Open Access Article
Open Access ArticleEcological HPLC method for analyzing an antidiabetic drug in real rat plasma samples and studying the effects of concurrently administered fenugreek extract on its pharmacokinetics
Nada S. Abdelwahab a,
Amani Morsib,
Yasmine M. Ahmedc,
Hossam M. Hassan
a,
Amani Morsib,
Yasmine M. Ahmedc,
Hossam M. Hassan *de and
Asmaa M. AboulMagd
*de and
Asmaa M. AboulMagd *a
*a
aPharmaceutical Chemistry Department, Faculty of Pharmacy, Nahda University (NUB), Beni-Suef, Egypt. E-mail: asmaa.aboulmaged@nub.edu.eg; Tel: +20-01223334785
bAnalytical Chemistry Department, National Organization of Drug Control and Research (NODCAR), Giza, Egypt
cPharmacology & Toxicology Department, Faculty of Pharmacy, Nahda University (NUB), Beni-Suef, Egypt
dPharmacognosy Department, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt. E-mail: hossam.mokhtar@nub.edu.eg; Tel: +20-01065605018
ePharmacognosy Department, Faculty of Pharmacy, Nahda University (NUB), Beni-Suef, Egypt
First published on 25th January 2021
Abstract
Currently, the total number of diabetic people worldwide is constantly increasing. Metformin (MET) is known to be a first-line antidiabetic drug with varied, wide-reaching applications. Concurrent administration of phytomedicines such as fenugreek extract with synthetic drugs is very common. It is reported that concomitant administration of fenugreek extract with metformin maintains lower blood glucose levels than metformin alone. In this work, an ecofriendly RP-HPLC method was established to study and compare the pharmacokinetics of metformin with and without the contemporary administration of fenugreek extract using rat as an animal model. In the developed method, a solvent mixture of 0.5 mM KH2PO4 solution![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (65
methanol (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v) was used as a mobile phase and guaiphenesin was used as an internal standard. The plasma concentration–time curve was plotted, and non-compartmental pharmacokinetic analysis was performed using PKSolver. The results of the pharmacokinetic study showed that concurrent administration of fenugreek significantly increased the bioavailability of metformin and doubled the time required to reach the peak plasma concentration (Tmax). Moreover, the volume of drug distribution decreased by about 70%, while its rate of clearance decreased by about 55.96%. Accordingly, the administration of fenugreek in combination with metformin significantly affected the pharmacokinetics of metformin, and this combination will be very useful in controlling blood glucose levels in diabetic patients. The greenness of the method was assessed using the Analytical Eco-Scale, Analytical Method Volume Intensity (AMVI), and National Environmental Method Index (NEMI), and all results affirmed that the method can be considered to be ecological.
35, v/v) was used as a mobile phase and guaiphenesin was used as an internal standard. The plasma concentration–time curve was plotted, and non-compartmental pharmacokinetic analysis was performed using PKSolver. The results of the pharmacokinetic study showed that concurrent administration of fenugreek significantly increased the bioavailability of metformin and doubled the time required to reach the peak plasma concentration (Tmax). Moreover, the volume of drug distribution decreased by about 70%, while its rate of clearance decreased by about 55.96%. Accordingly, the administration of fenugreek in combination with metformin significantly affected the pharmacokinetics of metformin, and this combination will be very useful in controlling blood glucose levels in diabetic patients. The greenness of the method was assessed using the Analytical Eco-Scale, Analytical Method Volume Intensity (AMVI), and National Environmental Method Index (NEMI), and all results affirmed that the method can be considered to be ecological.
1. Introduction
Type 2 diabetes mellitus is a non-insulin-dependent metabolic disorder in which the body becomes tolerant to the response of insulin.1 Management of patients with type 2 diabetes is possible with oral hypoglycemic pharmaceutical drugs. Diverse chemical classes have been developed as oral hypoglycemic agents that have various mechanisms of action, among which biguanides (metformin, MET) are the most widely used drugs.2 MET can be adjudged to be a first line oral hypoglycemic; it does not cause hypoglycemia or weight gain. Maintaining long-term glycemic control in diabetes treatment is a challenge; hence, concurrent administration of metformin and herbal medicines may achieve a synergistic effect and satisfactory blood glucose levels.3 After extensive literature review, different methods have been published for analysis of MET alone in different samples (plasma, urine, liver, brain, kidney, and muscles), including HPLC,4–9 hydrophilic interaction liquid chromatography (HILIC),10 LC/MS/MS,11–14 HILIC/MS,15,16 and GC/MS.17 Likewise, MET in combination with different drugs was determined in different plasma samples by HPLC,18–23 LC/MS/MS,24–31 and HILIC/MS32,33 methods.Recently, scientists have become concerned with applying the principles of green chemistry to different analytical methods in order to reduce hazards to human health and environment. Different metrics are now used for the assessment of the environmental impact of different analytical methods, such as the Analytical Eco-Scale,34 Analytical Method Volume Intensity (AMVI),35 and the National Environmental Method Index (NEMI).36
On the other hand, the co-administration of herbal medicines with conventional drugs may alter their pharmacokinetic or pharmacodynamic properties. Therefore, studying the herb–drug interactions has become a necessity.
Trigonella foenum-graecum Linn is an annual plant belonging to the family Fabaceae; it is commonly called fenugreek or methi, and it is used as a condiment in Mediterranean countries and the Indian sub-continent. Trigonella seeds have recently been used as a medicinal herb and as a flavoring agent to imitate the taste of maple.37 Moreover, Trigonella is known for its hypocholesterolemic, antibacterial, anticancer, hepatoprotective, lactation-aiding, and antidiabetic effects.38
The hypoglycemic effect of fenugreek seeds has been studied in many animal model systems39 as well as in humans, both in insulin-dependent diabetes mellitus (IDDM) patients40,41 and in non-insulin-dependent diabetes mellitus (NIDDM) patients.42 The therapeutic role of Trigonella seed powder in type-1 diabetes can be attributed to changes in glucose and lipid metabolizing enzyme activities to normal values.43 The various antidiabetic properties of Trigonella seed powder and extract and their active principles may be attributed to different constituents, such as steroid saponins, the fiber content in the seeds,44–46 furostanol saponins called trigoneosides, glycoside D, trigofoenoside A,47 and steroidal sapogenins such as diosgenin and yamogenin.48 Recently, it was reported that 4-hydroxyisoleucine,49 a novel amino acid in fenugreek extract, facilitates insulin secretion. It was also published that fenugreek seed fibers slow the rate of postprandial glucose absorption. Fenugreek is known for its emulsifying effect due to its high fiber, protein and gum content.50
This work aims to study how the concomitant use of fenugreek extract affects the pharmacokinetics of the oral hypoglycemic MET using rats as the experimental animal model. This pharmacokinetic study was carried out through the development of a green RP-HPLC method after simple sample pretreatment steps. Additionally, the method was validated according to FDA51 requirements. The most noteworthy feature of this study is that fenugreek extract can be a promising complimentary herbal medicine to the traditional hypoglycemic MET for satisfactory diabetes control; hence, this combination may reduce MET requirements and thus prevent long-term complications. The work in this manuscript was then extended to estimate the greenness of the developed chromatographic method using the previously mentioned tools. All the obtained results confirmed that the method fulfilled the necessary requirements to be an ecological method. The proposed method can be used as an economic alternative tool to previously published LC/MS methods because the required instruments and chemicals are less expensive than those required for the LC/MS method; additionally, no tedious sample purification steps are required for the suggested RP-HPLC method compared to those used in published LC/MS methods. On the other hand, this method possesses the sensitivity and selectivity required to quantify the studied drug in real plasma samples.
2. Experimental
2.1. Instruments
• For powdering of the plant seeds. Jet mill machinery, Mill Jet, Mill Classifier (ALPA) (1–1000 V, ISO9001, CE, Shandong, China).
• For extract evaporation. A rotary evaporator (Buchi Rotavapor R-300, Cole-Parmer, Vernon Hills, IL, USA).
• For taking different volumes from the separated plasma samples. Rongtai variable volume micropipettes (0.1–100 μL and 100–1000 μL) (Mainland, Shanghai, China).
• For mixing the taken plasma samples. A 250 VM vortex mixer (Hwashin, Seoul, Korea).
• For centrifugation and removal of the precipitated plasma protein. A 80-2C low-speed electric centrifuge (Zjmzym, China), 4000 rpm and 12 tubes × 20 mL.
• For chromatographic separation. A Dionex Ultimate 3000 UHPLC equipped with an auto-sampler, quaternary solvent delivery pump and diode array detector (Germany). The software used was Chromeleon software. The stationary phase used was a ZORBAX Eclipse Plus® C18 column with dimensions of 250 × 4.6 mm, 5 μm (California, USA). On the other hand, the used mobile phase was a mixture of 0.5 mM KH2PO4 solution
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (65
methanol (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v) which was filtered through a 0.45 μm millipore membrane filter.
35, v/v) which was filtered through a 0.45 μm millipore membrane filter.
• For sample weighting. A digital balance (Sartorius, Germany).
2.2. Chromatographic conditions
Separation was performed on a stationary phase of a 250 × 4.6 mm, 5 μm ZORBAX Eclipse Plus® C18 column using a mobile phase consisting of 0.5 mM KH2PO4 solution![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (65
methanol (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v), maintaining a constant flow rate at 1.7 mL min−1. The UV detector was set at 235 nm. The column temperature was adjusted to 25 °C, and guaiphenesin was the selected internal standard. The injection volume was 30 μL and the run time was 7.5 min.
35, v/v), maintaining a constant flow rate at 1.7 mL min−1. The UV detector was set at 235 nm. The column temperature was adjusted to 25 °C, and guaiphenesin was the selected internal standard. The injection volume was 30 μL and the run time was 7.5 min.
2.3. Materials and reagents
2.4. Solutions
The prepared solutions were then stored at −20 °C until the time of analysis.
2.5. Experimental animals
Mature female Wistar Albino rats (18 rats) with weights ranging from 200 to 250 g were used during this work. These rats were acquired from “Nahda University in Beni-Suef (NUB) Animal House”. For adaption and in order to scrutinize the variations in the pharmacokinetic parameters of the treated groups in comparison to the control group, the rats were set aside in the animal room for 14 days before being used in this work. Guidelines stated by “Nahda University Animal House” and approved by the Pharmacology and Toxicology Department, Faculty of Pharmacy, Nahda University in Beni-Suef (NUB) were followed for handling the experimental animals.2.6. Extraction of plant material
The plant seeds (1 kg) were obtained from a commercial market (Harraz store, Beni-Suef, Egypt). Dr Ahmed M. Sayed, Pharmacognosy Department, Faculty of Pharmacy Nahda University in Beni-Suef (NUB), examined and identified the purchased seeds; after that, they were finely powdered by small laboratory jet mill machinery. Extraction was then carried out by maceration without agitation using 70% ethanol (1–1.5 L, 3×, seven days each) at room temperature; then, the extract was concentrated under vacuum at 45 °C using a rotary evaporator to obtain 60 g crude extract. The crude extract was maintained at 4 °C until use for biological investigation.2.7. Preparation of calibration standards and quality control samples (QC) and construction of the calibration curve
Into a series of test tubes, 0.5 mL of rat blank plasma was spiked with different volumes (2.5–150 μL) of metformin, transferred from its working solution (100 μg mL−1). To each sample, 250 μL of guaiphenesin (internal standard (IS)) was added using its previously prepared working solution (100 μg mL−1), and the volume was then adjusted to 2 mL with methanol. The prepared samples were mixed for one minute using a vortex mixer, and the precipitated plasma protein was removed by centrifugation at for 4000 rpm for 10 min. The net supernatant liquid of each sample was totally transmitted to a new test tube and then evaporated to dryness. The deposit of every sample was then reconstituted in 0.5 mL mobile phase mixture to prepare metformin samples in the concentration range of 0.5–30 μg mL−1 that contained 50 μg mL−1 IS. From each sample, 30 μL was introduced into the HPLC system (three times for each); then, the chromatographic conditions were followed. The peak area ratio (peak area of metformin/peak area of IS) was recorded for each sample; then, the calibration curve was plotted, from which the regression equation was calculated.Additionally, three quality control samples were prepared (2, 15, and 28 μg mL−1) by the method adopted for calibration standards and were then used for validation of the proposed method by following the chromatographic conditions mentioned previously.
2.8. Drug administration and collection of plasma samples
Full-grown female Wistar Albino rats (18 rats, 200–250 g) were randomly divided into three groups (n = 6). Group (I) was the blank group, which received vehicle only, group (II) received an oral dose of 300 mg kg−1 metformin which was dissolved in saline,52 and group (III) received a combined dose of 300 mg kg−1 metformin and 500 mg kg−1 fenugreek extract which was suspended in saline.53 0.5 mL blood samples were withdrawn from the retro-orbital plexus of each rat into separate heparinized tubes at different time intervals (0.25, 0.5, 1, 2, 4, 7, and 24 h) after administration. The blood was immediately centrifuged at 3000 rpm for 10 min for separation of blood plasma, which was then collected into another clean tube and then maintained at −20 °C until analysis.2.9. Preparation of the collected plasma samples
The stored frozen plasma samples were thawed to room temperature, and 0.5 mL of each sample was accurately transferred to a clean centrifuge tube. 250 μL of IS working solution (0.1 mg mL−1) was accurately added, and then the volume was set to 2 mL with methanol. The samples were mixed for one minute; after that, centrifugation was carried out for 10 min. The pure supernatant of each prepared sample was accurately transferred to a clean test tube and then evaporated to dryness. The residue was reconstituted with 0.5 mL mobile phase mixture. The instructions of the developed method were then followed, and the concentration of the administered drug in each sample was calculated by substitution in the previously computed regression equation.2.10. Pharmacokinetic study
The previously calculated concentrations of metformin in the collected plasma samples at different time intervals from group II and III were used to plot the mean plasma concentration–time curves for metformin (300 mg kg−1) and metformin + fenugreek extract (300 mg kg−1 + 500 mg kg−1). Then, non-compartmental pharmacokinetic analysis was performed using PKSolver (a freely available menu-driven add-in program for Microsoft Excel)54 to obtain different pharmacokinetic parameters.3. Results and discussion
This article aims to provide clinical data regarding the effect of concurrent administration of fenugreek extract on the pharmacokinetics of the widely used drug metformin through development of a RP-HPLC method using rats as an animal model.3.1. Method optimization
During method optimization, different factors were studied in order to achieve complete separation between metformin and the plasma matrix within a short analysis time. The difficulty in the optimization of the developed method was increasing the retention time of metformin in order to separate it from the plasma peak because it is a highly polar component and thus is poorly retained in the stationary phase. Different columns were tried, including C18 XBridge® (Massachusetts, USA) with dimensions of 25 cm × 4.6 mm, 5 μm particle size, C18 ZORBAX Eclipse Plus® (250 × 4.6 mm, 5 μm, California, USA), C8 XBridge® (Massachusetts, USA) with dimensions of 25 cm × 4.6 mm, 5 μm particle size, and C8 ZORBAX Eclipse Plus® (250 × 4.6 mm, 5 μm, California, USA) columns, in a trial to improve the retention of metformin; no significant difference was observed between the separation efficiencies of these stationary phases. Optimization was then continued using the C18 ZORBAX Eclipse Plus® (250 × 4.6 mm, 5 μm) column. Different mobile phase mixtures were then tested, starting with the previously reported mobile phase solvent mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mM KH2PO4 solution with different ratios (from 60
10 mM KH2PO4 solution with different ratios (from 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 40
40 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, v/v) adjusting the pH of the aqueous phase at different pH values (from 3.8 to 7), and pumping the mobile phase mixture at different flow rates (from 0.5 to 2 mL min−1). In all the trials, the plasma matrix interfered with the early eluted metformin peak. All these trials were then repeated using methanol as an organic modifier that is greener than acetonitrile; unfortunately, unresolved plasma matrix and metformin peaks resulted. The 10 mM KH2PO4 solution was then replaced with water. It was noted that the retention time of metformin was significantly increased when using water as an aqueous phase, while very low retention was observed when using 10 mM KH2PO4 solution. After that, lower concentrations of KH2PO4 solution were tried (5 mM, 1 mM, and 0.5 mM) with different ratios of methanol. Complete separation between the plasma matrix and metformin was observed on using a mobile phase mixture of 0.5 mM KH2PO4 solution
60, v/v) adjusting the pH of the aqueous phase at different pH values (from 3.8 to 7), and pumping the mobile phase mixture at different flow rates (from 0.5 to 2 mL min−1). In all the trials, the plasma matrix interfered with the early eluted metformin peak. All these trials were then repeated using methanol as an organic modifier that is greener than acetonitrile; unfortunately, unresolved plasma matrix and metformin peaks resulted. The 10 mM KH2PO4 solution was then replaced with water. It was noted that the retention time of metformin was significantly increased when using water as an aqueous phase, while very low retention was observed when using 10 mM KH2PO4 solution. After that, lower concentrations of KH2PO4 solution were tried (5 mM, 1 mM, and 0.5 mM) with different ratios of methanol. Complete separation between the plasma matrix and metformin was observed on using a mobile phase mixture of 0.5 mM KH2PO4 solution![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (65
methanol (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v). Additionally, the mobile phase was pumped at different rates (from 0.5 to 2 mL min−1), and a flow rate of 1.7 mL min−1 was selected as the rate at which complete chromatographic separation within a suitable analysis time was obtained. Scanning was carried out at different wavelengths (210, 225, 235, and 254 nm); the optimum sensitivity with minimum noise was attained on scanning at 235 nm.
35, v/v). Additionally, the mobile phase was pumped at different rates (from 0.5 to 2 mL min−1), and a flow rate of 1.7 mL min−1 was selected as the rate at which complete chromatographic separation within a suitable analysis time was obtained. Scanning was carried out at different wavelengths (210, 225, 235, and 254 nm); the optimum sensitivity with minimum noise was attained on scanning at 235 nm.
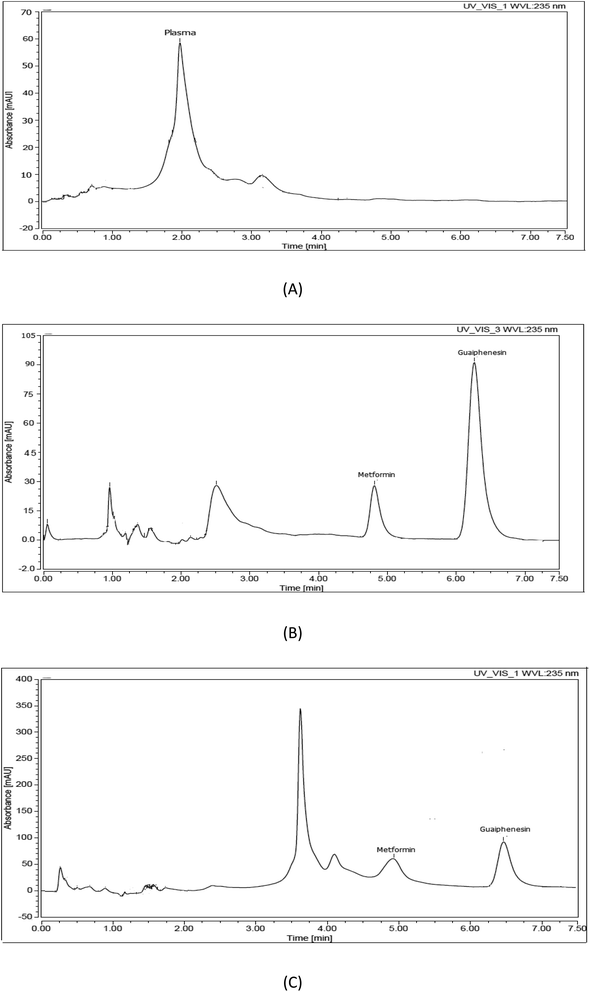
Using an internal standard during development of a bio-analytical method is very useful to enhance the accuracy, precision, and robustness of the developed method. Additionally, it is very important to compensate for the variability during the preparation and analysis of samples as well as to correct for the loss in the analyte of interest during sample preparation. Selection of a suitable internal standard is a challenge because it should have the same chromatographic behavior as the analyte and at the same time be completely resolved from both the analyte and the plasma peaks. Additionally, it should have similar chemical and physical character to the analyte of interest to be extracted by the same method. Different internal standards were tested, such as atenolol, metoclopramide, olanzapine, ibuprofen, and guaiphenesin. Guaiphenesin was the most suitable internal standard based on its chromatographic behavior relative to the separated peaks of both the plasma matrix and metformin. Finally, complete separation of the plasma matrix, metformin, and guaiphenesin was achieved within 7.5 min, with retention time values of 2–4, 4.9, and 6.3 min, respectively (Fig. 1).
The method of extraction of the parent drug from the collected plasma samples is very critical because it affects the sensitivity and selectivity of the method. The plasma protein precipitation method was chosen because it is a simple and inexpensive method. Different organic solvents were tested (acetonitrile, ethanol, and methanol). The highest extraction recovery for both the drug and the internal standard as well as the maximum protein precipitation resulted when using methanol.
3.2. Method validation
In order to test the validity of the developed bio-analytical method, guidelines recommended by the US Food and Drug Administration (FDA)51 were followed. Three quality control samples (QCs) were prepared and used during testing of the validity of the method (low QC (LQC), middle QC (MQC), and high QC (HQC) samples).| y = 0.1937x + 0.0890 |
The lower limit of quantitation was selected to be 0.5 μg mL−1, and this limit was chosen on the basis that it was the analyte concentration that gave a reproducible response which was at least five times that of the blank.
| Concentrationa (μg mL−1) | Intraday | Interday | ||||
|---|---|---|---|---|---|---|
| Recovery% | RSD% | Bias%b | Recovery% | RSD% | Bias%b | |
| a Average of 5 experiments.b Bias = [(measured concentration − nominal concentration)/nominal concentration] × 100. | ||||||
| 2.00 (LQC) | 100.99 | 0.27 | 0.99 | 100.30 | 0.57 | 0.30 |
| 15.00 (MQC) | 93.18 | 1.60 | −6.82 | 94.44 | 4.72 | −5.56 |
| 28.00 (HQC) | 99.30 | 0.08 | −0.70 | 99.30 | 0.15 | −0.70 |
3.3. Results of the pharmacokinetic study
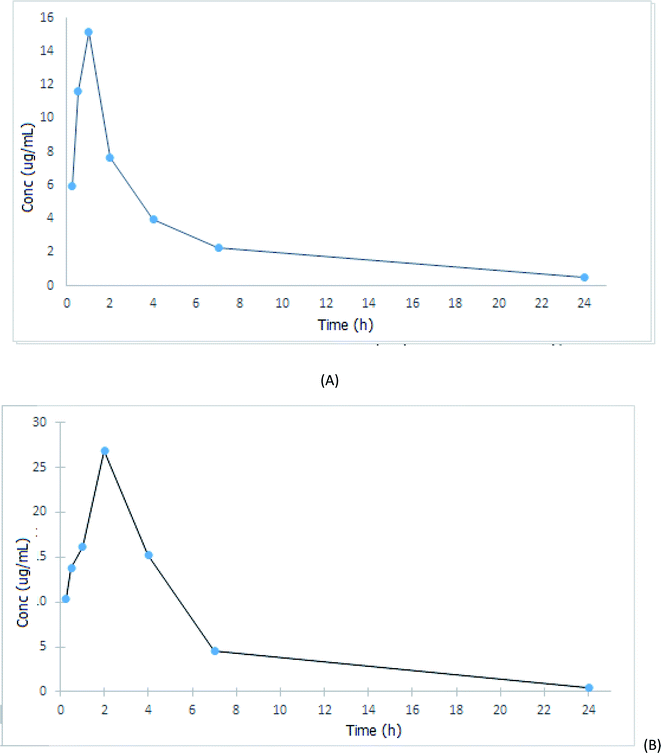
After drug administration either alone (group II) or with fenugreek extract (group III), the concentrations of the administered drug in the collected plasma samples were calculated using the previously computed regression equation; then, the mean plasma concentration–time plot was recorded (Fig. 2). Moreover, PKSolver was then used to perform the non-compartmental pharmacokinetic analysis. As shown in Fig. 2, metformin was rapidly absorbed after oral administration and appeared in plasma with an easily detectable concentration within 15 min. On the other hand, the calculated pharmacokinetic parameters given in Table 4 showed that the bioavailability of metformin was significantly increased by its concomitant administration with fenugreek extract, which was proven by the higher values of the maximum drug plasma concentration (Cmax) and area under the curve (AUC). It was found that Cmax of group III increased by 74.68% compared to that of group II, while its AUC0–t increased by about 148.55%. As a result of the increasing metformin absorption, its apparent volume of distribution was significantly decreased on using fenugreek extract. These alternations in the metformin pharmacokinetics may be due to the emulsifying effects of the fiber, protein and gum contents of fenugreek, which may increase the solubility of the drug, leading to a remarkable increase in its bioavailability. On the other hand, the time required to reach the peak plasma concentration of metformin (Tmax) was significantly influenced by the concomitant administration of the extract (Tmax of the metformin group = 1 h and of the metformin + extract group = 2 h); meanwhile, the half-life (T1/2) and the clearance rate of metformin decreased by 33.90% and 70.93%, respectively, in the metformin and extract co-administered group compared to the metformin-administered group.| Parameter | Unit | Metformin (300 mg kg−1) | Metformin + fenugreek extract, 300 + 500 (mg kg−1) |
|---|---|---|---|
| T1/2 | h | 2.95 | 1.94 |
| Tmax | h | 1.00 | 2.00 |
| Cmax | μg mL−1 | 15.14 | 26.90 |
| AUC0–t | μg mL−1 h−1 | 42.82 | 106.43 |
| AUC0–inf | μg mL−1 h−1 | 52.60 | 119.32 |
| Mean residence time (MRT) | h | 4.01 | 3.54 |
| Volume of distribution (Vd/F) | L | 24.25 | 7.05 |
| Clearance (Cl/F) | L h−1 | 5.70 | 2.51 |
From the results of the pharmacokinetic study discussed above, one can conclude that fenugreek can be considered to be a promising complimentary option for diabetes control when concurrently used with the first-line hypoglycemic metformin. It will increase the extent of absorption of metformin, leading to greater hypoglycemic effects and, hence, better blood glucose control.
3.4. Greenness assessment of the developed method
Recently, the concept of green chemistry has grown massively worldwide; it involves the exclusion or the reduction of hazardous, corrosive, toxic, bio-accumulative solvents and the resulting wastes. Different tools are now used to evaluate the greenness of an analytical method. Method greenness assessment can be carried out using the Analytical Eco-Scale, Analytical Method Volume Intensity (AMVI), and National Environmental Method Index (NEMI).The Analytical Eco-Scale depends on giving penalty points to any element that does not coincide with green analysis. The penalty points concerning different parameters in the developed analytical method (the used reagents and instruments) are calculated; then, the summation of these penalty points is subtracted from 100 to obtain the Eco-Scale score. A method is considered an ideal ecological method if it has an Eco-Scale score of 100, excellent if the score exceeds 75, acceptable if it is more than 50, and inappropriate if it is less than 50. As shown from the results in Table 5, the Analytical Eco-Scale of the developed method was calculated and found to be 87, revealing that the method can be classified as an excellent ecofriendly method.
| Parameters | Developed HPLC methoda | Penalty points | |
|---|---|---|---|
a Mobile phase consisted of 0.5 mM KH2PO4 solution![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (65 methanol (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v). 35, v/v). |
|||
| Reagents | PP of solvent = subtotal PP × number of pictogram × signal word | 0.5 mM KH2PO4 solution | |
| Consumed volume = 8.29 mL | 0 | ||
| Subtotal PP = 1 [solvent <10 mL] | |||
| Signal word= 0 none | |||
| No. of pictogram = 0 | |||
| Consumed volume = run time × flow rate × solvent percentage in system | Methanol | ||
| Consumed volume = 4.46 mL | 6 | ||
| Subtotal PP = 1 [solvent <10 mL] | |||
| Signal word = 2 danger [more severe hazard = 2] | |||
| No. of pictogram =3 | |||
| Instruments | Energy | ≤1.5 kW h per sample | 1 |
| Occupational hazard | Analytical process hermetization | 0 | |
| Centrifuge | 1 | ||
| Wastes | >10 mL | 5 | |
| Total penalty points | 12 | ||
| Analytical eco-scale total score | 87 | ||
Likewise, AMVI is a method which is based upon measuring the total consumed volume of solvent and the waste created from the proposed method. The AMVI for the proposed method was calculated and was found to be 157.25 (Table 6), ensuring that this method has limited negative impact on both human health and the environment.
| Parameters | Developed HPLC method |
|---|---|
| Solvent consumption method (mL) | 114.75 |
| Flow rate | 1.7 |
| Run time | 7.5 |
| No. of injection for one full analysis | 9 |
| No. of potential analytes | 1 |
| Solvent consumption sample preparation (mL) | 42.50 |
| Standard prep. volume (mL) | 10 |
| No. of standard preps. | 2 |
| Sample prep. volume (mL) | 2.5 |
| No. of sample preps. | 9 |
| Total method solvent consumption | 157.25 |
| Analytical method volume intensity | 157.25/1 = 157.25 |
| % Consumption method | 72.97% |
| % Consumption preparation | 27.03% |
Moreover, on evaluating the greenness profile of the developed HPLC method using the National Environmental Method Index (NEMI), it was noted that the chemicals used (KH2PO4 and methanol) are considered to be non-PBT (persistent, bio-accumulative, and toxic) and non-hazardous. The pH of the mobile phase was >2 and <12; hence, it was not corrosive. Furthermore, the waste produced was calculated and was found to be 12.75 g per sample (<50). Because the developed method succeeded in achievement of the four quadrants of the greenness profile (Fig. 3), the method can be considered to be ecologically harmless.
4. Conclusion
For the first time, a novel ecological RP-HPLC method was developed and optimized for in vivo analysis of MET and to study the effect of concurrent administration of fenugreek extract on the pharmacokinetics of the studied drug. The high fiber, protein, and gum contents of fenugreek may act as emulsifying agents that increase metformin solubility, leading to a significant increase in its bioavailability. The combination of fenugreek extract and metformin can be considered as an auspicious treatment for satisfactory diabetes control, which may lead to reduced MET requirements and hence minimize the expected long-term complications of metformin use. Furthermore, the developed RP-HPLC method has the advantage of being a highly sensitive and green method with minimal hazardous environmental effects. Moreover, it requires a short analysis time and simple protein precipitation method for sample pretreatment.Ethical committee approval
Ethical committee approval was attained by the Committee of Ethics for Scientific Research on Living Organisms, Faculty of Pharmacy, Nahda University in Beni-Suef (NUB). The approval number was (NUB-019-020).Author contributions
Nada S. Abdelwahab, Hossam M. Hassan and Asmaa M. AboulMagd contributed to the idea. Yasmine M. Ahmed carried out the in vivo study on the rats. Hossam M. Hassan aided the extraction and preparation of the plant. Nada S. Abdelwahab, Amani Morsi, Hossam M. Hassan, and Asmaa M. AboulMagd shared the practical analysis plan and performed the practical analysis. All the authors shared in the writing and revising the manuscript.Funding
This work is self funded.Conflicts of interest
Authors declare that they have no conflict of interest.Acknowledgements
The authors express their appreciation and thanks to Dr Ahmed M. Sayed, Assistant Professor, Pharmacognosy Department, Nahda University (NUB), Beni-Suef, Egypt, for his kind help in identifying the fenugreek plant seeds.References
- V. George and K. Sudhesh, Insulin action enhancers for the management of Type 2 diabetes mellitus, Expert Opin. Pharmacother., 2000, 1, 1413–1421 CrossRef.
- T. L. Lemke, D. A. Williams, V. F. Roche and S. W. Zito, Foye's Principles of Medicinal Chemistry, Lippincott Williams & Wilkins, 6th edn, 2008, p. 855 Search PubMed.
- B. Patel, N. P. Jivani and A. Khodakiya, Drug interaction: Can we make them advantageous for a human being, Int. J. Pharm. Res. Dev., 2012, 4, 8–15 Search PubMed.
- V. D. A. Ningrum, A. Wibowo, I. Fuaida, Z. Ikawati, A. H. Sadewa and M. Robikhul Ikhsan, Validation of an HPLC-UV method for the determination of metformin hydrochloride in spiked-human plasma for the application of therapeutic drug monitoring, Res. J. Pharm. Technol., 2018, 11, 2197–2202 CrossRef.
- Y. Harahap, K. Dianpratami, M. Wulandari and R. Rahmawati, Validation of metformin hydrochloride in human plasma by HPLC-photo diode array (PDA) for application of bioequivalence study, J. Life Sci., 2012, 6, 20–27 Search PubMed.
- V. Porta, et al., HPLC-UV determination of metformin in human plasma for application in pharmacokinetics and bioequivalence studies, J. Pharm. Biomed. Anal., 2008, 46, 143–147 CrossRef CAS.
- H. P. Chhetri, P. Thapa and A. Van Schepdael, Simple HPLC-UV method for the quantification of metformin in human plasma with one step protein precipitation, Saudi Pharm. J., 2014, 22, 483–487 CrossRef.
- Y. M. Rebecca, V. Sudha and A. K. H. Kumar, Validated high performance liquid chromatography method for the determination of metformin in human plasma and its application to pharmacokinetic study, J. Chromatogr. Sep. Tech., 2019, 2, 119–124 Search PubMed.
- N. Sher, N. Fatima, S. Perveen and F. A. Siddiqui, Simultaneous determination of anti-diabetic drugs, Braz. J. Pharm. Sci., 2019, 55, e17394 CrossRef.
- A. Liu and S. P. Coleman, Determination of metformin in human plasma using hydrophilic interaction liquid chromatography-tandem mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 3695–3700 CrossRef CAS.
- N. Koseki, H. Kawashita, M. Niina, Y. Nagae and N. Masuda, Development and validation of high selective quantitative determination of metformin in human plasma by cation exchanging with normal-phase LC/MS/MS, J. Pharm. Biomed. Anal., 2005, 36, 1063–1072 CrossRef CAS.
- D. Michel, M. Casey Gaunt, T. Arnason and A. El-Aneed, Development and validation of fast and simple flow injection analysis-tandem mass spectrometry (FIA-MS/MS) for the determination of metformin in dog serum, J. Pharm. Biomed. Anal., 2015, 107, 229–235 CrossRef CAS.
- J. G. Swales, R. Gallagher and R. M. Peter, Determination of metformin in mouse, rat, dog and human plasma samples by laser diode thermal desorption/atmospheric pressure chemical ionization tandem mass spectrometry, J. Pharm. Biomed. Anal., 2010, 53, 740–744 CrossRef CAS.
- C. Kiran, W. Jianmei, X. Yong, W. Ali, W. Linshu, D. Xiaowei, Y. C. Eric, L. Ran and Y. Shao-Hua, Determination of metformin bio-distribution by LC-MS/MS in mice treated with a clinically relevant paradigm, PLoS One, 2020, 15(6), e0234571 CrossRef.
- W. Zhang, F. Han, H. Zhao, Z. J. Lin, Q. M. Huang and N. Weng, Determination of metformin in rat plasma by HILIC-MS/MS combined with Tecan automation and direct injection, Biomed. Chromatogr., 2012, 26, 1163–1169 CrossRef CAS.
- A. Liu and S. P. Coleman, Determination of metformin in human plasma using hydrophilic interaction liquid chromatography–tandem mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 3695–3700 CrossRef CAS.
- E. Uçaktürk, The development and validation of a gas chromatography-mass spectrometry method for the determination of metformin in human plasma, Anal. Methods, 2013, 5, 4723–4730 RSC.
- K. Balamurugan, M. Kirtimaya and R. Suresh, Simultaneous estimation of linagliptin and metformin HCl in human plasma by RP-HPLC method, Int. Res. J. Pharm., 2019, 10, 167–170 CAS.
- A. Shakoor, A. Adnan and M. Ahmed, Simultaneous determination of metformin and vildagliptin by HPLC in human plasma: application to pharmacokinetic studies, Lat. Am. J. Pharm., 2019, 38, 1416–1423 CAS.
- M. M. Sebaiy, S. M. El-Adl, M. M. Baraka and A. A. Hassan, Rapid RP-HPLC method for simultaneous estimation of metformin, pioglitazone, and glimepiride in human plasma, Acta Chromatogr., 2020, 32, 16–21 CAS.
- M. C. Ranetti, M. Ionescu, L. Hinescu, E. Ionică, V. Anuţa, A. E. Ranetti, C. E. Stecoza and C. Mircioiu, Validation of a HPLC method for the simultaneous analysis of metformin and gliclazide in human plasma, Farmacia, 2009, 57, 728–735 CAS.
- C. Yardimci, N. Ozaltin and A. Gurlek, Simultaneous determination of rosiglitazone and metformin in plasma by gradient liquid chromatography with UV detection, Talanta, 2007, 72, 1416–1422 CrossRef CAS.
- A. Shakoora, M. Ahmedb, R. Ikramc, S. Hussaina, A. Tahird, B. M. Janc and A. Adnan, Stability-indicating RP-HPLC method for simultaneous determination of metformin hydrochloride and vildagliptin in tablet and biological samples, Acta Chromatogr., 2020, 32, 39–43 Search PubMed.
- M. S. Elgawish, S. Nasser, I. Salama, A. M. Abbasc and S. M. Mostafaa, Liquid chromatography tandem mass spectrometry for the simultaneous determination of metformin and pioglitazone in rat plasma: Application to pharmacokinetic and drug–drug interaction studies, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2019, 1124, 47–57 CrossRef CAS.
- X. Y. Zhang, Y. Peng, P. Wan, L. Yin, G. J. Wang and J. G. Sun, Simultaneous determination and pharmacokinetic study of metformin and pioglitazone in dog plasma by LC-MS-MS, J. Chromatogr. Sci., 2014, 52, 52–58 CrossRef CAS.
- B. Jagadeesh, D. V. Bharathi, C. Pankaj, V. S. Narayana and V. Venkateswarulu, Development and validation of highly selective and robust method for simultaneous estimation of pioglitazone, hydroxypioglitazone and metformin in human plasma by LC-MS/MS: application to a pharmacokinetic study, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2013, 930, 136–145 CrossRef CAS.
- S. R. Polagani, N. R. Pilli, R. Gajula and V. Gandu, Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC-MS/MS and its application to a human pharmacokinetic study, J. Pharm. Anal., 2013, 3, 9–19 CrossRef CAS.
- N. Li, Y. Deng, F. Qin, J. Yu and F. Li, Simultaneous quantification of metformin and glipizide in human plasma by high-performance liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic study, Biomed. Chromatogr., 2013, 27, 191–196 CrossRef CAS.
- S. Mowaka, E. F. Elkady, M. M. Elmazar and B. Ayoub, Enhanced LC-MS/MS determination of alogliptin and metformin in plasma: application to a pharmacokinetic study, Microchem. J., 2017, 130, 360–365 CrossRef CAS.
- D. Mohamed, M. S. Elshahed, T. N. Aboutaleb and N. O. Zakaria, Novel LC–MS/MS method for analysis of metformin and canagliflozin in human plasma: application to a pharmacokinetic study, BMC Chem., 2019, 13, 82–92 CrossRef.
- T. Wattamwar, A. Mungantiwar, S. Halde and N. Pandita, Development of simultaneous determination of empagliflozin and metformin in human plasma using liquid chromatography–mass spectrometry and application to pharmacokinetics, Eur. J. Mass Spectrom., 2020, 26, 117–130 CrossRef CAS.
- A. M. I. Mohamed, F. A. Mohamed, S. Ahmed and Y. A. Mohamed, An efficient hydrophilic interaction liquid chromatographic method for the simultaneous determination of metformin and pioglitazone using high-purity silica column, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, 997, 16–22 CrossRef CAS.
- R. Pontarolo, A. C. Gimenez, T. M. G. Francisco, R. P. Ribeiro, F. L. D. Pontes and J. C. Gasparetto, Simultaneous determination of metformin and vildagliptin in human plasma by a HILIC-MS/MS method, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 965, 133–141 CrossRef CAS.
- A. Gałuszka, P. Konieczka, Z. M. Migaszewski and J. Namieśnik, Analytical Eco-Scale for assessing the greenness of analytical procedures, Trends Anal. Chem., 2012, 37, 60–72 CrossRef.
- R. Hartman, R. Helmy, M. Al-Sayah and C. J. Welch, Analytical Method Volume Intensity (AMVI): A green chemistry metric for HPLC methodology in the pharmaceutical industry, Green Chem., 2011, 13, 934–939 RSC.
- E. A. Abdelaleem and N. S. Abdelwahab, Green chromatographic method for analysis of some anti-cough drugs and their toxic impurities with comparison to conventional methods, Saudi Pharm. J., 2018, 26, 1185–1191 CrossRef.
- S. K. Jain, Ethnobotanical uses of plants, in dictionary of Indian folk medicine and ethnobotany, Deep Publications, India, 1991, p. 182 Search PubMed.
- S. A. Wani and P. Kumar, Fenugreek: A review on its nutraceutical properties and utilization in various food products, J. Saudi Soc. Agric. Sci., 2018, 17, 97–106 Search PubMed.
- L. Ali, A. K. Azad Khan, Z. Hassan, M. Mosihuzzaman and N. Nahar, et al., Characterization of the hypoglycemic effects of Trigonella foenum graecum seed, Planta Med., 1995, 61, 358–360 CrossRef CAS.
- R. D. Sharma, Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects, Nutr. Res., 1986, 6, 1353–1364 CrossRef.
- R. D. Sharma, T. C. Raghuram and N. S. Rao, Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes, Eur. J. Clin. Nutr., 1990, 44, 301–306 CAS.
- R. D. Sharma, A. Sarkar and D. K. Hazra, Use of fenugreek seed powder in the management of non-insulin dependent diabetes mellitus, Nutr. Rep., 1996, 16, 1331–1339 Search PubMed.
- J. Raju, D. Gupta, A. R. Rao, P. K. Yadava and N. Z. Baquer, Trigonella Foenum Graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes, Mol. Cell. Biochem., 2001, 224, 45–51 CrossRef CAS.
- P. R. Petit, Y. D. Sauvaire, D. M. Hillaire-Buys, O. M. Leconte and Y. G. Baissac, et al., Steroid saponins from fenugreek seeds, Extraction, purification and pharmacological investigation on feeding behavior and plasma cholesterol, Steroids, 1995, 60, 674–680 CrossRef CAS.
- R. Moorthy, K. M. Prabhu and P. S. Murthy, Studies on the isolation and effect of orally active hypoglycemic principle from the seeds of fenugreek (Trigonella Foenum Graecum), Diabetes Bull., 1989, 9, 69–72 Search PubMed.
- Y. Sauvaire, Y. Baissac, O. Leconte, P. Petit and G. Ribes, Steroid saponins from fenugreek and some of their biological properties, Adv. Exp. Med. Biol., 1996, 405, 37–46 CrossRef CAS.
- M. Yoshikawa, T. Murakami, H. Komatsu, N. Murakami, J. Yamahara and H. Matsuda, Medicinal Foodstuffs. IV. Fenugreek Seed. (1): Structures of trigoneosides Ia, Ib, IIa, IIb, IIIa and IIIb, new furostanol saponins from the seeds of Indian Trigonella Foenum Graecum L, Chem. Pharm. Bull., 1997, 45, 81–97 CrossRef CAS.
- W. G. Taylor, J. L. Elder, P. R. Chang and K. W. Richards, Micro determination of diosgenin from fenugreek (Trigonella Foenum Graecum) seeds, J. Agric. Food Chem., 2000, 48, 5206–5210 CrossRef CAS.
- P. Sridevi, G. A. Lakshmi, K. Vunutha, K. Akshitha, K. Mahesh and M. Bhagavan Raju, Dipeptide synthesis and evaluation of antidiabetic activity of 4-hydroxyisoleucine from fenugreek seeds, Pharm. Bioprocess., 2017, 5, 44–53 Search PubMed.
- K. Srinivasan, Plant foods in the management of diabetes mellitus: Spices as potential antidiabetic agents, Int. J. Food Sci. Nutr., 2005, 56, 399–414 CrossRef CAS.
- FDA, Guidance for Industry Bio-analytical Method Validation Guidance for Industry Bio-analytical Method Validation, 2013 Search PubMed.
- K. Akahane, K. Ojima, A. Yokoyama, T. Inoue, S. Kiguchi, S. Tatemichi, H. Takeda and Y. Imai, Effects of combination of mitiglinide with various oral antidiabetic drugs in streptozotocin-nicotinamide-induced type 2 diabetic rats and Zucker fatty rats, Clin. Exp. Pharmacol. Physiol., 2017, 44, 1263–1271 CrossRef CAS.
- D. Sureshkumar, S. Begum, N. M. Johannah, B. Maliakel and I. M. Krishnakumar, Toxicological evaluation of a saponin-rich standardized extract of fenugreek seeds (FenuSMART®): Acute, sub-chronic and genotoxicity studies, Toxicol. Rep., 2018, 5, 1060–1068 CrossRef CAS.
- Y. Zhang, M. Huo, J. Zhou and S. Xie, An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel, Comput. Meth. Prog. Biomed., 2010, 99, 306–314 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |