Open Access Article
Open Access ArticleBoosting charge separation and nitrogen vacancies in graphitic carbon nitride by implanted strontium vanadate for highly efficient photocatalytic reduction of hexavalent chromium
Yuzhi Zhou,
Shi-Zhao Kang ,
Lixia Qin and
Xiangqing Li
,
Lixia Qin and
Xiangqing Li *
*
Shanghai Institute of Technology, China. E-mail: xqli@sit.edu.cn
First published on 29th April 2021
Abstract
Strontium vanadate nanoparticles embedded graphitic carbon nitride (g-C3N4/Sr2V2O7) was facilely prepared in situ via a hydrothermal method. It was shown that the Sr2V2O7 nanoparticles implanted into g-carbon nitride had a small size and high distribution. Importantly, compared with some other photocatalysts, the as-prepared g-C3N4/Sr2V2O7 nanohybrid showed excellent photocatalytic activity for reduction of Cr(VI), and as high as 99% efficiency for Cr(VI) reduction (100 mg L−1) was reached within 8 min. Moreover, its activity was hardly changed after five cycles, demonstrating that the developed g-C3N4/Sr2V2O7 nanohybrid was highly stable and promising an efficacious disposal of Cr(VI) in water. It was confirmed that the improved charge separation owing to more nitrogen vacancies in the hybrid was the main reason for the improved performance of the g-C3N4-Sr2V2O7 nanohybrid.
Introduction
Nowadays, heavy metal ions in water have received extensive attention because of their serious harm to human and animal health. Among these ions, Cr(VI) is one of the most commonly found toxic ions in wastewater,1 which seriously threatens ecological systems and could cause great damage to human health and induce many diseases such as cancer, dermatitis, liver damage, and inherited gene deficiency.2 Therefore, eliminating Cr(VI) from aquatic environments has drawn much attention all around the world.In those technologies for Cr(VI) removal,3 photocatalytic technology plays a dominant role in solving the problem of environmental pollution because of its simple operation that is clean, and has high repeatability.4 As a significant organic semiconductor photocatalyst, g-C3N4 has been widely used in H2 evolution,5 pollutants degradation,6 CO2 reduction,7 and other fields due to its facile preparation, nontoxicity, excellent electronic properties. Nevertheless, the photocatalytic activity of single g-C3N4 is generally undesirable as a result of fast e−/h+ recombination.8
Several strategies have been developed to facilitate separation and transfer of photoinduced charges, such as doping, coupling with other semiconductors.9 For instance, 95% of Cr(VI) can be removed by porous (P,Mo)-g-C3N4 prepared using a polycondensation strategy under sunlight irradiation for 120 min.10 A phosphorus-doped g-C3N4/SnS synthesized via hydrothermal method can completely remove 100 mg L−1 Cr(VI) after irradiation for 60 min.11 However, tedious synthetic process12 or quick photocorrosion of some metal sulfides restricts their application in Cr(VI) removal. Moreover, the stability of some common photocatalysts and the degradation rate towards Cr(VI) are still unsatisfying. Therefore, it is highly challenging to develop effective and reusable g-C3N4-based materials for photocatalytic reduction of Cr(VI).
Metal vanadates possessing high thermal stability have received significant interest due to their potential application in chemical sensors, transparent conductors and photocatalysts.13 As one of the important vanadates, strontium vanadate is widely used in the field of white-light-emitting devices and microwave-dielectrics.14 While there is few application in photocatalysis. Interestingly, the result of electronic structure calculation confirms that the valence band of Sr2V2O7 is mostly supported by oxygen atoms, and the bottom of the conduction band is mostly composed of the d orbitals of vanadium atoms.14 And the 3d orbital of vanadium is generally situated below the analogous d orbitals of some transition metals, and the bottom of conduction band is decreased.15 Therefore, Sr2V2O7 could be an excellent candidate for e−/h+ separation if a heterojunction system composed of g-C3N4 and Sr2V2O7 was smartly constructed in that it would greatly boost the charge transmission.
Herein, by hydrothermal method, small sized Sr2V2O7 is evenly in situ incorporated in g-C3N4. The as-prepared g-C3N4/Sr2V2O7 nanohybrid is characterized, and its performance is evaluated by photocatalytic reduction of Cr(VI). Moreover, the durability and stability of the g-C3N4/Sr2V2O7 hybrid are examined by consecutive cycling experiment. The research could pave a new way for fabricating highly active and reusable semiconductor photocatalysts for the removal of heavy metal ions in wastewater.
Experimental
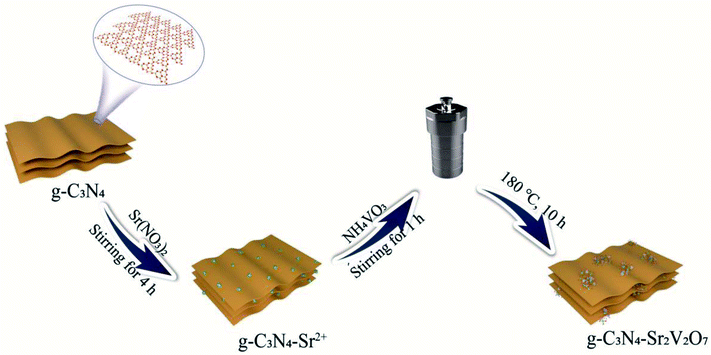
Synthesis of the g-C3N4-Sr2V2O7
g-C3N4 (0.075 g) prepared by the reported method16 was dispersed into DI water (140 mL). Sr(NO3)2 (10 mL, 0.01 mol L−1) was slowly dropped into the g-C3N4 dispersion, and stirred for 4 h. Then the separated solid (g-C3N4-Sr2+) was mixed with 70 mL DI water, and NH4VO3 (5 mL, 0.0132 mol L−1) was put in and continuously reacted for another 1 h. After that, the mixture was put into a stainless Teflon-lined autoclave and heated at 180 °C for 10 h. It was naturally cooled to 25 °C. The solid (g-C3N4-Sr2V2O7 nanohybrid) was washed thoroughly with DI water, and dried (Scheme 1).Characterizations
Crystal structure of the samples was performed using Bruker D8 Advance X-ray diffractometer (XRD) equipped with Cu Kα irradiation (Germany). The interaction in materials were measured on a Thermo ESCALAB 250 X-ray photoelectron spectrometer (XPS) with a monochromatic X-ray source (Al Kα hν = 1486.6 eV) (USA). The light adsorption property was measured with a UV-3900 spectrophotometer (Japan). The morphology was observed by transmission electron microscopy (TEM, JEM-1400F, JEOL, Japan). The element maps were analyzed by a scanning electron microscope (SEM, S-3400N, Hitachi, Japan). The electron paramagnetic resonance (EPR) measurements were carried out on a Bruker ESP A300 spectrometer (Germany). The photoluminescence (PL) spectra were measured with an FL-4600 fluorescence spectrophotometer (Japan).Photocatalytic measurement
Cr(VI) (with K2Cr2O7 as Cr(VI) resource) reduction with the g-C3N4-Sr2V2O7 nanohybrid as a photocatalyst was conducted in a quartz reactor under a 300 W xenon lamp. In order to reach the adsorption/desorption equilibrium, in each photocatalytic test, before irradiation, the mixture containing K2Cr2O7 (represented by Cr(VI), 40 mL, 100 mg L−1) aqueous solution and tartaric acid (30 mg) as well as g-C3N4-Sr2V2O7 nanohybrid (10 mg) was stirred continuously for 1 h. During photocatalytic reaction, the residual Cr(VI) in the solution was monitored by a UV-vis spectrophotometer. Its concentration was obtained according to the absorbance of Cr(VI) at 540 nm and Lambert Beer's law.Result and discussion
The characterization of the nanohybrid
