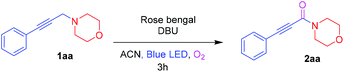Photoredox-catalyzed chemoselective aerobic Cα–H oxidation of propargylamines: synthesis of substituted 2-ynamide and oxazolo[2,3-a]isoquinolinone derivatives†
Mandapati
Bhargava Reddy
 ,
Nalladhambi
Neerathilingam
and
Ramasamy
Anandhan
,
Nalladhambi
Neerathilingam
and
Ramasamy
Anandhan
 *
*
Department of Organic Chemistry, University of Madras, Guindy Campus, Chennai-600 025, Tamil Nadu, India. E-mail: ananthanramasamy@gmail.com
First published on 6th November 2020
Abstract
An efficient approach for visible-light-induced chemoselective aerobic Cα–H oxidation of propargylamines via molecular oxygen as an oxidant using rose bengal as a photoredox catalyst is reported. The photochemical protocol was employed for the direct oxygenation of Cα–H 2-propynyl-tertiary amines to 2-ynamides and further cyclization of oxazolo[2,3-a]isoquinolinone derivatives from phenylpropynyltetrahydroisoquinoline was established.
Introduction
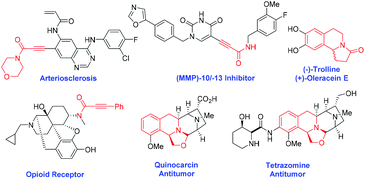
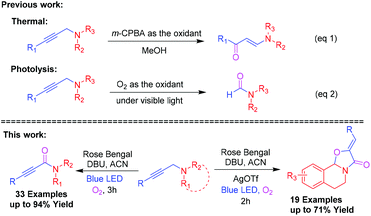
Ynamides are versatile intermediates in organic transformation1 for the construction of many biologically active natural products2 and heterocycles.3 In particular, natural product agonists, arteriosclerosis, [MMP]-10/-13-inhibitor and opioid receptor contains 2-ynamide motifs.4,5 Moreover, oxazolo[2,3-a]tetrahydroisoquinolines are a unique class of heterocyclic core structures found in many isoquinoline alkaloids.6 They have a wide range of biological and pharmacological properties including antibacterial activity, antioxidant activity, anti-hypertensive activity and anti-mycobacterial activity.7 Besides, they also act as synthons for several biologically active molecules such as (−)-trolline, (+)-oleracein E, quinocarcin, and trazomine (Fig. 1).8Propargylamines are versatile building blocks for the synthesis of a wide range of nitrogen heterocyclic compounds9 consisting of a highly reactive amine and alkyne moiety on the backbone. In the oxidation reaction, mostly the oxidizing agent reacts with its nitrogen to give N-oxides10 and Wacker-type oxidation of acetylenes into 1,2-diketones occurs.11 J. Chen et al. have investigated the oxidation of propargylamines to enaminones by the use of m-CPBA as the oxidant (Scheme 1, eqn (1)).12 Later on, W. Ji et al. reported a visible-light-mediated oxidative formylation of N,N-di(prop-2-yn-1-yl)anilines with molecular oxygen in the absence of a photosensitizer (Scheme 1, eqn (2)).13 Although the Cα–H oxidation reaction of propargylamines is unknown in the literature, it's of high demand in synthetic transformations.
So far, many strategies of Cα–H oxidation have been reported for the synthesis of amides directly from amines by the use of stoichiometric oxidants such as iodosobenezne, PhCO3tBu, tBuOOH, RuO2/NaIO4etc.14 In addition to these, many transition metal [M = Ru/Au/Cu/Fe/Mn, etc.] complexes have also been employed for the direct Cα–H oxidation.15 However, these oxidation conditions are not attractive from the economic and environmental perspectives and also limited to amides.
