Open Access Article
Open Access ArticleEfficient one-pot synthesis and dehydrogenation of tricyclic dihydropyrimidines catalyzed by OMS-2-SO3H, and application of the functional-chromophore products as colorimetric chemosensors†
Neda Mardazad,
Alireza Khorshidi * and
Abdollah Fallah Shojaei
* and
Abdollah Fallah Shojaei
Department of Chemistry, Faculty of Sciences, University of Guilan, 41335-1914, Rasht, Iran. E-mail: khorshidi@guilan.ac.ir; Fax: +9801333367262; Tel: +989113397159
First published on 29th March 2021
Abstract
An efficient and convenient one-pot multicomponent reaction (MCR) for the synthesis and dehydrogenation of tricyclic dihydropyrimidine derivatives, catalyzed by –SO3H functionalized octahedral manganese oxide molecular sieves (OMS-2-SO3H) as a novel solid acid catalyst, is reported. All of the organic products and the catalyst were unambiguously characterized with conventional techniques including Fourier-transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), X-ray diffraction analysis (XRD), 1H NMR, and 13C NMR spectroscopy. The targeted dehydrogenated chromophore compounds were successfully used as colorimetric chemosensors for detection of transition metals in aqueous solution. For example, 1-[4-(4-hydroxy-3-methoxy-phenyl)-2-methyl-benzo[4,5]imidazo[1,2-a]pyrimidin-3-yl]-ethanone (7d), exhibited high sensitivity and selectivity toward detection of Cr3+ over a panel of other transition metal cations. The interference of foreign ions was found to be negligible. It was found that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of Cr3+ and 7d is responsible for the color change of the solution from ochre to brown. These newly devised chemosensors can also exhibit significant wavelength shifts (up to 100 nm) when used as pH indicators. 7d for example, showed a vivid and sharp color change from pink to yellow in the pH range of 4 to 6.
1 complex of Cr3+ and 7d is responsible for the color change of the solution from ochre to brown. These newly devised chemosensors can also exhibit significant wavelength shifts (up to 100 nm) when used as pH indicators. 7d for example, showed a vivid and sharp color change from pink to yellow in the pH range of 4 to 6.
Introduction
The design and development of chemosensors have expanded substantially in last few decades. Design and synthesis of new chemosensors for d-metal ions, i.e. transition metals including heavy metal ions, have been of great interest for chemists in recent years as they play important roles in the areas of chemical,1,2 biological,3,4 and environmental systems.5,6 Among the chemosensors, the naked eye ones, i.e. the colorimetric chemosensors, are most significant because they don't involve either any costly instrument or pre-treatment of the sample,7–12 i.e., analyte.We live in a toxic globe. Every day we are exposed to several toxic metallic species and chemicals, food additives, pesticides, industrial wastes, etc. The term “hazardous metal” has been used as a general term for those metals which possess potential toxicity against human and/or environment.13 In spite of having toxic effects, some d-metal ions are beneficial also, particularly in small quantities (trace metals) and are nutritionally indispensable for a healthy life.
Among the metal ions, Cr3+ ion performs a crucial role in a wide range of biochemical processes, which is essential for good health in moderate intake. However, it is toxic at high concentrations.14–16
For a stable human or animal diet, intake amount of 50–200 μg of Cr3+ is necessary. However, the presence of Cr3+ in excess amount deteriorates the environment and can also spoil the cellular structures.17,18 The mechanism by which Cr3+ affects human metabolism is based on modulation of the action of insulin through glucose tolerance factors (GTF), thereby activating certain enzymes and stabilizing proteins and nucleic acids. A deficiency of chromium in the human body would lead to a variety of diseases, including diabetes and cardiovascular disease. Recently, Cr(III) has also been shown to adversely affect cellular structures, although it's in vivo toxicity observed is much lower than that of Cr(VI).19,20 Several biological functions depend directly or indirectly on the proper concentration and oxidation states of chromium to maintain the homoeostatic mechanism. Deficiency or overdose of chromium can lead to serious diseases, including Alzheimer's, Huntington's and Parkinson's disease.21,22
It is clear that the rational design and synthesis of efficient chemosensors for the selective detection of d-metal ions is of great importance for the purpose of public health and environment. Here we report a simple and easy to make imidazole-based chemosensor for Cr3+ based on the proton transfer signalling mode. Chromophores are functional π-electron systems that lay the molecular foundation of modern organic electronics and solar energy conversion with tailored small molecules.23 Hence, the efficient and rapid preparation of these novel functional organic molecules with specific photophysical and electrochemical properties remains an ultimate goal and challenge for researchers both in organic chemistry and materials science. In particular, multicomponent processes exactly tackle these synthetic challenges and, therefore, they have received considerable interest both in academia and industry, predominantly for synthesizing biologically active molecules.24–27 By definition, multicomponent reactions (MCRs) are a type of convergent organic reactions in which three or more precursors react in a one-pot fashion without isolation and purification of intermediates. These reactions have demonstrated a remarkable impact on the synthesis of complex products, with high atom economy and molecular diversity. In addition, the MCRs are straightforward for the synthesis of compounds with biological or pharmacological properties which is highly attractive for the pharmaceutical and agrochemical industries, among others.28,29 In the past decades, several MCRs have been well developed and widely used in natural product synthesis and drug discovery.30–32 Therefore, the development of MCRs for construction of biologically interesting molecules continues to attract considerable attention for their obvious atom- and step-economy. The multi-functionalized dihydropyrimidine scaffold (DHPMs) represents a heterocyclic system of remarkable pharmacological efficiency. In the past decades, a broad range of biological effects, including antiviral, antitumor, antibacterial, and anti-inflammatory activities, has been ascribed to these partly reduced pyrimidine derivatives.33 Dihydropyrimidines, the products of the Biginelli reaction, are widely used in the pharmaceutics as calcium channel blockers, antihypertensive agents, and alpha-1-a-antagonists.34–36 It has been suggested that the substituent effect can be attributed to intramolecular hydrogen bonding between the–alkoxy oxygen and the proton of the pyrimidine ring NH moiety.37
Among dihydropyrimidine derivatives, the benzo[4,5]imidazo[1,2-a]-pyrimidines have been reported to have a variety of biological activities, such as antineoplastic activity,38 protein kinase inhibitory,39 T cell activation,40 TIE-2 and/or VEGFR2 inhibitory activities.41
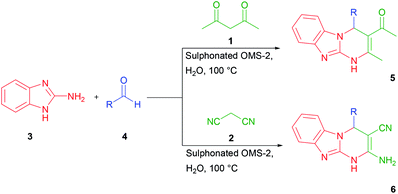
The known methods of the synthesis of benzo[4,5]imidazo[1,2-a]-pyrimidines suffer from some drawbacks including low yield, prolonged reaction time, and consumption of organic solvents. So, there is an urgent need to develop an alternative protocol for efficient and convenient MCRs synthesis of this type of heterocyclic compounds (Scheme 1).
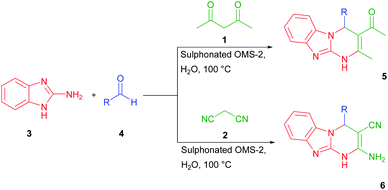 | ||
| Scheme 1 Synthesis of benzo[4,5]imidazo[1,2-a]pyrimidines (5) and 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazole (6). | ||
Porous manganese oxide natural materials, on the other hand, are found as manganese nodules, and when dredged from the ocean floors have been used as excellent adsorbents of metals, for example from electroplating wastes, and have been shown to have excellent catalytic activity.42–44 Variable pore size materials have been synthesized using structure directors by a variety of synthetic methodologies. Catalytic performance of these OMS materials have been shown to be related to the redox cycling of various oxidations states of manganese such as Mn2+, Mn3+, and Mn4+.45 Many concepts including nonstoichiometry, defects, oxygen vacancies, and intermediates are fundamental to many of the syntheses, characterization, and applications such as fuel cells, adsorption, sensors, batteries, and so on.46–48 A key phase that shows outstanding electrochemical and catalytic properties is the 2 × 2 synthetic cryptomelane or OMS-2 structure.49–51
In this contribution, we report the first modification of OMS-2 with chlorosulfonic acid to form –SO3H functionalized OMS-2 (OMS-2-SO3H). The idea was inspired by the excellent performance of solid acid catalysts based on –SO3H functionalized silica materials as heterogeneous catalysts.52–54
Results and discussion
Catalyst characterization
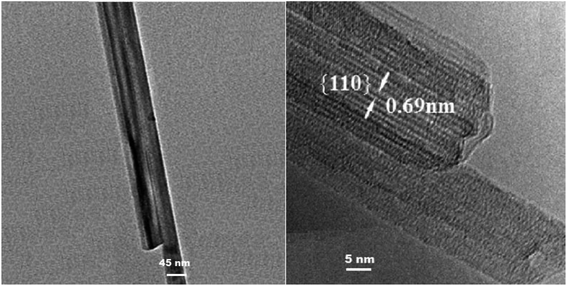
Sulfonated OMS-2 was subjected to XRD analysis to identify its crystal structure and crystallinity after sulfonation. Fig. S1† shows the XRD patterns for the OMS-2 and its sulfonated form. The observed reflections at 2θ values of 12°, 17.8°, 28.5°, 37.4°, 41.6°, 49.7°, 56°, 59.9°, 65.2°, and 69.3° can be indexed to the 110, 200, 310, 211, 301, 411, 600, 521, 602, and 541 planes of Q-phase cryptomelane-type manganese oxide (JCPDS card no. 29-1020) for both samples. To study the morphological changes of OMS-2 after sulfonation, electron microscopy imaging (SEM) was used (Fig. 1). SEM micrographs revealed a rod-shaped morphology for the OMS-2 sample, as anticipated. After sulfonation, however, surface of the nano-rods became rough and non-porous. High-resolution transmission electron microscope (HRTEM) imaging (Fig. 2), on the other hand, showed that OMS-2-SO3H is consisted of almost uniform nano-rods of about 45 nm in diameter. Fig. 2, right, shows that the fibres lie on the (110) plane. One can also note that the lattice fringes are separated by 0.69 nm. These findings are in agreement with the reported characteristics of the parent OMS-2.Nitrogen adsorption–desorption (BET) analysis showed that the specific surface area of the catalyst was changed from 93 m2 g−1 to 202.21 m2 g−1. This increase in the surface area is in agreement with the SEM observations, and is in line with the previous reports.55 It should be noted that the sulfonated sample was highly hygroscopic, and its high hydrophilicity may result in formation of a layer of water on its surface.
To further characterize the synthesized samples, FT-IR spectroscopy was used (Fig. S2†). In the FT-IR spectrum of OMS-2-SO3H, the observed peaks can be attributed as follows: stretching and bending vibrations of the adsorbed water: 3415 and 1635 cm−1; symmetric and asymmetric stretching vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: 1286 and 1178 cm−1; stretching vibration of S
O: 1286 and 1178 cm−1; stretching vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: 1067 cm−1; C–S stretching vibrations: 695 cm−1; MnO6 vibrations: 735, 594 and 535 cm−1. It is also evident that the intensity of the peak at about 3400 cm−1 has been increased, which is a direct indication of the inclusion of –SO3H functionality.
O: 1067 cm−1; C–S stretching vibrations: 695 cm−1; MnO6 vibrations: 735, 594 and 535 cm−1. It is also evident that the intensity of the peak at about 3400 cm−1 has been increased, which is a direct indication of the inclusion of –SO3H functionality.
Acidity determination
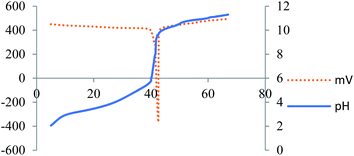
Quantification of the acidity of the OMS-2-SO3H was achieved by potentiometric titration with 0.1 N NaOH. The corresponding titration curve is depicted in Fig. 3. The acidity was found to be 119.66 mmol –SO3H per g of the solid acid catalyst.Optimization of the reaction conditions
Initially, we studied the three-component condensation reaction of 2-aminobenzimidazole 3 (2 mmol), 9-anthracenecarboxaldehyde 4a (2 mmol), and acetylacetone 1 (2 mmol) in presence of OMS-2-SO3H (10 mol%) in 2 mL H2O at 100 °C for 1 h to give the desired product 5a in 96% yield. However, only 20% yield of the target product 5a was observed when the mixture was stirred under similar conditions in the absence of the catalyst even after 4 h (Table 1, entry 1). Encouraged by the result, we further investigated the best reaction conditions by using different amounts of OMS-2-SO3H. An increase in the quantity of the catalyst from 0 mol% to 10 mol%, not only decreased the reaction time from 4 h to 1 h, but also increased the product yield from 20% to 96% (Table 1, entries 1–3). However, the yield did not increase when excess amount (20 mol%) of the catalyst was used under the same conditions. Therefore, 10 mol% of the OMS-2-SO3H was considered as the optimized amount of the catalyst.As shown in Table 2, solvent had an impact on the yield of the target product 5a (30–50%). The obtained yields in THF, DCM, toluene, and DMF (Table 2, entries 1, 2, 4 and 5) were indicative of the effect of the solvent nature on the reaction. Obviously, protic solvents such as EtOH and H2O resulted in better yields (88 and 96%, Table 2, entries 6 and 7). Thus, H2O was selected as the solvent for all of the subsequent reactions. Furthermore, in order to optimize the reaction temperature, the target multicomponent reaction was carried out in the range of 40–100 °C (Table 2, entries 7–10). It was found that elevated temperature had a significant effect on the reaction yield.
| Entry | Solvent | Temp. (°C) | Time (h) | Yield of 5ab (%) |
|---|---|---|---|---|
| a Conditions: 2-aminobenzimidazole 3 (2 mmol), 9-anthracenecarboxaldehyde 4a (2 mmol), acetylacetone 1 (2 mmol), OMS-2-SO3H (0.1 mmol, 10 mol%), solvent (2 mL).b Isolated yields. | ||||
| 1 | THF | 100 | 4 | 30 |
| 2 | Toluene | 100 | 4 | 50 |
| 3 | MeCN | 100 | 4 | 60 |
| 4 | DCM | 100 | 4 | 30 |
| 5 | DMF | 100 | 4 | 45 |
| 6 | EtOH | 100 | 1 | 88 |
| 7 | H2O | 100 | 1 | 96 |
| 8 | H2O | 40 | 7 | 20 |
| 9 | H2O | 60 | 6 | 50 |
| 10 | H2O | 80 | 4 | 65 |
To examine the generality and scope of this OMS-2-SO3H catalyzed reaction, we used a series of aldehydes in the title reaction (Table 3). In all cases, the substrates reacted smoothly to give the corresponding benzo[4,5]imidazo[1,2-a]pyrimidines and 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitriles in good yields (Table 3, entries 1–8). Most importantly, aromatic aldehydes carrying either electron-donating or electron-withdrawing substituents could react efficiently to give the desired products without significant differences. The reaction was tolerant to a variety of functional groups, such as –OCH3, –OH, –NO2, and Br.
| Entry | 1 or 2b | R | Product | Time (min) | Yieldc (%) |
|---|---|---|---|---|---|
| a Conditions: 2-aminobenzimidazole 3 (2 mmol), aldehyde 4 (2 mmol), and acetylacetone 1 (2 mmol) or malononitrile 2 (2 mmol), OMS-2-SO3H (0.1 mmol, 10 mol%), H2O (2 mL) at 100 °C.b Acetylacetone (1) and malononitrile (2).c Isolated yields. | |||||
| 1 | 1 | Anthracen-9-yl | 5a | 45 | 96 |
| 2 | 1 | 2-NO2C6H4 | 5b | 90 | 84 |
| 3 | 1 | 5-Br-2-OHC6H3 | 5c | 60 | 89 |
| 4 | 1 | 4-OH-3-CH3OC6H3 | 5d | 60 | 88 |
| 5 | 2 | Anthracen-9-yl | 6a | 30 | 90 |
| 6 | 2 | 2-NO2C6H4 | 6b | 30 | 87 |
| 7 | 2 | 5-Br-2-OHC6H3 | 6c | 20 | 94 |
| 8 | 2 | 4-OH-3-CH3OC6H3 | 6d | 10 | 94 |
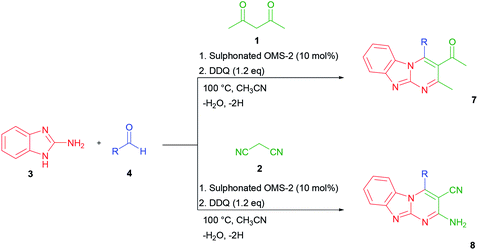
Tricyclic dihydropyrimidine derivatives containing an H atom in the meso position were expected to be able to easily oxidize to the corresponding conjugated products. It was also expected that the resulting conjugated compounds might display interesting transition metal cation binding and sensing properties. The choice of oxidized form of tricyclic dihydropyrimidine derivatives as the chromogenic-sensing molecule was mainly based on the fact that this conjugated skeleton could act not only as a color-reporting group but also as a binding affinity control group containing an acidic H-bond donor moiety and a basic H-bond acceptor moiety. Interestingly, after a series of optimization experiments, a one-pot method was devised for the synthesis and in situ oxidation of benzo[4,5]imidazo[1,2-a]pyrimidines (5) and 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitriles (6) to the corresponding dehydrogenated products (7 and 8). The optimized conditions are summarized in Table 4.
| Entry | 1 or 2b | R | Product | Time (min) | Yieldc (%) |
|---|---|---|---|---|---|
| a Conditions: 2-aminobenzimidazole 3 (2 mmol), aldehyde 4 (2 mmol), and acetylacetone 1 (2 mmol) or malononitrile 2 (2 mmol), OMS-2-SO3H (0.1 mmol, 10 mol%), DDQ (1.2 eq.), H2O (2 mL) at 100 °C.b Acetylacetone (1) and malononitrile (2).c Isolated yields. | |||||
| 1 | 1 | Anthracen-9-yl | 7a | 140 | 78 |
| 2 | 1 | 2-NO2C6H4 | 7b | 150 | 75 |
| 3 | 1 | 5-Br-2-OHC6H3 | 7c | 150 | 68 |
| 4 | 1 | 4-OH-3-CH3OC6H3 | 7d | 120 | 80 |
| 5 | 2 | Anthracen-9-yl | 8a | 110 | 72 |
| 6 | 2 | 2-NO2C6H4 | 8b | 110 | 65 |
| 7 | 2 | 5-Br-2-OHC6H3 | 8c | 100 | 77 |
| 8 | 2 | 4-OH-3-CH3OC6H3 | 8d | 90 | 69 |
As a representative example, the synthesis of 7d is shown in Scheme 2. The oxidized product 7d was prepared in 80% yield by condensation of 2-aminobenzimidazole 3, 4-hydroxy-3-methoxybenzaldehyde 4d, and acetylacetone 1 with DDQ in CH3CN.
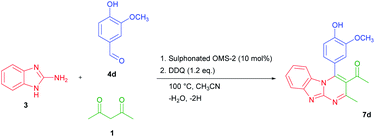 | ||
| Scheme 2 Synthesis of 1-[4-(4-hydroxy-3-methoxy-phenyl)-2-methyl-benzo[4,5]imidazo[1,2-a]pyrimidin-3-yl]-ethanone (7d). | ||
According to the obtained results, a plausible mechanism for the synthesis of dihydropyrimidine derivatives 5a–d and 6a–d catalyzed by the OMS-2-SO3H is outlined in Scheme 3. At the first step, aldehydes 4 can be activated by the OMS-2-SO3H solid acid, mainly through –SO3H groups, to afford the Knoevenagel condensation product of aldehydes 4 and acetylacetone (1) or malononitrile (2) C–H acid as intermediates (II) or (II′), respectively. Then, condensation of these intermediates with 2-aminobenzimidazole (3) produces Michael acceptor intermediates (III) or (III′), respectively, in the presence of OMS-2-SO3H. Finally, the activated intermediates (III) or (III′) are involved in the intramolecular Michael addition and subsequent tautomerization to afford tricyclic dihydropyrimidine derivatives 5a–d and 6a–d, respectively.
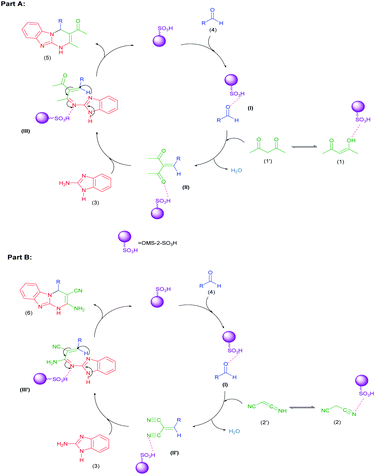 | ||
| Scheme 3 A plausible mechanism for the synthesis of dihydropyrimidine derivatives 5a–d (Part A) and 6a–d (Part B) in the presence of the OMS-2-SO3H catalyst. | ||
To demonstrate the effectiveness of OMS-2-SO3H, it was compared with some of the previously reported and published procedures (Table 5). The results clearly illustrate the superiority of this protocol against many of the others in terms of the product yield, reaction time and using a green solvent.
| Entry | Catalyst | Conditions | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | KAl(SO4)2·12H2O | Ethanol, reflux | 210 | 86 | 56 |
| 2 | p-TSA | Solvent-free, 80 °C | 30 | 93 | 57 |
| 3 | Triethylamine | Ethanol, reflux | 180 | 60 | 58 |
| 4 | MgO | CH3CN, reflux | 45 | 75 | 59 |
| 5 | Thiamine hydrochloride (VB1) | H2O, reflux | 180 | 90 | 60 |
| 6 | SBA-IL | Solvent-free, 150 °C | 40 | 87 | 61 |
| 7 | TBBDA | Solvent-free, 100 °C | 80 | 88 | 62 |
| 8 | H3PO4–Al2O3 | Solvent-free, 100 °C | 85 | 90 | 63 |
| 9 | Sulfamic acid | Solvent-free, 85 °C | 480 | 88 | 64 |
| 10 | OMS-2-SO3H | H2O, 100 °C | 45 | 96 | This work |
To study the properties of the synthesized products, their behaviour as a potential pH indicator was evaluated. Many dyes have been used as pH indicators due to the reversible protonation–deprotonation processes. It was found that our synthesized products could act as pH indicators in the pH range of 4 to 6 with an extremely sharp and clear colour change. The colour changes during this pH range are attributed to an intramolecular charge transfer mechanism resulting from the push–pull effect of the electron-donating amine substituent. The colour transition of compound 7d is depicted in Fig. 4.
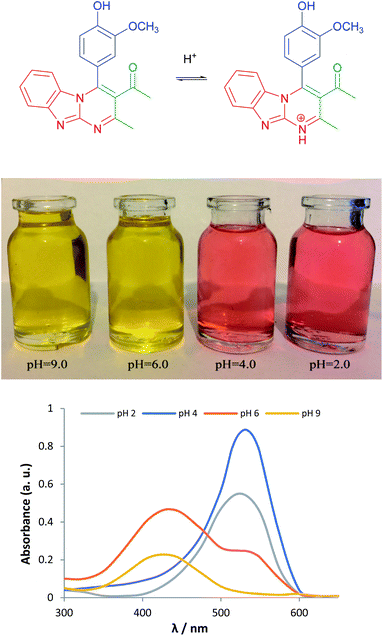 | ||
| Fig. 4 (Top) and (middle) pH indication using 7d, (bottom) spectral changes of 7d (5 × 10−6 M, EtOH 70%) at different pH. | ||
Table 6 compares the transition range of our products with other pH indicators in the pH range 4–6.65
| Transition range, pH | Indicator | Colour change | Composition of indicator's solution, % |
|---|---|---|---|
| 4.0–6.0 | Dyes 7a–d, 8a–d | Pink-yellow | 0.1 (in 70% ethanol) |
| 4.3–6.3 | Alizarin (red) S | Yellow-pink | 0.1 (in water) |
| 4.4–6.2 | Methyl red | Red-yellow-orange | 0.1 (in 96% ethanol) |
| 4.5–6.2 | Methyl red sodium salt | Red-yellow-orange | 0.05 (in water) |
| 4.7–6.3 | Bromophenol red | Yellow-purple | 0.1 (in 20% ethanol) |
| 4.8–6.4 | Chlorophenol red | Yellow-purple | 0.1 (in 20% ethanol) |
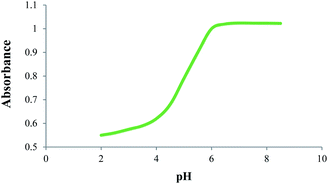
From the data listed in Table 6, it is clear that the synthesized dyes exhibited quite acceptable properties to be used as indicators. A dilute solution of 7d (5 × 10−6 M, EtOH 70%), presented a pink colour and displayed a strong absorption band at 430 nm, which may be attributable to the intramolecular charge transfer (ICT) arising from the π-extended conjugated structure of this compound. Upon addition of dilute acid (0.1 M HCl) to this solution, however, the absorption band was red-shifted to higher wavelengths around 530 nm, along with a distinctive colour change to yellow. Fig. 4 shows the spectroscopic behaviour of 7d at different pH.
To determine the colour transition range of the indicator, absorption of the solution was measured at 531 nm with gradual addition of dilute alkali solution (NH4OH). The result is shown in Fig. 5. As is apparent, a sharp colour transition was observed in the pH range of 4.0–6.0, which is comparable to methyl red.
Encouraged by these results, we decided to study the ability of our products in sensing transition metal cations. It was found that these dehydrogenated chromophore compounds interact with transition metal cations resulting to distinct spectral changes. For example, compound 7d exhibited hypsochromic effects in presence of some metal cations including Ca2+, Cr3+, Co2+ and Cu2+. The most intense colour change was observed for Cr3+ in acetonitrile solution. This result indicates that 7d can serve as a ‘naked eye’ Cr3+ indicator. The UV-vis spectrum of 7d (5 × 10−6 M) and Cr3+ (1.25 × 10−4 M) solutions in CH3CN, before and after mixing, is shown in Fig. 6.
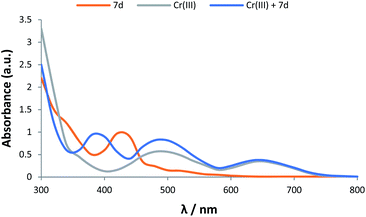 | ||
Fig. 6 UV-vis spectra of 7d (5 × 106 M), Cr3+ (1.25 × 104 M), and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 7d and Cr3+ solutions in acetonitrile. 1 mixture of 7d and Cr3+ solutions in acetonitrile. | ||
As shown in Fig. 6, Cr3+ shows classic transitions of a d3 cation corresponding to transitions from the 4A2 (F) ground state to the 4T1 (F) and 4T1 (P) excited states of the metal cation. After mixing 7d and Cr3+ solutions, a blue shift of about 40 nm for the 420 nm band of 7d was observed that might be due to N-bonding of 7d to the metal centre.
The spectral response of 7d to other transition metal cations (Ca2+, Fe3+, Ni2+, Cr3+, Co2+, Cu2+, Zn2+, Cd2+, Mn2+, Hg2+ and Sn2+) are depicted in Fig. 7.
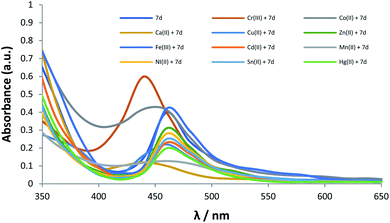 | ||
| Fig. 7 The spectral response of 7d to other transition metal cations (Ca2+, Fe3+, Ni2+, Cr3+, Co2+, Cu2+, Zn2+, Cd2+, Mn2+, Hg2+ and Sn2+). | ||
In Fig. 8 effect of foreign metal ions on the fluorescence intensity of the 7d + Cr3+ system is presented. There was almost no interference for the detection of Cr3+ in the presence of alkali and alkaline earth metal ions (Na+, K+, Mg2+, Ca2+), and several other transition metal ions (Mn2+, Fe3+, Co3+, Ni2+, Cu2+, Zn2+, Sn2+, Hg2+). In this study, 7d = 10−6 mol L−1, [Cr3+] = 10−6 mol L−1 and the foreign metal ions are present 10 times of the [Cr3+] i.e. 10−5 mol L−1. All these observations indicate that 7d exhibits a highly preferential binding of Cr3+ over other metal ions. The combined results revealed that 7d can be used to detect Cr3+ without interference from other metal ions, and is a good chemosensor for detection of Cr3+.
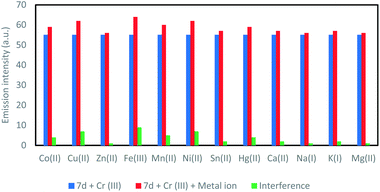 | ||
| Fig. 8 Interference of foreign cations. [blue: 7d + Cr3+; red: 7d + Cr3+ + other metal ion; green: resulting interference]. | ||
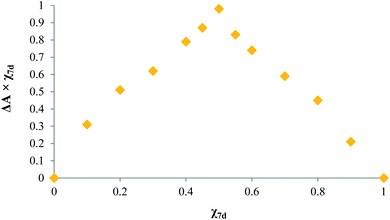
In order to gain an insight into the interaction mechanism between the cation and the receptors, a similar experiment with Cr3+ was conducted in acetonitrile at acidic pH (pH 2.0). In this case, the blue-shifted band was not observed upon addition of the metal, revealing that no interaction took place due to the fact that the imidazole moiety of 7d was N-protonated. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the complex as indicated by the changes in the colorimetric response of 7d, in the presence of the varying concentrations of Cr3+ (Fig. 9), is also in agreement with N-coordination of the metal.
1 stoichiometry of the complex as indicated by the changes in the colorimetric response of 7d, in the presence of the varying concentrations of Cr3+ (Fig. 9), is also in agreement with N-coordination of the metal.
Conclusions
In summary, a series of newly oxidized tricyclic dihydropyrimidine derivatives were synthesized one-pot, under heterogeneous catalysis of OMS-2-SO3H as a newly devised solid acid catalyst. The organic products were used as colorimetric probes for the detection of transition metal ions in aqueous solution. Formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of the chemosensor and cation was found to be responsible for the observed colour changes. The excellent selectivity of the synthesized chemosensors may provide a suitable platform for application in biological monitoring, for example, in tracking of Cr3+. The obtained dehydrogenated dihydropyrimidines were also capable to be used as pH indicators based on the proton transfer signaling mode. The outcome of our research is expected to pave the way for the study of other properties of dehydrogenated dihydropyrimidines.
1 complexes of the chemosensor and cation was found to be responsible for the observed colour changes. The excellent selectivity of the synthesized chemosensors may provide a suitable platform for application in biological monitoring, for example, in tracking of Cr3+. The obtained dehydrogenated dihydropyrimidines were also capable to be used as pH indicators based on the proton transfer signaling mode. The outcome of our research is expected to pave the way for the study of other properties of dehydrogenated dihydropyrimidines.
Experimental
Melting points were measured by an electro-thermal engineering micro-melting point apparatus and are uncorrected. NMR spectra were recorded on an INOVA 500 MHz instrument using DMSO-d6. TLC was performed on GF254 silica gel plates (Yantai Huiyou Inc., China). Voltammetry and pH measurements were performed using a Selecta lab device model pHW300 Microprocessor. The catalyst was characterized using X-ray diffraction (XRD). Diffractograms were recorded on a Panalytical X'Pert PRO MPD diffractometer using Cu Kα irradiation (λ = 1.54059 Å). Scanning Electron Microscopy (SEM) was performed on a LEO 1430VP instrument. High-resolution transmission electron microscopy (HRTEM) was performed on a FEI TEC9G20 instrument. BET surface area analysis was performed using nitrogen sorption on a Micrometrics PHS-1020 accelerated surface area system. Samples were degassed at 200 °C for 12 h prior to experiments which were carried out at 77 K. The specific surface area of the material was determined by the BET method. All of the FT-IR and NMR spectra are available in the ESI.†Catalyst preparation
Potassium containing OMS-2 (K-OMS-2) was synthesized using a variation of earlier reported procedures.55,66–68 Briefly, to a solution of 59.0 mmol MnSO4·H2O in 33 mL distilled water, 55.5 mmol of HNO3 was added. The obtained solution was added dropwise to another solution consisting of 42.0 mmol KMnO4 and 100 mL of distilled water, by using a dropping funnel, under vigorous stirring. The resulting grayish mixture was refluxed at 110 °C for 24 h, upon which the black precipitate was filtered, washed with distilled water until neutral, oven-dried at 120 °C overnight, and ground to fine powder.This powder (5 g) was then dispersed in 75.0 mL of dry dichloromethane by means of a magnetic stirrer and while stirring at 700 rpm, chlorosulfonic acid (1.0 g, 8.55 mmol in 10.0 mL of CH2Cl2) was added dropwise over a period of 90 min at room temperature. The evolved HCl was eliminated by suction. Then the sulfonated OMS-2 was separated by sedimentation and washed several times with dichloromethane and dried under vacuum at 70 °C.
General procedure for the synthesis of benzo[4,5]imidazo[1,2-a]pyrimidines 5 or 2-amino-4-substituted-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitriles 6
A mixture of 2-aminobenzimidazole 3 (2 mmol), aldehyde 4 (2 mmol), acetylacetone 1 and OMS-2-SO3H (35 mg, 0.1 mmol) in water (2 mL) was heated to reflux for 45–90 min. Also, a mixture of 2-aminobenzimidazole 3 (2 mmol), aldehyde 4 (2 mmol), malononitrile 2 (2 mmol) and OMS-2-SO3H (35 mg, 0.1 mmol) in water (2 mL) was heated to reflux for 10–30 min. After completion of the reaction (TLC), the solid was filtered off, washed with water and recrystallized from methanol (5 mL) to yield pure product 5 or 6.General procedure for the oxidative dehydrogenation
2-aminobenzimidazole 3 (2 mmol), aldehyde 4 (2 mmol), acetylacetone 1 or malononitrile 2 (2 mmol), and OMS-2-SO3H (35 mg, 0.1 mmol) in acetonitrile (2 mL) was heated to reflux until the disappearance of the starting reagents were complete (monitored by TLC). After completion of the first step, DDQ (1.2 mmol) solution in acetonitrile was added dropwise and slowly to the solution. This reaction was stirred for 1.5–2.5 h and gave a dark red precipitate. After completion of the oxidation step (indicated by TLC), the mixture was purified by short column chromatography (n-hexane/EtOAc, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to provide the desired products.
4) to provide the desired products.
Competition experiments
To prepare the solutions of metal ions, the chloride salts such as (Ca2+, Fe3+, Ni2+, Cr3+, Co2+, Cu2+, Zn2+, Cd2+, Mn2+, Hg2+ and Sn2+) were dissolved in acetonitrile to afford 1.25 × 10−4 M solutions. A 5 × 10−6 M solution of 7d was prepared in acetonitrile. After mixing them for a few second, UV-vis spectra were taken at room temperature.pH titration
Compound 7d (0.87 mg, 0.0025 mmol) was dissolved in ethanol (8 mL), dilute alkali solution NH4OH (0.01 M) was slowly added to the solution. After mixing them for a few minutes, UV-vis absorption spectra were taken at room temperature.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Partial support of this study by Research Council of University of Guilan is gratefully acknowledged.References
- L. Ding, P. Rahimi, R. Hawkins, S. Bhatt and Y. Shi, Appl. Catal., A, 2009, 371, 121–130 CrossRef CAS
.
- J. Li, Q. Wang, Z. Guo, H. Ma, Y. Zhang, B. Wang, D. Bin and Q. Wei, Sci. Rep., 2016, 6, 23558 CrossRef CAS PubMed
.
- P. T. Lieu, M. Heiskala, P. A. Peterson and Y. Yang, Mol. Aspects Med., 2001, 22, 1–87 CrossRef CAS
.
- L. H. Yuen, R. M. Franzini, S. S. Tan and E. T. Kool, J. Am. Chem. Soc., 2014, 136, 14576–14582 CrossRef CAS PubMed
.
- P. Bühlmann, E. Pretsch and E. Bakker, Chem. Rev., 1998, 98, 1593–1688 CrossRef PubMed
.
- N. Zhang, R. Qiao, J. Su, J. Yan, Z. Xie, Y. Qiao, X. Wang and J. Zhong, Small, 2017, 13, 1604293 CrossRef PubMed
.
- D. Zhang, M. Zhang, Z. Liu, M. Yu, F. Li, T. Yi and C. Huang, Tetrahedron Lett., 2006, 47, 7093–7096 CrossRef CAS
.
- K. K. Upadhyay, S. Upadhyay, K. Kumar and R. Prasad, J. Mol. Struct., 2009, 927, 60–68 CrossRef CAS
.
- O. G. Nikolaeva, E. N. Shepelenko, K. S. Tikhomirova, Y. V. Revinskii, A. D. Dubonosov, V. A. Bren and V. I. Minkin, Mendeleev Commun., 2016, 26, 402–404 CrossRef CAS
.
- Y.-T. Wang, S. Hu, Y. Zhang, H. Gong, R. Sun, W. Mao, D.-H. Wang and Y. Chen, J. Photochem. Photobiol., A, 2018, 355, 101–108 CrossRef CAS
.
- G. Dhaka, N. Kaur and J. Singh, Supramol. Chem., 2015, 27, 654–660 CrossRef CAS
.
- A. Caballero, R. Martínez, V. Lloveras, I. Ratera, J. Vidal-Gancedo, K. Wurst, A. Tárraga, P. Molina and J. Veciana, J. Am. Chem. Soc., 2005, 127, 15666–15667 CrossRef CAS PubMed
.
- T. Vamerali, M. Bandiera and G. Mosca, Environ. Chem. Lett., 2010, 8, 1–17 CrossRef CAS
.
- J. B. Vincent, Nutr. Rev., 2000, 58, 67–72 CrossRef CAS PubMed
.
- D. A. Eastmond, J. T. Macgregor and R. S. Slesinski, Crit. Rev. Toxicol., 2008, 38, 173–190 CrossRef CAS PubMed
.
- R.-G. Lv, S.-W. Chen and Y. Gao, Heterocycl. Commun., 2017, 23, 389–394 CAS
.
- J. Kováčik, P. Babula, B. Klejdus and J. Hedbavny, J. Agric. Food Chem., 2013, 61, 7864–7873 CrossRef PubMed
.
- D. A. Brose and B. R. James, Environ. Sci. Technol., 2010, 44, 9438–9444 CrossRef CAS PubMed
.
- A. K. Singh, V. K. Gupta and B. Gupta, Anal. Chim. Acta, 2007, 585, 171–178 CrossRef CAS PubMed
.
- K. Huang, H. Yang, Z. Zhou, M. Yu, F. Li, X. Gao, T. Yi and C. Huang, Org. Lett., 2008, 10, 2557–2560 CrossRef CAS PubMed
.
- D. Liu, T. Pang, K. Ma, W. Jiang and X. Bao, RSC Adv., 2014, 4, 2563–2567 RSC
.
- Y. Lei, Y. Su and J. Huo, J. Lumin., 2011, 131, 2521–2527 CrossRef CAS
.
- T. J. J. Müller, in Functional Organic Materials, 2006, ch. 5, pp. 179–223, DOI:10.1002/9783527610266
.
- R. O. Rocha, M. O. Rodrigues and B. A. D. Neto, ACS Omega, 2020, 5, 972–979 CrossRef CAS PubMed
.
- L. Levi and T. J. J. Müller, Chem. Soc. Rev., 2016, 45, 2825–2846 RSC
.
- F. K. Merkt and T. J. J. Müller, Isr. J. Chem., 2018, 58, 889–900 CrossRef CAS
.
- T. J. J. Müller, Drug Discovery Today: Technol., 2018, 29, 19–26 CrossRef PubMed
.
- D. Insuasty, J. Castillo, D. Becerra, H. Rojas and R. Abonia, Molecules, 2020, 25, 505 CrossRef PubMed
.
- L. M. Ramos, M. O. Rodrigues and B. A. D. Neto, Org. Biomol. Chem., 2019, 17, 7260–7269 RSC
.
- P. S. G. Nunes, H. D. A. Vidal and A. G. Corrêa, Org. Biomol. Chem., 2020, 18, 7751–7773 RSC
.
- L. Cala, P. Villar, Á. R. de Lera, F. J. Fañanás, R. Álvarez and F. Rodríguez, Chem. Sci., 2020, 11, 9181–9190 RSC
.
- L. Zeng, B. Huang, Y. Shen and S. Cui, Org. Lett., 2018, 20, 3460–3464 CrossRef CAS PubMed
.
- C. Oliver Kappe, Tetrahedron, 1993, 49, 6937–6963 CrossRef
.
- N. Kaur, K. Kaur, T. Raj, G. Kaur, A. Singh, T. Aree, S.-J. Park, T.-J. Kim, N. Singh and D. O. Jang, Tetrahedron, 2015, 71, 332–337 CrossRef CAS
.
- C. O. Kappe, Eur. J. Med. Chem., 2000, 35, 1043–1052 CrossRef CAS PubMed
.
- C. Oliver Kappe, W. M. F. Fabian and M. A. Semones, Tetrahedron, 1997, 53, 2803–2816 CrossRef
.
- B. J. Broughton, P. Chaplen, P. Knowles, E. Lunt, S. M. Marshall, D. L. Pain and K. R. H. Wooldridge, J. Med. Chem., 1975, 18, 1117–1122 CrossRef CAS PubMed
.
- A. A.-m. Abdel-hafez, Arch. Pharmacal Res., 2007, 30, 678 CrossRef CAS PubMed
.
- J. J. Nunes, X. Zhu, P. Amouzegh, C. Ghiron, D. N. Johnson and E. C. Power, US Pat., US7504396B2, 2009 Search PubMed
.
- J. J. Nunes, X. T. Zhu, P. Amouzegh, C. Ghiron, D. N. Johnston and E. C. Power, WO Pat., 005009443, 2005
.
- M. Cheung, P. A. Harris, M. Hasegawa, S. Ida, K. Kano, N. Nishigaki, H. Sato, J. M. Veal, Y. Washio and R. I. West, WO Pat., 2002044156, 2002
.
- J. Yuan, K. Laubernds, Q. Zhang and S. L. Suib, J. Am. Chem. Soc., 2003, 125, 4966–4967 CrossRef CAS PubMed
.
- Y. Wang, X. Meng, G. Chen and P. Zhao, Catal. Commun., 2018, 104, 106–111 CrossRef CAS
.
- X. Meng, Y. Wang, Y. Wang, B. Chen, Z. Jing, G. Chen and P. Zhao, J. Org. Chem., 2017, 82, 6922–6931 CrossRef CAS PubMed
.
- S. L. Suib, Acc. Chem. Res., 2008, 41, 479–487 CrossRef CAS PubMed
.
- M. Bibes and A. Barthelemy, IEEE Trans. Electron Devices, 2007, 54, 1003–1023 CAS
.
- D. M. D'Alessandro and F. R. Keene, Chem. Soc. Rev., 2006, 35, 424–440 Search PubMed
.
- K. Ragavendran, D. Vasudevan, A. Veluchamy and B. Emmanuel, J. Phys. Chem. B, 2004, 108, 16899–16903 CrossRef CAS
.
- S. L. Suib, J. Mater. Chem., 2008, 18, 1623–1631 RSC
.
- L. Yun, Y. Li, C. Zhou, L. Lan, M. Zeng, M. Mao, H. Liu and X. Zhao, Appl. Catal., A, 2017, 530, 1–11 CrossRef CAS
.
- Z. Sihaib, F. Puleo, J. M. Garcia-Vargas, L. Retailleau, C. Descorme, L. F. Liotta, J. L. Valverde, S. Gil and A. Giroir-Fendler, Appl. Catal., B, 2017, 209, 689–700 CrossRef CAS
.
- F. Mardani, A. Khorshidi and S. Gholampoor, ChemistrySelect, 2019, 4, 8015–8020 CrossRef CAS
.
- A. Khorshidi and S. Shariati, Chin. J. Catal., 2015, 36, 778–784 CrossRef CAS
.
- A. Khorshidi, S. Shariati and M. Abootalebi, J. Serb. Chem. Soc., 2016, 81, 1069–1079 CrossRef CAS
.
- N. N. Opembe, C. K. King’ondu and S. L. Suib, Catal. Lett., 2012, 142, 427–432 CrossRef CAS
.
- R. K. Ali and B. Fahimeh, Lett. Org. Chem., 2011, 8, 631–636 CrossRef
.
- M. V. Reddy, J. Oh and Y. T. Jeong, C. R. Chim., 2014, 17, 484–489 CrossRef CAS
.
- B. Insuasty, A. Salcedo, A. Rodrigo, J. Quiroga, M. Nogueras and A. Sánchez, Heterocycl. Commun., 2002, 8, 287–292 CAS
.
- H. Sheibani and M. Babaie, Russ. Chem. Bull., 2013, 62, 2202–2208 CrossRef CAS
.
- J. Liu, M. Lei and L. Hu, Green Chem., 2012, 14, 840–846 RSC
.
- L. Seyedakbari, G. M. Ziarani, A. Badiei, M. Yadavi, P. Hajiabbasi and A. A. Soorkic, Res. Chem. Intermed., 2013, 64, 832–837 CAS
.
- M. Abedini, F. Shirini, M. Mousapour and O. Goli Jolodar, Res. Chem. Intermed., 2016, 42, 6221–6229 CrossRef CAS
.
- H. R. Shaterian, N. Fahimi and K. Azizi, Res. Chem. Intermed., 2014, 40, 1879–1898 CrossRef CAS
.
- C.-S. Yao, S. Lei, C.-H. Wang, C.-X. Yu, Q.-Q. Shao and S.-J. Tu, Chin. J. Chem., 2008, 26, 2107–2111 CrossRef CAS
.
- A. G. Püntener, R. Pedrazzi, T. Clausen, Klaus Hunger, W. Bauer, M. Filosa and E. Ross, in Industrial Dyes Chemistry, Properties, Applications, ed. Klaus Hunger, Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 1st edn 2002, ch. 5, pp. 427–541, DOI:10.1002/3527602011
.
- N. N. Opembe, Y.-C. Son, T. Sriskandakumar and S. L. Suib, ChemSusChem, 2008, 1, 182–185 CrossRef CAS PubMed
.
- Y.-F. Shen, R. P. Zerger, S. L. Suib, L. McCurdy, D. I. Potter and C.-L. O'Young, J. Chem. Soc., Chem. Commun., 1992, 1213–1214, 10.1039/C39920001213
.
- N. N. Opembe, C. K. King’ondu, A. E. Espinal, C.-H. Chen, E. K. Nyutu, V. M. Crisostomo and S. L. Suib, J. Phys. Chem. C, 2010, 114, 14417–14426 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray diffraction patterns, FTIR, 1H NMR, and 13C NMR spectra of all of the synthesized materials. See DOI: 10.1039/d1ra01005k |
| This journal is © The Royal Society of Chemistry 2021 |