Open Access Article
Open Access ArticlePromotion in solid phase reaction of Pt/SiOx bilayer film by electron-orbital-selective-excitation
H. Yasuda *ab,
K. Satoab,
S. Ichikawa
*ab,
K. Satoab,
S. Ichikawa ab,
M. Imamurac,
K. Takahashi
ab,
M. Imamurac,
K. Takahashi c and
H. Moria
c and
H. Moria
aResearch Center for Ultra-High Voltage Electron Microscopy, Osaka University, Ibaraki, Osaka 567-0047, Japan. E-mail: yasuda@uhvem.osaka-u.ac.jp
bDivision of Materials and Manufacturing Science, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan
cSynchrotron Light Application Center, Saga University, Honjo 1, Saga 840-8502, Japan
First published on 4th January 2021
Abstract
A thermally impossible positive free energy reaction can proceed by electron-orbital-selective excitation. When the Si 2p core level is photo-excited in Pt/SiOx bilayer films, Coulomb repulsion at the final two-hole state localized in the valence band by an interatomic Auger transition induces dissociation of the O atom and formation of a Si–Pt bond. Consequently, Pt2Si silicide is formed by a positive free energy reaction. Under a single particle excitation of the valence band, low probability of the coexistence of the two-hole state for picosecond order suppresses to allow the reaction to proceed.
Atomic species-, atomic site- and electron orbital-selective electronic excitation can be induced in materials irradiated with energy variable photons. Atomic displacements by such selective photo-excitations are different from thermal diffusion and are controlled precisely for individual atomic elements. Consequently, specific kinds of atomic bond may be broken or formed also in the solid phase as represented by a Coulomb explosion in molecules.1,2 It has been found by our group's previous research that Pt2Si silicide is formed when bilayer films consisting of SiOx oxide and Pt metal kept at room temperature are excited by electrons or photons using a transmission electron microscope or synchrotron light source, respectively.3,4 Those results show that a reaction by the Si–O bond breaking and Si–Pt bond formation takes place simultaneously by an electronic excitation.
The formation enthalpy change of this reaction is as follows.5,6 Si + O2 = SiO2, for this reaction the formation enthalpy is ΔH298 = −912 kJ mol−1, and Si + 2Pt = Pt2Si, for this reaction the formation enthalpy is ΔH298 = −186 kJ mol−1. Therefore, SiO2 + 2Pt = Pt2Si + 2O (O2), for this reaction the formation enthalpy is ΔH298 = 726 kJ mol−1. No spontaneous reaction proceeds at the ground state, because this silicide formation reaction is increasing the formation enthalpy.
If the constituent atoms are at the excited states, the atoms act as different atoms from the ground state. The formation enthalpies and entropies at the excited state is expected to become remarkably different from physical property values given by thermodynamics at the ground state. In order to discuss the reaction under photo-irradiation, it is necessary to consider the essential difference from the thermochemical reaction. The characteristic of photochemical reactions is that only atoms and molecules that have absorbed photon energy contribute to the reaction, the reaction can be controlled spatially and temporally, and the lifetime of the excited state is extremely short. The electronic states of excited atoms and molecules are different from those of the ground state. Therefore, the structure of a substance produced by absorbing photons is often different from produced by a thermochemical reaction. It is important to make the effective use of these characteristics of photochemical reactions.
In the present research, we have focused on how to respond the Pt2Si silicide formation reaction, under the condition of being Si 2p core level is excited or not, using photon energy variable synchrotron light source. The effect of electron-orbital-selective excitation on the positive formation enthalpy thermodynamic reaction at the excited state may be discussed from the knowledge obtained here.
The Pt/SiOx bilayer films were prepared in a vacuum chamber connected with an analysis chamber for photoelectron spectroscopy (PES). SiOx was first evaporated from a filament to form the thin film on a highly oriented pyrolytic graphite (HOPG) substrate of 5 × 5 mm square sample with a thickness of 1 mm. Then Pt was evaporated from the other filament to form the Pt/SiOx bilayer film. The thickness of SiOx and Pt film are estimated to be approximately 10 nm and 1 nm, respectively. The source materials of SiOx and Pt were a chunk and wire supplied from Furuuchi Chemical Corporation, respectively. The temperature of the substrate was kept at room temperature, and the base pressure was around 10−6 Pa. The specimen in the vacuum was transferred from the preparation chamber to analysis chamber.
The chemical and valence states in the Pt/SiOx bilayer films were measured by synchrotron-radiation PES. The synchrotron-radiation PES measurements were carried out at room temperature with the incident photon energies of 140 or 80 eV and the photo-current of approximately 400 pA at the BL13 VLS beam line in SAGA Light Source facility. The temperature of the substrate was kept at room temperature, and the base pressure was around 10−8 Pa.
Photo-irradiation experiments have been carried out using the same synchrotron light source. The photon energies were 140 and 80 eV, and the photo current was approximately 200 nA. Changes in the core level electronic structures associated with photo-irradiation have been investigated in situ PES. The photocurrents during photo-irradiation and PES measurements were intentionally changed. During photo-irradiation the photocurrent was increased in order to improve the efficiency of the solid-phase reaction which is a cooperative phenomenon. On the other hand, the optimum photocurrent for PES measurements was set in consideration of the sensitivity of the detector.
Changes in Pt 4f core level spectra in the Pt/SiOx bilayer films by photo-irradiation are shown in Fig. 1(a). The Pt 4f core level spectra consist of two peaks 4f5/2 and 4f7/2 due to spin–orbit splitting. Focused on the Pt 4f7/2 peaks, the peak energy is 72.2 eV in both specimens as prepared and 80 eV photo-irradiated. On the other hand, in 120 eV photo-irradiated specimen, the Pt 4f7/2 peak shifts to higher energy of 72.8 eV. The Pt 4f core level spectra of Pt metal, Pt2Si and PtSi silicide are indicated in the same binding energy scale, as a reference.7 The Pt 4f7/2 peaks in Fig. 1(a) are compared to those in Fig. 1(b). The peak energy 72.2 eV in both specimens of as prepared and 80 eV photo-irradiated as shown in Fig. 1(a) is located between the peak energy 71.3 eV of Pt metal and the peak energy 72.2 eV of Pt2Si as shown in Fig. 1(b). This result on high energy shift of the Pt 4f7/2 peak indicates that the bonding between Pt and Si atom is locally formed, though a stoichiometric silicide is not enough formed in Pt/SiOx bilayer films as prepared. It is also shown that the reaction does not proceed during 80 eV photo-irradiation. On the other hand, Pt 4f7/2 peak energy of 140 eV photo-irradiated specimen, 72.8 eV is the same as that of Pt2Si silicide referred in Fig. 1(b), and it indicates formation of Pt2Si by the reaction.
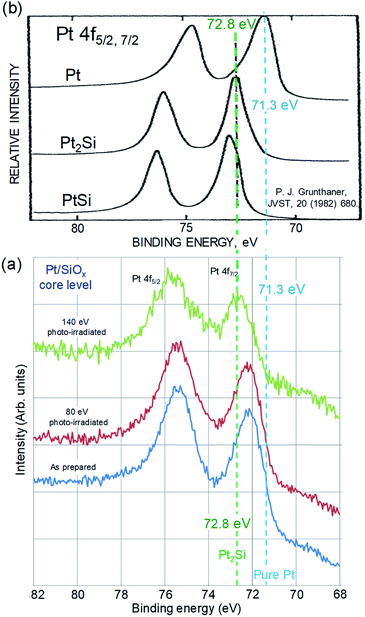 | ||
| Fig. 1 (a) Changes in Pt 4f core level spectra in the Pt/SiOx bilayer films by photo-irradiation, (b) the Pt 4f core level spectra of Pt metal, Pt2Si and PtSi silicide indicated as a reference.7 | ||
Fig. 2 shows valence band spectra of SiOx film, Pt/SiOx bilayer film as prepared, and Pt/SiOx bilayer film photo-irradiated at 80 eV and 140 eV. The spectrum consists of three peaks. In the SiOx film, the valence band maximum is about 1 eV from Fermi level (EF), and peaks consisting of dehybridized Si 3p states, hybridized Si 3sp states, and dehybridized Si 3s states are observed at 4.0 eV, 7.3 eV, and 10.8 eV, respectively. In the Pt/SiOx bilayer film as prepared, some peaks are shifted to the low energy side. The peaks observed at 3.5 eV, 6.7 eV, and 10.8 eV are a localized nonbonding Pt 5d band, a bonding counterpart of Si 3p–Pt 5d hybridization, and a dehybridized Si 3 s states, respectively. This result indicates that the Si–Pt bonds form in the Pt/SiOx bilayer film as prepared, and which is consistent with the change in the Pt 4f core level spectra shown in Fig. 1. In the Pt/SiOx bilayer film photo-irradiated at 80 eV, no change is observed in the peak energy from Pt/SiOx bilayer film as prepared, but the shoulder peak observed at 1.0 eV becomes slightly clearer than the spectrum in the bilayer film as prepared. This peak corresponds to antibonding counterpart of Si 3p–Pt 5d hybridization. On the other hand, in the Pt/SiOx bilayer film photo-irradiated at 140 eV, the peak energy due to the localized nonbonding Pt 5d band shifts from 3.5 eV to 3.8 eV.
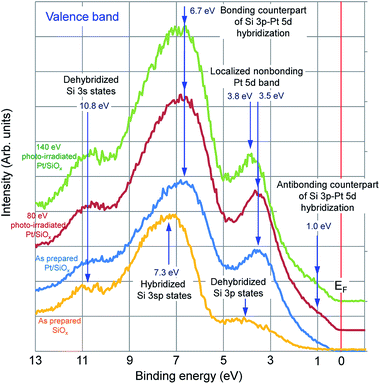 | ||
| Fig. 2 Valence band spectra of SiOx film, Pt/SiOx bilayer film as prepared, and Pt/SiOx bilayer film photo-irradiated at 80 eV and 140 eV. | ||
According to the previous literature,8,9 this peak shift consistent with the movement expected on the basis of the decreased number of Pt–Pt bonds, giving rise to less screening and Coulomb correlation effects in the metal bonds. The number of Pt–Pt bonds is reduced in the Pt/SiOx bilayer film photo-irradiated at 140 eV than that in the bilayer film as prepared. For example, number of Pt neighbors around Si atoms is 8 in Pt2Si, and consequently greater charge is lost from Pt by the larger number of Pt–Si bonds. It is suggested that the contribution of loss of Pt–Pt bonds associated with the formation of Pt2Si plays an important role in the peak shifts of the localized nonbonding Pt 5d band and the Pt 4f core level. This result is also confirmed by the change in the densities of states in the valence band by semi-empirical LCAO calculations.10,11 As mentioned above, from the results shown in Fig. 1 and 2, it became evident that Pt2Si was produced in the Pt/SiOx bilayer film photo-irradiated at 140 eV, and this result was consistent with structural analysis results obtained by transmission electron microscopy.4
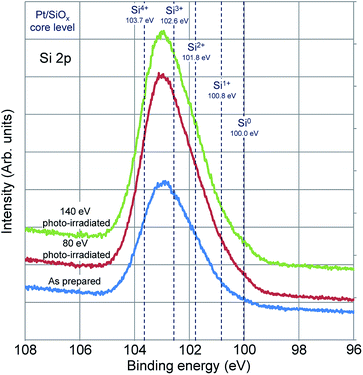
Changes in Si 2p core level spectra in the Pt/SiOx bilayer films by photo-irradiation are shown in Fig. 3. In all the specimens of as-prepared, 80 eV photo-irradiated, and 140 eV photo-irradiated specimen, no changes are in the spectral line shapes. The energies corresponding from zerovalent (Si0) to tetravalent (Si4+) of Si are indicated by broken lines in the figure. This result shows that the local bonding between Pt and Si atom is also formed in Pt/SiOx bilayer films as prepared, because the Pt 4f7/2 peak energy is the same in both specimens as prepared and 80 eV photo-irradiated specimen. In all the specimens of as-prepared, 80 eV photo-irradiated, and 140 eV photo-irradiated, Si valence is from trivalent (Si3+) to Si4+.
In order to investigate detail changes in Si valence, pure SiOx film was photo-irradiated. Changes in Si 2p core level spectra in SiOx films by photo-irradiation are shown in Fig. 4(a). The spectra consist of two peaks. The peak energies are 102.8 eV at the main peak and 100.0 eV at the sub peak in the specimen as prepared. The main peak shifts to higher energy of 103.4 eV in both of 80 eV and 140 eV photo-irradiated specimen, and the sub peak remains the peak energy of 100.0 eV, but the intensity increases. Fig. 4(b) shows subtracted spectra of as prepared specimen from that of the photo-irradiated specimens. Decrease of Si3+ and increase of Si4+ and Si0 are recognized in both spectra of 80 eV and 140 eV photo-irradiated specimen.
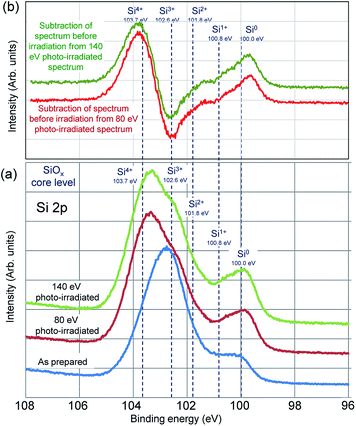 | ||
| Fig. 4 (a) Changes in Si 2p core level spectra in pure SiOx films by photo-irradiation, (b) subtracted spectra of as prepared specimen from that of the photo-irradiated specimens. | ||
In Fig. 4, when SiOx film is photo-irradiated, the change in the valence of Si clearly shows that Si0 and Si4+ increase with decreasing of Si3+. The results in Fig. 3 show that no change in the valence of Si is recognized when the Pt/SiOx bilayer film is photo-irradiated. The Si 2p peak due to formation of Pt–Si bonds between Si0 generated in the photo-irradiating SiOx and Pt broken from Pt–O bonds appears near 100 to 101 eV. On the other hand, the peak intensity at about 104 eV increases because of formation of Si4+ at the same time. After photo-irradiation, the peak intensity increases without energy change with the formation of Pt–Si bonds, and additionally the Si4+ peak intensity increases with the formation of oxides. Consequently, no peak shape changes regardless of reaction of silicide formation.
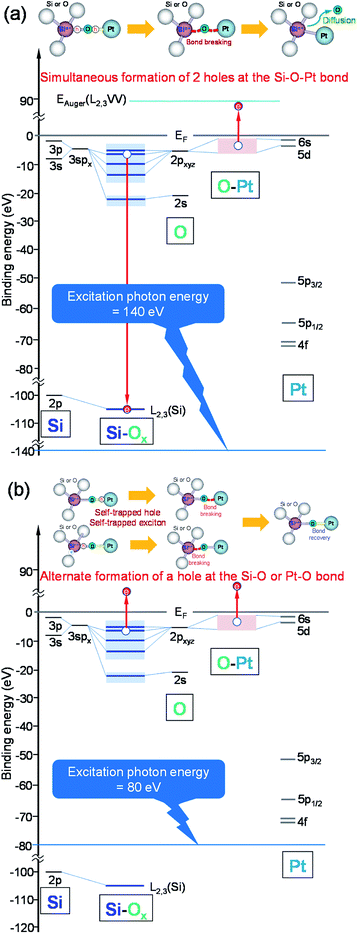
From the experiments mentioned above, it was evident that 140 eV photo-irradiation is required to form Pt2Si by reaction of Pt with SiOx. The photon energy dependence of the formation of Pt2Si by interfacial reaction of Pt/SiOx bilayer films will be discussed in the following. It is a well-known that the core level cannot be excited unless the excitation energy is larger than the binding energy of the core level. Two kinds of excitation energies, 140 eV and 80 eV excite the Si 2p core level of Pt/SiOx bilayer films or not. The relaxation process after core excitation is a competitive process consisting of X-ray fluorescence and Auger electron emission. It is known that the yield of Auger transition is higher than that of fluorescent X-ray in the excitation relaxation process of light elements such as Si and O. On the other hand, in a heavy element such as Pt, the X-ray fluorescence yield is dominant in the excitation relaxation processes. When the Si 2p core level is not excited, no Si core-valence-valence (LVV) Auger electron emission exists. Consequently, the probability that two holes are simultaneously present in the valence band in the final state of single-electron excitation is remarkably low. On the other hand, when the Si 2p core level is excited, two holes can exist in the valence band at the final state due to the emission of Si LVV Auger electrons. We have not experimentally confirmed the presence of two holes in the valence band, but it is a well-known fact. The presence of two holes at the same time for a short time contributes to bond breaking and will promote the reaction. The presence of two holes due to single electron excitation of the valence band has low temporal identity, which reduces the probability of contributing to bond breaking and promotion of the reaction. These processes are schematically shown in Fig. 5.
Fig. 5(a) shows a schematic illustration of an interatomic process of Auger transition in local Si–O–Pt molecular orbital levels of Pt/SiOx bilayer films by 140 eV photo-irradiation. The interatomic Auger transition is the following Auger electron emission process. The Si atom is ionized in the 2p core level by photon to transfer to the excited state. The subsequent recombination of the ionized 2p core hole of Si atom with the valence electron of O atom relaxes the system. The energy set free by the transition is taken on by the valence electrons of the O atom to be ejected. It is known that the probability of the interatomic Auger transition is approximately 31% of the total Auger transition.12,13 In the interatomic Auger transition which takes place within the lifetime of 10−12 s, the final two-hole state in the valence band is simultaneously localized on the O atom. Coulomb repulsion by the two-hole localization on the O atom bring about the bond instability. Both of simultaneous Si–O and O–Pt bond breaking induces a dissociation of O atom to recombine Si and Pt atom for Pt2Si formation, as illustrated in Fig. 5(a). On the other hand, Fig. 5(b) shows a schematic illustration of a single particle excitation of the valence band by 80 eV photo-irradiation. Formation of self-trapped hole and self-trapped exciton in the Si–O–Pt bond are alternately induced and is low probability of the coexistence to break the bonds of O atom.14 In the alternate formations of a hole in the Si–O and O–Pt bond of valence band, Pt–Si bond may be difficult to form.
From the above discussion, it was revealed that the Si 2p core level excitation play an important role in the formation of Pt2Si silicide by reaction of Si and Pt atom in the interface of Pt/SiOx bilayer films. Coulomb repulsion at final two-hole state localized in the valence band by Auger transition from the Si 2p core level excitation can induce simultaneous Si–O and O–Pt bond breaking and a dissociation of O atom to form the Si–Pt bond preferentially.
The candidate mechanisms to proceed a positive enthalpy reaction at room temperature have been elucidated from the relaxation process of electron-orbital-selective electronic excitation. One of the characteristic electronic configurations to destabilize the chemical bonds by a core level electronic excitation is localization of two-hole state in the valence band. A final state of Auger transitions may become a driving force of such reactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was, in part, supported by Advanced Research Network for Ultra-Microscopic Science from the Ministry of Education, Culture, Sports, Science and Technology, Japan.Notes and references
- A. Hishikawa, A. Matsuda, M. Fushitani and E. J. Takahashi, Phys. Rev. Lett., 2007, 99, 258302-1–258302-4 CrossRef.
- T. Endo, H. Fujise, Y. Kawachi, A. Ishihara, A. Matsuda, M. Fushitani, H. Konoc and A. Hishikawa, Phys. Chem. Chem. Phys., 2017, 19, 3550–3556 RSC.
- J.-G. Lee, T. Nagase, H. Yasuda and H. Mori, J. Appl. Phys., 2015, 117, 194307-1–194307-8 Search PubMed.
- K. Sato, H. Yasuda, S. Ichikawa, M. Imamura, K. Takahashi, S. Hata, S. Matsumura, S. Anada, J.-G. Lee and H. Mori, Acta Mater., 2018, 154, 284–294 CrossRef CAS.
- JANAF Thermochemical Tables, Michigan, US Department of Commerce/National Bureau of Standards/Institute for Applied Technology, 1965-68, Juni 1971, Suppliments 1974, 1975.
- L. Topor and O. J. Kleppa, Z. Metallkd., 1986, 77, 65–71 CAS.
- P. J. Grunthaner and F. J. Grunthaner, J. Vac. Sci. Technol., 1982, 20, 680–683 CrossRef CAS.
- P. Morgen, M. Szymonski, J. Onsgaard and B. Jorgensen, Surf. Sci., 1988, 197, 347–362 CrossRef CAS.
- R. T. Fryer and R. J. Lad, J. Alloys Compd., 2016, 682, 216–224 CrossRef CAS.
- I. Abbati, L. Braicovich, B. De Michelis, O. Bisi and R. Rovetta, Solid State Commun., 1981, 37, 119–122 CrossRef CAS.
- H. Bentmann, A. A. Demkov, R. Gregory and S. Zollner, Phys. Rev. B, 2008, 78, 205302-1–205302-9 CrossRef.
- E. H. S. Burhop, Proc. R. Soc. London, Ser. A, 1935, 148, 272–284 CAS.
- R. Hertlein, R. Weissmann and K. Müller, Surf. Sci., 1978, 77, 118–130 CrossRef CAS.
- A. Shluger and E. Stefanovich, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 42, 9664–9673 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |