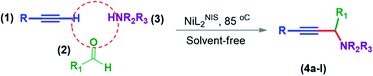Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biradical o-iminobenzosemiquinonato(1−) complexes of nickel(II): catalytic activity in three-component coupling of aldehydes, amines and alkynes†
Mina Nasibipoura,
Elham Safaei *a,
Ali Moaddeli
*a,
Ali Moaddeli a,
Marziyeh Sadat Masoumpour
a,
Marziyeh Sadat Masoumpour b and
Andrzej Wojtczakc
b and
Andrzej Wojtczakc
aDepartment of Chemistry, College of Sciences, Shiraz University, 71454, Shiraz, Iran
bDepartment of Chemistry, Estahban Higher Education Center, Estahban 74519-44655, Iran
cNicolaus Copernicus University, Faculty of Chemistry, 87-100 Torun, Poland
First published on 6th April 2021
Abstract
The six-coordinated bis-o-iminosemiquinone complex, NiL2BIS, in which LBIS is the o-iminosemiquinone 1-electron oxidized form of the tridentate o-aminophenol benzoxazole-based ligand H2LBAP, was synthesized and characterized. The crystal structure of the complex reveals octahedral geometry with a NiN4O2 coordination sphere in which Ni(II) has been surrounded by two tridentate LBIS ligands. This compound exhibits (SNi = 1) with both spin and orbital contribution to the magnetic moment and antiferromagnetic coupling between two electrons on two LBIS ligands which results in a triplet spin ground state (S = 1). The electronic transitions and the electrochemical behavior of this open-shell molecule are presented here, based on experimental observations and theoretical calculations. The electrochemical behavior of NiL2BIS was investigated by cyclic voltammetry and indicates ligand-centered redox processes. Three-component coupling of aldehydes, amines and alkynes (A3-coupling) was studied in the presence of the NiL2BIS complex, and the previously reported four-coordinated bis-o-iminosemiquinone NiL2NIS. Furthermore, among these two o-iminobenzosemiquinonato(1−) complexes of Ni(II) (NiL2NIS and NiL2BIS), NiL2NIS was found to be an efficient catalyst in A3-coupling at 85 °C under solvent-free conditions and can be recovered and reused for several cycles with a small decrease in activity.
Introduction
Ligands have the ability of reverse accepting and donating electrons and, therefore, modified redox transformations can be assumed as “electron reservoir” ligands. This property allows electron reservoir ligands to store redox equivalents and consequently, has generated important interest in their multi-electron reactivity features.2One of the classes of ligands which has electron reservoir ability, is redox-active ligands with different oxidation states. When some of these ligands are coordinated to some metal centers and produce complexes, both the metal and the ligand lack defined oxidation states. It means that the mentioned ligands bound to the metal ion could have a different oxidation state and significantly influence the oxidation state of the metal center. These ligands are known as (redox) non-innocent ligands. The interesting part in complexes of non-innocent ligands is that, the frontier orbitals of transition-metal and ligand are close in energy which leads to powerful mixing between these orbitals and it is difficult to assign the oxidation states to metal and ligand components alone.
The existence of redox-active ligands with different oxidation states and the cooperation of these ligands with the metal ion center causes the tuning of oxidation states of the central metal which is the key requirement to reach both developed catalytic activity and improved applicability of the overall complex in catalytic and enzymatic reactions.3
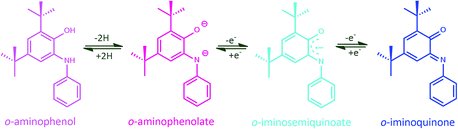
Among the numerous kinds of non-innocent ligands, o-amidophenolates due to their archetypal coordination abilities and spectroelectrochemical properties, have attracted significant attention. This non-innocent ligand can exist in the completely reduced closed-shell aromatic mono- or dianions of o-aminophenolate, or organic, open-shell radical (Srad = 1/2) of one-electron oxidized o-iminobenzosemiquinone or the closed-shell neutral fully oxidized o-iminobenzoquinone (Scheme 1).4
Therefore, designing and synthesis of the o-aminophenol ligand complexes as unique examples of non-innocent ligands, have been studied considerably and up to now, a number of examples of transition metal complexes (Cu, Pd, Ni, Ir, Ru, Os, Mo, V, Fe, Co, Mn) with two o-iminobenzoquinone ligands have been synthesized and characterized due to their structure properties and magnetism.5
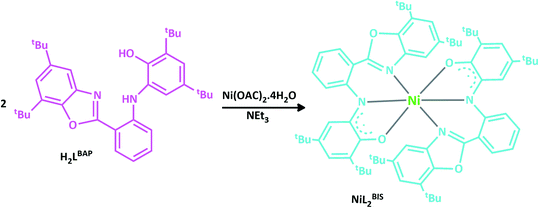
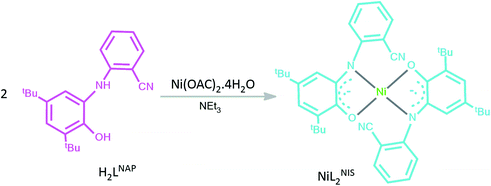
We present here the synthesis and characterization of nickel(II) complex, NiL2BIS, which combines two o-aminophenol benzoxazole-based ligands that acquire a non-innocent character (Scheme 2). NiII central atom in this complex and in some similar reported complexes of CuLBISX, X = (Cl, OAC),6a X=(Br−, I−, N3−, NO3−)6b and Cu(NNOISQ)6c is supported by deprotonated (o-iminosemiquinone) form of H2LBAP.
Reaction of Ni(OAC)2.4H2O with o-aminophenol H2LBAP in acetonitrile and in the presence of NEt3 leads to the formation of the desired complex.
As a part of our ongoing effort we try to investigate the catalytic activity of NiL2BIS complex and the previously reported four-coordinated bis-o-iminosemiquinone of NiL2NIS 1 (in which LNIS is the bidentate o-iminosemiquinone 1-electron oxidized by 2-amino-4,6-di-tert-butyl-phenol ligand of LNAP), (Scheme 3), and the comparison between these two Ni(II) iminosemiquinone complexes in synthesis of propargylamines from three-component coupling of aldehydes, amines and alkynes, A3-coupling reactions (Scheme 4).
 | ||
| Scheme 3 The structure of H2LNAP and the associated NiIIL2NIS complex.1 | ||
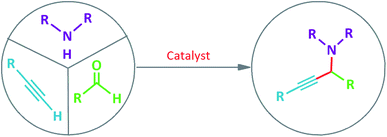
Propargylamines are useful building blocks for the synthesis of numerous nitrogen-containing heterocyclic compounds, and also important intermediates for preparation of natural complex products and active bio-molecules.7 Typically, propargylamines are synthesized by the nucleophilic addition of a metal alkynylide to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N electrophiles which often needs stoichiometric value of extremely active organometallic reagents like Grignard reagents,8 organolithium9 and organozinc reagents.10 Therefore, it is less attractive in terms of low endurance of functional groups, operational complexity and harsh reaction conditions.
N electrophiles which often needs stoichiometric value of extremely active organometallic reagents like Grignard reagents,8 organolithium9 and organozinc reagents.10 Therefore, it is less attractive in terms of low endurance of functional groups, operational complexity and harsh reaction conditions.
For the last decade, transition metal catalyzed coupling of aldehyde, alkyne, and amine that is usually referred as A3-coupling, has received greater attention due to its atom economy, step efficiency, and high chemical selectivity.11 This reaction was recommended to be carried out via the addition of in situ generated metal-alkynylide to iminium ion, that is formed in situ, via a reaction between amine and aldehyde and water molecule is the only side product.
Transition metal complexes, particularly coinage metal complexes (Ag, Cu and Au), and also In, Zn, Ni, Fe, Ir, Co, Mn, Bi, Hg and Cd have been established as the catalysts for this reaction, among which, there is an increasing interest for Ni catalysts due to their abundance and low costs. In this regard we decided to study the catalytic activity of two Ni(II) complexes for the mentioned reaction and compare their efficiency in A3-coupling based on our observations.
Results and discussion
Synthesis and general characterization of NiL2BIS
The o-aminophenol H2LBAP, was synthesized and purified according to the literature by the reaction of 2-amino benzyl amine and 3,5-di-tert-butyl-quinone (DTBQ) in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio.6a The complex NiL2BIS was synthesized in good yield, by stirring NiII(OAc)2·4H2O, H2LBAP and Et3N in CH3CN at room temperature. Slow evaporation from 1
1 molar ratio.6a The complex NiL2BIS was synthesized in good yield, by stirring NiII(OAc)2·4H2O, H2LBAP and Et3N in CH3CN at room temperature. Slow evaporation from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 MeOH/CH2Cl2 afforded NiL2BIS narrow cubic crystals.
2 MeOH/CH2Cl2 afforded NiL2BIS narrow cubic crystals.
The identification of the complex was confirmed by elemental analysis, IR and single-crystal X-ray structural analysis, temperature-dependent magnetic studies and cyclic voltammetry studies.
In the IR spectrum of the NiL2BIS complex, the sharp and strong νO–H and νN–H stretch band of the ligand disappears, which confirms the LBIS ligation to the Ni(II) center. All the vibrations of the ligand, ![[small nu, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e1dd.gif) = 1164 cm−1 (C–N stretching),
= 1164 cm−1 (C–N stretching), ![[small nu, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e1dd.gif) = 1614 cm−1 (C
= 1614 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching)
C stretching) ![[small nu, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e1dd.gif) = 1470 (C
= 1470 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching) and the tert-butyl groups bands at
N stretching) and the tert-butyl groups bands at ![[small nu, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e1dd.gif) = 2962 cm−1 exist in the IR spectrum of the complex, that confirms the presence of the ligand in the structure of complex (Fig. S1†).
= 2962 cm−1 exist in the IR spectrum of the complex, that confirms the presence of the ligand in the structure of complex (Fig. S1†).
Crystal structure of NiL2BIS complex
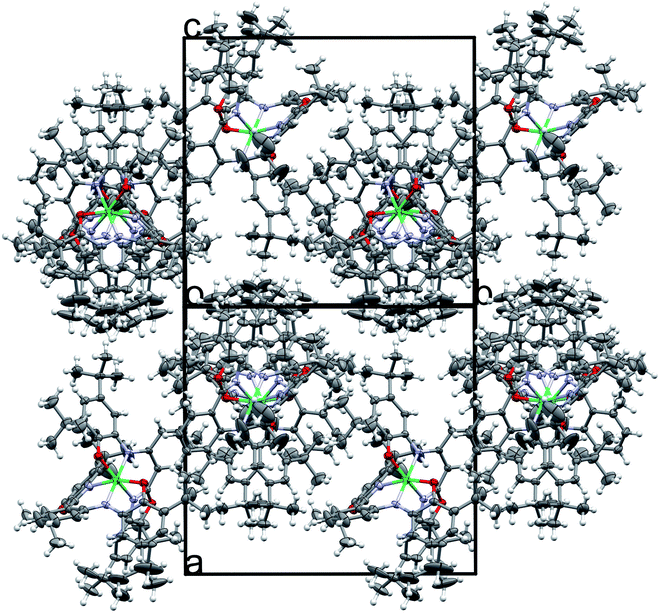
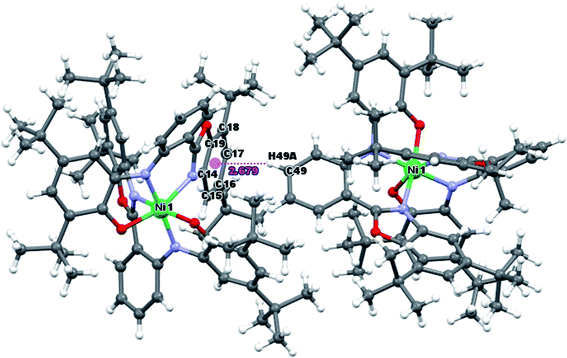
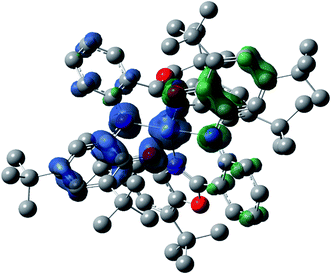
In Matte crimson crystals of NiL2BIS complex, the asymmetric unit of the reported structure consists of the single molecule of NiIIL2BIS (Fig. 1). The voids are found in the crystal lattice (solvent accessible voids, 18.4% of the unit cell) with no interpretable electron density (Fig. 2) (see Methods). The diffraction experiments and the structure refinement are summarized in Table 1. The selected bond lengths and angles are given in Table 2. The complete crystallographic data, bond lengths and angles and torsion angles are given in Table S1, S2 and S3 respectively.†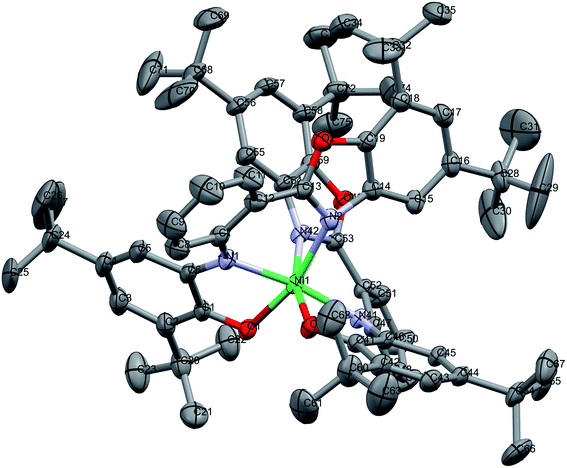 | ||
| Fig. 1 Molecular structure of NiIIL2BIS, H atoms have been omitted for clarity. Thermal ellipsoids are set at 30% probability. | ||
| Empirical formula | C70 H88 N4 Ni O4 |
| Formula weight | 1108.15 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| Unit cell dimensions | a = 18.1423(6), b = 17.5195(5), c = 23.9371(9) |
| α = 90, β = 106.001(3), γ = 90 | |
| Volume | 7313.5(4) Å3 |
| Z | 4 |
| Temperature | 293(2) K |
| Density (calculated) | 1.006 Mg/m3 |
| Crystal size | 0.793 × 0.564 × 0.517 mm3 |
| Absorption coefficient | 0.308 mm−1 |
| Reflections collected | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 213 213 |
| Independent reflections | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 548 [R(int) = 0.0442] 548 [R(int) = 0.0442] |
| Goodness-of-fit on F2 | 1.040 |
| Final R indices [I > 2 sigma(I)] | R1 = 0.0614, wR2 = 0.1426 |
| R indices (all data) | R1 = 0.1017, wR2 = 0.1725 |
| a Symmetry transformations used to generate equivalent atoms: #1 − x, −y, −z. | |||
|---|---|---|---|
| Ni1–N41 | 2.0193(19) | C2–C3 | 1.375(4) |
| Ni1–N1 | 2.027(2) | C4–C5 | 1.368(5) |
| Ni1–O41 | 2.0425(18) | C44–C45 | 1.375(4) |
| Ni1–O1 | 2.0466(19) | O1–C1 | 1.286(3) |
| Ni1–N2 | 2.143(2) | O41–C41 | 1.283(3) |
| Ni1–N42 | 2.153(2) | C6–N1 | 1.355(4) |
| N41–Ni1–N1 | 171.41(9) | C46–N41 | 1.363(3) |
| O1–Ni1–N2 | 163.62(8) | N1–C7 | 1.398(4) |
| N42–Ni1–O41 | 164.88(8) | N41–C47 | 1.394(3) |
| O1–Ni1–N1 | 80.16(8) | C53–N42–Ni1 | 120.77(16) |
| O41–Ni1–O1 | 102.55(8) | C54–N42–Ni1 | 131.18(17) |
The complex has two LBIS ligands coordinated to the central Ni(II) in the tridentate manner. Each ligand forms the coordination bonds via the phenolate O1, imine N1 and benzoxazole N2, with O1 and N2 positioned trans in the coordination sphere. Therefore, in the reported complex the octahedral coordination sphere NiN4O2 is found. Due to the relative rigidity of the LBIS molecules, two ligands form the structures approximately perpendicular to each other. The shortest coordination bonds are formed by imine N1 and N41 atoms of both ligands (Table 2). The phenolate O1 and O41 form slightly longer Ni–O bonds, the distances being 2.0466(19) and 2.0426(18) Å, respectively. The Ni bonds to benzoxazole N2 and N42 are longer by 0.14 Å from the ones formed by amine N1/N41. The angle between bonds formed by imine atoms N41–Ni1–N1 is 171.41(9)°. The other trans angles O1–Ni1–N2 163.62(8) and O41–Ni1–N42 164.88(8)° differ significantly from the expected 180°. The angles between the coordination bonds formed by atoms in cis positions range from N1–Ni1–O1 80.16(8)° to O41–Ni1–O1 102.55(8)° (Table 2). These values indicate significant deformation of the coordination octahedron, that can be attributed to the rigidity of the tridentate LBIS ligand.
The valence geometry of both LBIS ligands is almost identical. In both phenolic rings, bond lengths C2–C3 and C4–C5, and their equivalents range from C4–C5 1.368(5) to C44–C45 1.375(4) Å, indicating the localization of double bonds in these positions. These bonds are significantly shorter than other C–C bonds in the phenolic rings, which range from 1.427(5) to 1.466(4) Å and reveal significant contributions of single bonds (Table 2). The O1–C1 and O41–C41 bonds lengths of 1.286(3) and 1.283(3) Å, respectively, reveal their double rather than single bond character. The C6–N1 and C46–N41 bonds are 1.355(4) and 1.363(3) Å, respectively, and are shorter by 10σ than N1–C7 and N41–C47. Such distribution of the double bonds in the o-aminophenole fragment corresponds to o-iminosemiquinoate form of both LBIS ligands. That form seems to be consistent with both the bond distribution and the neutral charge of the complex with NiII center.
The tridentate coordination of LBIS causes the conformational adjustments resulting in the lack of planarity of the ligand. The additional factor affecting the conformation is the presence of bulky tBu substituents at the benzoxazole moieties. Their spatial arrangement in the complex molecule results in the intramolecular interactions of their methyl groups C34 to C69 and C31 to C74 (Fig. 1). The observed twist of both ligands can be quantified with the dihedral angles between best planes of the phenolic and benzoxazole moieties, being 58.82(12) and 46.53(12)° for ligand 1 and 2, respectively. The dihedral angles between the central phenyl ring and phenolic and benzoxazole rings are 51.66(17), 34.89(15)° and 49.01(14), 29.19(13)° for O1–O2 and O41–O42 ligands, respectively.
The intramolecular π⋯π interactions are detected. The benzoxazole five-membered heterocyclic moieties form the gravity centers Cg⋯Cg 3.8891(16) Å interaction. That results in the interactions of two benzoxazole moieties with the distance Cg⋯Cg of 3.8770(14) Å, with the dihedral angle between their planes being 39.21(10)°.
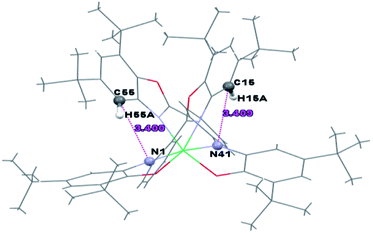
The intermolecular C–H⋯π interactions are detected between C49–H49A and C14–C19[3/2 − X, 1/2 + Y, 1/2 − Z] six-membered ring of benzoxazole, with the H⋯Cg distance 2.68 Å and C–H⋯Cg angle 158°. For the interactions of the C49–H49A group with the whole benzoxazole[3/2 − X, 1/2 + Y, 1/2 − Z] moiety, the corresponding values are 2.65 Å and C–H⋯Cg angle 160° (Fig. 3).
The intramolecular hydrogen bonds are shown in Table 3.
| a Symmetry transformations used to generate equivalent atoms. | |||||
|---|---|---|---|---|---|
 |
D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | <(DHA) |
| C(15)–H(15A)⋯N(41) | 0.93 | 2.70 | 3.409(4) | 133.7 | |
| C(55)–H(55A)⋯N(1) | 0.93 | 2.68 | 3.400(4) | 134.7 | |
Magnetic susceptibility measurements
Variable-temperature magnetic susceptibility measurement for crystalline samples of the ligand H2LBAP and NiL2BIS complex was carried out with an applied magnetic field of 1000 Oe in the temperature range 1.8–300 K (Fig. 4).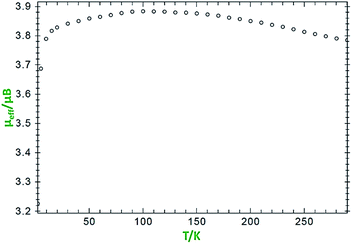 | ||
| Fig. 4 Variation of effective magnetic moment (μeff) with variation in temperature for the complex NiL2BIS. | ||
The effective magnetic moment values of NiL2BIS change from 3.7 BM (at 1 K) to 3.88 BM (at 125 K) and 3.78 (at 300 K). These values differ from that of the spin-only moment, which amounts to 2.83 BM. This difference between the measured and calculated values results from spin-orbital coupling and displays positive and commonly large deviations from the spin-only contribution of 2.83 BM. The reported compound exhibits (SNi = 1) because of the NiII center and antiferromagnetic coupling of both tridentate LBIS ligand radicals coordinated to Ni(II) ion whose spin alignment seems to be [(↑)–(↑↑)–(↓)] (Scheme 5). It indicates that the nickel complex exists in an octahedral triplet ground state. The ground state configuration of Ni(II) ion in a regular octahedral field is 3A2g(t62ge2g) and it will be paramagnetic with two unpaired electrons.12
Electrochemistry
The cyclic voltammetry (CV) electrochemical behavior of complex NiIIL2BIS has been recorded in CH2Cl2 solution containing 0.10 M NBu4ClO4 as supporting electrolyte at a glassy carbon as working electrode, and an Ag/AgNO3 reference electrode at room temperature.The electrochemical behavior of the complex NiIIL2BIS is similar to the previously studied o-iminobenzoquinone based complexes of Ni(II),1 owing to the presence of three one-electron ligand-centered redox transitions on the voltammogram (Fig. 5 and Table 4). The ligand-centered voltammograms are observed in the positive potential range showing radical-ligand based iminobenzosemiquinone/iminobenzoquinone (NiIIL2BIS/NiIILBISLBIQ and NiIILBISLBIQ/NiIIL2BIQ) redox couples and voltammograms observed in the negative potential range corresponding to iminobenzosemiquinone/amidophenoxide redox couples (NiIIL2BIS/NiIILBISLBAP) (Scheme 6).
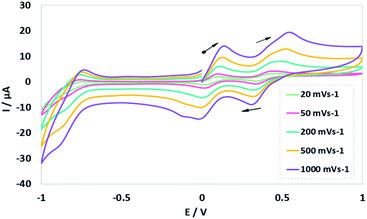 | ||
| Fig. 5 Cyclic voltammograms of NiL2BIS. Conditions: 1 mM complex, 0.1 M NBu4ClO4, scan rate 20, 50, 200, 500, 1000 mV s−1, CH2Cl2, 298 K. | ||
| Compound | E1/21/V | E1/23/V | E1/23/V |
|---|---|---|---|
| a The potential reported here is the average of anodic and cathodic peak potentials for a reversible process or the peak potential for an irreversible process. | |||
| NiL2BIS | −0.7 | 0.16 | 0.56 |
This process corresponds to the following equations (eqn (1)–(3)):
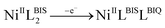 | (1) |
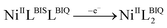 | (2) |
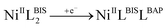 | (3) |
Electronic spectroscopy
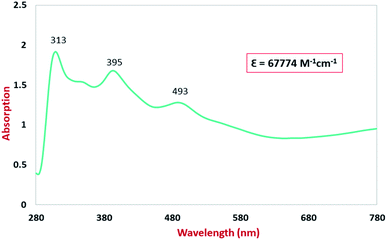
The UV-Vis/NIR spectra for the studied compound in CH2Cl2 solution were recorded in the range of 280–780 nm. The electronic spectrum of the ligand H2LBAP is shown in Fig. S2† and the electronic spectrum of the complex NiIIL2BIS is shown in Fig. 6. NiIIL2BIS exhibits four absorption bands in the visible, near infrared and near ultraviolet regions (Fig. 6). A ligand π → π* charge transfer was found for the complex in the ultraviolet field of the electronic spectra (313 nm). The electronic absorption band in region 395 nm is consistent with iminosemiquinone (π)-to-Ni–(dπ*), ligand-to-metal charge-transfer (LMCT) transition. The broad electronic absorption band in the region around 493 nm corresponded to metal-to-ligand (MLCT) charge transfer. Also, the extinction coefficient of the ligand and the Ni-complex have been extracted by the plot of the absorption maxima at a selected wavelength versus varied concentrations reply to Beer–Lambert law.We did the fluorescence experiment for the H2LBAP ligand and NiIIL2BIS complex. The ligand was emissive and the complex was non-emissive. The emission spectrum of the ligand is given in Fig. S2.†
Computational details
Description of the NiIIL2BIS structure
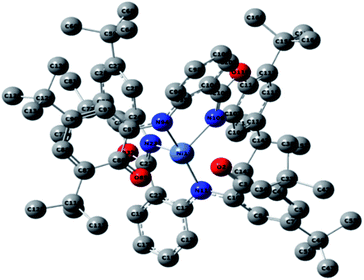
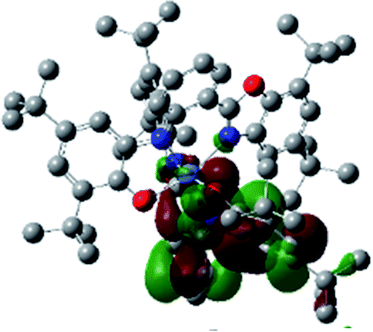
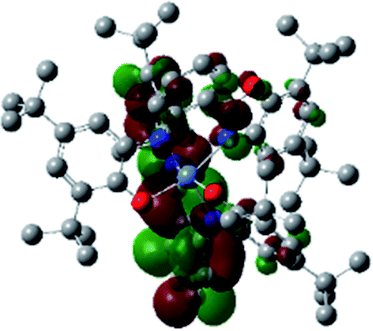
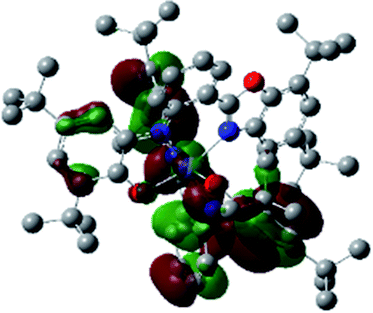
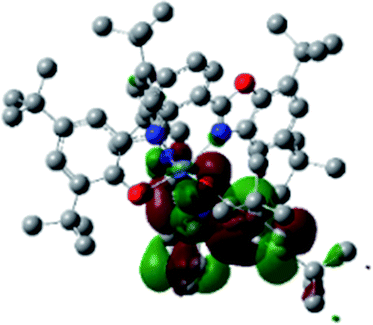
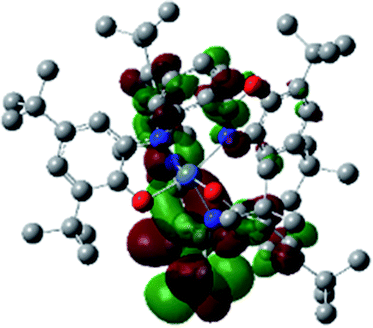
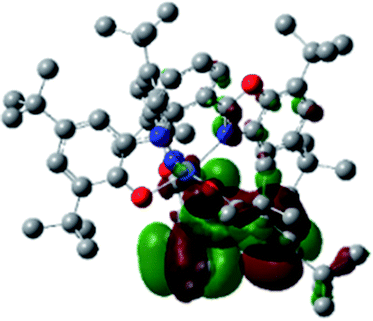
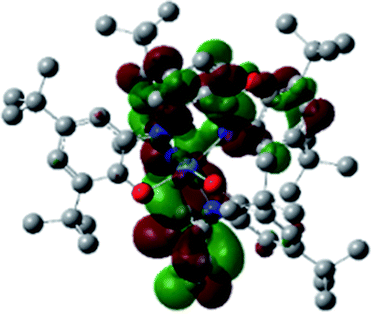
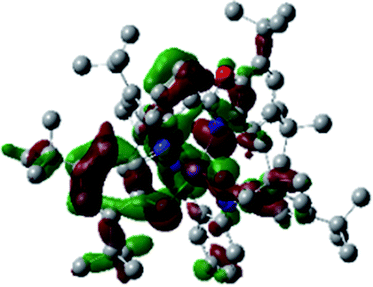
Optimized geometries were confirmed to be minima by the frequency analysis. The DFT calculation shows that triplet NiII octahedral complex, NiIIL2BIS, has the lowest energy. The singlet electronic structure is 33.4 kcal mol−1 higher in energy with respect to the triplet solution.The optimized structure of NiII octahedral complex is shown in Fig. 7 and in Table S4.† Some of the selected bond lengths are included. The root-mean-square deviation (RMSD) for the bond lengths from the crystal structure are in the order of 0.02 Å. Therefore, the optimized geometry of the complex is in good agreement with the experimental structure from X-ray crystallography. The C–O, C–N and aryl C–C bond distances of the redox-active fragments of both ligands are similar to the iminosemiquinone (LBIS)1− oxidation state reported by Brown.13 The predicted spin density for the NiIIL2BIS complex (Fig. 8) also shows the delocalization of α electron density over one ligand and β electron density over another ligand in agreement with the iminosemiquinone (LBIS)1− oxidation state for both ligands.
Theoretical analysis of the UV-Vis transitions for NiIIL2BIS
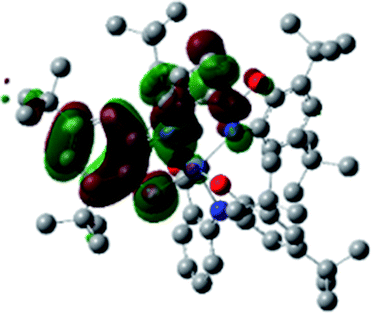
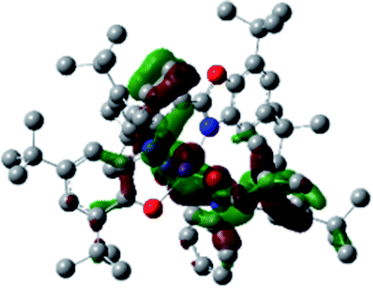
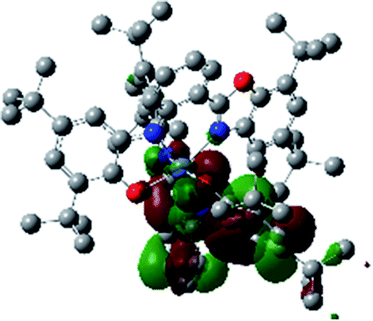
A detailed theoretical study of the UV-Vis electronic absorption spectrum of NiIIL2BIS complex was carried out using the TD-DFT approach to gain insight into the spectral features of the complex. In order to get an adequate comparison between the theoretical and experimental spectra, we performed self-consistent reaction field (SCRF) calculations in the presence of a solvent using the Polarizable Continuum Model (PCM).14 The solvent chosen was that used for the experimental spectrum, that is dichloromethane (CH2Cl2), with a dielectric constant ε = 8.94. The wavelengths, excitation energies, and oscillator strengths of the absorption bands calculated with the TD-DFT methodology are displayed in Table 5. For each transition, molecular orbitals with major contribution are also mentioned in Table 5. The nature of most of the TD-DFT transitions involve the excited states, that are not dominated by one singly excited configuration. For analyzing the nature of such electronic transitions, a Natural Transition Orbital (NTO) analysis15,16 has been performed as implemented in Gaussian 16 and the dominant NTO orbital pairs were calculated. The most important advantage of this procedure is that an electronic transition is readily attributed, for the majority of cases, to (at most) two NTO pairs (one occupied and one virtual) that qualitatively depict the electron density reorganization due to the excitation, thus facilitating the assignment of the transition nature. Table 6 gives the NTO pairs describing the transitions for the NiIIL2BIS complex. The two predicted lower energy bands are assigned as ligand-to-ligand charge transfer (LLCT) transitions (T14 and T34 states). These transitions can be identified as LLCT within the aromatic system of two ligands (π → π* transition). The highest-energy band (T61 state) has mixed character and the electron density migrates form metal-to-ligand (d → π* transition) or ligand to ligand (π → π* transition) groups (mixed MLCT/LLCT state).| Exp. λmax/nm | Tra | Major contribution | Energy/eV (nm) | Oscillator strength | Assignment |
|---|---|---|---|---|---|
| a Tr = transition number as obtained in the TD-DFT calculation output. | |||||
| 493 | 14 | HOMO(α) → LUMO+1(α) (41%) | 2.47 (501) | 0.1144 | LLCT |
| HOMO−3(β) → LUMO(β) (37%) | |||||
| 395 | 34 | HOMO−1(α) → LUMO+1(α) (10%) | 3.22 (384) | 0.0941 | LLCT |
| HOMO(α) → LUMO+3(α) (18%) | |||||
| HOMO−1(β) → LUMO+1(β) (24%) | |||||
| 313 | 61 | HOMO−15(β) → LUMO(β) (28%) | 3.92 (316) | 0.0376 | MLCT/LLCT |
| HOMO−14(β) → LUMO(β) (19%) | |||||
| HOMO−12(β) → LUMO(β) (12%) | |||||
Catalytic activity of NiIIL2BIS and NiIIL2NIS in A3-coupling reactions
General reaction of catalytic activity of NiIIL2BIS and NiIIL2NIS in A3-coupling reactions is shown in Scheme 7. This reaction was optimized for the various parameters such as temperature, solvent and catalyst loading.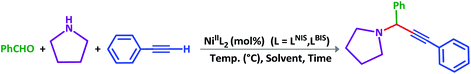 | ||
| Scheme 7 General reaction for testing the catalytic activity of NiIIL2BIS and NiIIL2NIS in A3-coupling reactions. | ||
The reaction temperature was initially optimized by performing the model reaction of benzaldehyde, pyrrolidine and phenylacetylene under solvent-free conditions at different temperatures (Table 7 entries 1–5). The model reaction was screened at RT in the presence of 2 mol% NiL2NIS catalyst, but a good yield was not obtained during 5 h. As the reaction temperature increased, the reaction time decreased, and the best result was obtained at 85 °C. With increasing temperature up to the optimized temperature (85 °C), a decrease in the yield of desired product was observed, that is related to the evaporation of the volatile precursors from the reaction media in solvent free conditions and also increasing by-products of the reaction.
| Entry | Catalyst | Temp. (°C) | Catalyst amount | Solvent | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|
| a All reactions were carried out with benzaldehyde (1 mmol), pyrrolidine (1.1 mmol), phenylacetylene (1.2 mmol). | ||||||
| 1 | NiL2NIS | RT | 2 mol% | — | 5 | 35 |
| 2 | NiL2NIS | 50 | 2 mol% | — | 3 | 43 |
| 3 | NiL2NIS | 70 | 2 mol% | 57 | ||
| 4 | NiL2NIS | 85 | 2 mol% | — | 2 | 86 |
| 5 | NiL2NIS | 100 | 2 mol% | — | 2 | 80 |
| 6 | NiL2NIS | 85 | 1 mol% | — | 3 | 67 |
| 7 | NiL2NIS | 85 | 3 mol% | — | 2 | 83 |
| 8 | Catalyst-free | 85 | — | — | 10 | NR |
| 9 | NiL2NIS | 100 | 2 mol% | PhCH3 | 4 | — |
| 10 | NiL2NIS | 80, reflux | 2 mol% | CH3CN | 4 | — |
| 11 | NiL2NIS | 65, reflux | 2 mol% | THF | 2 | 54 |
| 12 | NiL2BIS | 80 | 2 mol% | — | 3 | — |
| 13 | NiL2BIS | 90 | 3 mol% | — | 4 | 11 |
| 14 | NiL2BIS | 100 | 3 mol% | — | 4 | 15 |
| 15 | NiL2BIS | 100 | 4 mol% | — | 4 | 24 |
| 16 | NiL2BIS | 65, reflux | 4 mol% | THF | 4 | 10 |
To optimize the catalyst load, the model reaction was performed in the presence of various amounts of the catalyst 1–3 mol%. According to the results, 2 mol% NiL2NIS catalyst shows the best efficiency (Table 7, entries 4, 6–8). The effect of various solvents was also monitored by performing the model reaction in the presence of 2 mol% NiL2NIS catalyst (Table 7, entries 9–11).
Then we tried to investigate the A3-coupling reaction for the other NiII complex, NiL2BIS. It is noteworthy to mention that, this reaction did not happen in any measurable amount (Table 7, entries 12–16), which is due to the lack of free space in the coordination sphere of the six-coordinated NiL2BIS complex.
It other words, in the solution medium this complex keeps its stability and departure of ligands does not occur.
Also, a catalyst recycle experiment (Table 8) was done. The catalyst was recovered by centrifugation and the reaction was carried out for three cycles with a slight decrease in activity.
| Run | Time (h) | Yield (%) |
|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), pyrrolidine (1.1 mmol), phenylacetylene (1.2 mmol), NiL2NIS (2 mol%), solvent free, 85 °C. | ||
| 1 | 2 | 86 |
| 2 | 2 | 81 |
| 3 | 2 | 70 |
Encouraged by the optimization results, we turned our attention to various aldehydes and amines. Interestingly, various aldehydes reacted effectively with pyrrolidine, morpholine and piperidine.
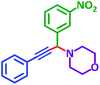
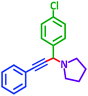
As exemplified in Table 9, this protocol is rather general for a wide variety of electron-rich as well as electron-deficient aromatic aldehydes and also various secondary amines. Results show the reaction was performed faster for the aldehydes bearing the electron-withdrawing group such as –NO2. In addition, pyrrolidine has the highest activity among the used amines (Table 9, entries e and g).
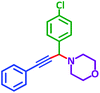
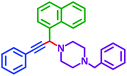
| Substrate (2) | Product (4) | Timeb | Yieldc | Substrate (2) | Product (4) | Timeb | Yieldc | ||
|---|---|---|---|---|---|---|---|---|---|
| Substrate (3) | Substrate (3) | ||||||||
| a Reaction conditions: aldehyde (1 mmol), phenylacetylene (1.2 mmol), amine (1.1 mmol), NiL2NIS (2 mol%) at 85 °C under solvent-free conditions.b Reactions time is based on the consumption of aldehyde monitored by TLC.c Isolated yield. | |||||||||
| a |  |
 |
2 h | 86% | g | 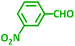 |
 |
2 h | 78% |
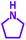 |
 |
||||||||
| b |  |
 |
2 h | 81% | h |  |
 |
4 h | 80% |
 |
 |
||||||||
| c |  |
 |
3 h | 83% | i |  |
 |
3 h | 84% |
 |
 |
||||||||
| d | 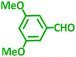 |
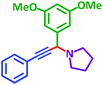 |
3 h | 88% | j |  |
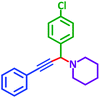 |
3 h | 86% |
 |
 |
||||||||
| e |  |
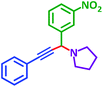 |
1 h | 75% | k |  |
 |
4 h | 86% |
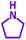 |
 |
||||||||
| f |  |
 |
3.5 h | 79% | l | 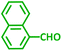 |
 |
4 h | 88% |
 |
 |
||||||||
Then we tried to compare the activity of our catalyst, NiL2NIS, and other catalysts which had been studied for A3-coupling in the literature (Table 10).17a–g To the best of our knowledge, there is only one more report of the Ni-catalyzed three-component coupling of aldehyde, alkyne and amine (Table 10, entry 1).
| Catalyst (amount) | Reaction condition: T (h)/temp. °C/solvent | AA (%) | Ref. |
|---|---|---|---|
| a (pip = (2-picolyliminomethyl)pyrrole anion). | |||
| Ni–Y zeolite (20 mg) | 4/80/— | 97 | 17a |
| Cyclohexanecarbaldehyde/morpholine/phenylacetylene | |||
| CuI2(pip)2a (0.4 mol%) | 2/110/toluene | 86 | 17b |
| Pyrrolidine/benzaldehyde/phenylacetylene | |||
| Ag/Ni-MOF (0.3 mol%) N2 (1 atm) | 30 min/80/MeCN | 93 | 17c |
| Pyrrolidine/aldehyde/phenylacetylene | |||
| CuI (10 mol%) | 12/100/PEG-400 | 92 | 17d |
| Pyrrolidine/benzaldehyde/phenylacetylene | |||
| Cu(OTF)2 (10 mol%) | 6/80/toluene | 92 | 17e |
| Pyrrolidine/methyl aldehyde/n-hexynyle | |||
| Au NPs (10 mol%) | 5/75/ACN | 97 | 17f |
| Piperidine/benzaldehyde/phenylacetylene | |||
| CuCl/succinic acid (20 mol%) | 3/50/DCM | 85 | 17g |
| Piperidine/benzaldehyde/1-phenyl-2-trimethylsilylacetylene | |||
| NiL2NIS (2 mol%) | 2/85/— | 86 | This work |
| Pyrrolidine/benzaldehyde/phenylacetylene | |||
Also, the mechanism involved in our Ni complex-catalyzed A3-coupling reaction can be different from the reaction mechanism which is dominant in other complex-catalyzed cross-coupling reactions due to the ability of NiL2NIS to present radical species.
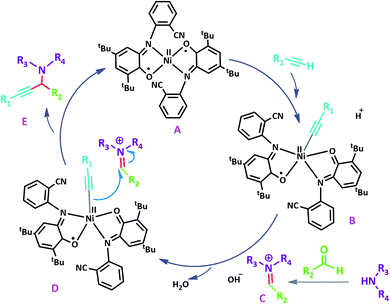
Both Ni0 and NiII species are used as Ni sources in Ni-catalyzed cross-couplings and Ni0 sources are generally considered as the catalytically active ones. While the easiest way is to use Ni0 reagents like Ni-(COD)2 and Ni(PPh3)4,18 these nickel sources are difficult to handle because of the high air sensitivity and thermal instability. Alternatively, NiII complexes are more convenient as pre-catalysts in terms of their availability and easy handling. Nevertheless, these NiII catalysts should be activated in situ with some additives19 such as base, I2 or PPh3 or used as bimetallic systems of Ni(II)/M(Zn(0), Mn(0), Cu(I), Ag(I). In some cases, Ni complex itself can act as the active catalyst (Table 10, entry 1)19a by cleavage of one of the oxo bridges of the ligand and generating an initial nickel(II) acetylide intermediate in A3-coupling reactions. Also, our Ni complex NiL2NIS can easily undergo a switch among the different oxidation states of the ligand easily. This event supports the non-innocent behavior of o-aminophenol ligand that can act as an “electron reservoir” and accept and donate electrons in a reverse catalytic cycle, result in a high reactivity. This behavior of NiL2NIS is well utilized in the homo-coupling of phenyl acetylene.1
A plausible reaction pathway is proposed as shown in Scheme 8. In the present work NiL2NIS itself acts as the active catalyst generating an initial nickel(II) acetylide intermediate B by the reaction between a Ni(II) center in the NiL2NIS complex with a terminal alkyne. One of the non-innocent iminosemiquinone LNIS ligands undergoes changes in its oxidation state and iminosemiquinonate/iminoquinonate form of the NiII complex, [NiIILNISLNIQ]+, (B), is achieved and keeps the total oxidation state unchanged. Moreover, complexation of this nickel acetylide intermediate with iminium ion, C, which is generated in situ from the aldehyde and amine, provides complex D and Ni acetylide complex coordinated with an iminium ion. Finally, the addition of acetylide to the iminium ion within the coordination sphere of nickel(II) gives propargylamine product E and regenerates the nickel catalyst A for the subsequent reactions.
Experimental
Material and method
All reagents were acquired via commercial sources unless stated otherwise. 3,5-DTBQ (3,5-di-tert-butylcyclohexa-3,5-diene-1,2-dione) was synthesized from the procedure reported in literature.20 The ligand H2LBAP was synthesized following the reported procedure.6aElemental analyses (C, H, and N) were done by the Elementar, Vario EL III. Fourier transform infrared spectroscopy with KBr pellets was performed on a FT IR Bruker Vector 22 instrument. NMR spectra were performed at 400 MHz on a Bruker DRX spectrometer in CDCl3 solution. UV-Vis absorbance spectra was recorded by using a CARY 100 Bio spectrophotometer. Cyclic voltammetry (CV) was carried out on a PAR-263A potentiometer. The cell was prepared with an Ag wire reference electrode, a glassy carbon working electrode, and a Pt counter electrode with 0.1 M NBu4ClO4 (TBAP) solutions in CH2Cl2. Ferrocene was used as an internal standard. The magnetic measurements were achieved with the use of a Quantum Design SQUID magnetometer MPMS-XL between 1.8 and 290 K with a dc applied field of 1000 Oe. Measurement was done on polycrystalline sample of 35 mg for NiL2BIS. Sample for X-band measurement was placed in 4 mm outer-diameter sample tubes with sample volumes of ∼300 μL.
All catalytic reactions were monitored by TLC (thin layer chromatography) and all yields refer to isolated products. 1H NMR spectra were recorded in CDCl3 on a Bruker DRX-400 AVANCE (400 MHz for 1H and 100 MHz for 13C) spectrometer.
Synthesis
NiIIL2BIS: synthesis of the ligand (H2LBAP), 2,4-di-tert-butyl-6-(2-(5,7-di-tert-butylbenzo [d] oxazol-2-yl) phenylamino) phenol, used in this work has previously been reported by our group.6aTo a magnetically stirred mixture of H2LBAP (0.524 g; 1 mmol) and Et3N in acetonitrile (5 mL), NiII(OAc)2·4H2O (0.248 g, 1 mmol) was added drop-wise and the resulting mixture was stirred for 3 h. The dark green precipitate that formed was collected by filtration, washed with cold MeOH and single crystals of suitable dimensions for X-ray analysis obtained from recrystallization of a concentrated solution of the microcrystalline solid in MeOH–CH2Cl2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) mixture. Yield: (78%). Anal. Calcd (%) for C49H68NiN3O4: C 74.02 (74.93), H 8.51 (8.81), N 4.83 (4.86). νmax(KBr)/cm−1: 2962 (C–H), 1614 (C
1; v/v) mixture. Yield: (78%). Anal. Calcd (%) for C49H68NiN3O4: C 74.02 (74.93), H 8.51 (8.81), N 4.83 (4.86). νmax(KBr)/cm−1: 2962 (C–H), 1614 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1470 (C
C), 1470 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1268 (C–O), 1164 (C–N) (Fig. S1†).
N), 1268 (C–O), 1164 (C–N) (Fig. S1†).
X-ray crystallography
The X-ray data were collected with the Oxford Sapphire CCD diffractometer, at 293(2) K using MoKα radiation λ = 0.71073 Å. The structure was solved by direct methods with SHELXT and refined with the full-matrix least-squares method on F2 with the use of SHELX2017 program package.21 The analytical absorption correction was applied by CrysAlis 171.38.43 package of programs.22 The large voids are found in the structure, covering 18.4% of the unit cell volume, but no significant density peaks have been located there. For the solvent accessible volume 1344 Å3, 24 electrons were found. Therefore, to account for the disordered solvent contribution to the structure factors, the SQUEEZE (Version 160617) program was used.23 The hydrogen atom positions were determined from the difference maps, and all hydrogen atoms were constrained during refinement. Four tBu groups (C24, C28, C32 and C68) revealed the rotational disorder. The attempt to define the discrete odel forthat disorder has given no improvement in the model quality. Therefore, the SIMU constraints for the disordered tBu groups and ISOR constraint the C25 methyl group were used in the final refinement. A summary of the crystal data and refinement details for compound 1 are given in Table 1. The structural data have been deposited at the Cambridge Crystallographic Data Center: (CCDC no. 2035886).Computational details
Geometry optimization was performed on the Ni complex using the hybrid B3LYP method, with LANL2DZ basis set for nickel and 6-31G* basis set for all other atoms, using the program Gaussian 16.24General procedure for the synthesis of propargylamine derivatives
In a typical reaction benzaldehyde (1 mmol), pyrrolidine (1.1 mmol), phenylacetylene (1.2 mmol) and catalyst, NiIIL2NIS, (2 mol%, 19 mg) were added into a 5 mL round bottom flask and were stirred at 85 °C. The progress of the reaction was monitored by TLC. After completion of the reaction, n-hexane (2 mL) was added to the reaction mixture and the solution was centrifuged at 3000 rpm for 2 min. Finally, the excess of solvent was removed under reduced pressure to give the corresponding product. Further purification was achieved by thin layer chromatography on silica gel using n-hexane/ethyl acetate. Then, the recovered catalyst was reused in recycle experiment of A3 coupling reaction of benzaldehyde, pyrrolidine and phenylacetylene. Physical and spectroscopic data for selected compounds are given in Fig. S3 to S16†).Conclusion
Complex NiIIL2BIS was synthesized and characterized in the current work. A combination of experimental and theoretical studies is used to investigate the electronic properties of this nickel complex. The synthesis of NiIIL2BIS neutral complex was achieved by ligation of the o-iminosemiquinone 1-electron oxidized form of the tridentate o-aminophenol benzoxazole-based ligand H2LBAP and characterized by X-ray crystallography. The bond lengths of the LBIS ligand indicate that is found in the semiquinone form. This observation is also supported by the electronic configuration as determined by density functional theory calculations.Then the catalytic activity of this complex, NiL2BIS, and the previously reported complex, NiL2NIS1 in three-component coupling of aldehydes, amines and alkynes (A3-coupling) was investigated and the four-coordinated NiL2NIS complex was found to be significantly more efficient catalyst due to the non-innocent o-aminophenol ligand that acts as an “electron reservoir” and can accept and donate electrons in the C–H activation stage of catalytic cycle resulting a high reactivity in A3-coupling reaction. This procedure is also environmentally friendly and is done under solvent-free conditions as it does not require any organic solvents. Good yields and mild reaction conditions are other remarkable advantages of this process. The catalyst can be readily recovered and reused for three cycles with a slight decrease in activity.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
E. Safaei is appreciate Shiraz University for financial support. A. Wojtczak acknowledges the partial support of the BRAIN Center of the Excellence in Research at the N. Copernicus University.References
- M. Nasibipour, E. Safaei, M. S. Masoumpour and A. Wojtczak, RSC Adv., 2020, 10, 24176–24189 RSC.
- (a) B. De Bruin, P. Gualco, N. D. Paul, in Ligand Design in Metal Chemistry: Reactivity and Catalysis, John Wiley & Sons, 2016, pp. 176–204 Search PubMed; (b) W. Kaim, Inorg. Chem., 2011, 50, 9752–9765 CrossRef CAS PubMed; (c) O. R. Luca and R. H. Crabtree, Chem. Soc. Rev., 2013, 42, 1440–1459 RSC; (d) V. Lyaskovskyy and B. de Bruin, ACS Catal., 2012, 2, 270–279 CrossRef CAS; (e) W. I. Dzik, J. I. van der Vlugt, J. N. H. Reek and B. de Bruin, Angew. Chem., Int. Ed., 2011, 50, 3356–3358 CrossRef CAS PubMed; (f) P. J. Chirik and K. Wieghardt, Science, 2010, 327, 794–795 CrossRef CAS PubMed.
- (a) D. L. J. Broere, R. Plessius and J. I. van der Vlugt, Chem. Soc. Rev., 2015, 44, 6886–6915 RSC; (b) P. J. Chirik, Inorg. Chem., 2011, 50, 9737–9740 CrossRef CAS PubMed; (c) A. I. Poddel’sky, V. K. Cherkasov and G. A. Abakumov, Coord. Chem. Rev., 2009, 253, 291–324 CrossRef; (d) L. A. Berben, B. de Bruin and A. F. Heyduk, Chem. Commun., 2015, 51, 1553–1554 RSC.
- (a) C. N. Verani, S. Gallert, E. Bill, T. Weyhermüller, K. Wieghardt and P. Chaudhuri, Chem. Commun., 1999, 17, 1747–1748 RSC; (b) P. Chaudhuri, C. N. Verani, E. Bill, E. Bothe, T. Weyhermüller and K. Wieghardt, J. Am. Chem. Soc., 2001, 123, 2213–2223 CrossRef CAS PubMed; (c) H. Chun, C. N. Verani, P. Chaudhuri, E. Bothe, E. Bill, T. Weyhermüller and K. Wieghardt, Inorg. Chem., 2001, 40, 4157–4166 CrossRef CAS PubMed; (d) H. Chun, T. Weyhermüller, E. Bill and K. Wieghardt, Angew. Chem., Int. Ed., 2001, 40, 2489–2492 CrossRef CAS.
- (a) P. Sarkar, M. K. Mondal, A. Sarmah, S. Maity and C. Mukherjee, Inorg. Chem., 2017, 56, 8068–8077 CrossRef CAS PubMed; (b) J. Jacquet, E. Salanouve, M. Orio, H. Vezin, S. Blanchard, E. Derat, M. Desage-El Murr and L. Fensterbank, Chem. Commun., 2014, 50, 10394–10397 RSC; (c) M. Nasibipour, E. Safaei, G. Wrzeszcz and A. Wojtczak, New J. Chem., 2020, 44, 4426–4439 RSC; (d) K. M. Conner, A. L. Perugini, M. Malabute and S. N. Brown, Inorg. Chem., 2018, 57, 3272–3286 CrossRef CAS PubMed; (e) P. Sarkar, S. Ghorai, G. C. Paul, M. Khannam, S. Barman and C. Mukherjee, Inorg. Chim. Acta., 2020, 502, 119340 CrossRef CAS; (f) M. Bubrin, A. Paretzki, R. Hübner, K. Beyer, B. Schwederski, P. Neugebauer, S. Záliš and W. Kaim, ýZ. Anorg. Allg. Chem., 2017, 643, 1621–1627 CrossRef CAS.
- (a) S. E. Balaghi, E. Safaei, L. Chiang, E. W. Y. Wong, D. Savard, R. M. Clarke and T. Storr, Dalton Trans., 2013, 42, 6829–6839 RSC; (b) S. E. Balaghi, E. Safaei, M. Rafiee and M. H. Kowsari, Polyhedron, 2012, 47, 94–103 CrossRef CAS; (c) E. Safaei, H. Bahrami, A. Wojtczak, S. Alavi and Z. Jagličić, Polyhedron, 2017, 122, 219–227 CrossRef CAS; (d) R. Saeedi, E. Safaei, Y. I. Lee and J. Lužnik, Appl. Organomet. Chem., 2019, 33, e4781 CrossRef.
- (a) R. K. Arigela, R. Kumar, S. Samala, S. Gupta and B. Kundu, Eur. J. Org. Chem., 2014, 2014, 6057–6066 CrossRef CAS; (b) C. E. Meyet and C. H. Larsen, J. Org. Chem., 2014, 79, 9835–9841 CrossRef CAS PubMed; (c) G. Naresh, R. Kant and T. Narender, Org. Lett., 2014, 16, 4528–4531 CrossRef CAS PubMed; (d) A. Ranjan, R. Yerande, P. B. Wakchaure, S. G. Yerande and D. H. Dethe, Org. Lett., 2014, 16, 5788–5791 CrossRef CAS PubMed; (e) Y. Xia, L. Y. Chen, S. Lv, Z. Sun and B. Wang, J. Org. Chem., 2014, 79, 9818–9825 CrossRef CAS PubMed; (f) O. P. Pereshivko, V. A. Peshkov, J. Jacobs, L. Van Meervelt and E. V. Van der Eycken, Adv. Synth. Catal., 2013, 355, 781–789 CrossRef CAS; (g) A. Monleón, G. Blay, L. R. Domingo, M. C. Muñoz and J. R. Pedro, Chem.–Eur. J., 2013, 19, 14852–14860 CrossRef PubMed.
- B. L. Chen, B. Wang and G. Q. Lin, J. Org. Chem., 2010, 75, 941–944 CrossRef CAS PubMed.
- (a) P. Kaur, G. Shakya, H. Sun, Y. Pan and G. Li, Org. Biomol. Chem., 2010, 8, 1091–1096 RSC; (b) K. B. Aubrecht, M. D. Winemiller and D. B. Collum, J. Am. Chem. Soc., 2000, 122, 11084–11089 CrossRef CAS.
- (a) L. Zani, S. Alesi, P. G. Cozzi and C. Bolm, J. Org. Chem., 2006, 71, 1558–1562 CrossRef CAS PubMed; (b) G. Huang, Z. Yin and X. Zhang, Chem.–Eur. J., 2013, 19, 11992–11998 CrossRef CAS PubMed; (c) G. Huang, Z. Yin and X. Zhang, Chem.–Eur. J., 2013, 19, 12579 CrossRef CAS; (d) G. Blay, E. Ceballos, A. Monleón and J. R. Pedro, Tetrahedron, 2012, 68, 2128–2134 CrossRef CAS; (e) W. Yan, B. Mao, S. Zhu, X. Jiang, Z. Liu and R. Wang, Eur. J. Org. Chem., 2009, 2009, 3790–3794 CrossRef.
- (a) E. Loukopoulos, M. Kallitsakis, N. Tsoureas, A. Abdul-Sada, N. F. Chilton, I. N. Lykakis and G. E. Kostakis, Inorg. Chem., 2017, 56, 4898–4910 CrossRef CAS PubMed; (b) S. I. Sampani, V. Zdorichenko, M. Danopoulou, M. C. Leech, K. Lam, A. Abdul-Sada, B. Cox, G. J. Tizzard, S. J. Coles, A. Tsipis and G. E. Kostakis, Dalton Trans., 2020, 49, 289–299 RSC; (c) K. Peewasan, M. P. Merkel, O. Fuhr, C. E. Anson and A. K. Powell, RSC Adv., 2020, 10, 40739–40744 RSC; (d) N. Uhlig, W. J. Yoo, L. Zhao, C. J. Li, in Modern Alkyne Chemistry: Catalytic and Atom- Economic Transformations, ed. B. M. Trost and C. J. Li, Wiley-VCH, Weinheim, 2014, pp. 239–268 Search PubMed; (e) G. Abbiati and E. Rossi, Beilstein J. Org. Chem., 2014, 10, 481 CrossRef PubMed; (f) V. A. Peshkov, O. P. Pereshivko and E. V. Van der Eycken, Chem. Soc. Rev., 2012, 41, 3790–3807 RSC.
- (a) R. C. Khulbe, R. P. Singh and Y. K. Bhoon, Transition Met. Chem., 1983, 8, 59–61 CrossRef CAS; (b) A. HASSAAN, J. Islamic Acad. Sci., 1990, 3, 269–272 Search PubMed.
- S. N. Brown, Inorg. Chem., 2012, 51, 1251–1260 CrossRef CAS PubMed.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed.
- A. V. Luzanov, A. A. Sukhorukov and V. É. Umanskii, Theor. Exp. Chem., 1976, 10, 354–361 CrossRef.
- R. L. Martin, J. Chem. Phys., 2003, 118, 4775–4777 CrossRef CAS.
- (a) K. Namitharan and K. Pitchumani, Eur. J. Org. Chem., 2010, 2010, 411–415 CrossRef; (b) H. B. Chen, Y. Zhao and Y. Liao, RSC Adv., 2015, 5, 37737–37741 RSC; (c) S. Wang, X. He, L. Song and Z. Wang, Synlett, 2009, 2009, 447–450 CrossRef; (d) Q. Zhang, J. X. Chen, W. X. Gao, J. C. Ding and H. Y. Wu, Appl. Organomet. Chem., 2010, 24, 809–812 CrossRef CAS; (e) C. E. Meyet, C. J. Pierce and C. H. Larsen, Org. Lett., 2012, 14, 964–967 CrossRef CAS PubMed; (f) M. Nasrollahzadeh and S. M. Sajadi, RSC Adv., 2015, 5, 46240–46246 RSC; (g) I. M. de Oliveira, D. C. Pimenta, J. Zukerman-Schpector, H. A. Stefani and F. Manarin, New J. Chem., 2018, 42, 10118–10123 RSC.
- (a) M. Zembayashi, K. Tamao, J. I. Yoshida and M. Kumada, Tetrahedron Lett., 1997, 18, 4089–4091 CrossRef; (b) J. D. Crowley, S. M. Goldup, N. D. Gowans, D. A. Leigh, V. E. Ronaldson and A. M. Z. Slawin, J. Am. Chem. Soc., 2010, 132, 6243–6248 CrossRef CAS PubMed; (c) K. M. Miller, C. Molinaro and T. F. Jamison, Tetrahedron: Asymmetry, 2003, 14, 3619–3625 CrossRef CAS.
- (a) X. H. Fan and L. ,M. Yang, Eur. J. Org. Chem., 2011, 2011, 1467–1471 CrossRef; (b) M. Wang, X. Yuan, H. Li, L. Ren, Z. Sun, Y. Hou and W. Chu, Catal. Commun., 2015, 58, 154–157 CrossRef CAS; (c) C. Chen and L. M. Yang, Tetrahedron Lett., 2007, 48, 2427–2430 CrossRef CAS; (d) V. Percec, J. Y. Bae and D. H. Hill, J. Org. Chem., 1995, 60, 1060–1065 CrossRef CAS; (e) J. C. Galland, M. Savignac and J. P. Genêt, Tetrahedron Lett., 1999, 40, 2323–2326 CrossRef CAS; (f) S. Saito, M. Sakai and N. Miyaura, Tetrahedron Lett., 1996, 37, 2993–2996 CrossRef CAS; (g) S. Saito, S. Oh-tani and N. Miyaura, J. Org. Chem., 1997, 62, 8024–8030 CrossRef CAS PubMed; (h) A. M. Oertel, V. Ritleng and M. J. Chetcuti, Organometallics, 2012, 31, 2829–2840 CrossRef CAS; (i) T. Shimasaki, Y. Konno, M. Tobisu and N. Chatani, Org. Lett., 2009, 11, 4890–4892 CrossRef CAS PubMed; (j) A. H. Christian, P. Müller and S. Monfette, Organometallics, 2014, 33, 2134–2137 CrossRef CAS; (k) F. Alonso, P. Riente and M. Yus, Arkivoc, 2008, 4, 8–15 Search PubMed; (l) S. Ghosh in A Mechanistic Insight into the Nickel-Catalyzed Homocoupling Reaction of Terminal Alkynes, Technische Universität Kaiserslautern, 2018, p. 208 Search PubMed; (m) A. P. Bhat and B. R. Bhat, Appl. Organomet. Chem., 2014, 28, 383–388 CrossRef CAS.
- T. M. Khomenko, O. V. Salomatina, S. Yu. Kurbakova, I. V. Il’ina, K. P. Volcho, N. I. Komarova, D. V. Korchagina, N. F. Salakhutdinov and A. G. Tolstikov, Russ. J. Org. Chem., 2006, 42, 1653–1661 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- CrysAlisPro 171.38.43 package of programs. Rigaku Oxford Diffraction, 2015.
- A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS PubMed.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, G. Petersson and H. Nakatsuji, et al., Gaussian 16, Revision A. 03, 2016.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2035886. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra10248b |
| This journal is © The Royal Society of Chemistry 2021 |