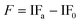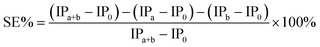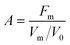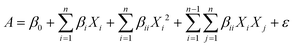Open Access Article
Open Access ArticleScreening of highly effective mixed natural antioxidants to improve the oxidative stability of microalgal DHA-rich oil
Fengwei Yina,
Xiaolong Suna,
Weilong Zhenga,
Xi Luoa,
Yingying Zhanga,
Longfei Yina,
Qiang Jiab and
Yongqian Fu *a
*a
aInstitute of Biomass Resources, Taizhou University, No. 1139 Shifu Road, Taizhou 318000, People's Republic of China. E-mail: bioengineer@163.com
bSeasons Biotechnology (Taizhou) Co., Ltd, Taizhou, People's Republic of China
First published on 27th January 2021
Abstract
Docosahexaenoic acid (DHA)-rich oil sourced from microalgae can easily become oxidized. The objective of this work was to screen the optimal natural antioxidant mixture for protecting DHA-rich oil. Different natural antioxidants, encompassing tea polyphenols, natural vitamin E, rosemary extract, licorice root antioxidant, ascorbyl palmitate and lecithin were tested individually and in combination in an accelerated oxidation process. Three antioxidants namely natural vitamin E, rosemary extract and ascorbyl palmitate with synergistic effects were chosen, and their concentrations were further optimized using response-surface methodology. The highest antioxidants activity of 16.1740 was obtained with a combination of 0.0224% vitamin E, 0.0259% rosemary extract and 0.0166% ascorbyl palmitate, which prolonged the time until oxidation induction to 20.21 days. The mixed natural antioxidants showed a similar antioxidant effect to 0.02% tert-butylhydroquinone and was better than 0.02% butylated hydroxyanisole. These data indicate that the mixed natural antioxidants optimized in this work can be directly applied in the protection of commercial microalgal DHA-rich oil.
1. Introduction
Docosahexaenoic acid (DHA) is one of the most important omega-3 polyunsaturated fatty acids (PUFAs), with proven effects against cardiovascular disease, hypertension, and diabetes.1 Most commercial DHA is still sourced from marine fish, but marine DHA-rich oil is becoming increasingly disfavored due to pollution, seasonal variation, and differences due to geographical location. In recent years, microalgal DHA has attracted much attention and become a new functional food additive that is widely popular among consumers.2As an oil product containing many PUFAs, one of the main problems in the application of microalgal oil in the market lies in its strong unpleasant flavor caused by oil oxidation.3 When oils are exposed to environmental factors during production and storage, inevitably, autoxidation reactions produce undesirable flavors and other forms of deterioration. The primary autoxidation products are hydroperoxides with no undesirable flavor. However, hydroperoxides are unstable and can be further decomposed to form aldehydes, ketones and other compounds called secondary oxidation products with detectable tastes and flavors. Notably, microalgal DHA-rich oil contains many different fatty acids, and the PUFA percentage can be as high as 70%.4 It was reported that oils with high ratios of PUFAs tend to be easily oxidized.5 Interestingly, microalgal oil also contains endogenous antioxidants such as carotenoids and phenolic compounds, which can play a certain role in antioxidant protection.6–8
However, the inherent quantities usually cannot provide sufficient protection during prolonged storage. Moreover, such intrinsic antioxidants can be substantially removed during the refining process. Therefore, one of the most effective and convenient strategies to improve the stability of microalgal oils is the addition of external antioxidants.9
Both natural and synthetic antioxidants have been studied and applied in the protection of oils against oxidation.10 Antioxidants have different mechanisms of action, whereby some act by eliminating free radicals in the oil peroxidation chain reaction, and others act as singlet oxygen quenchers that return active oxygen to the ground state. For example, quercetin has two pharmacophores within the molecule that can scavenge radicals.10 O'Sullivan et al. showed that rosemary extract can inhibit the formation of initial oxidation products.11 Typical and frequently used synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and tert-butylhydroquinone (TBHQ) have been approved for the preservation of oils.12 Although many synthetic antioxidants show high antioxidant efficiency at relatively low safe concentrations, due to the high requirements of food safety and increasing doubts surrounding the safety of synthetic products,13 the health food industry has to reduce the use of synthetic additives and replace them with natural substitutes.14
Individual antioxidants can be used alone to significantly improve the oxidative stability of oils, but this approach does not take advantage of the full scope of antioxidant protection or achieve the best antioxidant effect. This is because oil oxidation encompasses different stages, and antioxidants with different chemical structures can act via different modes of action or mechanisms.10 Therefore, in actual application, antioxidants are usually used in combination to take advantage of their different properties.15 For example, mixtures of phenolics and carotenoids at suitable ratios effectively enhance each other's antioxidant effects in synergy.16 Interestingly, the application of antioxidant mixtures can lead to the regeneration of some components of the mixture, as was observed in the mixture of quercetin and α-tocopherol, which increased the antioxidant ability of the system by regenerating α-tocopherol.17
With the rapidly developing commercial DHA production, it is essential to screen an efficient antioxidant or antioxidant mixture to improve the oxidative stability of DHA-rich oil during storage, extending its shelf life. Published literatures did a lot of work in exploring of synergistic effect of different antioxidants, it should be noticed that, response surface methodology (RSM) is a statistical experiment protocol for mathematical modeling, which has become an ideal strategy to optimize the dosage amount because it requires less experimental measurement, and provides statistical interpretation of data and interaction between variables. However, to the best of our knowledge, the synergistic effect has never been used in reported studies to screen optimal antioxidant combination using RSM. Therefore, this study focuses on screening potential natural antioxidants and designing their optimal combination through RSM, aiming to provide ideal mixed natural antioxidants for commercial products based on microalgal DHA-rich oil.
2. Materials and methods
2.1. Natural antioxidants and pretreatment of microalgal DHA-rich oil
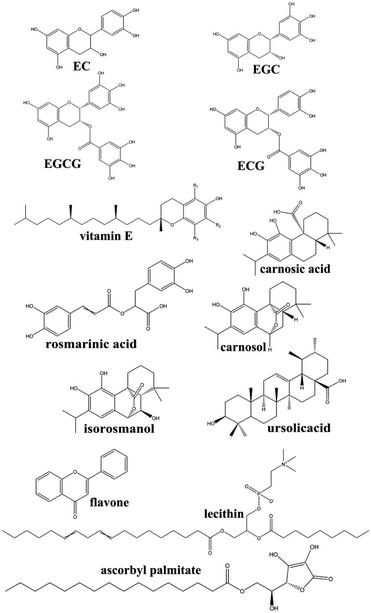
The microalgal DHA-rich oil, which was fermented using Schizochytrium sp., was obtained from Jiangsu Tiankai Biotechnology Co., Ltd. The crude oil was pretreated via a refining process encompassing the steps of degumming, alkaline refining, bleaching, and deodorizing.18 The final refined microalgal DHA-rich oil was applied for the antioxidant screening.Six different natural antioxidants, encompassing tea polyphenols, a mixture of polyhydroxy compounds, with the main active components were epicatechin (EC), epigallocatechin (EGC), epigallocatechin gallate (EGCG) and epicatechin gallate (ECG); natural vitamin E (α-, β-, γ-, δ-tocopherol); rosemary extract, in this study it was a lipid-soluble antioxidants mixture with the main active component were carnosic acid, rosmarinic acid, carnosol, ursolicacid, isorosmanol and so on; licorice root, with the main antioxidant is a mixture of flavonoids; ascorbyl palmitate; and lecithin. The chemical structures of main active components of antioxidants were shown in Fig. 1. Ascorbyl palmitate is regarded as a natural antioxidant because it's hydrolyzed to ascorbic and palmitic acid in the body.10
2.2. Detection of antioxidant capacity
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) in a 250 mL iodine flask. 0.5 mL saturated potassium iodide solution was added and reacted for 60 s, and then added 30 mL distilled water and 1 mL starch solution. Finally, the solution was titrated with 0.1 M sodium thiosulfate until the purple color disappeared.
60) in a 250 mL iodine flask. 0.5 mL saturated potassium iodide solution was added and reacted for 60 s, and then added 30 mL distilled water and 1 mL starch solution. Finally, the solution was titrated with 0.1 M sodium thiosulfate until the purple color disappeared.| PV (meq O2 per kg oil) = [(A − B) × C × 1000]/weight of sample (g) |
The induction period (IP) was considered as the time that is needed for the PV of the oil to reach 20 meq kg−1. This was in agreement with a general consideration that oil becomes rancid at PV values above 20 meq kg−1.20 The effects of antioxidants on oil oxidation were described by the stabilization factor (F).21
where IPa, IPb and IPa+b are the induction periods in the presence of antioxidant a, b, and their mixture, respectively. IP0 was the induction period without antioxidant. A positive value of SE% indicates synergism and a negative value indicate antagonism.
2.3. Optimizing the optimum ratio of natural antioxidants
Based on their synergistic effects, using Design Expert® software, a further response surface methodology (RSM) experiment was carried out to determine the optimum addition ratio of the mixed antioxidants. The activity of the mixed antioxidants was chosen as the response value for the RSM. Antioxidants with synergistic effects were selected as experimental factors for designing response surface optimization experiments. Antioxidants activity (A) is defined as a general parameter which unifies the effectiveness of the mixed antioxidants in terminating the autoxidation chain and reducing the oxidation rate.21where Fm is the stabilization factor with antioxidants, Vm and V0 were the oxidation rate with or without antioxidants, and the oxidation rate is the slope of the linear initiation stage of lipid oxidation based on the measurement of peroxide value, as was described in Asnaashari's study.21
On the basis of the synergistic effects investigations, a three-factor, three-level Box–Behnken experimental design was performed to optimize the added concentration. The 17 experiments were conducted randomly to establish a model for antioxidants activity. The experimental data obtained were fitted into a following second-order polynomial model:
2.4. Fatty acid profiles
Analysis of fatty acid composition was carried out using a GC-2020 gas chromatography (GC) system (Shimadzu, Japan) equipped with a DB-23 capillary column (60 m × 0.22 mm) and flame ionization detector (FID). The preparation of fatty acid methyl esters and the linear temperature-program for GC were the same as in our pervious study.242.5. p-Anisidine value
The p-anisidine value (p-AV) was determined according to the ISO 6885 method.2.6. Accelerated oxidation test
Accelerated oxidation of the oils was performed using the Schaal oven test.25 Specifically, 50 g of the refined oil was sampled into a 100 mL aluminum bottle with a cover, and was placed in a light-proof oven at 60 °C for accelerated oxidation. The oil was sampled every two days for analysis.2.7. Statistical analyses
The data was analyzed statistically using Statistical Product and Service Solutions (SPSS), and a t-test study for single means was carried out to determine the significantly differences (p < 0.05).3. Results and discussion
3.1. Effects of individual antioxidants on the stability microalgal oil
The oxidation of oils, especially those containing PUFAs, follows the three steps of initiation, propagation and termination, with the formation of primary and secondary oxidation products.26 Generally, the accumulation of primary products is the key to the formation of secondary oxidation products, and PV is applied as an index to measure the degree of oxidation in the early stage of oil oxidation.21 The PV of different antioxidants added individually to the same final concentration of 0.01 and 0.02% is shown in Fig. 2. Studies have shown that DHA-rich oil from Schizochytrium sp. contains endogenous antioxidants such as carotenoids and phenolic compounds.6–8 This might the reason for the relatively small oxidative activity of this oil. However, the endogenous antioxidants cannot fully protect the, and its PV increased fast at the beginning. In the accelerated oxidation experiment, the oxidation rate and PV of the oil increased rapidly, and the induction period was 9.74 d. With the addition of antioxidants, the rate of increase of PV was decreased. However, the antioxidant activity was not proportional to the concentration of added antioxidants, indicating that the protective effect of antioxidants is not a simple linear relationship with their dosage. Among the tested antioxidants, rosemary extract, tea polyphenols and ascorbyl palmitate showed the best protective effect. The antioxidant activity of rosemary extract is mainly attributed to the carnosic acid, carnosol, and rosmarinic acid compounds present in the mixture, such as carnosic acid is phenolic acid (Fig. 1) that presents effective antioxidant activity, acting as a hydrogen donor to free radical. A recent study showed that adding 0.02% (w/w) of rosemary was sufficient to stabilize flaxseed oil against oxidation,19 and was successfully applied as an antioxidant for sunflower oil.27 In the protection of DHA-rich oil, it has been reported that 0.5% rosemary extract showed a similar antioxidant effect to 200 ppm of the synthetic antioxidant butylated hydroxytoluene (BHT).28 It has been proved that tea polyphenols had stronger antioxidant activity than vitamin E and synthetic antioxidant butyl hydroxyanisole (BHA),29 similar to the results of this study. Moreover, the addition of 0.01% tea polyphenols prolonged the induction period of lipid oxidation to 13.96 d, which was 4.22 days longer than that of the control group. However, tea polyphenols are not easily soluble in oils, which might be solved through chemical modification such as esterification or acylation. For example, modified ascorbic acid–ascorbyl palmitate, is easy to dissolve in oil and showed strong antioxidant activity with a protection factor of 1.31 (Table 1). Low concentrations of soybean lecithin did not have an antioxidant effect on algal oil, but rather accelerated the oxidation. This might be related to the amount of lecithin added. Some studies indicated that below a concentration of 1% (w/w), there was not a good protective effect of lecithin against oxidation.30 Glycyrrhiza antioxidant contains flavonoids as the key antioxidant components. In terms of the protection factor (F) (Table 1), adding 0.02% of rosemary extract showed the highest value, indicating that rosemary extract had the best antioxidant activity as when used independently a single antioxidant.| Antioxidant | IP (d) | F |
|---|---|---|
| None | 9.74 | — |
| 0.01% rosemary extract | 12.94 | 1.33 |
| 0.02% rosemary extract | 14.31 | 1.47 |
| 0.01% vitamin E | 10.14 | 1.04 |
| 0.02% vitamin E | 10.29 | 1.06 |
| 0.01% tea polyphenols | 13.96 | 1.43 |
| 0.01% tea polyphenols | 13.94 | 1.43 |
| 0.01% licorice root antioxidant | 10.75 | 1.03 |
| 0.02% licorice root antioxidant | 10.96 | 1.13 |
| 0.01% ascorbyl palmitate | 11.63 | 1.19 |
| 0.02% ascorbyl palmitate | 12.84 | 1.31 |
| 0.01% lecithin | 7.82 | 0.80 |
| 0.02% lecithin | 9.09 | 0.93 |
3.2. Synergistic effects of different antioxidants
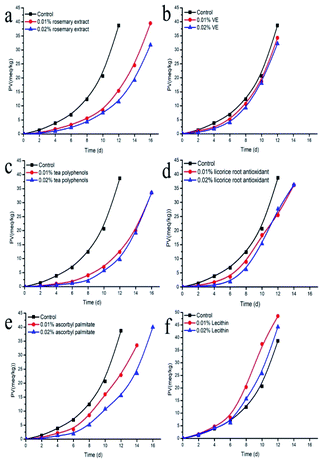
The single use of an antioxidant usually can have an antioxidant effect against a certain component, however, it cannot protect oil very well due to its limited physical and chemical properties. Mixed antioxidant combinations can complement each other, improving the performance of each component,15 and thereby reduce the cost of antioxidants. In order to select the best antioxidant combinations, the synergistic effects (SE) between the different tested natural antioxidants were investigated. The addition of a single antioxidant was 0.02% (w/w). As for antioxidant mixture, each antioxidant was 0.01% (w/w). Vitamin E is widely found in oil-rich plants and vegetables, and it plays an important role as a protective antioxidant in oils. It has been reported that ascorbic acid can regenerate vitamin E, thereby synergistically enhancing its antioxidant activity.31 Vitamin E reacts with free radicals to form tocopherol hydroxyl free compounds, and these compounds can also be reduced to vitamin E by ascorbyl palmitate. The main mechanisms of interaction and synergistic effect between vitamin E and ascorbyl palmitate should be: (1) ascorbyl palmitate can regenerate tocopherol oxygen free radicals to vitamin E; (2) ascorbyl palmitate reduces the consumption of vitamin E by scavenging oxygen free radicals. For example, the combined use of vitamin C and vitamin E enhanced its ability to scavenge free radicals and showed synergistic. Previous study showed that, tocopherol and ascorbyl palmitate exert a synergistic antioxidant effect in a number of systems and this interaction is based on radical exchange reactions between the two antioxidants.32 Liu et al. similarly showed that the combination of ascorbic acid and vitamin E enhanced the free-radical scavenging ability of the antioxidant system with a synergistic effect. In this study, the combination of ascorbyl palmitate and vitamin E showed a synergistic effect, with an improved antioxidant value of 57.75%. Rosemary extract can act as hydride donors to regenerate α-tocopherol, which helps improve the synergistic effect of the composite antioxidants.33 It had also been reported that rosemary extract could be combined with citric acid or ascorbyl palmitate, showing a synergistic effect in preventing the generation of hydrogen peroxide in sunflower seed oil.20 In this study, rosemary extract showed a certain synergistic effect with other antioxidants (Fig. 3), which might be related to the complex chemical constituents of rosemary extract (Fig. 1). The highest synergistic effect was obtained with vitamin E (32.96%) and ascorbyl palmitate (19.72%), respectively. Wada's studies have proved that the mixture of 0.02% α-tocopherol and 0.05% rosemary extract shows strong antioxidant activity in the protection of sardine DHA-rich oil, which prolongs the starting time of oil oxidation for 5 days.33 Carnosic acid is phenolic acid that presents effective antioxidant activity, acting as a hydrogen donor to free radical, therefore, rosemary extract can be used as a hydrogen atom donor to provide a hydrogen to α-tocopherol free radical, reducing α-tocopherol.34 Besides, previous study showed that the fish oil with tocopherols and rosemary extract rich in carnosic acid had the highest antioxidant activity due to the reduction of tocopheroxyl radicals by the carnosic acid.35 Ascorbyl palmitate can be used as both an antioxidant and a surfactant because it possesses a hydrophilic ascorbic acid and a lipophilic palmitic acid group. Ascorbyl palmitate preserves the strong reduction potential of ascorbic acid and can capture oxygen in the oil. Furthermore, it acts as a synergist of many antioxidants. In addition, it can block the free radical chain reaction of lipid peroxidation in the oil. However, not all antioxidant combinations showed synergistic effects. For example, the combination of some plant-derived phenols and α-tocopherol sunflower seed oil acted antagonistically.13 This might be due to the competition in the formation of free radicals of different active antioxidants in the oxidation process or the selectivity of the microenvironment between antioxidants. In addition, the effect of antioxidants does not usually increase with the concentration, and some antioxidants actually promote oxidation at high concentrations. For example, there was little synergistic effect between ascorbic acid and neutral phenols, or even antagonism. The synergistic effect of neutral phenol and ascorbic acid was inversely proportional to the concentration of ascorbic acid, and when the concentration of ascorbic acid was higher than 0.5 mg mL−1, the interaction changed to antagonism.36 Moreover, the effects of antioxidants might be different in different systems. For example, the combination of α-tocopherol with epicatechic acid or catechol showed a synergistic effect in oil-in-water type vegetable oil emulsions and phosphatidylcholinosome systems, but showed antagonistic effects in crude oil.37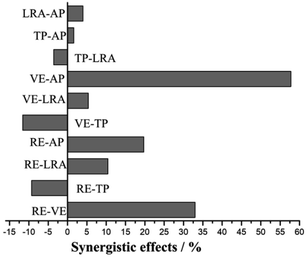 | ||
| Fig. 3 Synergistic effects between antioxidants. Licorice root antioxidant (LRA), ascorbyl palmitate (AP), tea polyphenols (TP), vitamin E (VE), rosemary extract (RE). | ||
We found that rosemary extract, vitamin E and ascorbyl palmitate had synergistic effects with each other and showed great antioxidant effect in actual application, therefore, the concentration of these three antioxidants were further optimized using response surface methodology.
3.3. Optimization of the mixed natural antioxidants using RSM
Basing on the results above, the antioxidants dosages were further optimized using RSM, the highest and lowest usage amounts of different antioxidants are as follows: vitamin E (X) 0.01–0.03%, ascorbyl palmitate (Y) 0.01–0.02%, rosemary extract (Z) 0.01–0.03%. The experimental results of antioxidant activity (A) obtained according to the Box–Behnken method were listed in Table 2. The analysis of variance of the response surface was shown in Table 3.| Run | X (%) | Y (%) | Z (%) | A |
|---|---|---|---|---|
| 1 | 0.02 | 0.015 | 0.02 | 15.73 |
| 2 | 0.02 | 0.015 | 0.02 | 15.65 |
| 3 | 0.02 | 0.01 | 0.01 | 11.38 |
| 4 | 0.01 | 0.02 | 0.02 | 14.04 |
| 5 | 0.02 | 0.015 | 0.02 | 15.82 |
| 6 | 0.01 | 0.015 | 0.01 | 12.71 |
| 7 | 0.02 | 0.02 | 0.03 | 15.15 |
| 8 | 0.01 | 0.01 | 0.02 | 10.74 |
| 9 | 0.03 | 0.01 | 0.02 | 12.80 |
| 10 | 0.02 | 0.015 | 0.02 | 15.42 |
| 11 | 0.03 | 0.015 | 0.03 | 15.68 |
| 12 | 0.02 | 0.015 | 0.02 | 15.81 |
| 13 | 0.01 | 0.015 | 0.03 | 13.63 |
| 14 | 0.02 | 0.02 | 0.01 | 11.60 |
| 15 | 0.02 | 0.01 | 0.03 | 11.25 |
| 16 | 0.03 | 0.02 | 0.02 | 13.01 |
| 17 | 0.03 | 0.015 | 0.01 | 11.77 |
| Source | Sum of squares | DF | Mean square | F-Value | P-Value |
|---|---|---|---|---|---|
| Model | 56.0865 | 9 | 6.231834 | 108.2063 | <0.0001 |
| X | 0.57245 | 1 | 0.57245 | 9.939724 | 0.0161 |
| Y | 7.277113 | 1 | 7.277113 | 126.356 | <0.0001 |
| Z | 8.507813 | 1 | 8.507813 | 147.7252 | <0.0001 |
| XY | 2.387025 | 1 | 2.387025 | 41.44706 | 0.0004 |
| XZ | 2.235025 | 1 | 2.235025 | 38.80781 | 0.0004 |
| YZ | 3.3856 | 1 | 3.3856 | 58.7858 | 0.0001 |
| X2 | 3.945364 | 1 | 3.945364 | 68.50525 | <0.0001 |
| Y2 | 18.0504 | 1 | 18.0504 | 313.4178 | <0.0001 |
| Z2 | 6.796506 | 1 | 6.796506 | 118.011 | <0.0001 |
| Residual | 0.403145 | 7 | 0.057592 | ||
| Lack of fit | 0.295825 | 3 | 0.098608 | 3.675301 | 0.120422 |
| R2 | 0.9929 | ||||
| Adj R2 | 0.9837 |
According to the analysis of variance of the response surface (Table 3), the F value of the model was 108.21, with a P < 0.0001, indicating that the established model had very high statistical significance. All parameters of the model and their interaction terms showed either significant (P < 0.05) or highly significant (P < 0.01) effects. The P value of the missing items of the model was 0.1204, i.e. it was not significant (P > 0.05), showing that the model had good applicability for predicting the variation. Therefore, the discreteness of the experimental data was caused by the pure error independent of the model. In addition, the determinant coefficient R2 of the model was 0.9929, which indicated that the model had very high significance and applicability for predicting the response, and that 99.29% of the overall change of the results came from the three variables studied. Similarly, the adjusted R2 was 0.9837, which was very close to R2, indicating the significance of the model. In general, R2 will increase with the addition of parameters to the model, but if the added parameters are meaningless, the adjusted R2 will decrease. Therefore, these two values should be close to each other to ensure that only meaningful parameters are included in the model. After multiple regression analysis of the experimental data, the second-order quadratic equation based on each factor was postulated as follows:
| A = 15.69 + 0.27X + 0.95Y + 1.03Z − 0.77XY + 0.75XZ + 0.92YZ − 0.97X2 − 2.07Y2 − 1.27Z2 |
It can be seen from the formula that all first-order coefficients are positive, indicating that the addition of all three antioxidants can promote the activity of the system, and the influence of each factor on the antioxidant activity was in the order rosemary extract > ascorbyl palmitate > vitamin E. Vitamin E can block the chain reaction of free radicals or inhibit the decomposition of peroxides to delay or prevent the oxidation of oils. However, the free radicals of vitamin E can be decomposed into hydrogen peroxide, which increases the peroxide free radicals in the oil when the amount of vitamin E is high.38 The P values of all the interaction items showed very high significance, indicating that the interaction of antioxidants had an important impact on the overall antioxidant activity of the system.
In order to better understand the effects of antioxidants and their interactions on the activity of the system, a three-dimensional response surface map was drawn, as shown in Fig. 4.
 | ||
| Fig. 4 3-D plots and interaction between different factors of response surface. (a) Ascorbyl palmitate–vitamin E; (b) rosemary extract–vitamin E; (c) ascorbyl palmitate–rosemary extract. | ||
The interaction between vitamin E and ascorbyl palmitate with the dosage of rosemary extract held constant is shown in Fig. 4a. As be seen from the three-dimensional graph, the activity of the system in the experimental range increased with the increase of ascorbyl palmitate concentration, and the speed of change was faster with a steep curve, which was further confirmed by the contour of the two-dimensional image. However, with the increase of vitamin E concentration, the system activity did not change significantly. This might be because the regeneration of α-tocopherol is the main interaction of the system components, and the ascorbyl palmitate in the system could meet the regeneration requirements, of α-tocopherol.
Fig. 4b shows the interaction between rosemary extract and vitamin E. With the increase of the final concentration of rosemary extract, the antioxidant activity of the system increased rapidly, indicating that rosemary extract had a greater impact on the system. When the concentration of rosemary extract exceeded 0.0259%, the curve tended to be smooth and the antioxidant activity of the oil tended to be stable. When rosemary extract was added to a certain amount, the antioxidant activity of the system increased with the increase of vitamin E concentration, indicating the obvious interaction between the two factors. This system is also suitable for the antioxidant protection of fish oil. For example, a study by Wada et al. indicated that the synergistic effect of rosemary extract and vitamin E could significantly improve the oxidative stability of DHA form fish oil.33
Fig. 4c shows the interaction between rosemary extract and ascorbyl palmitate. It can be seen from the figure that the interaction of the system is strongly dependent on the concentrations of the two antioxidants, and the antioxidant activity increased rapidly with the increased addition of the two antioxidants. In addition, it can be seen from the planar two-dimensional images that the contour lines of rosemary extract and ascorbyl palmitate are elliptical, indicating that their interaction is significant. When the dosage of the two additives reached the optimum system activity, the synergistic effect of the two antioxidants was not significant.
Based on the presented response surface analysis, it can be concluded that rosemary extract, vitamin E and ascorbyl palmitate contribute to the antioxidant activity of the system both individually and in combination. When ascorbyl palmitate is combined with tocopherol, the latter first reacts with free radicals to produce tocopherol free radicals. Tocopherol can be regenerated in the presence of ascorbyl palmitate. Fang et al. also confirmed that ascorbic acid could regenerate polyphenols, while polyphenols could regenerate vitamin E.39 Moreover, rosemary extract and ascorbyl palmitate (0.02%) could delay the loss of natural tocopherol during the rapeseed frying process.40
According to the fitted binary second-order equation, the optimum mixture with the highest antioxidant activity contained 0.0259% rosemary extract, 0.0224% vitamin E, and 0.0166% ascorbyl palmitate. At this ratio, the highest activity of the antioxidant system was 16.1740. In order to verify the adaptability and reliability of the model equation, the accelerated oxidation experiments of oil were carried out under the same conditions with the addition of the above proportion of antioxidants. The actual activity of the optimized antioxidant mixture was 15.9207, which was consistent with the predicted value of the model.
3.4. Application of mixed antioxidants
To further verify the protective effect of the antioxidant system on microalgal DHA-rich oil, we compared the optimized formulation with the synthetic antioxidants TBHQ and BHA. The changes of PV and p-AV of DHA-rich oil during accelerated oxidation were determined, and the fatty acid composition of the DHA-rich oil after oxidation with the different antioxidants was analyzed and compared.As can be seen in Fig. 5a, all three antioxidants have strong antioxidant activity against the development of rancidity in microalgal DHA-rich oil. During the induction period of lipid oxidation, the PV first increased slowly, but it began to increase rapidly after the 12th day. During this initial period, the lipid oxidation rate was very low. The mixed antioxidants showed similar effects to TBHQ and BHA. The induction periods of the mixed natural antioxidants, TBHQ and BHA were 20.21, 19.60 and 17.71 days, respectively, indicating that the mixed natural antioxidants and TBHQ had conferred similar oxidation stability to the oil. Subsequently, the oxidation of oil began to accelerate, and the antioxidant activity of the mixed antioxidants was lower than that of TBHQ. After 20 days, the PV was 20.23 meq kg−1 with the mixed antioxidants, while for TBHQ it was 18.36. However, the difference was not statistically significant (P > 0.05). When compared with BHA, the mixed antioxidants displayed higher antioxidant activity and the difference was significant (P < 0.05). Therefore, it can be concluded that the mixed antioxidants have similar antioxidant activity to TBHQ and higher than BHA.
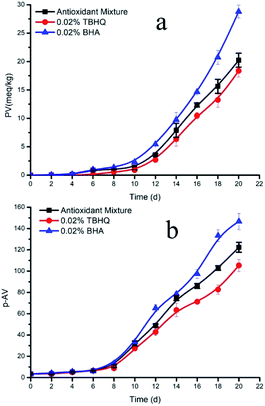 | ||
| Fig. 5 Changes of peroxide value (a) and p-anisidine value (b) of DHA oil with different antioxidants. | ||
Usually, the PV of oil reflects its oxidation rate or the protective ability of antioxidants, and the degree of oxidation of oil is therefore measured via the p-AV value. The change of the p-AV of the DHA-rich oil under accelerated oxidation conditions is shown in Fig. 5b. Generally, the p-AV showed an initial increase followed by a decrease and another increase in the course of preservation, resembling an undulating upward curve overall. However, in this experiment the p-AV increased continuously during the whole process, which may be caused by the rapid decomposition of hydrogen peroxide and the rapid accumulation of secondary oxidation products under accelerated oxidation conditions. Alternatively, this difference may be due to the long sampling interval, since the decline of p-AV in the interval time was not monitored. Nevertheless, it was clear from the graph that the growth rate was different in the interval time. The p-AV increased slowly during the induction period, and the increase became rapid when entering the oxidation period. The synthetic antioxidant TBHQ significantly reduced the growth rate of p-AV in the DHA-rich oil. Compared with TBHQ, the p-AV of the mixed natural antioxidants increased rapidly after entering the oxidation stage, and reached 122.35 after 20 days. As can be seen in the p-AV curve shown in Fig. 5b, the effect of different antioxidants on the secondary oxidation products was in the order TBHQ > mixed natural antioxidants > BHA. However, when compared with the p-AV of TBHQ (105.34), the mixed natural antioxidants had no significant difference (P = 0.07). Nevertheless, when compared with BHA, the difference was highly significant (P = 0.01), which showed that the protection against secondary oxidation conferred by the mixed natural antioxidants was similar to that of TBHQ, and was higher than that of BHA.
Table 4 lists the effects of different antioxidants on the fatty acids in the microalgal DHA-rich oil. The refined DHA-rich oil contains many different fatty acids, with total unsaturated fatty acids and DHA accounting for 64.49 and 44.37% of the total, respectively. After 10 days of accelerated oxidation, the fatty acid composition of the oil remained basically unchanged, which was similar to the increase of the PV, illustrating the protective effect of the antioxidants on the fatty acids of the oil. However, after 20 days of accelerated oxidation, there were significant differences in the fatty acid ratios. As shown in Table 4, after 20 days, the proportions of saturated fatty acids such as C14:0 (myristic acid), C16:0 (palmitic acid) and C18:0 (stearic acid) increased, while that of DHA and DPA (docosapentaenoic acid) decreased dramatically. This is likely caused by the accumulation of hydroperoxide in the oil and the weakened protection by the antioxidants with the prolongation of oxidation time. In this process, polyunsaturated fatty acids are prone to oxidation because of their unsaturated bonds.5 Conversely, the saturated fatty acids could remain stable during the oxidation process,41 resulting in a decrease of the proportion of unsaturated fatty acids and a corresponding increase of the proportion of saturated fatty acids. When comparing the effects of different antioxidants on the fatty acid composition of the oil, we found that the proportion of fatty acids after oxidation was similar when using mixed natural antioxidants and TBHQ, and the proportion of unsaturated fatty acids was higher than what was observed with BHA, which further confirmed that the optimized mixture of natural antioxidants had a good protective effect on the PUFAs in the DHA-rich microalgal oil. After 20 days of accelerated oxidation, the content of DHA was 42.44%, which was only 4.35% lower than in the initial oil.
| Fatty acid | Initial value | 10 days | 20 days | ||||
|---|---|---|---|---|---|---|---|
| MNAsa | TBHQ | BHA | MNAsa | TBHQ | BHA | ||
| a MNAs mixed natural antioxidants. | |||||||
| C14:0 | 7.51 ± 0.14 | 7.25 ± 0.06 | 7.66 ± 0.33 | 7.22 ± 0.41 | 8.41 ± 0.44b | 8.99 ± 0.47b | 10.46 ± 0.12a |
| C14:1 | 0.65 ± 0.04 | 0.62 ± 0.03 | 0.66 ± 0.04 | 0.67 ± 0.03 | 0.51 ± 0.01a | 0.48 ± 0.02a | 0.45 ± 0.01a |
| C16:0 | 22.45 ± 1.33 | 23.15 ± 1.23 | 21.64 ± 1.11 | 22.45 ± 0.45 | 25.74 ± 1.03b | 24.64 ± 1.87b | 28.66 ± 1.49a |
| C18:0 | 0.85 ± 0.01 | 0.77 ± 0.01 | 0.79 ± 0.02 | 0.74 ± 0.11 | 1.24 ± 0.04b | 1.21 ± 0.06b | 1.85 ± 0.17a |
| Squalene | 1.29 ± 0.07 | 1.49 ± 0.32 | 1.34 ± 0.15 | 1.14 ± 0.02 | 1.03 ± 0.06a | 0.96 ± 0.01a | 0.83 ± 0.02b |
| DPA | 18.18 ± 0.32 | 16.38 ± 1.22 | 17.68 ± 1.36 | 16.21 ± 0.54 | 14.21 ± 1.02ab | 15.64 ± 1.24a | 12.21 ± 0.14b |
| DHA | 44.37 ± 1.66 | 44.54 ± 1.14 | 44.55 ± 1.66 | 44.12 ± 0.38 | 42.44 ± 1.44a | 43.12 ± 2.12a | 40.44 ± 2.01b |
| Others | 4.7 ± 0.11 | 5.8 ± 0.05 | 5.68 ± 0.21 | 7.45 ± 0.06 | 6.42 ± 0.34a | 4.96 ± 0.38b | 5.1 ± 0.01b |
| ∑SFA% | 64.49 | 63.03 | 64.23 | 62.14 | 58.19 | 60.20 | 53.93 |
| ∑UFA% | 35.51 | 36.97 | 35.77 | 37.86 | 41.81 | 39.80 | 46.07 |
4. Conclusions
Adding antioxidant could slow down the oxidation rate of microalgal DHA-rich oil. However, using a single antioxidant cannot achieve the best antioxidant effect. Practical application tends to use mixed antioxidants with synergistic effects. Three nature antioxidants namely rosemary extract, ascorbyl palmitate and vitamin E presented synergies with each other and showed an excellent antioxidant effect. Based on the results, these three antioxidants were optimized by response-surface methodology. The optimized antioxidant combination encompassed 0.0259% rosemary extract, 0.0224% vitamin E, and 0.0166% ascorbyl palmitate. The mixture prolonged the oxidation induction to 20.21 days, which was 10.47 days longer than that of the control group. The optimized natural antioxidant mixture has similar antioxidant activity to 0.02% TBHQ, and exhibits stronger oxidation protection than BHA. This natural antioxidant mixture can be actually applied in micrialgal DHA-rich oil, helping to enhance the antioxidant ability of DHA oil and extend its shelf life.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Natural Science Foundation of Zhejiang Province (LGG20B060001 and LQ19B060003).Notes and references
- V. M. Ursin, J. Nutr., 2003, 133, 4271–4274 CrossRef CAS.
- G. Y. Sun, A. Simonyi, K. L. Fritsche, D. Y. Chuang, M. Hannink, Z. Z. Gu, C. M. Greenlie, J. K. Yao, J. C. Lee and D. Q. Beversdorf, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2018, 136, 3–13 CrossRef CAS.
- P. Karthik and C. Anandharamakrishnan, RSC Adv., 2016, 6, 3501–3513 RSC.
- F.-W. Yin, Y.-T. Zhang, J.-Y. Jiang, D.-S. Guo, S. Gao and Z. Gao, Process Biochem., 2019, 77, 1–7 CrossRef CAS.
- A. Ismail, G. Bannenberg, H. B. Rice, E. Schutt and D. Mackay, Lipid Technol., 2016, 28, 55–59 CrossRef CAS.
- M. Gaffney, R. O'Rourke and R. Murphy, Algal Res., 2014, 6, 195–200 CrossRef.
- A. R. Byreddy, Lipid Technol., 2016, 28, 68–70 CrossRef CAS.
- D. Sahin, U. H. Altindag and E. Tas, Process Biochem., 2018, 10–15 CrossRef CAS.
- S. Fereidoon and Z. Ying, Chem. Soc. Rev., 2010, 39, 4067–4079 RSC.
- N. V. Yanishlieva and E. M. Marinova, Eur. J. Lipid Sci. Technol., 2015, 103, 752–767 CrossRef.
- A. O'Sullivan, A. Mayr, N. B. Shaw, S. C. Murphy and J. P. Kerry, J. Aquat. Food Prod. Technol., 2005, 14, 75–94 CrossRef.
- M. H. Yang, H. J. Lin and Y. M. Choong, Food Res. Int., 2002, 35, 627–633 CrossRef CAS.
- M. S. Brewer, Compr. Rev. Food Sci. Food Saf., 2011, 10, 221–247 CrossRef CAS.
- F. Shahidi and P. Ambigaipalan, J. Funct. Foods, 2015, 18, 820–897 CrossRef CAS.
- Y. Wang, S. Liu, M. Yang, A. A. Taha, J. Wang and C. Ma, RSC Adv., 2020, 10, 14705–14713 RSC.
- H. W. Jiang, H. Y. Li, C. W. Yu, T. T. Yang, J. N. Hu, R. Liu and Z. Y. Deng, J. Food Sci., 2015, 80, C1162–C1169 CrossRef CAS.
- P. Pedrielli and L. H. Skibsted, J. Agric. Food Chem., 2002, 50, 7138–7144 CrossRef CAS.
- F. Yin, X. Sun, W. Zheng, X. Luo, C. Peng, Q. Jia and Y. Fu, J. Food Process. Preserv., 2020, 44, e14602 CAS.
- Y.-Z. Wang, S.-G. Fu, S.-Y. Wang, D.-J. Yang, Y.-H. S. Wu and Y.-C. Chen, LWT--Food Sci. Technol., 2018, 89, 210–216 CrossRef CAS.
- A. R. Hraš, M. Hadolin, Ž. Knez and D. Bauman, Food Chem., 2000, 71, 229–233 CrossRef.
- M. Asnaashari, R. Farhoosh and A. Sharif, Food Chem., 2014, 159, 439–444 CrossRef CAS.
- P. R. Quiroga, N. R. Grosso, A. Lante, G. Lomolino, J. A. Zygadlo and V. Nepote, Int. J. Food Sci. Technol., 2013, 48, 642–649 CrossRef CAS.
- E. R. Nedamani, A. S. Mahoonak, M. Ghorbani and M. Kashaninejad, J. Food Sci. Technol., 2015, 52, 4565–4571 CrossRef.
- F.-W. Yin, S.-Y. Zhu, D.-S. Guo, L.-J. Ren, X.-J. Ji, H. Huang and Z. Gao, Bioresour. Technol., 2019, 271, 118–124 CrossRef CAS.
- M. Homan and S. Fereidoon, J. Agric. Food Chem., 2008, 56, 4751 CrossRef.
- R. Guitard, J. F. Paul, V. Nardello-Rataj and J. M. Aubry, Food Chem., 2016, 213, 284–295 CrossRef CAS.
- G. N. Mezza, A. V. Borgarello, N. R. Grosso, H. Fernandez, M. C. Pramparo and M. F. Gayol, Food Chem., 2018, 242, 9–15 CrossRef CAS.
- M. Tsimidou, E. Papavergou and D. Boskou, Food Res. Int., 1995, 28, 431–433 CrossRef CAS.
- M. Koketsu and Y. I. Satoh, J. Food Lipids, 2010, 4, 1–9 CrossRef.
- A. Judde, P. Villeneuve, A. Rossignol-Castera and A. Le Guillou, J. Am. Oil Chem. Soc., 2003, 80, 1209–1215 CrossRef CAS.
- G. R. Buettner, Arch. Biochem. Biophys., 1993, 300, 535–543 CrossRef CAS.
- G. C. Beddows, C. Jagait and J. M. Kelly and, Food Chem., 2001, 73, 255–261 CrossRef.
- S. Wada and X. Fang, J. Food Process. Preserv., 1992, 16, 263–274 CrossRef CAS.
- X. Fang and S. Wada, Food Res. Int., 1993, 26, 405–411 CrossRef.
- W. Chaiyasit, D. J. Mcclements and E. A. Decker, J. Agric. Food Chem., 2005, 53, 4982–4988 CrossRef CAS.
- B. W. Bolling, Y. Y. Chen and C. Y. Chen, Int. J. Food Sci. Technol., 2014, 48, 2650–2658 CrossRef.
- Y. Jie, B. Eleonora Miquel, M. L. Andersen and L. H. Skibsted, Food Chem., 2012, 135, 2195–2202 CrossRef.
- D. H. Jho, S. M. Cole, E. M. Lee and N. J. Espat, Integr. Cancer Ther., 2004, 3, 98–111 CrossRef CAS.
- D. Fang, C. Wei-Feng and Z. Bo, Biochimie, 2008, 90, 1499–1505 CrossRef.
- M. H. Gordon and L. Kourkimskå, J. Sci. Food Agric., 2010, 68, 347–353 CrossRef.
- T. S. Kim, J. D. Yeo, Y. K. Ji, M. J. Kim and J. H. Lee, Food Chem., 2013, 138, 1792–1799 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |