Effects of additive NH3 with citric acid in the precursor and controlling the deposited thickness for growing molybdenum oxide crystals and nanorods†
Yukiko
Hirose
a,
Tohru
Sugahara
 *a,
Jun-ichi
Nakamura
*bc,
Nobuyuki
Harada
b and
Katsuaki
Suganuma
a
*a,
Jun-ichi
Nakamura
*bc,
Nobuyuki
Harada
b and
Katsuaki
Suganuma
a
aDepartment of Advanced Interconnection Materials, The Institute of Science and Industrial Research, Osaka University, 8-1 Mihogaoka, Ibaraki, Osaka 567-0047, Japan. E-mail: sugahara@sanken.osaka-u.ac.jp
bNippon Shokubai Research Alliance Laboratories, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan. E-mail: jun-ichi_nakamura@shokubai.co.jp
cNippon Shiokubai Co., Ltd, 5-8 Nishi Otabi-cho, Suita, Osaka 564-0034, Japan
First published on 9th October 2020
Abstract
In recent years, researchers have successfully established a simple method for the production of fine single crystal-like α-MoO3 nanorod structures on substrates using a solution metal–organic decomposition (MOD) method to deposit film coatings. However, to grow α-MoO3 nanorods by the MOD method, an accurate mechanism between α-MoO3 nanorod extension and precursor preparation (contents of precursor conditions) should be identified. Here, we demonstrated controllable α-MoO3 nanorods and plate-like α-MoO3 crystal growth based on as-deposited film thickness by using different citric acid (CA) and ammonia (NH3) ratios in the precursor solutions. The α-MoO3 nanorod growth mechanisms are discussed in detail with the precursor conditions and deposition film thickness; excellent results were achieved in controlling the film thickness by preparation of the precursor viscosities. In addition, the effects of the CA and NH3 amounts on the α-MoO3 nanorods and crystal growth were investigated. It was found that NH3 plays an effective role in delaying the decomposition timing of CA, promotes the growth of α-MoO3 nanorods, and reduces the growth and increase of plate-like α-MoO3 crystals. The study obtained excellent results for the growth extension of α-MoO3 nanorods and plate-like α-MoO3 crystals, controlled by film thickness as the role of CA and NH3. The demonstrated strictly controlled α-MoO3 nanostructure forming process can be achieved with the solution MOD method.
Introduction
In recent years, molybdenum (Mo) compounds have attracted considerable attention due to their properties, such as the various valences of Mo, the presence of many crystal structures, and the complex band structure. In particular, many reports in the past decade have been published due to broad applications, such as catalysts,1,2 electrodes,3,4 semiconductors,5,6 sensors,7–9 and organic,10–12 electrochromic,13,14 and photochromic devices.15 For example, Mo oxides are very useful as electronic catalysts owing to their high electron affinity. The Mo cation makes octahedral or tetrahedral coordination with anions such as oxygen, sulfur, and other chalcogens in compounds that are easy to distort and react with the other cations of various elements. The Mo complex compounds, therefore, have been widely investigated as cooperative catalysts,1 and α-MoO3 can be used as hybrid catalysts, which have been broadly investigated as cooperative catalysts with various elements. Moreover, Mo is well known for its various valences from divalent to hexavalent, and compounds containing oxygen in quadrivalent, pentavalent, and hexavalent states. Various types of Mo oxides are found in stoichiometric and non-stoichiometric oxides, such as MoO2(IV), Mo2O5(V), and MoO3(VI). Non-stoichiometric Mo oxides have oxygen vacancies, with average values of various types of suboxides between 6.0 and 4.0. Mo oxides have a variety of electron band structures and are expected to be useful from metals to wide-gap semiconductors.Nanostructured materials and two-dimensional materials of compounds, and oxides, are widely applied to next-generation functional clean energy applications16 such as electric devices and catalysts for chemical reactions17 due to the characteristic electronic structures and physical properties of the materials. In particular, nanostructured materials and two-dimensional materials of Mo compounds typified by MoO3−δ have excellent properties.18 More precisely, it is the electrode reaction that is sped up by the high ratio surface area and the abilities of quick electric accumulation and discharge. It is expected to be a new material for supercapacitors or lithium ion batteries (LIBs).19,20 Nanostructured Mo compounds have a large specific surface area and unique chemical and physical properties. Notably, the characteristic properties are crucial for these multiple electronic devices.
In addition, forming and controlling nanostructures is an essential issue; nanostructured Mo compounds have been fabricated by a wide variety of methods, such as hydrothermal synthesis,21 PVD,22 RF magnetron sputtering,23,24 thermal evaporation, and molecular beam epitaxy. However, these methods consume significant amounts of energy and material resources, implying that they are un-eco-friendly. In recent years, researchers have focused on the synthesis of fine and nanostructured oxide materials by the metal–organic decomposition (MOD) method.7,12,25–29 The MOD method consumes low energy, and is environment friendly, and it can contribute to the realization of a sustainable society.
We found that the α-MoO3 nanorods initially grew from spin-coated thin films after sintering at 673 K for several minutes. Subsequently, the α-MoO3 nanorods underwent a transition to the plate-like α-MoO3 phase, when continuously sintered for a long time (over approximately 20 min, as illustrated in Fig. SI-1, ESI†), which was discussed along with the physical properties by gas sensing, and chemical composition by XRD and TEM in a previous paper.7,25 However, the behavior of the seed layer thickness with sintering time and the transitions were uninvestigated in detail. When the experimental conditions, for example, molar ratio, mixing of starting materials, and spin coating, are changed for several reasons, the growth of α-MoO3 nanorod arrays and the transition timings are significantly different depending on the experimental restriction, as illustrated in Fig. SI-1 and SI-2 (ESI†). The molybdenum oxide thin films obtained after sintering at 673 K for 15 min (in Fig. SI-1, ESI†) from the same precursor and the same coating conditions were significantly different film structures from the other precursor and coating conditions after sintering for 15 min (Fig. SI-2, ESI†).
The α-MoO3 nanorod growth mechanism and relationship of the transition timing is assumed to depend on each experimental condition; the authors had investigated this with TG/DT analysis and concluded a critical role of the decomposition timing of CA in the precursor, but this was unclarified in the previous studies.7,25 Although the CA contributes to the growth of α-MoO3 nanorods, it has been uncontrolled precisely due to the lack of clarity in the details of the α-MoO3 nanorod growth mechanism. In addition, the thicknesses of the seed layers, which are the under layers of the α-MoO3 nanorods, have been insufficiently investigated with α-MoO3 nanorod growth.
In this study, the growth and elongation of α-MoO3 nanorods were investigated with the content effects of CA and NH3 on Mo in the precursor. The relationship between the α-MoO3 nanorods/plate-like α-MoO3 crystal growth and the as-deposited coating thickness are discussed with the seed layer thicknesses. In addition, here we demonstrated the formation of a transparent α-MoO3 nanorod thin-film sample in which the α-MoO3 nanorods can be extended up to one micrometer, and the seed layer thickness can be reduced by approximately 50 nm by using the processing method of this study. The molybdenum oxide thin-film structures, such as the α-MoO3 nanorod length, the seed layer thickness, and the plate-like α-MoO3 crystal numbers, were precisely controlled by several experimental parameters, such as the amount of CA and NH3 and the spin-coating thickness of the precursor.
Experimental
Ammonium molybdate (H8N2O4Mo), ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O), tri-ammonium citrate ((NH4)3C6H5O7), CA (C6H8O7), and N,N-dimethylformamide (DMF) (C3H7NO (10 mL)) purchased from Fujifilm Wako Pure Chemical, Japan were used in this experiment. Each pair of ammonium molybdate and CA, ammonium molybdate tetrahydrate and CA, tri-ammonium citrate adjusted ammonium molybdate tetrahydrate and CA was dissolved in 10 mL of DMF at the respective ratios to obtain solution precursors with Mo concentrations ranging from 0.4 to 1.0 M. These precursors were stirred and dissolved with magnetic stirring at room temperature for 1 day to 14 days until clear and homogeneous solutions were obtained.For the synthesis of Mo oxide nanocrystals, the precursor was spin-coated at 1000–5000 rpm (MIKASA SPINCOATER 1H-DX2, Tokyo, Japan) on a silica glass substrate after cleaning with N2 plasma for 90 s. The deposited thin film was sintered at 673 K for 15 min in an electric furnace (DENKEN KDF-P90, Kyoto, Japan) or a temperature of 373 K for 15 min (AS ONE Electric Oven NEXAS series, OFX-50, Tokyo, Japan) to obtain thin film samples.
The top and cross-section morphological studies were carried out using field-emission scanning electron microscopy (FE-SEM) (Hitachi SU8020, Tokyo, Japan). The values of viscosity were measured using a viscometer (TOKI SANGYIO VISCOMETER TV-25 Type L) at 298 K. The thickness after sintering at 373 K for 15 min was verified using a laser microscope (KEYENCE VK-9510).
Results and discussion
Fine almost single crystal α-MoO3 nanorods grown from substrates were investigated by XRD and TEM analyses in the previous study.25 The longest α-MoO3 nanorods were obtained at the molar ratio of molybdenum (Mo), CA, and ammonium (NH3) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (0.5
2 (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0) in the previous study.7,25 Because the effect between the ratios of CA in the precursors and the coating process properties will be discussed in detail, three kinds of precursors with ratios of 1
1.0) in the previous study.7,25 Because the effect between the ratios of CA in the precursors and the coating process properties will be discussed in detail, three kinds of precursors with ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in 0.5 M were prepared in this experiment. Table 1 summarizes the precursor viscosities and the other experimental conditions of each precursor. The precursor viscosities increased with increasing concentrations of CA in the precursors, as shown in Table 1. The viscosity of 1
2 in 0.5 M were prepared in this experiment. Table 1 summarizes the precursor viscosities and the other experimental conditions of each precursor. The precursor viscosities increased with increasing concentrations of CA in the precursors, as shown in Table 1. The viscosity of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is approximately 20 mPa s, two times as large as the 1
2 is approximately 20 mPa s, two times as large as the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 precursor, and the viscosity of 1
2 precursor, and the viscosity of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is 90 mPa s, which is nine times higher than that of the 1
2 is 90 mPa s, which is nine times higher than that of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 precursor.
2 precursor.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 M)
0.5 M)
Sample ratio/Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 NH3 |
Viscositya [mPa s] | Rotational speed of spin coatingb [rpm] | Thickness after drying 373 K [μm] | Nanorod lengthc [nm] | Seed layer thicknessc [nm] |
|---|---|---|---|---|---|
| a The viscosity of the precursors measured at 298 K and 1.1 mL under the ambient atmosphere. b Spin coating conditions are fixed at about 1 μm after drying at 373 K for 15 min. c The values of nanorod length and thickness are measured after sintering at 673 K for 15 min. | |||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
9.6 | 1500 | 1.1 | ∼400 | ∼200 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
18.8 | 2000 | 1.0 | ∼500 | ∼100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
89.2 | 3000 | 1.4 | ∼1000 | ∼50 |
To fix the thickness of the as-deposited precursor, spin coating was performed in a preliminary experiment with three types of precursors. The fixed spin-coating conditions were 1500, 2000, and 3000 rpm for each of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 precursors, respectively, which were determined to give approximately 1 μm of film thickness after sintering at 473 K.
2 precursors, respectively, which were determined to give approximately 1 μm of film thickness after sintering at 473 K.
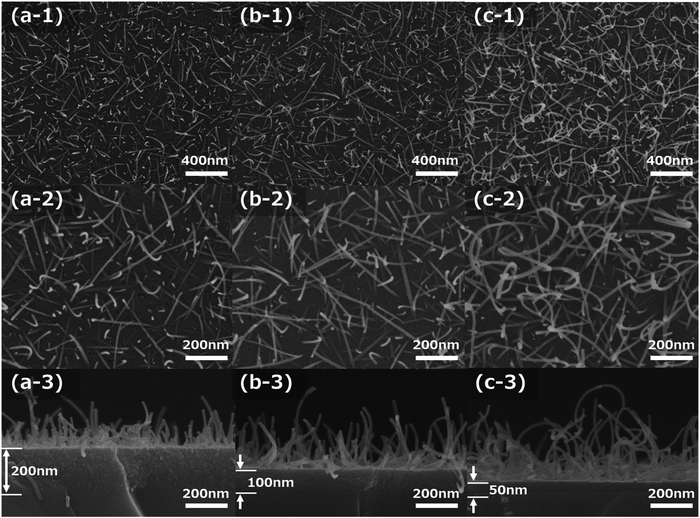
Fig. 1a-1–c-3 show the FE-SEM images of the molybdenum oxide thin-film samples obtained by growing on silica glass substrates after sintering at 673 K for 15 min, under the same conditions as in the preliminary experiment, that is, 1500 rpm of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 precursor, 2000 rpm of 1
2 precursor, 2000 rpm of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 precursor, and 3000 rpm of 1
2 precursor, and 3000 rpm of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 precursor, respectively. The top-view low magnification (×50
2 precursor, respectively. The top-view low magnification (×50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and high magnification (×100
000) and high magnification (×100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) FE-SEM images of the film samples are shown in Fig. 1a-1–c-1 and a-2–c-2, respectively. The cross-sectional FE-SEM images of the film samples are displayed in Fig. 1a-3–c-3. As Fig. 1a-1–c-3 illustrate, the α-MoO3 nanorods grew on the SiO2 glass substrate. The α-MoO3 nanorods grew almost uniformly on the surface of these samples, and the α-MoO3 nanorod length and the density in the film samples increased with increasing CA ratio in the precursors. The diameters of the α-MoO3 nanorods were almost the same at approximately 10 nm with changes in the other CA concentrations, as shown in the FE-SEM images in Fig. 1a-2–c-2. In addition, the α-MoO3 nanorod curved structures, and the curvatures are strong due to an increase in the CA ratio, as illustrated in Fig. 1a-2–c-2.
000) FE-SEM images of the film samples are shown in Fig. 1a-1–c-1 and a-2–c-2, respectively. The cross-sectional FE-SEM images of the film samples are displayed in Fig. 1a-3–c-3. As Fig. 1a-1–c-3 illustrate, the α-MoO3 nanorods grew on the SiO2 glass substrate. The α-MoO3 nanorods grew almost uniformly on the surface of these samples, and the α-MoO3 nanorod length and the density in the film samples increased with increasing CA ratio in the precursors. The diameters of the α-MoO3 nanorods were almost the same at approximately 10 nm with changes in the other CA concentrations, as shown in the FE-SEM images in Fig. 1a-2–c-2. In addition, the α-MoO3 nanorod curved structures, and the curvatures are strong due to an increase in the CA ratio, as illustrated in Fig. 1a-2–c-2.
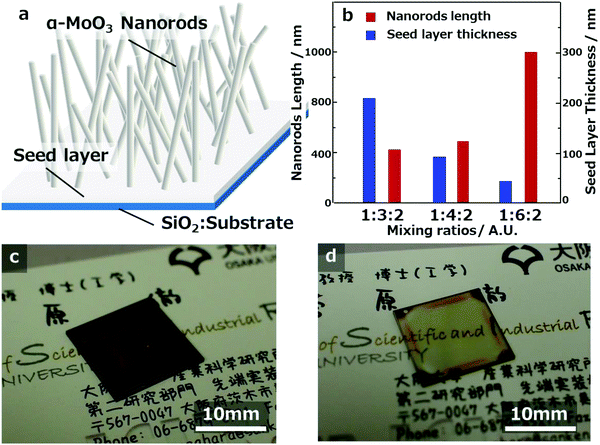
Fig. 2a shows the eye-catching schematic figure of the cross-sectional image of the molybdenum oxide thin-film samples. In the cross-sectional FE-SEM images of Fig. 1a-3–c-3, molybdenum oxide thin-film layers exist between the SiO2 substrate and the α-MoO3 nanorods structures, as shown in Fig. 2a. The layers are called seed layers because the α-MoO3 nanorods are grown from the seed layer, to decrease the thickness gradually with extending the sintering time, as shown in the FE-SEM images in Fig. SI-1 and SI-2 (ESI†). The thickness of the seed layers decreased with increasing CA ratio, and the total thickness of the seed layers and the α-MoO3 nanorod structure layers of these three samples look equivalent, as shown in Fig. 1a-3–c-3. It implies that the growth mechanism is strongly related to the as-deposited thickness (or volumes) of the precursors and the concentration of CA in the precursors.
From the top-view FE-SEM and cross-sectional FE-SEM images of Fig. 1, the lengths of the longest α-MoO3 nanorods and the thickness of the seed layer were measured and plotted in the graph of Fig. 2b. The data imply that the length of the α-MoO3 nanorods increased with increasing CA ratio. The longest lengths of the α-MoO3 nanorods were 400 nm at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 500 nm for 1
2, 500 nm for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1000 nm for 1
2, and 1000 nm for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. In contrast, the thicknesses of the seed layers decreased from approximately 200 nm for 1
2. In contrast, the thicknesses of the seed layers decreased from approximately 200 nm for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 100 nm for 1
2, 100 nm for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and up to 50 nm for 1
2, and up to 50 nm for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively, as the ratio of CA increased. Although the film thicknesses of these three samples were almost identical before sintering, the quantity of Mo included per unit volume is estimated to decrease with increasing CA ratio. This indicates that the length of the α-MoO3 nanorods increases with increasing CA ratio, despite the decrease in the amount of Mo per unit volume. This implies that the inclusion ratio of CA in the precursors has a significant influence on the growth and extension of α-MoO3 nanorods.
2, respectively, as the ratio of CA increased. Although the film thicknesses of these three samples were almost identical before sintering, the quantity of Mo included per unit volume is estimated to decrease with increasing CA ratio. This indicates that the length of the α-MoO3 nanorods increases with increasing CA ratio, despite the decrease in the amount of Mo per unit volume. This implies that the inclusion ratio of CA in the precursors has a significant influence on the growth and extension of α-MoO3 nanorods.
Owing to the almost fixed as-deposited thin-film thicknesses before sintering by spin coating, the α-MoO3 nanorod growth can be discussed with the seed layer thickness and CA amount in the precursors. When an extremely large amount of CA is included in the precursor, the seed layer may become thin, and the α-MoO3 nanorods are easily grown. On the other hand, the seed layers may become thick and the α-MoO3 nanorods are hardly grown when an extremely small amount of CA is present in the precursors. The α-MoO3 nanorods may grow from the seed layer, as described above, in parts of Fig. SI-1 and SI-2 (ESI†) because the seed layer thickness is related to the amount of CA in the precursor. The amount of CA played a significant role in the α-MoO3 nanorods growth, and the thicknesses of the seed layer decreased with α-MoO3 nanorods growth due to the use of CA and Mo compounds in the as-deposited precursor films.
To investigate the effect of film thickness, four types of samples were prepared with different spin-coating conditions using precursors with a fixed molar ratio of Mo, CA, and NH3 (0.4 mol L−1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
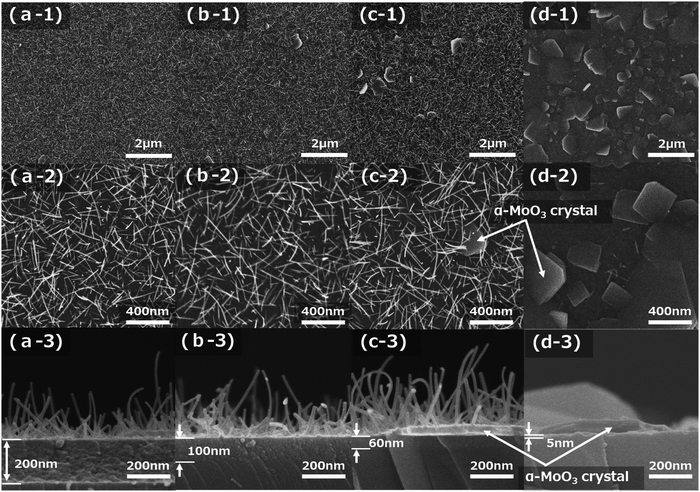
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Fig. 3a-1–d-3 show FE-SEM images after sintering at 673 K for 15 min of four kinds of molybdenum oxide thin-film samples obtained on silica glass substrates with spin-coating conditions of 1500, 2000, 2500, and 5000 rpm, respectively. The top-view low magnification (×10
2). Fig. 3a-1–d-3 show FE-SEM images after sintering at 673 K for 15 min of four kinds of molybdenum oxide thin-film samples obtained on silica glass substrates with spin-coating conditions of 1500, 2000, 2500, and 5000 rpm, respectively. The top-view low magnification (×10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and high magnification (×50
000) and high magnification (×50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) FE-SEM images of the film samples are shown in Fig. 3a-1–d-1 and a-2–d-2, respectively. The cross-sectional FE-SEM images of the film samples are displayed in Fig. 3a-3–d-3. The decrease in the number of α-MoO3 nanorods and the increase in the number of, plate-like α-MoO3 crystals, which are reported in the previous study,7,25 were observed with increasing spin-coating speeds, as shown in Fig. 3a-1–d-2. They are approximately 500 nm in length and the growth density of the α-MoO3 nanorods are almost the same as those shown in Fig. 3a-2–c-2. The size of the plate-like α-MoO3 crystals was observed from submicron to a few microns (Fig. 3d-1 and d-2). The thickness of the seed layers decreased with increasing spin-coating speeds, as shown in Fig. 3a-3–d-3. In particular, the seed layer thickness of the 5000 rpm sample is very thin (approximately 5 nm), with a few α-MoO3 nanorods and almost observing the plate-like α-MoO3 crystals (Fig. 3d-1 and d-2). Even though the thicknesses of the seed layers decreased from 200 nm to 60 nm, the length and diameter of the α-MoO3 nanorods were almost identical, as shown in Fig. 3a-3–c-3. Fig. 2c and d show that the color of the samples depends on the thickness of the seed layer.
000) FE-SEM images of the film samples are shown in Fig. 3a-1–d-1 and a-2–d-2, respectively. The cross-sectional FE-SEM images of the film samples are displayed in Fig. 3a-3–d-3. The decrease in the number of α-MoO3 nanorods and the increase in the number of, plate-like α-MoO3 crystals, which are reported in the previous study,7,25 were observed with increasing spin-coating speeds, as shown in Fig. 3a-1–d-2. They are approximately 500 nm in length and the growth density of the α-MoO3 nanorods are almost the same as those shown in Fig. 3a-2–c-2. The size of the plate-like α-MoO3 crystals was observed from submicron to a few microns (Fig. 3d-1 and d-2). The thickness of the seed layers decreased with increasing spin-coating speeds, as shown in Fig. 3a-3–d-3. In particular, the seed layer thickness of the 5000 rpm sample is very thin (approximately 5 nm), with a few α-MoO3 nanorods and almost observing the plate-like α-MoO3 crystals (Fig. 3d-1 and d-2). Even though the thicknesses of the seed layers decreased from 200 nm to 60 nm, the length and diameter of the α-MoO3 nanorods were almost identical, as shown in Fig. 3a-3–c-3. Fig. 2c and d show that the color of the samples depends on the thickness of the seed layer.
Using the methods employed in this study, the seed layer can be controlled without changing the growth length of α-MoO3 nanorods. The authors demonstrated that two kinds of seed layer thicknesses can be successfully coated with the spin-coating conditions of 1500 rpm and 2500 rpm. The α-MoO3 nanorod lengths are approximately 400 nm, as displayed in Fig. 2c and d and have seed layer thicknesses of approximately 200 nm and approximately 50 nm, respectively. The approximately 200 nm thick seed layer sample showed a transparent dark brown color, but the approximately 50 nm thick seed layer sample was transparent light brown. The results from these images imply that the seed layer thickness strongly correlated with the transparency, and related without α-MoO3 nanorod length and density.
Fig. 4 shows the number of α-MoO3 nanorods and plate-like α-MoO3 crystals, which were measured from the FE-SEM images in Fig. 3. The number of α-MoO3 nanorods per arbitrary unit area decreased sharply at approximately 40 nm of seed layer thickness. In contrast, the number of plate-like α-MoO3 crystals per arbitrary unit area increased sharply at almost identical seed layer thickness. These data imply that each of the numbers of α-MoO3 nanorods and plate-like α-MoO3 crystals are opposite in increasing or decreasing behavior at the critical seed layer thickness, as shown in Fig. 4. This indicates that we can control the number of α-MoO3 nanorods and/or plate-like α-MoO3 crystals by controlling the seed layer thickness.
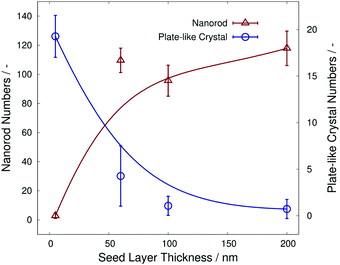 | ||
| Fig. 4 The α-MoO3 nanorod numbers and α-MoO3 plate-like crystal numbers as a function of each seed layer thickness. | ||
To investigate the effect of including the molar ratios of Mo, CA, and NH3 in the precursors, seven kinds of precursors were prepared in this experiment. The molar concentrations of the precursors were 0.5 M and 1.0 M, and the included molar ratios of Mo, CA, and NH3 were A(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), B(1
2), B(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), C(1
2), C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), D(1
2), D(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), E(1
2), E(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), F(1
1), F(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and G(1
1), and G(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively. Table 2 summarizes the mixing conditions of all precursor solutions used in this experiment, and the Mo contents shown as wt% in the solute and the other experimental conditions of each precursor. By fixing the Mo to NH3 ratios as 1
1), respectively. Table 2 summarizes the mixing conditions of all precursor solutions used in this experiment, and the Mo contents shown as wt% in the solute and the other experimental conditions of each precursor. By fixing the Mo to NH3 ratios as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, three kinds precursors of A(1
2, three kinds precursors of A(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), B(1
2), B(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and C(1
2), and C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were prepared, while the other three kinds of precursors, E(1
2) were prepared, while the other three kinds of precursors, E(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), F(1
1), F(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and G(1
1), and G(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), were prepared by fixing Mo
1), were prepared by fixing Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 as 1
NH3 as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The precursor D(1
1. The precursor D(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), with the same ratio as C(1
2), with the same ratio as C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), was prepared by the effect of the NH3 amount in the precursor using ammonium molybdate tetrahydrate. Because the same ratio of Mo
2), was prepared by the effect of the NH3 amount in the precursor using ammonium molybdate tetrahydrate. Because the same ratio of Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 (= 1
NH3 (= 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was adjusted in the precursor C(1
2) was adjusted in the precursor C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), triammonium citrate was added with ammonium molybdate tetrahydrate and CA.
2), triammonium citrate was added with ammonium molybdate tetrahydrate and CA.
| Sample name | Molar conc./Mo (M) | Weight conc. of Mo [wt%] | Sample ratio/Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 NH3 |
Rotational speed of spin coating [rpm] | Thickness after dryinga [μm] |
|---|---|---|---|---|---|
a Spin coating conditions are fixed at about 1 μm after drying at 373 K for 15 min.
b The sample D(Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 = 1 NH3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) is made from sample E(Mo 2) is made from sample E(Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 = 1 NH3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) + NH3 to prepare the same condition of C(Mo 1) + NH3 to prepare the same condition of C(Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 = 1 NH3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2).
|
|||||
A(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.5 | 7.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3000 | 1.4 |
B(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.5 | 10.7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2000 | 1.0 |
C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.5 | 13.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1500 | 1.1 |
D(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)b 2)b |
0.5 | 13.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1500 | 1.3 |
E(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.5 | 13.9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1150 | 1.3 |
F(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.0 | 13.9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3000 | 1.8 |
G(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.0 | 31.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1100 | 1.4 |
To fix the as-deposited film thickness, the spin-coating conditions were determined by preliminary experiments with seven kinds of precursors. Subsequently, the spin-coating conditions were fixed at 3000 rpm of the A(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) precursor, 2000 rpm of the B(1
2) precursor, 2000 rpm of the B(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) precursor, 1500 rpm of the C(1
2) precursor, 1500 rpm of the C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) precursor, 1500 rpm of the D(1
2) precursor, 1500 rpm of the D(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) precursor, 1150 rpm of the E(1
2) precursor, 1150 rpm of the E(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) precursor, 3000 rpm of the F(1
1) precursor, 3000 rpm of the F(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) precursor, and 1100 rpm of the G(1
1) precursor, and 1100 rpm of the G(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) precursor, respectively, which were determined to be approximately 1 to 1.8 μm after sintering at 473 K, as also displayed in Table 2.
2) precursor, respectively, which were determined to be approximately 1 to 1.8 μm after sintering at 473 K, as also displayed in Table 2.
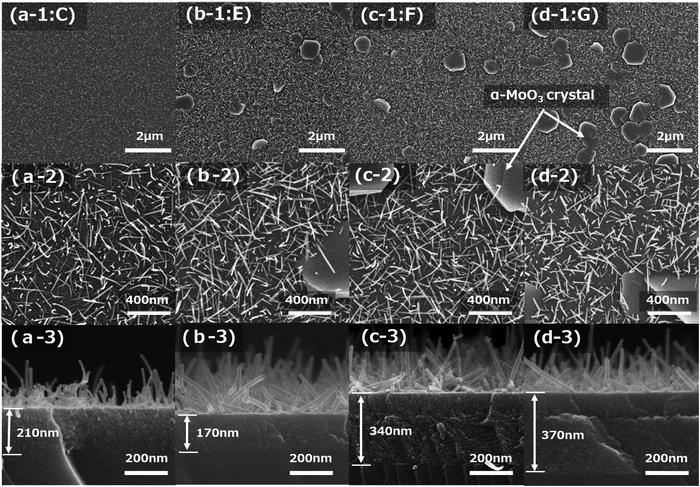
Fig. 5a-1–d-3 show FE-SEM images after sintering at 673 K for 15 min of four types of molybdenum oxide thin-film samples (C, E, F, and G), which were obtained on silica glass substrates with each spin-coating condition in Table 2. The top-view low magnification (×10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and high magnification (×50
000) and high magnification (×50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) FE-SEM images of the film samples are shown in Fig. 5a-1–d-1 and a-2–d-2, respectively. The cross-sectional FE-SEM images of the film samples are displayed in Fig. 5a-3–d-3.
000) FE-SEM images of the film samples are shown in Fig. 5a-1–d-1 and a-2–d-2, respectively. The cross-sectional FE-SEM images of the film samples are displayed in Fig. 5a-3–d-3.
The number of plate-like α-MoO3 crystals increased as the concentration of NH3 decreased. This is apparent in the comparison of Fig. 5a-1 and b-1. Similarly, the comparison of Fig. 5c-1 and d-1 shows that the number of plate-like α-MoO3 crystals increased with decreasing CA concentration. The comparison of Fig. 5a-2 and b-2 demonstrates that the lengths of the α-MoO3 nanorods are almost the same for different concentrations of NH3. Meanwhile, comparing Fig. 5c-2 with Fig. 5d-2 shows that the length of the α-MoO3 nanorods decreased with a decreasing concentration of CA. It is noteworthy that the seed layers of almost all the samples consisted of two layers, as shown in Fig. 5c-3 and d-3; however, the detailed mechanism was not determined in this study.
Fig. SI-3 (ESI†) shows top-view FE-SEM images of molybdenum oxide film samples with different NH3 concentrations of C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), D(1
2), D(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and E(1
2), and E(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). These samples were obtained on silica glass substrates after sintering at 673 K for 15 min, under the spin-coating conditions listed in Table 2. The α-MoO3 nanorods of sample E(1
1). These samples were obtained on silica glass substrates after sintering at 673 K for 15 min, under the spin-coating conditions listed in Table 2. The α-MoO3 nanorods of sample E(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were grown straight; on the other hand, the α-MoO3 nanorods of samples D(1
1) were grown straight; on the other hand, the α-MoO3 nanorods of samples D(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and C(1
2) and C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were grown curling, as shown in Fig. SI-3 (ESI†). The sample D(1
2) were grown curling, as shown in Fig. SI-3 (ESI†). The sample D(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was prepared by adding NH3 amounts from sample E(1
2) was prepared by adding NH3 amounts from sample E(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to adjust the same ratio of sample C(1
1) to adjust the same ratio of sample C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). It is presumed that the α-MoO3 nanorods underwent bending as the NH3 concentration increased; however, the NH3 role has not been clarified in this study.
2). It is presumed that the α-MoO3 nanorods underwent bending as the NH3 concentration increased; however, the NH3 role has not been clarified in this study.
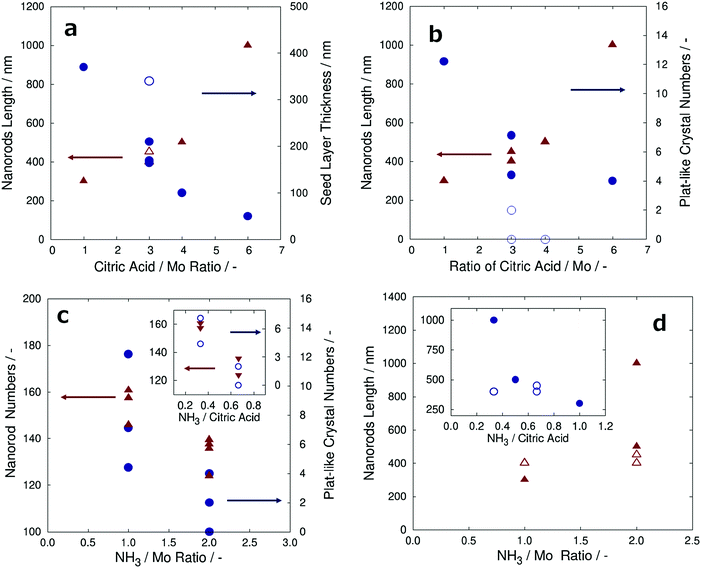
To better understand these experiments, the measured values of the α-MoO3 nanorods length, α-MoO3 nanorod numbers, seed layer thickness, and plate-like α-MoO3 crystal numbers are summarized with the amount ratio of NH3/Mo, CA/Mo, and NH3/CA, as shown in Fig. 6a–d. It is noteworthy that the number of α-MoO3 nanorods and plate-like α-MoO3 crystals were collected from the FE-SEM images divided into the grid of arbitrary unit area. Fig. 6a indicates the length of the α-MoO3 nanorods (triangles) and the thickness of the seed layer (circles) versus the ratio of CA to Mo. These data were measured by top-view FE-SEM and cross-sectional FE-SEM images from the total of seven samples in Table 2 (including Fig. 5). The length of the α-MoO3 nanorods increased and the thickness of the seed layer decreased, as the ratio of CA to Mo increased; this is shown in Fig. 6a. This result implies that α-MoO3 nanorods may not grow without the CA. Although the sample of the blue open circle symbol was three on the ratio of CA to Mo, the thickness of the seed layer was approximately 350 nm, deviating from the original tendency, which implies that its thickness may be observed around 200 nm. This is probably because the film thickness after sintering at 373 K is approximately 1.8 μm, which is thicker than those of the other samples, as shown in Table 2.
Fig. 6b shows the length of the α-MoO3 nanorods (triangles) and the number of plate-like α-MoO3 crystals per arbitrary unit area (circles) versus the ratio of CA to Mo. These data were collected from the top-view FE-SEM and cross-sectional FE-SEM images from a total of seven samples. As the ratio of CA to Mo increased, the number of plate-like α-MoO3 crystals per arbitrary unit area decreased, and the length of the α-MoO3 nanorods increased, as shown in Fig. 6b. More precisely, it is assumed that sufficient amount of CA is necessary to grow the α-MoO3 nanorods, while suppressing the growth of plate-like α-MoO3 crystals. The blue open circle symbol samples were 3–4 in the ratio of CA to Mo, and the plate-like α-MoO3 crystal numbers were 0–2, which deviated from the prospective tendency. Approximately 4–6 plate-like α-MoO3 crystals were observed in these samples that had the expected tendency. This result strongly implies the NH3 content effect with the ratio of the CA to Mo, suggesting the method to determine all parameters related to the ratio of amount of NH3 to that of CA.
To determine the effect of the amount of NH3 on the growth of α-MoO3 nanorods and plate-like α-MoO3 crystals, the ratio of NH3 to Mo and NH3 to CA in the precursors is discussed in this paragraph. Fig. 6c shows the number of α-MoO3 nanorods (triangles) and the number of plate-like α-MoO3 crystals per arbitrary unit area (circles) versus the ratios of NH3/Mo. These data were collected from the FE-SEM images of all the samples given in Table 2. As the ratio of NH3 to Mo increased, both the α-MoO3 nanorod numbers and plate-like α-MoO3 crystal numbers decreased, as shown in Fig. 6c. However, the number of plate-like α-MoO3 crystals is affected by the concentration of CA, as shown in Fig. 6b. To clarify the influence of the amount of NH3 separate from the effect of CA amount, the number of α-MoO3 nanorods and plate-like α-MoO3 crystals was segregated and plotted as a function of the ratio of NH3 to CA with a fixed ratio of CA to Mo in the inset graph of Fig. 6c. The inset graph of Fig. 6c demonstrates that the number of both α-MoO3 nanorods and plate-like α-MoO3 crystals decreased with an increase in the NH3 to CA ratio. These results suggest that not only the amount of CA but also the amount of NH3 has an effective role in the growth of both α-MoO3 nanorods and plate-like α-MoO3 crystals.
Fig. 6d shows plots of the length of α-MoO3 nanorods (triangles) as a function of the NH3 to Mo ratio. The lengths of the α-MoO3 nanorods seem to extend with an increase in the NH3 to Mo ratio, as shown in Fig. 6d. However, the length of the α-MoO3 nanorods increased with an increase in the amount of CA. To determine the influence of the amount of NH3, samples with the same ratio of CA to Mo are plotted with symbols of open triangles in Fig. 6d. These open triangle plots show that in spite of the increase in the NH3 to Mo ratio, the length of the α-MoO3 nanorods remained unchanged. Moreover, the α-MoO3 nanorod lengths are plotted as a function of the NH3 to CA ratio. It seems to decrease as the NH3 to CA ratio increases, as shown in the inset graph of Fig. 6d. However, samples with the same ratio of Mo to CA (open circle) are displayed, and the α-MoO3 nanorod lengths remained unchanged even if the NH3 to CA ratio increases. These results show that the intrinsic amount of NH3 does not affect the growth of α-MoO3 nanorods.
As a consequence, this has demonstrated that increasing the NH3 amount in the precursor has reducing effects on both the number of α-MoO3nanorods and plate-like α-MoO3 crystals without the effective growth of the length of the nanorods. Moreover, the plots of sample D(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) showed almost identical tendencies as sample C(1
2) showed almost identical tendencies as sample C(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in all of Fig. 6a–d. The results suggest that the NH3 concentration in the precursor can be controlled regardless of the ammonium molybdate reagent.
2) in all of Fig. 6a–d. The results suggest that the NH3 concentration in the precursor can be controlled regardless of the ammonium molybdate reagent.
Conclusions
The formation mechanism of the molybdenum oxide nanostructure, that is, nanorods and plate-like crystals on the seed layer by the MOD method, was examined in this study. The details of α-MoO3 nanorod growth in the molybdenum oxide thin films and the phase transition to plate-like α-MoO3 crystals of α-MoO3 nanorods were investigated, with the controlled precursor coating thickness under each spin-coating condition. The precursor viscosities were changed through the mixing ratios of the raw materials, such as molybdenum (Mo), citric acid (CA), ammonia (NH3), and solvent.The additive effect of CA in the precursor was investigated in detail with α-MoO3 nanorod growth. The result implied that the length of the grown α-MoO3 nanorods increased with an increase in the CA to Mo ratio, which implies that CA is an essential agent for α-MoO3 nanorod growth. In addition, the α-MoO3 crystal growth was examined considering the influence of the coating film thickness by changing the spin-coating conditions. The result implies that the phase transition from the α-MoO3 nanorods to the plate-like α-MoO3 crystals is highly dependent on the thickness of the as-deposited film coating. In optimizing the as-deposited coating thickness, only the α-MoO3 nanorods grew significantly; however, when the thickness of the coating was thin, the number of plate-like α-MoO3 crystals increased because of the occurrence of the phase transition of the α-MoO3 nanorods. When the film thickness was extremely thin after coating, almost all the α-MoO3 nanorods underwent the phase transition and formed a large number of plate-like α-MoO3 crystals.
The correlation between the seed layer thickness and the α-MoO3 crystal after sintering was also investigated. The number of α-MoO3 nanorods significantly increased with increasing seed layer thickness, but the number of plate-like α-MoO3 crystals sharply decreased at approximately 50 nm of the seed layer thickness after sintering at 673 K for 15 min. The results suggest that the phase transition from α-MoO3 nanorods to plate-like α-MoO3 crystals proceeded rapidly when the seed layer thickness was less than 50 nm after sintering. Based on these results and the fact that the α-MoO3 nanorods grew in the initial stage of the sintering, they underwent the phase transition to the plate-like α-MoO3 crystals in the second stage. This study implies that the as-deposited thicknesses after spin coating are important factors for the number of α-MoO3 nanorods and plate-like α-MoO3 crystals.
Furthermore, the effects of the growth of α-MoO3 nanorods and the transition to the plate-like α-MoO3 crystals were investigated with the amounts of CA and NH3 to Mo in the precursor. The large amount of NH3 to CA in the precursor suppressed the thermal decomposition of CA, and the α-MoO3 nanorods could be extended without the phase transition to the plate-like α-MoO3 crystals. The results of the experiments clarified that the CA role for α-MoO3 nanorod growth and extension, and the existence of NH3 delayed the decomposition timing of CA; consequently, the phase transition to plate-like α-MoO3 crystal timing could be controlled by changing the ratio of NH3 to CA in the precursor.
From these results, it is concluded that the α-MoO3 nanorod thin-film structures, that is, the α-MoO3 nanorod length, the seed layer thickness, and the number of plate-like α-MoO3 crystals, can be controlled by varying the amount of CA and NH3 in the precursor and the thickness of the spin-coated film. Moreover, using the methods proposed in this study, we demonstrated the fabrication of transparent thin films with α-MoO3 nanorods; the longest nanorod length was almost 1 μm. A detailed control technique to obtain a molybdenum oxide thin-film nanostructure was obtained in this research, which is a process to produce nanostructured thin films in a short time. This method may contribute to the development of various technologies for sustainable low energy societies in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
A part of this work is supported by Grant-in-Aid for Challenging Exploratory Research Grant Numbers “16K13637” and the Programs for JST CREST Grant Number of “JP MJCR19J1” from Japan Science and Technology Agency (JST). In addition, all the authors acknowledge funding of a part of this work from “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in “Network Joint Research Center for Materials and Devices” from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).References
- Z. Zhang, R. Yang, Y. Gao, Y. Zhao, J. Wang, L. Huang, J. Guo, T. Zhou, P. Lu, Z. Guo and Q. Wang, Novel Na2Mo4O13/α-MoO3 hybrid Material as Highly Efficient CWAO Catalyst for Dye Degradation at Ambient Conditions, Sci. Rep., 2014, 4, 1–9, DOI:10.1038/srep06797.
- Y. Liu, P. Feng, Z. Wang, X. Jiao and F. Akhtar, Novel Fabrication and Enhanced Photocatalytic MB Degradation of Hierarchical Porous Monoliths of MoO3 Nanoplates, Sci. Rep., 2017, 7(1), 1–12, DOI:10.1038/s41598-017-02025-3.
- J. Miao, F. X. Xiao, H. B. Yang, S. Y. Khoo, J. Chen, Z. Fan, Y. Y. Hsu, H. M. Chen, H. Zhang and B. Liu, Hierarchical Ni-Mo-S Nanosheets on Carbon Fiber Cloth: A Flexible Electrode for Efficient Hydrogen Generation in Neutral Electrolyte, Sci. Adv., 2015, 1(7), e150029, DOI:10.1126/sciadv.1500259.
- L. Zhang, S. Zheng, L. Wang, H. Tang, H. Xue, G. Wang and H. Pang, Fabrication of Metal Molybdate Micro/Nanomaterials for Electrochemical Energy Storage, Small, 2017, 13(33), 1–19, DOI:10.1002/smll.201700917.
- M. Choi, Y. J. Park, B. K. Sharma, S. R. Bae, S. Y. Kim and J. H. Ahn, Flexible Active-Matrix Organic Light-Emitting Diode Display Enabled by MoS2 Thin-Film Transistor, Sci. Adv., 2018, 4(4), 1–8, DOI:10.1126/sciadv.aas8721.
- M. Zhong, C. Shen, L. Huang, H. X. Deng, G. Shen, H. Zheng, Z. Wei and J. Li, Electronic Structure and Exciton Shifts in Sb-Doped MoS2 Monolayer, npj 2D Mater. Appl., 2019, 3, 1, DOI:10.1038/s41699-018-0083-1.
- S. Cong, T. Sugahara, T. Wei, J. Jiu, Y. Hirose, S. Nagao and K. Suganuma, Diverse Adsorption/Desorption Abilities Originating from the Nanostructural Morphology of VOC Gas Sensing Devices Based on Molybdenum Trioxide Nanorod Arrays, Adv. Mater. Interfaces, 2016, 3(14), 1–8, DOI:10.1002/admi.201600252.
- S. J. Xiao, X. J. Zhao, Z. J. Chu, H. Xu, G. Q. Liu, C. Z. Huang and L. Zhang, New Off-On Sensor for Captopril Sensing Based on Photoluminescent MoOx Quantum Dots, ACS Omega, 2017, 2(4), 1666–1671, DOI:10.1021/acsomega.7b00088.
- B. S. Archanjo, P. F. Siles, C. K. B. Q. M. Oliveira, D. L. Baptista and B. R. A. Neves, Characterization of Metal Oxide-Based Gas Nanosensors and Microsensors Fabricated via Local Anodic Oxidation Using Atomic Force Microscopy, Adv. Mater. Sci. Eng., 2013, 2013, 898565, DOI:10.1155/2013/898565.
- X. Y. Jiang, Z. L. Zhang, J. Cao, M. A. Khan, K. U. Haq and W. Q. Zhu, White OLED with High Stability and Low Driving Voltage Based on a Novel Buffer Layer MoOx, J. Phys. D: Appl. Phys., 2007, 40(18), 5553–5557, DOI:10.1088/0022-3727/40/18/007.
- M. T. Greiner and Z. H. Lu, Thin-Film Metal Oxides in Organic Semiconductor Devices: Their Electronic Structures, Work Functions and Interfaces, NPG Asia Mater., 2013, 5(7), 1–16, DOI:10.1038/am.2013.29.
- S. Cong, A. Hadipour, T. Sugahara, T. Wei, J. Jiu, S. Ranjbar, Y. Hirose, M. Karakawa, S. Nagao, T. Aernouts and K. Suganuma, Modifying the Valence State of Molybdenum in the Efficient Oxide Buffer Layer of Organic Solar Cells via a Mild Hydrogen Peroxide Treatment, J. Mater. Chem. C, 2017, 5(4), 889–895, 10.1039/c6tc04461a.
- A. Hasani, Le, Q. Van, T. P. Nguyen, K. S. Choi, W. Sohn, J. K. Kim, H. W. Jang and S. Y. Kim, Facile Solution Synthesis of Tungsten Trioxide Doped with Nanocrystalline Molybdenum Trioxide for Electrochromic Devices, Sci. Rep., 2017, 7(1), 1–10, DOI:10.1038/s41598-017-13341-z.
- Z. Lei, X. Yang, J. Dong and X. Yi, Novel Metastable Hexagonal MoO3 Nanobelts: Synthesis, Photochromic, and Electrochromic Properties, Chem. Mater., 2009, 21(23), 5681–5690, DOI:10.1021/cm9023887.
- H. El Moll, A. Dolbecq, I. M. Mbomekalle, J. Marrot, P. Deniard, R. Dessapt and P. Mialane, Tuning the Photochromic Properties of Molybdenum Bisphosphonate Polyoxometalates, Inorg. Chem., 2012, 51(4), 2291–2302, DOI:10.1021/ic202299d.
- X. Li, X. Yang, H. Xue, H. Pang and Q. Xu, Metal–Organic Frameworks as a Platform for Clean Energy Applications, EnergyChem, 2020, 2(2), 100027, DOI:10.1016/j.enchem.2020.100027.
- Q. Li, N. Li, J. An and H. Pang, Controllable Synthesis of a Mesoporous NiO/Ni Nanorod as an Excellent Catalyst for Urea Electro-Oxidation, Inorg. Chem. Front., 2020, 7(10), 2089–2096, 10.1039/d0qi00316f.
- I. A. de Castro, R. S. Datta, J. Z. Ou, A. Castellanos-Gomez, S. Sriram, T. Daeneke and K. Kalantar-zadeh, Molybdenum Oxides – From Fundamentals to Functionality, Adv. Mater., 2017, 29(40), 1–31, DOI:10.1002/adma.201701619.
- A. S. Aji, R. Nishi, H. Ago and Y. Ohno, High Output Voltage Generation of over 5 V from Liquid Motion on Single-Layer MoS2, Nano Energy, 2020, 68(October 2019), 104370, DOI:10.1016/j.nanoen.2019.104370.
- S. N. Lou, N. Yap, J. Scott, R. Amal and Y. H. Ng, Influence of MoO3 (110) Crystalline Plane on Its Self-Charging Photoelectrochemical Properties, Sci. Rep., 2014, 4, 7428, DOI:10.1038/srep07428.
- X. W. Lou and H. C. Zeng, Hydrothermal Synthesis of α-MoO3 Nanorods via Acidification of Ammonium Heptamolybdate Tetrahydrate, Chem. Mater., 2002, 14(11), 4781–4789, DOI:10.1021/cm0206237.
- R. Kaindl, B. C. Bayer, R. Resel, T. Müller, V. Skakalova, G. Habler, R. Abart, A. S. Cherevan, D. Eder, M. Blatter, F. Fischer, J. C. Meyer, D. K. Polyushkin and W. Waldhauser, Growth, Structure and Stability of Sputter-Deposited MoS2 Thin Films, Beilstein J. Nanotechnol., 2017, 8(1), 1115–1126, DOI:10.3762/bjnano.8.113.
- C. Liu, Z. Li and Z. Zhang, MoOx Thin Films Deposited by Magnetron Sputtering as an Anode for Aqueous Micro-Supercapacitors, Sci. Technol. Adv. Mater., 2013, 14(6), 065005, DOI:10.1088/1468-6996/14/6/065005.
- E. Elamurugu, P. Shanmugam, G. Gonçalves, N. Franco, E. Alves, R. Martins and E. Fortunato, The Electronic Transport Mechanism in Indium Molybdenum Oxide Thin Films RF Sputtered at Room Temperature, EPL, 2012, 97(3), 36002, DOI:10.1209/0295-5075/97/36002.
- S. Cong, T. Sugahara, T. Wei, J. Jiu, Y. Hirose, S. Nagao and K. Suganuma, Growth and Extension of One-Step Sol-Gel Derived Molybdenum Trioxide Nanorods via Controlling Citric Acid Decomposition Rate, Cryst. Growth Des., 2015, 15(9), 4536–4542, DOI:10.1021/acs.cgd.5b00790.
- T. Sugahara, L. Alipour, Y. Hirose, Y. Ekubaru, J. Nakamura, H. Ono, N. Harada and K. Suganuma, Formation of Metal-Organic Decomposition Derived Nanocrystalline Structure Titanium Dioxide by Heat Sintering and Photosintering Methods for Advanced Coating Process, and Its Volatile Organic Compounds’ Gas-Sensing Properties, ACS Appl. Electron. Mater., 2020, 2(6), 1670–1678, DOI:10.1021/acsaelm.0c00237.
- M. Karakawa, T. Sugahara, Y. Hirose, K. Suganuma and Y. Aso, Thin Film of Amorphous Zinc Hydroxide Semiconductor for Optical Devices with an Energy-Efficient Beneficial Coating by Metal Organic Decomposition Process, Sci. Rep., 2018, 8(1), 1–7, DOI:10.1038/s41598-018-27953-6.
- T. Araki, T. Sugahara, J. Jiu, S. Nagao, M. Nogi, H. Koga, H. Uchida, K. Shinozaki and K. Suganuma, Cu Salt Ink Formulation for Printed Electronics Using Photonic Sintering, Langmuir, 2013, 29(35), 11192–11197, DOI:10.1021/la402026r.
- T. Sugahara, Y. Hirose, S. Cong, H. Koga, J. Jiu, M. Nogi, S. Nagao and K. Suganuma, Sol-Gel-Derived High-Performance Stacked Transparent Conductive Oxide Thin Films, J. Am. Ceram. Soc., 2014, 97(10), 3238–3243, DOI:10.1111/jace.13116.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qm00535e |
| This journal is © the Partner Organisations 2021 |