Open Access Article
Open Access ArticleConstructing helical nanowires via polymerization-induced self-assembly†
Qiumeng Chena,
Yahui Lia,
Ming Liua,
Xuan Wub,
Jianliang Shen *ab and
Liangliang Shen
*ab and
Liangliang Shen *a
*a
aState Key Laboratory of Ophthalmology, Optometry and Vision Science, School of Ophthalmology and Optometry, School of Biomedical Engineering, Wenzhou Medical University, Wenzhou 325027, PR China
bEngineering Research Center of Clinical Functional Materials and Diagnosis & Treatment Devices of Zhejiang Province, Wenzhou Institute, University of Chinese Academy of Sciences, Xinsan Road, Longwan District, Wenzhou 325001, PR China. E-mail: shenjl@wiucas.ac.cn
First published on 1st March 2021
Abstract
While reliable strategies for constructing block copolymer (BCP) nanowires have been developed, helical nanowires are rarely reported in polymerization-induced self-assembly (PISA). Herein, in this work, a new strategy for constructing helical nanowires was developed via PISA mediated by a fluorinated stabilizer block. Ultralong nanowires with helical structure can be readily produced in a wide range of block compositions. In addition, the generality of this strategy was well testified by expanding monomer types. The achiral BCP nano-objects underwent a morphology transition from spheres to helical nanowires during aging. We believe this work will provide a general strategy for producing helical nanowires through PISA of achiral BCPs.
Introduction
Helix is a fascinating structural feature in nature due to its asymmetry, extraordinary aspect ratio, elasticity, aesthetic and chirality.1–3 In particular, helix is commonly adopted in biomacromolecules and life, such as DNA,4 collagen5 and tobacco mosaic virus.6 Inspired by nature, construction of helical nanostructures via self-assembly of synthetic block copolymers (BCPs) has attracted considerable attention.7,8 For example, Ho and co-worker elucidated the chirality transfer from molecular level to self-assembled helical phase of polylactide-containing BCP via the twisting and shifting.9 Xu et al. reported the successful preparation of helical toroids via self-assembly of polypeptide homopolymer and its BCP.10Over the past decade, polymerization-induced self-assembly (PISA) has been extensively investigated as a powerful strategy for the synthesis of BCP nanostructures with controllable size and morphology.11–18 During a typical PISA process, a stabilizer block is chain-extended by polymerizing a soluble monomer to produce amphiphilic BCPs, which in situ self-assemble into nano-objects with diverse morphologies. Recently, developing novel PISA strategies for constructing nanowires has attracted great research interest.19–22 For example, Guan et al. reported scalable production of nanowires via polymerization-induced hierarchical self-assembly of stilbene-containing BCPs.23 Boott et al. reported the formation of cylindrical micelles at high solids (up to 25%) via polymerization-induced crystallization-driven self-assembly.24 Despite great progress in preparation of BCP nanowires, synthesis of BCP helical nanowires via PISA has been rarely reported.
Recently, fluoropolymers have been extensively employed in PISA due to their unique properties, such as high lipophobicity, high chemical stability, low surface energy, and low refractive index.25,26 In an overwhelming majority of PISA formulations, fluorinated polymers are adopted as the core-forming blocks because of their outstanding solvophobicity. In particular, semi-fluorinated poly(meth)acrylates bearing side groups with high numbers of fluorocarbons exhibit excellent liquid crystalline nature and have been exploited in ordered self-assembly.27,28 For example, Huo and co-workers reported that cylindrical micelles could be readily prepared by dispersion of semi-fluorinated-containing monomer in a wide range of block compositions.29 Shen et al. reported on facile preparation of ellipsoidal morphologies through dispersion polymerization of heptadecafluorodecyl methacrylate.30 Despite these significant developments, using fluorinated polymers as the stabilizer blocks in PISA has been relatively undeveloped because of their poor solubility.
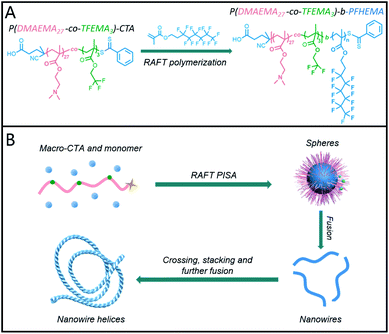
Jinnai and co-workers have demonstrated that a marginally solubilized block within a triblock copolymer can induce nanoparticle fusion, finally resulting in a morphology transition from spheres into helices.31 Considering the marginal solubility of short fluoropolymer in ethanol, we envision that inserting small amounts of fluorinated units within a stabilizer block can significantly improve the interaction between nanoparticles. Thus, the hierarchical self-assembly of BCP nanoparticles might be induced,32 producing helical nanowires. Herein, in this work, we aim to testify our hypothesis via PISA using a fluorinated stabilizer block. As demonstrated in Scheme 1A, a copolymer comprising of 2-(dimethylamino)ethyl methacrylate (DMAEMA) and 2,2,2-trifluoroethyl methacrylate (TFEMA) units was used to mediate the reversible addition–fragmentation chain transfer (RAFT) dispersion polymerizations of 2-(perfluorohexyl)ethyl methacrylate (FHEMA) in ethanol. The morphology of the resulting nano-objects was systematically investigated by dynamic light scattering (DLS), scan electron microscopy (SEM), and transmission electron microscopy (TEM).
Results and discussion
The fluorinated stabilizer block was firstly synthesized by RAFT homogeneous copolymerization of DMAEMA and TFEMA. The targeting degree of polymerization (DP) of DMAEMA and TFEMA was 45 and 5, respectively. The total monomer conversion was determined to be 60% by 1H NMR spectroscopy. After precipitation in petroleum ether and vacuum drying, pure polymer powder was obtained. The chemical structure of purified polymer powder was characterized by 1H NMR spectroscopy, which is shown in Fig. 1A. By comparing the integral ratio of signal e (δ = 4.09 ppm) and d (δ = 4.37 ppm), the actual repeated units of DMAEMA and TFEMA were determined to be 27 and 3, respectively. Gel permeation chromatography (GPC) analysis in Fig. 1B shows a narrow molecular weight distribution (Mw/Mn = 1.19) and good agreement between theoretical and experimental molecular weights (Mn,NMR = 5028 g mol−1, Mn,GPC = 5201 g mol−1). The TFEMA/DMAEMA ratio was deemed important because too much TFEMA units would lead to aggregation of the stabilizer blocks before dispersion polymerization. Therefore, the P(DMAEMA27-co-TFEMA3)-CTA ethanol solution was characterized by DLS to determine if the polymer was adequately dissolved in ethanol. DLS result in Fig. S1† indicated that no aggregates formed. As the inset image shown, the polymer solution is clear and transparent, indicating that no colloidal dispersion formed.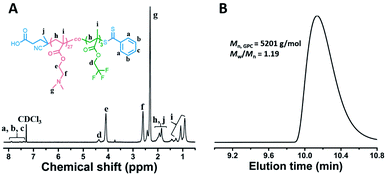 | ||
| Fig. 1 (A) 1H NMR spectrum of P(DMAEMA27-co-TFEMA3)-CTA in CDCl3; (B) GPC trace of P(DMAEMA27-co-TFEMA3)-CTA. | ||
Then, the dispersion polymerizations of FHEMA mediated by P(DMAEMA27-co-TFEMA3)-CTA were conducted at 20% solid content in ethanol. The polymerization conditions and results were summarized in Table 1. Near quantitative FHEMA conversions after 16 h were confirmed by 1H NMR spectroscopy. After precipitation and subsequent vacuum drying, pure P(DMAEMA27-co-TFEMA3)-b-PFHEMAn BCPs were obtained. Their BCP compositions were characterized by 1H NMR spectroscopy using a mixed solvent CDCl3/CF2ClCFCl2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v), which allowed efficient dissociation of PFHEMA block aggregation. As shown in Fig. 2, the signal e′ (δ = 4.37 ppm) increased with the increasing of targeting PFHEMA DP, indicating successful RAFT dispersion polymerizations.
2, v/v), which allowed efficient dissociation of PFHEMA block aggregation. As shown in Fig. 2, the signal e′ (δ = 4.37 ppm) increased with the increasing of targeting PFHEMA DP, indicating successful RAFT dispersion polymerizations.
| Feed ratioa | Solid (%) | Conv.b (%) | Dh,appc/nm | PDIc |
|---|---|---|---|---|
| a Molar ratio (P(DMAEMA27-co-TFEMA3)-CTA/FHEMA/AIBN).b Monomer conversions determined by 1H NMR spectroscopy in CDCl3. The corresponding peaks of HDFDMA completely disappeared in the 1H NMR spectra, indicating near quantitative monomer conversions (99%).c The average value of apparent hydrodynamic diameter (Dh,app) and the polydispersity index (PDI) of the nano-objects confirmed by DLS characterizations with the block copolymer concentration of 0.1% (1 mg mL−1). The left column: determined immediately after PISA synthesis; the right column: determined after aging for 7 days. | ||||
| 1/20/0.3 | 20 | 99 | 28–383 | 0.20–0.76 |
| 1/30/0.3 | 20 | 99 | 40–408 | 0.17–0.72 |
| 1/40/0.3 | 20 | 99 | 71–316 | 0.21–0.63 |
| 1/50/0.3 | 20 | 99 | 98–448 | 0.14–0.86 |
| 1/60/0.3 | 20 | 99 | 124–350 | 0.17–0.59 |
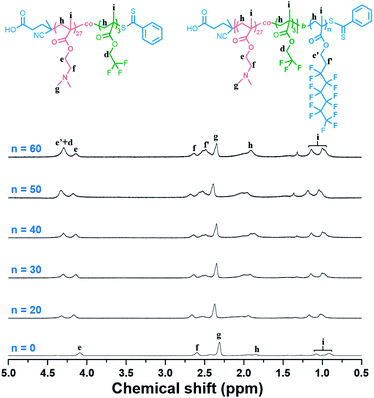 | ||
Fig. 2 1H NMR spectra of P(DMAEMA27-co-TFEMA3)-b-PFHEMAn in CDCl3/CF2ClCFCl2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). n = 0, 20, 30, 40, 50, and 60, respectively. 2, v/v). n = 0, 20, 30, 40, 50, and 60, respectively. | ||
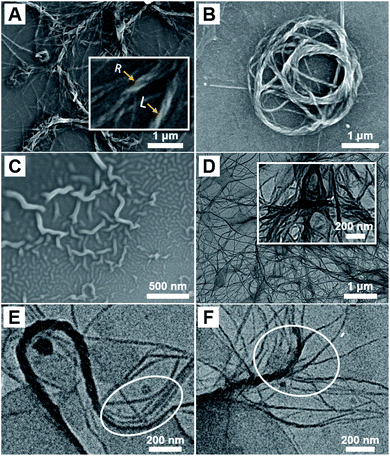
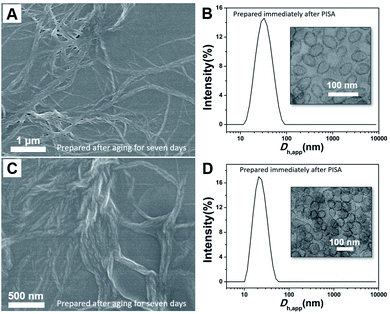
The morphology of the resulting P(DMAEMA27-co-TFEMA3)-b-PFHEMAn nano-objects was initially characterized by SEM. As shown in Fig. 3A, when the DP of PFHEMA was 20, helical nanowires with several micro-meters in length were observed. The handedness of the helical nanowires was confirmed based on SEM image. We observed that the handedness kept unchanged within an individual helix. The inset image in Fig. 3A indicated that there were both left- and right-handed ones in the same SEM sample. As demonstrated by circular dichroism analysis in Fig. S2,† no Cotton effect was observed for the helical nanowire ethanol solution. This is probably because the helical nanowires were resulted from the twist of the single nanowire without preferred twisting sense. After all, the BCPs synthesized in PISA exhibited no chirality and no chiral molecules were added in PISA synthesis as well. In addition, it can be observed that helical nanowires can fold into nest-like structures (Fig. 3B). Interestingly, spheres together with curved nanowires were observed in Fig. 3C, suggesting that the spheres seemed to undergo a fusion into helical nanowires. In addition to SEM characterizations, TEM analysis in Fig. 3D also indicated that the length of the helical nanowires extended to as long as several micro-meters. It can be clearly observed in Fig. 3E and F that the helical nanowires were multiple-strand helices, which consist of the twist of thinner nanowires. Note that the number of single nanowires in a helix varied between different helices. For example, the helix in Fig. 3E seems to consist of four thinner nanowires while the one in Fig. 3F has much more thinner nanowires. Therefore, the width and pitch of helix varied widely among the different helices in the same sample.
As the SEM image in Fig. 4A shown, helical nanowires can be still obtained when the DP of PFHEMA slightly increased to 30. TEM image in Fig. 4B also indicated the formation of micron-long helical nanowires. Interestingly, helical nanowires can be readily produced as the DP of PFHEMA finally increased to 60, which were shown in Fig. 4C–H. To the best of our knowledge, this is the first time that helical nanowires can be synthesized by PISA. Usually, the polymeric helical nanostructures were produced via self-assembly of chiral BCPs or induced by chiral molecules. Thus, the molecular chirality can be amplified by self-assembly, resulting helical nanostructures with supramolecular chirality. For instance, Lu et al. successfully fabricated double helices with controlled handedness via the self-assembly of an achiral BCP induced by D- and L-tartaric acid.33 Considering that no chiral factor was introduced in our PISA formulation, it can be rationally concluded that the helical nanostructure was not caused by chirality amplification. We speculated that the TFEMA unit within the stabilizer block led to the formation of helical nanowires. Dupont et al. reported successful preparation of double and triple helices through the self-assembly of an achiral ABC triblock copolymer.31 In their self-assembly system, the selected solvents are good for C block, poor for B block, and marginal for A block. A fusion mechanism for helices was proposed. Spheres formed initially and then fused into cylinders after aging for several days. During aging, the different cylinders underwent crossing, stacking and further fusion into helices. They described that the marginally solubilized block A functioned as glue between different cylinders to attract one another.
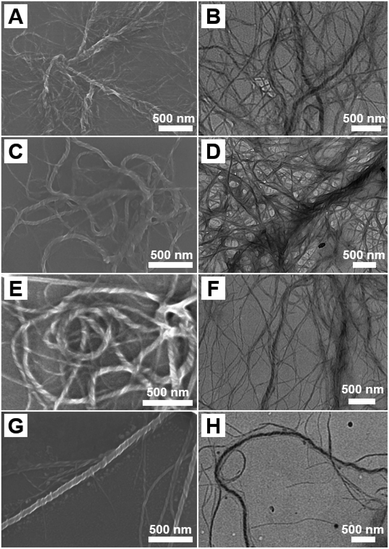 | ||
| Fig. 4 SEM and TEM micrographs of P(DMAEMA27-co-TFEMA3)-b-PFHEMAn helical nanowires: (A and B) n = 30; (C and D) n = 40; (E and F) n = 50; (G and H) n = 60. | ||
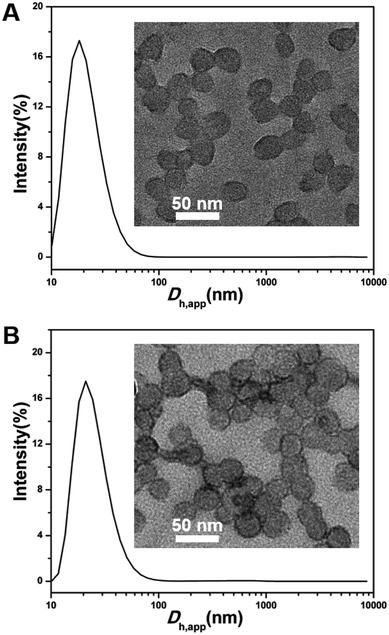
We carefully examined our experimental details. We found that both TEM and SEM analysis indicating helical nanowires were conducted seven days after PISA synthesis while the DLS analysis was performed immediately after PISA synthesis. As shown in Table 1, the P(DMAEMA27-co-TFEMA3)-b-PFHEMAn nano-objects exhibit an average apparent hydrodynamic diameter (Dh,app) from 28 to 124 nm with narrow distribution (polydispersity index (PDI) below 0.21). However, both Dh,app and PDI significantly increased after aging for several days. These DLS results seemed to indicate that spheres formed initially and then underwent a morphology transition into helical nanowires. In order to testify our hypothesis, PISA synthesis of P(DMAEMA27-co-TFEMA3)-b-PFHEMAn (n = 20 and 30, 20% solids in ethanol at 70 °C) was strictly repeated. DLS and TEM samples were prepared and analyzed immediately after PISA synthesis. As shown in Fig. 5, both DLS results and TEM images revealed that only spherical nanoparticles formed and no helical nanostructures were observed. As a control experiment, a dispersion polymerization of FHEMA with DP 30 was carried out using a PDMAEMA31 stabilizer block, of which the 1H NMR spectrum and GPC trace were shown in Fig. S3.† TEM analysis in Fig. S4† indiacted that spheres remained after aging for 7 days, which further confirmed that TFEMA units are key factors for the formation of helical nanowires. In fact, dispersion polymerizations of FHEMA mediated by a PDMAEMA stabilizer have been systematically investigated by Huo et al. and helical nanostructures were not observed.29
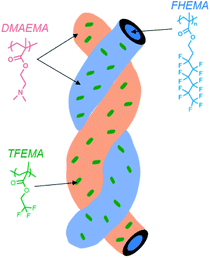
Very similar to the work of Dupont et al.,31 ethanol as the solvent in our PISA synthesis is good for PDMAEMA units, poor for PFHEMA block, and marginal for TFEMA units. Therefore, similar mechanism for the formation of helical nanowires can be proposed. Firstly, with the growth of the PFHEMA block, the resulting P(DMAEMA27-co-TFEMA3)-b-PFHEMAn BCPs initially in situ self-assembled into spheres during PISA synthesis. After aging for seven days, the as-synthesized spheres preferred to fuse into nanowires. Moreover, during aging, the different single nanowire underwent crossing, stacking and further fusion into helices induced by the marginally solubilized TFEMA units. To some extent, the TFEMA units randomly distributed within the stabilizer block functioned as glue between different nanowires to attract one another, which was depicted in Scheme 2. Thus, the nanowires aggregated and twisted into multiple helices so that the contact between TFEMA units and ethanol can be reduced.
To demonstrate the generality of our strategy for synthesizing helical nanowires, another two kinds of monomers were utilized in dispersion polymerizations mediated by P(DMAEMA27-co-TFEMA3) stabilizer block. The monomers were heptadecafluorodecyl methacrylate (HDFDMA) and lauryl methacrylate (LMA), respectively (Scheme S1†). Recently, HDFDMA, a semi-fluorinated methacrylate monomer bearing bulking and rigid side group is broadly adopted in the synthesis of one-dimensional nanostructures through PISA due to their excellent liquid crystalline nature. However, helical nanowires were never reported so far when using a homopolymer as the stabilizer block.29 In our work, a dispersion polymerization of HDFDMA using P(DMAEMA27-co-TFEMA3) as the stabilizer was conducted in ethanol at 20% solid content. As we expected, ellipsoidal nanoparticles formed initially and then underwent a morphology transition to helical nanowires after aging for seven days, which is shown in Fig. 6A and B. The size of ellipsoidal nanoparticles was statistically analyzed, which is shown in Fig. S5.† The formation of ellipsoidal nanoparticles can be attributed to the intrinsic ordering of PHDFDMA blocks.30,34–38 Similarly, a dispersion polymerization of LMA mediated by P(DMAEMA27-co-TFEMA3)-CTA was carried out in ethanol at 20% solid content. As shown in Fig. 6C and D, P(DMAEMA27-co-TFEMA3)-b-PLMA30 BCP nano-objects also exhibited a morphology evolution from spheres to helical nanowires during aging.
In summary, a new strategy for constructing helical nanowires was developed via dispersion polymerizations mediated by a fluorinated random copolymer stabilizer. Ultralong nanowires with helical structures can be readily produced in a wide range of block compositions. In addition, the generality of our strategy was well testified by expanding monomer types. It can be clearly observed that the helical nanowires were mostly multiple-strand helices, which consist of the twist of thinner nanowires. A fusion mechanism was proposed. Spheres initially formed during PISA and then underwent a fusion into helical nanowires after aging for days. We speculate that the marginally dissolved TFEMA units within the stabilizer block functioned as glue between different nanowires. Thus, the nanowires aggregated and twisted into multiple helices so as to reduce the contact between TFEMA units and ethanol. To the best of our knowledge, it is the first time to report helical nanowires in PISA. It should be noted that no chiral agent was introduced in our work. We believe this work will provide a general strategy for constructing nanowire helices through PISA of achiral BCPs.
Experimental
Materials
2,2′-Azobis(2-methylpropionitrile) (AIBN, 99%) was purchased from Sigma-Aldrich. Heptadecafluorodecyl methacrylate (HDFDMA, 98%), 2-(perfluorohexyl)ethyl methacrylate (FHEMA), 2-(dimethylamino)ethyl methacrylate (DMAEMA, 99%), 2,2,2-trifluoroethyl methacrylate (TFEMA, 98%) and lauryl methacrylate (LMA, 98%) were purchased from Shanghai Aladdin Bio-Chem Technology. CDCl3 (99.8%) and CF2ClCFCl2 (99.5%) were purchased from Shanghai Macklin Biochemical. All the monomers were passed through an Al2O3 column to remove the inhibitor prior to polymerizations. The chain transfer agent 4-cyanopentanoic acid dithiobenzoate (CPADB, 97%) was purchased from Shanghai Macklin Biochemical.Characterization
Monomer conversions and chemical structure of the purified polymers were analyzed by 1H NMR spectroscopy on a Bruker AV 400 MHz spectrometer. The core of the nanowire comprised of FHEMA blocks was hydrophobic, lipophobic and highly compact. Therefore, CF2ClCFCl2 was utilized as a cosolvent together with CDCl3 to sufficiently dissolve fluoro-containing block copolymer nanoparticles. GPC measurements were performed on a Waters Alliance e2695 GPC system equipped with a Styragel guard column (WAT054415, 30 × 4.6 mm), two Org separation columns consisting of D2500 (300 × 8 mm) and D5000 (300 × 8 mm). Detection was made with a 2414 refractive index detector (Waters Alliance), a Viscotek 302/305 UV detector (Malvern Instruments), and a Viscotek TDA 305-020 LALS/RALS detector (Malvern Instruments). Tetrahydrofuran (THF, HPLC grade) was used as the eluent at a flow rate of 1 mL min−1. To prepare GPC samples, 6–10 mg pure polymer powder was dissolved in 1 mL THF and filtered through a 0.20 μm Millipore filter. The apparent hydrodynamic diameter of the nano-objects was characterized by DLS analysis on a Malvern ZS90 with a He–Ne laser (633 nm, 4 mW) at a 90° angle. The as-synthesized nano-objects dispersions via PISA were diluted to 0.1% (1 mg mL−1) in a disposable cuvette using ethanol and analyzed immediately. The viscosity was 1.748 cP and the refractive index was 1.366. The temperature for all DLS characterizations was 25 °C and the equilibration time was 120 seconds. Autocorrelation functions were analyzed by the cumulants method to calculate the Z-average hydrodynamic diameter and polydispersity index (PDI). Circular dichroism analysis was conducted on a Chirascan Plus. The morphology of the nano-objects was characterized by TEM on a Jeol 200CX microscope (200 kV) and SEM on a SU8010 microscope. The polymer concentration for all the TEM and SEM samples is 0.2 mg mL−1. To prepare TEM samples stained by PTA, the nanoparticle-loaded grids were first dried for 15 minutes, followed by deposition of a small drop of PTA aqueous solution (0.5%). After 20 minutes, excess of PTA was washed away by water, and the TEM samples were dried overnight at 40 °C.Synthesis of macromolecular chain transfer agent P(DMAEMA27-co-TFEMA3)
CPADB (0.213 g, 0.762 mmol), DMAEMA (5.4 g, 34.28 mmol), TFEMA (0.642 g, 3.82 mmol), and AIBN (25.1 mg, 0.152 mmol) were added into a glass vial and subsequently dissolved in 7.3 mL 1,4-dioxane. After the solution was bubbled with nitrogen for 30 minutes in an ice bath, the glass vial was placed into an oil bath at 70 °C and stirred at 500 rpm. After 5 h of polymerization under nitrogen, the glass vial was removed from the oil bath and the resulting solution was exposed to air to terminate the polymerization. The total monomer conversion was determined to be 60% by 1H NMR spectroscopy. The pure polymer powder was obtained by precipitation in petroleum ether for three times and vacuum drying. The ratio of TFEMA units to DMAEMA units was determined by comparing the integral ratio of signal d (δ = 4.09 ppm) and e (δ = 4.37 ppm).Synthesis of macromolecular chain transfer agent PDMAEMA31
CPADB (0.213 g, 0.762 mmol), DMAEMA (6.0 g, 38.1 mmol), and AIBN (25.2 mg, 0.152 mmol) were added into a glass vial and subsequently dissolved in 7.25 mL 1,4-dioxane. After the solution was degassed with nitrogen for 30 minutes in an ice bath, the glass vial was placed into an oil bath at 70 °C and stirred at 500 rpm. After 5 h of polymerization under nitrogen atmosphere, the resulting solution was exposed to air to terminate the polymerization. The conversion of DMAEMA was determined to be 62% by 1H NMR spectroscopy. The pure polymer powder was obtained by precipitation in petroleum ether for three times and vacuum drying.Dispersion polymerizations mediated by P(DMAEMA27-co-TFEMA3)-CTA
The ethanolic synthesis of P(DMAEMA27-co-TFEMA3)-PFHEMA20 helical nanowires at 20% solid content was described as a representative example. In brief, P(DMAEMA27-co-TFEMA3)-CTA (0.08 g, 15.9 μmol), FHEMA (0.137 g, 318 μmol), and AIBN (0.52 mg, 3.2 μmol) were added to a glass vial and dissolved in 1.05 mL ethanol. In addition, 20 μL of DMF as inner standard agent was added into the polymerization mixture. Before polymerization, 20 μL of polymerization mixture was withdraw as the sample of 0 h for 1H NMR spectroscopy in CDCl3. After the solution was bubbled with N2 in an ice bath 5 minutes, the glass vial was placed into an oil bath at 70 °C with a stirring speed of 500 rpm. After 16 h of polymerization under nitrogen, the glass vial was removed from the oil bath and the solution was exposed to air to quench the radical. The resulting polymerization mixture was characterized again by 1H NMR spectroscopy, which was denoted as the sample of 16 h. FHEMA conversions can be calculated by comparing the integral ratio of DMF and FHEMA before and after polymerization. The corresponding peaks of FHEMA completely disappeared in the 1H NMR spectrum, indicating near quantitative monomer conversions. Purified P(DMAEMA27-co-TFEMA3)-b-PFHEMAn block copolymer was obtained by precipitation in petroleum ether and drying under vacuum. The BCPs compositions were characterized by 1H NMR spectroscopy in a mixture of CDCl3/CF2ClCFCl2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v).
2, v/v).
Author contributions
Qiumeng Chen, Yahui Li, and Ming Liu performed the experiments. Xuan Wu, Jianliang Shen and Liangliang Shen designed the project and performed the data analysis. Liangliang Shen wrote the paper. All authors discussed the results.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank financial support by the National Natural Science Foundation of China (51973161, 21604050), Wenzhou Medical University (KYYW201901, QTJ19035), and Natural Science Foundation of Zhejiang Province (LQ20B020009). We also thank the assistance of Instrumental Analysis and Research Centre (Wenzhou Institute, University of Chinese Academy of Sciences).References
- M. Liu, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304–7397 CrossRef CAS
.
- Y. Wang, J. He, X. Mu, D. Wang, B. Zhang, Y. Shen, M. Lin, C. Kübel, Y. Huang and H. Chen, ACS Nano, 2017, 11, 5538–5546 CrossRef CAS
.
- E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752–13990 CrossRef CAS
.
- J. D. Watson and G. S. Stent, The Double Helix: A Personal Account of the Discovery of the Structure of DNA, Atheneum, New York, 1980 Search PubMed
.
- B. Brodsky and J. A. M. Ramshaw, Matrix Biol., 1997, 15, 545–554 CrossRef CAS
.
- A. Klug, Philos. Trans. R. Soc., B, 1999, 354, 531–535 CrossRef CAS
.
- T. Wen, H.-F. Wang, M.-C. Li and R.-M. Ho, Acc. Chem. Res., 2017, 50, 1011–1021 CrossRef CAS
.
- R.-M. Ho, Y.-W. Chiang, S.-C. Lin and C.-K. Chen, Prog. Polym. Sci., 2011, 36, 376–453 CrossRef CAS
.
- R.-M. Ho, M.-C. Li, S.-C. Lin, H.-F. Wang, Y.-D. Lee, H. Hasegawa and E. L. Thomas, J. Am. Chem. Soc., 2012, 134, 10974–10986 CrossRef CAS
.
- P. Xu, L. Gao, C. Cai, J. Lin, L. Wang and X. Tian, Angew. Chem., Int. Ed., 2020, 59, 14281–14285 CrossRef CAS
.
- M. J. Derry, L. A. Fielding and S. P. Armes, Prog. Polym. Sci., 2016, 52, 1–18 CrossRef CAS
.
- X. Wang, L. Shen and Z. An, Prog. Polym. Sci., 2018, 83, 1–27 CrossRef CAS
.
- F. D'Agosto, J. Rieger and M. Lansalot, Angew. Chem., Int. Ed., 2020, 59, 8368–8392 CrossRef
.
- H. Sun, W. Cao, N. Zang, T. Clemons, G. Scheutz, Z. Hu, M. Thompson, Y. Liang, M. Vratsanos, X. Zhou, W. Choi, B. Sumerlin, S. Stupp and N. C. Gianneschi, Angew. Chem., Int. Ed., 2020, 59, 19136–19142 CrossRef CAS
.
- L. P. D. Ratcliffe, M. J. Derry, A. Ianiro, R. Tuinier and S. P. Armes, Angew. Chem., Int. Ed., 2019, 58, 18964–18970 CrossRef CAS
.
- F. Lv, Z. An and P. Wu, Nat. Commun., 2019, 10, 1–7 CrossRef CAS
.
- T. Lückerath, K. Koynov, S. Loescher, C. J. Whitfield, L. Nuhn, A. Walther, C. Barner-Kowollik, D. Y. W. Ng and T. Weil, Angew. Chem., Int. Ed., 2020, 59, 15474–15479 CrossRef
.
- X. Cheng, T. Miao, L. Yin, Y. Ji, Y. Li, Z. Zhang, W. Zhang and X. Zhu, Angew. Chem., Int. Ed., 2020, 59, 9669–9677 CrossRef CAS
.
- S. Guan and A. Chen, ACS Macro Lett., 2020, 9, 14–19 CrossRef CAS
.
- X. Zhang, S. Boisse, C. Bui, P.-A. Albouy, A. Brulet, M.-H. Li, J. Rieger and B. Charleux, Soft Matter, 2012, 8, 1130–1141 RSC
.
- X. S. Wang, G. Guerin, H. Wang, Y. S. Wang, I. Manners and M. A. Winnik, Science, 2007, 317, 644–647 CrossRef CAS
.
- A. M. Oliver, J. Gwyther, C. E. Boott, S. Davis, S. Pearce and I. Manners, J. Am. Chem. Soc., 2018, 140, 18104–18114 CrossRef CAS
.
- S. Guan, W. Wen, Z. Yang and A. Chen, Macromolecules, 2020, 53, 465–472 CrossRef CAS
.
- C. E. Boott, J. Gwyther, R. L. Harniman, D. W. Hayward and I. Manners, Nat. Chem., 2017, 9, 785–792 CrossRef CAS
.
- A. Vitale, R. Bongiovanni and B. Ameduri, Chem. Rev., 2015, 115, 8835–8866 CrossRef CAS
.
- T. Soulestin, V. Ladmiral, F. D. Dos Santos and B. Améduri, Prog. Polym. Sci., 2017, 72, 16–60 CrossRef CAS
.
- X. Li, B. Jin, Y. Gao, D. W. Hayward, M. A. Winnik, Y. Luo and I. Manners, Angew. Chem., Int. Ed., 2016, 55, 11392–11396 CrossRef CAS
.
- X. Li, Y. Gao, X. Xing and G. Liu, Macromolecules, 2013, 46, 7436–7442 CrossRef CAS
.
- M. Huo, D. Li, G. Song, J. Zhang, D. Wu, Y. Wei and J. Yuan, Macromol. Rapid Commun., 2018, 39, 1700840 CrossRef
.
- L. Shen, H. Guo, J. Zheng, X. Wang, Y. Yang and Z. An, ACS Macro Lett., 2018, 7, 287–292 CrossRef CAS
.
- J. Dupont, G. Liu, K. Niihara, R. Kimoto and H. Jinnai, Angew. Chem., Int. Ed., 2009, 48, 6144–6147 CrossRef CAS
.
- Y. Lu, J. Lin, L. Wang, L. Zhang and C. Cai, Chem. Rev., 2020, 9, 4111–4140 CrossRef
.
- X. Lu, J. Li, D. Zhu, M. Xu, W. Li and Q. Lu, Angew. Chem., Int. Ed., 2018, 57, 15148–15152 CrossRef CAS
.
- M. Huo, G. Song, J. Zhang, Y. Wei and J. Yuan, ACS Macro Lett., 2018, 7, 956–961 CrossRef CAS
.
- S. Guan, C. Zhang, W. Wen, T. Qu, X. Zheng, Y. Zhao and A. Chen, ACS Macro Lett., 2018, 7, 358–363 CrossRef CAS
.
- Y. Li, Q. Lu, Q. Chen, X. Wu, J. Shen and L. Shen, RSC Adv., 2021, 11, 1729–1735 RSC
.
- A. P. Soto, J. B. Gilroy, M. A. Winnik and I. Manners, Angew. Chem., Int. Ed., 2010, 49, 8220–8223 CrossRef
.
- Z. M. Hudson, C. E. Boott, M. E. Robinson, P. A. Rupar, M. A. Winnik and I. Manners, Nat. Chem., 2014, 6, 893–898 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00439e |
| This journal is © The Royal Society of Chemistry 2021 |