Open Access Article
Open Access ArticleRecycling of spent coffee grounds for useful extracts and green composites†
Yihao Leowab,
Pek Yin Michelle Yewa,
Pei Lin Cheea,
Xian Jun Loh a and
Dan Kai
a and
Dan Kai *a
*a
aInstitute of Materials Research and Engineering (IMRE), A*STAR, 2 Fusionopolis Way, #08-03 Innovis, 138634, Singapore. E-mail: kaid@imre.a-star.edu.sg
bDepartment of Materials Science and Engineering, National University of Singapore, 9 Engineering Drive 1, 117576, Singapore
First published on 13th January 2021
Abstract
Large amounts of spent coffee grounds (SCGs) are often discarded and there is a need to find alternative disposal methods due to environmental concerns. This project aims to develop sustainable materials by re-purposing spent coffee grounds (SCGs). Oil extraction was performed using different organic solvents and yielded approximately 10% coffee oil. Coffee oil contains potentially useful chemical compounds such as fatty acids and caffeine. They also exhibited antioxidant properties. Extracted SCGs (ESCGs) were blended with epoxy resin to form composites. ESCG composites displayed a general decrease in mechanical properties relative to epoxy. However, improvements were observed when comparing ESCG composites and SCG composites. The greatest improvement belongs to epoxy composite filled with acetone-ESCGs, where the tensile strength, flexural modulus and flexural strength increased to 23.4 MPa, 3.02 GPa and 42.9 MPa respectively. This study presents a way to exploit waste materials which contributes to the goal of sustainability.
Introduction
Coffee is one of the most popular beverages globally and a commodity in multiple countries.1 According to the International Coffee Organisation, economic trading of coffee has grown steadily over the years and the coffee industry is projected to continue growing. Approximately 50% of global coffee production is processed for soluble coffee.2 Being the second largest commodity traded worldwide after petroleum, coffee production and processing results in a large amount of by-products, such as coffee pulp, husks, silver skin and spent coffee grounds (SCGs).2,3 650 kg of SCGs are obtained from 1 ton of green coffee.3 Evidently, SCGs are one of the major by-products from the preparation of instant coffee and are often discarded as waste due to their lack of economic value.Conventional methods for eradicating SCGs include disposal as solid waste directly into landfill and sewage, which may cause pollution to the environment due to its toxic nature and organic compounds.4 The leached organic matter can threaten environmental and human health.4 However, landfill disposal is an ineffective solution, especially for small countries where land is already scarce. Another method of disposal is through incineration, which is also undesirable as the particulate matter generated may have a detrimental effect on air quality in the vicinity.3 These methods are highly destructive to the environment and thus highlight the need for better SCG waste management.
With greater environmental pressure to minimise pollution, many researchers have explored potential applications to fully utilise SCGs as bio-resources. Biochar catalyst for glucose isomerisation to fructose was demonstrated by Chen et al. by doping SCGs with melamine. The catalyst showed exceptional catalytic performance and adding acetone as cosolvent further improved its efficiency, yielding 14% fructose in 5 min.5 Cho et al. studied the use SCGs loaded with cobalt for the production of syngas and concluded pyrolysis under carbon dioxide as atmospheric gas results in the best condition for syngas manufacturing.6 They also evaluated the incorporation of zirconia into SCG pyrolysis and managed to double carbon monoxide production from 14.3 to 29.5 mol%, which is beneficial for fuel gas manufacturing with high carbon monoxide fraction.7 Numerous bio-refinery efforts to valorise SCGs have been summarised by Mata et al. and Banu et al. which include multiple combination of processes such as extraction, transesterification, hydrolysis, fermentation, and pyrolysis.8,9 SCGs can be converted into biofuels (biogas, bioethanol, bio-oil, biodiesel, hydrocarbon fuel, and fuel pellets) and valuable chemical compounds (adsorbents, bioactive compounds, biochar, compost, glycerine carotenoids, and polyhydroxyalkanotes).8–11 Phenolic compounds, caffeine, tannins and antioxidants are some compounds that can be extracted from SCGs for composting or fertiliser materials, and potential additives in the health and food industry.9–11 SCGs can also potentially be a filler material for composites.9,10
SCGs are composed of large quantities of organic compounds namely cellulose, hemicellulose, lignin and fatty acids.12,13 Cellulose and hemicellulose account for around 50 wt% whereas lignin and proteins accounts approximately 20 wt%.10 In general SCGs also contain 7 to 15 wt% of coffee oil depending on the species of coffee.14–16 Somnuk and his group studied the extraction of coffee oil using hexane, anhydrous ethanol, hydrous ethanol or methanol. Their optimised condition yielded 14.7 wt% coffee oil using hexane as the extraction solvent within 30.4 min.17 Coffee oil can be attained via microwave assisted extraction (MAE) as demonstrated by Hibbert and his team. Comparable amount of coffee oil, at 11.54 wt%, were collected via MAE, proving to be more efficient because it requires less time and solvent loss in contrast to Soxhlet extraction.18 Higher amounts of coffee oil can be extracted under different conditions and solvents. Caetano and his team managed to extract 6.3 to 28.3 wt% of coffee oil when experimenting with various extraction solvents and contact duration. 21.5 wt% coffee oil was extracted using 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 hexane/isopropanol mixture after 3 hours and they concluded that this method offers a good balance between extraction yield, solvent cost and energy consumed.19 Coffee oil comprises mainly monoglycerides, diglycerides, triglycerides and free fatty acids.18,20 Linoleic, palmitic, oleic and stearic acids are the most commonly found chemicals in the fatty acids.20 The remaining composition of coffee oil can be ascribed to unsaponifiable compounds such as waxes, phosphatides, tocopherols, sterols and diterpenes.20 Coffee oil also exhibits antioxidant activity, which are suitable for cosmetic or food industries.
50 hexane/isopropanol mixture after 3 hours and they concluded that this method offers a good balance between extraction yield, solvent cost and energy consumed.19 Coffee oil comprises mainly monoglycerides, diglycerides, triglycerides and free fatty acids.18,20 Linoleic, palmitic, oleic and stearic acids are the most commonly found chemicals in the fatty acids.20 The remaining composition of coffee oil can be ascribed to unsaponifiable compounds such as waxes, phosphatides, tocopherols, sterols and diterpenes.20 Coffee oil also exhibits antioxidant activity, which are suitable for cosmetic or food industries.
Since SCGs are widely available and there is a need for green materials, the idea to incorporate SCGs as a natural filler material for polymer composites has been widely explored. Existing research presented the use of different matrices such as polypropylene (PP), polylactide (PLA), polyvinyl alcohol (PVA), poly(butylene adipate-co-terephthalate) (PBAT), epoxy. Some studies involving SCGs in different matrices are as follows. García–García et al. reported the use of 20 wt% SCG as fillers in PP matrix. Four different treatments were presented and compared – untreated SCGs, palmitoyl chloride treated hydrophobized SCGs, (3-glycidyloxypropyl)trimethoxysilane silanised SCGs and lastly maleic anhydride (MA) grafted PP. They identified that the incorporation of untreated and treated SCGs in PP matrix results in a minor reduction in flexural strength but increases flexural modulus.21 Lee et al. evaluated the incorporation of SCG fillers and compared the results with respect to carbon black (CB) fillers. The fillers were added at varying compositions – 0, 1, 2, 3 wt% into PVA matrix. SCG-filled PVA exhibited improved tensile strength and Young's modulus relative to CB-filled PVA.22 Moustafa et al. demonstrated the use of 10, 20, 30, 50 wt% of SCG fillers incorporated in PBAT matrix coupled with 0 or 15 wt% of polyethylene glycol plasticisers. Results indicated that there was improved tensile properties for plasticised composites relative to unplasticised composites, but a reduction of elastic modulus.23
The goal of this paper is to provide an insight on how to exploit SCGs for coffee oil and as a filler material for bio-based composites. In addition, it aims to provide areas of exploration and possible applications through maximising the potential of SCGs. Coffee oil was extracted from SCGs via reflux extraction using four different solvents – hexane (H), tetrahydrofuran (T), acetone (A) and ethanol (E). The chemical composition of coffee oil (CO) extracted using different extraction solvents was evaluated. Antioxidant behaviour of these samples were also examined. The surface morphology of SCGs and extracted SCGs (ESCGs) were studied and thermal properties investigated. Therefore, SCGs and ESCGs constituting 30 wt% were then added as a filler material into epoxy resin to form a bio-based composite. The mechanical and thermal properties were investigated alongside fracture morphology.
Materials and methods
Materials
SCGs were sourced from the coffee machines in the pantry at Institute of Materials Research and Engineering (IMRE). The coffee beans in the machines are arabica coffee beans, roasted to a Full City Roast by a local company, BlackGold Coffee. SCGs were washed with room temperature deionised water to achieve a neutral pH level and to remove any contaminants along with water-soluble compounds. They were then oven dried at 110 °C overnight, followed by grinding using a commercial blender for 5 min. The oven dried fillers were approximately 200 to 500 μm. The extraction solvents employed in the reflux extraction process were 98.5% hexane (J.T.Baker), 100% tetrahydrofuran (VWR Chemicals), 99.5% acetone (Green Tropic Products Pte Ltd) and 99.98% ethanol absolute (VWR Chemicals). Slow-curing transparent Epofix epoxy and Epofix hardener system were purchased from Struers APS, Singapore. Epofix epoxy resin contains bisphenol-A-diglycidylether and Epofix hardener contains triethylenetetramine. The epoxy system used in the study was made from the standard weight ratio of 25 parts of epoxy to 3 parts of hardener.Reflux extraction of coffee oil
20 g of dry grounded SCGs and 100 ml of extraction solvent was added into a round bottom flask with a magnetic stirrer. The mixture was left to reflux for 24 h at 60 °C. The magnetic stirrer stirs at 350 revolutions per minute. After oil extraction, the solid and liquid phase were separated via vacuum filtration and the extraction solvent was distilled off via rotary evaporation. Residual extraction solvent was evaporated using a vacuum oven at 60 °C for 24 h. The variants of ESCGs were also vacuum oven dried under the same condition. Coffee oil yield was obtained with the assumption that all oil in SCGs was removed. Coffee oil variants were collected after oil extraction from SCGs using different extraction solvents. The coffee oil variants extracted using hexane, THF, acetone or ethanol which were labelled as H-CO, T-CO, A-CO and E-CO respectively. ESCG variants were obtained after oil extraction from SCGs using the same extraction solvents. ESCG variants after hexane, THF, acetone or ethanol extraction were labelled as H-ESCG, T-ESCG, A-ESCG and E-ESCG respectively.Coffee oil characterisation
Chemical composition analysis of coffee oil extracts was identified using Gas Chromatography-Mass Spectroscopy (GC-MS) on Aligent Technologies GC System (7890A) equipped with capillary column (ZB-5MSplus semi-standard non-polar) and Aligent Technologies triple axis detector (5975C). Automatic injection was made in spilt ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 at 250 °C injection temperature and 0.001 ml injection volume. Sample concentration used for injection was approximately 20.4 mg ml−1 in dichloromethane solvent and sample concentration introduced into GC column was approximately 1.8 mg ml−1. The interface temperature was 260 °C and ion source temperature was 250 °C. Oven temperature was programmed as follow: hold at 40 °C for 1 min, ramp up to 150 °C at 10 °C min−1, and from 150 °C ramp up to 250 °C at 20 °C min−1. The total run time for each sample was 60 min with a column flow rate of 1.5 ml min−1. The mass spectra were compared with NIST library for molecular structure confirmation.
10 at 250 °C injection temperature and 0.001 ml injection volume. Sample concentration used for injection was approximately 20.4 mg ml−1 in dichloromethane solvent and sample concentration introduced into GC column was approximately 1.8 mg ml−1. The interface temperature was 260 °C and ion source temperature was 250 °C. Oven temperature was programmed as follow: hold at 40 °C for 1 min, ramp up to 150 °C at 10 °C min−1, and from 150 °C ramp up to 250 °C at 20 °C min−1. The total run time for each sample was 60 min with a column flow rate of 1.5 ml min−1. The mass spectra were compared with NIST library for molecular structure confirmation.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) assay was used to evaluate the antioxidant activity of coffee oil extracted using different solvents. 60 μM DPPH solution was prepared in methanol (MeOH). Approximately 10 mg of each coffee oil variant was added into a series of 20 ml glass vials, followed by the addition of 20 ml 60 μM DPPH/MeOH into each vial. The DPPH free radical content was determined by measuring the absorbance at 517 nm at time intervals of 30 min, 1 h, 2 h, 4 h, 6 h, 24 h and 48 h. All coffee oil variants were prepared in triplicates. The antioxidant activity was represented by inhibition percentage of free radicals and was derived from the detection of absorbance change with respect to control solutions.
Preparation of epoxy, SCG and ESCG composite samples
The preparation of SCG and ESCG composite samples was conducted under room temperature. A total of 5 variants of dried SCGs or ESCGs are added into epoxy resin at 30 wt%. Each variant of SCG or ESCG composite sample amounted to 70 g and the mass ratio of resin to hardener is 25 to 3. First, 21 g of SCG or ESCG variant was added to 43.75 g of epoxy resin and hand mixed. Then, 5.25 g of hardener was added and further mixed to achieve a homogenous mixture. A control sample of epoxy is also prepared by hand mixing 62.5 g of epoxy resin and 7.5 g of hardener. Each mixture was transferred to silicone moulds adhering to the dimensions of tensile and flexural templates. The dimensions for tensile and flexural samples are in accordance to ISO 527-2 and ISO 178 standards respectively. They were left to set at room temperature for 24 h before removal.Characterisation of SCG and ESCG composite samples
The SCGs and different variants of ESCGs were analysed by Fourier Transform Infrared Spectroscopy (FTIR) using an infrared spectrometer (Spectrum 2000, PerkinElmer, USA). Each sample was subjected to 128 scans with a resolution of 2 cm−1 between 4000 and 400 cm−1. The matrix used was KBr and the samples were measured after pressing into a KBr disc.SCGs and ESCGs from different extraction solvents were examined morphologically using scanning electron microscopy (SEM) (JSM6700F, JEOL, Japan). The morphology of fracture surface from the epoxy, SCG and ESCG composite tensile samples were also examined. All inspected samples were coated by sputtering thin layers of gold before imaging.
Thermal degradation behaviour of SCG, ESCG variants and epoxy, SCG and ESCG composites were studied using thermogravimetric analysis (TGA) (Q500, TA Instruments, USA). Samples of approximately 15 mg were placed in standard alumina crucibles and subjected to heating from approximately 20 to 700 °C with a heating rate of 20 °C min−1 under nitrogen atmosphere.
Thermal stability behaviour of SCGs, ESCG variants and epoxy, SCG and ESCG composites were studied using differential scanning calorimetry (DSC) (Q100, TA Instruments, USA) equipped with auto cool accessory and calibrated with indium. Sample mass of approximately 5 mg was placed in standard aluminium crucibles and subjected to temperature ramp from −20 to 180 °C for SCG and ESCG variants, and −20 to 200 °C for epoxy, SCG and ESCG composites. The heating rate was at 20 °C min−1. Each sample is subjected to heating from −20 °C to maximum, held at maximum for 5 min, cooled from maximum to −20 °C and reheated from −20 °C to maximum under nitrogen atmosphere. Experimental data from the second heating run was obtained for analysis of glass transition temperature.
Mechanical testing
SCG and ESCG composites were subjected to tensile and flexural tests to evaluate their mechanical properties. Tensile tests were conducted using a universal testing machine (Instron 5569, USA) according to ISO 527-2 standard at crosshead speed of 1.00 mm min−1. Tensile tests were conducted until tensile failure. Similarly, flexural tests were conducted to flexural failure under same environmental conditions according to ISO 178 standard and at crosshead speed of 1.00 mm min−1 with span fixed at 40 mm. Data values from five replicated samples tested were used to obtain average values for both tensile and flexural properties.Results and discussion
Coffee oil extraction and chemical composition
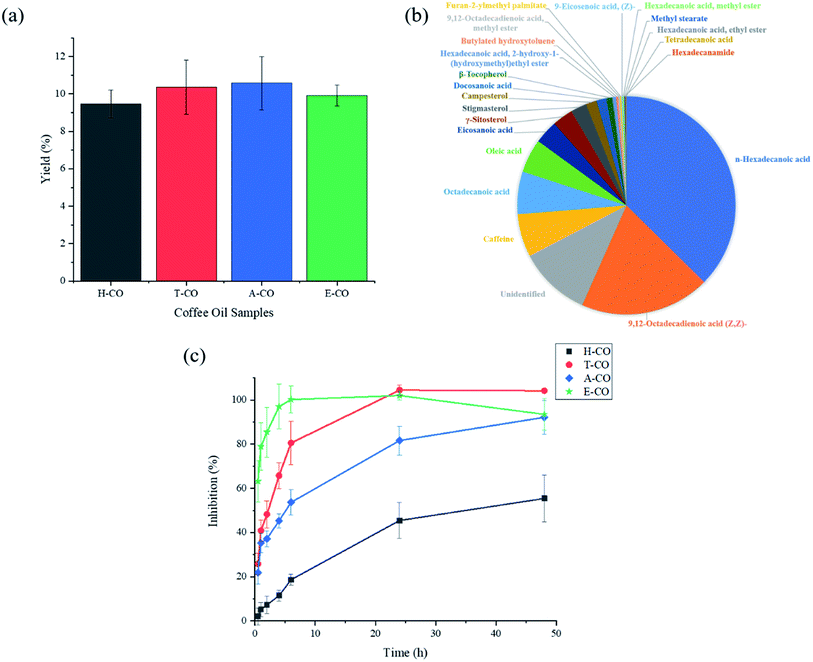
The effect of extraction using four different solvents on coffee oil yield is shown in Fig. 1(a). Yield of extracted coffee oil variants differs slightly but were generally similar at approximately 10%. The results fall within the range of coffee oil content for common coffee species reported.14,15 Our coffee oil yield is comparable to studies conducted by Somnuk et al. and Hibbert et al., however was half of what Caetano et al. achieved.17–19 Chemical composition of coffee oil variants was distinguished using GCMS analysis. Fig. 1(b) shows the proportion of chemical compounds that can be found in A-CO sample. Caffeine and some common chemical compounds present in larger quantities are tabulated in Table 1. Potentially useful antioxidant compounds were also included.| Compound name | H-CO (%) | T-CO (%) | A-CO (%) | E-CO (%) |
|---|---|---|---|---|
| n-Hexadecanoic acid | 42.28 | 26.80 | 37.39 | 28.38 |
| 9,12-Octadecadienoic acid (Z,Z)- | 22.05 | 14.33 | 19.27 | 15.49 |
| Octadecanoic acid | 6.83 | 3.68 | 6.30 | 6.03 |
| Caffeine | 0.15 | 2.63 | 6.37 | 5.70 |
| Butylated hydroxytoluene | 0.06 | 14.79 | 0.33 | 0.02 |
| Oleic acid | 5.22 | 5.29 | 5.01 | 6.07 |
| β-Tocopherol | 0.50 | 0.59 | 0.88 | 0.63 |
Relative amounts of fatty acids such as n-hexadecanoic acid (palmitic acid), 9,12-octadecadienoic acid (Z,Z)-(linoleic acid) and octadecanoic acid (stearic acid) identified corresponds with results presented by Couto et al.24 With the exception of T-CO, H-CO contains the most amount of fatty acids followed by A-CO and E-CO. This trend is attributed to the polarity of extraction solvents. Increasing order of extraction solvent polarity is as follows, hexane is the least polar followed by acetone then ethanol. Fatty acids are non-polar compounds due to their long hydrocarbon chain, thus non-polar solvents such as hexane are better suited for the extraction of fatty acids. The findings were coherent as generally non-polar solvents are better at lipid extraction than polar solvents. This is because non-polar solvent carries low or no charges. Hence, the extraction process is based on van der Waals forces of interactions between solvents and fatty acids. Thus a non-polar solvent will be better able to penetrate into the low polar matrix of SCGs and extract higher amounts of fatty acids.1 Increasing solvent polarity resulted in a slight decrease in fatty acid contents.
Palmitic acid and linoleic acid accounts for the largest proportion among chemical compounds found in coffee oil variants. Composition ranges from 26.80 to 42.28% for palmitic acid and 14.33 to 22.05% for linoleic acid across the coffee oil samples. Substantial content of palmitic acid is favourable as it exhibits anti-inflammatory property, as investigated by Aparna et al., and is abundantly used in topical medication for rheumatic symptoms.25 An inverse relationship between the consumption of linoleic acid and risk of coronary heart disease was established by Farvid et al.26 Coffee oil variants also contain 0.15 to 6.37% of caffeine. Consumption of caffeine in moderation can produce behavioural benefits which includes heightened alertness, fatigue reduction, better mental concentration and increased sense of energy.27
Antioxidant activity of coffee oil
Antioxidant compounds were also found in coffee oil samples. Butylated hydroxytoluene (BHT), β-tocopherol (vitamin E) and oleic acid are examples of antioxidant present. BHT exist at low amounts, 0.02 to 0.33%, with the exception of T-CO at 14.79%. The reason for high levels of BHT in T-CO is unclear, but is suspected to be related to the polarity and solubility of BHT in THF solvent. Multiple studies were conducted on the toxicity of BHT and concluded that BHT is safe to use at low concentration with potential anticancer property and other health benefits.28,29 Oleic acid is present at 5.01 to 6.07% and experimental results by Wang et al. indicated orally administered oleic acid is capable of reducing oxidative damage in vivo.30Antioxidant activity of coffee oil was investigated using DPPH assay. Free radical inhibition for different coffee oil samples were depicted in Fig. 1(c). E-CO exhibits the best antioxidant inhibition up to 24 h followed by T-CO, A-CO and H-CO. Rapid antioxidant activity can be observed from E-CO, whereby after 30 min, 63% inhibition is achieved and 100% inhibition is observed within 6 h. Initial antioxidant activity for T-CO and A-CO are similar at 26 and 22% respectively after 30 min. Antioxidant inhibition increased faster for T-CO and A-CO, with T-CO reaching 100% inhibition within 24 h for T-CO and 92% inhibition for A-CO after 48 h. H-CO after 30 min attained 2% inhibition and produced the lowest antioxidant activity at 55% inhibition after 48 h. High levels of antioxidant activity was observed in E-CO and T-CO, probably due to their higher content of oleic acid for E-CO at 6.07% and BHT for T-CO at 14.79%. With the exclusion of T-CO, DPPH assay designed for antioxidant testing suggests that more polar antioxidants compounds can be found in E-CO followed by A-CO and H-CO. The results also indicate that antioxidants are more easily extracted using polar solvents.
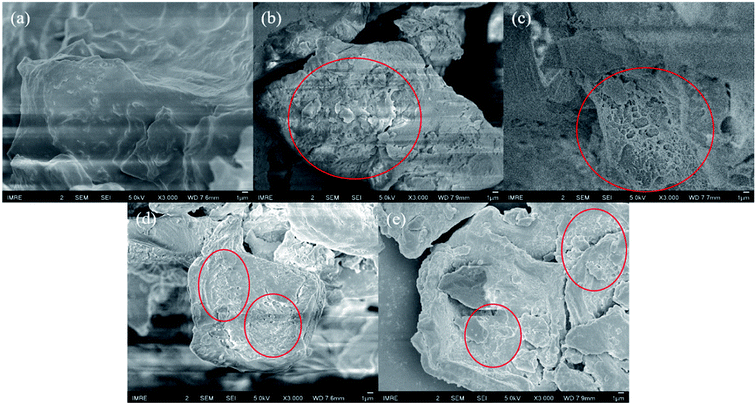
Surface morphology of SCG and ESCG variants
Surface morphology of SCG and ESCG variants were examined by SEM and presented in Fig. 2. The surface morphology of SCG before coffee oil extraction is relatively smooth compared to ESCG samples. Presence of coffee oil could lead to adhesion and aggregation of SCG particles which might affect the dispersion and mechanical properties of the composites. It is evident that after the extraction process, surface morphology of ESCG variants is rougher and more porous. The stirring motion could allow more collisions between SCG particles and increase solvent penetration, resulting in increased surface roughness and porosity. This change in surface morphology is also indicative of successful extraction of coffee oil that was previously trapped in SCGs. Collision between particles may also refine particle size, which could allow better dispersity in epoxy matrix.Chemical properties of SCG and ESCG variants
Chemical bond composition of SCG and ESCG variants were analysed using FTIR analysis. The FTIR spectra of SCG and ESCG variants are presented in Fig. 3(a). The spectra for all samples exhibited a broad peak from 3000 to 3750 cm−1, which corresponds to O–H and N–H bond stretching from the inter-molecular and intra-molecular hydrogen bonds of organic compounds. A minor peak at 3011 cm−1 is only present for SCG and coincide with H–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) bond stretching that is found in unsaturated fatty acids. The peaks at 2925 and 2855 cm−1 are attributed to asymmetric and symmetric C–H vibrations. Absorption peak at 1746 cm−1 belongs to the vibration of C
bond stretching that is found in unsaturated fatty acids. The peaks at 2925 and 2855 cm−1 are attributed to asymmetric and symmetric C–H vibrations. Absorption peak at 1746 cm−1 belongs to the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond stretching, commonly found in esters and is associated with lipids and fatty acids. Peaks 1650 and 813 cm−1 can be related to the presence of lignin.31 Presence of cellulose and hemicellulose can be identified by peaks 1378 and 1163 cm−1.31
O bond stretching, commonly found in esters and is associated with lipids and fatty acids. Peaks 1650 and 813 cm−1 can be related to the presence of lignin.31 Presence of cellulose and hemicellulose can be identified by peaks 1378 and 1163 cm−1.31
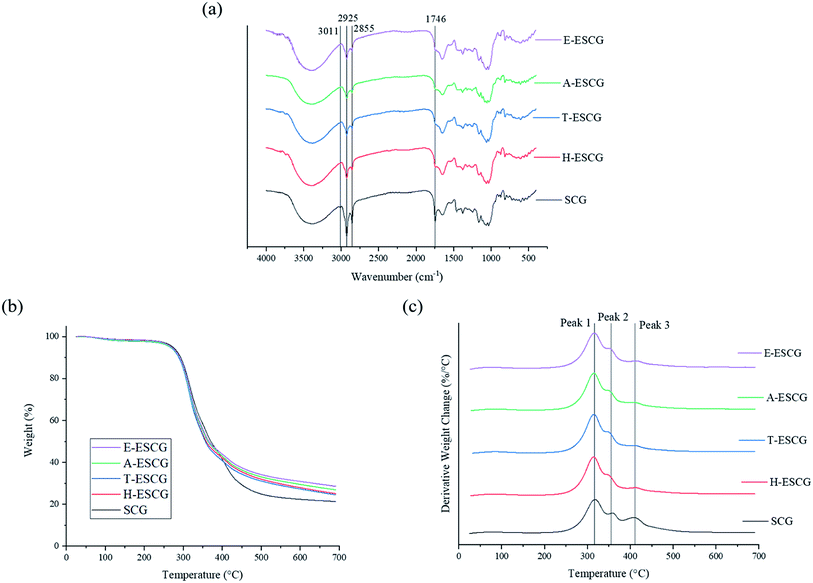 | ||
| Fig. 3 Characteristics of SCG and ESCG variants (a) FTIR spectra, (b) TGA weight percentage, (c) TGA derivative weight change. | ||
To evaluate the success of coffee oil extraction, a comparison between the absorbance spectrums of SCG and ESCG variants were analysed. The peak at 3011 cm−1 is completely removed for all ESCG variants, indicating successful removal of unsaturated fatty acids. Absorbance intensity for peak 2925 and 2855 cm−1 decreased significantly for all ESCG variants relative to SCG, which implies that the amount of alkyl groups in SCG is higher than ESCG variants. This is also demonstrative of successful coffee oil extraction. Comparing the absorbance peak magnitude at 1746 cm−1, ESCG variants displayed considerable lower intensity. The results can be attributed to a decrease in fatty acids, which is representative of effective coffee oil extraction. These observations were in agreement to those reported by Wu et al., where they found that hexane solvent extracted SCG displayed identical reductions in peaks by FTIR.32
Thermal properties of SCG and ESCG variants
Thermal and degradation properties of SCG and ESCG variants were studied by TGA analysis. The results are shown in Fig. 3(b), (c) and Table 2. Residue content at 700 °C for ESCG variants is more than SCGs. This implies that additional decomposition occurred only in SCGs and can potentially be the decomposition of coffee oil. The results suggest that initial oil embedded in SCGs were successfully extracted, leaving behind more residue. Minor weight loss can be observed from initial mass to around 150 °C. This observation could be due to the removal of bound moisture entrapped within the microstructure that cannot be simply removed at water boiling temperature.33,34 Thermal degradation occurs in three main stages as observed in Fig. 3(c). Compounds that undergo thermal degradation are mainly hemicellulose, cellulose and lignin. The first stage happens between 200 to 340 °C with maximum weight loss rate at peak 1 of approximately 316 °C. Degradation at this temperature range can be related to the pyrolysis of hemicellulose and it has been proven that hemicellulose decomposes at relatively lower temperature. The second stage occurs between 300 to 380 °C and at a maximum degradation rate evident at approximately 355 °C, peak 2. This finding can be associated with decomposition of cellulose which was confirmed to have a higher thermal stability. The third stage of degradation can be attributed to lignin decomposition with a maximum decomposition rate at approximately 411 °C, peak 3. This coincides closely with reported findings on thermal decomposition of lignin that takes place over a large range of temperature and maximum weight loss rate at 400 °C. Weight change of SCG at peak 3 is more evident than that of ESCG variants which could indicate decomposition of organic compounds such as fatty acids and proteins.23| Sample | Temperature at 95 wt% (°C) | Residue (wt%) | Peak 1 (°C) | Peak 2 (°C) | Peak 3 (°C) |
|---|---|---|---|---|---|
| SCG | 273 | 22.3 | 318 | 358 | 408 |
| H-ESCG | 269 | 27.9 | 315 | 343 | 412 |
| T-ESCG | 266 | 27.3 | 315 | 350 | 410 |
| A-ESCG | 268 | 29.5 | 315 | 345 | 410 |
| E-ESCG | 270 | 30.9 | 316 | 349 | 414 |
Surface morphology of epoxy, SCG and ESCG composites
To achieve satisfactory mechanical properties for composites, uniform dispersion and effective wetting of fillers in epoxy matrix is required alongside strong interfacial adhesion between the phases. SCGs or ESCG variants are considered a dispersed phase within the epoxy matrix phase. Fracture surface from tensile test samples of epoxy, SCG and ESCG composites were inspected using SEM and presented in Fig. 4. The filler particles can be identified from their irregular shape and are highlighted in red. From Fig. 4(a), fracture surface of epoxy is significantly smoother compared to surfaces of composites. Composites with SCG or ESCG fillers have an irregular fracture surface which is attributed to the disruption of matrix continuity by fillers. SCG particles tend to agglomerate and cluster together as seen in Fig. 4(b). The aggregation of SCG particles is the result of hydrogen bonding between SCG particles and coffee oil, thus reducing the dispersity of SCG particles in epoxy matrix. Inhabitation effect of coffee oil in SCGs may have led to the reduced adhesion between SCGs and epoxy matrix.32 These phenomena could potentially affect the mechanical properties of SCG composites negatively as it hinders effective stress distribution within the composite. After coffee oil extraction, the dispersion of ESCG particles were evidently more homogenous as depicted in Fig. 4(c)–(f). The improved distribution allows better interfacial interaction between ESCG particles and epoxy matrix, which can improve wetting and mechanical properties of ESCG composites. The improved properties of ESCG composites as compared to SCG composite is possibly due to the extraction process. The extraction of oil from SCGs modifies the integral structural components, primarily lignin, cellulose and hemicellulose of SCGs. Prior to the extraction, these polymers exist as tightly bounded structural component with very few exposed –OH groups to interact with. The removal of coffee oil and the extraction environment that SCGs were subjected to, unravels and loosens the polymers, thus exposing more –OH groups to the surround environment. These exposed –OH groups potentially react with epoxy and hardener as shown in Fig. 4(g). The interaction between the epoxy and ESCGs would result in a stronger composite.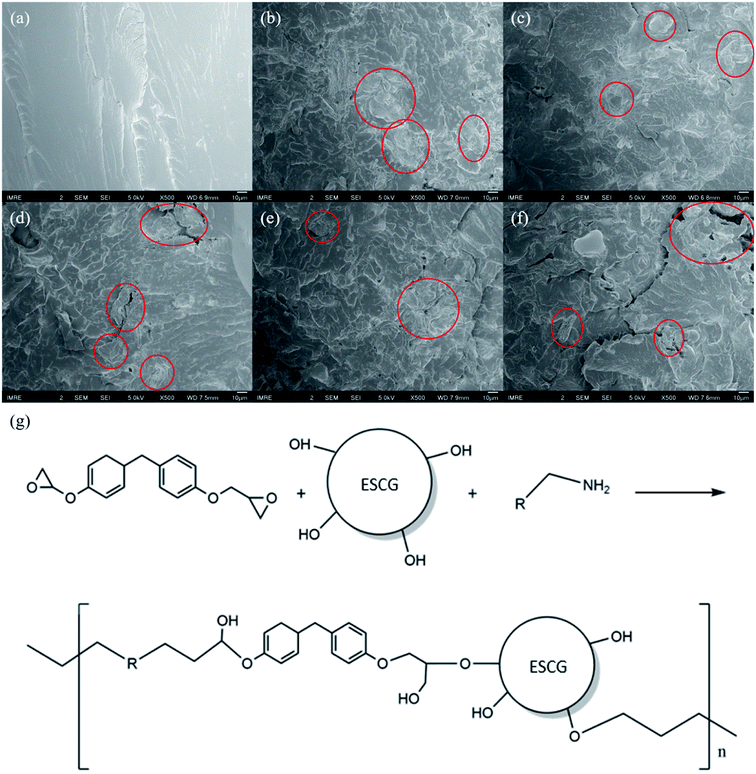 | ||
| Fig. 4 SEM images of composites, (a) epoxy, (b) SCG–epoxy, (c) H-ESCG–epoxy, (d) T-ESCG–epoxy, (e) A-ESCG–epoxy, (f) E-ESCG–epoxy, and (g) the chemical reaction between ESCG with epoxy and hardener. | ||
Mechanical properties of epoxy, SCG and ESCG composites
Mechanical properties of epoxy, SCG and ESCG variant composites are evaluated using tensile and flexural testing. The results were tabulated in Table 3. All samples exhibit a brittle fracture. Density of epoxy, SCG and ESCG variant composites ranges from 1074 to 1140 kg m−3.| Sample | Density (kg m−3) | Tensile properties | Flexural properties | ||||
|---|---|---|---|---|---|---|---|
| Young's modulus (GPa) | Stress at maximum (MPa) | Strain at break (%) | Young's modulus (GPa) | Stress at maximum (MPa) | Strain at break (%) | ||
| Epoxy | 1090 | 2.79 ± 0.53 | 36.4 ± 6.0 | 1.5 ± 0.5 | 2.48 ± 0.15 | 83.9 ± 9.4 | 4.1 ± 0.6 |
| SCG–epoxy | 1135 ± 7 | 2.94 ± 0.23 | 20.9 ± 3.4 | 0.8 ± 0.2 | 2.09 ± 0.24 | 33.0 ± 3.9 | 1.9 ± 0.3 |
| H-ESCG–epoxy | 1074 ± 4 | 3.13 ± 0.16 | 21.5 ± 5.6 | 0.7 ± 0.3 | 2.34 ± 0.14 | 38.6 ± 2.2 | 1.8 ± 0.2 |
| T-ESCG–epoxy | 1124 ± 10 | 3.04 ± 0.29 | 21.3 ± 3.9 | 0.8 ± 0.2 | 2.91 ± 0.17 | 42.8 ± 5.8 | 1.6 ± 0.2 |
| A-ESCG–epoxy | 1126 ± 11 | 2.94 ± 0.12 | 23.4 ± 2.8 | 0.8 ± 0.2 | 3.02 ± 0.06 | 42.9 ± 4.2 | 1.6 ± 0.1 |
| E-ESCG–epoxy | 1140 ± 9 | 2.80 ± 0.20 | 21.6 ± 4.5 | 0.8 ± 0.2 | 2.47 ± 0.20 | 37.0 ± 3.0 | 1.6 ± 0.2 |
Comparing the tensile properties of SCG and ESCG variant composites with epoxy, tensile modulus for the composites were higher than epoxy due to increase in surface interaction between filler particles and epoxy matrix. The greatest improvement from 2.79 to 3.13 GPa belongs to H-ESCG–epoxy. Addition of SCG and ESCG fillers greatly decrease the tensile strength of composites relative to epoxy, from 36.4 to approximately 21.7 GPa. The introduction of SCG and ESCG particles act as interfacial defects in the composite. Also, due to the difference in nature of filler particles and epoxy matrix, poor interfacial adhesion between hydrophobic epoxy matrix and hydrophilic SCG or ESCG particles can lead to reduced tensile strength. Tensile strains at break are approximately halved, 1.5 to 0.8%, for all composites compared to epoxy. This brittle behaviour is caused by the incorporation of fillers and hindered compatibility between filler particles and epoxy matrix.
ESCG-filled composites exhibit slight improvement in tensile strength relative to SCG-filled composites. The highest increase is attributed to A-ESCG–epoxy, from 20.9 to 23.4 MPa. This can be associated with successful oil removal and refined particles during the extraction process. The absence of oil along with reduced particle size could allow better homogeneity in the epoxy matrix, improving surface interaction between filler and matrix. In addition, previous tightly bonded structural components of SCGs could get loosened up, exposing more –OH bonding groups to surrounding environment. Upon incorporation of ESCGs into epoxy matrix, these extra –OH groups interact with the epoxy resin and hardener, resulting in greater integration and slightly enhanced tensile strength for ESCG composites.
Flexural modulus performance in comparison to epoxy for SCG–epoxy and H-ESCG–epoxy are lower at 2.09 and 2.34 GPa respectively, whereas T-ESCG–epoxy and A-ESCG–epoxy exhibit higher values at 2.91 and 3.02 GPa respectively. E-ESCG–epoxy has comparable flexural modulus with epoxy, approximately 2.48 GPa. Flexural strength is notably lower for all composites relative to epoxy. It decreases from 83.9 MPa to between 33.0 and 42.9 MPa. This reduction may be due to the incompatibility of hydrophilic filler and hydrophobic matrix, resulting in poor adhesion and stress transfer. Another important factor to consider is the aspect ratio of filler particles which relates to the reinforcing effect in composites. Fillers have a reinforcing effect when the aspect ratio of fillers exceed a numeric value of 6. This is attributed to preferential particle alignment which strengthens the mechanical properties of the material.21 As SCG and ESCG particles are highly irregular in size and shape, an aspect ratio of less than 2 is assigned.21 As a result, there is unlikely any particle alignment during formation of composites, hence lowering flexural strength of SCG and ESCG composites. Flexural strains at break for SCG and ESCG composites declined more than half as compared to epoxy, which is due to inadequate stress transfer arising from poor interfacial interactions between fillers and matrix. This creates areas of dispersed voids and consequently, promote crack propagation.
It is evident from Table 3 that flexural modulus for all ESCG composites increased after oil extraction in contrast to SCG composite. The most improvement can be seen in A-ESCG–epoxy, with an increase from 2.09 to 3.02 GPa. This observation can be explained by ESCG particles being more effective than SCG particles at restricting epoxy chain motion. Extraction of coffee oil led to improved homogenous dispersion of ESCG particles and enhanced interfacial adhesion between ESCG fillers and epoxy matrix, thus better resistance to flexural load deformation and stiffness. Flexural strength for ESCG composites is better than SCG composites. The greatest increment from 33.0 to 42.9 MPa belongs to A-ESCG–epoxy. The observation can be associated with improved interaction between ESCG fillers and epoxy matrix due to better particle dispersion which promotes stress transfer from epoxy matrix to ESCG fillers. Similarly, the oil removal process may improve ESCG integration by exposing more –OH groups that can provide more interaction sites with epoxy resin and hardener, hence improving the flexural strength of ESCG variant composites.
A comparison between 30 wt% and 50 wt% SCGs loading displayed a reduction in tensile strength performance from 20.9 to 13.9 MPa respectively ascribed to poor dispersion of incompatible SCGs in epoxy matrix. Moreover, flexural strength decreased from 33.0 to 28.2 MPa when SCGs loading increased and similarly, is due to the conflicting nature of SCGs and epoxy matrix. As depicted in Fig. S2,† SEM images from the fracture surface of the 50 wt% SCG–epoxy composite revealed multiple defects and highly aggregated SCGs arising from the increment in loading content. These incompatible particles and voids were introduced as interfacial defects that result in poorer mechanical properties. This observation is consistent in other studies. For example, Wu incorporated increasing amounts of SCGs into polylactide matrix and concluded that increasing SCGs has a negative impact on tensile strength.35
Many existing studies included the use of maleic anhydride (MA) as a compatibiliser agent to improve the bonding between fillers and matrix. Research conducted by Tarazona et al. reported on the addition of 35 wt% hydrogen peroxide treated SCG particles into epoxy resin coupled with MA led to an increase in tensile modulus from 4.4 to 9.5 MPa.36 A decrease in tensile strength, from 26.0 to 14.7 MPa, was also observed by Sohn et al. when 30 wt% untreated SCG fillers were added into BLOCK-polypropylene matrix with MA compatibiliser.37 The tensile testing results obtained were similar in comparison to our study. Relative to untreated SCG fillers, our investigation using solvent extraction for the removal of oil improved interfacial bonding between ESCGs and epoxy matrix without the use of coupling agents. This finding was comparable to Wu et al. whereby they established a positive relation that oil extraction of SCGs using hexane provided improvement to tensile and flexural properties when incorporated into PP matrix.32
Other types of natural fillers have been incorporated into thermoset matrices and outcome are consistent with our current study. Salasinska et al. investigated on the incorporation of finely grounded walnut shells of size 32 to 125 μm into the epoxy system. They reported that the walnut shell fillers have an aspect ratio close to 1, which is akin to the SCGs and ESCGs in our investigation. Research on the addition of 75 to 150 μm oil palm shell flour into thermosetting matrices was conducted by Nabinejad et al. The particle size of SCGs and ESCGs were between 200 to 500 μm, which were larger than that in the two studies. However, both studies achieved finer filler particles through milling process which might be more energy intensive compared to grinding by commercial blender for 5 min. Likewise, both groups presented a decrease in tensile strength and increase in tensile modulus which were observed in our composites.38,39
Thermal properties of epoxy, SCG and ESCG composites
Thermal degradation properties of epoxy, SCG and ESCG variant composites were determined using TGA analysis. Thermal stability of composites experienced a decrease upon the addition of SCG and ESCG fillers as indicated by the earlier onset of decomposition in Fig. 5(a). Relative to epoxy, there is no significant difference in thermal stability between the different composites. Remaining residue content was also significantly greater after the incorporation of fillers, which can be explained by the presence of SCGs or ESCGs having greater char content.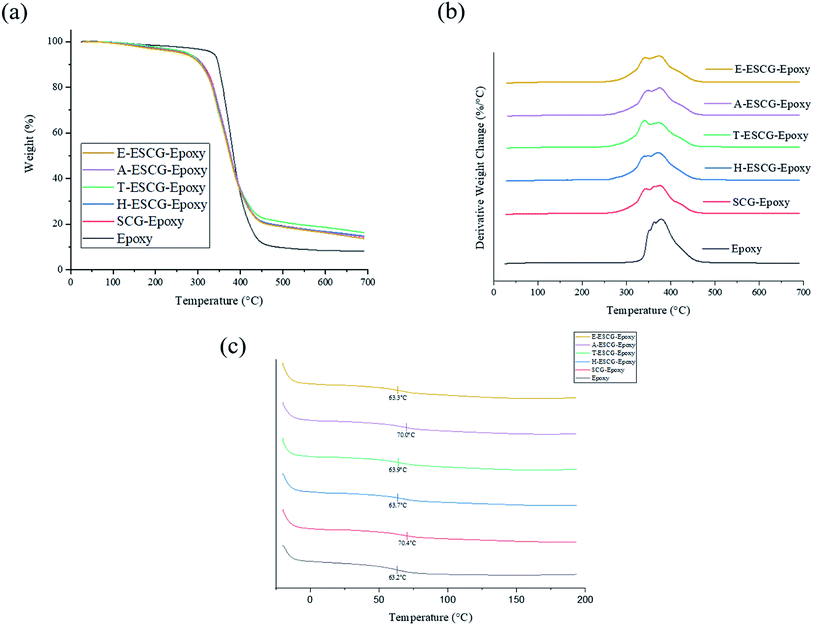 | ||
| Fig. 5 TGA curves of epoxy, SCG and ESCG composites, (a) weight percentage, (b) derivative weight change, (c) DSC curves of epoxy, SCG and ESCG composites. | ||
Glass transition temperature (Tg) of epoxy, SCG and ESCG variant composites were established using DSC analysis and marked out in Fig. 5(c). In comparison to epoxy, the addition of fillers did not drastically change the Tg except a slight increase for SCG–epoxy and A-ESCG–epoxy samples. This finding implies that glass transition characteristics and state of epoxy crosslinking remains fairly similar to epoxy even with the addition of fillers. It is also likely that SCGs and ESCGs does not have Tg in the temperature range of epoxy Tg. The increase in Tg to 70.0 °C for A-ESCG–epoxy suggests that A-ESCG particles are able to better interact with the crosslinking of epoxy matrix and restrict polymer chain rearrangement. This can be a probable explanation for the improved mechanical properties of A-ESCG–epoxy composite.
Conclusion
In this study, coffee waste has been recycled by extracting coffee oil and producing new composite material. There are many components of coffee oil which potential remains untapped. This study shows that more can be done to utilise SCGs, hence reducing waste in the environment. Here we demonstrated that SCGs can be used in a different manner via coffee oil extraction and as a filler material for composites. The addition of SCGs and ESCGs can strengthen mechanical properties of composites, specifically epoxy resin. Considering its similar appearance to dark wood, some possible applications of these newly form composites includes household furniture and decorative ornaments. This paper suggests a sustainable material application to recycle waste coffee grounds and provide insights towards material sustainability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors also gratefully acknowledge the financial support from CDA (Project No. CDA 202D800033), Agency for Science, Technology and Research (A*STAR).References
- I. Efthymiopoulos, P. Hellier, N. Ladommatos, A. Russo-Profili, A. Eveleigh, A. Aliev, A. Kay and B. Mills-Lamptey, Ind. Crops Prod., 2018, 119, 49–56 CrossRef CAS.
- P. S. Murthy and M. Madhava Naidu, Resour., Conserv. Recycl., 2012, 66, 45–58 CrossRef.
- S. I. Mussatto, E. M. S. Machado, S. Martins and J. A. Teixeira, Food Bioprocess Technol., 2011, 4, 661–672 CrossRef CAS.
- A. S. Fernandes, F. V. C. Mello, S. Thode Filho, R. M. Carpes, J. G. Honorio, M. R. C. Marques, I. Felzenszwalb and E. R. A. Ferraz, Ecotoxicol. Environ. Saf., 2017, 141, 30–36 CrossRef CAS.
- S. S. Chen, I. K. M. Yu, D.-W. Cho, H. Song, D. C. W. Tsang, J.-P. Tessonnier, Y. S. Ok and C. S. Poon, ACS Sustainable Chem. Eng., 2018, 6, 16113–16120 CrossRef CAS.
- D.-W. Cho, D. C. W. Tsang, S. Kim, E. E. Kwon, G. Kwon and H. Song, Bioresour. Technol., 2018, 270, 346–351 CrossRef CAS.
- D.-W. Cho, J. Park, G. Kwon, J. Lee, G.-J. Yim, W. Jung and Y.-W. Cheong, J. Hazard. Mater., 2020, 386, 121989 CrossRef CAS.
- T. M. Mata, A. A. Martins and N. S. Caetano, Bioresour. Technol., 2018, 247, 1077–1084 CrossRef CAS.
- J. Banu, S. Kavitha, Y. Kannah R, D. Kumar, M. Preethi, A. Atabani and G. Kumar, Bioresour. Technol., 2020, 302, 122821 CrossRef.
- J. McNutt and Q. He, J. Ind. Eng. Chem., 2019, 71, 78–88 CrossRef CAS.
- R. Campos-Vega, G. Loarca-Piña, H. A. Vergara-Castañeda and B. D. Oomah, Trends Food Sci. Technol., 2015, 45, 24–36 CrossRef CAS.
- L. F. Ballesteros, J. A. Teixeira and S. I. Mussatto, Food Bioprocess Technol., 2014, 7, 3493–3503 CrossRef CAS.
- D. Pujol, C. Liu, J. Gominho, M. À. Olivella, N. Fiol, I. Villaescusa and H. Pereira, Ind. Crops Prod., 2013, 50, 423–429 CrossRef CAS.
- S. K. Karmee, Waste Manag., 2018, 72, 240–254 CrossRef CAS.
- D. N. Raba, D. R. Chambre, D. M. Copolovici, C. Moldovan and L. O. Copolovici, PLoS One, 2018, 13, e0200314 CrossRef.
- R. W. Jenkins, N. E. Stageman, C. M. Fortune and C. J. Chuck, Energy Fuels, 2014, 28, 1166–1174 CrossRef CAS.
- K. Somnuk, P. Eawlex and G. Prateepchaikul, Agric. Nat. Resour., 2017, 51, 181–189 Search PubMed.
- S. Hibbert, K. Welham and S. H. Zein, SN Appl. Sci., 2019, 1, 1467 CrossRef.
- N. Caetano, V. Silva and T. Mata, Chem. Eng. Trans., 2012, 267–272 Search PubMed.
- I. Efthymiopoulos, P. Hellier, N. Ladommatos, A. Kay and B. Mills-Lamptey, Waste Biomass Valorization, 2019, 10, 253–264 CrossRef CAS.
- D. García-García, A. Carbonell, M. D. Samper, D. García-Sanoguera and R. Balart, Composites, Part B, 2015, 78, 256–265 CrossRef.
- H. K. Lee, Y. G. Park, T. Jeong and Y. S. Song, J. Appl. Polym. Sci., 2015, 132, 42043 Search PubMed.
- H. Moustafa, C. Guizani and A. Dufresne, J. Appl. Polym. Sci., 2017, 134, 44498 CrossRef.
- R. M. Couto, J. Fernandes, M. D. R. G. da Silva and P. C. Simões, J. Supercrit. Fluids, 2009, 51, 159–166 CrossRef CAS.
- V. Aparna, K. V. Dileep, P. K. Mandal, P. Karthe, C. Sadasivan and M. Haridas, Chem. Biol. Drug Des., 2012, 80, 434–439 CrossRef CAS.
- M. S. Farvid, M. Ding, A. Pan, Q. Sun, S. E. Chiuve, L. M. Steffen, W. C. Willett and F. B. Hu, Circulation, 2014, 130, 1568–1578 CrossRef CAS.
- D. Perrone and A. Farah, in Caffeine: Chemistry, The Royal Society of Chemistry, 2012, pp. 103–129, 10.1039/9781849734752-00103.
- G. M. Williams, M. J. Iatropoulos and J. Whysner, Food Chem. Toxicol., 1999, 37, 1027–1038 CrossRef CAS.
- W. A. Yehye, N. A. Rahman, A. Ariffin, S. B. Abd Hamid, A. A. Alhadi, F. A. Kadir and M. Yaeghoobi, Eur. J. Med. Chem., 2015, 101, 295–312 CrossRef CAS.
- J. Wang, Y. Zhang, Z. Fang, L. Sun, Y. Wang, Y. Liu, D. Xu, F. Nie and R. Gooneratne, Biol. Trace Elem. Res., 2019, 190, 95–100 CrossRef CAS.
- R. Musule, E. Alarcón-Gutiérrez, E. P. Houbron, G. M. Bárcenas-Pazos, M. del Rosario Pineda-López, Z. Domínguez and L. R. Sánchez-Velásquez, J. Wood Sci., 2016, 62, 537–547 CrossRef CAS.
- H. Wu, W. Hu, Y. Zhang, L. Huang, J. Zhang, S. Tan, X. Cai and X. Liao, J. Mater. Sci., 2016, 51, 10205–10214 CrossRef CAS.
- N. Zarrinbakhsh, T. Wang, A. Rodriguez-Uribe, M. Misra and A. K. Mohanty, BioResources, 2016, 11(3), 7637–7653 CrossRef CAS.
- Z. Al-Hamamre, S. Foerster, F. Hartmann, M. Kröger and K. Martin, Fuel, 2012, 96, 70–76 CrossRef CAS.
- C.-S. Wu, Polym. Degrad. Stab., 2015, 121, 51–59 CrossRef CAS.
- E. R. T. Tarazona, L. S. Oliveira, J. C. Rubio and A. S. Franca, 8th International Conference on Mechanical and Aerospace Engineering (IMCMAE), 2017, pp. 21–24 Search PubMed.
- J. Sohn, Y. Ryu, C.-S. Yun, K. Zhu and S. Cha, Sustainability, 2019, 11, 1720 CrossRef CAS.
- K. Salasinska, M. Barczewski, R. Górny and A. Kloziński, Polym. Bull., 2018, 75, 2511–2528 CrossRef CAS.
- O. Nabinejad, D. Sujan, M. E. Rahman and I. J. Davies, J. Cleaner Prod., 2017, 164, 1145–1156 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09379c |
| This journal is © The Royal Society of Chemistry 2021 |