Open Access Article
Open Access ArticleOn-demand retrieval of cells three-dimensionally seeded in injectable thioester-based hydrogels†
Shohei Ishikawaa,
Hiroyuki Kamata *a,
Ung-il Chungabc and
Takamasa Sakai
*a,
Ung-il Chungabc and
Takamasa Sakai *ac
*ac
aDepartment of Bioengineering, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan. E-mail: sakai@tetrapod.t.u-tokyo.ac.jp; kamata@tetrapod.t.u-tokyo.ac.jp
bCenter for Disease Biology and Integrative Medicine, School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan
cDepartment of Materials Engineering, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan
First published on 5th July 2021
Abstract
Scaffold systems that can easily encapsulate cells and safely retrieve them at the desired time are important for the advancement of cell-based medicine. In this study, we designed and fabricated thioester-based poly(ethylene glycol) (PEG) hydrogels with injectability and on-demand degradability as new scaffold materials for cells. Hydrogels can be formed in situ within minutes via thioester cross-linking between PEG molecules and can be degraded under mild conditions in response to L-cysteine molecules through thiol exchange occurring at the thioester linkage. Various cell experiments, especially with sucrose, which enables the adjustment of the osmotic pressure around the cells, showed that the damage to the cells during encapsulation and degradation was minimal, indicating the capability of on-demand retrieval of intact cells. This hydrogel system is a versatile tool in the field of cell-based research and applications such as tissue regeneration and regenerative medicine.
Synthetic three-dimensional (3D) extracellular matrices (ECMs) are one of the core technologies in the field of cell-based medicine.1 In particular, injectable hydrogels, which can be injected as a liquid, molded into any desired shape, and have physical properties (e.g., elasticity, flexibility, and water content) similar to those of biological tissues, are convenient for both in vivo applications and in vitro studies.2 On-demand degradation of the formed hydrogels after functioning as a scaffold is challenging but necessary for some applications.3,4 One such potential application is regenerative medicine, where cells are artificially differentiated in a 3D environment, and the differentiated cells are purified and transplanted with scaffolds.5 Such degradable hydrogels are also important as research tools for drug discovery or molecular biology, where drug stimuli are applied to cells in a 3D environment, and the stimulated cells can then be retrieved and evaluated by means of conventional techniques. Several hydrogel scaffolds have been developed to realize on-demand degradation; however, typical stimuli include the following, each of which has unresolved challenges: (1) hydrolysis;6–8 degradation of typical hydrolyzable linkages is very slow at neutral pH and biological temperatures, necessitating a change in pH or an increase in temperature; (2) enzymatic degradation;9,10 for instance, collagenase can degenerate the ECM produced by cells, and residual enzymes can cause cell death, posing problems for protein-related analysis; (3) UV-induced cleavage;11 potential damage to cells and DNA cannot be ignored.12 As such, there is no available tool that can encapsulate cells in a 3D manner, mold them into any shape, and retrieve the cells at any given time with minimal damage to the cells. To address this gap in the knowledge, herein, we propose an injectable hydrogel platform that enables both 3D encapsulation and on-demand retrieval of cells, which would potentially be beneficial for cell-based therapy where cells are used with scaffolds.
The hydrogels designed and fabricated here are based on completely synthetic poly(ethylene glycol) (PEG), which is often used as a base material for biomaterials because of its good biocompatibility.13,14 In particular, hydrogels assembled with 4-arm PEGs are widely used by researchers worldwide because of their highly predictable physical properties.15–18 However, PEG itself is not easily degraded under normal physiological conditions; therefore, on-demand degradation cannot be realized without a special mechanism. To overcome this problem, we introduced thioester linkages into cross-linking points between the PEG molecules. Thioester cross-linking can be readily achieved by mixing two types of aqueous solutions of PEG molecules bearing either sulfhydryl or succinimidyl groups at the ends,19,20 hereafter called Tetra-PEG–SH and Tetra-PEG–NHS, respectively (Fig. 1A). The resulting thioester bonds can be cleaved by simply applying a neutral solution of L-cysteine (L-cys), leading to macroscopic dissolution of the hydrogels (Fig. 1B). The degradation of PEG hydrogels cross-linked with thioester using L-cys is unique in that it does not involve stimuli that may damage cells, such as heat, pH changes, or UV irradiation. Similar approaches have been explored in some medical fields;21,22 however, currently, little information is available on their potential as an ECM. Although safe on-demand degradation and cell retrieval have been demonstrated in some self-healing hydrogels based on dynamic covalent bonding,21 such bonding is, in principle, susceptible to dilution, and the spatial and temporal stability of the scaffold in water-rich environments remains a concern.
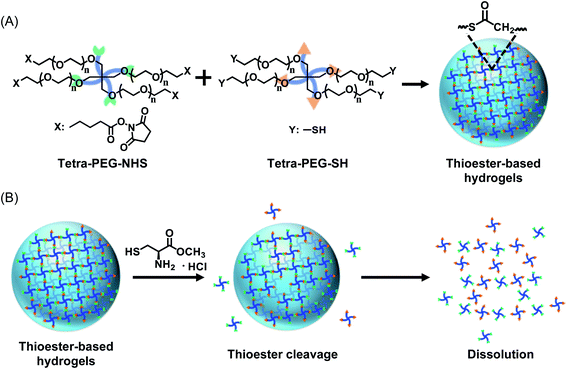 | ||
| Fig. 1 Conceptual drawings of thioester-based hydrogels. (A) Cross-linking chemistry involved in the formation of hydrogels. (B) Degradation of hydrogels by the addition of L-cys. | ||
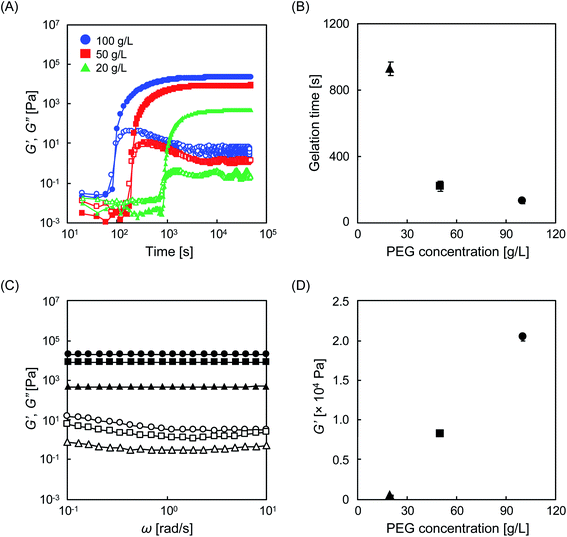
Prior to experiments with cells, the physical properties of the designed materials were evaluated. To demonstrate the injectability, the storage modulus (G′) and loss modulus (G′′) were evaluated by real-time rheological measurements immediately after mixing the two aqueous PEG solutions. In this study, we performed experiments with aqueous PEG solutions at different concentrations (20, 50, and 100 g L−1). Regardless of the concentration of PEG, G′ was smaller than G′′ in the time range of 0 to 100 s, indicating that the mixture is injectable during this time period, and can be administered via a syringe to any location while forming any shape (Fig. 2A and S1†). As time elapsed, a time point emerged at which G′ and G′′ overlapped that is technically defined as the gelation time (tgel). The tgel varied depending on the PEG concentration, with values of 930, 220, and 130 s, for 20, 50, and 100 g L−1, respectively (Fig. 2B). This gelation reaction is based on the formation of thioester bonds between Tetra-PEG–SH and Tetra-PEG–NHS molecules, as previously reported.19,20 To verify this, we performed FT-IR measurements. The characteristic but very weak peak of the S–H stretching at 2600–2550 cm−1 (not observed in Tetra-PEG–NHS) disappeared after the gelation reaction (Fig. S2†), which indirectly suggests the formation of thioester bonds between Tetra-PEG–SH and Tetra-PEG–NHS molecules. The obtained viscoelastic profile was comparable to that of existing injectable hydrogels, which are already used in medical sealants,23 drug carriers,24 and cell scaffolds,25–27 suggesting that this hydrogel could also be suitable as an injectable material. We have shown that in addition to PEG concentration, tgel is dependent on the pH, because, theoretically, a higher degree of protonation of the sulfhydryl group minimizes nucleophilic attack on the succinimidyl group.28 For instance, compared to the gelation behavior at pH 8.2, a longer time is required to form hydrogels at lower pH (Fig. S3†). Frequency sweep tests showed that G′ > G′′ in the angular frequency range of 0.1 to 10 Hz for all tested hydrogels (Fig. 2C), which is a characteristic feature of typical chemical hydrogels, indicating that the cross-linked structure is practically permanent. As the index of elasticity, G′ was approximately 20 kPa for the 100 g L−1 hydrogel, but this value decreased to 8 and 0.4 kPa for the 50 g L−1 and 20 g L−1 hydrogels, respectively (Fig. 2D). It is noteworthy that these values correspond to the range of those for biological soft tissues.29 The effect of the pH of the buffer solution was also examined. The G′ values of the hydrogels prepared at pH 6.7 and 7.8 were 14 and 19 kPa, respectively (Fig. S3C and D†), slightly lower than those obtained at pH 8.2; however, since hydrogels were formed at all tested pH values, they can be used for cell experiments that often involve neutral buffers. This slight decrease is explained by the inactivation of the succinimidyl group, which is prone to hydrolysis, due to a long tgel in aqueous buffers.8
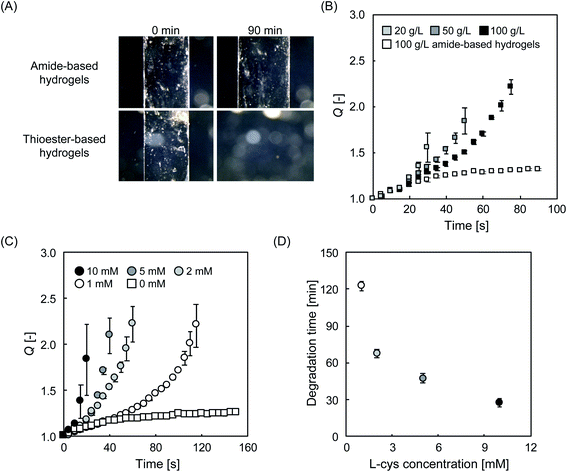
The on-demand degradability of the designed hydrogels was investigated. To evaluate the degradation profile, L-cys was added as a cleavage agent at various concentrations to cylindrical hydrogels in phosphate buffer, and their swelling behavior was carefully observed. In the presence of L-cys, the thioester-linked hydrogels dissolved completely after 90 min (Fig. 3A). In contrast, the amide-linked hydrogel prepared as the control group swelled slightly, but did not dissolve over the same time range. This result strongly suggests that L-cys selectively cleaved the thioesters in the hydrogels. The thioester bond is known to be cleaved via the thiol–thioester exchange reaction (Fig. S4†), not only by L-cys as demonstrated here but also by other thiol compounds with a sulfhydryl group.20 According to the proposed mechanism, when the gel degrades, sulfhydryl groups are generated. However, no characteristic peaks of –SH were observed in the measurement of the degradation products (Fig. S5†). One of the reasons why we failed to observe such peaks may be that the oxidation proceeded in the air and disulfide formation occurred. It is also possible that the reaction was not completed, and –SH groups were not generated in sufficient quantities for detection during FT-IR analysis. Since the degree of swelling (Q) of hydrogels in water is affected by the number of effective cross-linking points,8 the cleavage can be evaluated from a different perspective by observing Q over time. Here, we recorded the change in Q of a hydrogel consisting of amide bonds and the counterparts consisting of thioester bonds at different PEG concentrations. In a phosphate buffer with 5 mM L-cys at pH 7.4 and 25 °C (Fig. 3B), the hydrogel consisting of amide bonds (100 g L−1) swelled gradually, reached an equilibrium state, and remained stable thereafter in the range of Q < 1.5. As these results correspond well with previously reported findings,8 and therefore the experimental system is considered to be reasonable. In stark contrast, the thioester-based hydrogels (20, 50, and 100 g L−1) swelled at faster rates, eventually leading to complete dissolution within 40, 60, and 80 min, respectively (Fig. S6†). The longer degradation time (tdeg) with higher PEG concentrations can be attributed to the fact that, in the current system, the increase in PEG concentration decreases the critical cross-link density to maintain the gel state, resulting in broadening of the gel region in the sol–gel phase diagram.30,31 Similar experiments were performed by fixing the PEG concentration at 100 g L−1 while varying the concentration of L-cys. The tdeg decreased with increasing L-cys content (Fig. 3C and D). A similar trend was observed for the changes in pH and temperature (Fig. S7†).
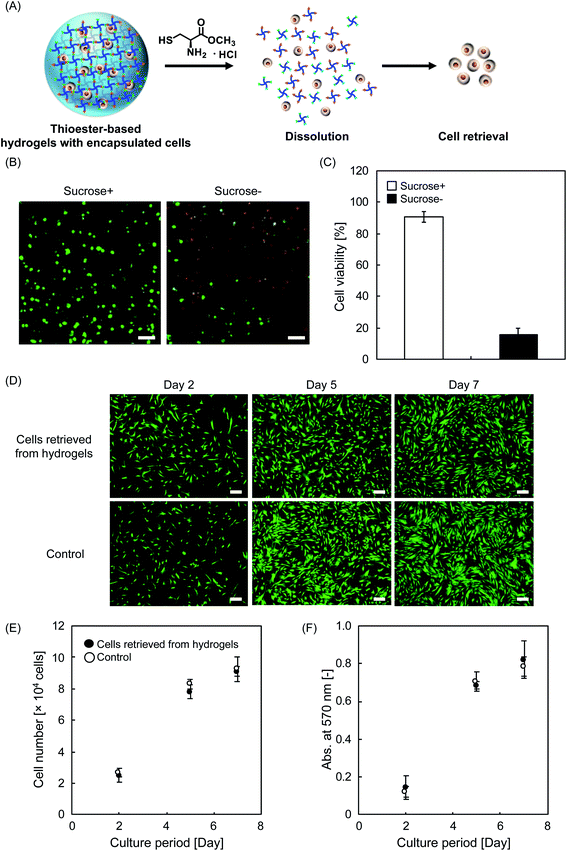
For biological applications, cells can be suspended prior to gelation in this system, where the resulting hydrogel serves as a 3D scaffold for the cells. When needed, the hydrogel could be dissolved by L-cys to retrieve the cells (Fig. 4A). Here, we used human mesenchymal stem/stromal cells (hMSCs), which have recently undergone remarkable development in the field of regenerative medicine because of their multipotency and paracrine effects.29,32,33 First, hMSCs were three-dimensionally encapsulated in hydrogels, and a live/dead assay was then performed to demonstrate cytocompatibility (Fig. 4B). In the absence of special efforts, cell viability was low after encapsulation in hydrogels with a PEG concentration of 100 g L−1. We hypothesized that this low cell viability was due to the difference in osmotic pressure inside and outside the cells suspended in the hydrogels. To protect encapsulated cells from such severe conditions, we added 10% sucrose, which is known to regulate the osmotic pressure between cells and their external environment34 and is often used to prevent the formation of ice crystals that can cause apoptosis during freezing and thawing of cells.35 Even in the presence of sucrose, the physical properties (e.g., tgel and G′) did not significantly change (Fig. S8†), and importantly, the cell viability improved significantly as expected (Fig. 4B and C). To quantitatively evaluate biocompatibility, confocal laser scanning microscopy (CLSM) images of hydrogels with three-dimensionally encapsulated hMSCs were recorded. Counting the number of live cells revealed that the cell viability was as low as 16% in the group without sucrose (Fig. 4C). In marked contrast, the group treated with sucrose showed a very high cell viability of 90%. This protective effect of sucrose was also effective in a variety of solutions, such as Tetra-PEG–NHS, where its activated ester can be cytotoxic in water (Fig. S9A†). The hMSCs encapsulated in the hydrogels were found to survive at a high viability of approximately 90% for at least 120 min (Fig. S9B†). Before the cell retrieval experiments, the effect of L-cys on cell viability was evaluated. Some cells exposed to an aqueous solution containing L-cys were observed as dead cells with a viability of approximately 80%, as determined by trypan blue staining (Fig. S10†), indicating that L-cys can be cytotoxic under certain experimental conditions, which is consistent with previous reports.36 This may be one of the reasons why the current mechanism (i.e., degrading hydrogels with thioester linkages) has not been widely used for cell retrieval. In contrast, in an aqueous solution of L-cys with sucrose, no such tendency of cell death was observed; rather, high viability was maintained. As such, the addition of sucrose can ensure high cell viability during the process of degradation induced by L-cys. Furthermore, in the presence of both L-cys and sucrose, the hydrogel containing the cells was incubated at 37 °C for 60 min, and the encapsulated cells were collected. The retrieved cells were re-seeded onto 48-well plates, and their proliferation was evaluated. Fluorescence images revealed that the hMSCs had qualitatively similar proliferation profiles throughout the cell culture period, regardless of their origin, that is, from hydrogels or not (Fig. 4D). To evaluate the results more quantitatively, we counted the number of cells and compared the mitochondrial activity between the two groups. In both conditions, the data were comparable (Fig. 4E and F), suggesting that the retrieved cells were intact throughout the processes of encapsulation and degradation. The encapsulated (or subsequently retrieved) cells in the system may potentially be utilized for further biological applications where safe on-demand degradation is required, such as the bioanalysis of differentiated cells,37 coculture with other types of cells (e.g., xenogeneic cells),38 and transplantation as a cell–matrix composite to repair tissue defects.39 These are the areas where conventional scaffolding systems fail due to cellular damage caused by a non-neutral pH,7 high temperature,40 and UV irradiation.11
Conclusions
Scaffold systems that can three-dimensionally encapsulate cells while allowing the cells to be retrieved with minimal damage are extremely rare. With the proposed system, cells can be easily encapsulated three-dimensionally into hydrogels that can be molded into any desired shape or injected as a cell-scaffold composite. The cells can be retrieved by simply applying L-cys compounds. We demonstrated that throughout the process from encapsulation to retrieval, the proliferation activity of the cells was not impaired, indicating that damage to the cells was minimal. This system is expected to be widely used as a basic technology in the future in all fields where cells are used.Author contributions
Conception and design: SI, HK; development of methodology: SI, HK; analysis and interpretation of data: SI, HK; writing, review, and/or revision of the manuscript: SI, HK, UC, and TS.Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) (grant number 20J01344) and Transformative Research Areas B (grant number 20H05732).References
- M. P. Lutolf and J. A. Hubbell, Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering, Nat. Biotechnol., 2005, 23, 47–55, DOI:10.1038/nbt1055.
- L. Yu and J. Ding, Injectable Hydrogels as Unique Biomedical Materials, Chem. Soc. Rev., 2008, 37, 1473–1481, 10.1039/b713009k.
- Q. Xu, C. He, Z. Zhang, K. Ren and X. Chen, Injectable, Biomolecule-Responsive Polypeptide Hydrogels for Cell Encapsulation and Facile Cell Recovery through Triggered Degradation, ACS Appl. Mater. Interfaces, 2016, 8, 30692–30702, DOI:10.1021/acsami.6b08292.
- P. J. LeValley, R. Neelarapu, B. P. Sutherland, S. C. Dasgupta, J. Kloxin and A. M. Kloxin, Photolabile Linkers: Exploiting Labile Bond Chemistry to Control Mode and Rate of Hydrogel Degradation and Protein Release, J. Am. Chem. Soc., 2020, 142, 4671–4679, DOI:10.1021/jacs.9b11564.
- D. Seliktar, Designing Cell-Compatible Hydrogels for Biomedical Applications, Science, 2012, 336, 1124–1128, DOI:10.1126/science.1214804.
- M. B. Browning, S. N. Cereceres, P. T. Luong and E. M. Cosgriff-Hernandez, Determination of the in vivo Degradation Mechanism of PEGDA Hydrogels, J. Biomed. Mater. Res., Part A, 2014, 102, 4244–4251, DOI:10.1002/jbm.a.35096.
- S. Ishikawa, K. Iijima, D. Matsukuma, M. Iijima, S. Osawa and H. Otsuka, An Interpenetrating Polymer Network Hydrogel with Biodegradability Through Controlling Self-assembling Peptide Behavior with Hydrolyzable Cross-linking Networks, Mater. Today Adv., 2021, 9, 100131, DOI:10.1016/j.mtadv.2021.100131.
- X. Li, Y. Tsutsui, T. Matsunaga, M. Shibayama, U. Chung and T. Sakai, Precise Control and Prediction of Hydrogel Degradation Behavior, Macromolecules, 2011, 44, 3567–3571, DOI:10.1021/ma2004234.
- R. Tian, X. Qiu, P. Yuan, K. Lei, L. Wang, Y. Bai, S. Liu and X. Chen, Fabrication of Self-Healing Hydrogels with On-Demand Antimicrobial Activity and Sustained Biomolecule Release for Infected Skin Regeneration, ACS Appl. Mater. Interfaces, 2018, 10, 17018–17027, DOI:10.1021/acsami.8b01740.
- H. Kamata, S. Ashikari-Hada, Y. Mori, A. Azuma and K. Hata, Extemporaneous Preparation of Injectable and Enzymatically Degradable 3D Cell Culture Matrices from an Animal-Component-Free Recombinant Protein Based on Human Collagen Type I, Macromol. Rapid Commun., 2019, 40, 1900127, DOI:10.1002/marc.201900127.
- M. Villiou, J. I. Paez and A. D. Campo, Photodegradable Hydrogels for Cell Encapsulation and Tissue Adhesion, ACS Appl. Mater. Interfaces, 2020, 12, 37862–37872, DOI:10.1021/acsami.0c08568.
- R. P. Sinha and D. P. Häder, UV-induced DNA Damage and Repair: A Review, Photochem. Photobiol. Sci., 2002, 1, 225–236, 10.1039/b201230h.
- B. V. Slaughter, S. S. Khurshid, O. Z. Fisher, A. Khademhosseini and N. A. Peppas, Hydrogels in Regenerative Medicine, Adv. Mater., 2009, 21, 3307–3329, DOI:10.1002/adma.200802106.
- J. Zhu, Bioactive Modification of Poly(ethylene glycol) Hydrogels for Tissue Engineering, Biomaterials, 2010, 31, 4639–4656, DOI:10.1016/j.biomaterials.2010.02.044.
- T. Sakai, T. Matsunaga, Y. Yamamoto, C. Ito, R. Yoshida, S. Suzuki, N. Sasaki, M. Shibayama and U. Chung, Design and Fabrication of a High-Strength Hydrogel with Ideally Homogeneous Network Structure from Tetrahedron-like Macromonomers, Macromolecules, 2008, 41, 5379–5384, DOI:10.1021/ma800476x.
- K. Nishi, K. Fujii, Y. Katsumoto, T. Sakai and M. Shibayama, Kinetic Aspect on Gelation Mechanism of Tetra-PEG Hydrogel, Macromolecules, 2014, 47, 3274–3281, DOI:10.1021/ma500662j.
- S. P. Zustiak and J. B. Leach, Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties, Biomacromolecules, 2010, 11, 1348–1357, DOI:10.1021/bm100137q.
- T. E. Brown and K. S. Anseth, Spatiotemporal Hydrogel Biomaterials for Regenerative Medicine, Chem. Soc. Rev., 2017, 46, 6532–6552, 10.1039/c7cs00445a.
- C. Ghobril, K. Charoen, E. K. Rodriguez, A. Nazarian and M. W. Grinstaff, A Dendritic Thioester Hydrogel Based on Thiol–Thioester Exchange as a Dissolvable Sealant System for Wound Closure, Angew. Chem., Int. Ed., 2013, 52, 14070–14074, DOI:10.1002/anie.201308007.
- M. D. Konieczynska and M. W. Grinstaff, On-Demand Dissolution of Chemically Cross-Linked Hydrogels, Acc. Chem. Res., 2017, 50, 151–160, DOI:10.1021/acs.accounts.6b00547.
- W. Huang, Y. Wang, Z. Huang, X. Wang, L. Chen, Y. Zhang and L. Zhang, On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing, ACS Appl. Mater. Interfaces, 2018, 10, 41076–41088, DOI:10.1021/acsami.8b14526.
- J. Hu, Y. Chen, Y. Li, Z. Zhou and Y. Cheng, A Thermo-degradable Hydrogel with Light-tunable Degradation and Drug Release, Biomaterials, 2017, 112, 133–140, DOI:10.1016/j.biomaterials.2016.10.015.
- Y. Liu, H. Meng, S. Konst, R. Sarmiento, R. Rajachar and B. P. Lee, Injectable Dopamine-Modified Poly(ethylene glycol) Nanocomposite Hydrogel with Enhanced Adhesive Property and Bioactivity, ACS Appl. Mater. Interfaces, 2014, 6, 16982–16992, DOI:10.1021/am504566v.
- N. K. Singh and D. S. Lee, In situ Gelling pH- and Temperature-Sensitive Biodegradable Block Copolymer Hydrogels for Drug Delivery, J. Controlled Release, 2014, 193, 214–227, DOI:10.1016/j.jconrel.2014.04.056.
- T. C. Tseng, L. Tao, F. Y. Hsieh, Y. Wei, I. M. Chiu and S. H. Hsu, An Injectable, Self-Healing Hydrogel to Repair the Central Nervous System, Adv. Mater., 2015, 27, 3518–3524, DOI:10.1002/adma.201500762.
- S. Ishikawa, K. Iijima, D. Matsukuma, Y. Asawa, K. Hoshi, S. Osawa and H. Otsuka, Interpenetrating Polymer Network Hydrogels via a One-Pot and in situ Gelation System Based on Peptide Self-Assembly and Orthogonal Cross-Linking for Tissue Regeneration, Chem. Mater., 2020, 32, 2353–2354, DOI:10.1021/acs.chemmater.9b04725.
- S. Ishikawa, D. Matsukuma, K. Iijima, M. Iijima, S. Osawa and H. Otsuka, N-Hydroxysuccinimide Bifunctionalized Triblock Cross-Linker Having Hydrolysis Property for a Biodegradable and Injectable Hydrogel System, ACS Biomater. Sci. Eng., 2019, 5, 5759–5769, DOI:10.1021/acsbiomaterials.9b00218.
- M. Kurakazu, T. Katashima, M. Chijiishi, K. Nishi, Y. Akagi, T. Matsunaga, M. Shibayama, U. Chung and T. Sakai, Evaluation of Gelation Kinetics of Tetra-PEG Gel, Macromolecules, 2010, 43, 3935–3940, DOI:10.1021/ma100176f.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Matrix Elasticity Directs Stem Cell Lineage Specification, Cell, 2006, 126, 677–689, DOI:10.1016/j.cell.2006.06.044.
- I. Fujinaga, T. Yasuda, M. Asai, U. Chung, T. Katashima and T. Sakai, Cluster Growth from a Dilute System in a Percolation Process, Polym. J., 2020, 52, 289–297, DOI:10.1038/s41428-019-0279-z.
- Y. Yoshikawa, N. Sakumichi, U. Chung and T. Sakai, Connectivity Dependence of Gelation and Elasticity in AB-type Polymerization: An Experimental Comparison of the Dynamic Process and Stoichiometrically Imbalanced Mixing, Soft Matter, 2019, 15, 5017–5025, 10.1039/C9SM00696F.
- S. Gobaa, S. Hoehnel, M. Roccio, A. Negro, S. Kobel and M. P. Lutolf, Artificial Niche Microarrays for Probing Single Stem Cell Fate in High Throughput, Nat. Methods, 2011, 8, 949–955, DOI:10.1038/nmeth.1732.
- R. Hass, C. Kasper, S. Böhm and R. Jacobs, Different Populations and Sources of Human Mesenchymal Stem Cells (MSC): A Comparison of Adult and Neonatal Tissue-derived MSC, Cell Commun. Signaling, 2011, 9, 12, DOI:10.1186/1478-811X-9-12.
- K. DeCourcy and B. Storrie, Osmotic Swelling of Endocytic Compartments Induced by Internalized Sucrose is Restricted to Mature Lysosomes in Cultured Mammalian Cells, Exp. Cell Res., 1991, 192, 52–60, DOI:10.1016/0014-4827(91)90156-O.
- K. Pollock, G. Yu, R. Moller-Trane, M. Koran, P. I. Dosa, D. H. McKenna and A. Hubel, Combinations of Osmolytes, Including Monosaccharides, Disaccharides, and Sugar Alcohols Act in Concert During Cryopreservation to Improve Mesenchymal Stromal Cell Survival, Tissue Eng., Part C, 2016, 22, 999–1008, DOI:10.1089/ten.tec.2016.0284.
- R. S. H. Chang, J. C. W. Lee, S. Pedron, B. A. C. Harley and S. A. Rogers, Rheological Analysis of the Gelation Kinetics of an Enzyme Cross-linked PEG Hydrogel, Biomacromolecules, 2019, 20, 2198–2206, DOI:10.1021/acs.biomac.9b00116.
- B. Çelebi, A. E. Elçin and Y. M. Elçin, Proteome Analysis of Rat Bone Marrow Mesenchymal Stem Cell Differentiation, J. Proteome Res., 2010, 9, 5217–5227, DOI:10.1021/pr100506u.
- M. S. K. Chong, J. Lim, J. Goh, M. W. Sia, J. K. Y. Chan and S. H. Teoh, Cocultures of Mesenchymal Stem Cells and Endothelial Cells As Organotypic Models of Prostate Cancer Metastasis, Mol. Pharmaceutics, 2014, 11, 2126–2133, DOI:10.1021/mp500141b.
- H. Park, Y. Jin, J. Shin, K. Yang, C. Lee, H. S. Yang and S. Cho, Catechol-Functionalized Hyaluronic Acid Hydrogels Enhance Angiogenesis and Osteogenesis of Human Adipose-Derived Stem Cells in Critical Tissue Defects, Biomacromolecules, 2016, 17, 1939–1948, DOI:10.1021/acs.biomac.5b01670.
- X. Li, S. Kondo, U. Chung and T. Sakai, Degradation Behavior of Polymer Gels Caused by Nonspecific Cleavages of Network Strands, Chem. Mater., 2014, 26, 5352–5357, DOI:10.1021/cm502480f.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01934a |
| This journal is © The Royal Society of Chemistry 2021 |