheres for excellent Na storage in sodium-ion batteries, Inorg. Chem. Front., 2018, 5, 2462–2471 RSC.
- Z. Z. Zhang, Y. C. Du, Q. C. Wang, J. Y. Xu, Y. N. Zhou, J. C. Bao, J. Shen and X. S. Zhou, A yolk-shell-structured FePO4 cathode for high-rate and long-cycling sodium-ion batteries, Angew. Chem., Int. Ed., 2020, 59, 17504–17510 CrossRef CAS.
- J. Y. Xu, E. L. Gu, Z. Z. Zhang, Z. H. Xu, Y. F. Xu, Y. C. Du, X. S. Zhu and X. S. Zhou, Fabrication of porous Na3V2(PO4)3/reduced graphene oxide hollow spheres with enhanced sodium storage performance, J. Colloid Interface Sci., 2020, 567, 84–91 CrossRef CAS.
- L. Q. Xu, J. Y. Li, C. Liu, G. Q. Zou, H. S. Hou and X. B. Ji, Research progress in inorganic solid-state electrolytes for sodium-ion batteries, Acta Phys.-Chim. Sin., 2020, 36, 1905013 Search PubMed.
- S. W. Fan, G. D. Li, F. P. Cai and G. Yang, Synthesis of porous Ni-doped CoSe2/C nanospheres towards high-rate and long-term sodium-ion half/full batteries, Chem. – Eur. J., 2020, 26, 8579–8587 CrossRef CAS.
- J. Chen, A. Q. Pan, Y. P. Wang, X. X. Cao, W. C. Zhang, X. Z. Kong, Q. Su, J. D. Lin, G. Cao and S. Liang, Hierarchical mesoporous MoSe2@CoSe/N-doped carbon nanocomposite for sodium ion batteries and hydrogen evolution reaction applications, Energy Storage Mater., 2019, 21, 97 CrossRef.
- M. H. Luo, H. X. Yu, F. Y. Hu, T. T. Liu, X. Cheng, R. T. Zheng, Y. Bai, M. Shui and J. Shu, Metal selenides for high performance sodium ion batteries, Chem. Eng. J., 2020, 380, 122557 CrossRef CAS.
- G. H. Newman and L. P. Klemann, Ambient temperature cycling of an Na-TiS2 cell, J. Electrochem. Soc., 1980, 127, 2097–2099 CrossRef CAS.
- T. Zheng, G. D. Li, L. X. Zhao, Y. X. Shen and X. G. Meng, Flowerlike Sb2S3/PPy microspheres used as anode material for high-performance sodium-ion batteries, Eur. J. Inorg. Chem., 2018, 10, 1224–1228 CrossRef.
- T. Zheng, G. D. Li, J. H. Dong, Q. Q. Sun and X. G. Meng, Self-assembled Mn-doped MoS2 hollow nanotubes with significantly enhanced sodium storage for high-performance sodium-ion batteries, Inorg. Chem. Front., 2018, 5, 1587–1593 RSC.
- Y. P. Gao, X. Wu, K. J. Huang, L. L. Xing, Y. Y. Zhang and L. Liu, Two-dimensional transition metal diseleniums for energy storage application: a review of recent developments, CrystEngComm, 2017, 19, 404–418 RSC.
- X. S. Wang, Z. H. Yang, C. Wang, D. Chen, R. Li, X. X. Zhang, J. T. Chen and M. Q. Xue, Buffer layer enhanced stability of sodium-ion storage, J. Power Sources, 2017, 369, 138–145 CrossRef CAS.
- Y. Lu, L. Yu and X. W. Lou, Nanostructured conversion-type anode materials for advanced lithium-ion batteries, Chem, 2018, 4, 972–996 CAS.
- J. F. Mao, T. F. Zhou, Y. Zheng, H. Gao, H. K. Liu and Z. P. Guo, Two-dimensional nanostructures for sodium-ion battery anodes, J. Mater. Chem. A, 2018, 6, 3284–3303 RSC.
- H. Y. Wu, X. Zhang, Q. H. Wu, Y. Han, X. Y. Wu, P. L. Ji, M. Zhou, G. W. Diao and M. Chen, Confined growth of 2D MoS2 nanosheets in N-doped pearl necklace-like structured carbon nanofibers with boosted lithium and sodium storage performance, Chem. Commun., 2020, 56, 141–144 RSC.
- Q. Zhou, L. Liu, Z. F. Huang, L. G. Yi, X. Y. Wang and G. Z. Cao, Co3S4@polyaniline nanotubes as high-performance anode materials for sodium ion batteries, J. Mater. Chem. A, 2016, 4, 5505–5516 RSC.
- J. L. Yang, S. N. Xiao, X. H. Cui, W. R. Dai, X. Lian, Z. K. Hao, Y. Zhao, J. S. Pan, Y. Zhou, L. Wang and W. Chen, Inorganic-anion-modulated synthesis of 2D nonlayered aluminum-based metal-organic frameworks as carbon precursor for capacitive sodium ion storage, Energy Storage Mater., 2020, 26, 391–399 CrossRef.
- S. T. Liu, D. Li, G. J. Zhang, D. D. Sun, J. S. Zhou and H. H. Song, Two-dimensional NiSe2/N-Rich Carbon Nanocomposites Derived from Ni-Hexamine Frameworks for Superb Na-Ion Storage, ACS Appl. Mater. Interfaces, 2018, 10, 34193–34201 CrossRef CAS.
- Y. Y. He, M. Luo, C. F. Dong, X. Y. Ding, C. C. Yin, A. M. Nie, Y. N. Chen, Y. T. Qian and L. Q. Xu, Coral-like NixCo1−xSe2 for Na-ion battery with ultralong cycle life and ultrahigh rate capability, J. Mater. Chem. A, 2019, 7, 3933 RSC.
- Y. J. Zhai, L. Q. Xu and Y. T. Qian, Ce-doped α-FeOOH nanorods as high-performance anode material for energy storage, J. Power Sources, 2016, 327, 423–431 CrossRef CAS.
- M. Yousaf, Y. J. Chen, H. Tabassum, Z. P. Wang, Y. S. Wang, A. Y. Abid, A. Mahmood, N. Mahmood, S. J. Guo, R. P. S. Han and P. Guo, A dual protection system for heterostructured 3D CNT/CoSe2/C as high areal capacity anode for sodium storage, Adv. Sci., 2020, 7, 1902907 CrossRef CAS.
- Y. Li, Y. H. Xu, Z. H. Wang, Y. Bai, K. Zhang, R. Q. Dong, Y. N. Gao, Q. Ni, F. Wu, Y. J. Liu and C. Wu, Stable carbon-selenium bonds for enhanced performance in tremella-like 2D chalcogenide battery anode, Adv. Energy Mater., 2018, 8, 1800927 CrossRef.
- P. Ge, S. J. Li, L. Q. Xu, K. G. Zou, X. Gao, X. Y. Gao, G. Q. Zou, H. S. Hou and X. B. Ji, Hierarchical hollow-microsphere metal-selenide@carbon composites with rational Surface engineering for advanced sodium storage, Adv. Energy Mater., 2019, 9, 1803035 CrossRef.
- S. Y. Lu, H. Wu, J. W. Hou, L. M. Liu, J. Li, C. J. Harris, C. Y. Lao, Y. Z. Guo, K. Xi, S. J. Ding, G. X. Gao, A. K. Cheetham and R. V. Kumar, Phase boundary engineering of metal-organic-framework-derived carbonaceous nickel selenides for sodium-ion batteries, Nano Res., 2020, 13, 2289–2298 CrossRef CAS.
- Y. Y. Wand, H. S. Fan, B. H. Hou, X. H. Rui, Q. L. Ning, Z. Cui, J. Z. Gao, Y. Yang and X. L. Wu, Ni1.5CoSe5 nanocubes embedded in 3D dual N-doped carbon network as advanced anode material in sodium-ion full cells with superior low temperature and high-power properties, J. Mater. Chem. A, 2018, 6, 22966 RSC.
- X. J. Xu, J. Liu, J. W. Liu, L. Z. Ouyang, R. Z. Hu, H. Wang, L. C. Yang and M. Zhu, A general metal-organic framework (MOF)-derived selenidation strategy for in situ carbon-encapsulated metal selenides as high-rate anodes for Na-ion batteries, Adv. Funct. Mater., 2018, 28, 1707573 CrossRef.
- B. H. Hou, Y. Y. Wang, D. S. Liu, Z. Y. Gu, X. Feng, H. S. Fan, T. F. Zhang, C. L. Lv and X. L. Wu, N-doped carbon-coated Ni1.8Co1.2Se4 nanoaggregates encapsulated in N-doped carbon nanoboxes as advanced anode with outstanding high-rate and low-temperature performance for sodium-ion half/full batteries, Adv. Funct. Mater., 2018, 28, 1805444 CrossRef.
- X. Ou, J. Li, F. H. Zheng, P. Wu, Q. C. Pan, X. H. Xiong, C. H. Yang and M. L. Liu, In situ X-ray diffraction characterization of NiSe2 as a promising anode material for sodium ion batteries, J. Power Sources, 2017, 343, 483 CrossRef CAS.
- S. W. Fan, G. D. Li, G. Yang, X. Guo and X. H. Niu, NiSe2 nanooctahedra as anodes for high-performance sodium-ion batteries, New J. Chem., 2019, 43, 12858–12864 RSC.
- Q. H. Zhang, X. D. Zhang, W. He, G. G. Xu, M. M. Ren, J. H. Liu, X. N. Yang and F. Wang, In situ fabrication of Na3V2(PO4)3 quantum dots in hard carbon nanosheets by using lignocelluloses for sodium ion batteries, J. Mater. Sci. Technol., 2019, 35, 2396–2403 CrossRef.
- V. Augustyn, P. Simon and B. Dunn, Pseudocapacitive oxide materials for high-rate electrochemical energy storage, Energy Environ. Sci., 2014, 7, 1597–1614 RSC.
- J. Wang, J. Polleux, J. Lim and B. Dunn, Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles, J. Phys. Chem. C, 2007, 111, 14925–14931 CrossRef CAS.
- X. Chen, N. Q. Zhang and K. N. Sun, Facile fabrication of CuO mesoporous nanosheet cluster array electrodes with super lithium-storage properties, J. Mater. Chem., 2012, 22, 13637–13642 RSC.
- J. Wang, L. Wang, C. Eng and J. Wang, Elucidating the irreversible mechanism and voltage hysteresis in conversion reaction for high-energy sodium-metal sulfide batteries, Adv. Energy Mater., 2017, 7, 1602706 CrossRef.
- X. F. Ge, S. H. Liu, M. Qiao, Y. C. Du, Y. F. Li, J. C. Bao and X. S. Zhou, Enabling superior electrochemical properties for highly efficient potassium storage by impregnating ultrafine Sb nanocrystals within nanochannel-containing carbon nanofibers, Angew. Chem., Int. Ed., 2019, 58, 14578–14583 CrossRef CAS.
- Y. X. Huang, Z. H. Wang, Y. Jiang, S. J. Li, Z. H. Li, H. Q. Zhang, F. Wu, M. Xie, L. Li and R. J. Chen, Hierarchical porous Co0.85Se@reduced graphene oxide ultrathin nanosheets with vacancy-enhanced kinetics as superior anodes for sodium-ion batteries, Nano Energy, 2018, 53, 524–535 CrossRef CAS.
- L. Wang, J. S. Zhao, X. M. He and C. R. Wan, Kinetic investigation of sulfurized polyacrylonitrile cathode material by electrochemical impedance spectroscopy, Electrochim. Acta, 2011, 56, 5252–5256 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qi01172j |
| This journal is © the Partner Organisations 2021 |