XRD result of the as-prepared nanohybrid is displayed in Fig. 1A, a peak at 27.4° corresponds to (002) crystal plane of g-C3N4.17 While the diffraction peaks appeared at 2θ = 33.2° and 45.3° are originated from the (215) and (1012) planes of Sr2V2O7, respectively (JCPDS No. 32-1268),18 demonstrating that the Sr2V2O7 is bound in the g-C3N4. In addition, the peaks of Sr2V2O7 are weaker, which could be attributed to lower content (about 0.12 wt% measured by XPS), small size or high dispersion of Sr2V2O7 in the nanohybrid. The morphology of the as-synthesized sample is studied by SEM and TEM. It shows some thicker sheet-like structures (Fig. 1B–D). The thickness of the blocky structure in SEM is about 0.92 μm measured by Digital Micrograph 3.9, and the thinner sheet-like structure is about 15.40 nm as observed in TEM image. Besides the sheet-like structure, no other morphology or crystal lattices are displayed. It is suggested that a small amount of Sr2V2O7 is evenly distributed in the g-C3N4, which has little influence on the morphology of g-C3N4. The result is consistent with that in XRD. The Sr2V2O7 in the sample is further identified by SEM mapping and XPS followed.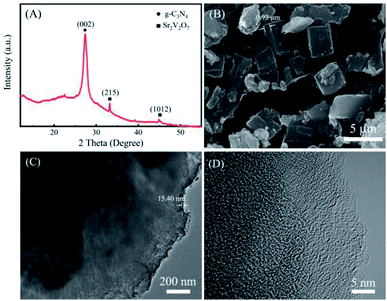 | ||
| Fig. 1 The structure and morphology of the g-C3N4-Sr2V2O7 nanohybrid. (A) XRD, (B) SEM, (C) TEM, (D) HRTEM. | ||
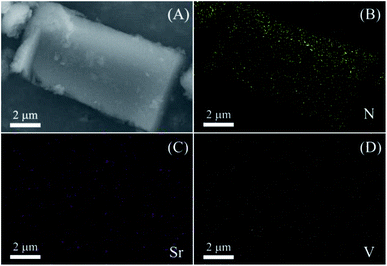
To further confirm the elemental distribution in the nanohybrid, the energy-filtered SEM image and elemental mappings for the nanohybrid are taken. In Fig. 2, N, Sr and V can be observed in their mappings, which are matched with the theoretical composition of the obtained g-C3N4-Sr2V2O7 nanohybrid. N element comes from g-C3N4, Sr and V elements come from the embedded Sr2V2O7. Moreover, as displayed in Fig. 2C and D, the density of Sr and V is obviously lower than N, and the distribution of Sr and V mostly conforms to that of the N, indicating that the Sr2V2O7 is relatively evenly implanted in the g-C3N4 hybrid.
According to the XPS shift in binding energy, the interaction among materials can be investigated. To confirm the interaction between Sr2V2O7 and graphitic carbon nitride, XPS analysis is performed. In Fig. 3A, C, N, O, Sr and V elements in the g-C3N4-Sr2V2O7 can be observed, which is consistent with those in Fig. 2. In C 1s spectrum of pristine g-C3N4 (Fig. 3B), the peaks at 287.68 and 284.38 eV are assigned to sp2 hybridized carbon atom and the surface carbon, respectively.19 The peak corresponding to C–N in the g-C3N4-Sr2V2O7 is shifted toward lower binding energy relative to pristine g-C3N4. In Fig. 3C, the signals of N 1s in pristine g-C3N4 can be fitted into three peaks at 400.73, 399.18, and 398.18 eV, which are assigned to N–H, N–C3, and C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, respectively.20 When Sr2V2O7 is embedded into the graphitic carbon nitride, the peaks of C–N
C, respectively.20 When Sr2V2O7 is embedded into the graphitic carbon nitride, the peaks of C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–H slightly shift towards lower binding energy, while the peak of the N–C3 shifts towards higher energy, which are attributed to strong interaction between strontium vanadate and graphitic carbon nitride in the nanohybrid. However, the low signal-to-noise ratio of Sr and V spectra is observed in Fig. 3D and E, which could be attributed to the low content and lower sensitivity of Sr and V.
C and N–H slightly shift towards lower binding energy, while the peak of the N–C3 shifts towards higher energy, which are attributed to strong interaction between strontium vanadate and graphitic carbon nitride in the nanohybrid. However, the low signal-to-noise ratio of Sr and V spectra is observed in Fig. 3D and E, which could be attributed to the low content and lower sensitivity of Sr and V.
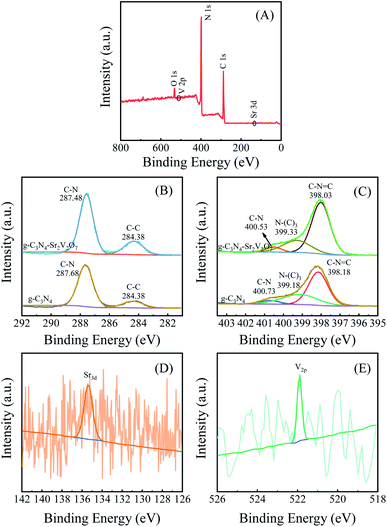 | ||
| Fig. 3 (A) Survey XPS of the g-C3N4-Sr2V2O7. The contrasting HRXPS of C 1s (B) and N 1s (C) in g-C3N4-Sr2V2O7 and g-C3N4. The HRXPS of Sr 3d (D) and V 2p (E) in g-C3N4-Sr2V2O7. | ||
Photocatalytic Cr(VI) reduction
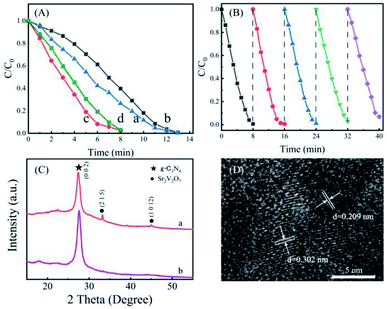
Performance of the prepared g-C3N4-Sr2V2O7 nanohybrid is investigated by photocatalytic reduction of Cr(VI). Due to low content (only 0.12 wt%) of Sr2V2O7 in the g-C3N4, its role in the composite photocatalyst is similar to a co-catalyst. Therefore, in photocatalytic measurement, the photocatalytic activity of the g-C3N4-Sr2V2O7 nanohybrid was mainly compared with that of g-C3N4. Fig. 4A shows the time course of C/C0 of Cr(VI) (C0 and C are the concentrations of Cr(VI) at adsorption–desorption equilibrium and irradiated for t time, respectively). It is reported that tartaric acid can show photocatalytic activity for Cr(VI) reduction.21 In this experiment, without any photocatalysts, only with tartaric acid, it needs about 13 min for complete reduction of Cr(VI). However, after pure g-C3N4 was introduced in the solution, its photocatalytic rate is lower than that only with tartaric acid, especially in the first ten minutes. Surprisingly, after the g-C3N4-Sr2V2O7 is added, under same conditions, 100% Cr(VI) was quickly reduced within 8 min. The reduction time of Cr(VI) is decreased about 5 min compared with that of pure g-C3N4. When the content of Sr2V2O7 is decreased from 0.12 wt% to 0.06 wt%, as shown in Fig. 4A(d), photocatalytic activity of nanohybrid is decreased. However, it is still higher than that of pure g-C3N4. It is indicated that the photocatalytic performance of graphitic carbon nitride can be effectively improved by embedded strontium vanadate.A good photocatalyst, besides its activity, its stability is also an important parameter that needs to be considered in practical applications. Therefore, as an active photocatalyst for Cr(VI) removal, the reusability of the g-C3N4-Sr2V2O7 hybrid is evaluated by five successive recycling tests. In the experiment, after the first photocatalytic reaction, the solution was removed by centrifugation, and the fresh Cr(VI) solution (100 mg L−1, 40 mL) was added for the next cycle. As displayed in Fig. 4B, after 5 recycles, the photocatalytic performance of the nanohybrid is changed little, and almost 100% Cr(VI) is reduced in each recycle. TEM and powder XRD after recycling experiment were measured. As shown in Fig. 4C and D, after recycling experiment, the intensity of diffraction peaks of Sr2V2O7 in the nanohybrid is decreased. It is deduced that some Sr2V2O7 fall off the nanohybrid during recycling. In the TEM image of the g-C3N4-Sr2V2O7 nanohybrid after recycling experiment, some crystal planes ((1012), (215)) corresponding to Sr2V2O7 are shown, which cannot be observed in TEM image of the nanohybrid before recycling experiment (Fig. 1D). It is indicated that some Sr2V2O7 particles grow up, and the crystallinity is improved during recycling. The two aspects are opposite to the photocatalytic activity. Subsequently, the photocatalytic activity of nanohybrid changes little after five recycling. The result demonstrates that the g-C3N4-Sr2V2O7 nanohybrid in situ prepared by hydrothermal method is with relatively higher stability, and is an effective and potential material for photocatalytic Cr(VI) removal.
For photocatalytic reduction of Cr(VI), many materials can be used as photocatalysts. As shown in Table 1, using the g-C3N4-Sr2V2O7 hybrid as the photocatalyst, in a shorter time (8 min), Cr(VI) reduction can be completed. Moreover, the Cr(VI) with higher concentration can be reduced by less dosage of the g-C3N4-Sr2V2O7 nanohybrid. It further demonstrates that the hybrid is a more active photocatalyst for Cr(VI) removal.
| References | Materials | Dosage of catalyst (mg mL−1) | The concentration of Cr(VI) (mg L−1) | Time (min) and efficiency (%) | Light source |
|---|---|---|---|---|---|
| Ref. 22 | TiO2/CN/rGO | 0.5 | 100 | 240 min, 97% | 300 W Xe lamp |
| Ref. 23 | Biochar-g-C3N4 microspheres | 40 | 50 | 240 min, 100% | 300 W Xe lamp |
| Ref. 24 | Br-doped g-C3N4 | 1 | 20 | 120 min, 62% | 300 W Xe lamp |
| Ref. 10 | (P,Mo)-CN | 1.25 | 100 | 120 min, 95% | 300 W Xe lamp |
| Ref. 25 | CaFe2O4/g-C3N4/CNT | 1 | 10 | 120 min, 97% | 300 W Xe lamp |
| Ref. 26 | CNT@MoS2/SnS2 | 1 | 50 | 90 min, 100% | 300 W Xe lamp |
| Ref. 11 | P-CN/SnS | 0.5 | 100 | 60 min, 100% | 300 W Xe lamp |
| Ref. 27 | γ-CD/MoS2/g-C3N4 | 0.4 | 10 | 50 min, 81% | 300 W Xe lamp |
| Ref. 28 | g-C3N4@bio-Fe(III)-hydroxysulfate | 0.2 | 20 | 20 min, 100% | 300 W Xe lamp |
| In this paper | g-C3N4-Sr2V2O7 | 0.25 | 100 | 8 min, 99% | 300 W Xe lamp |
To explore the reason for the improved photocatalytic performance in the g-C3N4-Sr2V2O7, the light adsorption property of the samples is studied. Clearly, in Fig. 5A, the g-C3N4 shows an absorption edge at ca. 468 nm. The corresponding band gap is 2.76 eV (Fig. 5B), which is in agreement with the reported result.29 And the nanohybrid displays comparable light-absorption, absorption edge is at 459 nm, and the corresponding band gap is 2.84 eV, showing that light adsorption and band gap of graphitic carbon nitride is changed little by the incorporation of Sr2V2O7.
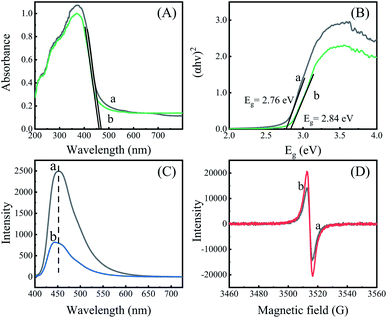 | ||
| Fig. 5 (A) Solid diffuse reflectance spectra; (B) Tauc plots; (C) fluorescence spectra, λex = 380 nm; (D) EPR spectra. (a) g-C3N4, (b) g-C3N4-Sr2V2O7. | ||
In addition, fluorescence spectra are measured in order to explore charge separation in the nanohybrid. Generally, a higher emission indicates more e−/h+ recombination and poorer photocatalytic performance.30 It can be seen in Fig. 5C, g-C3N4 shows a high emission at 453 nm, while the g-C3N4-Sr2V2O7 exhibits a weaker emission. Besides, an about 9 nm blue-shift is seen in the emission spectrum of the nanohybrid, suggesting that the embedding of strontium vanadate in the graphitic carbon nitride plays an important role in inhibiting recombination of e−/h+ pairs due to strong interaction between strontium vanadate and graphitic carbon nitride. It is very advantageous to increase photocatalytic performance of the g-C3N4-Sr2V2O7 nanohybrid.
To better substantiate and comprehend the charge separation in the nanohybrid, the EPR measurements are performed. In Fig. 5D, only one single Lorentzian line at g = 2.003 is shown for the two samples. It is ascribed to nitrogen vacancies.31 In the nanohybrid, the EPR signal is increased compared with that of the graphitic carbon nitride, suggesting more nitrogen vacancies generated in the nanohybrid. According to the results in Fig. 5, it is concluded that more nitrogen vacancies generated in the g-C3N4-Sr2V2O7 could be the intrinsic reason for increased charge separation and photocatalytic performance in the nanohybrid.
Conclusions
g-C3N4-Sr2V2O7 nanohybrid was smartly in situ synthesized by hydrothermal method. The nanohybrid with intimate contact showed fast and highly efficient removal of Cr(VI). Moreover, its photocatalytic performance was hardly changed after five successive recycling tests. The lower fluorescence and increased EPR signals achieved in the nanohybrid evidenced that highly efficient charge separation originated from more nitrogen vacancies in the nanohybrid was the main reason for the increased photocatalytic performance in the g-C3N4-Sr2V2O7. The work will provide an idea for designing a greatly preferable and recyclable photocatalyst for the removal of other heavy metal ions in wastewater.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 21771125, 21301118 and 21305092).References
- V. E. Pakade, N. T. Tavengwa and L. M. Madikizela, RSC Adv., 2019, 9, 26142–26164 RSC.
- S.-S. Wu, W.-C. Hou and D. K. Wang, Environ. Sci.: Nano, 2020, 7, 2399–2409 RSC.
- C. Yang, T. Ju, X. Wang, Y. Ji, C. Yang, H. Lv, Y. Wang, W. Dong, F. Dang, X. Shi, W. Wang and Y. Fan, RSC Adv., 2020, 10, 10612–10623 RSC.
- S. M. El-Sheikh, A. B. Azzam, R. A. Geioushy, F. M. El Dars and B. A. Salah, J. Alloys Compd., 2020, 13, 157513–157524 Search PubMed.
- P. Zhou, X. Meng, L. Li and T. Sun, J. Alloys Compd., 2020, 827, 154259–154265 CrossRef CAS.
- X. Bai, Y. Li, L. Xie, X. Liu, S. Zhan and W. Hu, Environ. Sci.: Nano, 2019, 6, 2850–2862 RSC.
- L. Zhao, Z. Zhao, Y. Li, X. Chu, Z. Li, Y. Qu, L. Bai and L. Jing, Nanoscale, 2020, 12, 10010–10018 RSC.
- K. Wang, Y. Li, J. Li and G. Zhang, Appl. Catal., B, 2020, 263, 117730–117739 CrossRef CAS.
- J. Lin, J. Hu, C. Qiu, H. Huang, L. Chen, Y. Xie, Z. Zhang, H. Lin and X. Wang, Catal. Sci. Technol., 2019, 9, 336–346 RSC.
- D. Chen, J. Liu, Z. Jia, J. Fang, F. Yang, Y. Tang, K. Wu, Z. Liu and Z. Fang, J. Hazard. Mater., 2019, 361, 294–304 CrossRef CAS PubMed.
- H. Sun and S.-J. Park, Appl. Surf. Sci., 2020, 531, 147325 CrossRef CAS.
- C. Yang, Z. Xue, J. Qin, M. Sawangphruk, S. Rajendran, X. Zhang and R. Liu, J. Phys. Chem. C, 2019, 123, 4795–4804 CrossRef CAS.
- G. Zhan, W. C. Ng, S. N. Koh and C.-H. Wang, ACS Sustainable Chem. Eng., 2018, 6, 2292–2301 CrossRef CAS.
- A. Sharma, M. Varshney, K.-H. Chae and S. O. Won, RSC Adv., 2018, 8, 26423–26431 RSC.
- S. Zhan, F. Zhou, N. Huang, Y. Yin, M. Wang, Y. Yang and Y. Liu, J. Mol. Catal. A: Chem., 2015, 401, 41–47 CrossRef CAS.
- X. Zhao, Y. Zhang, X. Zhao, X. Wang, Y. Zhao, H. Tan, H. Zhu, W. Ho, H. Sun and Y. Li, ACS Appl. Mater. Interfaces, 2019, 11, 27934–27943 CrossRef CAS PubMed.
- B. Li, Q. Fang, Y. Si, T. Huang, W.-Q. Huang, W. Hu, A. Pan, X. Fan and G.-F. Huang, Chem. Eng. J., 2020, 397, 125470–125482 CrossRef CAS.
- S. K. Gupta, K. Sudarshan and R. M. Kadam, Mater. Des., 2017, 130, 208–214 CrossRef CAS.
- K. Wang, L. Jiang, X. Wu and G. Zhang, J. Mater. Chem. A, 2020, 8, 13241–13247 RSC.
- H. Hu, J. Hu, X. Wang, J. Gan, M. Su, W. Ye, W. Zhang, X. Ma and H. Wang, Catal. Sci. Technol., 2020, 10, 4712–4725 RSC.
- Z. Xu, Y. Yu, D. Fang, J. Liang and L. Zhou, Mater. Chem. Phys., 2016, 171, 386–393 CrossRef CAS.
- G. Li, Y. Wu, M. Zhang, B. Chu, W. Huang, M. Fan, L. Dong and B. Li, Ind. Eng. Chem. Res., 2019, 58, 8979–8989 CrossRef CAS.
- Q. Jin, G. Xie, X. Cai, X. Hu, H. Wang, G. Qiu, W. Wang, D. Zhou, H. Huo, X. Tan and Y. Zhao, RSC Adv., 2020, 10, 6121–6128 RSC.
- M. Wang, Y. Zeng, G. Dong and C. Wang, Chin. J. Catal., 2020, 41, 1498–1510 CrossRef CAS.
- F. Liu, S. Dong, Z. Zhang, X. Li, X. Dai, Y. Xin, X. Wang, K. Liu, Z. Yuan and Z. Zheng, RSC Adv., 2019, 9, 25750–25761 RSC.
- J. Wan, P. Xue, R. Wang, L. Liu, E. Liu, X. Bai, J. Fan and X. Hu, Appl. Surf. Sci., 2019, 483, 677–687 CrossRef CAS.
- Z. Wu, X. He, Y. Xue, X. Yang, Y. Li, Q. Li and B. Yu, Chem. Eng. J., 2020, 399, 125747–125758 CrossRef CAS.
- X. Wang, Y. Xie, X. Chen, X. Zhou, W. Hu, P. Li, Y. Li, Y. Zhang and Y. Wang, Chem. Eng. J., 2020, 398, 125632–125644 CrossRef CAS.
- X. Wu, H. Ma, W. Zhong, J. Fan and H. Yu, Appl. Catal., B, 2020, 271, 118899–118906 CrossRef CAS.
- M. Zhou, G. Dong, F. Yu and Y. Huang, Appl. Catal., B, 2019, 256, 117825–117835 CrossRef CAS.
- X. a. Dong, J. Li, Q. Xing, Y. Zhou, H. Huang and F. Dong, Appl. Catal., B, 2018, 232, 69–76 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |