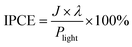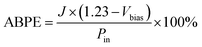Open Access Article
Open Access ArticleEfficient charge separation and transfer of a TaON/BiVO4 heterojunction for photoelectrochemical water splitting†
Na Liab,
Yi Jiang *a,
Xiaodi Wanga,
Chongyang Hua,
Wenchao Jianga,
Siyuan Lia and
Lixin Xia
*a,
Xiaodi Wanga,
Chongyang Hua,
Wenchao Jianga,
Siyuan Lia and
Lixin Xia *ab
*ab
aCollege of Chemistry, Liaoning University, Shenyang 110036, Liaoning, China. E-mail: jiangyi@lnu.edu.cn; lixinxia@lnu.edu.cn
bDepartment of Chemical and Environmental Engineering, Yingkou Institute of Technology, Yingkou, 115014, Liaoning, China
First published on 8th April 2021
Abstract
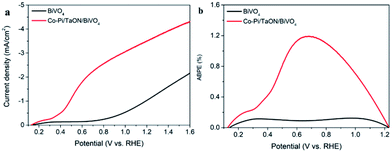
The separation and transfer of photogenerated electron–hole pairs in semiconductors is the key point for photoelectrochemical (PEC) water splitting. Here, an ideal TaON/BiVO4 heterojunction electrode was fabricated via a simple hydrothermal method. As BiVO4 and TaON were in well contact with each other, high performance TaON/BiVO4 heterojunction photoanodes were constructed. The photocurrent of the 2-TaON/BiVO4 electrode reached 2.6 mA cm−2 at 1.23 V vs. RHE, which is 1.75 times as that of the bare BiVO4. TaON improves the PEC performance by simultaneously promoting the photo-generated charge separation and surface reaction transfer. When a Co-Pi co-catalyst was integrated onto the surface of the 2-TaON/BiVO4 electrode, the surface water oxidation kinetics further improved, and a highly efficient photocurrent density of 3.6 mA cm−2 was achieved at 1.23 V vs. RHE. The largest half-cell solar energy conversion efficiency for Co-Pi/TaON/BiVO4 was 1.19% at 0.69 V vs. RHE, corresponding to 6 times that of bare BiVO4 (0.19% at 0.95 V vs. RHE). This study provides an available strategy to develop photoelectrochemical water splitting of BiVO4-based photoanodes.
Introduction
At present, the shortage of fossil fuels and increase of global warming have become very serious social and environmental problems. As a green energy source, solar energy is widely distributed. Hydrogen is also a type of clean energy with a high heating value. Therefore, the use of solar energy to produce hydrogen has attracted widespread attention.1 Building a photoelectrochemical (PEC) cell to mimic the photosynthesis system is an effective method to realize the conversion of solar energy into hydrogen energy.2–6 However, in a PEC system, the water oxidation reaction half reaction occurring in the photoanode involves light capture, charge separation, and complex surface reaction with multiple electron and proton transfer. Therefore, designing an efficient photoanode to accelerate the water oxidation process becomes the key to the development of PEC cells.In recent years, semiconductors based on metal oxides, such as BiVO4, TiO2, and WO3, have been widely used as photoanode materials.7–11 BiVO4, an n-type semiconductor with a band gap of 2.4 eV, is considered as one of the most promising alternative materials.12 Under the irradiation of a sunlight AM 1.5 G, the solar-to-hydrogen efficiency (STH) can reach 9.2%,13 which is close to the solar-powered water decomposition technology target (STH efficiency 10%).14 However, the severe recombination of the electron–hole pair of BiVO4 limits its development. Different strategies were adopted to enhance the PEC performance of BiVO4,15,16 such as elemental doping, nanostructure modification, loading cocatalysts and heterojunction construction. Among them, the construction of semiconductor heterojunctions with different materials is an effective approach to improve the PEC performance by enhancing the separation efficiency of photo-generated charges. Although there were numerous reports on heterojunction systems for BiVO4-based semiconductors, such as CaFe2O4/BiVO4,17 V2O5/BiVO4,18 WO3/BiVO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19–21 and TiO2/BiVO4,22,23 the construction of a high-efficiency heterojunction photoanode still faces great challenges.
19–21 and TiO2/BiVO4,22,23 the construction of a high-efficiency heterojunction photoanode still faces great challenges.
Here, TaON/BiVO4 heterojunction photoanodes were constructed, which exhibited a greatly improved PEC water oxidation activity compared to the bare BiVO4 photoanode. The photocurrent of the 2-TaON/BiVO4 photoanode reached 2.6 mA cm−2 at 1.23 V vs. RHE, which was 1.75-times that of BiVO4. In order to enhance the surface water oxidation kinetics of the photoanode, the Co-Pi water oxidation catalyst was immobilized on the photoanode, and the PEC performance was further promoted. A high photocurrent density of 3.6 mA cm−2 was obtained for Co-Pi/TaON/BiVO4 at 1.23 V vs. RHE.
Experimental section
Fabrication of the BiVO4 photoanode
A BiVO4 electrode was prepared according to the electrodeposition method reported in literature.24 150 mL of deionized water was taken in a small beaker, and nitric acid was added to adjust the pH to 1.7. Then, 2.91 g of bismuth nitrate and 9.96 g of potassium iodide were added to the above mixture, and sonicated for about 30 min to fully dissolve. The above solution was mixed with an ethanol solution containing 0.23 M of p-benzoquinone, and sufficiently stirred to prepare an electrodeposition solution. An appropriate amount of the above-mentioned deposition solution was placed in a small beaker, and a three-electrode cell was used for electrodeposition (FTO was used as a working electrode, Pt was used as a counter electrode, and Ag/AgCl was used a reference electrode). At room temperature, after depositing for about 300 s at an applied bias of −0.1 V vs. Ag/AgCl, we obtained a BiOI film, which was washed with deionized water and blown with nitrogen. Then, a DMSO solution-containing 0.2 M acetylacetonoxane was added dropwise to the deposited layer, and calcined at 450 °C for 2 h (2 °C min−1) to convert BiOI into BiVO4. Finally, it was immersed in a 1 M aqueous solution of sodium hydroxide for about 10 min in order to remove excess V2O5, rinsed with deionized water, and blown dry with nitrogen for use.Fabrication of the TaON/BiVO4 photoanode
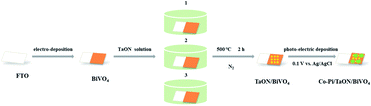
TaON nanoparticles were prepared by heating Ta2O5 particles at 1123 K for 15 h under NH3 flow (20 mL min−1).25 A certain amount of TaON (1, 0.02 g; 2, 0.04 g; 3, 0.06 g) was added to 100 mL of deionized water, sonicated to make a sol, and the BiVO4 photoanodes were separately immersed in different concentrations TaON sol at 60 °C for 1 h, and calcined at 500 °C for 2 h under N2 flow.Fabrication of the Co-Pi/TaON/BiVO4 photoanode
The Co-Pi cocatalyst was loaded by photoelectron deposition according to literature.26 The 2-TaON/BiVO4 photoanode was immersed in a phosphate buffer solution-containing a certain concentration of Co(NO3)2·6H2O. Deposition was carried out at an applied bias of 0.1 V vs. Ag/AgCl and the sun illumination (100 mA cm−2) for 300 s.Characterization of photoanodes
The XRD pattern was measured used a Bruker D8 QUEST. The SEM images were recorded on a SU8000 Schottky field emission scanning electron microscope (SFE-SEM) equipped with a Rontec EDX system. The UV-Vis absorbance spectra were recorded on a Lambda 35 UV-Vis spectrophotometer. X-ray photoelectron spectra (XPS) were recorded on a Thermo Scientific ESCALAB 250 instrument (150 W, spot size of 500 μm and Al Kα radiation at 1486.6 eV) to obtain the surface elements. Photoluminescence (PL) spectroscopy measurement was performed on a Cary Eclipse spectrophotometer.Photoelectrochemical measurements
The PEC performance tests of the electrodes were carried out using a standard three-electrode cell using an Ag/AgCl (3.5 M KCl) as the reference electrode, a platinum wire as the counter electrode and samples as the working electrode. The electrolyte used was a 0.1 M potassium buffer solution (pH 7.0) that was a mixture of mono- and dibasic hydrophosphates KH2PO4 and K2HPO4. The light intensity of the solar simulator (AM 1.5 G) was calibrated to 100 mW cm−2. The effective surface area of the electrodes was 1 cm2. The electrochemical impedance spectroscopy (EIS) was measured with the range from 0.1 Hz to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. The Mott–Schottky curves were obtained under dark conditions, and the potential range was from −0.6 V to +1.0 V vs. Ag/AgCl.
000 Hz. The Mott–Schottky curves were obtained under dark conditions, and the potential range was from −0.6 V to +1.0 V vs. Ag/AgCl.
The incident photon-to-current efficiency (IPCE) at each wavelength was determined at 1.23 V vs. RHE using monochromatic light illumination from a 300 W Xe arc lamp and neutral density filters simulating sunlight. The IPCE values have been calculated as follows:
The applied bias photon-to-current efficiency (ABPE) was calculated from a J–V curve using the following equation:
The total water oxidation photocurrent JH2O was determined by the following expression:
| JH2O = Jabs × ηsep × ηtrans |
To obtain ηtrans, Na2SO3 was added into the 0.1 M PBS electrolyte, which was an efficient hole scavenger. Therefore, the ηtrans efficiency equal to 1, and the photocurrent could be described as JNa2SO3 = Jabs × ηsep. So, the ηsep could be described as:
| ηsep = JNa2SO3/Jabs. |
The ηtrans was calculated using the equation:
| ηtrans = JH2O/JNa2SO3 |
Results and discussion
BiVO4 films were fabricated via the electrochemical-deposition method. TaON nanoparticles were loaded onto the BiVO4 surface, as shown in Scheme 1. By controlling the amount of TaON nanoparticles, different TaON/BiVO4 electrodes were fabricated (denoted as 1-TaON/BiVO4, 2-TaON/BiVO4 and 3-TaON/BiVO4, respectively). The microstructure of the pristine BiVO4 is shown in Fig. 1a. A porous structure could be seen clearly in the whole area, which guaranteed the combination with TaON nanoparticles. The SEM images of TaON/BiVO4 electrodes containing different amounts of TaON are shown in Fig. 1. The distribution density of TaON nanoparticles increased with the amount of TaON (Fig. 1b–d). The crystal structures of the TaON, BiVO4 and TaON/BiVO4 samples were tested by X-ray diffraction (XRD) patterns, as shown in Fig. S1,† confirming that TaON has been successfully modified on the BiVO4 electrode.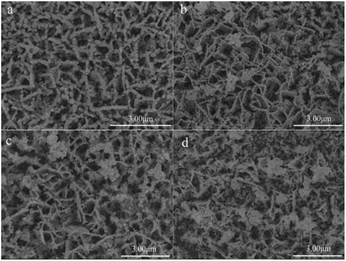 | ||
| Fig. 1 SEM images of different electrodes. (a) BiVO4, (b) 1-TaON/BiVO4, (c) 2-TaON/BiVO4, (d) 3-TaON/BiVO4. | ||
X-ray photoelectron spectroscopy (XPS) was performed to investigate the chemical states of the TaON/BiVO4 electrodes. Ta, O, N, Bi and V elements were detected and shown in Fig. 2. Compared to the pristine BiVO4, the characteristic signals of TaON can be observed in the spectrum of the TaON/BiVO4 electrode. The characteristic peaks of N 1s and Ta 4p are shown in Fig. 2a. The binding energies of 529.7 eV and 532.0 eV are assigned to the O 1s of BiVO4, and the O 1s peak of TaON appears at 530.6 eV (Fig. 2b). The binding energies of 159.1 eV and 164.4 eV were assigned to the 4f7/2 and 4f5/2 of the Bi element, confirming that the Bi element was present as Bi3+ (Fig. 2c).27 The binding energies for the V 2p3/2 and V 2p1/2 located at 516.6 and 524.2 eV were typical values for V5+ (Fig. 2d).28 The Bi 4f and V 2p peaks of the TaON/BiVO4 electrode shifted positively compared to that of the BiVO4 electrode, indicating an interaction between TaON and BiVO4.
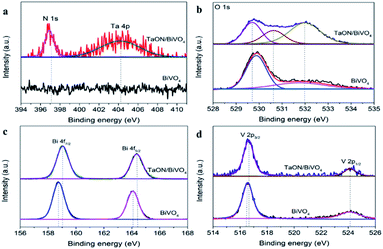 | ||
| Fig. 2 XPS spectra of BiVO4 and TaON/BiVO4 electrodes: (a) N 1s, Ta 4p, (b) O 1s, (c) Bi 4f, (d) V 2p. | ||
The PEC measurements of BiVO4 and TaON/BiVO4 were carried out using a three-electrode system under simulated solar light 100 mW cm−2. The linear sweep voltammetry (LSV) curves of the sample electrodes are shown in Fig. 3a. The photocurrent density of TaON/BiVO4 electrodes was significantly higher than that of the bare BiVO4 electrode. The 2-TaON/BiVO4 photoanode showed the highest photocurrent density, reaching 2.6 mA cm−2 at 1.23 V vs. RHE, which was 1.75-times that of bare BiVO4. However, the photocurrent density of 3-TaON/BiVO4 was lower than that of 2-TaON/BiVO4, suggesting that excessive TaON nanospheres hindered the charge transfer. The applied bias photon-to-current efficiencies (ABPEs) of BiVO4 and TaON/BiVO4 electrodes were calculated by the LSV curves (Fig. 3b). The maximum value of the 2-TaON/BiVO4 electrode reached 0.61% at 0.74 V, about 3-times of the BiVO4 electrode (0.19% at 0.95 V).
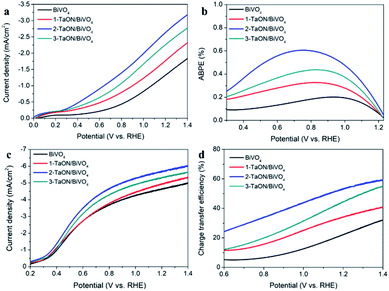 | ||
| Fig. 3 (a) LSV curves in the PBS solution, (b) ABPE values, (c) LSV curves in the PBS solution containing 0.1 M Na2SO3 and (d) the charge transfer efficiencies of different electrodes. | ||
In order to measure the charge recombination of the TaON/BiVO4 electrodes, 0.1 M Na2SO3 was added into the electrolyte as a hole scavenger for the PEC measurement.29 The introduction of Na2SO3 can eliminate the surface charge recombination. The PEC measurement results are shown in Fig. 3c. Compared to the bare BiVO4 electrode, all the TaON/BiVO4 electrodes showed higher photocurrent, which indicated that the combination of the two semiconductors produced a better charge separation. The 2-TaON/BiVO4 electrode showed the highest photocurrent density of 5.6 mA cm−2 at 1.23 V vs. RHE. Based on the LSV curves, the separation and surface charge transfer efficiencies of the sample electrodes are calculated and shown in Fig. S4† and 3d, respectively. All the TaON/BiVO4 electrodes exhibited higher ηsep and ηtrans than the bare BiVO4. 2-TaON/BiVO4 showed the highest values. These results indicated that the construction of TaON/BiVO4 could promote both charge separation and surface charge transfer.
The incident photon-to-current conversion efficiency (IPCE) values are shown in Fig. S2.† In the visible spectrum, TaON/BiVO4 showed a higher IPCE value than BiVO4. At 520 nm, the IPCE value dropped to 0, which was consistent with the absorption spectra (Fig. S3†).
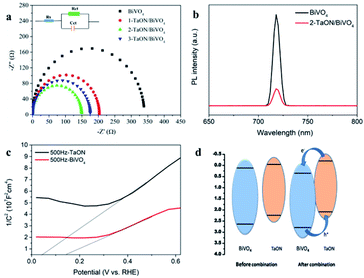
In order to understand the charge transfer characteristics of TaON/BiVO4 and BiVO4 electrodes, electrochemical impedance spectroscopy (EIS) tests were performed in 0.1 M PBS at 1.23 V. Smaller semicircles represent better charge transfer capabilities and faster surface reaction kinetics. As shown in Fig. 4a, the BiVO4 electrode exhibited the largest semicircle among all electrodes, indicating the highest interface charge transfer barrier. Three sample TaON/BiVO4 electrodes showed smaller charge transfer resistance than the BiVO4 electrode, further confirming that TaON was beneficial to improve the charge transfer. The interface charge transfer resistance (Rct) of the sample electrodes is shown in Table S1.† 2-TaON/BiVO4 showed the smallest charge transfer resistance, which was in accordance with the photocurrents.
To investigate the charge transfer behavior between BiVO4 and TaON, photoluminescence (PL) measurements were performed. As shown in Fig. 4b. BiVO4 electrode exhibited a high-intensity characteristic emission, suggesting an obvious radiative charge recombination. However, the TaON/BiVO4 electrode exhibited much lower emission intensity. The obvious photoluminescence quenching for TaON/BiVO4 electrode fully proved the formation of TaON/BiVO4 heterojunctions.
The Mott–Schottky plots of BiVO4 and TaON were also measured. As shown in Fig. 4c, it can be seen that the CB positions of BiVO4 and TaON are 0.15 eV and 0.05 eV. The band gap width of BiVO4 and TaON obtained from the absorption spectrum were 2.41 eV and 2.16 eV, respectively (Fig. S5†). The valence band positions of BiVO4 and TaON can be calculated by the formula using ECB = EVB − Eg, which were 2.56 eV and 2.21 eV. The CB edge potential of TaON was more negative than that of BiVO4. Thus, a difference of band potentials existed between the two materials, and a contact electric field was built at the interface of the TaON and BiVO4. When electrons and holes were photogenerated, as shown in Fig. 4d, driven by the contact electric field, electrons transferred from TaON to BiVO4 and holes transferred from BiVO4 to TaON, thereby leading to an enhancement both in photogenerated charge separation and transfer.
In order to further enhance the PEC performance, Co-Pi, which is well-used as highly efficient catalyst in PEC water oxidation, was equipped onto the TaON/BiVO4 electrode. The SEM image of the Co-Pi/TaON/BiVO4 electrode showed that Co-Pi appeared to be discontinuous particles (Fig. 5a). The EDS-mapping (Fig. 5b–h) suggested the existence of Bi, V, O, Ta, N, Co, and P elements, indicating the successful fabrication of the Co-Pi/TaON/BiVO4 electrode. According to the LSV curves shown in Fig. 6a, an obviously increased photocurrent density was obtained for the Co-Pi/TaON/BiVO4 photoanode, achieving 3.6 mA cm−2 at 1.23 V vs. RHE, which was about 3-times higher than that of the BiVO4 electrode. During a potentiostatic electrolysis at 0.8 V vs. RHE, the bare BiVO4 electrode always exhibited very low photocurrent density. For Co-Pi/TaON/BiVO4 high activity and stability was observed for more than 6000 s (Fig. S6†). The half-cell photoconversion efficiency of the Co-Pi/TaON/BiVO4 electrode achieved 1.19% at 0.69 V (Fig. 6a), approximately 6-times compared to that of BiVO4 electrode.
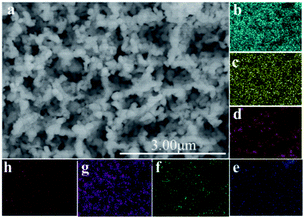 | ||
| Fig. 5 SEM image of the Co-Pi/TaON/BiVO4 electrode (a) and the EDS-mapping element of the Co-Pi/TaON/BiVO4 electrode. (b) Bi, (c) V, (d) O, (e) Ta, (f) N, (g) Co, (h) P. | ||
Conclusions
In summary, we have successfully fabricated TaON/BiVO4 heterojunction electrodes by integrating TaON nanoparticles onto BiVO4 electrodes. The as-prepared TaON/BiVO4 electrodes exhibited significantly higher performances for PEC water oxidation. The 2-TaON/BiVO4 electrode showed the highest photocurrent, reaching 2.6 mA cm−2 at 1.23 V vs. RHE, 1.75 times that of the bare BiVO4 electrode. In order to further increase the photocurrent, a Co-Pi water oxidation catalyst was attached onto the TaON/BiVO4 electrode. A high photocurrent density as high as 3.6 mA cm−2 (1.23 V vs. RHE) was obtained, and the half-cell photoconversion efficiency achieved 1.19% at 0.69 V, approximately 6-times that of the BiVO4 photoanode. This study provides a promising route to achieve highly efficient PEC water splitting in designing solar-to-fuel conversion devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21671089), the Liaoning Revitalization Talents Program (XLYC1807210), the Natural Science Foundation of Liaoning Province of China (2020-YKLH-22) and the Scientific Research Fund of Liaoning Provincial Education Department (L2020002).Notes and references
- M. E. El-Khouly, E. El-Mohsnawy and S. Fukuzumi, J. Photochem. Photobiol., C, 2017, 31, 36–83 CrossRef CAS.
- P. D. Frischmann, K. Mahata and F. Wurthner, Chem. Soc. Rev., 2013, 42, 1847–1870 RSC.
- J. Qi, W. Zhang and R. Cao, Adv. Energy Mater., 2018, 8, 1701620 CrossRef.
- R. L. Charles and M. B. Bart, Acc. Chem. Res., 2016, 49, 1121–1129 CrossRef PubMed.
- Y. Yi, N. Shuwen, H. Dongdong, L. Tianyu, W. Gongming and L. Yat, Adv. Energy Mater., 2017, 7, 1700555 CrossRef.
- W. Wei, X. Meigui, X. Xiaomin, Z. Wei and S. Zongping, Angew. Chem., Int. Ed., 2019, 5, 136–152 Search PubMed.
- M. Nurul Aida, S. Javad, I. Aznan Fazli, K. Muhammad Najib, J. Muhammad Fareez Amir Mohd, N. Mohamad Firdaus Mohamad, A. Nurul Affiqah, Z. Di, S. S. Jagdeep and T. Mohd Asri Mat, Mater. Res. Bull., 2020, 125, 110779 CrossRef.
- Z. Bo, Z. Haipeng, W. Zeyan, Z. Xiaoyang, Q. Xiaoyan, D. Ying, L. Yuanyuan, W. Peng, L. Yingjie and H. Baibiao, Appl. Catal., B, 2017, 211, 258–265 CrossRef.
- G. Qiqian, S. Xiaojuan, Y. Xijia, W. Liying, L. Xuesong, Z. Xueyu, D. Lianfeng and L. Wei, New J. Chem., 2019, 43, 8551–8556 RSC.
- S. Bo, S. Tielin, L. Zhiyong, T. Zirong, Z. Jianxin and L. Guanglan, RSC Adv., 2016, 6, 110120–110126 RSC.
- D. Paula, L. Tânia, M. Laura, A. Luísa and M. Adélio, Phys. Chem. Chem. Phys., 2016, 18, 5232–5243 RSC.
- K. C. Jason, G. Sheraz, M. T. Francesca, C. Le, L. Yi-Sheng, G. Jinghua, W. A. Joel, Y. Junko and D. S. Ian, J. Phys. Chem. C, 2015, 119, 2969–2974 CrossRef.
- P. Yuriy, T. Ivan, M. Kazuma, U. Jin, K. Yutaka, K. Sonya, M. Kikuo, S. Takeyoshi, M. Takuya, F. Daisuke, T. Masahiro, K. Michio and K. Takehiko, Sci. Rep., 2015, 8, 11141 Search PubMed.
- A. P. Blaise, D. B. Jesse, C. S. Linsey, J. F. Arnold, C. Zhebo, G. D. Todd, D. J. Brian, N. B. Kevin, N. B. George, A. Shane, W. Heli, M. Eric and F. J. Thomas, Energy Environ. Sci., 2013, 6, 1983–2002 RSC.
- H. Baobing, L. Yuchuan, H. Xing and X. Zailai, J. Mater. Chem. A, 2018, 6, 22277–22286 RSC.
- S. Xia, Z. Yu and J. Jiang, Chem. Commun., 2019, 55, 4813–4816 RSC.
- K. Eun Sun, K. Hyun Joon, M. Ganesan, K. Jae Young, J. Ji-Wook and L. Jae Sung, ACS Appl. Mater. Interfaces, 2014, 6, 17762–17769 CrossRef PubMed.
- J. Su, X.-X. Zou, G.-D. Li, X. Wei, C. Yan, Y.-N. Wang, J. Zhao, L.-J. Zhou and J.-S. Chen, J. Phys. Chem. C, 2011, 115, 8064–8071 CrossRef CAS.
- K. Ding, B. Chen, Z. Fang, Y. Zhang and Z. Chen, Phys. Chem. Chem. Phys., 2014, 16, 13465–13476 RSC.
- Y. Pihosh, I. Turkevych, K. Mawatari, T. Asai, T. Hisatomi, J. Uemura, M. Tosa, K. Shimamura, J. Kubota, K. Domen and T. Kitamori, Small, 2014, 10, 3692–3699 CrossRef CAS PubMed.
- S. J. Hong, S. Lee, J. S. Jang and J. S. Lee, Energy Environ. Sci., 2011, 4, 1781–1787 RSC.
- Y. B. Cheng, S. J. Yang, W. H. Cho and J. J. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 20032–20039 CrossRef PubMed.
- M. Xie, X. Fu, L. Jing, P. Luan, Y. Feng and H. Fu, Adv. Energy Mater., 2014, 4, 1300995 CrossRef.
- K. Tae Woo and C. Kyoung-Shin, Science, 2014, 343, 990–994 CrossRef PubMed.
- M. Higashi, K. Domen and R. Abe, Energy Environ. Sci., 2011, 4, 4138–4147 RSC.
- K. Z. Diane, C. Maurin, S. Kevin, G. Michael and R. G. Daniel, Energy Environ. Sci., 2011, 16, 1759–1764 Search PubMed.
- D. Long, G. Shuai, Z. Suiyi, X. Dongfang, Z. Leilei, H. Mingxin and Y. Xia, Catal. Commun., 2011, 16, 250–254 CrossRef.
- G. Yingna, Y. Xia, M. Fengyan, L. Kexin, X. Lei, Y. Xing and G. Yihang, Appl. Surf. Sci., 2009, 256, 2215–2222 Search PubMed.
- K. D. Zhong, S. Choi and R. D. Gamelin, J. Am. Chem. Soc., 2011, 133, 18370–18377 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00974e |
| This journal is © The Royal Society of Chemistry 2021 |