Open Access Article
Open Access ArticleA novel nano-lanthanum complex: synthesis, characterization and application as a macrofuran chemosensor in pharmaceutical, biological and environmental samples†
Sheta M. Sheta *a,
Mohkles M. Abd-Elzahera and
Said M. El-Sheikh
*a,
Mohkles M. Abd-Elzahera and
Said M. El-Sheikh b
b
aInorganic Chemistry Department, National Research Centre, 33, El-Behouth St., Dokki, Giza, 12622, Egypt. E-mail: dr.sheta.nrc@gmail.com; Fax: +20-02-33370931; Tel: +20 1009697356
bNanomaterials and Nanotechnology Department, Central Metallurgical R & D Institute, Cairo, 11421, Egypt
First published on 4th March 2021
Abstract
Macrofuran is widely used as an antibiotic for the treatment of urinary tract infections. Nevertheless, it is prohibited due to toxicity and environmental concerns. The development of a fast, simple, and cost-effective approach for the determination of macrofuran antibiotic (MFA) is still a challenge. Herein, we report a chemosensor based on a nano-lanthanum complex derived from phenylenediamine. The physicochemical properties and structure of the prepared complex were confirmed using different spectroscopic tools such as X-ray diffraction (XRD), scanning electron microscopy equipped with EDX, elemental analysis, Fourier transform-infrared (FT-IR) spectroscopy, UV-vis spectroscopy, mass spectroscopy and photoluminescence (PL). The nano-lanthanum complex was found to be chemically stable, highly sensitive and selective to MFA, without interference from other common antibiotics. The limit of detection for MFA was 0.025 ng mL−1, over a linear concentration range of 0.02–30.0 ng mL−1, with a correlation coefficient of 0.994. The nano-lanthanum complex can be used successfully as a promising chemosensor for MFA determination in pharmaceutical formulation and different biological samples (whole blood–serum–plasma). In addition, this approach will protect human beings from the environmental hazards of antibiotics through the detection of the low limit of MFA. Meanwhile, the mechanism of interaction between the nano-lanthanum complex and MFA was studied and investigated.
Introduction
Macrofuran (MFA), a trade name for nitrofurantoin (NFT) antibiotic, is the most commonly used antibiotic in our country. The chemical structure is shown in Fig. S1.† It is a synthetic antimicrobial broad-spectrum antibiotic (nitrofuran family), used for treating the cases of urinary tract infections due to its sensitivity toward various Gram (+/−) bacteria (strains of E. coli, Enterococci, S. aureus and certain species of Klebsiella, Enterobacter and Proteus bacteria).1,2 It is used not only locally, but also worldwide for humans and animals. For example, it is used as a treatment for antibacterial and anti-protozoan infections, and preventing their growth promotion in the additive feed for aquaculture and livestock.3,4Unfortunately, some of the metabolites of the nitrofuran family antibiotics exhibit potential teratogenicity, cytotoxicity and environmental hazards. In addition, some of these antibiotics have a lot of side effects on pregnant women and cause some hepatic issues after short/long treatment courses.1 Therefore, the European Union (EU), USA and few other countries have prohibited the use and administration of any nitrofuran products like furazolidone in 1995 (ref. 3 and 5).
Due to the broad applications of NFT antibiotics as well as their vital disadvantages, many research studies desired for NFT and metabolization. Moreover, a large number of researchers are interested in the innovation and development of fast, simple, specific and sensitive analytical techniques and approaches for the detection of traces of these types of antibiotics. There are many analytical techniques for NFT detection such as electrochemical sensors,1,3,6 voltammetric method,5 spectrofluorimetric method,7,8 flow injection-spectrophotometry,9 chemiluminescence approach,10 surface enhanced Raman spectroscopy,11 and UHPLC-DAD assay.12 Nevertheless, most of the reported methods have many advantages and limitations.
On the other hand, the metal complexes especially at the nanoscale and nanomaterials generally have a lot of properties and advantages.13–18 They have a relatively large surface area with respect to particle size.19 In addition, numerous nanomaterials are highly magnetic, semi-conductive, excellent adsorbent materials, and have promising physicochemical properties. These make the nanomaterials very selective and sensitive tools for the detection of organic molecules and can be easily interacted with a biological target more efficiently than bulk materials.20 Lanthanum complexes and MOFs vital examples for metal complexes, incubated in a considerable analytical application. For example, it is used for the detection of ascorbic acid in pharmaceutical preparations,21 as chemosensors for nitrofurans,22 iron(III),23 and for anthelmintic drugs.24
In the present work, a novel nano-lanthanum complex was prepared via a simple reaction of lanthanum chloride with 1,2-phenylenediamine. The complex produced was characterized using many analytical techniques and then used as a chemosensor for pharmaceutical, biological and environmental applications. The results revealed that the photoluminescence (PL) emission spectrum of the nano-lanthanum complex was adeptly enhanced as the concentration of macrofuran antibiotic (MFA) increased. Therefore, the nano-lanthanum complex could be used profitably as a promising chemosensor for the determination of MFA concentration. No interference from some biological molecules and commonly administered drugs and even nitro-antibiotics with similar structures to MFA was observed. Additionally, a comparison between the previously published works with the present approach is performed. The comparison revealed that the detection of MFA based on the nano-lanthanum complex is faster, simpler, inexpensive, highly sensitive, more selective, portable, and has a wide linear range and lower detection limit than previous reports (at 0.025 ng mL−1 to 0.10 nM MFA) and is easier to operate. Additionally, the present approach is applicable in different real samples such as biological samples (serum, plasma and urine samples) and pharmaceutical formulation. The analytical and statistical reproducibility, repeatability, accuracy and precision of the developed chemosensor are investigated as well as the mechanism of enhancement. The chemical stability of the prepared nano-lanthanum complex was also investigated.
Results and discussion
Nano-lanthanum complex characterization
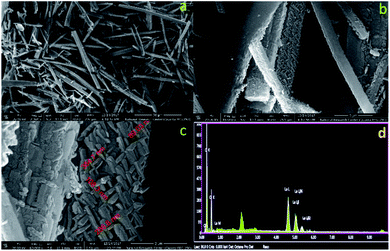
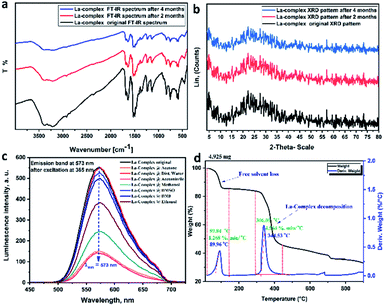
The nano-lanthanum complex was prepared via a simple reaction of lanthanum chloride with 1,2-phenylenediamine according to Scheme S1.† The obtained orange-red precipitate was filtered, washed and finally dried. The structure was elucidated based on the obtained analytical data and discussed as follows:![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C.28,29 The bands between 1129 and 1055 cm−1 are attributed to C–H. The band at 584 cm−1 is assigned to a mixture of the covalent and ionic bonding of lanthanum ion with nitrogen ν(La–N).30–33
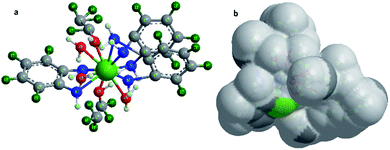
C.28,29 The bands between 1129 and 1055 cm−1 are attributed to C–H. The band at 584 cm−1 is assigned to a mixture of the covalent and ionic bonding of lanthanum ion with nitrogen ν(La–N).30–33Based on the above discussed physical, analytical and spectral data, we can deduce the 3D structure of the nano-lanthanum complex and the advanced molecular surface as represented in Fig. 2a and b, respectively.
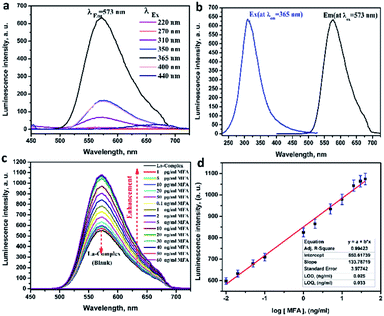
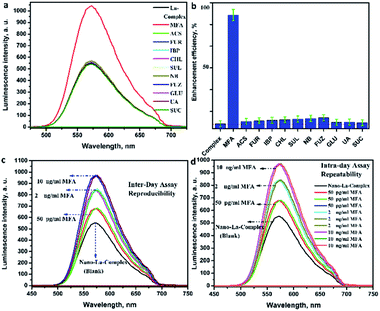
The nano-lanthanum complex was used as a spectrofluorimetric chemosensor for MFA. The PL spectrum (at λex = 365 nm) of the nano-lanthanum complex (10 mM) was investigated against different concentrations of MFA and the data is presented in Fig. 4c. As shown in Fig. 4c, the PL intensity spectrum of the nano-lanthanum complex was remarkably enhanced as the MFA concentration increased from 1 pg mL−1 to 60 ng mL−1. These results prove that the nano-lanthanum complex could be considered as a spectrofluorimetric chemosensor for MFA detection.
The calibration plot shows a linear relationship over a range of 0.02–30.0 ng mL−1. The fitting equations can be expressed as:
Nano-lanthanum PL intensity = 580.617 + 133.787![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[MFA] with r2 = 0.994. log[MFA] with r2 = 0.994. |
The LOD for the nano-lanthanum spectrofluorimetric chemosensor was found to be 0.025 ng mL−1, whereas the LOQ was 0.033 ng mL−1. A summary of the regression data analysis of PL is presented in Table S4.† From the above discussion and table data, the lower values of LOD and LOQ and wide linear range for the proposed chemosensor confirm its extreme sensitivity. Furthermore, the LOD and LOQ values are better and lower than the other previously published articles and the data are summarized in Table 1.
| Method | Linear detection range | LOD | Ref. |
|---|---|---|---|
| Electrochemical sensor based on lanthanum molybdate nanospheres | 0.01–144.0 μM | 72.00 nM | 1 |
| Electrochemical sensor based on reduced graphene oxide/Fe3O4 | 0.005–100.0 μM | 1.14 nM | 3 |
| Voltammetric method | 1.0–1000 nM | 0.15 nM | 5 |
| Electrochemical sensor based on molecularly imprinted copolymer | 0.001–0.05 μM; | 0.30 nM | 6 |
| 0.100–1.0 μM | |||
| Spectrofluorimetric method | 0.085–1.01 μM | 8.00 nM | 7 |
| Label-free photoluminescence assay | 0.05–4.00 μM | 30.00 nM | 8 |
| Flow injection-spectrophotometry | 0.021–1.27 nM | 8.03 nM; | 9 |
| 20.28 nM | |||
| Electrogenerated chemiluminescence | 10.0–100.0 μM | 0.60 μM | 10 |
| Surface enhanced Raman spectroscopy | 2.11–42.25 μM | 0.211 μM | 11 |
| UHPLC-DAD assay | 0.211–5.28 nM; | 0.114 nM; | 12 |
| 0.017–0.85 nM | 0.19 nM | ||
| Nano-lanthanum complex chemosensor | 0.02–30.0 ng mL−1 to (0.08–126.8 nM) | 0.025 ng mL−1 to (0.10 nM) | The present work |
The analysis of the output table data shows that the calculated relative error percent (RE%) values were between 1.0 and 1.003%; intra-day; and 0.943 and 1.004%; inter-day, which implies excessive accuracy, repeatability and reducibility of the proposed methodology. Moreover, the evaluated values of mean (X) were in target, the standard deviation (SD) were between 1.77 and 2.16%; intra-day; and 1.54 and 3.14%; inter-day, the coefficient of variation (CV) values are between 0.003 and 3.12%; intra-day; and 1.16 and 4.07%; inter-day. The extra-low values of SD as well CV imply the excellent precision, reducibility and repeatability of suggested methodology.
| Sample | Spiked MFAa (ng mL−1) | Founda (ng mL−1) | RSD% | Recovery% |
|---|---|---|---|---|
| a Each reading was repeated three times; RSD, relative standard deviation. | ||||
| Serum | 0.01 | 0.01 | 3.25 | 100.00 |
| 1.00 | 0.999 | 1.47 | 99.90 | |
| 30.00 | 30.40 | 3.25 | 101.30 | |
| Plasma | 0.01 | 0.009 | 3.57 | 90.00 |
| 1.00 | 0.972 | 2.57 | 97.20 | |
| 30.00 | 29.63 | 2.89 | 98.77 | |
| Urine | 0.01 | 0.011 | 2.12 | 105.00 |
| 1.00 | 0.97 | 0.998 | 97.30 | |
| 30.00 | 30.56 | 2.14 | 101.90 | |
The statistical evaluations for pharmaceutical formulation samples were carried out by the calculation of the percent recoveries (RC%) and relative standard deviation (RSD%). These data demonstrate that the average RC ± RSD% are 100.4 ± 2.66% for serum samples, 95.3 ± 3.01% for plasma samples, 101.4 ± 1.75% for urine samples, 95.44 ± 2.25%. The data obtained above demonstrate that the proposed methodology is applicable and effective for the quantification of MFA in different biological samples and pharmaceutical formulation. Moreover, this approach will be a potential critical analytical tool in the future, which will help, address and monitor a vital environmental issue and human health issue related to MFA quantification.
Experimental
Materials & instruments
See the (ESI file†) for details.Procedure
The pharmaceutical formulation (macrofuran capsules, 100 mg) i.e. five capsules from macrofuran were opened and carefully weighed under an inert atmosphere. A precise equivalent weight to 100 mg of macrofuran was transferred to a 50 mL beaker and dissolved efficiently in distilled water. Then, the solution was filtered and transferred to a volumetric flask (100 mL). The flask was filled to the mark with distilled water to obtain the stock solution from which the working solutions were prepared.
Conclusions
This work presents a spectrofluorimetric approach based on a novel promising nano-lanthanum complex, which was prepared and well characterized. The morphology of the prepared complex appeared to be uniform rods like those of the palm tree with an external surface consisting of nano-rods with an average size of 108 nm. Moreover, the chemical stability results showed significant stability of the nano-lanthanum complex. Therefore, the nano-lanthanum complex could be used profitably as a promising chemosensor for the determination of MFA concentration in pharmaceutical formulation and different biological samples (serum, plasma, and whole blood). The statistical evaluation of the proposed approach, in general, was fit for the purpose. The LOD for MFA was 0.025 ng mL−1, over a linear concentration range 0.02–30.0 ng mL−1, with r2 of 0.994. Comparison of the current work with the other published reports revealed this approach to be fast, simple, inexpensive, more sensitive, extremely selective, not time-consuming, with lower LOQ/LOD, wider dynamic linear ranges and applicability for different types of biological samples and pharmaceutical formulation. The results revealed a promising crucial tool for monitoring and quantification of MFA, which is a vital environmental and public human health issue.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support of the National Research Centre (NRC) Foundation of the internal project no. (E120908).Notes and references
- B. Karuppaiah, R. Ramachandran, S.-M. Chen, S. Wan-Ling and J. Y. Wan, New J. Chem., 2020, 44, 46–54 RSC.
- R. Gleckman, S. Alvarez and D. W. Joubert, Am. J. Hosp. Pharm., 1979, 36, 342–351 Search PubMed.
- B. He and J. Li, Anal. Methods, 2019, 11, 1427–1435 RSC.
- M. Vass, K. Hruska and M. Franek, Vet. Med. (Praha)., 2008, 53, 469–500 CrossRef.
- D. Dechtrirat, P. Yingyuad, P. Prajongtat, L. Chuenchom, C. Sriprachuabwong, A. Tuantranont and M. Tang, Microchim. Acta, 2018, 185, 261–270 CrossRef PubMed.
- M. Roushani and Z. Rahmati, J. Iran. Chem. Soc., 2019, 16, 999–1006 CrossRef CAS.
- T. S. Belal, J. Fluoresc., 2008, 18, 771–780 CrossRef CAS.
- Y. Wang, T. Chen, Q. Zhuang and Y. Ni, Talanta, 2018, 179, 409–413 CrossRef CAS PubMed.
- H. Hadi and M. Mouayed, J. Assoc. Arab Univ. Basic Appl. Sci., 2017, 24, 74–80 Search PubMed.
- N. Taokaenchan, T. Tangkuaram, P. Pookmanee, S. Phaisansuthichol, S. Kuimalee and S. Satienperakul, Biosens. Bioelectron., 2015, 66, 231–237 CrossRef CAS.
- Y. Zhang, Z. Yu, Z. Yue, J. Gao, S. Wu, Z. Zhang and G. Li, J. Raman Spectrosc., 2019, 50, 1094–1102 CrossRef CAS.
- R. A. Wijma, K. E. J. Hoogtanders, S. Croes, J. W. Mouton and R. J. M. Brüggemann, J. Pharm. Biomed. Anal., 2019, 174, 161–167 CrossRef CAS.
- S. M. Sheta, S. M. El-Sheikh and M. M. Abd-Elzaher, Appl. Organomet. Chem., 2019, 33, e5069 CrossRef.
- S. M. Sheta, S. M. El-Sheikh, M. M. Abd-Elzaher, S. R. Salem, H. A. Moussa, R. M. Mohamed and I. A. Mkhalid, Appl. Organomet. Chem., 2019, 33, e5249 CrossRef CAS.
- S. Liu, L. Wang, J. Tian, Y. Luo, G. Chang and A. M. Asiri, Chempluschem, 2012, 77, 23–26 CrossRef CAS.
- L. H. Abdel-Rahman, A. M. Abu-Dief, R. M. El-Khatib and S. M. Abdel-Fatah, Bioorg. Chem., 2016, 69, 140–152 CrossRef CAS.
- M. A. Akl, E. R. El-gharkawy, N. A. El-mahdy, S. M. El-sheikh and S. M. Sheta, Dalton Trans., 2020, 49, 15769–15778 RSC.
- M. Alhaddad and S. M. Sheta, ACS Omega, 2020, 5, 28296–28304 CrossRef CAS.
- S. M. Sheta, S. M. El-Sheikh and M. M. Abd-Elzaher, Dalton Trans., 2018, 47, 4847–4855 RSC.
- S. M. Sheta, M. A. Akl, E. Saad and E. R. H. El-gharkawy, RSC Adv., 2020, 10, 5853–5863 RSC.
- F. A. O. Olgun, D. Ozyurt, K. I. Berker, B. Demirata and R. Apak, J. Sci. Food Agric., 2014, 94, 2401–2408 CrossRef CAS.
- F. Zhang, H. Yao, T. Chu, G. Zhang, Y. Wang and Y. Yang, Chem.–Eur. J., 2017, 23, 10293–10300 CrossRef CAS PubMed.
- S. M. Sheta, S. M. El, S. Mohkles, M. A. Elzaher and A. R. Wassel, Appl. Organomet. Chem., 2019, 33, e4777 CrossRef.
- S. M. Derayea, A. A. Hamad, D. M. Nagy, D. A. Nour-eldeen, H. Refat, H. Ali and R. Ali, J. Mol. Liq., 2018, 272, 337–343 CrossRef CAS.
- K. Henrick, D. L. Kepert, E. Shewchuk, K. R. Trigwell and S. B. Wild, Aust. J. Chem., 1974, 27, 727–739 CrossRef CAS.
- S. M. Sheta, S. M. El-sheikh, D. I. Osman, A. M. Salem, O. I. Ali, F. A. Harraz, W. G. Shousha, M. A. Shoeib, S. M. Shawky and D. D. Dionysiou, Dalton Trans., 2020, 49, 8918–8926 RSC.
- A. S. Basaleh and S. M. Sheta, Anal. Bioanal. Chem., 2020, 412, 3153–3165 CrossRef CAS PubMed.
- B. M. Omkaramurthy and G. Krishnamurthy, Inorg. Nano-Met. Chem., 2019, 49, 375–384 CrossRef CAS.
- D. K. Yadav, R. Gupta, V. Ganesan, P. K. Sonkar and M. Yadav, ChemElectroChem, 2018, 5, 2612–2619 CrossRef CAS.
- A. I. Othman, I. R. Kaiss and A. H. Huda, Res. J. Chem. Environ., 2019, 23, 43–49 Search PubMed.
- A. S. Shawali, S. Elsheikh and C. Párkányi, J. Heterocycl. Chem., 2003, 40, 207–212 CrossRef CAS.
- A. S. Shawali, A. A. Elghandour and S. M. El-sheikh, J. Prakt. Chem., 2000, 342, 96–99 CrossRef CAS.
- S. M. El-shiekh, M. M. Abd-Elzaher and M. Eweis, Appl. Organomet. Chem., 2006, 20, 505–511 CrossRef CAS.
- K. Bikshalu, V. S. K. Reddy, P. C. S. Reddy and K. V. Rao, Int. J. Adv. Syst. Soc. Eng. Res., 2014, 4, 12–15 Search PubMed.
- S. M. Sheta, S. M. El-sheikh and M. M. Abd-elzaher, Anal. Bioanal. Chem., 2019, 411, 1339–1349 CrossRef CAS PubMed.
- A. S. Basaleh and S. M. Sheta, J. Inorg. Organomet. Polym. Mater., 2021 DOI:10.1007/s10904-021-01888-4.
- Y. Wu, W. Wu, L. Zou, J. Feng, C. Gu, B. Li, S. R. Batten, R. Yadav and A. Kumar, Inorg. Chem. Commun., 2016, 70, 160–163 CrossRef CAS.
- S. Pal, A. Bhunia, P. P. Jana, S. Dey, J. Möllmer, C. Janiak and H. P. Nayek, Chem.–Eur. J., 2015, 21, 2789–2792 CrossRef CAS PubMed.
- Y. Ito, K. Matsuda and Y. Kanemitsu, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 033309 CrossRef.
- M. M. Abd-Elzaher, M. A. Ahmed, A. B. Farag, M. S. Attia, A. O. Youssef and S. M. Sheta, Sens. Lett., 2017, 15, 977–981 CrossRef.
- M. M. Abd-Elzaher, M. A. Ahmed, A. B. Farag, M. S. Attia, A. O. Youssef and S. M. Sheta, Sens. Lett., 2017, 15, 525–530 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra10116h |
| This journal is © The Royal Society of Chemistry 2021 |