In recent years, visible light-mediated metal-free photoredox catalysts have been found as a powerful tool in many organic transformations,16 which offer cost-effective, mild reaction conditions and functional group tolerance and also provide an alternative to transition metal-based photocatalysts. Moreover, transition metal-free photocatalysts are capable of activating O2 by photoinduced electron transfer (PET) processes and this activated molecular oxygen is useful for Cα–H oxidation reactions.17 Recently, S. Das and co-workers demonstrated the use of visible light-mediated metal-free photoredox catalysts for Cα–H oxidation of tertiary amines to amides using oxygen as an oxidant.18 Recently, our group reported a visible-light-driven Cu(I)-catalysed aerobic oxidative C(sp)–S coupling reaction.19 Based on all the information furnished above, we wish to report an efficient approach for visible light initiated chemoselective aerobic Cα–H oxidation of 2-propynyl-tertiaryamines in the presence of highly reactive nucleophilic amine and alkyne groups via molecular O2 as an oxidant using rose bengal as a photoredox catalyst and further, to disclose a cyclization reaction for the synthesis of oxazolo[2,3-a]isoquinolinone derivatives.
Results and discussion
To start with, investigation of the Cα–H amine oxidation of 4-(3-phenylprop-2-yn-1-yl)morpholine 1aa (0.5 mmol) was done in the presence of rose bengal (RB) (2 mol%) and DBU (0.5 mmol) with an O2 atmosphere in ACN under blue LED irradiation to afford the ynamide 2aa in 94% isolated yield. Inspired by the result, three other photosensitizers such as 9-mesityl-10-methylacridinium tetrafluoroborate (Acr+-Mes), methylene blue, and rhodamine B were examined. However, they led to lower yields of 71%, 35%, and 15%, respectively (Table 1, entries 2–4). Other bases, such as K2CO3, Et3N, and 2,6-lutidine were found to be inferior to DBU (Table 1, entries 5–7). Screening solvents such as DCE, acetone, MeOH, and ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O provided only a moderate yield of 2aa (Table 1, entries 8–11). Control experiments (Table 1, entries 12–15) revealed that rose bengal, DBU, visible light and O2 are essential for the current Cα–H amine oxidation reaction.
H2O provided only a moderate yield of 2aa (Table 1, entries 8–11). Control experiments (Table 1, entries 12–15) revealed that rose bengal, DBU, visible light and O2 are essential for the current Cα–H amine oxidation reaction.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Variation from the standard conditions | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 0.5 mmol (1aa) and 2 mol% of catalyst and 0.5 mmol DBU in 4 mL of solvent were irradiated with blue light in the presence of an O2 atmosphere. b Yields were determined after purification of the compound. n.r. = no reaction. | ||
| 1 | None | 94 |
| 2 | Acr+-Mes instead of RB | 71 |
| 3 | Methylene blue instead of RB | 35 |
| 4 | Rhodamine B instead of RB | 15 |
| 5 | K2CO3 instead of DBU | Trace |
| 6 | Et3N instead of DBU | 35 |
| 7 | 2,6-Lutidine instead of DBU | 25 |
| 8 | DCE instead of ACN | 60 |
| 9 | Acetone instead of ACN | 75 |
| 10 | MeOH instead of ACN | 24 |
| 11 | ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of ACN 1) instead of ACN |
10 |
| 12 | Under air | 84 |
| 13 | Absence of DBU | n.r. |
| 14 | Absence of rose bengal | n.r. |
| 15 | Absence of light | n.r. |
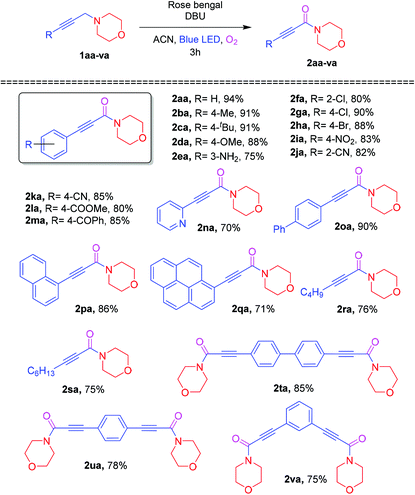
Encouraged by the optimization results for the Cα–H oxidation reaction, the substrate scopes of the different substituted aryl groups were investigated and the results are shown in Table 2. A propargylamine bearing electron-donating substituents (4-Me, 4-tBu, 4-OMe and 3-NH2,) on aromatic rings underwent the Cα–H oxidation with molecular oxygen, affording the corresponding ynamides 2ba–2ea in 75–91% yields. Furthermore, a propargylamine containing an electron-withdrawing group, including 2-Cl, 4-Cl, 4-Br, 4-NO2, 2-CN, 4-CN, 4-COOMe, and 4-COPh, at the aromatic rings had a smooth reaction with molecular oxygen to furnish the corresponding ynamides 2fa–2ma in 85–90% yields. Overall, para-substituted substrates delivered higher yields than ortho and meta-substituted substrates. Moreover, the oxidation reaction of biphenyl, naphthalene and pyrene at the terminal position of the triple bonds (1oa–1qa) also furnished the corresponding ynamides (2oa–2qa) in 71–90% yields. Besides, the heteroaryl 1na and aliphatic alkyne substituted 1ra and 1sa were examined to react with molecular oxygen, providing the ynamide products 2na, 2ra and 2sa in 70%, 76% and 75% yields, respectively. Next, the 1,4- and 1,3-bis propargylamines 1ta, 1ua and 1va also effectively underwent oxidation to provide bis-ynamides (2ta–2va) in good yields.
| a Standard conditions. Yields were determined after purification of the compound. |
|---|
|
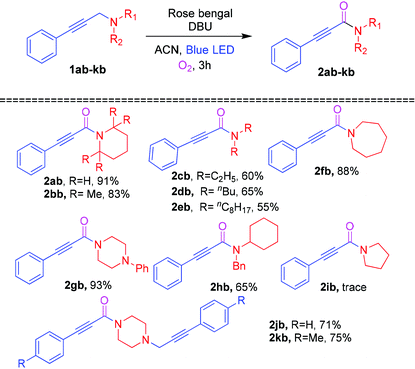
To expand the substrate scope, a variety of amines were examined as substrates for the photosensitized reaction with oxygen, and the results are shown in Table 3. To our delight, a series of alicyclic (1ab, 1bb, 1fb and 1gb) and acyclic (1cb–1eb) amines smoothly underwent the Cα–H oxidation reaction to furnish the corresponding ynamides (2ab–2hb) in 60–93% yields. In contrast, propargylamine 1ib gave a trace amount of ynamides 2ib. Next, 1,4-bis-propargylamines 1jb and 1kb were examined for the oxidation reaction affording the mono oxidative ynamides 2jb and 2kb in 71% and 75% yields, respectively.
| a Standard conditions. Yields were determined after purification of the compound. |
|---|
|
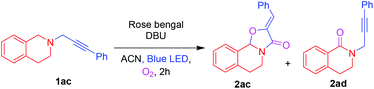
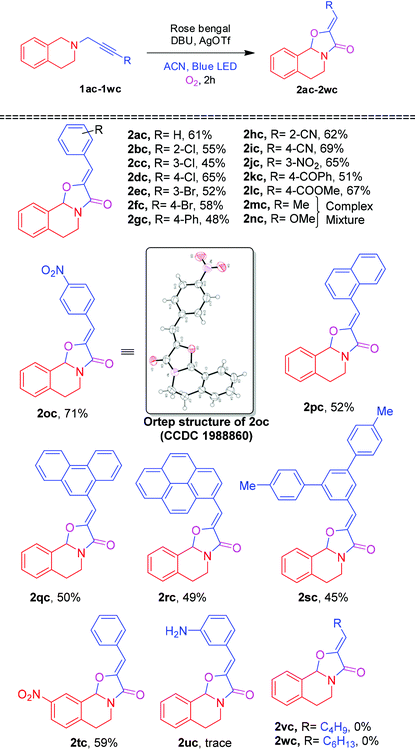
On further utilization of this protocol, we demonstrated the photocatalytic cyclization reaction for the novel synthesis of (Z)-2-benzylidene-5,6-dihydro-2H-oxazolo[2,3-a]isoquinolin-3(10bH)-one 2ac from phenylpropynyl-tetrahydroisoquinoline 1ac shown in Table 4. Initially, the oxidative cyclization reaction of 1ac was investigated in the presence of rose bengal (2 mol%) and DBU (0.3 mmol) under an O2 atmosphere in ACN under blue LED irradiation to afford the oxazolo[2,3-a]isoquinolinone 2ac and 3,4-dihydroisoquinolinone 2ad in 45% and 25% yields, respectively. Among the screened solvents (Table 4, entries 1–4), ACN was found to be the best one. A slight enhancement of the yield of 2ac was observed by the addition of CuCl (5 mol%). However, after the addition of different amounts of AgOTf (Table 4, entries 6 and 7), 10 mol% was proved to improve the yield of 2ac maximum up to 61%. Moreover, addition of AgOAc and AgBr provided a lower yield of 2ac (Table 4, entries 8 and 9). When the reaction was performed without blue LED irradiation, no reaction was observed.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Variation from the standard conditions | 2ac (%) | 2ad (%) |
|---|---|---|---|
| a Reaction conditions: 0.3 mmol (1ac) and 2 mol% of catalyst, AgOTf 10 mol% and 0.3 mmol DBU in 4 mL of solvent were irradiated with blue light in the presence of an O2 atmosphere. b Yields were determined after the purification of the compound. n.r. = no reaction. | |||
| 1 | None | 45 | 25 |
| 2 | EtOH instead of ACN | 35 | 15 |
| 3 | DCE instead of ACN | 38 | 17 |
| 4 | DMF instead of ACN | 41 | 25 |
| 5 | Addition of CuCl (5 mol%) | 47 | 23 |
| 6 | Addition of AgOTf (5 mol%) | 56 | 15 |
| 7 | Addition of AgOTf (10 mol%) | 61 | Trace |
| 8 | Addition of AgOAc (10 mol%) | 54 | 18 |
| 9 | Addition of AgBr (10 mol%) | 51 | 20 |
| 10 | Without blue LED | n.r | n.r. |
After optimizing reaction conditions, we next focused our attention on exploring the scope of the photocatalytic cyclization reaction with various alkyne substituted tetrahydroisoquinolines. As shown in Table 5, a wide range of alkynes with different substituents (R = 2-Cl, 3-Cl, 4-Cl, 3-Br, 4-Br, 4-Ph, 2-CN, 4-CN, 3-NO2, 4-NO2, 4-COPh & 4-COOMe) were subjected to the photocatalytic cyclization reaction, furnishing the corresponding oxazolo[2,3-a]isoquinolinones 2ac–2lc and 2oc in 45–71% yields. However, alkynes (R = Me and OMe) also underwent a cyclization reaction, generating a complex mixture of products 2mc and 2nc (inseparable), respectively.20 Napthalene, anthracene, pyrene and meta-terphenyl substituted alkynes were also tolerated in the cyclization reaction, affording 2pc–2sc in 45–52% yields. Nitro bearing tetrahydroisoquinoline 1tc also smoothly underwent the photocatalystic cyclization affording the product 2tc in 59% yield. In contrast, under the optimized conditions, 3-aminophenylacetylene and n-alkyl substituted alkynes 1uc, 1vc and 1wc failed to produce the corresponding oxazolo[2,3-a]isoquinolinones 2uc, 2vc and 2wc.
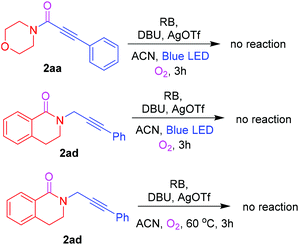
To gain mechanistic insights, a number of control experiments were carried out (Scheme 2). First, ynamide 2aa was tested for further Cα–H oxidation, and no reaction was observed. To understand the possible formation of the reaction intermediate, 2ad was tested for further Cα–H oxidation with and without blue LED light; however, the corresponding cyclized product 2ac was not observed.
To recognize the reactive oxygen species and the mechanism of the Cα–H oxidation reaction, several quenching experiments were carried out, as shown in Table 6. First, subjecting radical scavengers, namely 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO) or tert-butyl hydroperoxide (TBHP) under the optimized conditions afforded a trace yield of 2aa, implying that the reaction proceeds via a radical pathway. Next, the singlet oxygen quencher 1,4-diazabicyclo[2.2.2]octane and the superoxide radical anion quencher benzoquinone failed to produce the 2-ynamide 2aa, due to the presence of both singlet oxygen and superoxide radical anions. Moreover, addition of CuCl2 dramatically suppressed the yield, thereby revealing that the single electron process is also involved. Hence the quenching reactions indicate the involvement of both singlet oxygen (1O2) and superoxide radical anions (O2−˙) in the photocatalytic Cα–H oxidation.
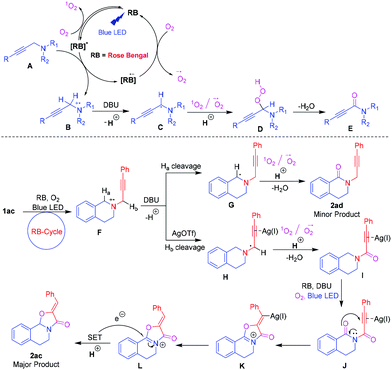
Based on the above and quenching experiment results and literature reports,21 a plausible mechanism is proposed in Scheme 3. Initially, Rose Bengal (RB) was excited upon irradiation of visible light to form [RB]*. The [RB]* underwent reductive quenching with A to generate a nitrogen radical cation B and [RB]−˙. In parallel, [RB]* reacts with O2 to form singlet oxygen (1O2) by photoinduced energy transfer (PET) and it regenerates [RB]. Subsequently, [RB]−˙ reduces O2 to form a superoxide radical anion (O2−˙) by single electron transfer (SET) and recycles the photoredox [RB].21 The resulting radical cation B loses a proton in the presence of a base to give the aminomethyl radical C. The in situ generated 1O2 and O2−˙ reacted with intermediate C to generate hydroperoxy intermediate D. Finally, intermediate D was converted to the observed ynamide E. Similarly, [RB]* underwent reductive quenching with propargylamine 1ac to generate a nitrogen radical cation F. The formed F loses Ha in the presence of DBU followed by a reaction with 1O2 and O2−˙ to give a minor product 2ad. In another way, the AgOTf coordinated electron-deficient triple bond of the radical cation loses Hb in the presence of DBU followed by a reaction with 1O2 and O2−˙ to produce product I. The intermediate I underwent further oxidation reaction under the same protocol to produce imides J. The imides J then underwent nucleophilic attack with activated carbonyl oxygen, affording cyclized iminium K. Finally, the RB cycle induces a single electron transfer (SET) process,22 which donates an electron to the iminium L to afford the desired major product 2ac.
Conclusions
In conclusion, we have developed a new protocol for visible light-mediated chemoselective Cα–H oxidation of propargylamines in the presence of highly reactive amine and alkyne groups under molecular oxygen and rose bengal as a photoredox catalyst. This photochemical protocol was employed for the synthesis of 2-ynamides from propargylamines and further cyclization for the synthesis of oxazolo[2,3-a]isoquinolinone analogs from phenylpropynyltetrahydroisoquinoline was achieved. The quenching, control experiment and mechanistic studies indicated the involvement of singlet oxygen and superoxide radical anions via photoinduced energy transfer (PET)/single electron transfer (SET) between the photoredox catalyst and oxygen.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank DST-SERB for a research grant (vide Grant No: EEQ/2018/001129) and DST-FIST for providing NMR and HRMS facilities to the Department of Organic Chemistry. The authors also thank the Department of Chemistry, IIT Madras for X-ray crystallographic analysis. MBR and NN acknowledge DST-SERB, New Delhi, for a fellowship.Notes and references
- (a) M. Lecomte and G. Evano, Harnessing the Electrophilicity of Keteniminium Ions: A Simple and Straightforward Entry to Tetrahydropyridines and Piperidines from Ynamides, Angew. Chem., Int. Ed., 2016, 55, 4547–4551 CrossRef CAS; (b) L. L. Baldassari, A. De la Torre, J. Li, D. S. Lüdtke and N. Maulide, Ynamide Preactivation Allows a Regio- and Stereoselective Synthesis of alpha,beta-Disubstituted Enamides, Angew. Chem., Int. Ed., 2017, 56, 15723–15727 CrossRef CAS; (c) R. N. Straker, Q. Peng, A. Mekareeya, R. S. Paton and E. A. Anderson, Computational ligand design in enantio- and diastereoselective ynamide [5 + 2] cycloisomerization, Nat. Commun., 2016, 7, 10109 CrossRef CAS.
- (a) K. A. Dekorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang and R. P. Hsung, Ynamides: A Modern Functional Group for the New Millennium, Chem. Rev., 2010, 110, 5064–5106 CrossRef CAS; (b) C. Eibl, L. Munoz, I. Tomassoli, C. Stokes, R. L. Papke and D. Gündisch, The 3,7-Diazabicyclo[3.3.1]Nonane Scaffold for Subtype Selective Nicotinic Acetylcholine Receptor Ligands. Part 2: Carboxamide Derivatives with Different Spacer Motifs, Bioorg. Med. Chem., 2013, 21, 7309–7329 CrossRef CAS.
- (a) X. Xie, X. Lu, Y. Liu and W. Xu, Palladium(II)-Catalyzed Synthesis of α-Alkylidene-γ-Butyrolactams from N-Allylic 2-Alkynamides. Total Syn-thesis of (±)-Isocynodine and (±)-Isocynometrine, J. Org. Chem., 2001, 66, 6545–6550 CrossRef CAS; (b) H. Peng and G. Liu, Palladium-Catalyzed Tandem Fluorination and Cyclization of Enynes, Org. Lett., 2011, 13, 772–775 CrossRef CAS; (c) B. Prabagar, N. Ghosh and A. K. Sahoo, Cyclization and Cycloisomerization of π-Tethered Ynamides: An Expedient Synthetic Method to Construct Carbo- and Heterocycles, Synlett, 2017, 28, 2539–2555 CrossRef CAS.
- C. Zhang, J. Liu and C. Xia, Palladium-N-Heterocyclic Carbene (NHC)-Catalyzed Synthesis of 2-Ynamides via Oxidative Aminocarbonylation of Alkynes with Amines, Catal. Sci. Technol., 2015, 5, 4750–4754 RSC.
- N. Senn, M. Ott, J. Lanz and R. Riedl, Targeted Polypharmacology: Discovery of a Highly Potent Non-Hydroxamate Dual Matrix Metalloproteinase (MMP)-10/-13 Inhibitor, J. Med. Chem., 2017, 60, 9585–9598 CrossRef CAS.
- (a) X. Wu, Z.-H. Zhu, H. He, L. Ren, C.-F. Zhu and Y.-G. Li, Construction of 1,3-Oxazolidines through a Three-Component [3 + 2] Cycloaddition of Tetrahydroisoquino-lines, Aldehydes, and Ethyl Ketomalonate, J. Org. Chem., 2020, 85(9), 6216–6224 CrossRef CAS; (b) E. D. Bergmann, The Oxazolidines, Chem. Rev., 1953, 53, 309–352 CrossRef CAS.
- (a) D. Jack and R. Williams, Chemistry and Biology of the Tetrahydroisoquinoline Antitumor Antibiotics, Chem. Rev., 2002, 102, 1669–1730 CrossRef; (b) L. Yu, W. Zhou and Z. Wang, Synthesis and in vitro antibacterial activity of oxazolidine LBM-415 analogs as peptide deformylase inhibitors, Bioorg. Med. Chem. Lett., 2011, 21, 1541–1544 CrossRef CAS.
- (a) R. M. Williams, T. Glinka, M. E. Flanagan, R. Gallegos, H. Coffman and D. Pei, Cannizzaro-based O2-dependent cleavage of DNA by quinocarcin, J. Am. Chem. Soc., 1992, 114, 733–740 CrossRef CAS; (b) F. Tomita, K. Takahashi and T. Tamaoki, Quinocarcin, a novel antitumor antibiotic. 3. Mode of ac-tion, J. Antibiot., 1984, 37, 1268–1272 CrossRef CAS.
- (a) K. Lauder, A. Toscani, N. Scalacci and D. Castagnolo, Synthesis and Reactivity of Propargylamines in Organic Chemistry, Chem. Rev., 2017, 117, 14091–14200 CrossRef CAS; (b) S. Arshadi, E. Vessally, L. Edjlali, R. Hosseinzadeh-Khanmiri and R. Ghorbani-Kalhor, N-Propargylamines: versatile building blocks in the construction of thiazole cores, Beilstein J. Org. Chem., 2017, 13, 625–638 CrossRef CAS.
- S. L. Jain and B. Sain, Ruthenium catalyzed oxidation of tertiary nitrogen compounds with molecular oxygen: an easy access to N-oxides under mild conditions, Chem. Commun., 2002, 1040–1041 RSC.
- (a) W. Ren, Y. Xia, S.-J. Ji, Y. Zhang, X. Wan and J. Zhao, Wacker-Type Oxidation of Alkynes into 1,2-Diketones Using Molecular Oxygen, Org. Lett., 2009, 11, 1841–1844 CrossRef CAS; (b) X. Zhu, P. Li, Q. Shi and L. Wang, Thiyl radical catalyzed oxidation of diarylalkynes to α-diketones by molecular oxygen under visible-light irradiation, Green Chem., 2016, 18, 6373–6379 RSC.
- J. Chen, R. Properzi, D. P. Uccello, J. A. Young, R. G. Dushin and J. T. Starr, One-Pot Oxidation and Rearrangement of Propargylamines and in Situ Pyrazole Synthesis, Org. Lett., 2014, 16, 4146–4149 CrossRef CAS.
- W. Ji, P. Li, S. Yang and L. Wang, Visible-light-induced oxidative formylation of N-alkyl-N-(prop-2-yn-1-yl)anilines with molecular oxygen in the absence of an external photosensitizer, Chem. Commun., 2017, 53, 8482–8485 RSC.
- (a) R. M. Moriarty, R. K. Vaid, M. P. Duncan, M. Ochiai, M. Inenaga and Y. Nagao, Hypervalent iodine oxidation of amines using iodosobenzene: Synthesis of nitriles, ketones and lactams, Tetrahedron Lett., 1988, 29, 6913–6916 CrossRef CAS; (b) X. F. Wu, C. B. Bheeter, H. Neumann, P. H. Dixneuf and M. Beller, Lewis acid-catalyzed oxidation of benzylamines to benzamides, Chem. Commun., 2012, 48, 12237–12239 RSC; (c) J. B. Feng, H. Neumann, A. Pews-Davtyan, P. Langer and M. Beller, A general and practical oxidation of alcohols to primary amides under metal-free conditions, Green Chem., 2013, 15, 1956–1961 RSC.
- (a) C. J. Legacy, A. Wang, B. J. O'Day and M. H. Emmert, Iron-Catalyzed C-alpha-H Oxidation of Tertiary, Aliphatic Amines to Amides under Mild Conditions, Angew. Chem., Int. Ed., 2015, 54, 14907–14910 CrossRef CAS; (b) J. R. Khusnutdinova, Y. Ben-David and D. Milstein, Oxidant-Free Conversion of Cyclic Amines to Lactams and H2 Using Water As the Oxygen Atom Source, J. Am. Chem. Soc., 2014, 136, 2998–3001 CrossRef CAS; (c) E. R. Klobukowski, M. L. Mueller, R. J. Angelici, L. K. Woo, R. J. Angelici and L. K. Woo, Conversions of Cyclic Amines to Nylon Precursor Lactams Using Bulk Gold and Fumed Silica Catalysts, ACS Catal., 2011, 1, 703–708 CrossRef CAS; (d) T. Nanjo, E. C. de Lucca and M. C. White, Late-Stage Oxidation of Aliphatic C–H Bonds in Amide-Containing Molecules, J. Am. Chem. Soc., 2017, 139, 14586–14591 CrossRef CAS.
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS; (b) N. A. Romero and D. A. Nicewicz, Organic Photoredox Catalysis, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS.
- (a) W. Schilling, D. Riemer, Y. Zhang, H. Nareh and S. Das, Metal-Free Catalyst for Visible-Light-Induced Oxidation of Unactivated Alcohols Using Air/Oxygen as an Oxidant, ACS Catal., 2018, 8, 5425–5430 CrossRef CAS; (b) Y. Jin, L. Ou, H. Yang and H. Fu, Visible-Light-Mediated Aerobic Oxidation of N-Alkylpyridinium Salts under Organic Photocatalysis, J. Am. Chem. Soc., 2017, 139, 14237–14243 CrossRef CAS; (c) N. A. Romero, K. A. Margrey, N. E. Tay and D. A. Nicewicz, Site-selective arene C-H amination via photoredox catalysis, Science, 2015, 349, 1326–1330 CrossRef CAS; (d) W. Schilling, Y. Zhang, R. Daniel and S. Das, Visible-Light-Mediated Dearomatisation of Indoles and Pyrroles to Pharmaceuticals and Pesticides, Chem. – Eur. J., 2020, 26, 390–395 CrossRef CAS; (e) Y. Zhang, W. Schilling, R. Daniel and S. Das, Metal-free photocatalysts for the oxidation of non-activated alcohols and the oxygenation of tertiary amines performed in air or oxygen, Nat. Protoc., 2020, 15, 822–839 CrossRef CAS.
- Y. Zhang, D. Riemer, W. Schilling, J. Kollmann and S. Das, Visible-Light-Mediated Efficient Metal-Free Catalyst for α-Oxygenation of Tertiary Amines to Amides, ACS Catal., 2018, 8, 6659–6664 CrossRef CAS.
- M. Bhargava Reddy and R. Anandhan, Visible light initiated amino group ortho-directed copper(I)-catalysed aerobic oxidative C(sp)–S coupling reaction: synthesis of substituted 2-phenylbenzothiazoles via thia-Wolff rearrangement, Chem. Commun., 2020, 56, 3781–3784 RSC.
- Although the compound seems to be single on TLC and column chromatography, the 1H NMR spectrum shows a mixture of inseparable compounds.
- (a) P. C. C. Lee and M. A. J. Rodgers, Laser flash photokinetic studies of rose bengal sensitized photodynamic interactions of nucleotides and dna, Photochem. Photobiol., 1987, 45, 79–86 CrossRef CAS; (b) T. Shen, Z. G. Zhao, Q. Yu and H. J. Xu, Photosensitized reduction of benzil by heteroatom-containing anthracene dyes, J. Photochem. Photobiol., A, 1989, 47, 203–212 CrossRef CAS.
- (a) A. Trowbridge, D. Reich and M. J. Gaunt, Multicomponent synthesis of tertiary alkylamines by photocatalytic olefin-hydroaminoalkylation, Nature, 2018, 561, 522–527 CrossRef CAS; (b) N. J. Flod'en, A. Trowbridge, D. Willcox, S. M. Walton, Y. Kim and M. J. Gaunt, Streamlined Synthesis of C(sp3)-Rich N-Heterospirocycles Enabled by Visible-Light-Mediated Photocatalysis, J. Am. Chem. Soc., 2019, 141, 8426–8430 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data for all new compounds, and the crystal data of compound 2oc. CCDC 1988860. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0qo01220c |
| This journal is © the Partner Organisations 2021 |