Open Access Article
Open Access ArticleBiofabricated silver nanoparticles exhibit broad-spectrum antibiofilm and antiquorum sensing activity against Gram-negative bacteria
Faizan Abul Qais a,
Iqbal Ahmad
a,
Iqbal Ahmad *a,
Mohammad Altafbc,
Salim Manoharadas
*a,
Mohammad Altafbc,
Salim Manoharadas c,
Basel F. Al-Rayesc,
Mohammed Saeed Ali Abuhasild and
Yaser Ayesh Almaroaie
c,
Basel F. Al-Rayesc,
Mohammed Saeed Ali Abuhasild and
Yaser Ayesh Almaroaie
aDepartment of Agricultural Microbiology, Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh, UP 202002, India. E-mail: ahmadiqbal8@yahoo.co.in; Fax: +91-571-2703516; Tel: +91-571-2703516
bDepartment of Chemistry, College of Science, King Saud University, PO Box 2455, Riyadh, 11451, Saudi Arabia
cCentral Laboratory, College of Science, King Saud University, PO Box 2455, Riyadh, 11451, Saudi Arabia
dDepartment of Food Science and Nutrition, College of Agriculture and Food Science, King Saud University, Riyadh, Saudi Arabia
eDepartment of Biology, College of Science, Umm Al-Qura University, Makkah 673, Saudi Arabia
First published on 13th April 2021
Abstract
The emergence and spread of antimicrobial resistance (AMR) among bacterial pathogens have created a global threat to human health and the environment. Targeting the quorum sensing (QS) linked virulent traits of bacteria is considered to be a novel approach for addressing the problem of AMR. In this study, green synthesized silver nanoparticles (AgNPs-MK) were evaluated for the inhibition of the formation of biofilms and quorum sensing controlled virulence factors against three Gram negative bacteria. Remarkable inhibition (>80%) of QS-mediated violacein production was recorded in C. violaceum 12472. Up to 90% inhibition of the QS-mediated virulent traits of S. marcescens MTCC 97 was observed. The virulence factors of P. aeruginosa PAO1 also decreased in a dose dependent manner in the presence of AgNPs-MK. Moreover, the development of biofilms of C. violaceum 12472, S. marcescens MTCC 97, and P. aeruginosa PAO1 was reduced by 87.39, 81.54, and 71.34%, respectively. Biofilms on glass surfaces were remarkably reduced, with less aggregation of bacterial cells and the reduced formation of extra polymeric substances. The findings clearly show the efficacy of AgNPs-MK against the development of biofilms and the QS mediated virulent traits of Gram negative bacterial pathogens. AgNPs-MK may be further exploited for the development of alternative antimicrobial agents after careful scrutiny in animal models for the management of bacterial infections, especially for topical applications.
1. Introduction
In the last few decades, there has been a tremendous increase in the incidence and emergence of multi-drug resistance (MDR) among bacterial pathogens, both in developing and developed countries.1 Today, the diseases caused by infectious microbes have become the major cause of global mortality and morbidity after cancer and cardiovascular diseases.2 These MDR pathogens or “superbugs” are now considered to be an epidemiological concern and antimicrobial resistance (AMR) worsens the treatment of infectious diseases by reducing the therapeutic efficacy of antibiotics.3,4 The situation has become so alarming that if no action is taken, AMR will become a major cause of mortality, even surpassing cancer in the next few decades.5,6 AMR not only poses a burden on public health systems, but is also problematic for livestock and the environment. The development of AMR has also complicated the management of other chronic diseases. The first six or seven decades of the 20th century is considered as the golden era for the discovery of antibiotics and this period resulted in the discovery of 70% of all antibiotics reported to date. A timeline of antibiotic discovery shows a large gap or void for almost four decades, during which only a few antibiotics were discovered that exhibit a novel mechanism of action.7 The antibiotic selection pressure is one of the key drivers in the development of AMR among microbial pathogens.8,9 The selection pressure is developed by the sub-judicious and unprescribed use of antibiotics in human health care, livestock and the environment. Now, even the latest generation of antibiotics can no longer be trusted for long term applications because of the risk of development of resistance. Therefore, there are two major problems that need to be addressed in antibacterial drug discovery; one is the development of new therapeutic antimicrobials with a novel mode of action, and the other is to prevent the development of AMR against the discovered antimicrobials. This has created a need for the development of alternative anti-infective strategies to combat AMR.Targeting quorum sensing (QS) regulated virulence factors and biofilm development are considered as promising anti-infective drug targets to combat the AMR problem.10 QS is a microbial cell to cell communication system in which microbes express their certain genes only when their population reaches a certain threshold limit. Bacteria synthesize and secrete certain signal molecules called autoinducers (AIs). The concentration of AIs increases with the increase in the bacterial population. When the population density of bacteria reaches a certain limit, a certain set of genes are expressed by transcriptional regulation.11 The AIs in Gram negative bacteria are N-homoserine lactones (AHLs), and autoinducer (AI) peptides in Gram positive bacteria. Many virulent traits of bacteria, as well as the production of antibiotic degrading enzymes, are controlled via QS. The previous assumption about the growth of bacteria was that they live in a planktonic state, but later it was found that most bacteria live in complex structures called biofilms. Biofilms are comprised of microbial communities and extracellular polymeric substances (EPS) that act as a protective barrier.12 In the biofilm mode of growth, the expression of some phenotypes of bacteria are different from those in planktonic growth. The importance of biofilms can be understood by considering that the National Institute of Health (NIH) estimates that nearly 80% of microbial infections are encouraged and established by biofilms.13,14 The vast majority of infections are caused by the development of biofilms, either by pathogenic or opportunistic pathogens.15,16
A new approach in the discovery of antimicrobials is to target the bacterial QS and biofilms using green synthesized nanoparticles. Previous reports have indicated that silver nanoparticles may prove to be useful as alternative antimicrobial agents.17 Nanotechnology has gained vast attention in scientific research owing to the possibly of its application in medicine, bioremediation, diagnostics, agriculture and so on.18 Many materials produce better biological effects in the nano form compared to their bulk state, mainly because they exhibit different physical and chemical properties at this scale. It is anticipated that nanotechnology has possible applications in many disciplines of health care, such as for use as novel drugs, in drug delivery, diagnostics, and improved biomaterials (medical devices). There are certain risks associated with the use of nanoparticles in medicine that include toxicity to the host's system. There are numerous approaches for the synthesis of metal nanoparticles, such as biological, chemical, and physical methods. The major disadvantages of the chemical methods are the formation of hazardous and non-biodegradable byproducts that are toxic to the environment. The ‘green synthesis’ approach is gaining significant attention as it minimizes waste production and uses safer (non-toxic) solvents/materials, making this approach more environmentally friendly.19
Previously, many studies have demonstrated the efficacy of green synthesized nanoparticles against drug resistant bacteria.17 In this study, the aqueous extract from M. koenigii was used to synthesize silver nanoparticles (AgNPs-MK). The effect of AgNPs-MK was tested for the broad-spectrum inhibition of biofilm formation and QS mediated virulence factors of three Gram negative bacteria, viz. C. violaceum 12472, P. aeruginosa PAO1, and S. marcescens MTCC 97.
2. Materials and methods
2.1. Materials and reagents
Azocasein and elastin congo red (ECR) were purchased from Sigma Aldrich, USA. Trichloroacetic acid (TCA), orcinol and bacteriological culture media were procured from HiMedia Laboratories, Mumbai, India. 2,3,5-Triphenyltetrazolium chloride (TTC) was purchased from SRL Pvt. Ltd.2.2. Synthesis of AgNPs-MK
The silver nanoparticles (AgNPs-MK) were synthesized using an aqueous extract of Murraya koenigii. Detailed information on the AgNPs-MK synthesis and the characterization data has been published previously.20 Briefly, an aqueous extract of M. koenigii leaves was prepared by dissolving 5 g plant material in 100 ml double distilled water. For the synthesis of the silver nanoparticles, 0.5 ml of the extract was mixed with 20 ml AgNO3 solution (1 mM) and placed on a magnetic stirrer for 4 h. The colour of the reaction mixture changed to dark brown indicating the formation of silver nanoparticles. The nanoparticles (MK-AgNPs) were harvested by centrifugation and washed thrice with distilled water. The particles were then dried in an oven at 60 °C and stored for further use. The nanoparticles were characterized using UV-vis spectroscopy, X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), scanning electron microscopy, and energy dispersive X-ray (EDX) analysis. The addition of the extract to silver nitrate solution resulted in a colour change of the reaction mixture. AgNPs-MK exhibited a surface plasmon resonance (SPR) band at 410 nm, this indicated the formation of silver nanoparticles. TEM analysis revealed that the size of the AgNPs-MK ranged from 5 to 20 nm. Moreover, most of the nanoparticles were spheroidal in shape and a few nanoparticles exhibited an anisotropic morphology. The average size of the AgNPs-MK calculated from the XRD data was 13.54 nm.2.3. Determination of minimum inhibitory concentrations
The minimum inhibitory concentration (MIC) of the AgNPs-MK against tested bacterial strains was determined using the microbroth dilution method using TTC as a growth indicator dye.21 The AgNPs-MK (10 μl) were added to 190 μl Luria Bertani broth in a 96-well microtiter plate and were diluted two-fold to give treatments of varying concentrations. The diluted culture (100 μl, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) of bacteria from the log phase was added as an inoculum in each well. The microtitre plate was incubated overnight at the respective optimum growth temperatures of each bacterium. TTC (20 μl, 2 mg ml−1) was added to each well and the plate was incubated at 37 °C for 30 min in the dark. The wells were examined for colour change. The development of a pink/red colour indicated the presence of actively growing cells, while no colour change showed a lack of bacterial growth. Wells showing no change in colour were spotted on Luria-Bertani (LB) agar plates to further confirm the growth inhibition.
50) of bacteria from the log phase was added as an inoculum in each well. The microtitre plate was incubated overnight at the respective optimum growth temperatures of each bacterium. TTC (20 μl, 2 mg ml−1) was added to each well and the plate was incubated at 37 °C for 30 min in the dark. The wells were examined for colour change. The development of a pink/red colour indicated the presence of actively growing cells, while no colour change showed a lack of bacterial growth. Wells showing no change in colour were spotted on Luria-Bertani (LB) agar plates to further confirm the growth inhibition.
2.4. Quantitative determination of the violacein pigment in C. violaceum 12472
The quantitative assessment of violacein production was performed following the standard procedure.22 In short, C. violaceum 12472 was grown in the absence and presence of varying sub-MICs of AgNPs-MK for 18 h at 30 °C. The grown culture (1 ml) was centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 5 min to separate out the insoluble pigment (violacein) and microbial cells. The pellet obtained was resuspended in DMSO in a volume of 1 ml and vortexed vigorously (5 min) to dissolve the pigment. The suspension was again centrifuged to spin down the debris from the bacterial cells. The supernatant was collected and its absorbance was recorded at 585 nm using a UV-2600 spectrophotometer, Shimadzu, Japan.
000 rpm) for 5 min to separate out the insoluble pigment (violacein) and microbial cells. The pellet obtained was resuspended in DMSO in a volume of 1 ml and vortexed vigorously (5 min) to dissolve the pigment. The suspension was again centrifuged to spin down the debris from the bacterial cells. The supernatant was collected and its absorbance was recorded at 585 nm using a UV-2600 spectrophotometer, Shimadzu, Japan.
2.5. Assays for the inhibition of the virulence factors of S. marcescens MTCC 97
Various QS regulated virulence factors of S. marcescens MTCC 97 were determined in the presence and absence of AgNPs-MK at sub-minimum inhibitory concentrations (MICs). The assays used for determination of individual virulence factors are briefly described below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to pellet down the bacterial cells. The pellet was dissolved in 1 ml of acidified ethanol (96 ml ethanol + 4 ml 1 M HCl) by vigorous vortexing for 5 min. The sample was again centrifuged to remove the debris and the absorbance of the supernatant was recorded at 534 nm using a UV-2600 spectrophotometer.
000 rpm for 5 min to pellet down the bacterial cells. The pellet was dissolved in 1 ml of acidified ethanol (96 ml ethanol + 4 ml 1 M HCl) by vigorous vortexing for 5 min. The sample was again centrifuged to remove the debris and the absorbance of the supernatant was recorded at 534 nm using a UV-2600 spectrophotometer.2.6. Determination of the virulence factors of P. aeruginosa PAO1
Various QS regulated virulence factors of P. aeruginosa PAO1 were examined in the absence and presence of AgNPs-MK. The methods used for determination of individual virulence factors are briefly described below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to pellet down the bacterial cells. Pyocyanin present in the cell free supernatant was extracted in chloroform (3 ml) by vortexing and the aqueous phase was discarded. The organic (chloroform) phase was re-extracted in 1.2 ml of 0.2 N HCl. The absorbance of the aqueous phase was recorded at 520 nm using a UV-2600 spectrophotometer against 0.2 N HCl as blank.
000 rpm for 5 min to pellet down the bacterial cells. Pyocyanin present in the cell free supernatant was extracted in chloroform (3 ml) by vortexing and the aqueous phase was discarded. The organic (chloroform) phase was re-extracted in 1.2 ml of 0.2 N HCl. The absorbance of the aqueous phase was recorded at 520 nm using a UV-2600 spectrophotometer against 0.2 N HCl as blank.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min) to remove the insoluble azocasein and the absorbance of the supernatant was recorded at 400 nm using a UV-2600 spectrophotometer.
000 rpm for 5 min) to remove the insoluble azocasein and the absorbance of the supernatant was recorded at 400 nm using a UV-2600 spectrophotometer.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min) and the absorbance was recorded at 495 nm using a UV-2600 spectrophotometer.
000 rpm for 5 min) and the absorbance was recorded at 495 nm using a UV-2600 spectrophotometer.2.7. Assays to measure biofilm inhibition
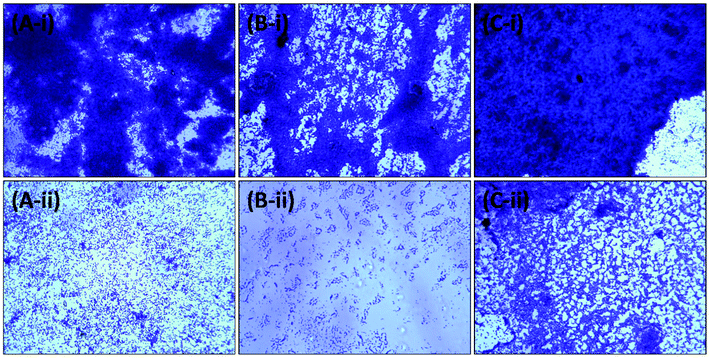
2.7.2.1. Light microscopy. Briefly, 60 μl of cultures of test bacteria grown overnight were inoculated into 24-well culture plates containing 3 ml of culture media. Sterile glass coverslips (1 × 1 cm) and the respective highest sub-MICs of the AgNPs-MK were added to the wells. The control group was not subjected to any treatment. After 24 h of incubation, the loosely bound cells were removed from the coverslips by gentle washing with phosphate buffer and air dried for 20 min at room temperature. The coverslips containing the biofilms were stained with 0.1% w/v crystal violet solution for 15 min. The excess dye was washed away and the slides were finally air dried for 30 min. The biofilms were visualized under a light microscope (Olympus BX60, Model BX60F5, Olympus Optical Co. Ltd Japan) equipped with a colour VGA camera (Sony, Model no. SSC-DC-58AP, Japan). The images were captured at 40× magnification.
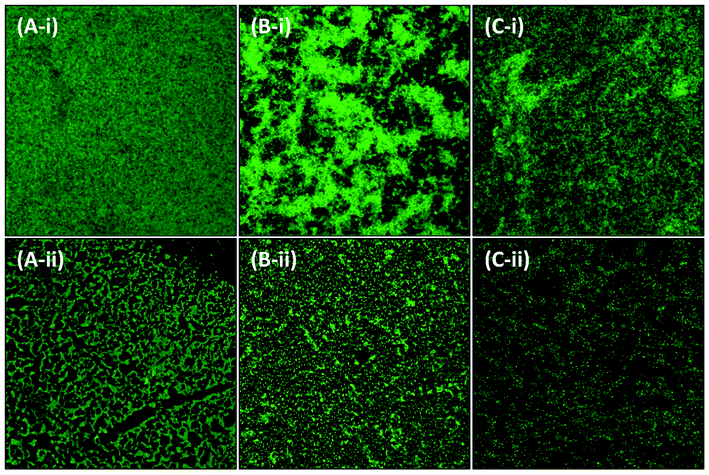
2.7.2.2. Confocal laser scanning microscopy. The biofilms were formed on glass coverslips in the absence and presence of AgNPs-MK as mentioned in the light microscopy section. After 24 h of incubation, the loosely bound cells were removed from the coverslips by gentle washing with sterile phosphate buffer and air dried for 20 min at room temperature. The coverslips were then stained with 0.1% acridine orange for 20 min and air dried for 60 min at room temperature in the dark. The biofilms were visualized using Zeiss LSM780, at University Sophisticated Instrumentation Facility (USIF), AMU, Aligarh, and images were captured at 63× magnification.
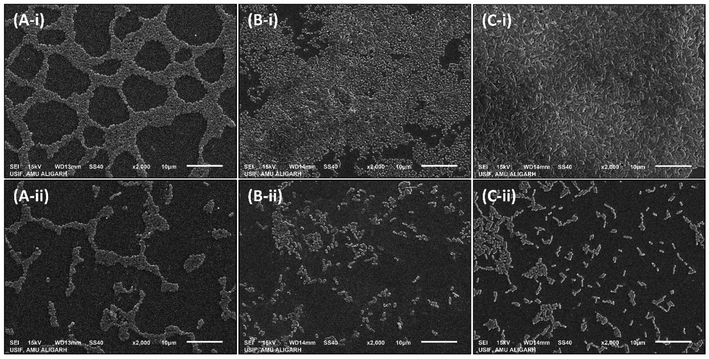
2.7.2.3. Scanning electron microscopy. The biofilms were formed on glass coverslips in the absence and presence of AgNPs-MK as mentioned in the light microscopy section. The coverslips were washed to remove loosely adhered cells and air dried. The biofilms were fixed with glutaraldehyde (2.5% in 50 mM phosphate buffer) at 4 °C for 24 h. The cells in biofilms were dehydrated using an increasing gradient (20 to 100%) of ethanol. The glass coverslips were air dried and coated with gold before visualization. The biofilms were visualized and the images were captured using a JEOL-JSM 6510 LV at USIF, AMU, Aligarh.
2.8. Statistical analysis
All experiments were performed as three independent replicates and the data presented is the mean with standard deviation. The statistical significance of the data was determined by calculating the P-value using the t-test with respect to control. A P-value of less than 0.05 with respect to the control was considered as significant and is denoted with *.3. Results and discussion
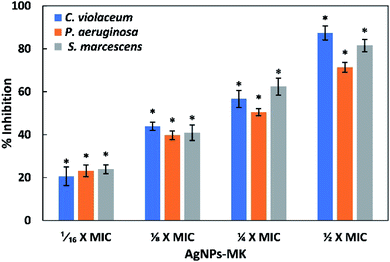
3.1. MICs of AgNPs-MK
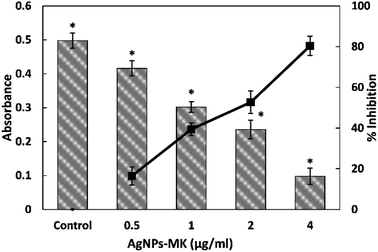
The MICs of the AgNPs-MK against C. violaceum 12472, P. aeruginosa PAO1, and S. marcescens MTCC 97 were found to be 8, 16, and 16 μg ml−1, respectively. The effect of AgNPs-MK on biofilm formation and QS regulated virulence factors was tested below the inhibitory concentrations (sub-MIC).3.2. Effect of AgNPs-MK on the QS-mediated virulence factors of C. violaceum 12472
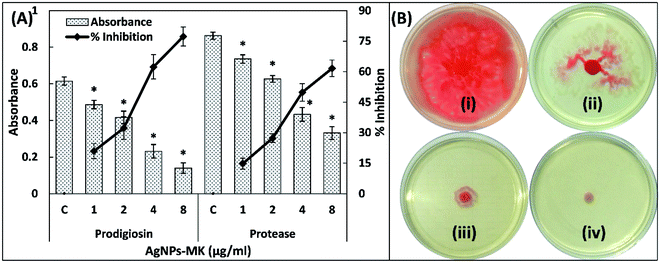
3.3. Inhibition of the virulence factors of S. marcescens MTCC 97 by AgNPs-MK
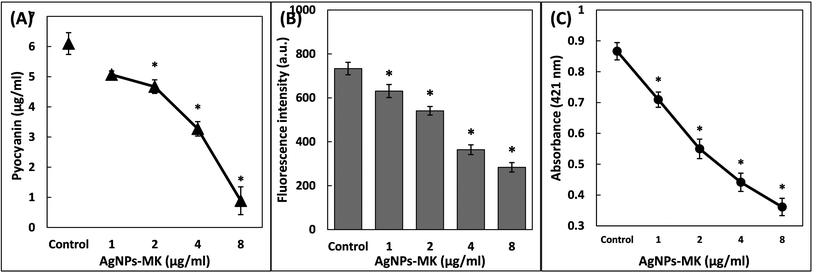
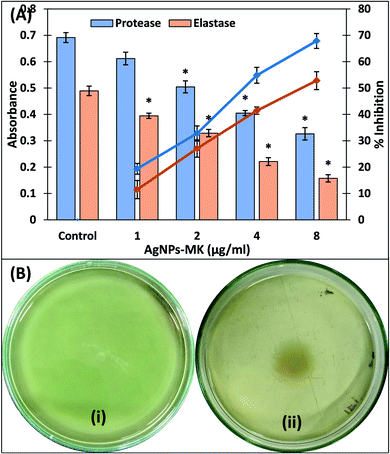
3.4. Inhibition of the virulence factors of P. aeruginosa PAO1 by AgNPs-MK
3.5. Inhibition of the development of biofilms by AgNPs-MK
A previously published study reported that nanocrystalline silver particles significantly decreased the viability of bacterial cells within biofilms of P. aeruginosa and up to 90% of bacterial cells within biofilms were killed, even at lower doses of silver nanoparticles. The study concluded that the possible application of silver nanoparticles in dressings may improve the wound by killing the bacterial cells residing in biofilms.58 The decrease in the viability of bacterial cells in biofilms has been confirmed by double staining (propidium iodide and Con A-FITC) in which the 3D structure of the biofilm was disrupted and a decrease in the glycocalyx matrix was found by the treatment of silver nanoparticles.59 Therefore, the data clearly validated the broad-spectrum antibiofilm activity of the AgNPs-MK synthesized using M. koenigii extract against Gram negative bacterial pathogens.
4. Conclusions
Greenly synthesized AgNPs-MK remarkably reduced the multiple QS-regulated functions of Gram negative bacterial pathogens, such as P. aeruginosa PAO1, S. marcescens MTCC 97, and C. violaceum 12472. An inhibition of violacein pigment production in C. violaceum 12472 of more than 80% was recorded. The QS-controlled virulent traits of S. marcescens MTCC 97 were also reduced by up to 90% upon treatment with AgNPs-MK. The nanoparticles also inhibited the virulence factors of P. aeruginosa PAO1 in a dose-dependent manner at sub-MICs. The formation of biofilms of all test bacteria was decreased by more than 70% at their highest respective sub-MICs. Moreover, the formation of biofilms on the surfaces of glass coverslips was remarkably inhibited, and the aggregation and formation of EPS were almost not visible. In conclusion, greenly synthesized AgNPs-MK could be exploited for the treatment of topical/skin infections. Moreover, they may also be used for coating the surfaces of medical implants/devices to prevent bacterial adherence and the development of biofilms. Further in vivo assays are needed to evaluate the therapeutic efficacy against infections caused by drug resistant bacterial pathogens.Abbreviations
| AgNPs-MK | Silver nanoparticles synthesized using M. koenigii extract |
| AHL | Acyl homoserine lactone |
| AI | Autoinducer |
| CLSM | Confocal laser scanning microscopy |
| ECR | Elastin congo red |
| QS | Quorum sensing |
| SEM | Scanning electron microscopy |
Data availability
The data that support the findings of this study are available from the corresponding author (IA) upon reasonable request.Conflicts of interest
The authors declare that there are no conflicts of interest. We wish to confirm that there are no known conflicts of interest associated with this publication.Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group project number RG-1439-076. FAQ is thankful to CSIR [File no. 09/112(0626)2k19 EMR] for providing SRF.References
- P. Vikesland, E. Garner, S. Gupta, S. Kang, A. Maile-Moskowitz and N. Zhu, Acc. Chem. Res., 2019, 52, 916–924 CrossRef CAS.
- WHO, The Top 10 Causes of Death, 2018 Search PubMed.
- S. Shakoor, J. A. Platts-Mills and R. Hasan, Infect. Dis. Clin. North Am., 2019, 33, 1105–1123 CrossRef.
- S. Andleeb, M. Majid and S. Sardar, in Antibiotics and Antimicrobial Resistance Genes in the Environment, Elsevier, 2020, pp. 269–291 Search PubMed.
- P. Dadgostar, Infect. Drug Resist., 2019, 12, 3903–3910 CrossRef CAS PubMed.
- WHO, No Time to Wait: Securing the Future from Drug-Resistant Infections, 2019 Search PubMed.
- I. Ahmad, F. A. Qais, Samreen, H. H. Abulreesh, S. Ahmad and K. P. Rumbaugh, in Antibacterial Drug Discovery to Combat MDR, Springer Singapore, Singapore, 2019, pp. 1–21 Search PubMed.
- W. C. Albrich, D. L. Monnet and S. Harbarth, Emerg. Infect. Dis., 2004, 10, 514–517 CrossRef PubMed.
- K. Miller, J. Antimicrob. Chemother., 2002, 49, 925–934 CrossRef CAS PubMed.
- P. Piewngam, J. Chiou, P. Chatterjee and M. Otto, Expert Rev. Anti Infect. Ther., 2020, 18, 499–510 CrossRef CAS PubMed.
- D. G. Davies, M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton and E. P. Greenberg, Science, 1998, 280, 295–298 CrossRef CAS PubMed.
- D. Lopez, H. Vlamakis and R. Kolter, Cold Spring Harbor Perspect. Biol., 2010, 2, a000398 Search PubMed.
- R. M. Donlan, Clin. Infect. Dis., 2001, 33, 1387–1392 CrossRef CAS PubMed.
- B. Schachter, Nat. Biotechnol., 2003, 21, 361–365 CrossRef CAS.
- N. Martins and C. F. Rodrigues, J. Clin. Med., 2020, 9, 722 CrossRef.
- I. Lasa, J. L. del Pozo, J. R. Penadés and J. Leiva, An. Sist. Sanit. Navar., 2005, 28, 163–175 CAS.
- F. A. Qais, M. S. Khan and I. Ahmad, in Biotechnological Applications of Quorum Sensing Inhibitors, ed. V. C. Kalia, Springer Singapore, Singapore, 2018, pp. 227–244 Search PubMed.
- M. Valcárcel and Á. I. López-Lorente, Trac. Trends Anal. Chem., 2016, 84, 1–2 CrossRef.
- J. Singh, T. Dutta, K.-H. Kim, M. Rawat, P. Samddar and P. Kumar, J. Nanobiotechnol., 2018, 16, 84 CrossRef CAS.
- F. A. Qais, A. Shafiq, H. M. Khan, F. M. Husain, R. A. Khan, B. Alenazi, A. Alsalme and I. Ahmad, Bioinorg. Chem. Appl., 2019, 2019, 4649506 Search PubMed.
- J. Eloff, Planta Med., 1998, 64, 711–713 CrossRef CAS.
- C. Matz, P. Deines, J. Boenigk, H. Arndt, L. Eberl, S. Kjelleberg and K. Jurgens, Appl. Environ. Microbiol., 2004, 70, 1593–1599 CrossRef CAS.
- H. Slater, M. Crow, L. Everson and G. P. C. Salmond, Mol. Microbiol., 2003, 47, 303–320 CrossRef CAS.
- R. Salini and S. K. Pandian, Pathog. Dis., 2015, 73, ftv038 Search PubMed.
- D. W. Essar, L. Eberly, A. Hadero and I. P. Crawford, J. Bacteriol., 1990, 172, 884–900 CrossRef CAS.
- R. Ankenbauer, S. Sriyosachati and C. D. Cox, Infect. Immun., 1985, 49, 132–140 CrossRef CAS PubMed.
- F. M. Husain, I. Ahmad, A. S. Al-Thubiani, H. H. Abulreesh, I. M. AlHazza and F. Aqil, Front. Microbiol., 2017, 8, 727 CrossRef.
- E. Kessler, M. Israel, N. Landshman, A. Chechick and S. Blumberg, Infect. Immun., 1982, 38, 716–723 CrossRef CAS PubMed.
- G. A. O'Toole and R. Kolter, Mol. Microbiol., 1998, 30, 295–304 CrossRef.
- C. Fuqua, M. R. Parsek and E. P. Greenberg, Annu. Rev. Genet., 2001, 35, 439–468 CrossRef CAS PubMed.
- F. A. Qais, A. Shafiq, I. Ahmad, F. M. Husain, R. A. Khan and I. Hassan, Microb. Pathog., 2020, 144, 104172 CrossRef PubMed.
- J.-R. Wei and H.-C. Lai, Int. J. Med. Microbiol., 2006, 296, 117–124 CrossRef CAS PubMed.
- P. Goluszko, B. Nowicki, E. Goluszko, S. Nowicki, A. Kaul and T. Pham, FEMS Microbiol. Lett., 1995, 133, 41–45 CrossRef CAS PubMed.
- G. Y. Liu and V. Nizet, Trends Microbiol., 2009, 17, 406–413 CrossRef CAS.
- G. W. Lau, D. J. Hassett, H. Ran and F. Kong, Trends Mol. Med., 2004, 10, 599–606 CrossRef CAS PubMed.
- R. Srinivasan, L. Vigneshwari, T. Rajavel, R. Durgadevi, A. Kannappan, K. Balamurugan, K. Pandima Devi and A. Veera Ravi, Environ. Sci. Pollut. Res., 2018, 25, 10538–10554 CrossRef CAS.
- R. Coria-Jiménez, C. Zárate-Aquino and O. Ponce-Ponce, Folia Microbiol., 2004, 49, 321–326 CrossRef PubMed.
- V. Braun, H. Gunther, B. Neub and C. Tautz, Arch. Microbiol., 1985, 141, 371–376 CrossRef CAS PubMed.
- Y. Kida, H. Inoue, T. Shimizu and K. Kuwano, Infect. Immun., 2007, 75, 164–174 CrossRef CAS PubMed.
- A. Alagely, C. J. Krediet, K. B. Ritchie and M. Teplitski, ISME J., 2011, 5, 1609–1620 CrossRef CAS PubMed.
- L. E. P. Dietrich, A. Price-Whelan, A. Petersen, M. Whiteley and D. K. Newman, Mol. Microbiol., 2006, 61, 1308–1321 CrossRef CAS PubMed.
- J. L. Fothergill, S. Panagea, C. A. Hart, M. J. Walshaw, T. L. Pitt and C. Winstanley, BMC Microbiol., 2007, 7, 45 CrossRef PubMed.
- T. Das, S. K. Kutty, R. Tavallaie, A. I. Ibugo, J. Panchompoo, S. Sehar, L. Aldous, A. W. S. Yeung, S. R. Thomas, N. Kumar, J. J. Gooding and M. Manefield, Sci. Rep., 2015, 5, 8398 CrossRef CAS PubMed.
- M. E. Peek, A. Bhatnagar, N. A. McCarty and S. M. Zughaier, Interdiscip. Perspect. Infect. Dis., 2012, 2012, 1–10 CrossRef PubMed.
- C. Cox and P. Adams, Infect. Immun., 1985, 48, 130–138 CrossRef CAS PubMed.
- M. E. Davey, N. C. Caiazza and G. A. O'Toole, J. Bacteriol., 2003, 185, 1027–1036 CrossRef CAS PubMed.
- T. Köhler, L. K. Curty, F. Barja, C. van Delden and J. C. Pechère, J. Bacteriol., 2000, 182, 5990–5996 CrossRef PubMed.
- R. S. Smith, B. H. Iglewski and H. Barbara, Curr. Opin. Microbiol., 2003, 6, 56–60 CrossRef CAS.
- B. R. Singh, B. N. Singh, A. Singh, W. Khan, A. H. Naqvi and H. B. Singh, Sci. Rep., 2015, 5, 13719 CrossRef PubMed.
- P. A. Bejarano, J. P. Langeveld, B. G. Hudson and M. E. Noelken, Infect. Immun., 1989, 57, 3783–3787 CrossRef CAS PubMed.
- H. Yu, X. He, W. Xie, J. Xiong, H. Sheng, S. Guo, C. Huang, D. Zhang and K. Zhang, Can. J. Microbiol., 2014, 60, 227–235 CrossRef CAS.
- S. A. Beatson, C. B. Whitchurch, A. B. T. Semmler and J. S. Mattick, J. Bacteriol., 2002, 184, 3598–3604 CrossRef CAS PubMed.
- S. Wang, S. Yu, Z. Zhang, Q. Wei, L. Yan, G. Ai, H. Liu and L. Z. Ma, Appl. Environ. Microbiol., 2014, 80, 6724–6732 CrossRef PubMed.
- A. Pompilio, V. Crocetta, S. De Nicola, F. Verginelli, E. Fiscarelli and G. Di Bonaventura, Front. Microbiol., 2015, 6, 951 Search PubMed.
- J. C. Nickel, I. Ruseska, J. B. Wright and J. W. Costerton, Antimicrob. Agents Chemother., 1985, 27, 619–624 CrossRef CAS PubMed.
- S. G. Ali, M. A. Ansari, H. M. Khan, M. Jalal, A. A. Mahdi and S. S. Cameotra, Bionanoscience, 2018, 8, 544–553 CrossRef.
- K. Ali, B. Ahmed, S. Dwivedi, Q. Saquib, A. A. Al-Khedhairy and J. Musarrat, PLoS One, 2015, 10, 1–20 Search PubMed.
- V. Kostenko, J. Lyczak, K. Turner and R. J. Martinuzzi, Antimicrob. Agents Chemother., 2010, 54, 5120–5131 CrossRef CAS PubMed.
- M. A. Ansari, H. M. Khan, A. A. Khan, S. S. Cameotra and M. A. Alzohairy, Indian J. Med. Microbiol., 2015, 33, 101 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |