Open Access Article
Open Access ArticleDistribution and behaviour of 233Pa in 2LiF–BeF2 molten salt
Zhongqi Zhao†
 abc,
Jifeng Hu†abc,
Zhiqiang Chengabc,
Junxia Gengabc,
Wenxin Liab,
Qiang Douab,
Jingen Chenab,
Qingnuan Li*ab and
Xiangzhou Cai*ab
abc,
Jifeng Hu†abc,
Zhiqiang Chengabc,
Junxia Gengabc,
Wenxin Liab,
Qiang Douab,
Jingen Chenab,
Qingnuan Li*ab and
Xiangzhou Cai*ab
aShanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China
bCentre of Excellence TMSR Energy System, Chinese Academy of Sciences, Shanghai 201800, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 15th February 2021
Abstract
Distribution and behavior of 233Pa, essential in the thorium–uranium nuclear fuel cycle, were studied in 2LiF–BeF2 (66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 mole%, FLiBe) molten salt by γ-ray spectrometry. The experiments showed that 233Pa deposited slightly on the surface of graphite crucible. The addition of Hastelloy and metallic lithium decreased the 233Pa specific activity in the salt by 1 to 2 orders of magnitude rapidly. Analysis indicated that reductive deposition of 233Pa was responsible for the rapid decrease of 233Pa specific activity in the salt. Additional experiments strongly supported the mechanism of reductive deposition of 233Pa induced by Hastelloy and metallic lithium. In view of the large deposition of 233Pa on Hastelloy, the possible influence of fissile nuclide 233U produced from 233Pa decay on the operation of thorium-based molten salt reactor was discussed.
34 mole%, FLiBe) molten salt by γ-ray spectrometry. The experiments showed that 233Pa deposited slightly on the surface of graphite crucible. The addition of Hastelloy and metallic lithium decreased the 233Pa specific activity in the salt by 1 to 2 orders of magnitude rapidly. Analysis indicated that reductive deposition of 233Pa was responsible for the rapid decrease of 233Pa specific activity in the salt. Additional experiments strongly supported the mechanism of reductive deposition of 233Pa induced by Hastelloy and metallic lithium. In view of the large deposition of 233Pa on Hastelloy, the possible influence of fissile nuclide 233U produced from 233Pa decay on the operation of thorium-based molten salt reactor was discussed.
Introduction
In the context of increasing global warming, nuclear energy is gaining more and more attention as a low-carbon clean energy. Adequate supply of nuclear resources is an important premise of sustainable development of nuclear energy. Thorium, which is made up of fertile nuclide 232Th only, is 3 to 4 times more abundant than uranium and is widely distributed in nature.1 Similarly to 238U, 232Th can absorb a neutron and then convert into fissile nuclide 233U after two sequential β− decays:
 | (1) |
More importantly, the thorium–uranium fuel cycle has a number of advantages, such as higher conversion to fissile nuclide in thermal reactors, much less minor actinides formed than in the uranium-plutonium fuel cycle, and intrinsic proliferation resistance. Therefore, the thorium–uranium fuel cycle has potential application in nuclear power.1
However, the intermediate nuclide 233Pa in the 232Th–233U conversion chain has a relative longer half-life of 27 days than the 2.35 days half-life of 239Np in the uranium-plutonium fuel cycle, leading to its high equilibrium concentration in reactor. The combination of the high 233Pa concentration with its large thermal neutron capture cross section (about 43 barns) causes the undesirable double loss of neutrons and fissile nuclide, resulting in the decrease of reactor efficiency and 233U breeding factor. An effective way to remedy these defects is removing 233Pa from the reactor in time, and then return it into the reactor after decay to 233U.1 Obviously, this way is easier to achieve in liquid fuel reactor than in solid fuel reactor.
Molten salt reactor (MSR) is a unique liquid fuel reactor among the Advanced Generation IV Reactors.2 The main feature of MSR is the use of the molten fluoride salt as the fuel carrier and the reactor coolant simultaneously. MSR has the common advantages of Generation IV Reactors, including the higher energy conversion rate derived from high outlet temperature, and high safety and reliability. Moreover, MSR also gains a number of specific benefits that the other solid fuel reactors may not provide, e.g. the feasibility of on-line processing for the irradiated fuels. By contacting the fuel salt with the helium gas flow in the pump bowl,3 the noble gas fission products (the high neutron toxic 135Xe in particular) can be continuously removed without shutting down the reactor. By using specialized on-line processing facilities, the key nuclide 233Pa can be removed from the MSR in batch mode. All of these on-line processes considerably improve the neutron economy, fuel utilization rate and 233U breeding factor. Consequently, MSR is extremely suitable for nuclear application of thorium resources, and thorium-based molten salt reactor has become a hot spot in the development of MSR.4
The world's first MSR, aircraft reactor experiment (ARE) with 2.5 MW power, was built by Oak Ridge National Laboratory (ORNL) at USA in 1954,5 and the construction of a 10 MWt molten-salt reactor experiment (MSRE) was started in 1961. After reached criticality in 1966, the MSRE has been operated with 235U and 233U as fuel successively and separately for 4 years.6 Meantime, ORNL commenced the conceptual design of the molten salt breeder reactor (MSBR) on the basis of MSRE.7 Although ORNL realized the importance of the separation of Pa, unfortunately, the total projects concerning MSRE and MSBR were terminated in 1973. In the entire operation of MSRE, the fertile nuclide 232Th had never been added into the fuel. Therefore, no information about the behavior of Pa in MSR has ever been reported.
Shanghai Institute of Applied Physic (SINAP) has been engaged in the project on the development of thorium based molten salt reactor (TMSR), supported by Chinese Academy of Sciences for years.8 Radiochemistry laboratory in SINAP has made a great effort to devote the experimental investigation of pyrochemistry processes for irradiated fuel from TMSR. Recently, the research on the radiochemistry of fission products and nuclear fuel in molten salts has been launched at a laboratory level. In this article the preliminary studies on distribution and behaviour of Pa in FLiBe molten salt were reported.
Experimental
The materials, equipment and methods employed in this work have been described in our previous work.9 Briefly, UF4 and ThF4 powders loaded separately in sealed acrylic (polymethyl methacrylate) boxes were irradiated for about 3 days with neutrons source driven by a 15 MeV electron linear accelerator.10 After cooling for about 20 hours, about 0.2 g UF4/ThF4 powder was weighted precisely and mounted on a counting plate for γ-spectrum measurement separately to obtain the original inventory of radionuclides. The irradiated ThF4, UF4 and LiF–BeF2 eutectic salt (66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 mole%) with mass ratio of 2
34 mole%) with mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 were mixed well in a graphite crucible, followed by heating in a furnace. The temperature was enhanced to 650 °C and maintained for at least 24 hours until the mixture was completely melted.
26 were mixed well in a graphite crucible, followed by heating in a furnace. The temperature was enhanced to 650 °C and maintained for at least 24 hours until the mixture was completely melted.
To obtain the specific activity of given nuclides (Bq g−1 salt), a small portion of the salt sample was taken by a quartz tube with 5 mm inner diameter, followed by weighed and measured for γ-ray spectra. The measurement of specific activity was performed each day in order to observe the variation of the specific activity within the entire experimental period. To examine the distribution and behavior of Pa in the presence of Hastelloy (primary structural materials of MSR), a piece of Hastelloy C276 with surface area of 830 mm2 was immersed into the salt. After at least 8 hours, the specific activity of 233Pa was determined in the same manner. Similar procedure was repeated to examine the variation of the specific activity of 233Pa resulted from the interaction of ThF4-containing molten salt with 20 mg metallic lithium. The salt was cooled to room temperature after the experiments terminated, and the solidified molten salts at different depth were measured with γ-ray spectrometer to obtain the distribution of radionuclides. All of the transfer of samples and chemical treatment were carried out in a homemade argon covered glove box.
The γ-ray measurements of irradiated UF4/ThF4 powder and molten salt samples were carried out by a calibrated HPGe detector. The counting time varied from 0.5 hours to 24 hours, depending on the activity of interested nuclides. The γ-ray spectra were analyzed by GammaVision program provided by ORTEC Co. Assignments of radioactive nuclides were made mainly on the basis of their characteristic energies of γ-rays. If necessary, half-life and concordance with other γ-rays emitted by the presumed nuclide were used to help the assignments of nuclides. The basic nuclear data used for nuclide assignment and activity calculation were provided by ENDF/B-VII.1 (USA, 2011), and listed in Table 1. The uncertainties in activities experimentally determined in this work were standard deviations, including mainly the statistic errors in the γ-ray counts, a 3–4% error in detector efficiency, and a 5% error in sample geometry. The typical uncertainty for 233Pa was around 8–12%.
| Nuclide | Energy/keV | Intensity/% | Energy/keV | Intensity/% | Energy/keV | Intensity/% |
|---|---|---|---|---|---|---|
| 233Pa | 311.9 | 38.50 | 300.1 | 6.63 | 340.5 | 4.45 |
| 237U | 208.1 | 21.20 | 59.5 | 34.50 | 164.6 | 1.86 |
| 239Np | 277.6 | 14.44 | 228.2 | 11.14 | 209.8 | 3.42 |
| 228Ac | 911.2 | 26.20 | 969.0 | 15.90 | 338.3 | 11.40 |
Results and discussion
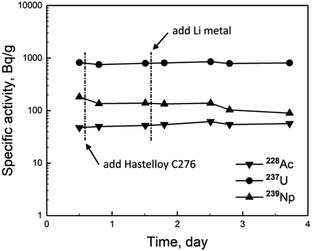
ORNL found that the redox potential of the molten salt increased gradually with the operation of MSRE,11 and high redox potential would promote the corrosion of the Hastelloy reactor vessel. Consequently, ORNL managed to suppress the increase of redox potential by frequent addition of metallic beryllium reductant into the molten salt.11 Thus, the study on Pa behavior in FLiBe molten salt was to examine the Pa distribution and its variation after interaction with Hastelloy and metal reductant (Hastelloy C276 and Li in this work separately). The examination was achieved mainly via the measurement of the specific activity of 233Pa in the salt by γ-ray spectrometer.In our previous work,9 it was found that the specific activities of the fission products 131I, 140Ba, 140La, 141Ce and 143Ce remained well a constant during a four-day experimental period. As indicated by ORNL, these nuclides belonged to the salt-seeking fission products, which dissolved quantitatively and existed steadily in molten salt.11,12 Thus the experimental result above was in agreement with that expected. In the present work, the specific activities of several selected actinides (228Ac, 237U and 239Np) during the experimental period were determined and showed in Fig. 1. Here 239Np was produced via 238U(n,γ) 239U followed by β decay, and 237U was formed by 238U(n,2n) 237U, while 228Ac is a daughter nuclide of the 232Th decay chain. Similarly to the salt-seeking fission products, the specific activities of these actinides also kept well a constant (after correction for decay). Furthermore, there were no significant changes in their activities even though after the addition of Hastelloy C276 and metal lithium, with the exception of 239Np, for which there was a certain decrease observed after addition of Hastelloy C276. These results implied high stability of these actinides in FLiBe molten salt and their behavior was basically independent on redox potential of the salt.
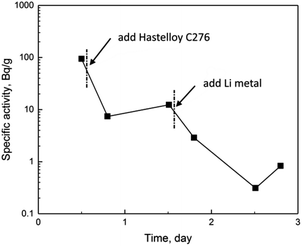
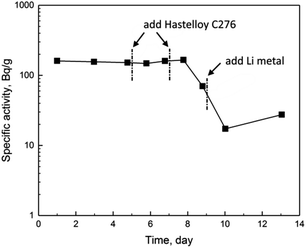
The specific activities of 233Pa determined in four-day experiment were shown in Fig. 2. After the mixture of irradiated UF4, ThF4 and FLiBe was melt completely, the specific activity of 233Pa in the salt was determined to be 94.1 Bq g−1. Comparing with the specific activity of 100.3 Bq g−1 salt determined as the inventory of the irradiated ThF4 sample, the about 6% difference seemed to indicate a slight deposition of Pa on graphite crucible. Differently from Pa, the deposition of Nb could reach 16% in a similar condition, as reported in our previous work.9
It was more or less surprising that the specific activity of 233Pa underwent greater variation compared with those of actinides mentioned above. As shown in Fig. 2, the specific activity of 233Pa decreased immediately by one order of magnitude after the addition of Hastelloy C276. After that, the specific activity of 233Pa decreased rapidly again by more than one order of magnitude when metallic lithium reductant was added. Dramatic variation of 233Pa activity was undesirable, and the behavior of Pa following the interaction with Hastelloy and lithium metal needed further examination.
To confirm the great variation of the specific activity of 233Pa mentioned above, similar test was carried out in LiF–NaF–KF (46.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.5
11.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 mole%, FLiNaK) molten salt. Variation of the specific activities of 233Pa determined in eight-day experiment was indicated in Fig. 3. As can be seen, Pa exhibited slightly better stability in the FLiNak salt than that in FLiBe salt. The addition of the first piece of Hastelloy C276 did not caused immediately decrease of 233Pa activity. Only when the second piece of Hastelloy C276 was immersed into the FLiNaK salt, the specific activity of 233Pa began to decrease obviously. Following the addition of metallic lithium, the specific activity of 233Pa also rapidly decreased by about one order of magnitude. Despite the degree of decrease in 233Pa specific activity was different in the two different molten salt systems, it was certain that both Hastelloy C276 and metallic lithium were able to reduce the specific activity of 233Pa in molten salt significantly.
42 mole%, FLiNaK) molten salt. Variation of the specific activities of 233Pa determined in eight-day experiment was indicated in Fig. 3. As can be seen, Pa exhibited slightly better stability in the FLiNak salt than that in FLiBe salt. The addition of the first piece of Hastelloy C276 did not caused immediately decrease of 233Pa activity. Only when the second piece of Hastelloy C276 was immersed into the FLiNaK salt, the specific activity of 233Pa began to decrease obviously. Following the addition of metallic lithium, the specific activity of 233Pa also rapidly decreased by about one order of magnitude. Despite the degree of decrease in 233Pa specific activity was different in the two different molten salt systems, it was certain that both Hastelloy C276 and metallic lithium were able to reduce the specific activity of 233Pa in molten salt significantly.
 | ||
| Fig. 3 Variation of specific activity for 233Pa in FLiNaK molten salt after addition of Hastelloy and metallic lithium. | ||
Although no study on the distribution and behavior of Pa in MSRE was reported by ORNL, the scientists at ORNL made great efforts to develop the separation techniques of Pa for processing the irradiated fuels from MSR,7,13–15 as a part of the conceptual design of MSBR. After evaluation for various separation techniques, a preferred protactinium isolation method, the reductive extraction method was recommended for further development.7 This processing method involved the selective distribution of Pa between salt and metallic bismuth containing reducing reagent, such as thorium and lithium metal.7,14,15 The isolation principle of the reductive extraction is relatively straightforward, and described as following:
| PaF8(salt)4− + 4Li(Bi) = 4Li(salt)+ + Pa(Bi) + 8F(salt)− | (2) |
The separation factor of Pa between the liquid bismuth containing lithium and the FLiBe salt could reach as high as 1200.7.
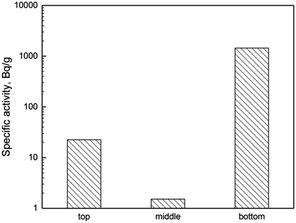
Similar process based on the reduction of Pa would also occur in this experiment. Following addition of the metallic lithium reductant, Pa was reduced into insoluble metal state. Because there was no molten metal phase existing, the insoluble metal Pa granules would settle down and accumulate in the bottom of the salt in graphite crucible. At the end of experiment, the specific activities of 233Pa in different depth of solidified FLiBe molten salt were determined and showed in Fig. 4. As indicated, the majority of Pa was accumulated in the bottom of salt indeed, which experimentally supported the reductive deposition mechanism induced by metallic lithium.
Besides, the minority of Pa was observed at the top of molten salt rather than in the middle part. To explain this phenomenon, the distribution pattern of the noble-metal fission products was referred. As reported by ORNL, noble-metal fission products, including Nb, Mo, Ru, Sb and Te, were easily reduced into the form of black metal granules and distributed widely inside of MSRE. Because of non-wetting by the molten salt, these black granules were removed from the salt into gas–solution interface or the bottom of salt.11,12,14,16 Thus the slight accumulation of Pa in upper salt and the intense enrichment of Pa in lower salt, shown in Fig. 4, were in agreement with the distribution of noble-metal fission products to some extent.
Since metallic lithium is able to reduce the activity of 233Pa in salt via reductive deposition, it is reasonable to conjecture that the similar reductive deposition induced by Hastelloy C276 might be responsible for the decrease of 233Pa activity in salt. The Hastelloy C276 consisted of metallic Fe, Ni, Cr and Mo elements, among which metal Cr is most active in chemical redox properties. According to ORNL, UF4 in reactor could be reduced to UF3 by Cr metal:11,12,14,15
| Cr(metal) + 2UF4(salt) = CrF2(salt) + 2UF3(salt) | (3) |
In addition, the noble-metal fluorides appeared to be less stable in fuel salt, and might be reduced to metal state by the Cr in Hastelloy,11,14,15 via a direct reaction (where M referred to the noble-metal element):
| nCr(metal) + 2MFn(salt) = nCrF2(salt) + 2M(metal) | (4) |
| nUF3(salt) + MFn(salt) = nUF4(salt) + M(metal) | (5) |
The reduced noble metals would be removed from salt and deposited on the surfaces of various structural materials.11,14,15 Therefore, similar to noble-metal fission products, 233Pa might be reduced and deposited on the surface of Hastelloy C276.
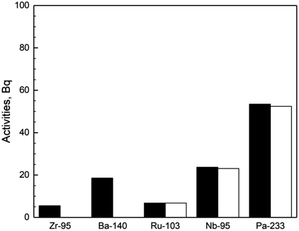
To confirm the assumption above, the immersed Hastelloy C276 was taken out from the salt after the current experiments terminated, and subjected to γ-ray spectrum analysis. The activities of some selected fission products, 95Zr, 95Nb, 140Ba, 103Ru and 233Pa on Hastelloy C276 detected experimentally were showed in Fig. 5. Undoubtedly, 95Zr and 140Ba, the typical salt-seeking fission products, would not be reduced and deposited on Hastelloy C276, so their existence on Hastelloy C276 (showed in black column in Fig. 5) should be attributed to adhesion of the molten salt. By using the same manner described in our previous work,9 the contribution resulted from the adhesion of the molten salt could be eliminated for all of five nuclides mentioned above. After that, the activities on Hastelloy C276, resulted truly from reduction and deposition were also showed in Fig. 5 for 95Zr, 95Nb, 140Ba, 103Ru and 233Pa (showed in white column in Fig. 5).
As shown in Fig. 5, the activities of 95Zr and 140Ba on Hastelloy C276 totally came from the adhesion of salt. On the contrary, the activities of 95Nb, 103Ru and 233Pa on Hastelloy C276 almost completely came from reductive deposition, with little activities being attributed to the adhesion of salt. Consequently, the conclusion could be made that 233Pa behaved as 95Nb and 103Ru, the typical noble-metal fission products, rather than 95Zr and 140Ba, the salt-seeking fission products. In other words, 233Pa, similar to the noble-metal fission products, was able to be reduced directly or indirectly by Hastelloy C276 and deposited on Hastelloy C276 itself, leading to the decrease of 233Pa activity in the salt. All these results strongly supported the conjecture that reductive deposition induced by Hastelloy was the reason responsible for the decrease of the specific activity of 233Pa in salt, and the majority of the reduced 233Pa would be deposited on Hastelloy C276 and the bottom of the salt.
Now that the decrease in specific activity of 233Pa in the salt was originated from the reductive deposition induced by Hastelloy C276 and metallic lithium, the remarkable difference in degree of 233Pa activity decrease between FLiBe and FLiNaK salts could be explained by their distinct influence on the redox potential of Pa4+/Pa pair, which can be expressed as:
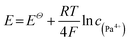 | (6) |
The lower Pa4+ ion concentration in FLiNaK salt moved the nominal potential of the Pa4+/Pa pair to negative direction, which meant the reduction of Pa is more difficult than in FLiBe salt. Different from FLiNaK salt, the higher Pa4+ ion concentration in FLiBe salt moved the nominal potential of the Pa4+/Pa pair to positive direction, leading to reduction of Pa more easier. So the specific activity of 233Pa in FLiBe salt was decreased more rapidly than that in FLiNaK salt after the addition of reductants. It can be seen that the remarkable difference in the decrease degree of 233Pa activity between FLiBe and FLiNaK salts could be reasonable explained by the reductive deposition mechanism. In reverse, the reasonable explanation further confirmed the significant role of reductive deposition responsible for the decrease of 233Pa specific activity in the salt.
Due to corrosion of FLiBe molten salt, Hastelloy has been selected as most proper structural materials of the reactor vessel of MSR. However, the deposition of Pa on Hastelloy C276 or its localized concentration in salt revealed in this work would generate a series of impact on operation and control of TMSR, such as neutron economy, reactor power, burnup, fuel conversion and breeding etc. More importantly, if 233Pa is deposited as a form of granules on Hastelloy reactor vessel, the fissile nuclide 233U produced by 233Pa decay would be cumulated partially on the surface of Hastelloy. Because of the irradiation of thermal neutron with high flux, the accumulated 233U would undergo unexpected fission. The release of extra fission energy would generate undesirable high temperature in localized locus on the Hastelloy reactor vessel, resulting probably in severe nuclear accident. Similar accident had happened indeed when ORNL carried out the development of a aqueous homogenous reactor HRE-2 with UO2SO4 solution in early stage.17 Therefore, 233Pa behavior in TMSR must be studied in more detail, especially its deposition induced by Hastelloy N, and the possible safety threats should be evaluated carefully.
Conclusions
The distribution and behavior of Pa in FLiBe molten salt was investigated mainly by measuring the activity of 233Pa in the salt. The studies indicated that Pa deposited slightly on the surfaces of graphite crucible. Similar to the noble-metal fission products, 233Pa would be removed rapidly from the FLiBe molten salt and deposited on the surface of Hastelloy C276 and the bottom of FLiBe salt after the Hastelloy C276 and metallic lithium were added into the system. The reason leading to the deposition was explained well by reductive deposition mechanism induced by Cr metal in Hastelloy C276 and metallic lithium. If the similar reduction–deposition process occurs in TMSR, a number of issues, related with operation, control, and even possible safety threats of TMSR, would be evoked. So the distribution and behavior of Pa in FLiBe molten salt should be studied in more detail.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the “Strategic Priority Research Program” and “Frontier Science Key Program” of the Chinese Academy of Sciences (Grant No. XDA02030000 and QYZDYSSW-JSC016).References
- IAEA, Thorium Fuel Cycle - Potential Benefits and Challenges, International Atomic Energy Agency, Vienna, 2005 Search PubMed.
- USDOE, Philos. Rev., 2002, 66, 239–241 Search PubMed.
- F. N. Peebles, Removal of Xe-135 from circulating fuel salt of the MSBR by mass transfer to helium bubbles, Oak Ridge National Lab., Tenn., 1968 Search PubMed.
- L. Mathieu, D. Heuer, R. Brissot, C. Garzenne, C. L. Brun, D. Lecarpentier, E. Liatard, J. M. Loiseaux, O. Meplan and E. Merle-Lucotte, Prog. Nucl. Energy, 2006, 48, 664–679 CrossRef CAS.
- W. B. Cottrell, H. E. Hungerford, J. K. Leslie and J. L. Meem, Operation of the aircraft reactor experiment, Oak Ridge National Lab., Tenn., 1955 Search PubMed.
- R. C. Robertson, MSRE design and operations report. Part I. Description of reactor design, Oak Ridge National Lab., Tenn., 1965 Search PubMed.
- R. C. Robertson, Conceptual design study of a single-fluid Molten-Salt Breeder Reactor, Oak Ridge National Lab., Tenn., 1971 Search PubMed.
- M. Jiang, H. Xu and Z. Dai, Bulletin of Chinese Academy of Sciences, 2012, 27, 366–374 Search PubMed.
- Z. Cheng, Radiochim. Acta, 2020 DOI:10.1515/ract-2020-0057.
- H. Wang, J. Chen, X. Cai, Z. Lin and Y. Ma, et al., Hejishu, 2014, 37, 120–124 Search PubMed.
- R. E. Thoma, Chemical Aspects of MSRE operations, Oak Ridge National Lab., Tenn., 1971 Search PubMed.
- E. L. Compere, S. S. Kirslis, E. G. Bohlmann, F. F. Blankenship and W. R. Grimes, Fission product behavior in the Molten Salt Reactor Experiment, Oak Ridge National Lab., Tenn., 1975 Search PubMed.
- L. M. Ferris, J. C. Mailen, J. J. Lawrence, F. J. Smith and E. D. Nogueira, J. Inorg. Nucl. Chem., 1970, 32(6), 2019–2035 CrossRef CAS.
- W. R. Grimes, Chemical research and development for Molten-Salt Breeder Reactors, Oak Ridge National Lab., Tenn., 1967 Search PubMed.
- L. E. McNeese, Engineering development studies for Molten-Salt Breeder Reactor processing No. 3, Oak Ridge National Lab., Tenn., 1971 Search PubMed.
- R. J. Kedl, Migration of a class of fission products (noble metals) in the Molten-Salt Reactor Experiment, Oak Ridge National Lab., Tenn., 1972 Search PubMed.
- A. M. Weinberg, The First Nuclear Era: The Life and Times of a Technological Fixer, American Institute of Physics, 1994 Search PubMed.
Footnote |
| † Zhongqi Zhao and Jifeng Hu are co-first authors of the article. |
| This journal is © The Royal Society of Chemistry 2021 |