Open Access Article
Open Access ArticleChromatographic separation of rare earths from aqueous and ethanolic leachates of NdFeB and SmCo magnets by a supported ionic liquid phase†
Dženita Avdibegović and
Koen Binnemans
and
Koen Binnemans *
*
Department of Chemistry, KU Leuven, Celestijnenlaan 200F, P. O. Box 2404, B-3001 Leuven, Belgium. E-mail: Koen.Binnemans@kuleuven.be
First published on 19th February 2021
Abstract
The separation of rare-earth elements (REEs) from other components of end-of-life NdFeB and SmCo magnets was investigated by column chromatography. A carboxylic-acid functionalized supported ionic liquid phase (SILP) was studied as a stationary phase. The magnets were firstly leached with a dilute aqueous or ethanolic hydrochloric acid solution at room temperature. Leaching of REEs from a NdFeB magnet was similarly efficient with both lixiviants, but the REEs were more efficiently leached from a SmCo magnet with the ethanolic lixiviant. The SILP exhibited a high affinity towards trivalent cations of REEs, which were successfully recovered from the aqueous and ethanolic leachates of magnets. Divalent cations of iron and cobalt, which were the major components of the acidic aqueous leachates of magnets, were rejected by the SILP. Iron and cobalt were present as negatively charged chloro complexes in the ethanolic leachates of magnets, and were not recovered by the cation-exchanging SILP. A versatile column chromatography method is developed, suitable for the separation of REEs from iron and cobalt, either from aqueous or ethanolic leachates of permanent magnets.
Introduction
Permanent magnets comprising rare-earth elements (REEs) have high magnetic energy products.1 These magnets are important for energy-related applications, such as electric vehicles and wind turbines. At present, REEs are mainly produced from ore deposits in China and this monopoly creates a supply risk.2,3 Recycling of REEs from end-of-life consumer products could contribute to alleviate the supply risk of REEs.4–6 The most common REE permanent magnets are NdFeB and SmCo magnets, and they typically contain 20–30 wt% of REEs. Selective extraction of REEs over iron or cobalt is a major challenge in recycling of NdFeB and SmCo magnet waste. The separation of REEs from other elements in magnets has been widely studied and a variety of recycling methods have been developed.5,7–13 They include pyrometallurgical, hydrometallurgical or solvometallurgical methods.14–20 All separation methods come with advantages and disadvantages, and some are only applicable to a certain type of magnet. For instance, hydrometallurgical processes for the separation of REEs from spent magnets operate at room temperature, the processing steps are relatively simple and similar to those encountered in production of REEs from ores. However, in hydrometallurgical processes, usually the REEs magnets are firstly unselectively leached with an acid. Iron, which is often one of the major elements in the REEs magnets, leaches into the solution as iron(II) which is stable until pH of 6, and it is difficult to efficiently separate it from REEs by precipitation, due to a co-precipitation of REEs.5,20 Upon exposure to air iron(II) can be oxidized to iron(III) which can be separated from REEs by precipitation at pH around 2. However, air oxidation of iron(II) to iron(III) is kinetically slow at acidic pH.5 Nevertheless, hydrometallurgical methods can be applied to recover REEs irrespective of the composition of magnets. This is very important because the spent REEs magnets are not collected separately, and economic recycling processes have to focus on recovery of REEs from both, NdFeB and SmCo magnets, within one process.21 The vast majority of the previously performed studies on the separation of REEs from magnets covered one type of magnets, either NdFeB or SmCo. Moreover, the stream of minor components in magnets (e.g. aluminum) or even major components such as zirconium in SmCo magnets is often not studied. However, to adequately estimate the advantages and limitations of a separation process it is important to consider all components of magnets.In the present study, the separation of REEs from other components in leachates of the most common REEs permanent magnets (NdFeB and SmCo) has been investigated using a supported ionic liquid phase (SILP) as the stationary phase in ion-exchange chromatography. Ion-exchange processes can be relatively easily implemented and controlled in flow systems to perform separations of desired elements. The SILP studied here was previously designed and used for the recovery and separation of scandium from bauxite residue leachates.22 The studies showed that the SILP exhibits good affinity also for other REEs, even in the presence of high concentrations of iron. Therefore, the potential of the SILP in separating REEs from major components (iron and cobalt in particular) in the leachates of NdFeB and SmCo magnets by dilute aqueous hydrochloric acid solution is studied here. The separation of REEs from other components of the magnets (e.g. aluminium, nickel, chromium, copper and zirconium) is discussed as well. Moreover, the performance of the SILP for separating the REEs from other components of the magnets is also investigated by a solvometallurgical route. Solvometallurgy is a branch of extractive metallurgy that uses green organic solvents instead of water to improve the selectivity of processes.23 In non-aqueous solvent extraction processes for the separation of REEs, the mutual solubility and the poor phase disengagement of the less polar organic phase comprising the extractant and the more polar organic phase comprising the components that need to be separated can be issues. For instance, the applicability of ethanol in non-aqueous solvent extraction for the separation of REEs is limited due to the miscibility of ethanol with common extractants (e.g. tri-n-butyl phosphate) and diluents. Solid ion-exchangers, such as the SILP, which can be packed in a chromatography column usually do not suffer from such drawbacks. Thus, ion exchange could be implemented as a separation process after the solvometallurgical leaching of REEs magnets, which includes solvents that are not suited for the solvent extraction, such as ethanol. Previous studies show that ethanol can improve the selectivity of ion-exchange processes. A selective uptake of desired metal ions is especially important for chromatography processes involving sorbents that exhibit relatively small sorption capacities, such as the SILP. If the process is not selective enough, untargeted elements can easily saturate the sorption sites of the sorbent, thus diminish its relevance to industry. Moreover, from a toxicological and environmental point of view, ethanol is considered as a green solvent.24 Therefore, apart from the separation of REEs from other elements in the aqueous leachates, their separation from the ethanolic hydrochloric acid leachates of NdFeB and SmCo magnets by the SILP is investigated as well in this paper.
Experimental
Chemicals
The samples of NdFeB and SmCo magnets were kindly provided by Magneti Ljubljana – D.D. (Slovenia). They were never magnetized because they were rejected after quality control. Acetic acid (99.8%), sodium hydroxide (98%), 2,9-dimethyl-1,10-phenanthroline (neocuproine, 99%) were purchased from Fisher Scientific (Merelbeke, Belgium). Nitric acid (65%, HNO3), standard solutions (1000 μg mL−1) of aluminum, boron, cobalt, copper, chromium, dysprosium, iron, gadolinium, gallium, manganese, nickel, neodymium, samarium, terbium, praseodymium, zirconium and holmium were purchased from Chem-Lab NV (Zedelgem, Belgium). Hydrochloric acid (37% HCl), ethanol (99.5%, EtOH), betaine hydrochloride (99%), 1,10-phenanthroline (>99%) and triethylamine (99%), iron(II) chloride (98%) were purchased from Acros Organics (Geel, Belgium). Polystyrene-divinylbenzene (PS-DVB) sulfonyl chloride resin (0.91 mmol g−1, 200–400 mesh) was purchased from RappPolymere (Tübingen, Germany). Trifluoromethanesulfonamide (98%) was purchased from J&K Scientific GmbH (Pforzheim, Germany). Dichloromethane (DCM) (p.a.), and acetone (p.a.) were purchased from Fisher Chemical (Loughborough, UK). The supported ionic liquid phase (SILP) betainium sulfonyl(trifluoromethanesulfonylimide) poly(styrene-co-divinylbenzene) [Hbet-STFSI-PS-DVB] (ESI, Fig. S1†) was synthesized according to a previously described method.22 The chemicals were used as received, without any further purification.Equipment
Magnets were ground in a disc mill (DM 200, Retsch, Germany) and ball mill (Pulverisette Premium 7, Fritsch, Germany). X-ray powder diffraction (XRD) patterns of the magnet powders were collected on a Bruker D2 PHASER X-ray. The procedure on characterisation of magnets is described in more detail in the ESI.† Leaching experiments were performed using Thermo Fisher shaker (Type 462-0355). UV-VIS absorption spectra of the leachates of magnets were measured on an Agilent Cary 6000i spectrophotometer and analyzed with Cary WinUV software. The sample preparation for the UV-VIS analysis can be found in the ESI.† A fraction collector CF-2 (Spectrum Laboratories, Inc.) equipped with a drop sensor and the IPC 8-channel peristaltic pump (ISMATEC) was used for sampling during the chromatography studies. Concentrations of elements in solutions were measured by an inductively coupled plasma-optical emission spectrometer (ICP-OES) (PerkinElmer Avio 500) equipped with an axial/radial dual plasma view and a GemCone High Solids nebulizer, which is suitable for analysis of samples with high content of organic matter. The standard solutions and all samples were prepared by dilution with 2 wt% HNO3. Holmium (5 ppm) was used as an internal standard.Digestion and leaching of magnets
In order to determine the composition of the magnets, 50 mg of magnet sample was digested in triplicate with 4 mL of 4 mol L−1 of HNO3 at room temperature. The fraction of elements in the magnets was calculated according to the following formula:
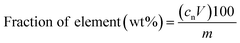 | (1) |
The mass concentration of elements (mg L−1) in the digested samples of NdFeB or SmCo magnets is cn, corrected by the dilution factor for ICP-OES measurement. The volume of the digested sample (0.004 L) is V, and the mass (50 mg) of the digested magnet is m.
Leaching of the magnets was generally performed by adding 50.0 mL of a 0.7 mol L−1 HCl solution in water or in ethanol to 0.625 g of magnet powder (<400 microns). It should be noted here that 0.7 mol L−1 HCl in ethanol was prepared by diluting the concentrated aqueous HCl (37 wt%) by ethanol, and therefore the lixiviant was not completely anhydrous. The mixture was shaken for 24 hours on Thermo Fisher shaker at 250 rpm at room temperature. The leachates were filtered through 0.20 μm pore size syringe filters. The freshly prepared leachates were studied by column chromatography. For investigating the leaching efficiency with the two lixiviants as a function of time, the magnets were leached for 2, 4, 6.5, 19.5 and 24 hours, with a liquid-to-solid ratio of 80. The leaching efficiencies (%) of the major elements in the magnets were determined from the ratio of their concentrations in the samples collected after a certain period of time (ci) and their concentrations in the completely digested samples (c0, mg L−1) using the formula:
 | (2) |
Column chromatography studies
A gravity flow glass column (BIO-RAD) with a length of 30 cm and a diameter of 0.7 cm was used in chromatographic separation experiments. The column was packed with 2 g of the dry SILP [Hbet-STFSI-PS-DVB] by a wet packing method. The SILP was preconditioned with 0.3 mol L−1 HCl solution or with absolute ethanol prior to each experiment. All column chromatography experiments were conducted at room temperature. For the optimizing of the separation of REEs by elution, 10 mL of the aqueous or ethanolic leachate was flowed through the column with the SILP. Then, to assure the flow of the aqueous leachate through the column, 10 mL of 0.3 mol L−1 HCl solution in water was added. Likewise, elution with 10 mL of ethanol was applied after the ethanolic leachate. To avoid evaporation of ethanol, vials of samples were closed immediately after collection. The recovery of elements by the SILP was calculated based on their concentrations in fractions collected after the flow of the leachates and the corresponding eluents:
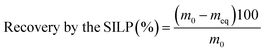 | (3) |
The mass of an element (mg) in the 10 mL of the feed is m0, and the sum of masses of the same element in the collected fractions (mg) is meq, calculated from the measured mass concentrations.
The elution of metals recovered by the SILP was then performed with 20 mL of 0.5 mol L−1 HCl, followed by 30 mL to 95 mL of 1.5 mol L−1 HCl. From the concentrations of elements in the collected fractions, the cumulative elution percentage was calculated using the formula:
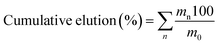 | (4) |
The mass of an element in the collected fractions is mn, which is calculated from the measured mass concentration, whereas m0 is the mass of the same element (mg) in the 10 mL of the feed. The flow rate of the leachates was 0.1 mL min−1 and that of the eluents was 0.5 mL min−1.
Results and discussion
Characterisation and leaching of NdFeB and SmCo magnets
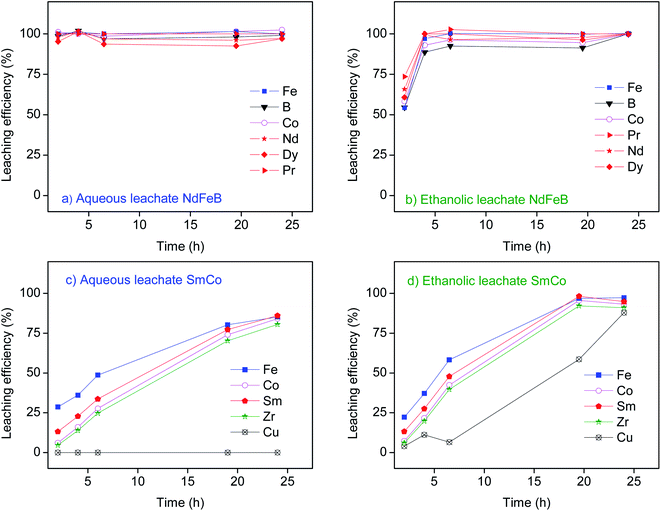
In order to determine the composition of the NdFeB and SmCo magnets, the powder samples (ESI, Fig. S2†) were digested with 4 mol L−1 HNO3 (Tables 1 and 2). The common components of the magnets can easily be dissolved in the applied media.13,25 The exception is, for instance, niobium, which typically forms an insoluble oxide in such acidic solutions unless a large amount of strongly complexing anion is added (e.g. with the mixture of HF/HNO3).26 However, niobium is only a trace constituent in the type of magnets used in the present study.9,10 Extensive studies on leaching of the magnets were previously reported in the literature.9,14,18,19,27 As the focus of the present study is on the chromatography separation of REEs from other major components of the magnets, leaching with HCl diluted in water or in ethanol was performed at room temperature (Fig. 1).| Elements | Fe | Nd | Pr | Dy | Co | B | Sm | Tb | Al | Total |
| Fraction of element (wt%) | 62.3 | 24.0 | 7.15 | 1.65 | 1.41 | 0.92 | 0.86 | 0.76 | 0.54 | 99.6 |
| Elements | Co | Fe | Sm | Cu | Zr | Ga | Al | Gd | Mn | Cr | Ni | Total |
| Fraction of element (wt%) | 46.0 | 23.9 | 20.6 | 5.14 | 2.70 | 0.37 | 0.22 | 0.08 | 0.03 | 0.03 | 0.02 | 99.1 |
The standard reduction potentials of the iron and neodymium redox couples are 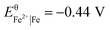 and
and 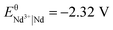 , and of cobalt and samarium
, and of cobalt and samarium 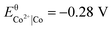 and
and 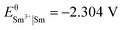 , respectively. The values indicate that the most abundant elements in the magnets can readily be dissolved by the dilute aqueous hydrochloric acid:25
, respectively. The values indicate that the most abundant elements in the magnets can readily be dissolved by the dilute aqueous hydrochloric acid:25
| 2REE + 6HCl → 2REECl3 + 3H2 | (5) |
| M + 2HCl → MCl2 + H2 | (6) |
REEs and iron were leached (>95%) from NdFeB magnet within 2 hours with the aqueous lixivant (Fig. 1a). With the ethanolic lixiviant the leaching efficiency of >95% was achieved after 4 hours (Fig. 1b). In the leaching process, the oxidized metals are transferred into solution forming solvated metal cations or anionic complexes of metal ions.28 The Gutmann donor number (DN) is a good measure of the ability of a solvent to solvate metal cations. Solvents with the higher DN exhibit stronger interactions with the dissolved metal cations. The DN of water is 33.0, and that of ethanol is 19.1.29 The experimental results (Fig. 1) indicate that the ionic species formed during the leaching of NdFeB magnet might be slightly less soluble in the ethanolic lixiviant than in the aqueous lixiviant. It appears that leaching of NdFeB magnet with the ethanolic lixiviant is rather a thermodynamically governed process. However, the solvation of the formed species only partly explains the observed differences, as the particular property of the solvent or a solution cannot serve as the sole parameter determining the leaching efficiency of the magnet.12
The highest leaching efficiency (about 85%) of samarium, cobalt and iron from the SmCo magnet with the aqueous lixiviant was achieved after 24 hours (Fig. 1c). Moreover, copper was not leached from the magnet with the aqueous lixiviant. Conversely, the vast majority of elements was leached from SmCo magnet within 19 hours and with more than 90% of leaching efficiencies with the ethanolic lixiviant (Fig. 1d). Additionally, the high leaching efficiency of copper (>88%) with ethanolic lixiviant was achieved after 24 hours of leaching. In solutions with low chloride concentrations copper is present as cupric ion (Cu2+), and its standard reduction potential of the redox couple 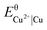 is +0.34 V.25 Therefore, the dissolution of metallic copper from SmCo magnet with the aqueous lixiviant (0.7 mol L−1 HCl) did not take place. However, it has been previously reported that at high chloride concentrations cuprous (Cu+) species such as CuCl, [CuCl2]−, [CuCl3]2− and [CuCl4]3− can form and that the contribution of cuprous ions to the ionisation potential is greater than that of cupric ions.30–33 Based on the UV-VIS absorption spectra of the ethanolic leachate of SmCo magnet (ESI, Fig. S3†), copper was found to be present in the form of cuprous ions in the leachate. According to the Bjerrum theory of ion pairs, lowering the dielectric constant of a solvent increases the forces between ions of opposite charge and therefore favours the formation of their complexes.34 The dielectric constant of ethanol (εr = 25.3) is lower than that of water (εr = 80.1).25 The formation of soluble cuprous chloride complexes was therefore promoted by the ethanolic lixiviant, which can explain the dissolution of copper from SmCo magnet and the overall better dissolution of SmCo magnet by the ethanolic lixiviant over the aqueous lixiviant. This is in contrast to the leaching of NdFeB magnet with the two lixiviants (Fig. 1). Copper is one of the most abundant elements in the SmCo magnet (Table 2), and therefore its leaching rate could affect the overall leaching of SmCo magnet by the tested lixiviants (Fig. 1). The NdFeB magnet does not contain a significant amount of copper which could hinder its leaching efficiency with the aqueous lixiviant. Moreover, the most abundant component in the NdFeB magnet is iron (Table 1), and in the SmCo magnet is cobalt (Table 2), and iron typically exhibits lower corrosion resistance in the acidic chloride media than cobalt.25 Thus, the NdFeB magnet was leached faster than the SmCo magnet, and especially with the aqueous lixiviant (Fig. 1).
is +0.34 V.25 Therefore, the dissolution of metallic copper from SmCo magnet with the aqueous lixiviant (0.7 mol L−1 HCl) did not take place. However, it has been previously reported that at high chloride concentrations cuprous (Cu+) species such as CuCl, [CuCl2]−, [CuCl3]2− and [CuCl4]3− can form and that the contribution of cuprous ions to the ionisation potential is greater than that of cupric ions.30–33 Based on the UV-VIS absorption spectra of the ethanolic leachate of SmCo magnet (ESI, Fig. S3†), copper was found to be present in the form of cuprous ions in the leachate. According to the Bjerrum theory of ion pairs, lowering the dielectric constant of a solvent increases the forces between ions of opposite charge and therefore favours the formation of their complexes.34 The dielectric constant of ethanol (εr = 25.3) is lower than that of water (εr = 80.1).25 The formation of soluble cuprous chloride complexes was therefore promoted by the ethanolic lixiviant, which can explain the dissolution of copper from SmCo magnet and the overall better dissolution of SmCo magnet by the ethanolic lixiviant over the aqueous lixiviant. This is in contrast to the leaching of NdFeB magnet with the two lixiviants (Fig. 1). Copper is one of the most abundant elements in the SmCo magnet (Table 2), and therefore its leaching rate could affect the overall leaching of SmCo magnet by the tested lixiviants (Fig. 1). The NdFeB magnet does not contain a significant amount of copper which could hinder its leaching efficiency with the aqueous lixiviant. Moreover, the most abundant component in the NdFeB magnet is iron (Table 1), and in the SmCo magnet is cobalt (Table 2), and iron typically exhibits lower corrosion resistance in the acidic chloride media than cobalt.25 Thus, the NdFeB magnet was leached faster than the SmCo magnet, and especially with the aqueous lixiviant (Fig. 1).
UV-VIS study of the leachates of NdFeB and SmCo magnets
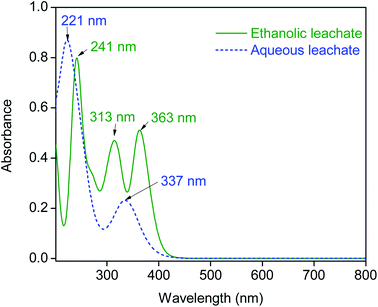
Previous studies showed that the recovery of metal ions by the SILP is taking place by proton exchange of the carboxylic group of the SILP with metal cations in the solution.35 Different ionic species of metals can form in aqueous and ethanolic leachates of magnets (vide supra). The change in speciation can impact the overall separation of elements in the leachates by the SILP. Insight into the speciation of components of leachates could be beneficial for optimization of the column chromatography separation process. Therefore the UV-VIS absorption spectra of major elements, such as iron and cobalt in the aqueous and ethanolic leachates of magnets were investigated.The absorption maxima at 221 nm and at 337 nm in the aqueous leachates of NdFeB (Fig. 2) and SmCo magnets (ESI, Fig. S4†) are observed, due to the dominant presence of iron in the form of ferrous ions (Fe2+).36,37 A similar optical absorption spectrum was recorded with the synthetic aqueous solution of iron(II) chloride (ESI, Fig. S5†). Therefore, iron was present in the aqueous leachates as a divalent cation which can be recovered by the SILP via a proton exchange mechanism. The prominent absorption maxima in the ethanolic leachates are observed at 241 nm, 313 nm and 363 nm. The same absorption spectrum was recorded with a synthetic solution of iron(II) chloride in ethanol (ESI, Fig. S5†). The absorption spectra of the ethanolic leachates (Fig. 2, ESI Fig. S4†) are very similar to that of the tetrachloroferrate(II) complex [FeCl4]2−.36,38 However, both iron(II) and iron(III) exhibit similar absorption spectra in ethanolic chloride solutions.37 Therefore, the presence of iron(II) was confirmed by the UV-VIS analysis in the presence of 1,10-phenanthroline ESI, (Fig. S6†).
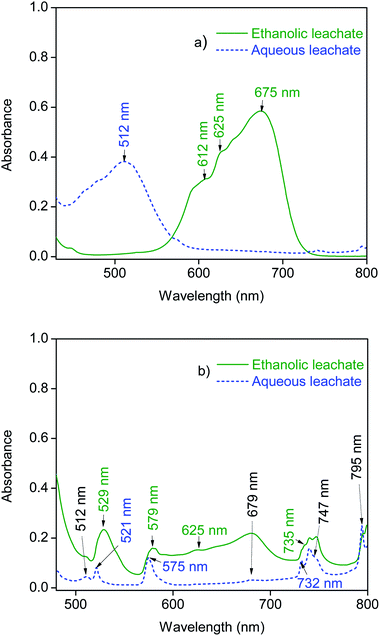
In order to measure the UV-VIS absorption spectra of cobalt undiluted as well as 50 times diluted leachates of the magnets were analysed. The absorption maxima in the UV-VIS absorption spectra of the aqueous leachates of both magnets were observed between 440 nm to 560 nm (Fig. 3). The octahedral species of cobalt ([Co(H2O)6]2+ and [CoCl(H2O)5]+) absorb in this spectral region.39,40 However, the absorption maxima in the ethanolic leachates of SmCo or NdFeB magnets were observed at the range from 580 nm to 720 nm (Fig. 3). This spectrum corresponds to the tetrahedral complex [CoCl4]2−.40 The results indicate that in the tested ethanolic solutions, cobalt is predominantly present in the form of an anionic complex. The spectra of cobalt are more prominent in the leachates of SmCo magnet (Fig. 3a), since they contained higher amount of cobalt than the leachates of NdFeB magnet (Tables 1 and 2).
Apart from the spectra of cobalt, additional absorption maxima were observed in the leachates of NdFeB magnets between 520 nm and 580 nm, and between 720 nm and 800 nm (Fig. 3b). These absorption maxima arise from the presence of neodymium chloride in the leachates.41 The absorbance maxima of neodymium in the ethanolic leachates were slightly shifted in comparison with the absorbance maxima in the aqueous leachate (e.g. from 521 nm to 529 nm, from 575 nm to 579 nm, from 732 to 735 nm). Neodymium is typically present in the form of hydrated Nd3+ in the absence of coordinating ligands. However, complexation of neodymium with chlorides can be enhanced by the increase in temperature and in chloride concentration.42 Similar observations of the shift of the absorption maxima were found in previous studies of neodymium chloride solutions in the presence of high concentrations of LiCl (5 mol L−1) at high temperatures.41 It was concluded that the two dominant species of neodymium in the tested solutions were Nd3+ and NdCl2+. Organic solvents can promote the formation of chloro complexes (vide supra). Therefore, the shift in the absorption maxima of neodymium towards the longer wavelength numbers (Fig. 3) might indicate an increased concentration of the NdCl2+ complex in the ethanolic leachate. However, the shift of the characteristic absorption maxima can also be caused by solvent effects on the UV-VIS spectra, such as the effect of the solvent polarity.43 Nevertheless, the UV-VIS absorption spectra do indicate that REEs are present as cationic species in both leachates of magnets (Fig. 3). It should be noted that the absorption maxima of cobalt partly overlaps with the absorption maxima of neodymium in the spectra of leachates of NdFeB magnet (Fig. 3b) (e.g. absorbance maxima of cobalt around 512 nm in the aqueous leachate and around 679 nm in the ethanolic leachate). Nevertheless, neodymium ions do not exhibit absorption maxima around 625 nm. Therefore that absorbance maxima in the ethanolic leachate of NdFeB magnet are attributed uniquely to the [CoCl4]2− complex. The change in the speciation of the studied metals in aqueous and ethanolic leachates of NdFeB and SmCo magnets can also be observed from the different colours of the solutions (ESI, Fig. S7 and S8†).
Separation of REEs from other elements in the leachates of permanent magnets by column chromatography
The leachates of magnets obtained after 24 hours of leaching with the aqueous and ethanolic lixiviants (Fig. 1) were investigated for the separation of REEs from other elements by column chromatography. The volume of 10 mL of aqueous or ethanolic leachate of magnets magnet was flowed through the column packed with the SILP. Then, the flow of the entire volume of the leachates through the column was assured by applying additional 10 mL of 0.3 mol L−1 HCl in water or by ethanol as eluents. The 0.3 mol L−1 HCl solution in water was used as the subsequent eluent since similar concentration of HCl was present in the tested aqueous leachates. Ethanol was tested as an alternative eluent for the separation of REEs from other elements, based on the potential formation of metal complexes in ethanol. Recoveries of elements by the SILP were then evaluated and the elution of the recovered elements by dilute HCl was further investigated, which is discussed in the following sub-sections.Separation of REEs from the leachates of NdFeB magnet
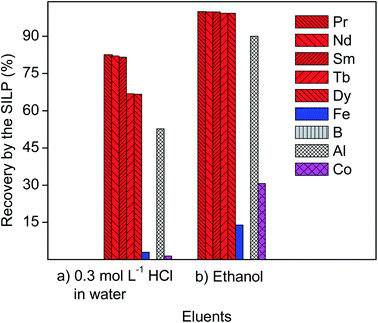
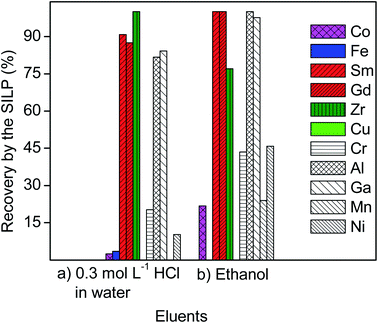
The recovery of REEs from the 10 mL of aqueous leachate of NdFeB magnet (Table 3) and after elution with 10 mL of 0.3 mol L−1 of HCl in water was in the range 67–83% (Fig. 4). The recovery yield of iron and cobalt, as well as of boron, from the aqueous leachate of NdFeB magnet was negligible. Around 52% of aluminum was recovered along with REEs.| Concentration (mg L−1) | Fe | Co | B | Al | Nd | Pr | Dy | Tb | Sm |
|---|---|---|---|---|---|---|---|---|---|
| Aqueous leachate | 7789 | 145 | 76 | 69 | 2776 | 892 | 224 | 95 | 69 |
| Ethanolic leachate | 7788 | 135 | 77 | 74 | 2546 | 724 | 200 | 83 | 52 |
Previous studies showed that the electrostatic interactions between the carboxylic group of the SILP with the metal cations increase with the increase of the charge of the metal cations.22 The divalent cations of iron and cobalt, and the trivalent cations of REEs are the dominant species in the aqueous leachate of NdFeB magnet (vide supra).25 Therefore, the SILP with the carboxylic functional group exhibited a higher affinity for more charged ions in solutions like the trivalent ions of REEs, over the doubly-charged ions like iron and cobalt. In aqueous solutions of hydrochloric acid, boron typically exists as boric acid, which is unlikely to be recovered by the carboxylic-acid-functionalized SILP. Moreover, among ions of the same charge, the ion exchange between the protons of the SILP will preferentially take place with metal ions with lower hydration enthalpy. REEs have the lowest hydration enthalpy among the trivalent ions in the leachate of the NdFeB magnet, for example aluminium, which was actually recovered less than REEs (Fig. 4). Additionally, the SILP exhibited a preference for light REEs over heavy REEs (Fig. 4) (the recovery yield of praseodymium, neodymium and samarium was approximately 83% versus the recovery yield of dysprosium and terbium of approximately 67%). The hydration enthalpies of the light REEs are lower than the hydration enthalpies of the heavy REEs. Therefore, the dehydration of the light REEs is more favourable than of the heavy REEs, resulting in the higher recovery yields of the light REEs. However, the protons of the acidic aqueous leachate of NdFeB magnet and of the subsequent eluent (0.3 mol L−1 HCl in water) competed with REEs in ion exchange by the SILP, and REEs were not completely recovered from the 10 mL of aqueous leachate (Fig. 4).
The recovery of REEs from the 10 mL of ethanolic leachate of NdFeB magnet (Table 3) and after elution with 10 mL of absolute ethanol was nearly quantitative (>99%) (Fig. 4). Around 90% of aluminium was recovered along with REEs from the ethanolic leachate. Aqueous and ethanolic leachates of NdFeB magnet were acidic, as an excess of hydrochloric acid was used to leach the magnets (vide supra). Therefore, the protons of hydrochloric acid in the leachates competed in the ion exchange process by the SILP with the positively charged metal ions during the flow of the leachates through the column. However, ethanol does not provide many protons that could compete with metal ions in the ion exchange process during the subsequent elution of the SILP, unlike when using 0.3 mol L−1 HCl as eluent. This could explain the higher recovery of REEs and aluminium from the ethanolic leachate, followed by elution with ethanol, than from the aqueous leachate (Fig. 4). The recovery yields of iron and cobalt were still much lower than of the REEs (around 14% and 31%, respectively), whereas boron was not recovered by the SILP from the ethanolic leachate. It is known that iron and cobalt have the tendency to form anionic chloro complexes in ethanol at even low concentration of chloride ions (e.g. 0.7 mol L−1), vide supra. These complexes cannot be recovered by the SILP, neither boron in the form of boric acid. Therefore, the recovery of iron, cobalt and boron from the ethanolic leachate and when using ethanol as eluent was highly supressed (Fig. 4). The formation of anionic iron(II) chloro complexes, for instance, in aqueous solutions can only be promoted by high concentrations of chlorides and/or by an increase in the temperature of solution (e.g. in solutions with chloride concentrations greater than 2 mol L−1 at temperatures higher than 60 °C).38 The recovery of iron and cobalt from the ethanolic leachate and after elution of the SILP by ethanol (Fig. 4) indicate that their conversion to the chloride complexes was not quantitative. The high concentration of iron in the ethanolic leachate (Table 3), might have been an issue for the quantitative formation of its anionic chloro complexes in the tested leachate with the chloride concentration of 0.7 mol L−1, since typically an excess of chlorides is required for their formation. Besides, the tested ethanolic lixiviant was not completely anhydrous (see the Experimental section). The presence of water might contribute to the formation of cationic species of iron(II) and cobalt(II) in the ethanolic leachate, which can be then prone to the cation exchange with protons of the SILP. Nevertheless, the REEs do not tend to form the anionic complexes in the ethanolic leachate of NdFeB magnet, which is also evident from their recovery yield by the SILP (Fig. 4). Therefore, ethanol appears to be a promising eluent for separating iron, cobalt and boron from REEs, without diminishing the recovery of REEs by the SILP.
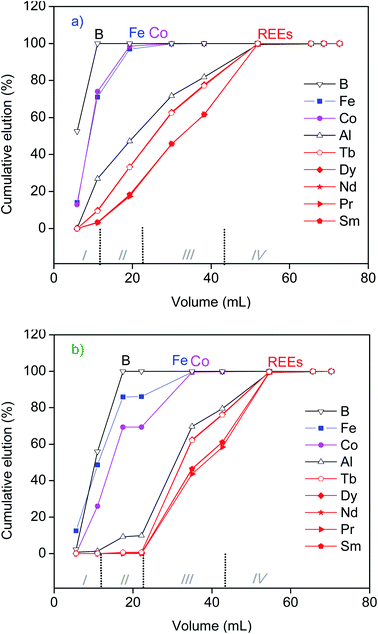
Further elution of the elements recovered by the SILP from the aqueous and ethanolic leachates of NdFeB magnet was performed with dilute aqueous HCl solutions (Fig. 5, ESI Fig. S9†). All iron and cobalt, along with the vast majority of aluminium were eluted by 0.5 mol L−1 HCl, regardless of the type of leachate (aqueous or ethanolic). However, a significant amount of REEs was also eluted at that point (e.g. up to 62% of neodymium, Fig. 5). Therefore, the separation of REEs from the base elements was mostly achieved during the flow of the leachate through the column packed with the SILP, and the subsequent elution by 0.3 mol L−1 HCl or by ethanol. Moreover, when eluting the SILP by 0.5 mol L−1 HCl after its elution by ethanol a decrease of the bed height of the SILP from approximately 14 cm to 9.6 cm was observed. This was due to the difference in swelling of the SILP in the two solvents. By further elution with the 1.5 mol L−1 HCl changes in the bed height were not observed. The changes in the bed height were not observed in the case of REEs separation from the aqueous leachate. A complete elution of REEs originating either from the aqueous or ethanolic leachate of NdFeB magnet was achieved by 1.5 mol L−1 HCl (Fig. 5). Fractions of REEs free from iron, boron and cobalt were obtained. Only a minor amount of aluminium was present among the base elements in the final collected fractions of REEs from both leachates.
Separation of REEs from leachates of SmCo magnet
The ethanolic leachate of SmCo magnet was more rich in the major elements (samarium, cobalt, iron, copper, zirconium) than the aqueous leachate (Table 4). The significant differences in the composition of the leachates of SmCo magnet were due to the difference in the leaching efficiencies between the two lixiviants (Fig. 1).The separation of REEs from the aqueous and ethanolic leachate of SmCo magnet was performed in the same manner as from the leachates of NdFeB magnet (vide supra). The recovery yields of samarium and gadolinium from the aqueous leachate of SmCo magnet and after elution with 10 mL of 0.3 mol L−1 of HCl were 91% and 87%, respectively. The recovery yield of divalent cobalt and iron was negligible (Fig. 6). Zirconium was quantitatively recovered from the aqueous leachate of the SmCo magnet. Copper was not leached with the aqueous lixiviant (Fig. 1 and Table 4) and therefore it was not present in the tested aqueous leachate of SmCo magnet. The results are in accordance with the hypothesis of the higher preference of the SILP for the more charged ions (vide supra). Moreover, the SILP exhibited an excellent affinity towards tetravalent zirconium (Fig. 6). A similar uptake preference of the more charged ions was observed among the minor elements in the aqueous leachate of SmCo magnet: trivalent chromium, aluminium and gallium were more efficiently recovered than divalent manganese and nickel (Fig. 6).
The recovery of samarium and gadolinium from the 10 mL of ethanolic leachate of the SmCo magnet and after elution with 10 mL of absolute ethanol was nearly quantitative (>99%) (Fig. 6). Conversely, the recovery of cobalt from the ethanolic leachate of SmCo magnet was around 22%, whereas iron was not recovered by the SILP (Fig. 6). Although copper was leached with the ethanolic solution of HCl (Fig. 1d), it was not recovered by the SILP (Fig. 6). The three elements (cobalt, iron and copper) were present in the form of anionic complexes in the ethanolic solutions (vide supra), which explains their low or negligible recovery from the ethanolic leachate and after elution with ethanol. Iron was less concentrated in the leachate of SmCo magnet than in the leachate of NdFeB magnet (Tables 3 and 4), and it was completely separated from REEs in SmCo leachate by elution with ethanol (Fig. 6). The results indicate that the concentration of iron in the ethanolic leachate of SmCo magnet was low enough to form chloro complexes, unlike in the ethanolic leachate of NdFeB magnet. The recovery yield of zirconium was lower from the ethanolic leachate (around 77%) than from the aqueous leachate of SmCo magnet (>99%) (Fig. 6). In the ethanolic leachate of SmCo magnet the concentration of samarium was higher than in the aqueous leachate (Table 4), and its recovery could have suppressed the recovery of zirconium from the ethanolic leachate.
Among the minor components of the ethanolic leachate of SmCo magnet, trivalent aluminium and gallium were the most efficiently recovered by the SILP, similarly as in the case of the aqueous leachate (Fig. 6). The concentration of divalent manganese and nickel in the ethanolic leachate was the lowest among all the elements (Table 4), and their uptake could easily be suppressed by other elements. However, the uptake of divalent manganese and nickel was slightly enhanced from the ethanolic leachate, in regards to their uptake from the aqueous leachate (Fig. 6). The lower acidity of ethanol than of the 0.3 mol L−1 of HCl could have enhanced the uptake of these ions (vide supra).
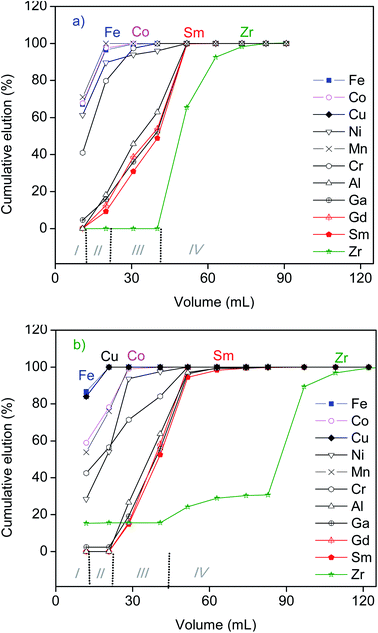
Further separation of REEs from the other elements recovered by the SILP from the leachates of SmCo magnet was investigated by the isocratic elution with dilute HCl (Fig. 7, ESI Fig. S10†). Cobalt, iron and vast majority of the minor elements were eluted from the column with 0.5 mol L−1 HCl (Fig. 7), analogously to the separation of REEs from the other major elements of NdFeB magnet (Fig. 5). Up to 40% and 58% of REEs from aqueous and ethanolic leachate, respectively, was eluted along with the other metals. A complete elution of samarium and gadolinium was achieved with 1.5 mol L−1 HCl (Fig. 7). The majority of zirconium (92%) was eluted together with REEs from the aqueous leachate of SmCo magnet (Fig. 7a). Only around 15% of zirconium was eluted along with REEs from the ethanolic leachate (Fig. 7b). The REEs were more concentrated in the ethanolic leachate of SmCo magnet, and they were more efficiently recovered from the ethanolic leachate than from the aqueous leachate (Table 4 and Fig. 6). Since the SILP exhibits lower affinity towards the trivalent REEs than towards tetravalent zirconium, the high amounts of REEs were eluted earlier than zirconium consuming the majority of the HCl in the eluent (Fig. 7b).
The vast majority of minor elements (chromium, manganese, nickel) were not recovered by the SILP. The amount that was recovered was eluted with 0.5 mol L−1 HCl, prior to a complete elution of REEs. Exceptionally, aluminium and gallium were completely eluted with 1.5 mol L−1 HCl, along with REEs. The trend can be explained by a combination of several effects (charge of ions, hydration enthalpies and their concentration), as already discussed in the previous section on the separation of minor elements from the leachates of NdFeB magnets (Fig. 5).
The uptake of REEs from SmCo and NdFeB magnets and their elution generally followed very similar trends (Fig. 5 and 7), indicating that the chromatography separation of REEs can be simultaneously performed from leachates of both types of permanent magnets. The highest separation between the REEs and the major elements of SmCo and NdFeB magnets was actually achieved due to the preferential recovery of REEs by the SILP. The isocratic elution by HCl of the recovered components from the leachates of magnets was performed to investigate if the poorly recovered elements such as iron or cobalt could be further separated from REEs. However, using only the isocratic elution with HCl did not result in their complete separation, as a part of the recovered REEs were co-eluted along with the other elements. In order to minimize the total volume of eluent, the elution of REEs recovered by the SILP could be more efficiently performed by more concentrated solutions of HCl (>1.5 mol L−1). The separation of REEs from iron by elution with phosphoric acid was previously demonstrated in a study on the separation of REEs from bauxite residue leachates by the SILP.22 Therefore, using eluents other than dilute HCl could improve even more the separation of REEs from other elements that are recovered by the SILP along with REEs, and even the separation of REEs into sub-groups. In the present study, the separation of REEs by the SILP from the leachates which were obtained without any pre-treatment of NdFeB and SmCo magnets (and thus rich in iron and cobalt) was performed as a proof-of-concept. The method could be extended to an integrated process in which major components can be partly removed,13,44,45 and then REEs can be further purified by the column chromatography. Previous studies have shown that the SILP has moderate sorption capacity.
It should be noted that REEs were recovered well by the SILP when using 10 mL of leachates (Fig. 4 and 6). The volume of leachates that can be applied on the column to efficiently recover the REEs depends on the total ion-exchange capacity of the SILP. Furthermore, the total ion-exchange capacity is related to the total amount of functional groups of the PS-DVB sulfonyl chloride resin, which is used for synthesising the SILP (vide supra, Experimental section). A SILP with a higher sorption capacity could be synthesised from a very high capacity sulfonyl chloride resin (>0.91 mmol g−1), thus enable a better throughput of leachates.
Conclusions
The lixiviants 0.7 mol L−1 HCl in water or in ethanol could efficiently (>95%) leach all components of the NdFeB magnet in 4 hours. However, REEs were not completely leached from the SmCo magnet with the aqueous lixiviant even after 24 hours. The ethanolic lixiviant enhanced the leaching of copper, which is a major constituent of SmCo magnets, and the overall leaching efficiency of metals from the SmCo magnet. The SILP is more selective for the trivalent REEs (e.g. 82% recovery of neodymium and 90% of samarium) over divalent iron and cobalt (<5%) in the aqueous leachates of NdFeB and SmCo magnets. The low pH of the subsequent eluent (0.3 mol L−1 HCl in water) enhanced the separation of REEs and other major components of the aqueous leachates. REEs were efficiently recovered (>99%) from the ethanolic leachates of NdFeB and SmCo magnets. The subsequent elution of the SILP with ethanol did not lower the recovery yield of REEs. Iron, cobalt and copper were mainly present in the form of anionic chloro complexes in the ethanolic leachates and were hence rejected by the cation-exchanging SILP. The results indicate that ethanol can be used as a suitable eluent for the separation of iron, cobalt and copper from REEs. Zirconium was recovered efficiently from either the aqueous or ethanolic leachate of the SmCo magnet. The minor elements of NdFeB and SmCo magnets that were recovered by the SILP from the aqueous or ethanolic leachates were generally co-eluted along with REEs. Nevertheless, the separation of REEs from elements like iron, cobalt, copper or boron by the SILP appears to be a versatile method, applicable to either NdFeB or SmCo magnets, after leaching with an aqueous or organic lixiviant.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Magneti Ljubljana – D. D (Slovenia) is acknowledged for providing the samples of NdFeB and SmCo magnets. The authors thank Dr Mehmet Ali Recai Önal for milling and sieving the magnets, for the XRD analysis of the SmCo magnets and for scientific discussions. Thupten Palden is acknowledged for his assistance in the XRD analysis of the NdFeB magnets. The research leading to these results received funding from the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Programme: Grant Agreement 694078 – Solvometallurgy for critical metals (SOLCRIMET). This publication reflects only the authors' view, exempting the community from any liability.References
- O. Gutfleisch, M. A. Willard, E. Brück, C. H. Chen, S. G. Sankar and J. P. Liu, Adv. Mater., 2011, 23, 821–842 CrossRef CAS.
- P. Emsbo, P. I. Mclaughlin, G. N. Breit, A. Edward and A. E. Koenig, Gondwana Res., 2015, 27, 776–785 CrossRef CAS.
- G. Charalampides, K. I. Vatalis and B. Apostoplos, Procedia Economics and Finance, 2015, 24, 126–135 CrossRef.
- K. Binnemans and P. T. Jones, J. Sustain. Metall., 2015, 4, 126–146 CrossRef.
- P. Venkatesan, T. Vander Hoogerstraete, K. Binnemans, Z. Sun, J. Sietsma and Y. Yang, ACS Sustainable Chem. Eng., 2018, 6, 9375–9382 CrossRef CAS.
- Y. Yang, C. Lan, L. Guo, Z. An, Z. Zhao and B. Li, Sep. Purif. Technol., 2020, 233, 116030 CrossRef CAS.
- P. Venkatesan, T. Vander Hoogerstraete, T. Hennebel, K. Binnemans, J. Sietsma and Y. Yang, Green Chem., 2018, 20, 1065–1073 RSC.
- Y. Yang, C. Lan, Y. Wang, Z. Zhao and B. Li, Sep. Purif. Technol., 2020, 230, 115870 CrossRef CAS.
- M. Orefice, H. Audoor, Z. Li and K. Binnemans, Sep. Purif. Technol., 2019, 219, 281–289 CrossRef CAS.
- M. A. R. Önal, C. R. Borra, M. Guo, B. Blanpain and T. Van Gerven, J. Sustain. Metall., 2015, 1, 199–215 CrossRef.
- X. Li, Z. Li, M. Orefice and K. Binnemans, ACS Sustainable Chem. Eng., 2019, 7, 2578–2584 CrossRef CAS.
- M. Orefice, A. Eldosouky, I. Škulj and K. Binnemans, RSC Adv., 2019, 9, 14910–14915 RSC.
- S. Van Loy, M. A. R. Önal, K. Binnemans and T. Van Gerven, Hydrometallurgy, 2020, 191, 105154 CrossRef CAS.
- M. Gergoric, C. Ravaux, B. M. Steenari, F. Espegren and T. Retegan, Metals, 2018, 8, 1–17 CrossRef.
- M. Orefice, A. Van den Bulck, B. Blanpain and K. Binnemans, J. Sustain. Metall., 2020, 6, 91–102 CrossRef.
- X. Xu, S. Sturm, Z. Samardzija, J. Scancar, K. Markovic and K. Zuzek Rozman, Green Chem., 2020, 22, 1105–1112 RSC.
- Y. Kataoka, T. Ono, M. Tsubota and J. Kitagawa, AIP Adv., 2015, 5, 117212 CrossRef.
- C. H. Lee, Y. J. Chen, C. H. Liao, S. R. Popuri, S. L. Tsai and C. E. Hung, Metall. Mater. Trans. A, 2013, 44, 5825–5833 CrossRef CAS.
- J. Kitagawa and R. Uemura, Sci. Rep., 2017, 7, 1–6 CrossRef CAS.
- P. Venkatesan, Z. H. I. Sun, J. Sietsma and Y. Yang, Sep. Purif. Technol., 2018, 191, 384–391 CrossRef CAS.
- T. Lorenz and M. Bertau, J. Cleaner Prod., 2020, 246, 118980 CrossRef CAS.
- D. Avdibegović, M. Regadío and K. Binnemans, RSC Adv., 2018, 8, 11886–11893 RSC.
- K. Binnemans and P. T. Jones, J. Sustain. Metall., 2017, 3, 570–600 CrossRef.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. Mcelroy, S. Abou-shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- D. R. Lide, CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, FL, 2005 Search PubMed.
- M. Nete, W. Purcell, E. Snyders and J. T. Nel, S. Afr. J. Chem., 2010, 63, 130–134 Search PubMed.
- F. Liu, A. Porvali, P. Halli, B. P. Wilson and M. Lundström, JOM, 2019, 72, 806–815 CrossRef.
- N. Sato, Corros. Sci., 1987, 27, 421–433 CrossRef CAS.
- M. Ansari Fard, G. H. Rounaghi, M. Chamsaz and K. Taheri, J. Inclusion Phenom. Macrocyclic Chem., 2009, 64, 49–56 CrossRef CAS.
- P. M. May, I. M. Ritchie and E. T. Tan, J. Appl. Electrochem., 1991, 21, 358–364 CrossRef CAS.
- K. Yoo, S. kyung Kim, J. chun Lee, M. Ito, M. Tsunekawa and N. Hiroyoshi, Miner. Eng., 2010, 23, 471–477 CrossRef CAS.
- H. R. Watling, Hydrometallurgy, 2014, 146, 96–110 CrossRef CAS.
- X. Li, W. Monnens, Z. Li, J. Fransaer and K. Binnemans, Green Chem., 2020, 22, 417–426 RSC.
- L. Al-Attar, A. Dyer and L. Contam, Reclam, 2007, 15, 427–436 Search PubMed.
- D. Avdibegović and K. Binnemans, Ind. Eng. Chem. Res., 2020, 59, 15332–15342 CrossRef.
- C. A. Heinrich and T. M. Seward, Geochim. Cosmochim. Acta, 1990, 54, 2207–2221 CrossRef CAS.
- W. Liu, B. Etschmann, J. Brugger, L. Spiccia, G. Foran and B. McInnes, Chem. Geol., 2006, 231, 326–349 CrossRef CAS.
- R. Zhao and P. Pan, Can. J. Chem., 2001, 79, 131–144 CAS.
- R. Lommelen, T. Vander Hoogerstraete, B. Onghena, I. Billard and K. Binnemans, Inorg. Chem., 2019, 58, 12289–12301 CrossRef CAS.
- G. Pass and H. Sutcliffe, The elements in the first transition series, in Practical Inorganic Chemistry, Springer, Dordrecht, 1974 Search PubMed.
- S. A. Stepanchikova and G. R. Kolonin, Russ. J. Coord. Chem., 2005, 31, 193–202 CrossRef CAS.
- C. H. Gammons, S. A. Wood and A. E. Williams-Jones, Geochim. Cosmochim. Acta, 1996, 60, 4615–4630 CrossRef CAS.
- L. Cui, F. Cheng and J. Zhou, Ind. Eng. Chem. Res., 2015, 54, 7534–7542 CrossRef CAS.
- M. A. R. Önal, C. R. Borra, M. Guo, B. Blanpain and T. Van Gerven, J. Rare Earths, 2017, 35, 574–584 CrossRef.
- M. A. R. Önal, S. Riaño and K. Binnemans, Hydrometallurgy, 2020, 191, 105213 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Figure of the SILP, XRD diffractograms of NdFeB and SmCo magnets, UV-VIS absorption spectra of copper(I) in the ethanolic leachate in the presence of neocuproine, UV-VIS absorption spectra of the aqueous and ethanolic synthetic solutions of iron(II), UV-VIS absorption spectra of the complex between iron(II) and 1,10-phenanthroline. Protocols for characterisation of magnets and sample preparation for the UV-VIS analysis are available. Figures of the aqueous and ethanolic leachates of the REEs magnets are available, and the chromatographic separation of REEs from the aqueous and ethanolic leachates of magnets with the mass concentration of elements in the fractions as a function of the volume of eluents. See DOI: 10.1039/d0ra09766g |
| This journal is © The Royal Society of Chemistry 2021 |