Open Access Article
Open Access ArticleAchieving deep desulfurization with inverse-micellar polyoxometalates and oxygen†
Jinghui Wu‡
abc,
Yue Li‡a,
Menting Jianga,
Yang Huo *b,
Xianze Wang
*b,
Xianze Wang *ab and
Xiaohong Wanga
*ab and
Xiaohong Wanga
aKey Lab of Polyoxometalate Science of Ministry of Education, Northeast Normal University, Changchun, 130024, China
bScience and Technology Innovation Center for Municipal Wastewater Treatment and Water Quality Protection, Northeast Normal University, Changchun 130117, China. E-mail: huo0814@outlook.com; wangxz940@nenu.edu.cn; Tel: +86-181-43082239
cKey Laboratory of Songliao Aquatic Environment, Ministry of Education, Jilin Jianzhu University, Changchun 130118, China
First published on 1st March 2021
Abstract
Designing green and efficient catalytic systems that can operate under mild conditions and utilize molecular oxygen as an oxidant for achieving deep desulfurization is highly desirable. In this study, an inverse-micellar polyoxometalate (POM) (NH4)5(CTA)6PMo4V8O40 (CTA = cetyltrimethylammonium), abbreviated as (CTA)PMo4V8, was designed and its activity in desulfurization was evaluated. Almost ∼100% of organic sulfur was removed in 8 h at 100 °C, using only flowing oxygen under atmospheric pressure. (CTA)PMo4V8 exhibited excellent activity in treating sulfur-containing compounds (dibenzothiophene (DBT), 4,6-dimethyldibenzothiophene (DMDBT), benzothiophene (BT) and thiophene) in real oils, i.e. diesel and FCC gasoline, affording clean oils with super-low sulfur content of 8.77 and 6.17 ppm, respectively. Furthermore, (CTA)PMo4V8 showed high activity in the oxidative desulfurization of real oils in the presence of oxygen and nitrogen (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Such inverse-micellar POMs could be reused at least six times without significant loss of activity due to their high stability.
1). Such inverse-micellar POMs could be reused at least six times without significant loss of activity due to their high stability.
1 Introduction
Although great efforts have been dedicated to developing desulfurization techniques for fossil fuels,1,2 methods that can achieve super-clean oils with a sulfur content of lower than 10 ppm are still needed due to strict regulations and environmental problems caused by the use of fossil fuels.3 Several desulfurization methods have been developed, including hydrodesulfurization,1 adsorption,2 extractive desulfurization,4,5 and oxidative desulfurization.6,7 In order to obtain super-clean oils, oxidative desulfurization (ODS) is a good choice, due to its advantages of high efficiency and low cost, especially for the removal of aromatic sulfur compounds such as dibenzothiophene (DBT), 4,6-dimethyldibenzothiophene (DMDBT), benzothiophene (BT) and thiophene. Recently, various ODS methods have been developed using H2O2, O2 or other oxidants, while O2 or air is more economical. Generally, achieving aerobic ODS under mild conditions is still a challenge. Moreover, the combination of oxidation of refractory aromatic sulfur and extraction of obtained sulfone in one unit is more efficient, cheap and environmentally benign.Polyoxometalates (POMs) have been widely investigated as potential catalysts in aerobic oxidation desulfurization (Table S1†). In this study, POMs and POM-supported hybrids were evaluated for their oxidation of DBT, BT and 4,6-DMDBT at 60–120 °C for 1.5–12 h, and were found to give 70–100% conversion. 100% conversion of DBT, 4,6-DMDBT, and BT was achieved using (NH4)5H6PMo4V8O40 at 100 °C for 6–11 h with TOF values of 6.76, 5.87, 5.07 h−1 (TOF = [converted sulfur]/[usage of POMs] × time (h−1)). Meanwhile, the oxidation of thiophene was successfully performed at 100 °C for 12 h using (NH4)5H6PMo4V8O40 with a TOF of 7.18 h−1. Compared to (NH4)5H6PMo4V8O40, H4PMo11VO40 exhibited lower activity or 17.3% removal efficiency under the same reaction conditions (Table S1†). This determined that POMs containing more vanadium atoms exhibited higher activity than those with fewer. In the aerobic oxidation of refractory aromatic sulfur compounds, there were difficulties in achieving mass transfer between solid POMs, gaseous O2 and substrates, which limited the oxidation rates. One strategy to overcome these drawbacks is to design an amphiphilic POM system, which uses a long-chain surfactant as a counter-ion to fabricate surfactant/POMs anion–cation hybrids.8,9 In organic solvents, such amphiphilic molecules can self-assemble to form inverse-micelles, which can concentrate O2 and substrates to enhance the reaction rates.10
In the course of our research work, (NH4)5H6PMo4V8O40 was found to be most active in the aerobic oxidation of DBT and its derivates. However, there were some difficulties in mass transfer between the solid catalyst, oil and oxygen. During our study on POM catalysis, it was found that amphiphilic POMs could concentrate organic substrates and oxygen around the active sites of POMs to enhance the activity.8,9
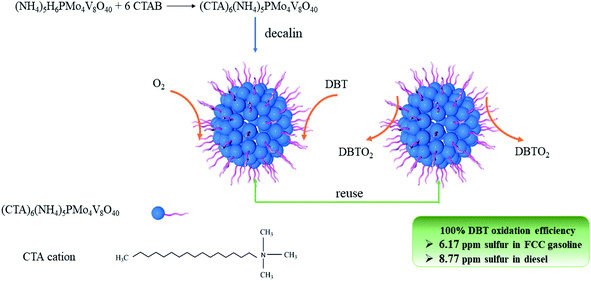
In order to solve the environmental problems caused by fossil fuels, there is an urgent need for a green and efficient catalytic system that can obtain super-clean oil under mild conditions, using molecular oxygen as an oxidant. Therefore, the amphiphilic POM (NH4)5(CTA)6PMo4V8O40 (CTA is cetyltrimethylammonium, abbreviated as (CTA)PMo4V8) was synthesized and was used as a catalyst in the ODS of organic sulfur compounds under atmospheric pressure. In previous research, the ODS of real fuels has rarely been reported. This process is important in the production of ultra-clean oils. (CTA)PMo4V8 showed high activity in treating diesel and FCC gasoline with 8.77 and 6.17 ppm sulfur content, which meets the VI standard of China.11 The regeneration and stability of (CTA)PMo4V8 was also assessed to determine its economic viability.
2 Experimental
Details about the materials and physical measurements are given in the ESI,† while the experimental methods are also described.3 Results and discussion
3.1 Synthesis and characterization
(CTA)PMo4V8 was characterized firstly by elementary analysis to give the elemental content as: Mo, 11.75%; P, 0.96%; V, 12.52%; C, 42.01%; N, 4.74%; H, 8.33%. These results are consistent with the calculated values of: Mo, 11.79%; P, 0.90%; V, 12.53%; C, 42.00%; N, 4.70%; H, 8.30%. They also correspond to the formula of (NH4)5(CTA)6PMo4V8O40, and give a P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo
Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V molar ratio of 1
V molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
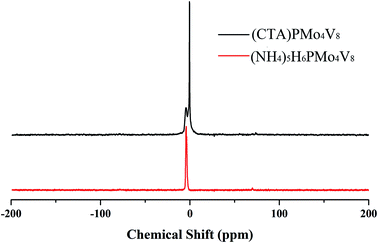
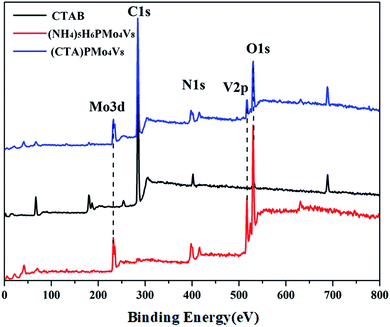
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, indicating that the Keggin anion is intact. Meanwhile, the carbon content and nitrogen content in the POM were about 42.01% and 4.74%, respectively, corresponding to 6 moles of CTA cation in the hybrid. The IR spectrum (Fig. S1†) of (CTA)PMo4V8 shows four characteristic peaks, at 1051 cm−1 (νasP–Oa), 930 cm−1 (νasMo–Od), 863 cm−1 (νasMo–Ob), and 743 cm−1 (νasMo–Oc), which are similar to its parent, (NH4)5H6PMo4V8 (1055, 932, 861 and 732 cm−1). The presence of these four characteristic peaks with slight shifts indicates that the Keggin structure is maintained and there is some interaction between (CTA)+ and PMo4V811−. The 31P MAS NMR of (CTA)PMo4V8 exhibits one peak at −4.43 ppm with a shoulder (Fig. 1). Compared to the peak of (NH4)5H6PMo4V8 at −4.44 ppm, it exhibits a slight shift and a shoulder due to some interaction between CTA+ and PMo4V811−. XPS was used to determine the composition and valence states of (CTA)PMo4V8 (Fig. 2). From the XPS results, it can be seen that Mo3d and V2p at 243.3 and 527.8 eV were found, corresponding Mo6+, V5+ and P5+, indicating the existence of the PMo4V8 anion in the hybrid. Meanwhile, C1s, O1s and N1s were also found in the XPS results due the existence of CTA+ and NH4+ cations.
8, indicating that the Keggin anion is intact. Meanwhile, the carbon content and nitrogen content in the POM were about 42.01% and 4.74%, respectively, corresponding to 6 moles of CTA cation in the hybrid. The IR spectrum (Fig. S1†) of (CTA)PMo4V8 shows four characteristic peaks, at 1051 cm−1 (νasP–Oa), 930 cm−1 (νasMo–Od), 863 cm−1 (νasMo–Ob), and 743 cm−1 (νasMo–Oc), which are similar to its parent, (NH4)5H6PMo4V8 (1055, 932, 861 and 732 cm−1). The presence of these four characteristic peaks with slight shifts indicates that the Keggin structure is maintained and there is some interaction between (CTA)+ and PMo4V811−. The 31P MAS NMR of (CTA)PMo4V8 exhibits one peak at −4.43 ppm with a shoulder (Fig. 1). Compared to the peak of (NH4)5H6PMo4V8 at −4.44 ppm, it exhibits a slight shift and a shoulder due to some interaction between CTA+ and PMo4V811−. XPS was used to determine the composition and valence states of (CTA)PMo4V8 (Fig. 2). From the XPS results, it can be seen that Mo3d and V2p at 243.3 and 527.8 eV were found, corresponding Mo6+, V5+ and P5+, indicating the existence of the PMo4V8 anion in the hybrid. Meanwhile, C1s, O1s and N1s were also found in the XPS results due the existence of CTA+ and NH4+ cations.
The morphology of (CTA)PMo4V8 in decalin was determined using SEM (Fig. S3†). It can be seen that spheres were found, with a size of 1.0 × 103 nm, showing that (CTA)PMo4V8 could self-assemble to form inverse-micelles. As shown in Fig. S4,† (CTA)PMo4V8 presents a long-range ordered regular arrangement in a parallel direction. A combination of elementary analysis, IR, 31P MAS NMR, XPS, SEM and TEM determined that (CTA)PMo4V8 maintained the Keggin structure, while the interaction between CTA+ and PMo4V811− confirmed its self-assembly to form inverse-micelles in decalin.
3.2 Catalytic properties
The activity of (CTA)PMo4V8 in the ODS of DBT was assessed using only flowing oxygen under atmospheric pressure (Fig. 3). It can be seen that (CTA)PMo4V8 could activate O2 to oxidize DBT in the temperature range of 80 °C to 100 °C. The maximum conversion of DBT was obtained as ∼100% at 4, 6, 7, and 8 h at different reaction temperatures of 100, 90, 85 and 80 °C, respectively. Based on these results, the activation energy (Ea) was calculated as 45.38 kJ mol−1 (Fig. S5†). Compared to (NH4)5H6PMo4V8, the Ea and TOF of (CTA)PMo4V8 for the aerobic oxidation of DBT were improved from 2690.49 kJ mol−1 and 6.76 h−1 to 45.38 kJ mol−1 and 13.89 h−1.7 This improvement might be due to the self-assembly of (CTA)PMo4V8 in decalin, as CTA+ cations wrap around active PMo4V811− cations, providing concentrated active sites for DBT (Scheme 1). Meanwhile, the adsorption of DBT by (CTA)PMo4V8 and (NH4)5H6PMo4V8 varied from 40.9% to 32.6% under the same reaction conditions, when using nitrogen instead of oxygen for 30 min at 100 °C. The selectivity of DBT oxidation was almost 100% to sulfone (DBTO2), as determined by GC-MS (Fig. S6†). The concentration of DBT also influenced the activity of (CTA)PMo4V8, as shown by increasing DBT concentration from 50 to 500 ppm (Fig. S7†), whereby 100% conversion was easily achieved at 50 ppm of DBT. Achieving 100% conversion at 50 ppm of DBT demonstrated the ability of (CTA)PMo4V8 to treat organic sulfur at low concentrations. Another advantage for (CTA)PMo4V8 is that produced DBTO2 can be repelled from the active sites of PMo4V8 by the hydrophobic surrounding of surfactant tails (Fig. S8†). From Fig. S8,† it can be seen that the obtained crystal of DBTO2 was found to be attached to the reactor, and needed to be separated using a decanting method.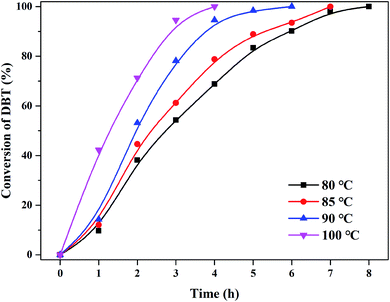 | ||
| Fig. 3 The effect of temperature from 80 °C to 100 °C on DBT removal in the presence of (CTA)PMo4V8 (0.05 mmol) with O2, 5 h. | ||
Meanwhile, (CTA)PMo4V8 showed wide tolerance to different substrates, including 4.6-DMDBT and BT (Fig. 4). Without any catalyst, no desulfurization of organic sulfur was found, showing the limitation of oxygen under such reaction conditions. It can be seen that the oxidation of various organic sulfur compounds depends on the sulfur electron density: 5.758, 5.758 and 5.760 corresponding to BT, DBT and 4.6-DMDBT, respectively. The activity followed the trend of DBT > 4.6-DMDBT > BT with a reaction time of 5 h. Up until now, there are few reports concerning the aerobic oxidation of thiophene (Table S1†). (CTA)PMo4V8 could activate O2 to oxidize thiophene with almost 100% conversion at elevated temperatures from 90 to 110 °C (Fig. 4).
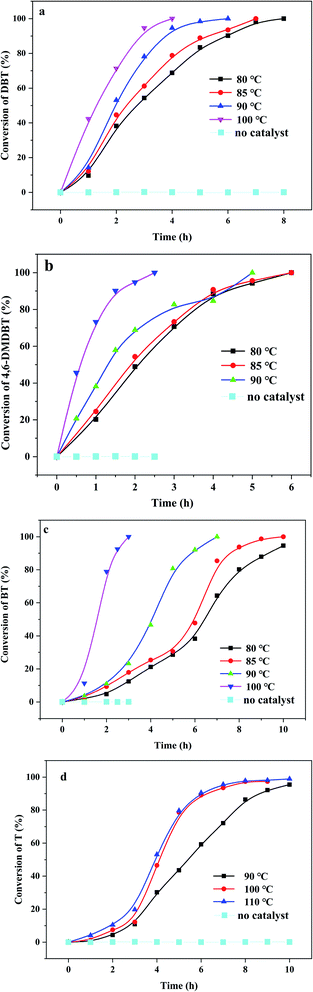 | ||
| Fig. 4 Different organic sulfur compounds in the presence of (CTA)PMo4V8 (0.03 mmol) with O2: (a) DBT; (b) 4,6-DMDBT; (c) BT; and (d) T. | ||
Based on the above results, (CTA)PMo4V8 was expected to be effective in the ODS treatment of real oils containing high amounts of sulfur compounds. The ODS of diesel (1282 ppm sulfur content) and FCC gasoline (996 ppm sulfur content) was performed under the reaction conditions of 6 mL of oil and 0.05 mmol of catalyst for 8 h with stirring (500 rpm) at 100 °C with flowing molecular oxygen at 10 mL min−1. Ultra-clean oils were obtained with sulfur content of 6.17 and 8.77 ppm, exhibiting 99.1% and 99.5% removal efficiency, respectively, using O2 as an oxidant under atmospheric pressure. In order to improve the safety, a mixture of oxygen and nitrogen with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used instead of pure oxygen. (CTA)PMo4V8 demonstrated 87.6% and 89.4% desulfurization efficiency in diesel and FCC gasoline treatment, respectively, demonstrating its potential application in the industry.
1 was used instead of pure oxygen. (CTA)PMo4V8 demonstrated 87.6% and 89.4% desulfurization efficiency in diesel and FCC gasoline treatment, respectively, demonstrating its potential application in the industry.
The reusability of a catalytic system is vital, and determines its usability in practical applications.12 Therefore, the spent catalyst was separated from the reaction system together with obtained DBTO2 by filtration after the completion of each run, and was washed with ethanol to separate DBTO2 for reuse. (CTA)PMo4V8 was found to be capable of catalyzing the oxidation of DBT for ten reaction cycles without a significant decrease of its activity (Fig. 5). The loss of (CTA)PMo4V8 was only about 8.3% of the initial amount after ten cycles. This loss of catalyst was due to operation during separation or washing treatment. It was confirmed that (CTA)PMo4V8 is relatively stable and recyclable under extremely mild conditions. The stability of (CTA)PMo4V8 was determined by IR, XPS and SEM (Fig. S9†), which showed that the spent catalyst (CTA)PMo4V8 displayed similar characteristics to the fresh one. The above results confirmed that (CTA)PMo4V8 remained intact during reaction and exhibits long durability and stability for practical applications.
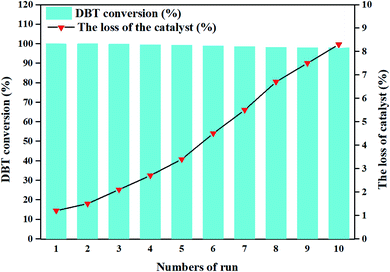 | ||
| Fig. 5 Reusability of (CTA)PMo4V8. Reaction conditions: DBT (500 ppm, 6 mL decalin), (CTA)PMo4V8 (0.05 mmol) at 100 °C with O2, 5 h. | ||
4 Conclusions
(CTA)PMo4V8 exhibits a high efficiency both in the oxidation of model oils and real oils, through the synergistic effect of concentrating DBT around active sites, activating O2, and repelling DBTO2 from PMo4V811− under atmospheric pressure. Almost 100% efficiency was achieved in the treatment of DBT, BT, 4.6-DMDBT and thiophane, while two kinds of ultra-clean oils with 8.77 and 6.17 ppm were obtained using (CTA)PMo4V8 and O2. (CTA)PMo4V8 showed high stability and excellent recycling performance, and great potential for desulfurization using O2 or a mixture of oxygen and nitrogen under atmospheric pressure.Author contributions
Jinghui Wu, Yang Huo and Xianze Wang conceived and designed the experiments; Jinghui Wu, Yue Li and Xianze Wang performed the experiments; Yue Li and Menting Jiang analyzed the data; Jinghui Wu and Yue Li contributed analysis tools; Jinghui Wu, Xianze Wang and Xiaohong Wang wrote the paper. Yang Huo and Xianze Wang have given their approval for the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51978134, 51908241, 51908109 and 51708094), the Jilin Provincial Science and Technology Department (20180414069GH), the Fundamental Research Funds for the Central Universities (2412020FZ012) and the Environmental Protection Scientific Research Project (JiHuanKeZi No. 2020-14).Notes and references
- A. J. Hernandez-Maldonado and R. T. Yang, J. Am. Chem. Soc., 2004, 126, 992–993 CrossRef CAS.
- R. T. Yang, A. J. Hernández-Maldonado and H. Frances, Science, 2003, 301, 79 CrossRef CAS.
- D. J. O'Rear, Lube base oils with improved stability, US pat., 09/966298, 2003 Search PubMed.
- M. Y. Chi, T. Su, L. L. Sun, Z. G. Zhu, W. P. Liao, W. Z. Ren, Y. C. Zhao and H. Y. Lü, Appl. Catal., B, 2020, 275, 119134 CrossRef CAS.
- P. S. Tam, J. R. Kittrell and J. W. Eldridge, Ind. Eng. Chem. Res., 1990, 29(3), 321–324 CrossRef CAS.
- R. Ghubayra, C. Nuttall, S. Hodgkiss, M. Craven, E. F. Kozhevnikova and I. V. Kozhevnikov, Appl. Catal., B, 2019, 253, 309–316 CrossRef CAS.
- M. Shi, D. Zhang, X. Yu, Y. M. Li, X. H. Wang and W. Yang, Fuel Process. Technol., 2017, 160, 136–142 CrossRef CAS.
- L. Hongying, W. Ren, W. Liao, C. Wei, L. Yang and Z. Suo, Appl. Catal., B, 2013, 138–139, 79–83 Search PubMed.
- N. Tang, Y. Zhang, F. Ling, H. Lu, Z. Jiang and C. Li, Chem. Commun., 2012, 48, 11647–11649 RSC.
- Y. Zhang, D. Li, Y. Chen, X. Wang and S. Wang, Appl. Catal., B, 2009, 86, 182–189 CrossRef CAS.
- National Development and Reform Commission, F.G.B.N.Y[S], 2017, No. 1665.
- D. M. Fernandes, A. D. S. Barbosa, J. o. Pires, S. S. Balula, L. Cunha-Silva and C. Freire, ACS Appl. Mater. Interfaces, 2013, 5, 13382–13390 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00428j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |